Summary
Objective
To study the effect of total hip replacement (THR) on serum cartilage oligomeric matrix protein concentration (sCOMP) and its correlation with joint loading during gait in patients with unilateral hip osteoarthritis.
Design
In this prospective multimodal (clinical, biomechanical, biochemical) study blood samples from 15 patients were taken before and up to three times after THR (7 days, 3 months and 1 year), each after a resting period of at least 30 min, for analysis of sCOMP. Gait analysis was performed before and 1 year after THR to determine hip and knee joint moments.
Results
Seven days after THR, sCOMP decreased significantly compared to the preoperative measurement (p < 0.001). Three months and 1 year postoperatively, sCOMP reverted to concentrations in the range of the preoperative value. One year postoperatively, a linear correlation between sCOMP and the maximum hip flexion moment was indicated in the first half of the stance phase on the unaffected side (r = −0.736, p = 0.024). No further correlations could be determined.
Conclusions
Surprisingly, the removal of a joint affected by osteoarthritis did not have a sustained effect on sCOMP. Both before and after THR there was no scientifically substantiated correlation between sCOMP and joint moments from gait analysis. Consequently, the examination of sCOMP is not useful to detect altered joint loads that may influence degenerative changes of adjacent joints after THR.
The registration number in the German Registry of Clinical Trials is DRKS00015053.
Keywords: COMP, Serum biomarker, Total hip arthroplasty, Osteoarthritis, Gait analysis, Joint loading
1. Introduction
Osteoarthritis (OA) is one of the leading causes of joint pain and chronic disability worldwide [1]. Patients with unilateral hip OA adopt characteristic gait patterns to reduce pain. Gait analysis has been used for decades to quantify these biomechanical abnormalities. Typical gait patterns of patients with hip OA include modified spatio-temporal gait parameters like reduced gait speed compared to healthy controls [2]. Kinetic gait parameters are also altered and patients present higher knee and hip joint loading in the non-affected limb compared to the affected limb before total hip replacement (THR) [[3], [4], [5], [6]]. Pathological joint loading during gait is a potential risk factor for initiation of OA and enhances its progression [7]. In particular, peak external knee adduction moments (KAM) are associated with initiation and progression of OA [7,8]. Moreover, several studies revealed that gait modifications in patients with hip OA result in abnormal loading on other joints of the lower extremities. As an example, patients flex their trunk more to the affected side in order to relieve pain [9,10]. The line of action of the ground reaction force shifts laterally as a result of increased lateral trunk displacement and therefore also has an influence on knee joint loading on the side of the affected hip [11,12]. A shift of the knee joint load from the medial to the lateral compartment could be shown in the affected limb in patients with unilateral hip OA [13]. Even after THR abnormal kinetics of the hip and knee have been discovered [6,[14], [15], [16]].
Irreversible joint damages are often already manifested at the time of first diagnosis using traditional radiographic measures. Besides the established, elaborated gait analysis, other methods might be useful to detect OA before irreversible joint cartilage damage occurs, to improve disease staging and to predict the course of disease [17,18]. Currently, extracellular matrix components that are released from articular cartilage, subchondral bone and synovial tissue, are used as biomarkers and monitored to quantify joint remodeling and disease progression. They are partially metabolized by the joint tissue and released as intact proteins or as fragments into biological fluids where they can be detected and quantified [[18], [19], [20], [21]]. Biomarkers can be classified into one or more of the following categories by using the “BIPED” classification: burden of disease, investigative, prognostic, efficacy of intervention and diagnostic [22]. For instance, the non-collagenous, non-proteoglycan pentameric glycoprotein cartilage oligomeric matrix protein (COMP), also known as thrombospondin 5, can especially be found in the extracellular matrix of articular cartilage [18,19,[23], [24], [25]]. It is mainly produced by chondrocytes and an important component in the organization of the collagenous cartilage matrix [23,25]. In addition, COMP has also been identified in other tissues such as ligaments, meniscus and vascular smooth muscle, but the respective contribution of these sources to overall sCOMP in the circulation is not known so far [26]. The release of the biomarker COMP is mechanosensitive and, therefore, elevated serum levels were detected with increased joint load, for example during walking and running even in healthy adults [[27], [28], [29], [30]]. COMP is also related to cartilage degradation during OA [[18], [19], [20],24,31]. The serum COMP concentration (sCOMP) increases with the number of affected joints as well as with the severity of OA [19]. On the other side, decreasing sCOMP was found in response to immobilization. Again, even healthy individuals show significantly decreased sCOMP after extended bed rest [32,33]. However, the time course of sCOMP after total joint replacement has never been analyzed systematically. Only Sharif et al. [34] discovered incidentally altered sCOMP after total knee replacement. It is therefore important to further investigate the influence of an artificial joint on sCOMP before and after THR.
