Summary
Objective
MicroRNAs (miRNAs) are being launched as biomarkers for various diseases, but a robust biomarker for articular cartilage pathology has yet to be discovered. Here we evaluate plasma extracellular vesicle (EV) miRNAs as possible biomarkers for osteoarthritis (OA).
Method
We compared miRNA levels found in plasma EVs from patients with OA with controls without OA using next generation sequencing (NGS) technique. The patient and control pairs were matched for age, gender and body mass index.
Results
23 pairs of patients and controls were included. Patients with OA differed significantly from controls in both clinical and radiological assessment of OA. We identified 177 canonical miRNAs in plasma EVs, but found no difference in miRNA levels between the two groups. Interestingly, the concentration of each miRNA in plasma EVs showed minimal difference between the participants, suggesting that the release of miRNAs in EVs from cells within the various organs is a tightly controlled process.
Conclusion
This is the first study using NGS in search of a miRNA biomarker in plasma EVs in OA. The levels of each plasma EVs miRNA were surprisingly similar for all participants. No plasma EVs miRNA can be used as a biomarker for OA.
Keywords: Articular cartilage, Osteoarthritis, microRNA, Extracellular vesicles, Biomarker
1. Introduction
To find a biochemical marker indicating persistent articular cartilage pathology is of paramount interest. The marker may identify people with increased risk of developing pathology and allow the monitoring of disease progression, enabling earlier treatment to reduce symptoms and prevent the development of osteoarthritis (OA) [1]. Further, the biomarker may enable better evaluation of treatment effects.
Since the discovery of small, non-coding double-stranded RNAs 25 years ago, microRNAs (miRNAs) have been investigated as possible biomarkers of disease [2]. MiRNAs are predominantly found in the cell cytoplasm, but are also released as stable molecules bound to proteins, lipoproteins or contained in extracellular vesicles (EVs). EVs are found in most body fluids, are thought to function as intercellular communication packages and are easily accessible for analysis [3].
MiRNAs have been reported as potential biomarkers for cancer and cardiovascular disease [3].
A few studies have also shown differential expression (DE) of miRNAs in serum or plasma between patients with OA and controls [[4], [5], [6], [7]]. The findings from these studies are inconsistent, but miR-885-5p was upregulated in OA patients in two studies [5,7]. However, to date no single miRNA or group of miRNAs has been accepted as robust biomarkers for OA. Plasma EVs are intercellular information packages, and their miRNA content has been shown to be different from miRNAs carried in serum or plasma [8,9]. We hypothesized that plasma EV miRNA levels might act as biomarkers for OA. To test this hypothesis, we compared miRNA levels of plasma EVs between OA patients and matched controls using next generation sequencing (NGS) technique. To the best of our knowledge, this is the first study comparing miRNAs from plasma EVs in persons with and without OA.
2. Methods
2.1. Participants
23 patients with OA and 23 controls without OA aged 42–72 years were recruited from the Musculoskeletal pain in Ullensaker Study (MUST) [10]. All participants underwent comprehensive clinical examination by trained physicians, x-rays of both knees, hips and hands and blood samples. The groups were paired and matched by age, sex and body mass index. All patients had either radiographic OA (Kellgren-Lawrence grade 2 or more) in hip(s) and/or knee(s) (n = 21) or previous joint prosthesis of one hip (n = 1) or one knee (n = 1). The controls did not fulfil the American College of Rheumatology criteria for hands, hips and knees, had no prosthesis in hips or knees, had no radiographic OA in their hips or knees (Kellgren-Lawrence grade 0). Further, the controls had maximum two hand joints with mild radiographic OA (Kellgren-Lawrence grade 2) and no or doubtful OA (Kellgren-Lawrence grade 0–1) in remaining hand joints. The evaluation of hip, knee and hand radiographs were done by trained readers with good to excellent reliability.
2.2. Informed consent and ethical approval
Prior to inclusion in the MUST study, all participants signed a written informed consent which stated that blood samples would be stored in a biobank for future analysis of associations between clinical characteristics and biomarkers. The procedures followed were in accordance with the ethical standards of the Norwegian Regional Ethics Committee (Ref. no: 2009/812a and 2009/1703a) and with the Helsinki Declaration of 1975, as revised in 2000.
