Abstract
Objective
Osteoarthritis (OA) is a painful degenerative disease of the whole joint structure, including articular cartilage, synovial fluid, and subchondral bone. Hyaluronic acid (HA), an anionic non-sulfated glycosaminoglycan, is commonly used for intra-articular (IA) treatment in OA, while bisphosphonates (BPs) are anti-resorptive drugs that act on the bone. Here, a novel conjugate with a covalent and hydrolysable linker between HA and alendronate (ALD) was designed as an attractive therapeutic strategy for IA drug delivery.
Design
The HA-ALD derivative was synthesized and tested in comparison with a simple mixture of HA and ALD for in vitro ALD release, rheological properties, cytotoxicity towards osteoblasts and chondrocytes and in an in vitro efficacy assay of OA inflammatory model on bovine cartilage explants.
Results
The structure of HA-ALD was elucidated exhibiting no depolymerization and efficient drug incorporation. The controlled ALD release in vitro was slower compared to the simple mixture of HA and ALD; moreover, the derivative showed calcium-tuned rheological properties. The absence of cytotoxicity towards osteoblasts and chondrocytes was shown for up to 7 days, and the viability of chondrocytes was confirmed by fluorescence microscopy. Finally, a reduction in collagen release and MMP-13 expression was measured in the OA inflammatory model.
Conclusion
This new HA-ALD derivative opens the door to a new approach for OA treatment, as it combines viscosupplementation and biological effects of HA with the pharmacological activity of BPs. Prolonged ALD release increased rheological properties and beneficial effect against cartilage degradation make it a promising IA therapy for OA.
Keywords: Hyaluronan, Bisphosphonate, Osteoarthritis, Intra-articular, Alendronate
Graphical abstract
Highlights
-
•
Development of an IA treatment to target OA pathogenesis rather than symptoms.
-
•
A novel hyaluronan-alendronate conjugate was synthesized with a reversible linker.
-
•
The drug delivery system confirmed slow alendronate release.
-
•
The efficacy was demonstrated in an osteoarthritis inflammatory model.
-
•
New intra-articular treatment: viscosupplementation and pharmacological activity.
1. Introduction
Osteoarthritis (OA) is a painful degenerative disease of the whole joint that leads to chronic pain and disability. In 2013, 242 million people suffered from OA of the hip and/or the knee worldwide, and the U.S. economic burden in 2003 was estimated to be approximately $128 billion [1].
OA progression involves all joint structures, including deterioration of articular cartilage, reduction of synovial fluid properties and remodelling of subchondral bone [2,3]. In detail, abnormal subchondral bone remodelling is characterized by altered architecture and flexibility with simultaneous hypomineralization of subchondral cancellous bone, sclerosis of the subchondral plate, thickening of the calcified cartilage layer and invasion with blood vessels [4]. These alterations can lead to sensory innervation [5], osteophyte development, endochondral ossification and the formation of microfractures, cysts, and bone marrow lesions. These changes occur as a result of biochemical crosstalk between subchondral bone osteoblasts and osteoclasts and articular cartilage chondrocytes [6].
Currently available OA treatments, such as oral non-steroidal drugs or intra-articular (IA) corticosteroids, target symptoms rather than the pathogenesis of knee OA [7]. On the other hand, expanding the criteria for total knee (and/or hip) arthroplasty would amplify the economic burden of OA while leading to greater failure rates and lower patient satisfaction. Hence, the search for more non-operative options as well as for treatments that address the underlying mechanisms of OA continues [8].
Hyaluronic acid (HA), a physiological component of synovial fluid and extracellular matrix, is commonly used for IA treatment in OA due to its unique viscoelastic and lubricant features. In addition, exogenous HA stimulates endogenous production, chondrocyte metabolism and the synthesis of cartilage matrix components with anti-inflammatory effects [9]. With the aim of expanding its use in several biomedical applications, a broad range of chemical modifications of this polysaccharide have been developed [10].
Bisphosphonates (BPs) are FDA-approved anti-resorptive drugs used to treat osteoporosis because they inhibit the recruitment and maturation of osteoclast precursors and the activity of mature osteoclasts at the bone level [11,12]. BPs have also been proposed as a potential disease-modifying treatment for OA [13] because of their ability to limit excessive bone remodelling, impede synovitis and block osteoclast-mediated pain pathways [14,15]. Alendronate (ALD), a nitrogen-containing BP, showed an in vitro reduction in the expression of the major metalloproteinases responsible for cartilage degradation, namely, ADAMTS-5 and MMP-13, while upregulating Col II expression in chondrocytes [16,17]. In OA animal models, BPs showed promising [[18], [19], [20]], although contradictory, results [21,22]. Although a recent meta-analysis of randomized controlled trials showed no effects of oral BPs on the progression of knee OA in patients with established disease [6], it is still under debate whether intervention with an anti-resorptive agent would be beneficial in the early stages [13].
Unfortunately, oral administration of BPs suffers from several limitations, such as poor bioavailability [23], nonspecific bone binding and multiple side effects [24]. To overcome undesirable side effects at the systemic level and modulate drug release, IA administration is an emerging strategy for the local treatment of OA. Despite these advantages, this strategy suffers from an increased risk of joint infections, discomfort for patients and different mechanisms of drug clearance and distribution from the synovial fluid [25,26]. However, direct IA administration of a drug delivery system (DDS) can minimize systemic side effects while allowing for higher and prolonged bioavailability of therapeutics in the joint environment [27].
In this study, we synthesized a novel chemical conjugate with a covalent and hydrolysable linker between ALD and HA, acting as a prodrug for the DDS strategy via IA administration. The drug release of this biomaterial was investigated, and the mechanical properties were characterized. We then performed in vitro studies to investigate the biocompatibility on chondrocytes and osteoblasts and the efficacy in an ex vivo cartilage inflammatory model of OA.
2. Materials and methods
2.1. Materials
Hyaluronic acid (HA) sodium salt (HA-Na) and HA tetrabutylammonium salt (HA-TBA) were provided by Fidia Farmaceutici S.p.A. (Abano Terme, Italy). Ultrapure water (UPW) was generated using a water treatment apparatus from Sartorius (Italy). ALD was purchased from TCI EUROPE N.V. (Belgium), and all other reagents were supplied by Sigma (Italy) unless specified and used without further purification.
