Abstract
Objective
We aimed to evaluate the association between inflammatory biomarkers in peripheral blood and severity of knee osteoarthritis (OA).
Methods
We performed a cross-sectional study in participants with frequent knee pain, evaluated radiographic and clinical severity. We measured inflammatory biomarkers: plasma (p) IL-1Ra, IL-1β, IL-18, serum (s) CD14, hsCRP and bone and cartilage biomarkers: urine (u) CTX-II, (s) HA, COMP, CTX-I, PIIANP. We assessed radiographic severity by Kellgren-Lawrence (KL) grading and Osteoarthritis Research Society International (OARSI) standardized scoring atlas; and clinical severity by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).
Results
139 participants (82% women, mean ± SD age: 55.5 ± 7.8 years) were included. (p) IL-1Ra was negatively associated with radiographic severity by KL grading (Spearman rho = −0.197, P = 0.021), osteophytes (Spearman rho = −0.217, P = 0.011), and joint space narrowing of index knee (Spearman rho = −0.172, P = 0.045); and KL sum score of both knees (Spearman rho = −0.180, P = 0.035), after adjustment for age, gender and body mass index (BMI). Other inflammatory markers were not associated with radiographic severity. Cartilage degradation markers (u) CTXII and (s) COMP were modestly associated with radiographic severity after adjustment. In multivariate models, (s) hsCRP and the bone and cartilage biomarkers, but not the inflammatory biomarkers, were associated with radiographic severity.
Conclusion
Among the inflammatory biomarkers in peripheral blood, IL-1Ra was negatively associated with radiographic severity in this early knee OA cohort.
Keywords: Osteoarthritis, Biomarkers, Radiography, Inflammation
1. Introduction
Osteoarthritis (OA) is a leading cause of disability that imposes huge burden to affected individuals, healthcare and social services worldwide [1]. Current therapy is directed at symptom relief using analgesics and joint replacement surgery for end-stage disease [2]. Inflammation has been shown to underlie the pathogenesis of OA [3]. Inflammatory changes with production of pro-inflammatory cytokines was a feature of synovial membranes from patients from early to late stages of OA [4]. Inflammatory cytokines in serum preceded radiographic changes of OA by many years and were shown to stimulate the release of degradative matrix metalloproteinases (MMPs) by synovial macrophages and chondrocytes [3,4]. Determining if inflammatory biomarkers are associated with a higher burden of OA is an important first step in biomarker discovery and gives insights into pathological inflammatory mechanisms underlying OA. This would be specifically meaningful for early stage knee OA where clinical interventions may have greater therapeutic efficacy and disease may be reversible.
Interleukin-1β (IL-1β), a major pathogenic cytokine in OA [5], was shown to induce the collagen-degrading and aggrecan-degrading enzymes including MMPs and A Disintegrin And Metalloproteinase with Thrombospondin motifs (ADAMTs) [6]. IL-1β and the co-secretary IL-18 were found to be regulators of cartilage degradation [7,8] and were also associated with the severity of knee OA [9,10]. Additionally, polymorphisms of the Interleukin-1 Receptor antagonist (IL-1Ra) gene (ILRN) and plasma (p) levels of IL-1Ra, the natural antagonist of IL-1β, were found to be associated with progression of knee OA [11,12].
IL-1β and other cytokines are produced by synovial macrophages, chondrocytes and synovial fibroblasts in the joint [13,14]. In an animal model with depleted synovial macrophages, the expression of MMP-3 was reduced [15]. Using a scintigraphic imaging technique via (99 m) Tc-EC20 (Etarfolatide) that binds to folate receptor-beta, activated but not resting macrophages can be detected in vivo [16]. The quantity of activated macrophages was positively associated with radiographic knee OA severity and joint symptoms [16]. On the other hand, soluble CD14 in synovial fluid was shown to be associated with the abundance of activated macrophages in synovium, and therefore is a marker of activated macrophages [17]. Additionally, soluble CD14 was associated with radiographic and clinical severity of knee OA, as well as with the radiographic progression of knee OA over time [17].
High-sensitivity C-reactive protein (hsCRP) is a well-known biomarker of systemic inflammation and was shown to be associated with synovitis and increased pain in OA. Two systematic reviews have shown that serum hsCRP was associated with OA pain and physical function, but not with prevalence or progression of radiographic OA [18,19].
Nevertheless, the evidence that supports the link between inflammation and OA severity is sparse and require further validation. Inflammatory mediators produced by articular tissue in OA can be reflected in peripheral blood [20]. Circulating blood leukocytes in patients with knee OA exhibit higher IL-1b gene expression [9] and soluble inflammatory cytokines including IL-1b, IL1Ra, IL-6 were found to be at higher levels in the peripheral blood from patients with joint effusions compared with controls (11). Although present at a lower concentration, serum levels of soluble inflammatory cytokines generally mirror levels in synovial fluid from the OA joints [17]. Soluble cytokines present in peripheral blood are more feasible to develop into biomarkers for use in clinical practice compared to that from synovial fluid. We hypothesize that in early knee OA, inflammatory biomarkers are associated with radiographic and clinical severity. In this cross-sectional study, we aim to evaluate the associations between peripheral blood inflammatory biomarkers IL-1β, IL-1Ra, IL-18, CD14 and hsCRP with radiographic severity in early knee OA. Our secondary aim was to evaluate the association between the inflammatory biomarkers and other known biomarkers involved in bone and cartilage turnover.
2. Materials and methods
2.1. Study design
We conducted a cross-sectional study of 139 participants with chronic knee pain at a single tertiary rheumatology centre at the Singapore General Hospital, Singapore. The study protocol was read and approved by the SingHealth Centralized Institutional Review Board (Ref: 2012/837/E) and informed consent was obtained from all participants prior to the start of the study.