Biomechanical changes using instrumented gait analysis and biochemical markers in body fluids such as COMP can be used to evaluate patients with OA in the research setting [35]. However, a correlation between gait pattern and sCOMP has not been investigated so far. Until now, only gait patterns of healthy volunteers were correlated with sCOMP [[36], [37], [38]]. Herger et al. [36], Denning et al. [37] and Firner et al. [38] found that with rising joint load sCOMP increases more strongly as a basis for investigating the relevance of the dose-response relationship between ambulatory load magnitude and load-induced changes in biomarkers involved in joint cartilage metabolism for the initiation and progression of articular cartilage disease such as OA. We are not aware of any study that analyzes the correlation of sCOMP and gait pattern in patients with unilateral hip OA. Therefore, the purpose of the present study was to identify and to compare these appropriate biomechanical and biochemical outcome measures before and after THR. This should create conditions to establish an approach that will allow screening of high-risk patients and an earlier diagnosis of OA than currently possible with traditional radiographic measures. Therefore, sCOMP was compared with already established biomechanical joint loading data from gait analysis. We hypothesized that (1) sCOMP would sustainably decrease after removal of the affected hip joint. In addition, we hypothesized that (2) before THR higher sCOMP would directly correlate with lower dynamic hip and knee joint loading of the affected limb since the load on this side is kept low by compensatory mechanisms [[3], [4], [5], [6]]. Consequently, joint loading of the non-affected side would positively correlate with sCOMP before THR. Finally, we hypothesized that (3) after removal of the affected hip joint, sCOMP would correlate with joint loading of the ipsilateral knee and contralateral hip and knee joint.
2. Methods
2.1. Participants
Fifteen patients diagnosed only with unilateral symptomatic hip OA, validated by conventional anterior/posterior and lateral pelvic radiographs (7 females, 8 males, age = 58.1 8.8 years, height = 1.74 0.08 m, weight = 80.3 16.2 kg, body mass index (BMI) = 26.3 4.5 kg/m2), from our university hospital voluntarily participated in the study (Table 1). All patients reported pain for the ipsilateral hip whereas the contralateral hip was free from any symptoms at the preoperative time point. At the time of all follow-up measurements all patients were pain-free. The period of prospective examination began before surgery and ended approximately 1 year after initial THR. Patients were included if THR was indicated, Kellgren-Lawrence-Score [39] was 2 and both standing and walking without assistive device was possible. Exclusion criteria were previous orthopedic surgery of lower extremities, earlier joint infection or inflammatory arthritis, OA of lower limb joints (except the affected hip joint), chronic or neuromuscular diseases and injury of lower extremities.
Table 1.
Anthropometric parameters of patients (mean with standard deviation in parenthesis).
| Characteristic | Patients, t0 (n = 15) | Patients, t3 (n = 10) |
|---|---|---|
| Gender, female/male | 7/8 | 6/4 |
| Age (years) | 58.1 (8.8) | 59.3 (10.5) |
| Height (m) | 1.74 (0.08) | 1.73 (0.06) |
| Body mass (kg) | 80.3 (16.2) | 82.3 (19.1) |
| Body mass index (kg/m2) | 26.3 (4.5) | 27.3 (5.1) |
t0 – 6.2 10.2 days before total hip replacement; t3 – 52.4 1.9 weeks after total hip replacement.
Prior to first examination, all participants gave informed consent. The study was accepted by the local medical ethics committee of the Goethe University Frankfurt in Germany (No. 497/15) and conducted in accordance with the Declaration of Helsinki. This is a prospective multimodal (clinical, biomechanical, biochemical) study with level of evidence II.
2.2. Gait analysis
In nine patients the gait pattern was analyzed within 1 week before THR (t0 = 3.2 1.8 days) and about 1 year postoperatively (t3 = 52.5 2.0 weeks) because five failed due to postoperative lack of interest and one did not meet the quality standards for gait analysis (a more detailed explanation can be found below). To collect spatio-temporal and kinematic data at 200 Hz an 8-camera MX T10 Vicon motion capture system (VICON Motion Systems, Oxford, UK) was used. Simultaneously, ground reaction forces were acquired at 1.000 Hz with two AMTI force plates (Advanced Mechanical Technology, Inc., Watertown, MA), localized in the mid of a 15 m long level walkway. A lower body protocol (called MA) was used as an aid for better reliability and accuracy when analyzing gait data [40]. In addition to the standardized Plug-in-Gait marker set [41], reflective markers were brought on the medial malleolus, medial femoral condyle and greater trochanter to ascertain joint centers of rotation for ankle, knee and hip. The hip joint center was calculated with a standardized geometrical prediction method using regression equations [42] which is frequently used in this field [43]. Patients were asked to walk barefoot in freely chosen gait velocity along the walkway.
After the analysis, 3D marker trajectories were reconstructed and missing frames filled by using the Vicon-Nexus software version 2.5 (Vicon Motion Systems, Oxford, UK). A Woltring filter was applied to spline smooth the data [44]. Altogether, five trials were examined for each subject and the mean value was taken for further analysis. Good quality of the marker trajectories and precise foot-force plate-contact were prerequisites for use of the trial. To exclude the potential effect of cross-talk - meaning that one joint rotation (e.g. flexion in the sagittal plane) is construed as another (e.g. adduction in the frontal plane) because of axis malalignment of the marker set - and in keeping with physiological and clinical standards, only patients having a knee varus/valgus ROM during gait 10° were included [45].
Means and standard deviations were calculated by a custom-made algorithm in Matlab R2018b (The MathWorks, Inc., Natick, MA). Moreover, using an inverse dynamic approach, external joint moments (normalized to body weight) were calculated from force plate data and mathematically derived joint centers. Hip and knee joint moments in the frontal and sagittal plane were analyzed in this study on the basis of clinical relevance [13,14,46]. The graphs of the KAM and external hip adduction moment (HAM) show a characteristic “m” or “double hump” wave form. Consequently, the following external joint moments in the frontal plane were defined as primary outcomes in the present study: First and second peak of KAM and HAM in first and second half of stance phase. In addition, the impulse of KAM and HAM (area under the curve) [36] was calculated. Regarding the sagittal plane, peak flexion moment in the first half of stance phase and peak extension moment in the second half of stance phase for the knee and hip joint were determined. All gait parameters were normalized to 100% stance phase, which is defined as the time between the moment of heel-strike (first ground contact) and toe off (last ground contact).