2.2. Synthesis and characterization of hyaluronic acid-alendronate conjugate (HA-ALD 4)
HA-ALD 4 was prepared by mixing a solution of alendronate tetrabutylammonium salt (ALD-TBA 1) and HA-TBA in the presence of 2-chloroethyl 1H-imidazole-1-carboxylate 2, hereafter also called the “linker”, in anhydrous DMSO. The synthesis and characterization of 1 and 2 are reported in the Supplementary Material.
To prepare HA-ALD, ALD-TBA (0.79 g; 1.61 mmol) was treated with a solution of 2 (0.34 g; 1.97 mmol) in anhydrous DMSO (20 mL) at 40 °C for 18 h under vigorous stirring. Then, the reaction mixture was added to a solution of HA-TBA (MW 500 kDa, 1.00 g; 1.61 mmol) in anhydrous DMSO (80 mL) and stirred at 40 °C for 48 h. The product was precipitated by slowly adding a saturated solution of sodium bromide (2 mL) and ethanol (500 mL). Finally, the product was recovered by filtration, solvated in UPW (500 mL), purified by means of an ultrafiltration cassette (Spectra/Por®, MWCO 20 kDa) against UPW at pH = 6 for 6 h and freeze-dried, affording the product as a white fluffy sponge (603 mg, yield 90%). The chemical characterization of HA-ALD is reported in paragraph 3.1 and in the Supplementary Material.
2.3. HA-ALD characterization
1H and 31P NMR spectra were acquired at 20 mg/mL in D2O (Bruker Avance operating at 400 MHz, Bruker Italia, Italy). DSmol (molar degree of substitution; ALD with respect to HA repeating unit) was calculated by HPLC analysis. Briefly, HA-ALD was digested in strongly acidic conditions (HCl 6.0 M at 165 °C for 6 h); after neutralization, a previously reported protocol [28] was adapted to derivatize ALD using FMOC-Cl. Quantification was performed against ALD standard solution, comparing absorbances at 266 nm (see SI for details).
2.4. In vitro ALD release assay
HA-ALD samples were dissolved in TRIS buffer (100 mM, pH = 7) at 20 mg/mL at room temperature for 1 h. The mixture of HA and ALD was prepared with 500 kDa HA at 18.2 mg/mL and ALD at 1.8 mg/mL. Samples were placed in a dialysis membrane (MWCO 20 kDa, SpectraPor, Fisher Scientific Italia, Italy) in 10 mL of TRIS buffer (100 mM, pH = 7) at 37 °C. Aliquots of 500 μL were taken at each time point for quantification of ALD in solution at each time point by analysing phosphorous content via ICP-OES analysis (PerkinElmer, Italy) at 213.5 nm (phosphorous specific wavelength), and the volume was replaced with fresh buffer. Samples were tested in triplicate.
2.5. Rheological measurements
Samples were prepared by dissolving HA-ALD (DSmol 16% mol/mol, 8.8% w/w) in TRIS buffer (20 mg/mL, 20 mM, pH = 7) at room temperature for 1 h. The mixture of HA and ALD was prepared with 500 kDa HA-Na at 20 mg/mL and ALD at 1.8 mg/mL. Viscoelastic moduli were recorded before and after the addition of a CaCl2 solution (final concentration 20 mM) with an MCR92 rheometer (Anton Paar GmbH, Graz, Austria) at 20 °C. The G′ (storage or elastic modulus) and G″ (loss or viscous modulus) were measured from 0.1 to 100 rad/s at a fixed strain value of 10% (an initial strain sweep with an oscillatory shear strain of increasing amplitude (γ) and a constant frequency of 1 Hz were applied to determine the region of linear response of the sample; for example, at 10%, the viscoelastic range was linear). In a typical experiment, 1.2 mL of solution or hydrogel was placed between the plates at a distance of 0.102 mm using a 1° 50 mm cone. All samples were freshly prepared before the measurements were taken.
2.6. Cell isolation and culture
Bovine articular chondrocytes were isolated from the femoral condyles and the patellofemoral groove of skeletally mature bovine stifles. Cartilage was manually minced and incubated for 1 h in DMEM/F-12 medium (Life Technologies, Italy) containing 100 U/mL penicillin/streptomycin (Gibco, Italy), 2.5 μg/mL amphotericin B (Gibco, Italy) and 0.4% (w/v) pronase (Sigma Aldrich, Italy) at 37 °C, followed by overnight digestion at 37 °C in DMEM/F-12 containing 0.1% (w/v) collagenase type II (Gibco, Italy), 2% FBS (v/v), amphotericin B and penicillin/streptomycin.
Undigested cartilage was removed using a 70 μm cell strainer (BD Falcon, Italy) followed by a wash step in PBS 1X (Euroclone, Italy). The isolated chondrocytes were counted, plated and incubated under standard culture conditions in DMEM/F-12 supplemented with 10% FBS, 100 U/mL penicillin/streptomycin and 50 μg/mL l-ascorbic acid (Sigma Aldrich, Italy). The medium was changed every 3 days. Cells were used for experiments at passage 1.
Human osteoblast (Saos-2, ATCC HTB-85) cells were maintained in McCoy’s 5A (Life Technologies, Italy) containing 10% FBS in a humidified incubator at 37 °C and 5% CO2.
2.7. Preparation of bovine cartilage biopsies
Articular cartilage was manually isolated using a scalpel from the femoral condyles and the patellofemoral groove of bovine stifles, obtained from a local butchery 1–2 days after killing. Cartilage biopsies were aseptically obtained using a Ø = 4 mm biopsy steel punch (Kay Medical, Italy). The isolated explants, weighing approximately 10–20 mg, were transferred to a 48-well plate and washed twice with 1X PBS containing 100 U/mL penicillin/streptomycin and 2.5 μg/mL amphotericin B and maintained in 500 μL of DMEM/F-12 supplemented with 2% FBS, penicillin/streptomycin and amphotericin B.
2.8. Cytotoxicity assay
The biocompatibility of the tested compounds was evaluated by means of a quantitative analysis according to the ISO 10993–5:2012 International Standard. For cell viability assays, Saos-2 osteoblasts and primary bovine chondrocytes were plated at a density of 1 × 104 cells per well in 96-well plates (Corning, USA). After 24 h of incubation under standard conditions, the cells were washed with PBS 1X (Life Technologies, Italy), and solutions of ALD and HA-ALD were added to reach a final conc. of 3, 6, 12, 25, 50 and 100 μM (four replicates were tested for each condition). The cells were incubated for 24 h, 3 days and 7 days, and the medium was then aspirated. The cells were washed again with 1X PBS, and 100 μL of complete medium containing 10% Alamar Blue (Life Technologies, Italy) was added. The cells were subsequently incubated for 4 h under standard culture conditions, and finally, the fluorescence was measured using a microplate reader (Nanoquant Infinite M200 Pro, Tecan Group Ltd, Switzerland) at an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Samples were tested in four replicates. Values of IC50, the concentration of sample required to inhibit cell growth by 50% in comparison with the growth of a cell control, were analysed using Origin 8.5.1 software (Origin Lab Corporation, USA) with a non-linear sigmoidal fit.
