Abstract
Objective
Describe “usual care” patterns of education, exercise, weight management, pain medication and other nonsurgical treatments for knee osteoarthritis (OA) in people recommended for nonsurgical care by an orthopaedic surgeon.
Methods
We used a telephone-administered questionnaire to capture treatments people with knee OA used over the three to six years after an orthopaedic surgeon recommended nonsurgical care. The primary outcome, guideline-consistent nonsurgical treatments, was an aggregate measure defined as using education, exercise, weight management, and at least one recommended medication. Secondary outcomes were first-line (education, exercise, and weight management) and guideline-inconsistent treatments (orthoses, opioids, hyaluronic acid, platelet rich plasma, and stem cell therapy). Multivariable robust Poisson regression assessed the association between participant characteristics and use of guideline-consistent, first-line and guideline-inconsistent treatments.
Results
479 people were invited and 250 participated (52%). Participants were 58% female with a mean age 66.2 years. Participants received education by a healthcare professional (64%), exercised regularly (74%), used weight management (38%), and used recommended pain medications (91%). All guideline-consistent nonsurgical treatments were used by 19% of participants, 19% of participants used first-line treatments, and 42% used guideline-inconsistent treatments. Over six years, 34% had another consult then underwent arthroplasty. Older participants were less likely to use any treatment. People without post-secondary education were less likely to use first-line treatments (RR 0.54, 95% CI: 0.30–0.96), and females were less likely to use guideline-inconsistent treatments (RR 0.62, 95% CI:0.47–0.81).
Conclusions
Nonsurgical usual care for people with knee OA was not consistent with international clinical guidelines.
Keywords: Osteoarthritis, Knee, Health services research, Health education, Exercise, Weight loss, Practice guideline
Abbreviations: OA, Osteoarthritis; TKR, Total knee replacement; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; OARSI, Osteoarthritis Research Society International; RR, Relative risks; CI, Confidence intervals
1. Introduction
Rising incidence of knee osteoarthritis (OA) creates significant burden on individuals and health systems [1,2]. International clinical guidelines recommend a stepped treatment approach focused on symptom management [[3], [4], [5], [6], [7], [8]]. All guidelines recommend education, exercise, and weight management as first-line treatments [[3], [4], [5], [6], [7], [8]], meaning the primary treatment in standard clinical practice [9], for everyone with knee OA. Education programs should include knowledge about OA as well as self-management techniques like goal setting, problem solving and coping strategies [10]. Exercise recommendations include physical activity, meaning any bodily movement [11], and therapeutic exercise which are specific movements prescribed for improving or maintaining OA symptoms [12]. Guidelines recommend physical activity dosages similar to general health recommendations [13]. Therapeutic exercise is more effective at improving symptoms than general physical activity [5]. All aerobic and strengthening therapeutic exercises are recommended because a superior exercise type or dosage has not been found [12]. Pharmaceutical pain management is provided as an adjunct when first-line treatments do not adequately relieve symptoms [9]. Cognitive behavioural therapy and gait aids are additional adjunctive therapies considered on a case-by-case basis [3]. Total knee replacement (TKR) is appropriate when nonsurgical (first-line, pharmaceutical, and other adjuncts) treatments are not sufficient for symptom management [14].
International evidence suggests first-line treatments for knee OA are underused in primary care while pharmaceutical and surgical treatments are overused [[15], [16], [17], [18], [19]]. Previous research investigated use of nonsurgical treatments over short periods [15,16,18,19], but knee OA is a chronic disease and long-term use of these services are unknown. In addition, prior research evaluated nonsurgical treatment use before referral to the orthopaedic surgeon, not people with knee OA who attend a consultation regarding TKR who are not surgical candidates (approximately 40% of those referred [20]). First-line and pharmaceutical treatments would typically be suggested to manage symptomatic knee OA for people who are not surgical candidates, but actual use of these services after consultation is unknown. We filled this knowledge gap by evaluating long-term use of nonsurgical treatments after an orthopaedic surgeon consultation.