2.3. Collection of plasma and storage
4 mL of whole blood was obtained from each participant. 500 μL of plasma was collected in EDTA tubes and stored at – 80º Celsius. Qiagen services were used for EV isolation and miRNA sequencing (Qiagen, Vedbaek, Denmark).
2.4. EV size measurement
EVs were isolated by centrifugation of plasma at 16 000×g for 5 min before purification using the exoRNeasy Serum Plasma Kit according to the protocol from the manufacturer (Qiagen). EVs size distribution was performed using the Zetasizer Nano ZS system according to the manual from the manufacturer (Malvern Panalytical, Malvern UK).
2.5. Western blotting
EVs from 4 mL plasma were isolated and eluted in 200 μl Buffer XE (Qiagen). 200 μl of the eluate was mixed with 200 μl of 2x Laemmli Sample Buffer (Sigma-Aldrich, St. Louis, MO), vortexed for 20 s and incubated at 98 °C for 10 min to denature proteins. 35 μl lysate was loaded onto a 4–20% gradient polyacrylamide gel (Biorad, Hercules, CA). Proteins were separated by gel electrophoresis, transferred to PVDF membranes using the TransBlot Turbo system (Biorad) and incubated with rabbit anti-human ALIX, rabbit anti-human TSG101 and rabbit anti-human CD81 antibodies (Abcam, Cambridge, UK). After washing, incubation with a horseradish peroxidase-conjugated horse anti-rabbit IgG (H + L) secondary antibody (Vector labs, Burlingame, CA) and a final washing step the bands were visualized using the myECL imager (Thermo Fisher Scientific, Waltham, MA). All antibodies were diluted in 1X TBS, 5% nonfat dry milk, 0.1% Tween 20.
2.6. Library preparation and NGS
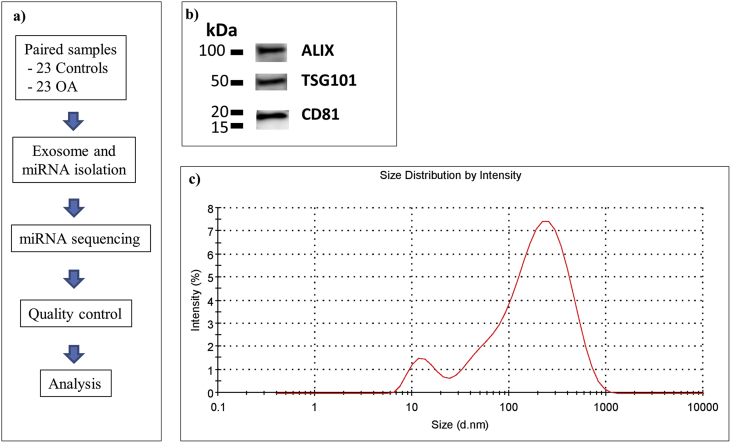
The library preparation was done using the QIAseq miRNA Library Kit (Qiagen). A number of spike-ins were added to the samples prior to RNA isolation. A total of 6 μL total RNA was converted into miRNA NGS libraries. Adapters containing unique molecular identifiers were ligated to the RNA before conversion to cDNA. The cDNA was amplified using PCR (22 cycles) and during the PCR indices were added. After PCR, the samples were purified. Library preparation quality control was performed using either Bioanalyzer 2100 (Agilent, Santa Clara, CA) or TapeStation 4200 (Agilent). All 46 samples formed adequate cDNA libraries and were sequenced. Based on quality of the inserts and the concentration measurements the libraries were pooled in equimolar ratios. The library pools were quantified using qPCR and sequenced on a NextSeq500 sequencing instrument according to the manufacturer instructions to a depth of 22 million reads per sample (average) with a single-end read of 51 nucleotides. Raw data were de-multiplexed and FASTQ files for each sample were generated using the bcl2fastq software (Illumina Inc., San Diego, CA). FASTQ data were checked using the FastQC tool. The RNA libraries construction and sequencing were performed on the Illumina platform with the NEBNext Multiplex Small RNA Library Prep Set for Ilumina (Illimina). All deep sequencing analyses were blinded, as those who prepared the samples and performed the data analysis were unaware of group affiliation. The study complied with the Minimum Information about a Microarray Experiment (MIAME) checklist. The workflow of the experiment is demonstrated in Fig. 1a.
Fig. 1.
a) Schematic overview of the experiments. b) Western blot of ALIX, TSG10 and CD81. c) Size distribution of the EVs. d - diameter, nm - nanometer.