2.9. Cell viability analysis
The viability of bovine articular chondrocytes was analysed using a Live & Dead viability/cytotoxicity kit (Thermo Fisher Scientific Inc., USA). Chondrocytes were plated at a density of 1 × 104 cells per well in flat black 96-well plates (Sarstedt, Germany). After 24 h of incubation under standard conditions (37 °C, 5% CO2), the cells were washed with PBS 1X (Life Technologies, Italy), and the ALD, HA + ALD mixture and HA-ALD solutions at six different dilutions (ALD final concentration: 100 μM, 50 μM, 25 μM, 12 μM, 6 μM, 3 μM) were added to the wells (three replicates were tested for each condition). Chondrocytes in DMEM/F-12 + 10% FBS were used as live controls, whereas chondrocytes treated with 70% methanol for 30 min were used as dead controls. The cells were incubated for 24 h, 3 days and 7 days, and then the medium was aspirated. The cells were washed with PBS 1X, and 100 μL of a 2 μM calcein-AM and 4 μM EthD-I solution in DMEM/F-12 w/o FBS was added. The plate was incubated for 20 min in the dark, and images were taken using an inverted fluorescence microscope (DMI8, Leica Microsystems, Germany). Samples were tested in three replicates. Live and dead cells were counted for each sample per condition, and finally, the mean number of viable cells per condition was normalized to the viability of untreated cells (control), which was set to 100%.
2.10. Collagen release assay and MMP-13 expression in an ex vivo bovine cartilage model
The cartilage inflammatory model has been adapted according to the protocol reported in the literature [29]. To induce a pro-inflammatory condition, the cytokines oncostatin M (OSM) (OriGene, Italy) and interleukin 1β (IL-1β) (Life Technologies, Italy) at 10 ng/mL were added to the explants. Biopsies were treated with solutions of HA, ALD, HA + ALD mixture or HA-ALD 4 to assess their ability to reduce the inflammatory effects of OSM and IL-1β. The final tested concentration was set to 0.5 mM in ALD (0.13 mg/mL for free ALD; 1.3 mg/mL for HA; 1.2 mg/mL for HA and 0.11 mg/mL for ALD in the HA + ALD mixture; 1.3 mg/mL for HA-ALD 4); three biopsies were tested for each condition. The culture period lasted for 21 days, and the culture medium was changed weekly. After 3 weeks, soluble collagen and MMP13 concentrations were determined in the supernatants by means of the Sircol collagen assay kit (Biocolor, UK) and Bovine Collagenase 3 ELISA kit (MyBioSource Inc., USA), respectively.
2.11. Statistical analysis
Data from collagen release and MMP-13 assays were first investigated with Grubbs’ test for outlier identification and then with one-way ANOVA with Tukey’s post hoc test, using GraphPad Prism 8.0 software (GraphPad Software, USA).
3. Results and discussion
3.1. Two-step one-pot reaction to synthesize HA-ALD conjugate
Several strategies have been explored for the development of BP conjugates for bone targeting and drug delivery. Different classes of polymers and types of linkers have been used and previously reviewed [30]. In particular, Ossipov and co-workers developed and extensively investigated an HA-based injectable hydrogel system with BPs covalently linked to the matrix for osteoporosis treatment [[31], [32], [33]]. The most used strategy to covalently link the BP moiety to the HA scaffold presents hydrazide or amide groups via EDC chemistry. In contrast, we designed a novel HA-ALD derivative with the presence of two cleavable bonds under physiological conditions, an ester and a carbamate moiety, to allow the release of BP from the macromolecular backbone. We developed a hydrolysable linker because biodegradability is a major concern in the choice of drug delivery vehicles for successful IA treatment [25]. Moreover, the derivative was designed to release HA, ALD and ethylene glycol [34].
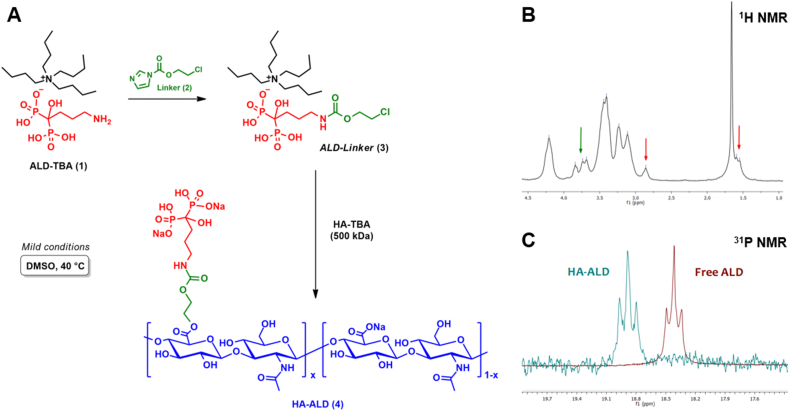
Briefly, ALD was conjugated to a 500 kDa HA backbone via a two-step one-pot reaction [35]. The first step involves a carbamoylation reaction between the primary amine of ALD and 2-chloroethyl 1H-imidazole-1-carboxylate 2 from a modified protocol reported by Liu et al. [36]. The formation of ALD-linker 3 was followed by LC-MS and confirmed via NMR analysis (Fig. S4, Supporting Material). The second step consists of the esterification of the HA carboxylic group through a nucleophilic substitution of the alkyl halide (Fig. 1A) [10].
Fig. 1.
Synthesis and NMR characterization of HA-ALD. (A) Overall reaction scheme. (B)1H NMR spectrum of HA-ALD 4 in D2O; diagnostic signals from spacer and alendronate are denoted by green and red arrows, respectively. (C) Superimposition of 31P NMR spectra in D2O for HA-ALD (cyan) and free ALD (dark red).
