Abstract
Objective
To conduct a network meta-analysis comparing all treatments for osteoarthritis (OA) pain in the Cochrane Library.
Design
The Cochrane Library and Epistemonikos were searched for randomized controlled trials (RCTs) about treatments for hip and knee OA. We constructed 17 broad categories, comprising drug treatments, exercise, surgery, herbs, orthotics, passive treatments, regenerative medicine, diet/weight loss, combined treatments, and controls. In addition to a full network analysis, we compared the direct/indirect effects, and studies with shorter-/longer follow-up. CINeMA software was used for assessing confidence in network meta-analysis estimates.
Results
We included 35 systematic reviews including 445 RCTs. There were 153 treatments for OA. In total, 491 comparisons were related to knee OA, less on hip OA, and only nine on hand OA. Six treatment categories showed clinically significant effects favoring treatment over control on pain. “Diet/weight loss” and “Surgery” had effect sizes close to zero. The network as a whole was not coherent. Of 136 treatment comparisons, none were rated as high confidence, six as moderate, 13 as low, and 117 as very low.
Conclusions
Direct comparison of different available treatment options for OA is desirable, however not currently feasible in practice, due to heterogeneous study populations and lack of clear descriptions of control interventions. We found that many treatments were effective, but since the network as a whole was not coherent and lacked high confidence in the treatment comparisons, we could not produce a ranking of effects.
Keywords: Osteoarthritis, Pain, Pharmacological, Interventions, Non-pharmacological, Network meta-analysis
Abbreviations: ACR, American College of Rheumatology; CINeMA, Confidence In the results of Network Meta-Analysis; DJW, Chinese Duhuo Jisheng Wan; EULAR, European Alliance of Associations for Rheumatology; NMAs, Network meta-analyses; NSAIDs, nonsteroidal anti-inflammatory drugs; OA, Osteoarthritis; OARSI, Osteoarthritis Research Society International; PRP, platelet-rich plasma; QoL, Quality of Life; RoB, Cochrane risk of bias; SKI 306X, Extract from a mixture of Clematis mandshurica, Trichosanthes kirilowii and Prunella vulgaris; TENS, Transcutaneous nerve stimulation; WOMAC, The Western Ontario and McMaster Universities Osteoarthritis Index
1. Introduction
Osteoarthritis (OA) is a disease affecting synovial joints. It involves structural alterations in the articular cartilage, subchondral bone, ligaments, capsule, synovial membrane, and periarticular muscles [1]. In a recent population-based cohort study, the estimated lifetime risk of symptomatic hand OA was 47.2% in women and 24.6% in men [2]. The corresponding lifetime risk for symptomatic knee OA is between 40 and 50% [3], and one in four people may develop symptomatic hip OA in his or her lifetime [4]. OA is a leading cause of disability in elders, and a source of high societal cost [1,5]. The medical cost of osteoarthritis in various high-income countries is estimated to account for 1% to 2·5% of the gross domestic product of these countries [6], with hip and knee joint replacements representing the major proportion of these health-care costs [1].
There is currently no cure for OA. According to guidelines from the American College of Rheumatology (ACR) [7] and the Osteoarthritis Research Society International (OARSI) [8], the core treatments for knee OA are patient education and self-management, land-based exercise (strengthening/cardio/balance/neuromuscular or mind-body), and dietary weight management for people with overweight or obesity. In addition, aquatic exercise, gait aids, topical and oral nonsteroidal anti-inflammatory drugs (NSAIDs), intraarticular steroid injections and tibiofemoral bracing for tibiofemoral are recommended for knee OA. The OARSI guideline strongly recommends topical NSAIDS, but conditionally recommends intraarticular injections, oral NSAIDs, proton pump inhibitors, and COX-2 inhibitors. For hip OA, the two guidelines recommend patient education, self-management, and land-based exercises as core interventions. Both guidelines recommend oral NSAIDs and mind-body exercises, whereas the ACR guidelines also strongly recommend gait aids, intraarticular glucocorticoid injections, and weight loss. For hand OA, the ACR and EULAR (European Alliance of Associations for Rheumatology) treatment recommendations state patient education, hand exercises, and orthoses as core interventions [7,9].
It can generally be stated that the guidelines are not consistent with regard to the recommended pharmacological and non-pharmacological interventions.
Thus, it is difficult for patients and clinicians to find the best treatment. In addition, most intervention studies only investigate one treatment. This can only answer questions like “does treatment X work better than placebo?” or “does treatment X work better than treatment Y?” Patients, on the other hand, want to know “which treatment works best for me?“.
Epistemonikos (epistemonikos.org) is the largest source of systematic reviews (SRs) relevant for health-decision making. In December 2021 it contained 332 SRs about interventions for OA. Concurrently, there were 9013 randomized controlled trials on treatment for OA in PubMed. Network meta-analysis is a relatively new statistical method for producing summary estimates of treatment effects across clinical trials [10]. Moreover, they offer some additional benefits: (1) indirect comparisons of treatments that have never been directly compared, (2) ranking of treatments according to effect estimates, and (3) drawing statistical strength from indirect comparisons by combining direct and indirect estimates of effect [10]. Earlier network meta-analyses were limited to pharmacological interventions [11,12], acupuncture and other physical treatments [13], non-steroidal anti-inflammatory drugs (NSAIDs) [14], glucosamine, diacerein, and NSAIDs [15,16], NSAIDs and opioids [17], or exercise [18]. Hence, there is a need for a network meta-analysis that includes all kinds of treatments for OA.
Cochrane reviews represent the gold standard for SRs of the effects of medical interventions. The aims of the present article were i) to conduct a network meta-analysis of the effects on pain of all treatments for OA published on the Cochrane Library, ii) to explore in more detail the effects of the core treatments for OA on pain, iii) to summarize the amount of randomized, controlled evidence on each treatment, iv) to identify research gaps, and v) to study whether it is feasible to conduct a network meta-analysis including all treatments for OA pain. We did not have a specific hypothesis, and the present study is to be regarded as hypothesis-generating.
2. Methods and materials
The present SR and frequentist network meta-analysis of interventions for OA was pre-registered in the PROSPERO database (CRD42019114700). We followed the PRISMA extension statement for reporting of incorporating network meta-analyses of health care interventions: checklist and explanations as far as possible [19]. Cases in which we deviated from this statement were listed separately (Supplementary material). This is an overview of overviews, and we have followed the Cochrane Handbook for Systematic Reviews of Interventions (Chapter V) [20].
Data were collected from SRs (Cochrane reviews and supplemented by English language reviews in the Epistemonikos database), and effect data and risk of bias assessments for each primary study from the SRs were extracted. We assumed that Cochrane reviews had high methodological quality. Two authors independently assessed the quality of the remaining reviews from Epistemonikos with the AMSTAR (A MeaSurement Tool to Assess systematic Reviews) tool [21].
The extraction of study data from reviews may result in selection bias because our inclusion criteria would not necessarily equal the inclusion criteria in the systematic reviews. Nonetheless, it was assumed that both we and all the review authors had the following PICO (Population-Intervention-Comparison-Outcome):
Population: Patients with knee, hip or hand OA.