2.2. Participants recruitment
We recruited participants (40−79 years old) from the community via advertisements, social media and referrals from SingHealth Polyclinics and the Singapore General Hospital. Participants contacted a recruitment hotline and were screened by a trained staff. Participants who had pain in at least one knee on most days during the past month were invited to a screening clinic visit. All participants were examined by an experienced rheumatologist (YYL) at the clinic. Study inclusion required positive response to question “Do you have pain, aching or stiffness of the knee on most days of the past month.” Participants were excluded if they had prior total knee replacement surgery; late stage OA defined as Kellgren-Lawrence (KL) grade 4 on radiography [21] as read by the recruiting rheumatologist or were planning for knee replacement surgery in the next 6 months; isolated patellofemoral joint involvement on radiography; significant joint injuries in the past one-year; and other joint diseases (rheumatoid arthritis, spondyloarthritis, Paget's disease, joint fractures, hyperparathyroidism, hyperthyroidism, hypothyroidism). Participants were classified into knee OA and no knee OA using the American College of Rheumatology (ACR) Clinical and Radiographic Criteria [22]. These criteria required knee pain, at least 1 of 3 of the following: i) older than 50 years old, ii) less than 30 min of knee stiffness or iii) crepitus, and a radiographic criteria of KL grade ≥2 on either knee. As the broader study protocol was to evaluate biomarkers in the synovial fluid and knee OA severity on magnetic resonance imaging (MRI), we excluded participants with contraindications to MRI, such as significant renal impairment, pregnancy, metallic implants in situ and claustrophobia. Participants with warfarin usage were also excluded due to an increase risk of bleeding after joint aspiration. The MRI and synovial fluid biomarkers data are outside the scope of the current study.
2.3. Clinical data collection
We collected clinical variables including age, gender, ethnic group, history of major joint injuries or surgeries, comorbidities (hypertension, hyperlipidemia, diabetes mellitus, and coronary artery disease) and current medications. We measured body weight and height in the clinic using an electronic weight scale and an ultrasonic height sensor (Avamech B1000 Series) without shoes and calculated the body mass index (BMI) in kg/m2. The index knee was defined as the more symptomatic knee, or the dominant knee if symptoms were equal on both sides. Clinical severity of knee OA was evaluated using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [23] of the index knee that comprises 24 items divided into 3 subscales − pain, stiffness and physical function. Each domain was standardized to obtain a score ranging from 0 to 100; higher scores reflected greater clinical severity.
2.4. Radiographic assessment
At recruitment, all participants underwent posteroanterior fixed-flexion weight-bearing knee radiography of both knees with a SynaFlexer lower limb positioning frame (Synarc). All radiographies were taken starting with a 10° caudal beam angle until the alignment of anterior and posterior margins of the tibial plateau within 1.2 mm was achieved [24]. The radiograph of each knee was scored by an experienced musculoskeletal radiologist (SBW) blinded to the clinical condition of the participant according to the KL grading (0–4) [21]. In addition, radiographic features of Osteophytes (OST) and Joint Space Narrowing (JSN) for each knee were scored in accordance to the Osteoarthritis Research Society International (OARSI) standardized atlas [25] by a rheumatologist (YYL) experienced in OA. Radiographic severity of knee OA was defined by the following radiographic scoring: i) KL grading (0–4), ii) OST (0–12), and iii) JSN (0–6) of the medial and lateral compartments of tibiofemoral (TF). The radiographic scores from both knees were summated to obtain a KL Sum Score (0–8), OST Sum Score (0–24) and JSN Sum Score (0–12) scores [26]. The summated scores were hypothesized to better reflect total disease burden compared to index knee scores and thus have a more relevant association with peripheral blood biomarkers. A total of 70 (25%) of radiographs were re-scored for KL grades, and 80 (30%) for OST and JSN by the original assessors 8−12 weeks apart and blinded to the original scores. The intraclass correlation coefficients (ICCs) of KL grade of TF compartment, OST and JSN were 0.75 (95% confidence interval, CI: 0.60–0.84), 0.91 (95% CI: 0.85–0.94) and 0.86 (95% CI: 0.74–0.92), respectively.
2.5. Biological sample collection and processing
We collected blood samples from each participant at recruitment at least 2 hours post-prandial using a needle and syringe. We then transferred the blood samples into BD Vacutainer® SST™ Tubes containing spray-coated silica and a polymer gel for serum separation and BD Vacutainer® K2 EDTA tubes. Blood samples were centrifuged at 3000 revolutions per minute for 15 min. Supernatant of serum and plasma was isolated from the gel tubes and EDTA tubes respectively. Biological samples were processed and aliquoted within 2 hours from collection and stored at −80 °C until analysis. The collection of urine samples was standardized as the second void of the day.
2.6. Biomarker measurement
We measured the following serum (s), plasma (p) and urine (u) biomarkers using commercially available enzyme-linked immunosorbent assay (ELISA) kits: (p) IL-1Ra, IL-1β, IL-18; (s) CD14, hsCRP, C-terminal telopeptides of Type I collagen (CTX-I), Type IIA Collagen N-Propeptide (PIIANP), Cartilage Oligomeric Matrix Protein (COMP), Hyaluronic Acid (HA); (u) C-Telopeptide of Type II Collagen (CTXII). Details on the function of biomarkers, manufacturers and sensitivity information of ELISA kits are summarized in Supplementary Table I. All dilution and measurements were performed according to the manufacturer's protocol and in duplicates to obtain the intra-assay coefficient of variations (CVs).
2.7. Statistical analysis
Parametric and non-parametric baseline characteristic data were described and analyzed using student t-test and chi-square test as appropriate. Majority of the biomarkers except PIIANP and IL-18 were not normally distributed and therefore the biomarkers were analyzed with non-parametric tests for consistency.
We assessed in the univariate analysis, the Spearman's Rho between various biomarkers with radiographic severity by KL, OST and JSN Index and Sum Scores; and clinical severity by WOMAC pain and function. We conducted partial correlations for each biomarker with radiographic and clinical severity of knee OA adjusted for age and sex in Model 1, and further adjusted for BMI in Model 2.
In the multivariable analysis, we assessed the associations between biomarkers and the radiographic severity of knee OA using linear regression, backwards elimination by likelihood ratio, and adjusted for age, gender and BMI.
A P-value < 0.05 (two-tailed) was regarded as statistically significant. Partial correlations were conducted using R 3.1.1 (R Core Team, Vienna, Austria) and the other analyses were conducted using SPSS version 23 (IBM Corp.).