After purification, the chemical structure of HA-ALD 4 was investigated through NMR analysis (Fig. 1B). Methylene protons of the ALD chain were detected at 2.9 ppm and at 1.7 ppm, which partially overlapped with acetamide protons of HA [37]. Methylene protons of the spacer between ALD and HA were found to be weakly detectable at 3.5 ppm and 3.9 ppm because of the overlap with the proton signals of the HA backbone; their presence was further confirmed by 1H,1H-COSY correlations and 1H,13C-HMQC short-range correlations (Figs. S10 and S11, Supporting Material). Moreover, a distinctive phosphorus peak at 18.8 ppm was observed in 31P NMR spectra (Fig. 1C), in accordance with the value reported by Kootala et al. for an HA-BP derivative [30]. It is possible to appreciate a slight shift of 0.5 ppm from the phosphorous signals of HA-ALD and the unbound ALD, confirming that the linkage does not involve the phosphonate moieties [36].
The molar degree of substitution (DSmol; ALD with respect to the HA repeating unit) was calculated by HPLC analysis on the dialysed sample and was equal to 16% mol/mol (Fig. S13, supporting material). Finally, no effect on the molecular weight reduction of the final product compared to the native polymer was observed by SEC analysis (Fig. S12, Supporting Material).
3.2. ALD release profile: reversible linkage
Small molecular drugs introduced into the IA space could easily and quickly be removed by blood vessels and lymphatics in a few hours [38]; instead, HA has a half-life of one day [39]. Ideal IA drug delivery platforms should offer controlled release of the therapeutic agent with extended bioavailability and joint retention [40].
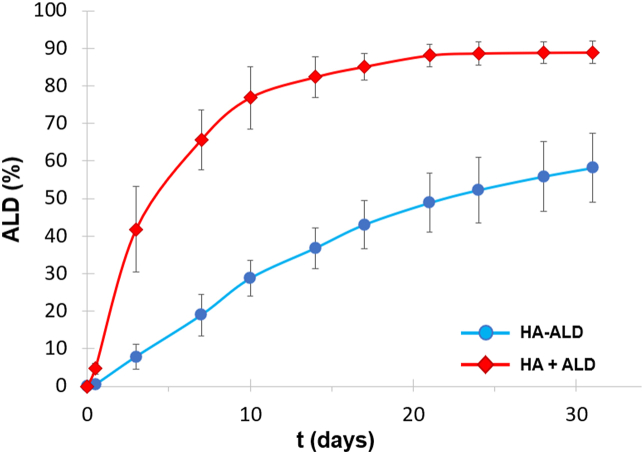
The ability of the macromolecular backbone to release ALD under physiological conditions was assessed in vitro (Fig. 2). In human SF, proteins with MW > 160 kDa are totally absent [41]; subsequently, the cut-off of a healthy synovial membrane is lower than 160 kDa. Samples of the conjugate HA-ALD and the physical mixture HA + ALD were placed in a 20 kDa dialysis and total released phosphorous was measured in the reservoir at different time points. Less than 30% of the total bound ALD was released from the conjugate within 10 days, while the mixture released almost 80% of the bisphosphonate content at the same time. The release profile of the drug in solution by HA-ALD confirmed the reversibility of the linkage and the potential application of the macromolecular DDS.
Fig. 2.
ALD release profile in 20 mM TRIS buffer, pH=7, at 37 °C. For each series, samples were prepared and measured in triplicate; data reported in the plot correspond to the mean ± SD.
3.3. Calcium ions modulate the viscoelastic properties
It is fundamental to investigate the rheological properties of HA hydrogels during the development of IA DDS and viscosupplements [3,42] because high shear forces are applied while being extruded through the needle and at the level of the joints. HA has a relatively short half-life (1–2 days in the tissue), and the use of unmodified HA is limited by a high degradation rate, poor mechanical properties, and rapid clearance [40]. For this reason, HA crosslinking is a common strategy to form mechanically and chemically improved hydrogels while retaining their biocompatibility and increasing their retention time in the joint space.
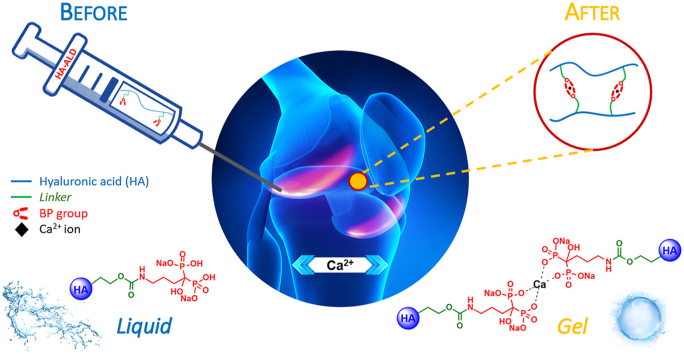
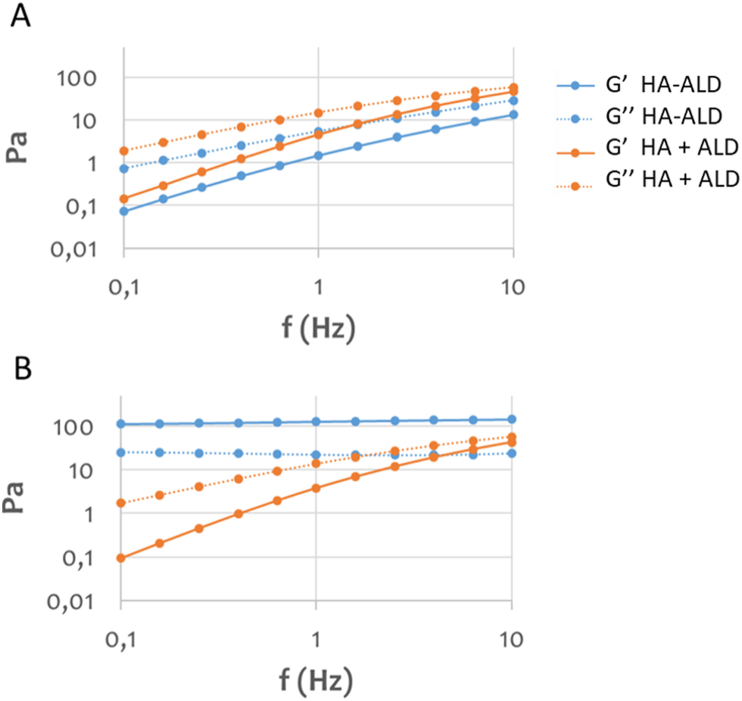
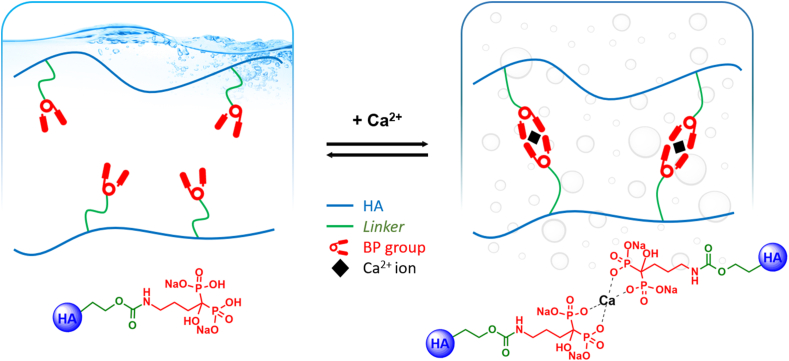
It is well known that BPs have a strong affinity for calcium and other divalent metals [30]. In a previous work, Nejadnik et al. [43] showed that a hydrogel of polymer-grafted BP reversibly bonded calcium ions. In this study, the viscoelastic modulus of the HA-ALD derivative was investigated and compared with the physical mixture of the two components, HA and free ALD. In the absence of calcium ions, the derivative showed elastic and viscous moduli comparable with those of the physical mixture (Fig. 3A). In the presence of calcium, the elastic and viscous moduli of HA-ALD increased, yielding a compact hydrogel in less than 30 s. This increment in viscoelastic moduli of two orders of magnitude at low frequencies (Fig. 3B) was afforded by the ionic crosslinking between the ALD moieties of different polymer chains, founding an intra- and intermolecular network (Fig. 4), with no appreciable effect on the polyanionic chains of HA and free ALD.