Intervention: Interventions for OA included in the Cochrane Library.
Comparison: placebo, no intervention, other intervention or standard treatment.
Outcome: pain If there were more than one measure of pain, we followed the hierarchy described in Fransen et al. [22]. In the pre-registered protocol, we planned to also include physical function, fatigue, patient global assessment of disease activity, quality of life, and adverse events as outcomes, but we started with pain. This was because pain was the most frequently reported outcome in the included systematic reviews, and most relevant from the patient perspective. In addition, space limitations did not allow for more than one outcome in this paper.
Inclusion criteria for SRs: Cochrane intervention reviews published 2013 (Issue 1)-2022 (Issue 22) based on randomized controlled trials of any intervention for osteoarthritis with pain as outcome. For older Cochrane reviews, we searched the Epistemonikos database for SRs on the same interventions published 2013–2021 (December 8). We only conducted searches in databases and did not contact authors.
Two reviewers independently searched the Cochrane Library and Epistemonikos for systematic reviews. We used the following search strategy for Cochrane reviews in December 2018: Restricting to title, abstract or keyword we entered the term ‘osteoarthritis”. Cochrane intervention reviews published during the latest 5 years (2013–2018, inclusive) were considered for inclusion. In Epistemonikos, we entered “osteoarthritis” in title or abstract with filter for publication year “last five years”, publication type “systematic review” and systematic review question “interventions”. In addition, we entered the names of those interventions that were registered in the Cochrane Library but not updated 2013 or later. We did a separate search for each intervention. The searches were updated until Issue 2 on February 12, 2022 (Cochrane) and February 4, 2022 (Epistemonikos). S6 Appendix lists the interventions in the Cochrane Library that were not updated after 2012 and the number of hits in Epistemonikos for the same interventions after 2012.
Because we expected to find a large number of treatment comparisons, we planned to construct categories of treatments. For pharmacological interventions we used the categorization reported by Gregori et a [11] and consulted a pharmacist at Diakonhjemmet Hospital for support on the categorization of pharmacological interventions and herbs. For the remaining interventions, we constructed categories after group discussions among all authors.
We report standardized mean differences as effect measure. If the reviews reported this, we used the numbers as reported. If mean differences were reported, we used the Campbell Effect Size Calculator (https://www.campbellcollaboration.org/research-resources/effect-size-calculator.html) or the calculator in RevMan to compute standardized mean differences with 95% confidence intervals. When risk ratios were reported [23] we used the reported numbers in each group to compute odds ratios using a calculator on the web (https://www.socscistatistics.com/biostatistics/default2.aspx) and converted them to SMDs (Standardized Mean Differences) using the following R code:
logOR < - log (odds ratio).
d_logOR < - logOR ∗ (sqrt(3)/pi).
d_logOR.
We extracted the risk of bias assessments for the included studies that were reported in the Cochrane and Epistemonikos reviews. If a review did not report risk of bias, we acquired the full texts of the primary studies and performed risk of bias assessments ourselves, using the original Cochrane risk of bias (RoB) domain set (random sequence generation, allocation concealment, blinding of patients and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, other bias). It was not seen as expedient to use the new risk of bias tool – RoB 224 as a large number of the included systematic reviews were conducted before this tool was published. If the risk of bias assessments were different (i.e. the review authors had added, collapsed, divided or excluded risk of bias domains), we tried to translate the risk of bias assessments to the standard set. If, e.g. “other bias” was not listed, we coded this as unclear. CINeMA (Confidence In the results of Network meta-analysis, see below) requires that within-study bias is coded in three categories (no concerns, some concerns, major concerns). In order to do the transformation from the seven risk of bias domains to the three required by CINeMA, we employed an algorithm adapted from Schwingshackl et al. [25]. Studies were classified as being at low risk of bias (if at least three domains were rated as low risk; and maximum one domain rated with a high risk of bias), high risk of bias (if at least two domains were rated as high risk), and moderate/unclear risk (all other studies).
2.1. Grading of confidence in the results
We employed CINeMA for grading the confidence in the results. CINeMA requires that researchers consider six domains: (a) within-study bias, (b) reporting bias, (c) indirectness, (d) imprecision, (e) heterogeneity, and (f) incoherence. The result is a report with confidence gradings for each treatment comparison in the network. The software is semi-automated and contains algorithms that produce assessments for each of the six domains. Within-study bias is e.g., computed as a weighted average of the three risk of bias categories. Imprecision, heterogeneity, and incoherence are assessed after the user has entered a minimal clinically important difference (MCID). Risk of bias due to imprecision, heterogeneity and incoherence is assessed considering the MCID and the 95% confidence intervals and prediction intervals as shown in Fig. 4 in Papakonstantinou et al. [26]. We used an MCID of 0.469 for our pain outcome. Angst et al. [27] reported this number as the SMD-equivalent MCID for improvement on a 0–100-point pain scale. We also interpreted effect sizes according to Cohen's categories for small, medium and large effect sizes [28].
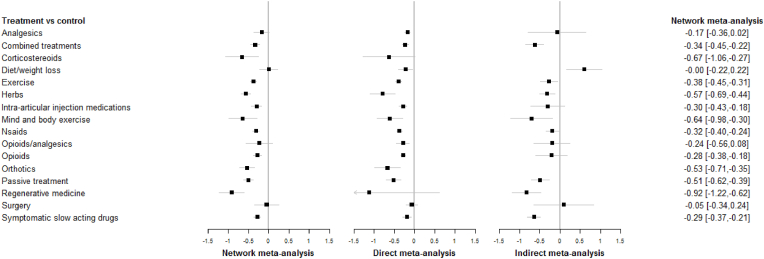
Fig. 4.
Forest plots of the network meta-analysis, direct meta-analysis, and indirect meta-analysis, all studies are included (negative numbers mean ‘favors treatment’ and positive numbers mean ‘favors control’). NSAIDS = Non-steroidal anti-inflammatory drugs.
2.2. Statistical analysis
Statistical analyses were performed in R. The frequentist network meta-analysis was performed using the package “netmeta” [29]. CINeMA also depends on netmeta. For studies with more than one comparison, we used all comparisons in each study. For studies with the same comparison at different follow-up times, all follow-ups were used. Because different follow-up estimates are not independent, we performed the following sensitivity analyses: (1) combined estimate across follow-up times for each comparison, (2), one randomly chosen follow-up time for studies with more than one follow-up, (3) shortest follow-up for each comparison, and (4) longest follow-up for each comparison (S4 Appendix). All sensitivity analyses produced similar results, and we present only the analyses that included all follow-up times. The results were presented using network graphs and forest plots. We performed sub-analyses that contrasted studies with less than 12 weeks follow-up (the median) with studies with more than 12 weeks follow-up. We compared the full network meta-analysis with analyses restricted to direct effects or indirect effects, respectively. The direct effect associated with a treatment was estimated using only those studies directly comparing that treatment with a control group. Here random-effects meta-analysis was used to combine the individual estimates. The indirect effect summarized the indirect evidence associated with the treatment vs. control comparison. This effect was estimated using network meta-analysis after removing all estimates directly comparing the treatment to a control group.