3. Results
3.1. Baseline characteristics of participants
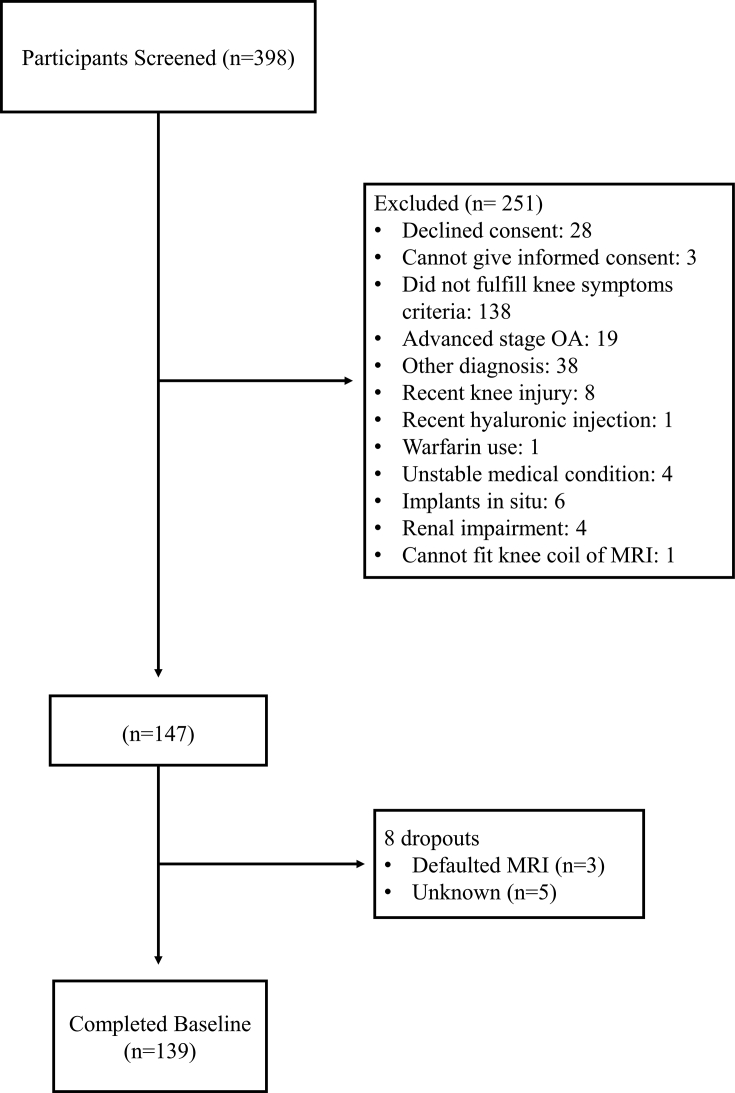
The details of recruitment of 139 participants are illustrated in Fig. 1. Baseline demographic and clinical characteristics of participants are summarized in Table 1. The mean ± standard deviation (SD) age and BMI of the 139 participants (82% women) were 55.5 ± 7.8 years and 26.0 ± 5.9 kg/m2 43.2% of these participants had knee OA according to the ACR clinical and radiographical classification criteria. Participants with knee OA had higher BMI and were more likely to have hypertension. Concentrations of inflammatory biomarkers (p) IL-1Ra, (p) IL-1β, (p) IL-18 and (s) CD14 were similar between the two groups, however (s) hsCRP was significantly higher in the knee OA group. As for the bone and cartilage biomarkers, only (s) COMP and (s) CTXI were significantly elevated in the knee OA group. The other bone and cartilage biomarkers tend to be higher in the knee OA group, but not statistically insignificant. The ranges of these biomarkers were comparable to other knee OA studies [10,11,27].
Fig. 1.
Flow diagram of participant recruitment. Legend: OA = Osteoarthritis; MRI = Magnetic Resonance Imaging; Other diagnosis includes suspected Sjogren's syndrome, systemic lupus erythematosus, inflammatory bowel disease, inflammatory arthritis, tendonitis/bursitis, muscle sprain, spine or back problems, suspected chondroid tumor, hypothyroidism.
Table 1.
Baseline characteristics of 139 participants. Knee osteoarthritis (KOA) was classified according to the American College of Rheumatology Clinical and Radiographic Criteria for KOA.
| Total (n = 139) | No KOA (n = 79) | KOA (n = 60) | P-value | |
|---|---|---|---|---|
| Female (%) | 82.0 | 79.8 | 85.0 | 0.424 |
| Age, yearsa | 55.5 ± 7.8 | 54.4 ± 7.8 | 56.9 ± 7.6 | 0.061 |
| Ethnicity (%) | ||||
| Chinese/Malay/Indian/Others | 81.3/10.1/7.2/1.4 | 81.0/8.9/7.6/2.5 | 81.7/11.7/6.7/0.0 | 0.609 |
| BMI, kg/m2a | 26.0 ± 5.9 | 24.6 ± 4.5 | 28.0 ± 6.9 | 0.001∗∗ |
| Comorbidities (%) | ||||
| Hypertension | 30.4 | 22.8 | 40.0 | 0.024∗ |
| Diabetes | 8.0 | 8.9 | 6.7 | 0.655 |
| Hyperlipidemia | 38.4 | 38.0 | 38.3 | 0.904 |
| Coronary artery disease | 2.9 | 3.8 | 1.7 | 0.466 |
| Highest KL grade of either knee (%) | ||||
| 0 | 22.6 | 49.4 | 0.0 | |
| 1 | 31.4 | 50.6 | 0.0 | |
| 2 | 27 | 0.0 | 65.0 | |
| 3 | 16.8 | 0.0 | 31.7 | |
| 4 | 2.2 | 0.0 | 3.3 | |
| WOMAC pain (0–100)a | 32.9 ± 18.8 | 29.8 ± 17.6 | 32.9 ± 19.5 | 0.332 |
| WOMAC function (0–100)a | 32.0 ± 19.9 | 30.1 ± 20.2 | 34.5 ± 19.4 | 0.201 |
| (p) IL-1Ra (pg/mL)a | 370.9 ± 220.3 | 373.0 ± 229.4 | 368.1 ± 209.7 | 0.897 |
| (p) IL-1β (%)b | 17.3 | 20.3 | 13.3 | 0.285 |
| (p) IL-18 (pg/mL)a | 181.1 ± 58.2 | 180.64 ± 60.8 | 181.8 ± 55.1 | 0.910 |
| (s) CD14 (ng/mL)a | 1280.9 ± 217.3 | 1280.3 ± 234.3 | 1281.6 ± 194.7 | 0.971 |
| (s) hsCRP (mg/L)a | 3.74 ± 4.48 | 2.8 ± 3.1 | 4.9 ± 5.6 | 0.006∗∗ |
| (s) HA (ng/mL)a | 52.4 ± 71.4 | 47.0 ± 59.2 | 59.5 ± 84.9 | 0.305 |
| (s) COMP (ng/mL)a | 1257.1 ± 338.1 | 1199.0 ± 333.9 | 1333.7 ± 330.9 | 0.019∗ |
| (u) CTXII (ng/mmol Cr)a | 522.0 ± 679.7 | 431.8 ± 540.3 | 640.7 ± 818.2 | 0.072 |
| (s) PIIANP (ng/mL)a | 3130.6 ± 1052.4 | 2982.7 ± 985.0 | 3325.4 ± 1113.6 | 0.057 |
| (s) CTXI (ng/mL)a | 0.25 ± 0.12 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.002∗∗ |
∗P < 0.05; ∗∗P < 0.01; χ2 test for proportions and student t-test for means testing.