2.3. Blood samples and COMP analysis
After a resting time for at least 30 min to minimize the influence of previous activity [27,47], venous blood samples were taken at the following four time points: t0 within 1 week (6.2 10.2 days) before THR, t1 7 days (7.0 0.5 days) postoperative, t2 3 months (14.0 1.8 weeks) postoperative and t3 about 1 year (52.4 1.9 weeks) postoperative. Blood samples from 15 patients were collected for the first three time points. Only ten patients completed the study with blood sampling at t3 because five failed due to lack of interest. Blood samples at the times t0 and t3 were taken after 30 min rest on the same day before gait analyses to avoid the influence of joint load and fatigue.
Serum was analyzed for COMP concentration as follows. After clotting for at least 15 min in the serum tube, samples were centrifuged for 10 min at 2670×g and stored aliquoted in Eppendorf tubes at −80 °C until further analysis. All samples were diluted 1:50 and analyzed in duplicates for each participant on the same assay plate to avoid inter-assay variation [32] using a commercial enzyme-linked immunosorbent assay (Human COMP ELISA kit, BioVendor, Brno, Czech Republic). The mean value for each patient at each time point was used for further exploration. Absolute sCOMP was used for statistical analysis. Relative sCOMP between two time points t and t' was calculated as (sCOMP(t')-sCOMP(t))/sCOMP(t)*100.
2.4. Statistical analysis
Statistical data analysis was performed with SPSS Version 25 (IBM Corporation, New York, NY, USA). The Shapiro-Wilk-test as well as the coefficient of skewness and kurtosis were used to confirm normal distribution of the analyzed parameters. To show time related effects of absolute sCOMP, one-way repeated measures analysis of variance (ANOVA) was used. In case of a significant main effect, post hoc pairwise comparisons (Bonferroni) were performed (hypothesis 1). The significance level was set at p ≤ 0.05.
Pearson product-moment correlation analyses (coefficient r) were performed to identify linear relationships between absolute sCOMP and joint moments (knee and hip) during gait in the affected and non-affected limb both pre- (hypothesis 2) and postoperative (hypothesis 3). Correlation values below 0.30 were interpreted as low, between 0.30 and 0.65 as medium and correlations above 0.65 as high [48].
3. Results
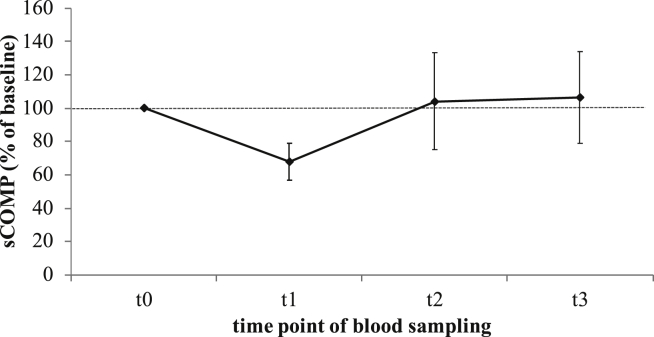
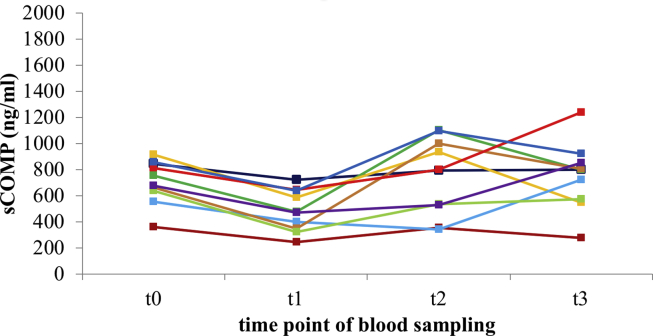
COMP data of all participating patients were normally distributed at all time points. Absolute sCOMP for each time point is summarized in Table 2. The course of sCOMP after THR for ten patients, normalized to baseline concentration before THR (t0), is illustrated in Fig. 1. Additionally, the absolute sCOMP for each of these ten patients at all sampling time points is shown in Fig. 2. Within 7 days after THR (t1) sCOMP decreased on average by 32.23%. Three months (t2) and 1 year (t3) after surgery the mean sCOMP went back to the range of baseline concentration (104.08 29.03% and 106.55 27.40% normalized to baseline concentration). ANOVA showed a significant time related effect of absolute sCOMP (p = 0.001). Pairwise comparisons of mean absolute sCOMP indicated a significant difference between time point t0 before and t1 after THR (p < 0.001; Table 3). Measured concentrations at t2 and t3 were also significantly higher than sCOMP at t1 (p = 0.049 and p = 0.011). Pairwise comparisons between t0, t2 and t3 did not result in significant differences.
Table 2.
Serum COMP concentrations (mean with standard deviation in parenthesis) before and 3 time points after total hip replacement (n = 15 patients at t0, t1, t2; n = 10 patients at t3).
| Time points | Absolute sCOMP (ng/ml) | 95% confidence interval |
|
|---|---|---|---|
| Lower limit | Upper limit | ||
| t0 | 718.96 (135.77) | 643.77 | 794.15 |
| t1 | 477.28 (147.27) | 395.73 | 558.84 |
| t2 | 732.92 (237.54) | 601.37 | 864.47 |
| t3 | 754.88 (255.07) | 572.41 | 937.34 |
sCOMP – serum cartilage oligomeric matrix protein concentration; THR – total hip replacement; t0 – 6.2 10.2 days before THR; t1 – 7.0 0.5 days after THR; t2 – 14.0 1.7 weeks after THR; t3 – 52.4 1.9 weeks after THR.