2.7. Statistical analysis
Mean, median and standard deviation were calculated for continuous variables. Categorical data were presented in frequencies and cumulative frequencies. The reads mapping to mature miRNAs sequences were normalised using trimmed mean of M-values (TMM) normalization and edgeR was used for DE analysis of paired samples. Filtering on sequencing depth normalised values was applied before accounting for composition bias. A threshold of 12 counts per million reads in at least half the number of samples was used, as this corresponds to 5 reads in the smallest sample which can be seen as detection boundary.
3. Results
16 females and 7 males with similar age (58.0 and 57.7) and BMI (27.6 and 27.1) were included in the two groups. The clinical and radiologic evaluations were significantly different between the two groups. Demographics and clinical characteristics of the study population are presented in Table 1.
Table 1.
Demographic information about the study population.
| Persons with OA | Persons without OA | p-value | |
|---|---|---|---|
| Sex, n (%) women | 16 (69.6) | 16 (69.6) | 1.0 |
| Age, mean (SD) | 58.0 (7.0) | 57.7 (7.3) | 0.97 |
| BMI, mean (SD) | 27.6 (3.6) | 27.1 (3.6) | 0.91 |
| Fulfil ACR criteria, n (%) | |||
| Hip | 3 (13.0) | 0 (0) | 0.23 |
| Knee | 19 (82.6) | 0 (0) | <0.001 |
| Hand | 11 (47.8) | 0 (0) | <0.001 |
| K-L sum scores (median, range) | |||
| Hip | 1 (0–3) | 0 (0) | 0.49 |
| Knee | 2 (0–4) | 0 (0) | <0.001 |
| Hand | NA | 1 (0–2) | NA |
BMI – body mass index, ACR – American College of Rheumatology clinical evaluation, K-L – Kellgren-Lawrence radiologic evaluation, OA – osteoarthritis, NA – not assessed.
A schematic representation of the experiments is shown in Fig. 1a. The average size of the EVs was 235 nm (range: 70–900 nm) (Fig. 1b). ALIX, TSG101 and CD81, generally found in EVs, were all present in the EVs (Fig. 1c).
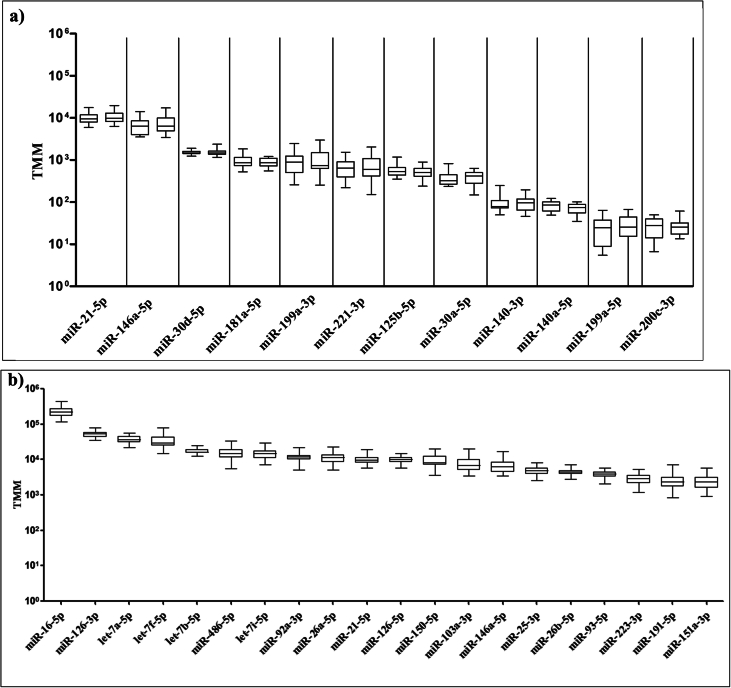
A total of 177 canonical miRNAs were detected in plasma EVs using our filtering criteria (Supplementary Table S1). However, a paired DE analysis did not reveal statistically significant differences in plasma EV miRNA levels between patients with OA and controls. A plot of twelve miRNAs known to be associated with OA [2] confirmed very similar miRNA levels in the two groups (Fig. 2a). The top 20 miRNAs for all 46 participants combined are plotted in Fig. 2b. They have all previously been detected in plasma EVs in other studies [11].