Fig. 3.
Rheological characterization of HA-ALD 4 and a mixture of HA and ALD. (A) Viscoelastic moduli of HA-ALD 4 and a mixture of HA and ALD in 100 mM TRIS buffer, pH = 7. (B) Viscoelastic moduli in 100 mM TRIS buffer, pH = 7, 20 mM CaCl2.
Fig. 4.
Schematic representation of physical crosslinking in the presence of calcium ions. The addition of calcium ions to HA-ALD 4 solution leads to the formation of a hydrogel. The same does not occur with the physical mixture of HA and ALD.
Notably, strong calcium binding may also interfere with the formation of calcium phosphate crystals involved in OA pathogenesis [44] and with the increase in the calcium phosphate complex, which is a potential catabolic mediator in the subchondral milieu and supports the pathogenic role of subchondral bone in the early stages of cartilage degeneration [16].
3.4. Cytotoxicity assay
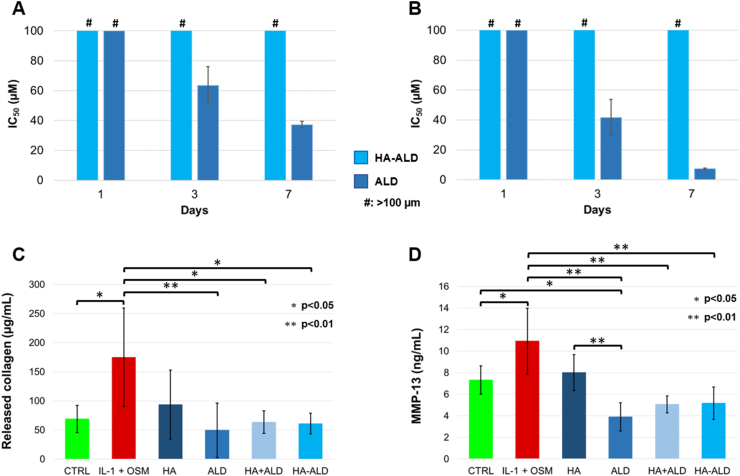
The HA-ALD derivative, once injected in the joint, would be in contact with several cell types in cartilage, synovium and bone. As a first step to guarantee the safety of this derivative, the cytotoxicity was evaluated on primary bovine chondrocytes (PBCs) and on Saos-2 osteoblasts in comparison to free ALD (Fig. 5A and B). According to the literature, a decrease in cell viability and proliferation was shown at 72 h with high concentrations (≥10 μM) of alendronate or, more generally, bisphosphonates on osteoblasts [45] and on chondrocytes in vitro [46]. No cytotoxicity was shown for either free alendronate or HA-ALD 16% mol/mol after one day. However, alendronate was shown to be cytotoxic to both chondrocytes and osteoblasts after 3 days, with an IC50 of 63.5 μM on bovine chondrocytes (Figs. 5A) and 41.7 μM on Saos-2 cells (Fig. 5B). As expected, the cytotoxicity of ALD in the mixture further increased after 7 days of incubation. In contrast, no cytotoxicity was observed for the test compound HA-ALD at any of the considered timepoints, likely due to the slow ALD release over time.
Fig. 5.
Biological in vitro tests. Cytotoxicity on (A) primary bovine chondrocytes (PBC) and on (B) Saos-2 osteoblasts, with #: >100 μM. (C) Collagen release and (D) MMP-13 expression from bovine cartilage biopsies with no treatment, exposed to IL-1β/OSM (10 ng/mL) without any treatment, or treated with HA, ALD, HA + ALD mixture or HA-ALD 4.