3. Results
3.1. Review selection
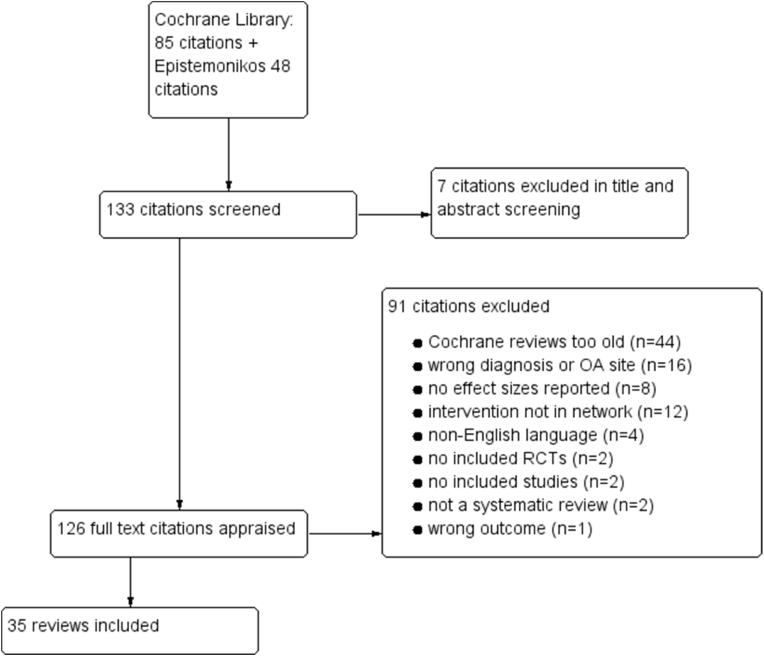
There were 85 systematic reviews on the Cochrane library in December 2018 with “osteoarthritis” in title, abstract or keywords. Forty-one were published during 2013–2018 and considered eligible for this network meta-analysis, whereas 44 were published in 2012 or earlier. After reading the full texts we included 22 Cochrane reviews [22,23,[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]]. An additional Cochrane review by Leopoldino et al. [50] fulfilled our inclusion criteria but did not add any further studies. A review by Moskal et al. [51] also fulfilled our inclusion criteria, but none of the interventions (Navigated versus conventional total knee arthroplasty) had been compared to any other intervention in the network. There were 48 systematic reviews in Epistemonikos published 2013 or later that had studied one of the 14 combinations of interventions and OA site that were included in outdated Cochrane reviews. After reading the full texts we included 13 reviews from Epistemonikos [15,[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. In total, we included 35 systematic reviews in our network meta-analysis (Table 1). After our initial search in December 2018, we included a Cochrane review by Toupin-April [48] published in Issue 5, 2019 and one by Palmer [44], published in Issue 7, 2019.
Table 1.
Included reviews.
| Author, Year | Found in | Interventions | OA site | Outcome measures | Methodological quality of non-Cochrane reviews (AMSTAR criteria fulfilled) |
|---|---|---|---|---|---|
| Bartels, 2016 [55] | Cochrane | Aquatic exercise | Hip + knee | Pain, disability, QoL | |
| Brouwer, 2014 [20] | Cochrane | Osteotomy | Knee | Pain, function, participant satisfaction, adverse events | |
| Cameron, 2013 [21] | Cochrane | Topical herbal therapies | OA | Pain, function, adverse events | |
| Cameron, 2014 [22] | Cochrane | Oral herbal therapies | OA | Pain, function, adverse events | |
| Da Costa, 2014 [23] | Cochrane | Oral or transdermal opioids | Hip + knee | Pain, function, adverse events | |
| Derry, 2016 [13] | Cochrane | Topical NSAIDs | OA | Clinical successa, adverse events | |
| Duivenvoorden, 2015 [24] | Cochrane | Braces and orthoses | Knee | Pain, function, QoL, global patient assessment, adverse events | |
| Fidelix, 2014 [25] | Cochrane | Diacerin | OA | Pain, function, QoL, adverse events | |
| Fransen, 2014 [27] | Cochrane | Exercise | Hip | Pain, function, QoL | |
| Fransen, 2015 [26] | Cochrane | Exercise | Knee | Pain, function, QoL | |
| Hall 2019 [42] | Epistemonikos | Diet-induced weight-loss + exercise | Knee | Pain, function, inflammatory biomarkers | 1-4, 6–10 (9 of 11) |
| Health Quality Ontario, 2014 [56] | Epistemonikos | Arthroscopic debribement | Knee | Pain, function | 3, 6–8, 10 (5 of 11) |
| Hurley, 2018 [28] | Cochrane | Exercise | Hip + knee | Pain, function | |
| Jevsevar, 2015 [57] | Epistemonikos | Viscosupplementation | Knee | Pain, WOMAC function | 3, 7–10 (5 of 11) |
| Jüni, 2015 [29] | Cochrane | Corticosteroids | Knee | Pain, function, QoL, adverse events | |
| Kongtharvonskul, 2015 [45] | Epistemonikos | glucosamine, Diacerin, and NSAIDS | Knee | Pain, function, adverse events | 2, 3, 6, 7, 9–10 (6 of 11) |
| Kroon, 2014 [30] | Cochrane | Self-management education | OA | Pain, function, QoL | |
| Li, 2013 [31] | Cochrane | Electromagnetic fields | OA | Pain, function, QoL, adverse events | |
| Machado 2015 [46] | Epistemonikos | Paracetamol (acetaminophen) | Hip + knee | 2-4, 6–10 (8 of 11) | |
| Manheimer, 2018 [32] | Cochrane | Acupuncture | Hip | Pain, function, QoL | |
| Meheux, 2016 [47] | Epistemonikos | Intra-articular platelet-rich plasma injections | Knee | Pain, function, stiffness | 1, 3, 6–8, 11 (6 of 11) |
| Newberry, 2017 [58] | Epistemonikos | Many different treatments | Knee | Pain, function, adverse events | 1- 9, 11 (10 of 11) |
| Østerås, 2017 [33] | Cochrane | Exercise | Hand | Pain, function, QoL, adverse events | |
| Palmer 2019 [34] | Cochrane | Surgical interventions | Knee OA | Pain, function, adverse events, QoL | |
| Puljak, 2017 [35] | Cochrane | Celecoxib | OA | Pain, function, QoL, adverse events | |
| Runhaar, 2017 [49] | Epistemonikos | Glucosamine | Knee + hip | Pain, function | 2-4, 6–11 (9 of 11) |
| Santos, 2015 [36] | Cochrane | Tapentadol | OA | Pain, adverse events | |
| Simental-Mendía, 2018 [59] | Epistemonikos | glucosamine and chondroitin | Knee | Pain, Total WOMAC | 6, 7, 9, 10 (4 of 11) |
| Singh, 2015 [37] | Cochrane | Chondroitin | OA | Pain, function, WOMAC, patient global assessment, adverse events | |
| Smith, 2016 [60] | Epistemonikos | NSAIDS and opioids | Knee | Pain | 2, 6, 7, 9–10 (5 of 11) |
| Sun, 2018 [61] | Epistemonikos | knee arthroplasty | Knee | WOMAC, Knee Society, Score, Range of Motion | 2, 6, 7, 9–10 (5 of 11) |
| Toupin-April 2019 [38] | Cochrane | Tramadol | OA | Pain, function, adverse events | |
| Verra, 2013 [39] | Cochrane | Retention vs sacrifice (surgical interventions) | Knee | Pain, WOMAC, Knee Society Function | |
| Zhang, 2016a [62] | Epistemonikos | Ultrasound | Knee | Pain, function, adverse events | 2-4, 6, 7, 9 (6 of 11) |
| Zhang, 2016b [63] | Epistemonikos | Chinese herbal medicine | Knee | Pain, WOMAC | 2, 3, 6–10 (7 of 11) |
Defined as at least a 50% reduction in pain, or an equivalent measure such as a ‘very good’ or ‘excellent’ global assessment of treatment, or ‘none’ or ‘slight’ pain on rest or movement, measured on a categorical scale.