BMI = Body Mass Index; KL = Kellgren and Lawrence grading system; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; (p) = plasma; (s) = serum; (u) = urine; IL = interleukin; CD = cluster of differentiation; hsCRP = High-sensitivity C-Reactive Protein; HA = Hyaluronic Acid; COMP = Cartilage Oligomeric Matrix Protein; CTXII = C-terminal crosslinked telopeptides of Type II collagen; PIIANP = Type IIA collagen N-Propeptide; CTXI = C-terminal crosslinked telopeptides of Type I collagen.
(mean ± SD).
Proportion of participants with detectable IL-1β levels.
As less than 75% (only 16.6%) of (p) IL-1β results were above the lower limit of detection (LLOD), (p) IL-1β was analyzed as categorical variable (0 = result below LLOD and 1 = result above LLOD).
3.2. Associations between inflammatory, bone and cartilage biomarkers
The inflammatory biomarkers were significantly associated with each other, but not with the bone and cartilage biomarkers (Supplementary Table II). Among the bone and cartilage markers, (u) CTXII was positively associated to (s) CTXI and (s) PIIANP. The inflammatory biomarkers (p) IL-1Ra, (p) IL-18 and (s) hsCRP, and (s) PIIANP were significantly associated with BMI.
3.3. Associations between biomarkers and knee osteoarthritis severity
We evaluated (p) IL-1Ra, (p) IL-1β, (p) IL-18, (s) CD14 and (s) hsCRP for association with radiographic severity according to KL, OST and JSN Index and Sum Scores (Table 2). A significant inverse association between (p) IL-1Ra with radiographic severity of knee OA using KL Index, KL sum, OST Index and JSN Index was revealed after adjustment for BMI.
Table 2.
Spearman's Rho correlations of bone and cartilage markers with severity of knee osteoarthritis.
| Biomarker |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammatory |
Osteophyte burden, synovitis |
Cartilage Degradation |
Cartilage Synthesis |
Bone Resorption |
|||||||
| (p) IL-1Ra | (p) IL-1βa | (p) IL-18 | (s) CD14 | (s) hsCRP | (s) HA | (s) COMP | (u) CTXII | (s) PIIANP | (s) CTXI | ||
| KL Index Score (0–4) | Unadjusted | 0.027 | −0.124 | 0.049 | 0.115 | 0.214∗ | 0.074 | 0.194∗ | 0.211∗ | 0.156 | 0.230∗ |
| Model 1 | 0.031 | −0.070 | 0.074 | 0.050 | 0.209∗ | 0.081 | 0.134 | 0.176∗ | 0.099 | 0.195∗ | |
| Model 2 | −0.197∗ | −0.046 | −0.044 | 0.083 | 0.116 | 0.115 | 0.090 | 0.194∗ | 0.023 | 0.149 | |
| KL Sum Score (0–8) | Unadjusted | 0.033 | −0.123 | 0.045 | 0.086 | 0.205∗ | 0.088 | 0.174∗ | 0.198∗ | 0.150 | 0.247∗∗ |
| Model 1 | 0.036 | −0.066 | 0.068 | 0.027 | 0.187∗ | 0.084 | 0.120 | 0.151 | 0.105 | 0.213∗∗ | |
| Model 2 | −0.180∗ | −0.051 | −0.060 | 0.065 | 0.078 | 0.120 | 0.053 | 0.180∗ | 0.022 | 0.166 | |
| OST Index Score (0–12) | Unadjusted | 0.028 | 0.046 | 0.226∗∗ | −0.025 | 0.192∗ | 0.042 | 0.262∗∗ | 0.254∗∗ | 0.224∗∗ | 0.072 |
| Model 1 | 0.015 | 0.016 | 0.248∗∗ | −0.063 | 0.188∗ | 0.009 | 0.264∗∗ | 0.238∗∗ | 0.198∗ | 0.060 | |
| Model 2 | −0.217∗ | 0.024 | 0.118 | −0.038 | 0.050 | 0.049 | 0.184∗ | 0.242∗∗ | 0.104 | −0.009 | |
| OST Sum Score (0–24) | Unadjusted | 0.076 | 0.030 | 0.231∗∗ | −0.039 | 0.210∗ | 0.011 | 0.262∗∗ | 0.223∗∗ | 0.207∗ | 0.061 |
| Model 1 | 0.064 | 0.014 | 0.258∗∗ | −0.072 | 0.207∗ | −0.019 | 0.249∗∗ | 0.223∗∗ | 0.183∗ | 0.041 | |
| Model 2 | −0.096 | 0.043 | 0.141 | −0.053 | 0.098 | 0.041 | 0.142 | 0.219∗ | 0.094 | −0.039 | |
| JSN Index Score (0–6) | Unadjusted | 0.005 | −0.171 | 0.125 | 0.040 | 0.276∗∗ | 0.077 | 0.111 | 0.164 | 0.154 | 0.166∗ |
| Model 1 | −0.012 | −0.165 | 0.138 | −0.033 | 0.279∗∗ | 0.100 | 0.033 | 0.176∗ | 0.079 | 0.126 | |
| Model 2 | −0.172∗ | −0.107 | 0.042 | −0.022 | 0.166 | 0.150 | −0.037 | 0.165 | −0.002 | 0.087 | |
| JSN Sum Score (0–12) | Unadjusted | 0.017 | −0.188∗ | 0.099 | −0.006 | 0.220∗ | 0.039 | 0.093 | 0.139 | 0.104 | 0.148 |
| Model 1 | 0.016 | −0.155 | 0.118 | −0.080 | 0.209∗ | 0.050 | 0.060 | 0.120 | 0.059 | 0.118 | |
| Model 2 | −0.123 | −0.119 | 0.035 | −0.051 | 0.127 | 0.085 | −0.007 | 0.133 | −0.033 | 0.069 | |
| WOMAC Pain | Unadjusted | 0.066 | 0.032 | −0.097 | 0.068 | −0.018 | 0.079 | −0.003 | 0.041 | 0.117 | −0.032 |
| Model 1 | 0.056 | 0.056 | −0.089 | 0.040 | −0.042 | 0.038 | 0.003 | −0.002 | 0.098 | −0.028 | |
| Model 2 | 0.017 | 0.054 | −0.153 | 0.048 | −0.113 | 0.022 | −0.002 | −0.003 | 0.080 | −0.051 | |
| WOMAC Function | Unadjusted | −0.101 | 0.070 | −0.045 | 0.049 | −0.157 | 0.010 | −0.026 | 0.171∗ | 0.065 | 0.119 |
| Model 1 | −0.119 | 0.154 | −0.045 | 0.043 | −0.181∗ | −0.008 | −0.070 | 0.090 | 0.031 | 0.111 | |
| Model 2 | −0.095 | 0.104 | −0.088 | 0.000 | −0.148 | 0.039 | −0.051 | 0.044 | 0.034 | 0.128 | |
∗P < 0.05; ∗∗P < 0.01.