3.5. Viability assay
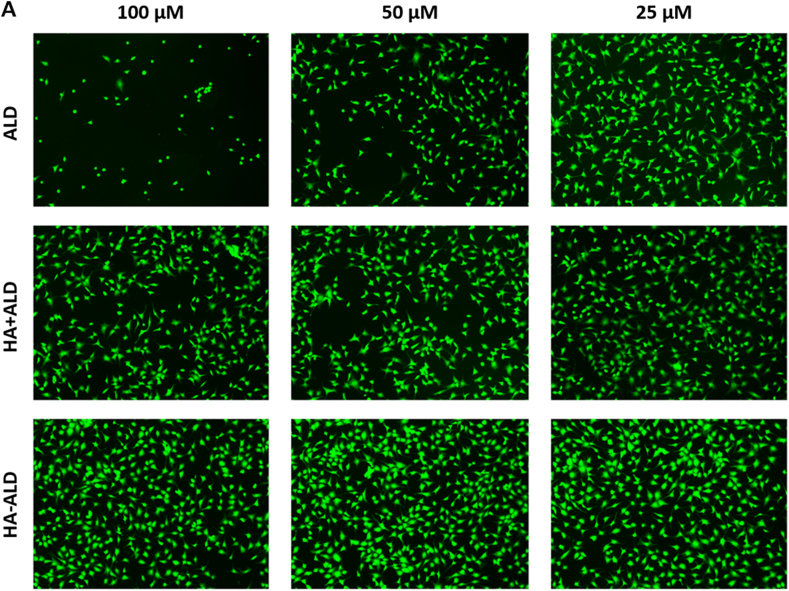
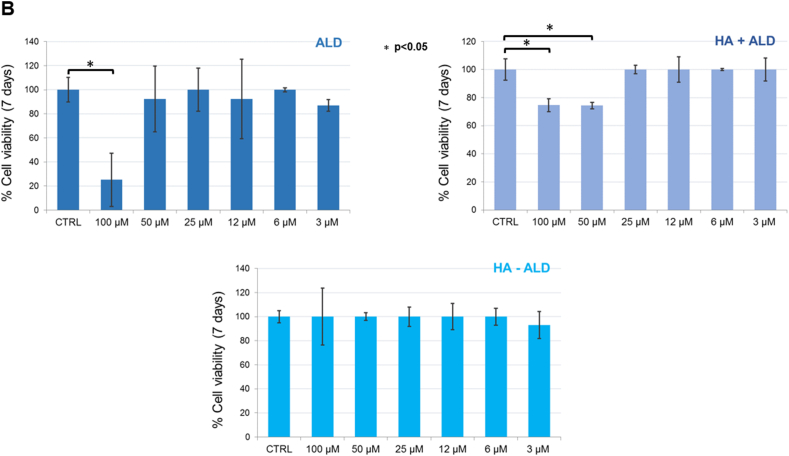
To further assess cell viability, a Live & Dead assay was performed on primary bovine chondrocytes at three time points. No decrease in the percentages of viable cells was shown for all dilutions of the tested compounds after 1 and 3 days (see SI). At 7 days, the highest ALD concentration (100 μM) showed a cytotoxic effect according to the Alamar blue assay, whereas the HA + ALD mixture at 100 and 50 μM concentrations exhibited a reduction in vitality compared to the control (Fig. 5, see SI for details). The HA-ALD compound showed the same results as the control for all tested conditions (Fig. 5). This trend is better displayed in Fig. 6, where the graphs show the percentages of viable cells counted for each dilution normalized to the control, whose cell viability was set at 100%. Finally, no signal was detected in the red channel for all tested solutions because the dead cells detached from the bottom of the well and were washed away after the assay washing steps.
Fig. 6.
Live/Dead assay. (A) Primary bovine chondrocytes (PBCs) stained with calcein-AM (2 μM) and ethidium homodimer-1 (4 μM) after seven days of incubation with six dilutions (100 μM, 50 μM, 25 μM, 12 μM, 6 μM, 3 μM) of ALD, HA + ALD mixture and HA-ALD solution. Magnification: 40 X. (B) Chondrocyte viability expressed as the percentage of viable cells for each tested condition normalized to the control, set at 100%.
3.6. In vitro efficacy assay in an OA inflammatory model
In OA, collagen and GAGs are lost during cartilage degradation, an effect exerted by several metalloproteinases, such as MMP-13 [47]. Additionally, collagen biosynthesis is a slow process [48]; thus, preserving collagen from cleavage should be a key target in OA management. The efficacy of the hyaluronan derivative was tested using an inflammatory model on bovine cartilage explants. Untreated bovine cartilage biopsies were used as controls; experimental groups included biopsies exposed to inflammatory cytokines (IL-1β and OSM) with or without treatment with HA, ALD, HA + ALD mixture or HA-ALD 4 for 3 weeks (Fig. 5C). A significant increase in soluble collagen release was detected in pro-inflammatory conditions compared to the untreated control, likely due to the induction of cartilage degradation enzymes. All the treatments, except for HA, led to a significant reduction of collagen release compared to the positive control, showing a beneficial effect against cartilage degradation through the reduction of collagen catabolism in the ex vivo model. These results correlated with the reduced expression of MMP-13 (Fig. 5D), in accordance with the previously reported effect of ALD treatment after IL-1β stimulation in vitro [16]. In these experiments, HA-ALD showed positive effects by reducing collagen release and MMP-13 expression under inflammatory conditions. Although these results showed no superior effect of the conjugate compared to the mixture, it is important to note that this inflammatory model tested a prolonged treatment in stationary conditions. This may limit the release effect of a macromolecular DDS. Furthermore, small molecules present a faster clearance rate compared to the macromolecular conjugates in the joint.
For these reasons, future in vivo studies will be focused on the effect and residence time of HA-ALD conjugate 4 to elucidate the potential of the macromolecular DDS for IA treatment of OA.
4. Conclusions
In this study, HA was functionalized with BP moieties to obtain a biocompatible and biodegradable drug-polymer derivative. Alendronate-conjugated hyaluronic acid was developed for the intra-articular treatment of OA, representing, to the best of our knowledge, the first macromolecular DDS with these two components. The synthesized HA-ALD adduct opens the door to a new approach for OA treatment, as it combines viscosupplementation and biological effects due to the presence of HA with the pharmacological activity of BPs. The obtained results suggest that HA-ALD could be beneficial in both cartilage degradation and restoration of subchondral bone function, as previously reported in the literature for HA and ALD. Finally, local administration and controlled BP release would likely overcome the drawbacks of ALD oral administration, such as nonspecificity and long-term toxic side effects.
Author contributions
Stefano Pluda: Conceptualization, Investigation, Writing – original draft. Riccardo Beninatto: Investigation, Methodology, Visualization, Writing – original draft. Matteo Soato: Investigation, Methodology, Visualization, Writing – original draft. Carlo Barbera: Investigation, Methodology, Visualization, Writing – original draft. Alba di Lucia: Investigation, Methodology, Visualization, Writing – original draft, Formal analysis. Lidia Fassina: Investigation. Filippo Gatti: Investigation. Cristian Guarise: Writing – review & editing. Devis Galesso: Writing – review & editing. Mauro Pavan: Conceptualization, Investigation, Writing – original draft, Formal analysis, Supervision.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to thank Prof. Giulia Licini and Prof. Stefano Mammi, University of Padova, for providing qualified assistance with NMR measurements.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2021.100159.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Osteoarthritis Research Society International Osteoarthritis: a serious disease, submitted to the U.S. Food and Drug Administration. December 1, 2016. https://www.oarsi.org/education/oarsi-resources/oarsi-white-paper-oa-serious-disease OARSI White Paper. Published May 9, 2018.