Fig. 1 is a flow chart of the inclusion process.
Fig. 1.
Flow chart.
3.1.1. Quality of the included systematic reviews
We assessed 13 reviews from Epistemonikos with the AMSTAR tool [21]. The number of fulfilled AMSTAR criteria for each review ranged from 4 to 10 on the 0–11 scale with mean of 6.5 and median of 6. The most common methodological problems were item no. 1: “Was an ‘a priori’ design provided?“, item no. 5: “Was a list of studies (included and excluded) provided?“, and item no. 11: “Was the conflict of interest included?” (Tables 1 and S7 Appendix).
3.1.2. Risk of bias assessment
The risk of bias assessments was reported according to the standard domains in the Cochrane Handbook in eight of the reviews. It was only possible to extract risk of bias at the study level (not at the outcome level). S7 Appendix lists the deviations from the standard that we found in the included reviews. Because some of the reviews had more than one deviation, the numbers add up to more than 35. S7 Appendix also shows the risk of bias assessments for all the 626 treatment comparisons.
Fig. 2 shows the within-study bias for all treatment comparisons. Of the 557 treatment comparisons included in the network, 293 were classified as low risk of bias, 111 as moderate risk, and 153 as high risk.
Fig. 2.
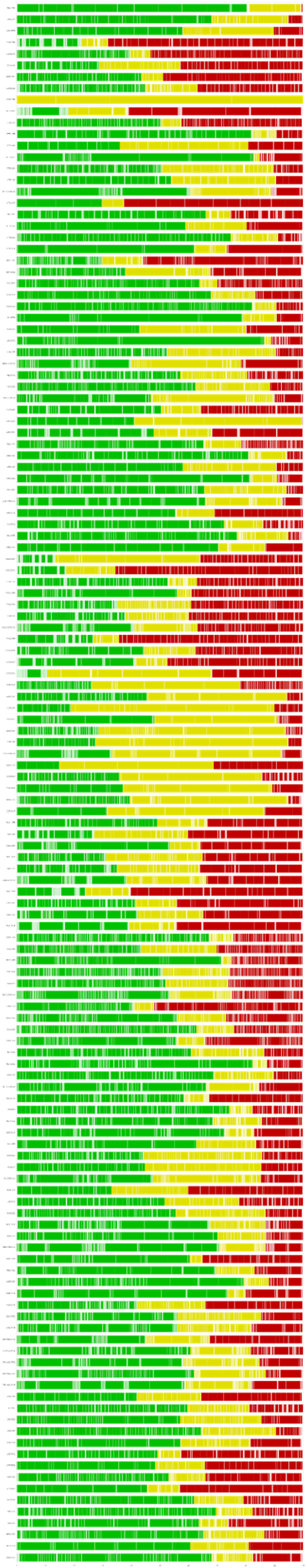
Within-study risk of bias for all treatment comparisons.
3.2. Confidence in the network estimates
S2 Appendix shows that none of the 136 comparisons were rated as “high confidence”. Among the mixed evidence, six comparisons were rated as moderate. Thirteen were rated as low confidence. The rest (n = 117) were rated as very low confidence. Among the indirect comparisons, all were rated as very low confidence. The network as a whole was not coherent with the Chi-squared statistic being 73.109 with 19° of freedom and p-value 0.000.
3.3. The broader categories of interventions
Table 2 shows the broad categories used for our main analyses. For each category, we list the specific interventions in that category as they were named in the SRs. Whenever the primary study reported specific doses, we report them in Table 2.
Table 2.
Broad categories and included specific interventions and doses.
| Broad category | Specific Interventions and dosesa (n = 153) |
|---|---|
| ANALGESICS |
|
| COMBINED TREATMENTS |
|
| CONTROL |
|
| CORTICOSTEROIDS |
|
| DIET/WEIGHT LOSS |
|
| EXERCISE |
|
| HERBS |
|
| INTRA-ARTICULAR INJECTION MEDICATIONS |
|
| MIND AND BODY EXERCISE |
|
| NSAIDs |
|
| OPIOIDS |
|
| OPIOIDS/ANALGESICS |
|
| ORTHOTICS |
|
| PASSIVE TREATMENT |
|
| REGENERATIVE MEDICINE |
|
| SURGERY |
|
| SYMPTOMATIC SLOW-ACTING DRUGS |
|
Doses were only reported for a small number of the interventions in the Cochrane/Epistemonikos reviews. We report these doses here.
3.4. Description of the network
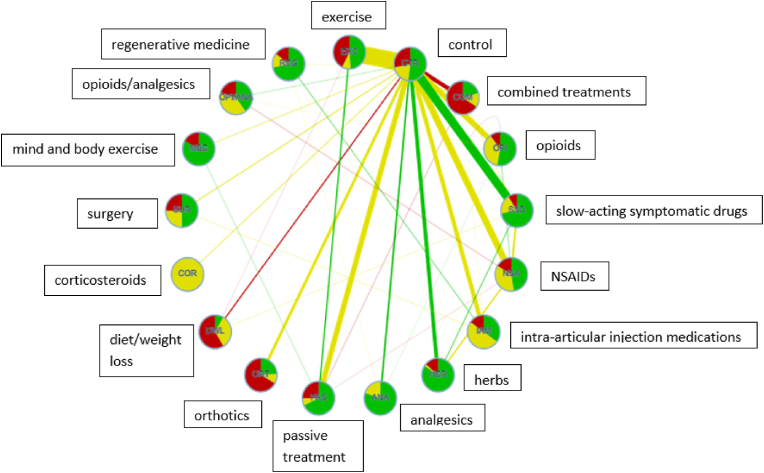
Fig. 3 represents the network graph. The line width represents number of studies comprising the respective comparisons. The line color represents the average RoB. The widest lines show that the most frequent direct comparisons were exercise versus control, symptomatic slow-acting drugs versus control, NSAIDs versus control, and passive treatments versus control. The nodes and lines are color-coded according to proportion of studies with low (green), moderate (yellow), and high (red) RoB. The studies on corticosteroids are, e.g. all of moderate risk of bias. Most treatments were directly compared to a control group and not with each other.
Fig. 3.
Network graph. The thickness of the lines represents how frequent the respective comparisons are.