Fig. 2.
a) Boxplots of 12 miRNAs associated with osteoarthritis and cartilage in controls (left) and patients (right). b) Boxplots of the 20 most abundant miRNAs. Controls and patients are shown as one group since there was no difference between the two groups. MiRNA – microRNA, OA – osteoarthritis, TMM – trimmed Mean of M-values.
4. Discussion
The most important finding of the present study was that miRNA plasma EV levels from patients with OA were not statistically different from controls using NGS technique. We identified 177 miRNAs in plasma EVs. All of the 20 most abundant miRNAs have been detected in plasma EVs in other studies [11,12], lending support to our observations.
So far, reports of miRNAs as a biomarker for OA have mainly been based on studies of animals, human chondrocytes and synovial fluid [13]. Although miRNA studies are rapidly increasing, the results are inconsistent. miR-16-5p has been shown to be expressed at higher levels in OA cartilage than in healthy cartilage, and was hypothesized to control development of OA [14]. miR-16-5p was the most abundant miRNA in this study, but we found no difference between the two groups (Fig. 2b). Lin et al. found miR-30d to be highly expressed in human articular chondrocytes [15], whereas Withrow and colleagues demonstrated that miR-200c is elevated in synovial fluid in OA patients compared with non-OA controls [16]. We identified both miR-30d and miR-200c, but no statistically significant differences were detected between persons with OA and without OA (Fig. 2a). miR-140 has previously been found to play a significant role in OA pathogenesis and is hailed as a potential biomarker for articular cartilage pathologies [2]. miR-140 was detected in our material (Fig. 2a), but was not among the 20 most abundant miRNAs (Fig. 2b).
MiRNAs are predominantly found in the cell cytoplasm, but are also reported in extracellular fluids bound to proteins, lipoproteins or contained in vesicles such as EVs. The role of circulating miRNAs as potential biomarkers for OA is relatively unexplored. To our knowledge, there are only four studies comparing levels of circulating miRNAs between OA patients and controls [[4], [5], [6], [7]]. Murata et al. compared concentrations of five different miRNAs in plasma and synovial fluid in patients with rheumatoid arthritis, knee OA and healthy controls [4]. They found that miR-16 and miR-132 were significant lower in OA patients compared with healthy controls, and stated that miR-132 could detect individuals with OA with 84% sensitivity. Beyer and colleagues analysed pooled serum samples from 13 OA patients who underwent hip or knee arthroplasty with pooled serum samples from 13 individuals without arthroplasty [5]. Using a microarray screen, they identified 12 miRNAs with DE. They then compared the levels of these miRNAs in 67 individuals with knee/hip arthroplasty with 749 individuals without, and identified three potentially predictive miRNAs for severe OA, namely let-7e, miR-454 and miR-885-5p. Ntoumou et al. used a microarray platform to compare serum miRNAs DE in 12 OA patients undergoing knee replacement surgery with 12 patients undergoing knee fracture repair surgery [6]. They found a significant downregulation of miR-33b-3p, miR-140-3p and miR-671-3p in OA serum compared to controls. Finally, Cuadra and associates compared circulating miRNAs in plasma from patients with primary knee OA with controls without clinical or radiological knee OA, also using a microarray platform [7]. They identified 12 elevated miRNAs in the OA group. As none of these studies investigated all possible miRNA sequences by NGS technology, and their results are largely non-overlapping, their usefulness as biomarkers of early OA remains to be proven.
The relative concentrations of miRNAs secreted in EVs are different from those found in the cell cytosol, and different from serum/plasma concentrations of miRNAs [17]. As EVs are thought to have a special role as intercellular messengers, we hypothesized that miRNA information about OA might be found in plasma EVs. In order not to introduce an a priori selection bias on our screening assay, we used NGS which picks up all possible miRNA sequences. The vast majority of the miRNAs found in the serum/plasma studies mentioned above and suggested to have a role as biomarkers were also found in plasma EVs, but with similar levels for patients and controls. Further, no other miRNAs were found to differ between the two groups. As we have investigated all possible miRNA sequences in a fairly large group (23 pairs) of OA patients and controls matched for age, sex and body mass index, the possibility of finding a miRNA biomarker for OA in plasma EVs must now be almost absent.