Fig. 1.
Relative sCOMP (mean with standard deviation) up to one year after total hip replacement (THR) normalized to sCOMP at baseline (before THR, n = 10 patients). sCOMP – serum cartilage oligomeric matrix protein concentration; t0 – 7.1 12.4 days before THR; t1 – 7.0 0.7 days after THR; t2 – 14.0 2.1 weeks after THR; t3 – 52.4 1.9 weeks after THR; sCOMPt0/t0 = 100%; sCOMPt1/t0 = 67.77 11.14%; sCOMPt2/t0 = 104.08 29.03%; sCOMPt3/t0 = 106.55 27.40%.
Fig. 2.
Absolute sCOMP for each patient up to one year after total hip replacement (n = 10 patients). sCOMP – serum cartilage oligomeric matrix protein; THR – total hip replacement; t0 – 7.1 12.4 days before THR; t1 – 7.0 0.7 days after THR; t2 – 14.0 2.1 weeks after THR; t3 – 52.4 1.9 weeks after THR; minimal sCOMPt0 = 361.94 ng/ml; maximal sCOMPt0 = 914.73 ng/ml.
Table 3.
Pairwise comparisons (Bonferroni) between concentrations of absolute sCOMP (n = 10 patients).
| p-Value | Mean difference | 95% confidence interval |
||
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| sCOMP (t0)/ | ||||
| sCOMP (t1) | <0.001* | 222.93 | 135.29 | 310.57 |
| sCOMP (t2) | 1.000 | −41.00 | −253.39 | 171.38 |
| sCOMP (t3) | 1.000 | −46.93 | −269.82 | 175.97 |
| sCOMP (t1)/ | ||||
| sCOMP (t2) | 0.049* | −263.93 | −526.57 | −1.29 |
| sCOMP (t3) | 0.011* | −269.86 | −479.58 | −60.14 |
| sCOMP (t2)/ | ||||
| sCOMP (t3) | 1.000 | −5.92 | −314.73 | 302.89 |
sCOMP – serum cartilage oligomeric matrix protein concentration; THR – total hip replacement; t0 – 7.1 12.4 days before THR; t1 – 7.0 0.7 days after THR; t2 – 14.0 2.1 weeks after THR; t3 – 52.4 1.9 weeks after THR; *Significant difference.
Correlations between absolute sCOMP and biomechanical parameters measured by gait analysis are shown in Table 4. All kinetic gait parameters of interest were normally distributed. Pearson product-moment correlation analyses between absolute sCOMP and joint moments at t0 before THR demonstrated neither a significant correlation for the affected nor for the non-affected side. Even after THR at t3 no linear correlations for the affected side were found, however a trend towards negative correlation between sCOMP(t3) and the maximum hip flexion moment in the first half of the stance phase could be observed (r = −0.652, p = 0.057). Regarding the non-affected side at t3 after THR, a significant negative correlation was demonstrated between sCOMP(t3) and the maximum hip flexion moment in the first half of the stance phase (r = −0.736, p = 0.024).
Table 4.
Pearson correlation coefficients (r) and p-Values (in parenthesis) for absolute sCOMP and kinetic gait parameters during the stance phase of gait (n = 9 patients).
| Preoperative |
Postoperative |
|||
|---|---|---|---|---|
| Affected side | Non-affected side | Affected side | Non-affected side | |
| Knee moments – sagittal plane | ||||
| Max. flexion first half of stance | 0.395 (0.293) |
0.490 (0.181) |
−0.003 (0.994) |
−0.386 (0.305) |
| Max. extension second half of stance | 0.041 (0.917) |
0.008 (0.984) |
0.263 (0.495) |
0.034 (0.931) |
| Knee moments – frontal plane | ||||
| Max. adduction first half of stance (1st KAM) | −0.285 (0.458) |
−0.087 (0.824) |
−0.254 (0.509) |
0.050 (0.898) |
| Max. adduction second half of stance (2nd KAM) | −0.272 (0.479) |
0.072 (0.854) |
−0.148 (0.704) |
0.259 (0.501) |
| Adduction impulse (AUC) | −0.252 (0.514) |
−0.059 (0.880) |
−0.137 (0.726) |
0.176 (0.651) |
| Hip moments – sagittal plane | ||||
| Max. flexion first half of stance | −0.276 (0.472) |
0.065 (0.868) |
−0.652 (0.057)+ |
−0.736 (0.024)* |
| Max. extension second half of stance | −0.249 (0.518) |
−0.305 (0.425) |
−0.332 (0.382) |
−0.174 (0.655) |
| Hip moments – frontal plane | ||||
| Max. adduction first half of stance (1st HAM) | 0.091 (0.815) |
0.301 (0.431) |
0.195 (0.614) |
−0.058 (0.883) |
| Max. adduction second half of stance (2nd HAM) | 0.087 (0.823) |
0.300 (0.433) |
0.017 (0.965) |
0.272 (0.479) |
| Adduction impulse (AUC) | 0.034 (0.930) |
0.286 (0.455) |
0.152 (0.697) |
0.317 (0.407) |
sCOMP – serum cartilage oligomeric matrix protein concentration; AUC – area under the curve; flexion – positive values; extension – negative values; adduction – positive values; abduction – negative values; *Significant correlation; +Trend towards a linear correlation.