3.5. Type of effect sizes
For 17 of the included reviews, we were able to use the effect sizes as reported (SMDs). For another 17 of the reviews, we computed SMDs from means and SDs, and for one systematic review (Smith 2016 60), we obtained the full texts from some of the primary studies and extracted the effects.
In total, we were able to extract 626 effect sizes from 445 randomized controlled trials from the available systematic reviews. There were 153 unique treatments and combinations of treatments, and after group discussions, we agreed on the final 17 broad categories. Some of the interventions did not fit in to any broad category and are listed under “Combined treatments”. All treatments and treatment combinations with suggested broad categories are listed in Table 2.
When applying the broader categories, we lost a few comparisons because they involved comparing two interventions of the same broad category (e.g. ‘celecoxib vs NSAIDs’ at the specific level became ‘NSAIDs vs NSAIDs’ at the broad category level.)
3.6. Results of the network meta-analysis
In addition to the network meta-analysis, we conducted two sub-analyses, one on direct effects, and one on indirect effects. The forest plot in Fig. 4 shows all three analyses. Almost all the main categories seemed to be effective according to the network meta-analysis, but only six of the categories had effect sizes exceeding our predefined MCID of 0.469 (corticosteroids, herbs, mind and body exercises, orthotics, passive treatment, and regenerative medicine. The effect of regenerative medicine came mainly from indirect comparisons. Exercise (131 studies) showed consistent small to moderate effects across network/direct/indirect analyses (SMDs: −0.38/-0.45/-0.31). Herbs (29 studies) also showed consistent moderate effects (SMDs: −0.57/-0.69/-0.44). The S1 & S4 & S5 Appendices show sub analyses for duration of follow-up.
3.7. Ranking of effects
Since none of the 136 comparisons were rated as “high confidence” and the network as a whole was not coherent, we have not produced a ranking of effects.
3.8. Follow-up duration
When the results were categorized by short and long follow-up, the clearest differences were seen for drug treatments. Analgesic studies with less than 12 weeks follow-up had a small effect (−0.19 (−0.36, −0.02), but studies with more than 12 weeks follow-up had a smaller effect −0.13 (−0.89, 0.63). This was even more pronounced for corticosteroids for which a large short-term effect (SMD: −0.97, 95% CI: −1.46, −0.48) was contrasted with a small long-term effect (SMD: −0.36, 95% CI: −0.98, 0.26). The same was true for NSAIDs (short-term: −0.4 (−0.48, −0.32), long-term: −0.06 (−0.23, 0.1)). Opioids, intra-articular injection medications, and symptomatic slow-acting drugs also showed a similar pattern with larger effects for short-term follow-up. Thus, in general, drugs seemed to have only short-term effects on OA pain. Herbs, on the other hand, had larger effects in the long term −0.79 (−0.99, −0.59) than in the short term −0.41 (−0.55, −0.27). The same was true for mind and body exercise (short-term: −0.55 (−1.05, −0.05), and long-term: −0.78 (−1.26, −0.29)). None of the studies on surgery had a follow-up of 12 weeks or less.
The analyses of studies focusing on knee OA only, closely mirrored the main analyses (data not shown). Regenerative medicine had the strongest effect, but both studies in this category were performed on knee OA. The six studies on surgery did not show any clinically significant effect on pain (SMD: −0.05, 95% CI: −0.36, 0.26). Most studies (n = 339) were performed on patients with knee OA (Table 3) or on a combination of knee and hip OA (n = 67). Few studies examined hip (n = 24) or hand (n = 6) OA, and nine studies only stated OA without specifying the site.
Table 3.
Distribution of studies and comparisons according to OA site.
| OA site | Number of studies | Number of comparisons | Percent of total comparisons |
|---|---|---|---|
| Hand | 6 | 9 | 1.4 |
| Hip | 24 | 28 | 4.5 |
| Hip + knee | 67 | 88 | 14.0 |
| Knee | 339 | 491 | 78.3 |
| OA | 9 | 10 | 1.6 |
| Total | 445 | 626 |
3.9. Summary of main findings
Table 4 is a summary of findings table for the treatment comparisons. The SMD for each comparison is shown along with its 95% CI. For each comparison, we graded the confidence. The comment column uses standard formulations from the GRADE Working Group [64]. We define an MCID as SMD> 0.469 according to Angst et al.‘s criterium [27]. Smaller differences are labelled “slight”. We label treatment differences of moderate confidence “probable”, and use “may” for comparisons of low confidence. Finally, effect sizes with very low confidence are not shown in Table 4. All effect sizes are shown in the league table in the supplementary material (S3 Appendix).
Table 4.
Summary of findings table for all treatment comparisons (n = 136).
| Comparison | SMD (95% CI) | Confidence | Comment |
|---|---|---|---|
| High confidence: We do not have high confidence in any treatment comparison | |||
| Moderate confidence (n = 6) | |||
| Analgesics vs regenerative medicine | 0.735 (0.394, 1.077) | Moderate | Analgesics is probably less effective than regenerative medicine. |
| Control vs mind body exercise | 0.641 (0.302, 0.980) | Moderate | Control is probably less effective than mind body exercise. |
| Control vs regenerative medicine | 0.909 (0.608, 1.209) | Moderate | Control is probably less effective than regenerative medicine. |
| Herbs vs slow-acting symptomatic drugs | −0.280 (−0.416, −0.143) | Moderate | Herbs is probably slightly more effective than slow-acting symptomatic drugs. |
| Intra-articular injection medications vs regenerative medicine | 0.620 (0.326, 0.914) | Moderate | Intra-articular injection medications are probably less effective than regenerative medicine. |
| Mind body exercise vs passive treatment | −0.134 (−0.475, 0.206) | Moderate | There is probably little or no difference between mind body exercise and passive treatment. |
| Low confidence (n = 13) | |||
| Analgesics vs control | −0.174 (−0.363, 0.016) | Low | There may be little or no difference between analgesics and control. |
| Analgesics vs opioids | 0.110 (−0.103, 0.324) | Low | There may be little or no difference between analgesics and opioids. |
| Control vs exercise | 0.378 (0.310, 0.446) | Low | Control may be slightly less effective than exercise. |
| Control vs herbs | 0.566 (0.445, 0.688) | Low | Control may be less effective than herbs. |
| Control vs intra-articular injection medications | 0.289 (0.163, 0.415) | Low | Control may be slightly less effective than Intra-articular injection medications. |
| Control vs NSAIDs | 0.320 (0.239, 0.401) | Low | Control may be slightly less effective than NSAIDs. |
| Control vs opioids | 0.284 (0.182, 0.385) | Low | Control may be slightly less effective than opioids. |
| Control vs opioids/analgesics | 0.239 (−0.083, 0.560) | Low | There may be little or no difference between control and opioids/analgesics. |
| Control vs passive treatment | 0.507 (0.391, 0.622) | Low | Control treatment may be less effective than passive treatment. |
| Diet/weight loss vs exercise | 0.377 (0.151, 0.604) | Low | diet/weight loss may be slightly less effective than exercise. |
| Herbs vs NSAIDs | −0.246 (−0.379, −0.114) | Low | Herbs may be slightly more effective than NSAIDs. |
| Intra-articular injection medications vs surgery | −0.239 (−0.548, 0.070) | Low | There may be little or no difference between intra-articular injection medications and surgery. |
| NSAIDs vs passive treatment | 0.187 (0.047, 0.327) | Low | NSAIDs may be slightly less effective than Passive treatment. |
| Very low confidence (n = 117) | |||
| All other treatment comparisons | NA | Very low | We have very little confidence in the effect estimates: The true effect is likely to be substantially different from the estimate of effect. |
Exercise may be slightly more effective than control and also slightly more effective than diet/weight loss. The category ‘Combined treatments’ including education, skills training and self-management was inconclusive. Furthermore, we cannot infer about the effectiveness of ‘Orthotics’ based on the present analysis.