Still, the study has limitations. The plasma samples used in this study were stored at −80º Celsius for up to 7 years before analysis were performed. This may have affected our findings. Although the collection and handling of blood samples were performed after a strict study protocol, any inconsistencies in these procedures may alter the levels of miRNAs. However, the exoRNeasy Serum Plasma Kit has been compared with ultracentrifugation and other EV isolation kits, and found to be the best, indicating that no other isolation strategy would have more reliable results [18,19]. The yield of purified EVs in plasma is small (less than 0.1 μg of total RNA in 1 mL of plasma), and deep sequencing techniques usually requires at least 1 μg of total RNA. As we utilized only 500 μL of plasma, this may have led to greater variability among our results. However, the spike-in sequences were found at expected and reproducible levels, and the number of miRNAs identified was similar to those found in other studies [4], suggesting that our observations are likely to truly represent the relative concentrations of miRNAs in plasma EVs. Also, we do not have information about OA in other joints, such as hands (OA patients), spine, feet and shoulders, and cannot exclude that patients or controls had OA at these sites. This is a limitation affecting many biomarker studies. However, for the references used in this manuscript [[4], [5], [6], [7]], at least we know that they had OA in the same joints (hips and/or knees) as the OA patients enrolled in the current study. Lastly, others have investigated long noncoding (lnc) RNAs in cartilage and found aberrant expression in OA [20]. The possible role played by lncRNA in the pathogenesis and as biomarkers of OA was not investigated in the present study, and remains to be fully determined.
As NGS has not yet been used in studies to identify circulating miRNA biomarkers for OA, there is still hope for this strategy. However, miRNAs are presumably released from all the cells in the body to eventually find their way into the blood stream, and miRNAs from cells of OA affected joints may just be too few to impact on the overall levels of circulating miRNAs. Indeed, the most remarkable observation presented here is the similarity in plasma EV miRNA levels between individuals, suggesting that this is a tightly controlled process.
5. Conclusion
This study is the first to compare circulating miRNAs in EVs in OA patients using NGS. We did not identify any plasma EV miRNAs that can potentially act as biomarkers for OA. Further research is necessary to identify a biomarker for early OA.
Contributions
TFA and TAK contributed equally and performed the literature search, interpreted the data, drafted and edited the article. IKH and MAR provided study material and gave critical review of the manuscript, ØBL gave critical review of the manuscript and provided funding and JEB launched the hypothesis of the study with study design, interpreted the data, gave critical review of the manuscript and provided funding. All authors made contributions to conception and design, was involved in the drafting and read and approved the final manuscript.
Role of the funding source
This project was funded by research funds from the Department of orthopaedic surgery – Kristiansund Hospital, Health Møre and Romsdal HF, Department of immunology – Oslo University Hospital, Rikshospitalet and the Norwegian Cartilage Project. The authors declare that the study sponsors were not involved in the study.
Data statement
The datasets generated and analysed during the current study are not publicly available due to the size of these datafiles but are available from the corresponding author on reasonable request.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
We thank the MUST study group for providing the blood samples used in the study, Qiagen services, 2950 Vedbaek, Denmark for conducting isolation, NGS and statistical analysis, Tor Åge Myklebust, Section for research, innovation, education and competence development, Health Møre and Romsdal HF for statistical consultation and Tore Geir Iversen, Oslo University Hospital, Institute for Cancer Research, Department of Molecular Cell Biology for size measurements.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2019.100018.
Contributor Information
Tommy Frøseth Aae, Email: tommy.aae@gmail.com.
Tommy Aleksander Karlsen, Email: tommy.a.karlsen@rr-research.no.
Ida K. Haugen, Email: ida.k.haugen@gmail.com.
May Arna Risberg, Email: m.a.risberg@nih.no.
Øystein Bjerkestrand Lian, Email: oystein.bjerkestrand.lian@helse-mr.no.