- 2.Hügle T., Geurts J. What drives osteoarthritis? synovial versus subchondral bone pathology. Rheumatology. 2017;56:1461–1471. doi: 10.1093/rheumatology/kew389. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls M., Manjoo A., Shaw P., Niazi F., Rosen J. Rheological properties of commercially available hyaluronic acid products in the United States for the treatment of osteoarthritis knee pain, clinical medicine insights: arthritis and musculoskeletal disorders. January 2018. https://doi:10.1177/1179544117751622 [DOI] [PMC free article] [PubMed]
- 4.Findlay D., Kuliwaba J. Bone–cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res. 2016;4:16028. doi: 10.1038/boneres.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu S., Zhu J., Zhen G., Hu Y., An S., Li Y., et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Invest. 2019;129:1076–1093. doi: 10.1172/JCI121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaysbrot E.E., Osani M.C., Musetti M.-C., McAlindon T.E., Bannuru R.R. Are bisphosphonates efficacious in knee osteoarthritis? A meta-analysis of randomized controlled trials, Osteoarthritis Cartilage. 2018;26:154–164. doi: 10.1016/j.joca.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Cutolo M., Berenbaum F., Hochberg M., Punzi L., Reginster J.-Y. Commentary on recent therapeutic guidelines for osteoarthritis. Semin. Arthritis Rheum. 2015;44:611–617. doi: 10.1016/j.semarthrit.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Losina E., Paltiel D., Weinstein A.M., Yelin E., Hunter D.J., Chen S.P., et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015;67:203–215. doi: 10.1002/acr.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L.H., Xue J.F., Zheng Z.Y., Shuhaidi M., Thu H.E., Hussain Z. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: a review of recent developments and critical appraisal of preclinical and clinical investigations, Int. J. Biol. Macromol. 2018;116:572–584. doi: 10.1016/j.ijbiomac.2018.05.068. [DOI] [PubMed] [Google Scholar]
- 10.Schanté C.E., Zuber G., Herlin C., Vandamme T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011;85:469–489. doi: 10.1016/j.carbpol.2011.03.019. [DOI] [Google Scholar]
- 11.Roelofs A.J., Thompson K., Ebetino F.H., Rogers M.J., Coxon F.P. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr. Pharmaceut. Des. 2010;16:2950–2960. doi: 10.2174/138161210793563635. https://doi:10.2174/138161210793563635 [DOI] [PubMed] [Google Scholar]
- 12.Reszka A.A., Rodan G.A. Mechanism of action of bisphosphonates. Curr. Osteoporos. 2003;1:45–52. doi: 10.1007/s11914-003-0008-5. [DOI] [PubMed] [Google Scholar]
- 13.Lane N. Bisphosphonates and OA — is there a bone and joint connection? Nat. Rev. Rheumatol. 2018;14:185–186. doi: 10.1038/nrrheum.2018.18. [DOI] [PubMed] [Google Scholar]
- 14.Qvist P., Bay-Jensen A.-C., Christiansen C., Dam E.B., Pastoureau P., Karsdal M.A. The disease modifying osteoarthritis drug (DMOAD): is it in the horizon? Pharmacol. Res. 2008;58:1–7. doi: 10.1016/j.phrs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Walsh D.A., Chapman V. Bisphosphonates for osteoarthritis. Arthritis Res. Ther. 2011;13:128. doi: 10.1186/ar3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cable M., Jackson N.M., Flynn J.C., Markel D.C. Alendronate reverses increases in MMP13 due to IL-1β stimulation in human chondrocytes in vitro. Current Orthopaedic Practice. 2015;26:336–342. https://doi 10.1097/BCO.0000000000000247 [Google Scholar]
- 17.Wu X., Yin Q., Zheng C., Xia H. Alendronate impact on Col II, MMP-13, and β-catenin in osteoarthritis rats. Int. J. Clin. Exp. Pathol. 2016;9:3618–3623. www.ijcep.com/ISSN:1936-2625/IJCEP0020211 [Google Scholar]
- 18.Mohan G., Perilli E., Parkinson I.H., Humphries J.M., Fazzalari N.L., Kuliwaba J.S. Pre-emptive, early, and delayed alendronate treatment in a rat model of knee osteoarthritis: effect on subchondral trabecular bone microarchitecture and cartilage degradation of the tibia, bone/cartilage turnover, and joint discomfort. Osteoarthritis Cartilage. 2013;21:1595–1604. doi: 10.1016/j.joca.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Siebelt M., Waarsing J.H., Groen H.C., Müller C., Koelewijn S.J., de Blois E., et al. Inhibited osteoclastic bone resorption through alendronate treatment in rats reduces severe osteoarthritis progression. Bone. 2014;66:163–170. doi: 10.1016/j.bone.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Hayami T., Pickarski M., Wesolowski G.A., Mclane J., Bone A., Destefano J., et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model, Arthritis Rheum. 2004;50:1193–1206. doi: 10.1002/art.20124. https://doi:10.1002/art.20124 [DOI] [PubMed] [Google Scholar]
- 21.Bagi C.M., Berryman E., Zakur D.E., Wilkie D., Andresen C.J. Effect of antiresorptive and anabolic bone therapy on development of osteoarthritis in a posttraumatic rat model of OA. Arthritis Res. Ther. 2015;17:315. doi: 10.1186/s13075-015-0829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers S.L., Brandt K.D., Burr D.B., O’Connor B.L., Albrecht M. Effects of a bisphosphonate on bone histomorphometry and dynamics in the canine cruciate deficiency model of osteoarthritis. J. Rheumatol. 1999;26:2645–2653. [PubMed] [Google Scholar]
- 23.Pazianas M., Abrahamsen B., Ferrari S., Russell R.G. Eliminating the need for fasting with oral administration of bisphosphonates. Therapeut. Clin. Risk Manag. 2013;9:395–402. doi: 10.2147/TCRM.S52291. https://doi:10.2147/TCRM.S52291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen M.R. The effects of bisphosphonates on jaw bone remodeling, tissue properties, and extraction healing, Odontology. 2011;99:8–17. doi: 10.1007/s10266-010-0153-0. [DOI] [PubMed] [Google Scholar]
- 25.Jones I.A., Togashi R., Wilson M.L., Heckmann N., Vangsness C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019;15:77–90. doi: 10.1038/s41584-018-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang M.L., Im G.-I. Drug delivery systems for intra-articular treatment of osteoarthritis. Expet Opin. Drug Deliv. 2014;11:269–282. doi: 10.1517/17425247.2014.867325. https://doi:10.1517/17425247.2014.867325 [DOI] [PubMed] [Google Scholar]