4. Discussion
To our best knowledge, this is the first network meta-analysis on all treatments for OA. From the 35 included reviews, we extracted data on 626 comparisons of 153 unique interventions from 445 RCTs. Most studies were on knee OA, but we also included studies on hip and hand OA. It is notable that there were only 9 treatment comparisons involving hand OA while this is the most prevalent type of OA. Six treatment categories showed clinically significant effects favoring treatment over control on pain, while “Diet/weight loss” and “Surgery” had effect sizes close to zero. Furthermore, drugs seemed to have only short-term effects on OA pain, whereas herbs as well as mind and body exercise had larger effects long term effects. The results are too a large degree in line with recommendations of ACR, OARSI and EULAR. Nonetheless, the network as a whole was not coherent, which reduces the confidence in all estimates of effect.
4.1. Results in the light of the current guidelines for OA
Exercise was the largest category (131 comparisons). The effects were large and consistent, both across direct/indirect estimates and follow-up time. This is in accordance with the EULAR, ACR and OARSI guidelines that recommend exercise as core treatments. These guidelines also recommend self-management and educational interventions, which were included in our category “Combined treatments” and showed small to moderate effects on pain. Mind and body exercises (hatha yoga, tai chi) also showed strong effects.
Weight loss is recommended in the EULAR, ACR and OARSI guidelines for people with knee and hip OA and overweight. However, the meta-analyses did not show any consistent effect on pain from dietary interventions. This may not be surprising because even if weight loss may impact pain and function, interventions aimed at weight loss have seldom succeeded in achieving clinically relevant reductions in body weight.
In light of the strong ACR recommendation against regenerative medicine, it is interesting that these interventions (with platelet rich plasma) showed the strongest effect. However, this category comprised only two studies. It is also noteworthy that the category “passive treatment”, which includes acupuncture and different kinds of manual therapies as well as electrotherapy revealed consistent and long-term effects. Most of these treatments are not recommended in recent guidelines, moreover, the ACR and OARSI guidelines recommend strongly against electrotherapy (transcutaneous electrical nerve stimulation (TENS)). Similarly, our results indicate strong effects of symptomatic slow-acting drugs such as chondroitin and glucosamine, which also are recommended strongly against by ACR and OARSI. Corticosteroids had a clinically significant effect, but only for less than 12 weeks follow-up. The “Herbs” category comprised 32 different interventions and showed a clinically significant effect when all studies were considered but is again recommended against in the OARSI recommendations.
Summing up the comparison between the results of the network meta-analysis and the guideline recommendations, there was consistent evidence for positive effects for exercise, self-management, and educational interventions. Our analysis shows that they are clinically relevant, and they are also recommended by the EULAR, ACR and OARSI guidelines. At the same time, weight loss, which is recommended by all three guidelines did not show clinical relevance in our analysis, most likely due to the inefficiency of the interventions to achieve weight loss. Inconsistencies were found with regard to clinically relevant effects in our analysis and recommendations against in guidelines for: regenerative medicine (ACR), electrotherapy (ACR and OARSI), symptomatic slow-acting drugs (ACR and OARSI), and Herbs (OARSI). For some therapies, which were shown to be effective and clinically relevant in the network meta-analysis such as acupuncture, manual therapies, and mind and body exercise, there is disagreement in the recommendations in the EULAR, OARSI and ACR guidelines.
When developing clinical guidelines, many aspects must be taken into account, such as clinical effectiveness (indicated by statistical effect, but not solely), the occurrence of adverse events in relation to clinical effect, availability, and health economics. Thus, it is not surprising that there are some inconsistencies between the guidelines with regard to how these aspects are evaluated and scored. Consequently, the guidelines are not consistent in their conditional recommendations for versus against a specific intervention. Moreover, some interventions with scientific evidence are not included which reduces their availability for patients. Even though the network meta-analysis methodology is mainly hypothesis generating, it provides an appropriate tool to safeguard that all therapies that are relevant for a certain disease are included and weighed against each other. We believe that this methodology can contribute to the development and optimization of evidence-based guidelines, which would greatly enhance good clinical treatment choices to the benefit of the patients.
4.2. Research gaps
Even though hand and hip OA are both highly prevalent, there are few randomized controlled trials on patients with these conditions compared to knee OA. Another research gap is on surgery versus placebo or no intervention/standard care. The existing studies on surgery have mostly compared different surgical protocols against each other. Although there are many studies on glucosamine and chondroitin, there is a lack of studies with low risk of bias. Future primary research warrants RCTs with lower risk of bias (blinding of assessors, better descriptions of interventions and control conditions and pre-registration of protocol). There is a need for studies that compare surgery with non-surgery and for studies of weight loss with per protocol analyses.
This review has several limitations. Firstly, we included only interventions in Cochrane reviews. Therefore, we might have missed treatments that are not found in any Cochrane review. Secondly, we did not read reports of primary studies in full text. Almost all information about the primary studies was extracted from the Cochrane reviews or from SRs in Epistemonikos. Because of this, we lack information about whether the “control” groups received no intervention, treatment as usual, or placebo. Thirdly, we did not look at effect modifiers such as severity of OA. Fourthly, a main assumption in network meta-analysis is that every patient should in principle be available for randomization to any of the interventions. Although this might be true, in practice there might be different groups of patients that were recruited to e.g. surgical interventions compared to e.g. the studies of treatment with herbs. If this is the case, then there is a risk of heterogeneity in the network meta-analysis with direct and indirect effects being unequal. Lastly, the categories and the treatments comprising them are open for debate. Especially, the category “Combined treatments” is heterogeneous, and the results of this category are difficult to interpret.
In conclusion, this is the first network meta-analysis to incorporate all treatments for OA pain. We have very low confidence in the ranking of effect estimates among the different treatment categories of this broad overview. Much of the reason for this low confidence is that the risk of bias in the primary studies is generally high and that the method of overviews of overviews tends to miss many details of these primary studies. We are, however, confident that we have included the best evidence provided by RCTs on the effect of treatments for OA in the Cochrane Library and Epistemonikos.
Author contributions
GS is responsible for the study idea and methodology, the writing and conceptualizing the initial draft, and for visualizing the results. JS and GS conducted the analyses. All authors contributed to the conceptualization and data curation of the study, the validation of the results, and to writing and critically reviewing the final version of the manuscript.
Funding
This work received no specific funding.