Model 1: Adjusted for age, gender. Model 2: Adjusted for age, gender, BMI.
KL = Kellgren and Lawrence grading system; OST = Osteophytes; JSN = Joint Space Narrowing; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; (p) = plasma; (s) = serum; (u) = urine; IL = interleukin; CD = cluster of differentiation; hsCRP = High-sensitivity C-Reactive Protein; HA = Hyaluronic Acid; COMP = Cartilage Oligomeric Matrix Protein; CTXII = C-terminal crosslinked telopeptides of Type II collagen; PIIANP = Type IIA collagen N-Propeptide; CTXI = C-terminal crosslinked telopeptides of Type I collagen.
IL-1β analyzed as categorical variable, 0 < Lower Limit of Detection and 1 ≥ Lower Limit of Detection.
(p)IL-18 and (s) hsCRP were positively associated with radiographic severity of knee OA according to KL, OST and JSN Index and Sum Scores in the univariable analysis, which persisted with adjustment with age and gender, but was not statistically significant after adjustment with BMI. (s) CD14 was not associated with radiographic severity.
The bone and cartilage biomarkers were positively associated with knee OA radiographic severity namely KL Index, KL sum, OST Index and OST sum. However only the cartilage degradation markers (u) CTXII and (s) COMP remained positively associated with knee OA radiographic severity after adjustment for age, gender and BMI.
For clinical severity, only (u) CTXII was positively associated with WOMAC function in the univariable analysis (Table 2), but became insignificant after controlling with age, gender and BMI. All the other biomarkers were not associated with clinical severity.
3.4. Analysis of biomarkers with knee osteoarthritis severity
In the multivariable analysis adjusted for age, gender, BMI and all biomarkers, (s) hsCRP, (s) HA and (s) CTXI were significantly associated with severity of KL sum score; (s) COMP was significantly associated with OST sum score; and lastly, (s) hsCRP and (u) CTXII were significantly associated with JSN sum score (Table 3).
Table 3.
Multivariable analysis of biomarkers with KL, Osteophytes and Joint Space Narrowing Sum Scores.
| Biomarker | β (95% CI) | P-value | |
|---|---|---|---|
| KL Sum Score (0−8) | Age | 0.179 (0.006–0.087) | 0.025∗ |
| Gender | 0.149 (−0.016–1.574) | 0.055 | |
| BMI | 0.265 (0.036–0.146) | 0.001∗∗ | |
| (s) hsCRP | 0.171 (0.006–0.148) | 0.033∗ | |
| (s) HA | 0.181 (0.001–0.009) | 0.018∗ | |
| (u) CTXII | 0.13 (0–0.001) | 0.096 | |
| (s) CTXI | 0.158 (0.075–5.212) | 0.044∗ | |
| OST Sum Score (0–24) | BMI | 0.396 (0.237–0.536) | 0.000∗∗ |
| (s) COMP | 0.192 (0.001–0.006) | 0.014∗ | |
| (u) CTXII | 0.153 (0–0.003) | 0.051 | |
| JSN Sum Score (0–12) | BMI | 0.276 (0.028–0.108) | 0.001∗∗ |
| (p) IL-1βa | −0.145 (−1.147–0.043) | 0.069 | |
| (s) hsCRP | 0.182 (0.007–0.111) | 0.027∗ | |
| (u) CTXII | 0.171 (0–0.001) | 0.034∗ |
∗P < 0.05; ∗∗P < 0.01.
In the multivariate models, important covariates were identified via backward elimination based on likelihood ratios. Clinical variables entered into the multivariate analysis: Age, Gender, BMI, (p) IL-1Ra, (p) IL-1β, (p) IL-18, (s) CD14, (s) hsCRP, (s) HA, (s) COMP, (u) CTXII, (s) PIIANP, (s) CTXI.
(p) = plasma; (s) = serum; (u) = urine; IL = interleukin; CD = cluster of differentiation; hsCRP = High-sensitivity C-Reactive Protein; HA = Hyaluronic Acid; COMP = Cartilage Oligomeric Matrix Protein; CTXII = C-terminal crosslinked telopeptides of Type II collagen; PIIANP = Type IIA collagen N-Propeptide; CTXI = C-terminal crosslinked telopeptides of Type I collagen.
IL-1β analyzed as categorical variable, 0 < Lower Limit of Detection and 1 ≥ Lower Limit of Detection.
4. Discussion
Our study demonstrated that plasma IL-1Ra was inversely associated with radiographic severity of knee OA among participants with frequent knee pain, after adjustment for BMI. Other inflammatory biomarkers were significantly associated with radiographic severity only in the univariate models. In the multivariable analysis, hsCRP, the bone and cartilage degradation biomarkers, together with BMI were significantly associated with radiographic severity, but not the other inflammatory biomarkers. All the biomarkers measured were not associated with clinical severity assessed by WOMAC scores after adjustment for age, gender and BMI.