4. Discussion
The purpose of the present longitudinal study was to examine for the first time the effect of THR on sCOMP in patients with unilateral hip OA. Secondly, sCOMP was compared with biomechanical joint loading using instrumented gait analysis before and after THR. The removal of a joint affected by OA did not have a sustained influence on sCOMP. After a drop of sCOMP 7 days postoperatively, sCOMP increased 3 months after THR back to the range of baseline concentration and remained at this level up to 1 year after surgery. Preoperatively, sCOMP did not depend on changes in joint moments caused by gait alterations. An unexpected negative correlation between sCOMP and maximum hip flexion moment of the unaffected side after THR was detected. Rather, other factors seem to have an influence on the release of COMP from cartilage.
We observed a significantly decreased sCOMP 7 days after THR, analyzed using an ELISA. These results partially confirmed our first hypothesis that sCOMP would permanently decrease after removal of the arthritic hip joint, since in OA COMP originates mainly from the affected joint and is mechanically released into the serum [20,31]. However, contrary to our hypothesis, 3 months and 1 year after THR we observed sCOMP in the range of baseline concentrations before THR. In order to explain this result, the relevant influences on the release of COMP are pointed out in the following. A certain basal level of sCOMP can be detected in healthy individuals. The majority of COMP is in its intact form and released as a result of normal loading of and extrusion from cartilage tissue [21]. Increased sCOMP in patients with OA has been described in several studies with the explanation that additional COMP is released in response to cartilage damage [[18], [19], [20],24,31]. As mentioned above, COMP is then released as both intact as well as fragmented protein [20,21]. Further degradation might occur in the serum. Since the antibodies used in different assays might target different parts of the COMP molecule, a comparison of absolute sCOMP is generally difficult. Further, different commercially available assays [19,20,31] report results in units [31] that cannot be converted like mg/l and U/l. Addison et al. [49] demonstrated a correlation of sCOMP with the total-body bone scintigraphy in patients with symptomatic OA. This means that sCOMP is related to total-body burden of OA. Clark et al. [19] described a dependence of sCOMP on the size and number of affected joints and severity of disease. If one refers these results to our study, sCOMP would have to decrease permanently after removal of the large affected hip joint in patients with unilateral hip OA.
The results of the present study suggest that there are further influences on the release of COMP from the extracellular cartilage matrix. Liphardt et al. [32,33] who investigated sCOMP in bed rest studies could detect a reduced sCOMP due to immobilization and thus lacking mechanical loading of the tissue. The decrease in sCOMP observed in our study 7 days after surgery may indicate that limited mobility and reduced mechanical loading during the first days after THR. COMP is not only released by cartilage damage in OA, but to some extent also by increased but physiological joint load during walking and running, as the release of COMP is mechanosensitive [[27], [28], [29], [30]]. For our results, this could mean that patients 3 months and 1 year after surgery are more mobile than 7 days postoperative because they are free from symptoms and pain. The increased mobility 3 months and 1 year postoperatively compared to the preoperative time compensates for the expected permanent decrease of sCOMP after removal of the arthritic hip. Thus, the same level of preoperative concentrations and those 3 months and 1 year postoperatively can be explained. Sharif and colleagues [34] reported increased sCOMP 3 months after total knee replacement (TKR), persisting for up to 12 months. This is in contrast to our study, even though we also did not detect a decrease or a difference in sCOMP 3 and 12 months postoperatively compared to the baseline measurement. The discrepancy between both studies could be explained as follows: First, Sharif and colleagues [34] examined patients with knee OA after TKR, in contrast to our study which examined patients with unilateral hip OA after THR. The contribution of COMP from the respective joint cartilage to the measured sCOMP is generally not known and may therefore be different for knee and hip joint. Second, in our study, blood samples were taken after a resting time of at least 30 min to minimize the influence of previous activity. Sharif and colleagues [34] did not specify a standardized resting period before blood samples were taken. This could be a reason for the postoperative increase in sCOMP in the study of Sharif and colleagues [34], because patients were free from symptoms and pain and therefore more mobile. Third, blood samples were only taken 3 months postoperatively and not after 7 days as in our study. These differences between both studies make it difficult to compare the results. In summary, the results show that sCOMP depended not only on OA but also on activity before blood sampling. In order to reduce the short-term impact of activity and immobilization on sCOMP in the present study design, patients rested at least 30 min before blood sampling [27,47]. Obviously, a restricted movement over 7 days after THR has a greater impact on the release of COMP into the serum than a resting time of 30 min immediately before blood sampling. On the other hand, the influence of fatigue after previous defined activity, which has not been taken into account in the present study, may have an influence on the COMP analysis and gait parameters. This aspect was outside the scope of this research and should therefore be considered in the design of future studies.
We could not find a significant correlation between sCOMP and joint moments during gait before THR. Therefore, the second hypothesis that a higher sCOMP before THR would directly correlate with a lower dynamic hip and knee joint loading of the affected side and with a higher dynamic hip and knee joint loading of the non-affected was refuted. Herger et al. [36] and Denning et al. [37] were able to demonstrate a load-induced increase in sCOMP in healthy volunteers. According to their results, the already described increased joint load on the unaffected joint before THR [[3], [4], [5], [6]] should lead preoperatively to increased sCOMP. Firner et al. [38] investigated the effect of increased external knee flexion moments on sCOMP in healthy volunteers. The participants ran on a treadmill with a passive and then with active knee orthoses. No significant differences were found between external knee flexion moments and sCOMP. Consequently, our study confirmed these results as sCOMP did not correlate with hip and knee joint moments in patients affected by OA.