Acknowledgements
Thanks to pharmacist Kirsten K. Viktil at Diakonhjemmet Hospital for valuable assistance in constructing categories for various herbs and medical substances.
Footnotes
Study registration: PROSPERO database CRD42019114700.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2022.100242.
Contributor Information
Geir Smedslund, Email: geir.smedslund@fhi.no.
Ingvild Kjeken, Email: ingvild.kjeken@diakonsyk.no.
Frauke Musial, Email: frauke.musial@uit.no.
Joseph Sexton, Email: joesexton@gmail.com.
Nina Østerås, Email: nina.osteras@medisin.uio.no.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Qin J., Barbour K.E., Murphy L.B., Nelson A.E., Schwartz T.A., Helmick C.G., et al. Lifetime risk of symptomatic hand osteoarthritis: the Johnston county osteoarthritis project. Arthritis Rheumatol. 2017;69:1204–1212. doi: 10.1002/art.40097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy L., Schwartz T.A., Helmick C.G., Renner J.B., Tudor G., Koch G., et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy L.B., Helmick C.G., Schwartz T.A., Renner J.B., Tudor G., Koch G.G., et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18:1372–1379. doi: 10.1016/j.joca.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 6.Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014;10:437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 7.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., et al. American College of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2019;72(2020):149–162. doi: 10.1002/acr.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Kloppenburg M., Kroon F.P., Blanco F.J., Doherty M., Dziedzic K.S., Greibrokk E., et al. Update of the EULAR recommendations for the management of hand osteoarthritis. Ann. Rheum. Dis. 2018;78(2019):16–24. doi: 10.1136/annrheumdis-2018-213826. [DOI] [PubMed] [Google Scholar]
- 10.Chaimani A., Caldwell D.M., Li T., Higgins J.P.T., S G. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.0. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. 2019. Chapter 11: undertaking network meta-analyses.www.training.cochrane.org/handbook (updated July 2019). Cochrane. Available from: [Google Scholar]
- 11.Gregori D., Giacovelli G., Minto C., Barbetta B., Gualtieri F., Azzolina D., et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564–2579. doi: 10.1001/jama.2018.19319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung S.Y., Jang E.J., Nam S.W., Kwon H.H., Im S.G., Kim D., et al. Comparative effectiveness of oral pharmacologic interventions for knee osteoarthritis: a network meta-analysis. Mod. Rheumatol. 2018;28:1021–1028. doi: 10.1080/14397595.2018.1439694. [DOI] [PubMed] [Google Scholar]
- 13.Corbett M.S., Rice S.J., Madurasinghe V., Slack R., Fayter D.A., Harden M., et al. Acupuncture and other physical treatments for the relief of pain due to osteoarthritis of the knee: network meta-analysis. Osteoarthritis Cartilage. 2013;21:1290–1298. doi: 10.1016/j.joca.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Costa B.R., Reichenbach S., Keller N., Nartey L., Wandel S., Juni P., et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- 15.Kongtharvonskul J., Anothaisintawee T., McEvoy M., Attia J., Woratanarat P., Thakkinstian A. Efficacy and safety of glucosamine, diacerein, and NSAIDs in osteoarthritis knee: a systematic review and network meta-analysis. Eur. J. Med. Res. 2015;20:24. doi: 10.1186/s40001-015-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X., Wu D., Sang L., Wang Y., Shen Y., Zhuang X., et al. Comparative effectiveness of glucosamine, chondroitin, acetaminophen or celecoxib for the treatment of knee and/or hip osteoarthritis: a network meta-analysis. Clin. Exp. Rheumatol. 2018;36:595–602. [PubMed] [Google Scholar]
- 17.Smith S.R., Deshpande B.R., Collins J.E., Katz J.N., Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis Cartilage. 2016;24:962–972. doi: 10.1016/j.joca.2016.01.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uthman O.A., van der Windt D.A., Jordan J.L., Dziedzic K.S., Healey E.L., Peat G.M., et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ Br. Med. J. (Clin. Res. Ed.) 2013;347:f5555. doi: 10.1136/bmj.f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 20.Pollock M., Fernandes R.M., Becker L.A., Pieper D., Hartling L. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. 2021. Chapter V: overviews of reviews.www.training.cochrane.org/handbook (updated February 2021). Cochrane. Available from: [Google Scholar]
- 21.Shea B.J., Grimshaw J.M., Wells G.A., Boers M., Andersson N., Hamel C., et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fransen M., McConnell S., Harmer A.R., Van der Esch M., Simic M., Bennell K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2015;1:CD004376. doi: 10.1002/14651858.CD004376.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derry S., Conaghan P., Da Silva J.A., Wiffen P.J., Moore R.A. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2016;4:CD007400. doi: 10.1002/14651858.CD007400.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z.R., Sun F., Zhan S.Y. [Risk on bias assessment: (2) Revised Cochrane risk of bias tool for individually randomized, parallel group trials (RoB2.0)] Zhonghua Liuxingbingxue Zazhi. 2017;38:1285–1291. doi: 10.3760/cma.j.issn.0254-6450.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Schwingshackl L., C A., Schwedhelm C., Toledo E., Pünsch M., Hoffman G., et al. Comparative effects of different dietary approaches on blood pressure in hypertensive and prehypertensive patients: a systematic review and network meta-analysis. Crit. Rev. Food Sci. Nutr. 2019;59:2674–2687. doi: 10.1080/10408398.2018.1463967. [DOI] [PubMed] [Google Scholar]
- 26.Papakonstantinou T., Nikolakopoulouy A., Higgins J., Egger M., Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell System. Rev. 2020;16 doi: 10.1002/cl2.1080. wileyonlinelibrary.com/journal/cl2 | 1 of 152020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angst F., Aeschlimann A., Angst J. The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. J. Clin. Epidemiol. 2017;82:128–136. doi: 10.1016/j.jclinepi.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. 2 Edition. LawrenceErlbaum Associates; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sicences. [Google Scholar]
- 29.Rücker G., Krahn U., König J., Efthimiou O., Schwartzer G. 2020. Netmeta: Network Meta-Analysis Using Frequentist Methods.https://CRAN.R-project.org/package=netmeta R package version 1.2-1. [Google Scholar]