To our knowledge, this is the first study that demonstrates an inverse association between IL-1Ra and radiographic severity of knee OA. This inverse association between (p) IL-1Ra and radiographic severity was revealed only after adjusting for BMI, suggesting that BMI has a significant interaction with IL-1Ra. IL-1Ra is a soluble anti-inflammatory cytokine that competes with IL-1β for binding to the IL-1 receptor [5]. A lower systemic concentration of IL-1Ra could therefore tilt the balance towards a pro-inflammatory environment in OA. Progression of OA was diminished in an animal OA model induced by meniscectomy and transfected with the IL1RN gene [28]. Polymorphisms of the IL1RN gene in humans predicted radiographic severity and progression of OA [8,29]. A cross sectional study of the Framingham cohort revealed associations for the highest levels of IL-1β production and the presence of knee osteophytes in women [30]. However, BMI was not controlled in the study. As BMI is the most important risk factor of knee OA [31,32], adjustment of BMI would be essential. Attur et al. showed that plasma IL-1Ra was associated with radiographic severity, and participants who had progression of JSN had a higher baseline level of IL-1Ra [11]. However, the association between IL-1Ra and radiographic severity became null after adjustment with age, gender and BMI, which hinted at an intriguing but yet undetermined relationship between IL-1Ra and BMI. In contrast to Attur's study, we showed that IL-1Ra was inversely associated with radiographic severity as opposed to a positive association. There may be a few possible reasons for the contrasting results. Firstly, the effect sizes of association between IL-1Ra and radiographic severity in both studies were small, suggesting that more research is needed on biomarkers related to radiographic and symptom severity. Secondly, the exact role of IL-1Ra in OA pathogenesis is unknown. Although IL-1β has been implicated as a pathogenic cytokine in OA while IL-1Ra is its natural antagonist, it is unclear whether IL-1Ra plays a role directly in the pathogenesis of OA or is merely a bystander. Furthermore, IL-1Ra was shown to be associated with obesity [29] and the presence of obesity would confound epidemiological studies on OA and inflammation. Obesity is associated with an inflammatory state and may increase the risk of knee OA via both an increase in mechanical loading and pressure, and/or effecting an inflammatory state [32]. In a population-based cohort that evaluated the association of IL1RN gene variants with radiographic progression of knee OA, BMI was associated with OA progression in participants carrying a specific IL1RN haplotype, but not in participants without that haplotype [29]. A deeper understanding on the mechanistic pathways of IL-1Ra and IL-1β in the pathogenesis of knee OA, and the interaction between a chronic, systemic inflammatory state in relation with obesity is needed.
IL-1Ra has been studied as a therapeutic target and showing positive results in preclinical studies [[33], [34], [35], [36]]. However, a randomized controlled trial (RCT) targeting a dual antibody neutralising IL-1α and IL-1β has failed to demonstrate the primary outcome target in knee OA [37]. Similarly, intra-articular IL-1β inhibitor, anakinra failed to show clinical efficacies in a RCT in patients with knee OA [38]. Although IL-1β has been implicated to play a major role in the pathogenesis of OA, data from clinical trial is casting doubts on whether IL-1β is as important in OA development as previously considered [39].
One problem of studying IL-1β is its tiny concentrations in the peripheral blood that is difficult to quantify. In our study, (p) IL-1β was detectable in only 16% of samples despite the use of a highly sensitive ELISA kit. Both IL-1β and IL-18 are activated by caspase-1, which is elevated in articular cartilage and synovium in knee OA [40,41]. Similar to IL-1β, IL-18 was also found to promote cartilage degradation and bone loss in knee OA [5]. Genetic variation in the IL18 promoter region was found to be associated with knee OA [42] and increased levels of IL-18 in peripheral blood and synovial fluid in patients with knee OA were positively associated with radiographic severity [43]. This suggests that IL-18, which is more readily detectable in peripheral blood, may serve as a surrogate for IL-1β. In our study however, IL-18 was only associated with radiographic severity of knee OA in the univariable analysis. Evaluation of IL-1β in synovial fluid with a highly sensitive measurement method may be valuable.
CD14, a marker of activated macrophages present in synovial fluid and blood was reported to be associated with severity and progression of radiographic knee OA [17]. In the current study, CD14 in the peripheral blood was not associated with symptomatic or radiographic severity of knee OA. One possible explanation could be that the peripheral blood cytokines are not representative of cytokines in joints with OA. Previous studies that demonstrated positive association between macrophages markers and IL-18 with knee OA severity were generally taken from synovial fluid, which better reflects the pathophysiological processes of an OA joint compared to peripheral blood. Our findings cognizant that these cytokines were more diluted in peripheral blood compared to those found in joints with OA. Peripheral blood biomarkers levels can also be influenced by number of joints with OA, and other systemic factors such as obesity. Another explanation for the difference in findings could be the use of a highly sensitive scintigraphic imaging in other studies that is capable of detecting signals of activated macrophages in joint capsule and synovium, as compared to only bone damage with radiography in the current study [10,44].
The National Institutes of Health (NIH) has recommended the “BIPEDs” biomarker classification as a systematic approach in the selection and usage of OA biomarkers. The “BIPEDs” stands for “Burden of Disease”, “Investigative”, “Prognostic”, , “Diagnostic and safety” [45]. Under this classification system, hsCRP and the bone and cartilage biomarkers that were shown to associate with radiographic knee OA severity in our study and can be identified as biomarkers of “Burden of Disease” that best predict the total burden of disease measured by the summative radiographic scores. This findings are consistent with the Foundation for the National Institutes of Health (FNIH) study, where the bone and cartilage biomarkers ((u) CTXII, (s) HA, (u) CTXIα and (u) crosslinked N-telopeptide of type I collagen (NTXI)) were found to be the best combination in predicting pain and progression of knee OA [27]. However, the development and qualification of “Prognostic markers” are of greater importance compared to biomarkers for “Burden of Diseases”. These inflammatory biomarkers, namely IL-1Ra, IL-18 and hsCRP, which have shown potential to be associated with the radiographic burden of disease in the current study, will be good candidates for further evaluation as “Prognostic” biomarkers in a longitudinal setting for their capacity to predict structural progression of OA.