With respect to our third hypothesis, 1 year after THR a significant correlation between sCOMP and maximum hip flexion moment for the non-affected side could be observed in the first half of the stance phase. One year after THR, COMP can no longer be released from the artificial hip joint. The measured sCOMP must have been released either from the remaining articular cartilage with unknown OA status or other tissues that contain COMP [26]. Increased production or degradation of COMP in cartilage may be the reason for a release into the blood serum [34]. This underlines the potential of COMP as a prognostic marker, as the degradation of articular cartilage begins before the onset of pain and radiological changes occur [17,18,35]. However, the gait pattern 1 year after THR is still altered and can therefore also influence sCOMP. Previous research described increased joint load of the non-affected side after THR [6,[14], [15], [16]]. Consequently, increased joint loading would result in a load-induced release of COMP into serum as previously described for healthy volunteers [36,37]. However, we were only able to observe a negative correlation. Therefore, our results provide no scientific evidence for a load-induced release of COMP in patients with unilateral hip OA treated with a THR.
The results of the present study should be interpreted carefully and in light of its limitations. First, we examined a small number of patients. However, we collected sufficient data at different time points to obtain obvious and significant differences in sCOMP. In addition, the increase of sCOMP 3 months after THR to baseline concentration was confirmed by a fourth measurement 1 year after THR in ten patients. To avoid inter-assay variation of sCOMP, samples were analyzed for each patient on the same plate. Second, due to the surprising course of sCOMP after THR, it would be interesting to know whether COMP is released as an intact protein or in form of specific fragments into the serum [20,21]. Third, although we adhered to a resting time of at least 30 min before each blood sampling, the interpretation of our results regarding OA is limited by the load dependence of sCOMP at the time of the first follow-up after THR. The use of a step counter during study participation would be a possibility to measure the long-term mobility of the participants in order to better incorporate this into the interpretation of sCOMP. Fourth, it would have been interesting to analyze COMP not only in serum but also in synovial fluid and collect blood samples at other times to find out exactly when sCOMP increases. This was not possible in our study for clinical-practical and ethical reasons and warrants further investigation. Fifth, the use of medication as well as the type and extent of physiotherapy were not documented during the study period.
In conclusion, the present longitudinal study was the first that analyzed the effect of THR on sCOMP at different postoperative time points. We were able to show that sCOMP initial decreased after THR and then increased again to the preoperative baseline concentration after THR. Our results indicate that the release of COMP is primarily influenced by mobility and that the removal of a hip joint affected by OA does not have a sustained influence on sCOMP. Due to the absence of substantiated correlation between sCOMP and biomechanical joint loading using instrumented gait analysis, the examination of COMP is not useful to detect altered joint loads during gait that may influence degenerative changes of adjacent joints after THR. Future studies are necessary to better understand the initial decrease in sCOMP immediately after THR and the subsequent and persistent increase to the preoperative level 1 year after THR. In particular, a potential OA progression in other joints could influence sCOMP within the one year follow up time. It is important to use the aforementioned approaches to improve study design to determine whether COMP is a suitable prognostic marker for degeneration of articular cartilage in patients with OA. The aim could be the screening of high-risk patients and an earlier diagnosis of OA. This makes it possible to develop more effective and specific therapeutic approaches for the treatment of patients before irreversible damage to articular cartilage occurs.
Author contributions
FS, FZ, SvD and AM concepted and designed the study; EE, FS and SvD acquired data; EE processed the blood samples; EE, FS and SvD processed and analyzed data of gait analysis; EE, FS and FZ interpreted data; EE and FS performed the statistical analysis; EE drafted the article; FS, FZ, SvD and AM revised it critically for important intellectual content; all authors approved the final and submitted version.
Role of the funding source
No contributors or sources of funding were involved in the study.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank all subjects for volunteering to participate in this study, Inna Schaible and Natalie Molotkov for their help in blood sample analysis, and Zoe Feja and Jana Holder for their help in gait data collection.
Contributor Information
Elisa Endres, Email: elisa.endres@icloud.com.
Stefan van Drongelen, Email: s.vandrongelen@friedrichsheim.de.
Andrea Meurer, Email: a.meurer@friedrichsheim.de.
Frank Zaucke, Email: f.zaucke@friedrichsheim.de.
Felix Stief, Email: f.stief@friedrichsheim.de.