- 30.Bartels E.M., Juhl C.B., Christensen R., Hagen K.B., Danneskiold-Samsoe B., Dagfinrud H., et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst. Rev. 2016;3:CD005523. doi: 10.1002/14651858.CD005523.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brouwer R.W., Huizinga M.R., Duivenvoorden T., van Raaij T.M., Verhagen A.P., Bierma-Zeinstra S.M., et al. Cochrane Database Syst Rev; 2014. Osteotomy for Treating Knee Osteoarthritis; p. CD004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron M., Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst. Rev. 2013:CD010538. doi: 10.1002/14651858.CD010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron M., Chrubasik S. Oral herbal therapies for treating osteoarthritis. Cochrane Database Syst. Rev. 2014:CD002947. doi: 10.1002/14651858.CD002947.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.da Costa B.R., Nuesch E., Kasteler R., Husni E., Welch V., Rutjes A.W., et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst. Rev. 2014:CD003115. doi: 10.1002/14651858.CD003115.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duivenvoorden T., Brouwer R.W., van Raaij T.M., Verhagen A.P., Verhaar J.A., Bierma-Zeinstra S.M. Cochrane Database Syst Rev; 2015. Braces and Orthoses for Treating Osteoarthritis of the Knee; p. CD004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fidelix T.S., Macedo C.R., Maxwell L.J., Fernandes Moca Trevisani V. Cochrane Database Syst Rev; 2014. Diacerein for Osteoarthritis; p. CD005117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fransen M., McConnell S., Hernandez-Molina G., Reichenbach S. Cochrane Database Syst Rev; 2014. Exercise for Osteoarthritis of the Hip; p. CD007912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurley M., Dickson K., Hallett R., Grant R., Hauari H., Walsh N., et al. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst. Rev. 2018;4:CD010842. doi: 10.1002/14651858.CD010842.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juni P., Hari R., Rutjes A.W., Fischer R., Silletta M.G., Reichenbach S., et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015:CD005328. doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroon F.P., van der Burg L.R., Buchbinder R., Osborne R.H., Johnston R.V., Pitt V. Self-management education programmes for osteoarthritis. Cochrane Database Syst. Rev. 2014:CD008963. doi: 10.1002/14651858.CD008963.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S., Yu B., Zhou D., He C., Zhuo Q., Hulme J.M. Electromagnetic fields for treating osteoarthritis. Cochrane Database Syst. Rev. 2013:CD003523. doi: 10.1002/14651858.CD003523.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Manheimer E., Cheng K., Wieland L.S., Shen X., Lao L., Guo M., et al. Acupuncture for hip osteoarthritis. Cochrane Database Syst. Rev. 2018;5:CD013010. doi: 10.1002/14651858.CD013010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osteras N., Kjeken I., Smedslund G., Moe R.H., Slatkowsky-Christensen B., Uhlig T., et al. Exercise for hand osteoarthritis. Cochrane Database Syst. Rev. 2017;1:CD010388. doi: 10.1002/14651858.CD010388.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer J.S., Monk A.P., Hopewell S., Bayliss L.E., Jackson W., Beard D.J., et al. Surgical interventions for symptomatic mild to moderate knee osteoarthritis. Cochrane Database Syst. Rev. 2019;7:CD012128. doi: 10.1002/14651858.CD012128.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puljak L., Marin A., Vrdoljak D., Markotic F., Utrobicic A., Tugwell P. Celecoxib for osteoarthritis. Cochrane Database Syst. Rev. 2017;5:CD009865. doi: 10.1002/14651858.CD009865.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos J., Alarcao J., Fareleira F., Vaz-Carneiro A., Costa J. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2015:CD009923. doi: 10.1002/14651858.CD009923.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh J.A., Noorbaloochi S., MacDonald R., Maxwell L.J. Chondroitin for osteoarthritis. Cochrane Database Syst. Rev. 2015;1:CD005614. doi: 10.1002/14651858.CD005614.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toupin April K., Bisaillon J., Welch V., Maxwell L.J., Juni P., Rutjes A.W., et al. Tramadol for osteoarthritis. Cochrane Database Syst. Rev. 2019;5:CD005522. doi: 10.1002/14651858.CD005522.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verra W.C., van den Boom L.G., Jacobs W., Clement D.J., Wymenga A.A., Nelissen R.G. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst. Rev. 2013:CD004803. doi: 10.1002/14651858.CD004803.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leopoldino A.O., Machado G.C., Ferreira P.H., Pinheiro M.B., Day R., McLachlan A.J., et al. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database Syst. Rev. 2019;2:CD013273. doi: 10.1002/14651858.CD013273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moskal J.T., Capps S.G., Mann J.W., Scanelli J.A. Navigated versus conventional total knee arthroplasty. J. Knee Surg. 2014;27:235–248. doi: 10.1055/s-0033-1360659. [DOI] [PubMed] [Google Scholar]
- 52.Hall M., Castelein B., Wittoek R., Calders P., Van Ginckel A. Diet-induced weight loss alone or combined with exercise in overweight or obese people with knee osteoarthritis: a systematic review and meta-analysis. Semin. Arthritis Rheum. 2019;48:765–777. doi: 10.1016/j.semarthrit.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Health Quality Ontario Arthroscopic debridement of the knee: an evidence update. Ontario health technol. assess. series. 2014;14:1–43. [PMC free article] [PubMed] [Google Scholar]
- 54.Jevsevar D., Donnelly P., Brown G.A., Cummins D.S. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. The Journal of bone and joint surgery. American. 2015;97:2047–2060. doi: 10.2106/JBJS.N.00743. [DOI] [PubMed] [Google Scholar]
- 55.Machado G.C., Maher C.G., Ferreira P.H., Pinheiro M.B., Lin C.W., Day R.O., et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meheux C.J., McCulloch P.C., Lintner D.M., Varner K.E., Harris J.D. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495–505. doi: 10.1016/j.arthro.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Newberry S.J., FitzGerald J., SooHoo N.F., Booth M., Marks J., Motala A., et al. Treatment of Osteoarthritis of the Knee: an Update Review. 2017. Treatment of osteoarthritis of the knee: an update review. Rockville (MD. [PubMed] [Google Scholar]
- 58.Runhaar J., Rozendaal R.M., van Middelkoop M., Bijlsma H.J.W., Doherty M., Dziedzic K.S., et al. Subgroup analyses of the effectiveness of oral glucosamine for knee and hip osteoarthritis: a systematic review and individual patient data meta-analysis from the OA trial bank. Ann. Rheum. Dis. 2017;76:1862–1869. doi: 10.1136/annrheumdis-2017-211149. [DOI] [PubMed] [Google Scholar]
- 59.Simental-Mendía M., Sánchez-García A., Vilchez-Cavazos F., Acosta-Olivo C.A., Peña-Martínez V.M., Simental-Mendía L.E. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol. Int. 2018;38:1413–1428. doi: 10.1007/s00296-018-4077-2. [DOI] [PubMed] [Google Scholar]
- 60.Smith S.R., Deshpande B.R., Collins J.E., Katz J.N., Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis and cartilage/OARS. Osteoarthritis Res. Soc. 2016;24:962–972. doi: 10.1016/j.joca.2016.01.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun X., Su Z. A meta-analysis of unicompartmental knee arthroplasty revised to total knee arthroplasty versus primary total knee arthroplasty. J. Orthop. Surg. Res. 2018;13:158. doi: 10.1186/s13018-018-0859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C., Xie Y., Luo X., Ji Q., Lu C., He C., et al. Effects of therapeutic ultrasound on pain, physical functions and safety outcomes in patients with knee osteoarthritis: a systematic review and meta-analysis. Clin. Rehabil. 2016;30:960–971. doi: 10.1177/0269215515609415. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W., Wang S., Zhang R., Zhang Y., Li X., Lin Y., et al. Evidence of Chinese herbal medicine Duhuo Jisheng decoction for knee osteoarthritis: a systematic review of randomised clinical trials. BMJ Open. 2016;26 doi: 10.1136/bmjopen-2015-008973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santesso N., Glenton C., Dahm P., Garner P., Akl E.A., Alper B., et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J. Clin. Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.