There are several limitations to the present study. Firstly, the cross-sectional study design makes it impossible to determine the causal relationship between the inflammatory biomarkers and knee OA. Secondly, given the small sample size of this study, the results arising from this study would have to be validated in a larger cohort. Thirdly, the associations we found between inflammatory biomarkers and OA severity were modest. We did not study cytokines at the tissue level (synovial fluid or biopsy), which should better represent the pathophysiology of OA as compared to cytokines in peripheral blood. In this study, we did not evaluate the immune cells in the peripheral blood which are the main source of inflammatory cytokines including IL-1Ra. We think that the soluble biomarkers in the peripheral blood that we have studied is a biological relevant moiety that represents either the burden of systemic inflammation or that diffused from the OA joints. Assay of soluble biomarkers in peripheral blood is the most feasible modality in clinical practice and can be more easily and reliably measured commercialized measurement kits. Moreover, we used radiographic severity as the main outcome which only reveal bone damage and is a late feature of OA [46]. It is therefore unsurprising that the bone and cartilage biomarkers were found to be the best biomarkers that predict these outcomes, instead of inflammatory biomarkers. On the other hand, MRI is a modality that reveals pathology of all joint tissues from cartilage, synovium, and meniscus to bone marrow. It would be of value to evaluate whether these inflammatory biomarkers are associated with the burden of synovitis and bone marrow lesions detected by MRI [47,48].
In summary, in this cross-sectional study in participants having predominantly early knee OA, bone and cartilage degradation markers best predict the radiographic burden of disease. We demonstrated that plasma IL-1Ra was significantly and inversely associated with radiographic severity of knee OA after adjustment for BMI. Other inflammatory biomarkers such as hsCRP, IL-18 and IL-1β were associated with radiographic severity in the univariable analysis and was not statistically significant after adjustment with BMI. This supports the role of inflammation in the pathogenesis of OA, and these biomarkers are potential candidates for evaluation of their ability to prognostic progression of knee OA in longitudinal studies.
Financial support
This study was supported by the National Medical Research Council, Singapore (NMRC/CSA-INV/0022/2017), Academic Medicine – Enhancing Training, Healthcare, Outcomes and Standards (AM-ETHOS), Duke-NUS Medical Student Research Fellowship and the Lee Foundation Grant.
Authors contributions
YYL conceptualized and designed the study; YYL, CAM, SNR and SBW acquired the data; CAM and JL performed the data analysis; all authors interpreted the data; CAM and YYL drafted the manuscript; all authors critically revised the manuscript; and approved the final version of manuscript.
Role of the funding source
The funding source has no role in the scientific design, data analysis and interpretation and any other part of scientific input to this manuscript.
Data sharing statement
This manuscript reported the original results of an observational study. Data for this study can be available on reasonable request sent to the corresponding author.
Ethical disclosure
The study protocol was read and approved by the SingHealth Centralized Institutional Review Board (Ref: 2012/837/E) and informed consent were obtained from all participants prior to the start of the study.
Declaration of Competing Interest
All authors (CAM, SNR, JL, SBW and YYL) have no financial disclosures or notable competing interests.
Acknowledgements
We thank all participants in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2020.100046.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAlindon T.E., Bannuru R.R., Sullivan M.C., Arden N.K., Berenbaum F., Bierma-Zeinstra S.M., et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Smith M.D., Triantafillou S., Parker A., Youssef P.P., Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J. Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 5.Schett G., Dayer J.-M., Manger B. Interleukin-1 function and role in rheumatic disease. Nat. Rev. Rheumatol. 2015;12:14–24. doi: 10.1038/nrrheum.2016.166. [DOI] [PubMed] [Google Scholar]
- 6.Koshy P.J., Lundy C.J., Rowan A.D., Porter S., Edwards D.R., Hogan A., et al. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 2002;46:961–967. doi: 10.1002/art.10212. [DOI] [PubMed] [Google Scholar]
- 7.Goldring M.B., Birkhead J.R., Suen L.F., Yamin R., Mizuno S., Glowacki J., et al. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J. Clin. Invest. 1994;94:2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attur M., Wang H.Y., Kraus V.B., Bukowski J.F., Aziz N., Krasnokutsky S., et al. Radiographic severity of knee osteoarthritis is conditional on interleukin 1 receptor antagonist gene variations. Ann. Rheum. Dis. 2010;69:856–861. doi: 10.1136/ard.2009.113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attur M., Belitskaya-Lévy I., Oh C., Krasnokutsky S., Greenberg J., Samuels J., et al. Increased interleukin-1? Gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011;63:1908–1917. doi: 10.1002/art.30360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denoble A.E., Huffman K.M., Stabler T.V., Kelly S.J., Hershfield M.S., McDaniel G.E., et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl. Acad. Sci. U.S.A. 2011;108:2088–2093. doi: 10.1073/pnas.1012743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attur M., Statnikov A., Samuels J., Li Z., Alekseyenko A.V., Greenberg J.D., et al. Plasma levels of interleukin-1 receptor antagonist (IL1Ra) predict radiographic progression of symptomatic knee osteoarthritis. Osteoarthritis Cartilage. 2015;23:1915–1924. doi: 10.1016/j.joca.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerkhof H.J.M., Doherty M., Arden N.K., Abramson S.B., Attur M., Bos S.D., et al. Large-scale meta-analysis of interleukin-1 beta and interleukin-1 receptor antagonist polymorphisms on risk of radiographic hip and knee osteoarthritis and severity of knee osteoarthritis. Osteoarthritis Cartilage. 2011;19:265–271. doi: 10.1016/j.joca.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Palmer G., Mezin F., Juge-Aubry C.E., Plater-Zyberk C., Gabay C., Guerne P.A. Interferon beta stimulates interleukin 1 receptor antagonist production in human articular chondrocytes and synovial fibroblasts. Ann. Rheum. Dis. 2004;63:43–49. doi: 10.1136/ard.2002.005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopa S., Leijs M.J., Moretti M., Lubberts E., van Osch G.J., Bastiaansen-Jenniskens Y.M. Arthritic and non-arthritic synovial fluids modulate IL10 and IL1RA gene expression in differentially activated primary human monocytes. Osteoarthritis Cartilage. 2015;23:1853–1857. doi: 10.1016/j.joca.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Blom A.B., van Lent P.L., Libregts S., Holthuysen A.E., van der Kraan P.M., van Rooijen N., et al. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 2007;56:147–157. doi: 10.1002/art.22337. [DOI] [PubMed] [Google Scholar]