References
- 1.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Constantinou M., Barrett R., Brown M., Mills P. Spatial-temporal gait characteristics in individuals with hip osteoarthritis: a systematic literature review and meta-analysis. J. Orthop. Sports Phys. Ther. 2014;44 doi: 10.2519/jospt.2014.4634. 291–B7. [DOI] [PubMed] [Google Scholar]
- 3.Shakoor N., Hurwitz D.E., Block J.A., Shott S., Case J.P. Asymmetric knee loading in advanced unilateral hip osteoarthritis. Arthritis Rheum. 2003;48:1556–1561. doi: 10.1002/art.11034. [DOI] [PubMed] [Google Scholar]
- 4.Foucher K.C., Hurwitz D.E., Wimmer M.A. Preoperative gait adaptations persist one year after surgery in clinically well-functioning total hip replacement patients. J. Biomech. 2007;40:3432–3437. doi: 10.1016/j.jbiomech.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz D.E., Hulet C.H., Andriacchi T.P., Rosenberg A.G., Galante J.O. Gait compensations in patients with osteoarthritis of the hip and their relationship to pain and passive hip motion. J. Orthop. Res. 1997;15:629–635. doi: 10.1002/jor.1100150421. [DOI] [PubMed] [Google Scholar]
- 6.Foucher K.C., Wimmer M.A. Contralateral hip and knee gait biomechanics are unchanged by total hip replacement for unilateral hip osteoarthritis. Gait Posture. 2012;35:61–65. doi: 10.1016/j.gaitpost.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Andriacchi T.P., Mündermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr. Opin. Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki T., Wada M., Kawahara H., Sato M., Baba H., Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann. Rheum. Dis. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reininga I.H., Stevens M., Wagenmakers R., Bulstra S.K., Groothoff J.W., Zijlstra W. Subjects with hip osteoarthritis show distinctive patterns of trunk movements during gait-a body-fixed-sensor based analysis. J. NeuroEng. Rehabil. 2012;9:3. doi: 10.1186/1743-0003-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer C.A., Corten K., Fieuws S., Deschamps K., Monari D., Wesseling M., et al. Biomechanical gait features associated with hip osteoarthritis: towards a better definition of clinical hallmarks. J. Orthop. Res. 2015;33:1498–1507. doi: 10.1002/jor.22924. [DOI] [PubMed] [Google Scholar]
- 11.Stief F., Böhm H., Ebert C., Döderlein L., Meurer A. Effect of compensatory trunk movements on knee and hip joint loading during gait in children with different orthopedic pathologies. Gait Posture. 2014;39:859–864. doi: 10.1016/j.gaitpost.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Mündermann A., Asay J.L., Mündermann L., Andriacchi T.P. Implications of increased medio-lateral trunk sway for ambulatory mechanics. J. Biomech. 2008;41:165–170. doi: 10.1016/j.jbiomech.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt A., Meurer A., Lenarz K., Vogt L., Froemel D., Lutz F., et al. Unilateral hip osteoarthritis: the effect of compensation strategies and anatomic measurements on frontal plane joint loading. J. Orthop. Res. 2017;35:1764–1773. doi: 10.1002/jor.23444. [DOI] [PubMed] [Google Scholar]
- 14.Stief F., Schmidt A., van Drongelen S., Lenarz K., Froemel D., Tarhan T., et al. Abnormal loading of the hip and knee joints in unilateral hip osteoarthritis persists two years after total hip replacement. J. Orthop. Res. 2018;36:2167–2177. doi: 10.1002/jor.23886. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu M.L., Lamontagne M., Beaulé P.E. Lower limb biomechanics during gait do not return to normal following total hip arthroplasty. Gait Posture. 2010;32:269–273. doi: 10.1016/j.gaitpost.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Bennett D., Ryan P., O'Brien S., Beverland D.E. Gait kinetics of total hip replacement patients-A large scale, long-term follow-up study. Gait Posture. 2017;53:173–178. doi: 10.1016/j.gaitpost.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Nepple J.J., Thomason K.M., An T.W., Harris-Hayes M., Clohisy J.C. What is the utility of biomarkers for assessing the pathophysiology of hip osteoarthritis? A systematic review. Clin. Orthop. Relat. Res. 2015;473:1683–1701. doi: 10.1007/s11999-015-4148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi X. Correlation of serum cartilage oligomeric matrix protein with knee osteoarthritis diagnosis: a meta-analysis. J. Orthop. Surg. Res. 2018;13:262. doi: 10.1186/s13018-018-0959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark A.G., Jordan J.M., Vilim V., Renner J.B., Dragomir A.D., Luta G., et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42:2356–2364. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Neidhart M., Hauser N., Paulsson M., DiCesare P.E., Michel B.A., Häuselmann H.J. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br. J. Rheumatol. 1997;36:1151–1160. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]
- 21.Lai Y., Yu X.-P., Zhang Y., Tian Q., Song H., Mucignat M.T., et al. Enhanced COMP catabolism detected in serum of patients with arthritis and animal disease models through a novel capture ELISA. Osteoarthritis Cartilage. 2012;20:854–862. doi: 10.1016/j.joca.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer D.C., Hunter D.J., Abramson S.B., Attur M., Corr M., Felson D., et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14:723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Hedbom E., Antonsson P., Hjerpe A., Aeschlimann D., Paulsson M., Rosa-Pimentel E., et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. Chem. 1992;267:6132–6136. [PubMed] [Google Scholar]