- 27.Gerwin N., Hops C., Lucke A. Intraarticular drug delivery in osteoarthritis. Adv. Drug Deliv. Rev. 2006;58:226–242. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Ptáček P., Klíma J., Macek J. Determination of alendronate in human urine as 9-fluorenylmethyl derivative by high-performance liquid chromatography. J. Chromatogr. B. 2002;767:111–116. doi: 10.1016/S0378-4347(01)00551-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen-An P., Andreassen K.V., Henriksen K., Karsdal M.A., Bay-Jensen A.-C. Investigation of chondrocyte hypertrophy and cartilage calcification in a full-depth articular cartilage explants model. Rheumatol. Int. 2013;33:401–411. doi: 10.1007/s00296-012-2368-6. [DOI] [PubMed] [Google Scholar]
- 30.Ossipov D.A. Bisphosphonate-modified biomaterials for drug delivery and bone tissue engineering. Expet Opin. Drug Deliv. 2015;12:1443–1458. doi: 10.1517/17425247.2015.1021679. https://doi:10.1517/17425247.2015.1021679 [DOI] [PubMed] [Google Scholar]
- 31.Kootala S., Ossipov D., van den Beucken J.J.J.P., Leeuwenburgh S., Hilborn J. Bisphosphonate-functionalized hyaluronic acid showing selective affinity for osteoclasts as a potential treatment for osteoporosis. Biomater. Sci. 2015;3:1197–1207. doi: 10.1039/C5BM00096C. [DOI] [PubMed] [Google Scholar]
- 32.Varghese O.P., Sun W., Hilborn J., Ossipov D.A. In Situ cross-linkable high molecular weight Hyaluronan−Bisphosphonate conjugate for localized delivery and cell-specific targeting: a hydrogel linked prodrug approach, J. Am. Chem. Soc. 2009;131:8781–8783. doi: 10.1021/ja902857b. https://doi:10.1021/ja902857b [DOI] [PubMed] [Google Scholar]
- 33.Yang X., Akhtar S., Rubino S., Leifer K., Hilborn J., Ossipov D.A. Direct ″click″ synthesis of hybrid bisphosphonate–hyaluronic acid hydrogel in aqueous solution for biomineralization. Chem. Mater. 2012;24:1690–1697. https://doi:10.1021/cm300298n [Google Scholar]
- 34.LaKind J.S., McKenna E.A., Hubner R.P., Tardiff R.G. A review of the comparative mammalian toxicity of ethylene glycol and propylene glycol, Crit. Rev. Toxicol. 1999;29:331–365. doi: 10.1080/10408449991349230. https://doi:10.1080/10408449991349230 [DOI] [PubMed] [Google Scholar]
- 35.Barbera C., Pavan M., Pluda S. WO2020079551-Conjugates of hyaluronic acid and aminobisphosphonates and the therapeutic use thereof. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020079551
- 36.Liu J., Jo J.-I., Kawai Y., Aoki I., Tanaka C., Yamamoto M., et al. Preparation of polymer-based multimodal imaging agent to visualize the process of bone regeneration. J. Contr. Release. 2012;157:398–405. doi: 10.1016/j.jconrel.2011.09.090. [DOI] [PubMed] [Google Scholar]
- 37.Alanne A.-L., Hyvönen H., Lahtinen M., Ylisirniö M., Turhanen P., Kolehmainen E., et al. Systematic study of the physicochemical properties of a homologous series of aminobisphosphonates. Molecules. 2012;17:10928–10945. doi: 10.3390/molecules170910928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsen C., Østergaard J., Larsen S.W., Jensen H., Jacobsen S., Lindegaard C., et al. Intra-articular depot formulation principles: role in the management of postoperative pain and arthritic disorders. J. Pharmacol. Sci. 2008;97:4622–4654. doi: 10.1002/jps.21346. [DOI] [PubMed] [Google Scholar]
- 39.Coleman P.J., Scott D., Ray J., Mason R.M., Levick J.R. Hyaluronan secretion into the synovial cavity of rabbit knees and comparison with albumin turnover. J. Physiol. 1997;503:645–656. doi: 10.1111/j.1469-7793.1997.645bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rai M.F., Pham C.T. Intra-articular drug delivery systems for joint diseases. Curr. Opin. Pharmacol. 2018;40:67–73. doi: 10.1016/j.coph.2018.03.013. https://doi:10.1016/j.coph.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thing M., Mertz N., Ågårdh L., Weng Larsen S., Østergaard J., Larsen C. Simulated synovial fluids for in vitro drug and prodrug release testing of depot injectables intended for joint injection. J. Drug Deliv. Sci. Technol. 2019;49:169–176. doi: 10.1016/j.jddst.2018.11.012. [DOI] [Google Scholar]
- 42.He Z., Wang B., Hu C., Zhao J. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloids and Surfaces B: Biointerfaces. 2017;154:33–39. doi: 10.1016/j.colsurfb.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Nejadnik M.R., Yang X., Bongio M., Alghamdi H.S., van den Beucken J.J.J.P., Huysmans M.C., et al. Self-healing hybrid nanocomposites consisting of bisphosphonated hyaluronan and calcium phosphate nanoparticles. Biomaterials. 2014;35:6918–6929. doi: 10.1016/j.biomaterials.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Ea H.-K., Chobaz V., Nguyen C., Nasi S., van Lent P., Daudon M., et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PloS One. 2013;8 doi: 10.1371/journal.pone.0057352. https://doi:10.1371/journal.pone.0057352 e57352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naidu A., Dechow P.C., Spears R., Wright J.M., Kessler H.P., Opperman L.A. The effects of bisphosphonates on osteoblasts in vitro, oral surgery, oral medicine, oral pathology. Oral Radiology, and Endodontology. 2008;106:5–13. doi: 10.1016/j.tripleo.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 46.Van Offel J.F., Schuerwegh A.J., Bridts C.H., Stevens W.J., De Clerck L.S. Effect of bisphosphonates on viability, proliferation, and dexamethasone-induced apoptosis of articular chondrocytes. Ann. Rheum. Dis. 2002;61:925–928. doi: 10.1136/ard.61.10.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldring M.B., Otero M., Plumb D.A., Dragomir C., Favero M., El Hachem K., et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cell. Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. https://doi:10.22203/ecm.v021a16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koide T., Nagata K. In: Brinckmann J., Notbohm H., Müller P.K., editors. vol. 247. Springer; Berlin, Heidelberg: 2005. Collagen biosynthesis; pp. 85–114. (Collagen. Topics in Current Chemistry). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.