- 16.Kraus V.B., McDaniel G., Huebner J.L., Stabler T.V., Pieper C.F., Shipes S.W., et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016;24:1613–1621. doi: 10.1016/j.joca.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daghestani H.N., Pieper C.F., Kraus V.B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheum. 2015;67:956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin X., Beguerie J.R., Zhang W., Blizzard L., Otahal P., Jones G., et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 2015;74:703–710. doi: 10.1136/annrheumdis-2013-204494. [DOI] [PubMed] [Google Scholar]
- 19.Kerkhof H.J.M., Bierma-Zeinstra S.M.A., Castano-Betancourt M.C., de Maat M.P., Hofman A., Pols HaP., et al. Serum C reactive protein levels and genetic variation in the CRP gene are not associated with the prevalence, incidence or progression of osteoarthritis independent of body mass index. Ann. Rheum. Dis. 2010;69:1976–1982. doi: 10.1136/ard.2009.125260. [DOI] [PubMed] [Google Scholar]
- 20.Sowers M., Jannausch M., Stein E., Jamadar D., Hochberg M., Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage. 2002;10:595–601. doi: 10.1053/joca.2002.0800. [DOI] [PubMed] [Google Scholar]
- 21.Kellgren J.H., Lawrence J.S. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Ann. Rheum. Dis. 1957;16 [PubMed] [Google Scholar]
- 22.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee; Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29 doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 23.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 24.Charles H.C., Kraus V.B., Ainslie M., Hellio Le Graverand-Gastineau M.P. Optimization of the fixed-flexion knee radiograph. Osteoarthritis Cartilage. 2007;15:1221–1224. doi: 10.1016/j.joca.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Altman R.D., Gold G.E. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 2007;15 doi: 10.1016/j.joca.2006.11.009. revised. [DOI] [PubMed] [Google Scholar]
- 26.McDaniel G., Renner J.B., Sloane R., Kraus V.B. Association of knee and ankle osteoarthritis with physical performance. Osteoarthritis Cartilage. 2011;19:634–638. doi: 10.1016/j.joca.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraus V.B., Collins J.E., Hargrove D., Losina E., Nevitt M., Katz J.N., et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA biomarkers consortium. Ann. Rheum. Dis. 2017;76:186–195. doi: 10.1136/annrheumdis-2016-209252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes J., Tardif G., Martel-Pelletier J., Lascau-Coman V., Dupuis M., Moldovan F., et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am. J. Pathol. 1999;154:1159–1169. doi: 10.1016/S0002-9440(10)65368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X., Kondragunta V., Kornman K.S., Wang H.Y., Duff G.W., Renner J.B., et al. IL-1 receptor antagonist gene as a predictive biomarker of progression of knee osteoarthritis in a population cohort. Osteoarthritis Cartilage. 2013;21:930–938. doi: 10.1016/j.joca.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraenkel L., Roubenoff R., LaValley M., McAlindon T., Chaisson C., Evans S., et al. The association of peripheral monocyte derived interleukin 1beta (IL-1beta), IL-1 receptor antagonist, and tumor necrosis factor-alpha with osteoarthritis in the elderly. J. Rheumatol. 1998;25:1820–1826. [PubMed] [Google Scholar]
- 31.Blagojevic M., Jinks C., Jeffery A., Jordan K.P. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Leung Y.Y., Allen J.C., Noviani M., Ang L.W., Wang R., Yuan J.M., et al. Association between body mass index and risk of total knee replacement, the Singapore Chinese Health Study. Osteoarthritis Cartilage. 2015;23:41–47. doi: 10.1016/j.joca.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsaid K.A., Ubhe A., Shaman Z., D'Souza G. Intra-articular interleukin-1 receptor antagonist (IL1-ra) microspheres for posttraumatic osteoarthritis: in vitro biological activity and in vivo disease modifying effect. J. Exp. Orthop. 2016;3:18. doi: 10.1186/s40634-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta S., Akhtar S., Porter R.M., Onnerfjord P., Bajpayee A.G. Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Res. Ther. 2019;21:238. doi: 10.1186/s13075-019-2003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caron J.P., Fernandes J.C., Martel-Pelletier J., Tardif G., Mineau F., Geng C., et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39:1535–1544. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier J.P., Caron J.P., Evans C., Robbins P.D., Georgescu H.I., Jovanovic D., et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- 37.Fleischmann R.M., Bliddal H., Blanco F.J., Schnitzer T.J., Peterfy C., Chen S., et al. A phase II trial of Lutikizumab, an anti-interleukin-1 alpha/beta dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheum. 2019;71:1056–1069. doi: 10.1002/art.40840. [DOI] [PubMed] [Google Scholar]
- 38.Chevalier X., Goupille P., Beaulieu A.D., Burch F.X., Bensen W.G., Conrozier T., et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61:344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 39.Vincent T.L. IL-1 in osteoarthritis: time for a critical review of the literature. F1000Res. 2019;8 doi: 10.12688/f1000research.18831.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Courties A., Gualillo O., Berenbaum F., Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23:1955–1965. doi: 10.1016/j.joca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Ghayur T., Banerjee S., Hugunin M., Butler D., Herzog L., Carter A., et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 42.Saha N., Moldovan F., Tardif G., Pelletier J.P., Cloutier J.M., Martel-Pelletier J. Interleukin-1β-converting enzyme/caspase-1 in human osteoarthritic tissues: localization and role in the maturation of interleukin-1β and interleukin-18. Arthritis Rheum. 1999;42:1577–1587. doi: 10.1002/1529-0131(199908)42:8<1577::AID-ANR3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 43.Inoue H., Hiraoka K., Hoshino T., Okamoto M., Iwanaga T., Zenmyo M., et al. High levels of serum IL-18 promote cartilage loss through suppression of aggrecan synthesis. Bone. 2008;42:1102–1110. doi: 10.1016/j.bone.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Hutton C.W., Higgs E.R., Jackson P.C., Watt I., Dieppe P.A. 99 mTc HMDP bone scanning in generalised nodal osteoarthritis. II. The four hour bone scan image predicts radiographic change. Ann. Rheum. Dis. 1986;45:622–626. doi: 10.1136/ard.45.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bauer D.C., Hunter D.J., Abramson S.B., Attur M., Corr M., Felson D., et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14:723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Amin S., LaValley M.P., Guermazi A., Grigoryan M., Hunter D.J., Clancy M., et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52:3152–3159. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 47.Atukorala I., Kwoh C.K., Guermazi A., Roemer F.W., Boudreau R.M., Hannon M.J., et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann. Rheum. Dis. 2016;75:390–395. doi: 10.1136/annrheumdis-2014-205894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanamas S.K., Wluka A.E., Pelletier J.P., Pelletier J.M., Abram F.O., Berry P.A., et al. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology. 2010;49:2413–2419. doi: 10.1093/rheumatology/keq286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.