Understanding what treatments people choose and how these strategies align with clinical guidelines can help heath systems design and implement new services to fill these evidence-practice gaps. We describe “usual care” patterns (the mixture of treatments that people attempt) in a cohort with knee OA who were not surgical candidates then conduct exploratory data analysis to identify participant characteristics associated with nonsurgical treatment use.
2. Methods
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting cross-sectional studies [21].
2.1. Study and design
This cross-sectional study was nested within a prospective cohort study (BEST-Knee) [22,23]. Participants attended a TKR consultation at a high-volume bone and joint central intake clinic with 25 orthopaedic surgeons in Edmonton, Alberta, Canada between October 27, 2014 and September 30, 2016. An orthopaedic surgeon confirmed knee OA as the primary diagnosis, but did not recommend TKR during this initial consultation. Participants were re-engaged between October 28, 2019, and February 3, 2020 and invited to participate in a survey capturing nonsurgical treatments used for OA symptom management since the initial orthopaedic consultation.
2.2. Participants
Participants were enrolled if they previously consented to participate in the BEST-Knee Study [22,23], had an orthopaedic surgeon diagnosis of knee OA, deemed inappropriate for surgical intervention during the initial orthopaedic consultation, were ≥30 years of age, had the ability to read and comprehend English, and understand and provide written consent to participate.
2.3. Data collection
We designed a telephone-administered questionnaire on REDCap, a secure web-based application designed to support data capture for research studies [24,25]. The questionnaire asked about socio-demographics, comorbidities, health professional visits, and OA treatments used after their initial orthopaedic consultation. Participants were asked, “what interventions have you tried to help manage your knee pain?” then selected from the following list: “medications,” “joint injections,” “strategies to manage your weight,” “exercise,” “physiotherapy,” “education about how to manage your knee without surgery,” “the use of a walking aid (i.e. cane, walker, Nordic walking poles, etc.),” “the use of joint protection (i.e. knee brace, orthotics, assistive devices),” “mental health supports,” “saw a different surgeon,” “other,” and “nothing.” Follow-up questions asked specific details about each type of intervention and current use. Comorbidities were identified by answering yes or no to the following list of conditions: “heart disease”, “heart attack (myocardial infarction)”, “high blood pressure”, “high cholesterol or lipids”, “stroke”, “asthma”, “chronic bronchitis”, “emphysema or chronic obstructive pulmonary disease”, “diabetes”, “kidney disease”, “liver disease”, “intestinal or stomach ulcer”, “rheumatoid arthritis”, “depression”, “low back pain”, and “other physical impairment which limits your activity”. Participants were contacted chronologically starting with the most recent orthopaedic surgeon consultation.
2.4. Outcomes
The primary outcome, guideline-consistent nonsurgical knee OA treatment use (yes or no), was an aggregate measure defined as having used education, exercise, weight management (if body mass index ≥25 kg/m2), and at least 1 recommended medication (oral or topical anti-inflammatory, acetaminophen, or corticosteroid injection) unless gastrointestinal or cardiovascular contraindications are reported. This treatment definition used the 2014 Osteoarthritis Research Society International (OARSI) guidelines for nonsurgical treatment of knee OA [3] to align with evidence-based recommendations that existed during the participant's initial orthopaedic consultation. Our definition also aligns with the 2019 OARSI guidelines because nonsurgical recommendations did not change from 2014 to 2019. If statements with Boolean operators (and/or) were used to create conditional expressions for the primary outcome and coding was verified by visually inspecting the dataset. Participants had guideline-consistent education if they reported that a registered healthcare professional (orthopaedic surgeon, family doctor, physiotherapist, chiropractor, naturopath, or other registered health professional) provided formal instruction about OA and self-management techniques. Guideline-consistent exercise was defined as self-reported use of any amount of any exercise requiring muscular contraction for health benefits or managing knee OA symptoms. Two definitions were used to define an adequate dose of exercise. A minimum exercise dosage to maintain physical function was defined as 55 min or more of moderate−to−vigorous intensity physical activity per week which aligns with evidence suggesting this dosage best predicts disability-free status over four years in people with knee OA [26]. A minimum exercise dosage for general health maintenance was defined as 150 min or more of moderate−to−vigorous intensity physical activity per week which aligns with current Canadian Society of Exercise Physiologist guidelines [27,28]. Guideline-consistent weight management was defined as attempted weight management reported by people with body mass index ≥25 kg/m2 as per the conventional cut-off point between normal and overweight categories [29]. All people <25 kg/m2 were defined as receiving guideline-consistent weight management if they did or did not report attempting weight management.