- 24.Saxne T., Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br. J. Rheumatol. 1992;31:583–591. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- 25.Tan K., Duquette M., Joachimiak A., Lawler J. The crystal structure of the signature domain of cartilage oligomeric matrix protein: implications for collagen, glycosaminoglycan and integrin binding. Faseb. J. 2009;23:2490–2501. doi: 10.1096/fj.08-128090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng S., Reddi A.H., Di Cesare P.E. Cartilage oligomeric matrix protein (COMP): a biomarker of arthritis. Biomark. Insights. 2009;4:33–44. doi: 10.4137/bmi.s645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mündermann A., Dyrby C.O., Andriacchi T.P., King K.B. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthritis Cartilage. 2005;13:34–38. doi: 10.1016/j.joca.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Mündermann A., Nüesch C., Klenk C., Billich C., Nickel T., Pagenstert G., et al. Changes in cartilage biomarker levels during a transcontinental multistage footrace over 4486 km. Am. J. Sports Med. 2017;45:2630–2636. doi: 10.1177/0363546517712945. [DOI] [PubMed] [Google Scholar]
- 29.Neidhart M., Müller-Ladner U., Frey W., Bosserhoff A.K., Colombani P.C., Frey-Rindova P., et al. Increased serum levels of non-collagenous matrix proteins (cartilage oligomeric matrix protein and melanoma inhibitory activity) in marathon runners. Osteoarthritis Cartilage. 2000;8:222–229. doi: 10.1053/joca.1999.0293. [DOI] [PubMed] [Google Scholar]
- 30.Niehoff A., Kersting U.G., Helling S., Dargel J., Maurer J., Thevis M., et al. Different mechanical loading protocols influence serum cartilage oligomeric matrix protein levels in young healthy humans. Eur. J. Appl. Physiol. 2010;110:651–657. doi: 10.1007/s00421-010-1529-0. [DOI] [PubMed] [Google Scholar]
- 31.Arends R.H.G.P., Karsdal M.A., Verburg K.M., West C.R., Bay-Jensen A.C., Keller D.S. Identification of serological biomarker profiles associated with total joint replacement in osteoarthritis patients. Osteoarthritis Cartilage. 2017;25:866–877. doi: 10.1016/j.joca.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Liphardt A.-M., Mündermann A., Andriacchi T.P., Achtzehn S., Heer M., Mester J. Sensitivity of serum concentration of cartilage biomarkers to 21-days of bed rest. J. Orthop. Res. 2018;36:1465–1471. doi: 10.1002/jor.23786. [DOI] [PubMed] [Google Scholar]
- 33.Liphardt A.-M., Mündermann A., Koo S., Bäcker N., Andriacchi T.P., Zange J., et al. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligometric matrix protein (COMP) during immobilization. Osteoarthritis Cartilage. 2009;17:1598–1603. doi: 10.1016/j.joca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Sharif M., Kirwan J.R., Elson C.J., Granell R., Clarke S. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50:2479–2488. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]
- 35.Emery C.A., Whittaker J.L., Mahmoudian A., Lohmander L.S., Roos E.M., Bennell K.L., et al. Establishing outcome measures in early knee osteoarthritis. Nat. Rev. Rheumatol. 2019;15:438–448. doi: 10.1038/s41584-019-0237-3. [DOI] [PubMed] [Google Scholar]
- 36.Herger S., Vach W., Liphardt A.-M., Egloff C., Nüesch C., Mündermann A. Dose-response relationship between ambulatory load magnitude and load-induced changes in COMP in young healthy adults. Osteoarthritis Cartilage. 2019;27:106–113. doi: 10.1016/j.joca.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Denning W.M., Becker Pardo M., Winward J.G., Hunter I., Ridge S., Hopkins J.T., et al. Ambulation speed and corresponding mechanics are associated with changes in serum cartilage oligomeric matrix protein. Gait Posture. 2016;44:131–136. doi: 10.1016/j.gaitpost.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Firner S., Willwacher S., de Marées M., Bleuel J., Zaucke F., Brüggemann G.-P., et al. Effect of increased mechanical knee joint loading during running on the serum concentration of cartilage oligomeric matrix protein (COMP) J. Orthop. Res. 2018;36:1937–1946. doi: 10.1002/jor.23859. [DOI] [PubMed] [Google Scholar]
- 39.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stief F., Böhm H., Michel K., Schwirtz A., Döderlein L. Reliability and accuracy in three-dimensional gait analysis: a comparison of two lower body protocols. J. Appl. Biomech. 2013;29:105–111. doi: 10.1123/jab.29.1.105. [DOI] [PubMed] [Google Scholar]
- 41.Kadaba M.P., Ramakrishnan H.K., Wootten M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990;8:383–392. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 42.Davis R.B., III, Õunpuu S., Tyburski D., Gage J.R. A gait data collection and reduction technique. Hum. Mov. Sci. 1991;10:575–587. doi: 10.1016/0167-9457(91)90046-Z. [DOI] [Google Scholar]
- 43.Stief F. In: Handbook of Human Motion. Müller B., Wolf S.I., Brüggemann G.P., Deng Z., McIntosh A., Miller F., et al., editors. Springer International Publishing; Basel: 2016. Variations of marker sets and models for standard gait analysis; pp. 1–18. [Google Scholar]
- 44.Woltring H.J. Representation and calculation of 3-D joint movement. Hum. Mov. Sci. 1991;10:603–616. doi: 10.1016/0167-9457(91)90048-3. [DOI] [Google Scholar]
- 45.Reinschmidt C., van den Bogert A., Lundberg A., Nigg B.M., Murphy N., Stacoff A., et al. Tibiofemoral and tibiocalcaneal motion during walking: external vs. skeletal markers. Gait Posture. 1997;6:98–109. doi: 10.1016/S0966-6362(97)01110-7. [DOI] [Google Scholar]
- 46.Ewen A.M., Stewart S., Gibson A., Kashyap S.N., Caplan N. Post-operative gait analysis in total hip replacement patients-a review of current literature and meta-analysis. Gait Posture. 2012;36:1–6. doi: 10.1016/j.gaitpost.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 47.Andersson M.L., Thorstensson C.A., Roos E.M., Petersson I.F., Heinegård D., Saxne T. Serum levels of cartilage oligomeric matrix protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC Muscoskel. Disord. 2006;7:98. doi: 10.1186/1471-2474-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen J. second ed. Lawrence Erlbaum Associates; Philadelphia: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 49.Addison S., Coleman R.E., Feng S., McDaniel G., Kraus V.B. Whole-body bone scintigraphy provides a measure of the total-body burden of osteoarthritis for the purpose of systemic biomarker validation. Arthritis Rheum. 2009;60:3366–3373. doi: 10.1002/art.24856. [DOI] [PMC free article] [PubMed] [Google Scholar]