Jan E. Brinchmann, Email: jan.brinchmann@rr-research.no.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Luyten F.P., Denti M., Filardo G., Kon E., Engebretsen L. Definition and classification of early osteoarthritis of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2012;20:401–406. doi: 10.1007/s00167-011-1743-2. [DOI] [PubMed] [Google Scholar]
- 2.Nugent M. MicroRNAs: exploring new horizons in osteoarthritis. Osteoarthr. Cartil. 2016;24:573–580. doi: 10.1016/j.joca.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Pritchard C.C., Cheng H.H., Tewari M. MicroRNA profiling: approaches and considerations. Nat. Rev. Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murata K., Yoshitomi H., Tanida S., Ishikawa M., Nishitani K., Ito H., et al. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2010;12:R86. doi: 10.1186/ar3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer C., Zampetaki A., Lin N.Y., Kleyer A., Perricone C., Iagnocco A., et al. Signature of circulating microRNAs in osteoarthritis. Ann. Rheum. Dis. 2015;74:e18. doi: 10.1136/annrheumdis-2013-204698. [DOI] [PubMed] [Google Scholar]
- 6.Ntoumou E., Tzetis M., Braoudaki M., Lambrou G., Poulou M., Malizos K., et al. Serum microRNA array analysis identifies miR-140-3p, miR-33b-3p and miR-671-3p as potential osteoarthritis biomarkers involved in metabolic processes. Clin. Epigenet. 2017;9:127. doi: 10.1186/s13148-017-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgonio Cuadra V.M., Gonzalez-Huerta N.C., Romero-Cordoba S., Hidalgo-Miranda A., Miranda-Duarte A. Altered expression of circulating microRNA in plasma of patients with primary osteoarthritis and in silico analysis of their pathways. PLoS One. 2014;9:e97690. doi: 10.1371/journal.pone.0097690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endzelins E., Berger A., Melne V., Bajo-Santos C., Sobolevska K., Abols A., et al. Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer. 2017;17:730. doi: 10.1186/s12885-017-3737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murillo O.D., Thistlethwaite W., Rozowsky J., Subramanian S.L., Lucero R., Shah N., et al. exRNA Atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell. 2019;177:463–477. doi: 10.1016/j.cell.2019.02.018. e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osteras N., Risberg M.A., Kvien T.K., Engebretsen L., Nordsletten L., Bruusgaard D., et al. Hand, hip and knee osteoarthritis in a Norwegian population-based study--the MUST protocol. BMC Muscoskelet. Disord. 2013;14:201. doi: 10.1186/1471-2474-14-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng L., Sharples R.A., Scicluna B.J., Hill A.F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanz-Rubio D., Martin-Burriel I., Gil A., Cubero P., Forner M., Khalyfa A., et al. Stability of circulating exosomal miRNAs in healthy subjects. Sci. Rep. 2018;8:10306. doi: 10.1038/s41598-018-28748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen L.T., Sharma A.R., Chakraborty C., Saibaba B., Ahn M.E., Lee S.S. Review of prospects of biological fluid biomarkers in osteoarthritis. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L., Jia J., Liu X., Yang S., Ye S., Yang W., et al. MicroRNA-16-5p controls development of osteoarthritis by targeting SMAD3 in chondrocytes. Curr. Pharmaceut. Des. 2015;21:5160–5167. doi: 10.2174/1381612821666150909094712. [DOI] [PubMed] [Google Scholar]
- 15.Lin L., Shen Q., Zhang C., Chen L., Yu C. Assessment of the profiling microRNA expression of differentiated and dedifferentiated human adult articular chondrocytes. J. Orthop. Res. 2011;29:1578–1584. doi: 10.1002/jor.21423. [DOI] [PubMed] [Google Scholar]
- 16.Withrow J., Murphy C., Duke A., Fulzele S., Hamrick M. ORS 2016 Annual Meeting. Orlando, Florida. 2016. Synovial fluid exosomal miRNA profiling of osteoarthritis patients and identification of synoviocyte-chondrocyte communication pathway. [Google Scholar]
- 17.Turchinovich A., Weiz L., Langheinz A., Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enderle D., Spiel A., Coticchia C.M., Berghoff E., Mueller R., Schlumpberger M., et al. Characterization of RNA from exosomes and other extracellular vesicles isolated by a novel spin column-based method. PLoS One. 2015;10:e0136133. doi: 10.1371/journal.pone.0136133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan S., Yeri A., Cheah P.S., Chung A., Danielson K., De Hoff P., et al. Small RNA sequencing across diverse biofluids identifies optimal methods for exRNA isolation. Cell. 2019;177:446–462. doi: 10.1016/j.cell.2019.03.024. e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang S.D., Lu J., Deng Z.H., Li Y.S., Lei G.H. Long noncoding RNAs in osteoarthritis. Jt. Bone Spine. 2017;84:553–556. doi: 10.1016/j.jbspin.2016.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.