Secondary outcomes evaluated prior use of first-line treatments and guideline-inconsistent treatments. First-line treatments are a subset of the primary outcome measure and defined as education, exercise, and weight management (if body mass index ≥25 kg/m2). Guideline-inconsistent treatments are defined as knee braces, foot orthotics, other orthoses, opioid use, and injections (hyaluronic acid, platelet rich plasma, and stem cell therapy) because 2014 guidelines suggested these interventions lack evidence, are of limited efficacy, and/or have an unfavourable risk profile (2019 guidelines made a similar statement). Knee braces, foot orthotics, and other orthoses were included in the definition for guideline-inconsistent treatments because these biomechanical interventions were the only interventions included in the 2014 guidelines [30], but removed in 2019 guidelines [3] due to inadequate efficacy and poor-quality evidence.
2.5. Sample size
King et al. found 39% of participants in the BEST-Knee study had not attempted all nonsurgical treatments prior to surgical referral to centralized clinics [20]. We estimated that a sample size of 250 participants would provide 90% power to detect a 20% minimum difference in participant characteristics, such as age and sex, between those who used and did not use guideline-consistent nonsurgical treatments if 40% of our sample used nonsurgical treatments and p-value was set at < 0.05.
2.6. Statistical methods
Participant characteristics were summarized using frequencies, medians and interquartile ranges or means and standard deviations, as appropriate. Continuous variable distributions were assessed for normality. Characteristics for respondents/non-respondents, the entire sample, and those who used or did not use guideline-consistent nonsurgical treatments, first-line treatments, and guideline-inconsistent nonsurgical treatments were compared using the Chi-square test, Fisher's exact test, or Student's t-test, as appropriate.
This study evaluates the combination of sex and gender using the term sex because pre-consult questionnaires did not separate gender (i.e., man, woman, and gender diverse people) from sex at birth (i.e., male or female). A race-based analysis was not possible because 91% of the sample identified as Caucasian.
Robust Poisson regression models enabled us to express associations between participant characteristics and health outcomes as relative risks [31] (RR)(the risk of a health event in one group divided by the risk of a health event in another group [32]). The following variables were assessed individually for association with primary and secondary outcomes: sex, age, level of education (post-secondary vs less), household income (> and < $60,000/year), marital status (married vs divorce/separated/widowed), living arrangement (living alone vs living with spouse/family/relatives), specific comorbidities (yes/no), number of comorbidities (0, 1, 2, and 3+), reason for nonsurgical recommendation reported by the orthopaedic surgeon (symptoms not severe enough, patient declined surgery, another treatment should be tried first and other reason), and whether the participant proceeded to surgery at a later date. Specific comorbidities were evaluated as some (i.e., heart disease, kidney disease, and gastrointestinal disease) may contraindicate use of guideline-consistent pharmaceuticals. The number of comorbid conditions was assessed to evaluate the overall burden of comorbidity. Variables were excluded from the model if sample size in either group was below 10. Robust Poisson regression models were built with all hypothesized variables and variables with statistically significant (p-value <0.05) association in univariate analysis. Variables were entered in the model using stepwise selection based on p-value (low-to-high). Likelihood ratios were assessed to determine which nested model performed best. Models produced similar results, so we reported models with all hypothesized variables. All RR in the robust Poisson regression are presented with 95% confidence intervals (CI) and two-sided p-value of 0.05 was considered statistically significant. A small fraction (4%) of our data was missing. We are reporting observed data only because we cannot conclude that our data was missing at random and exploratory multiple imputation made no appreciable difference to the primary outcome results.
Statistical analyses were performed using STATA (v15.1. College Station, Texas, USA). The study was approved by the Research Ethics Boards at the University of Calgary (REB 14-1294).
3. Results
3.1. Participants
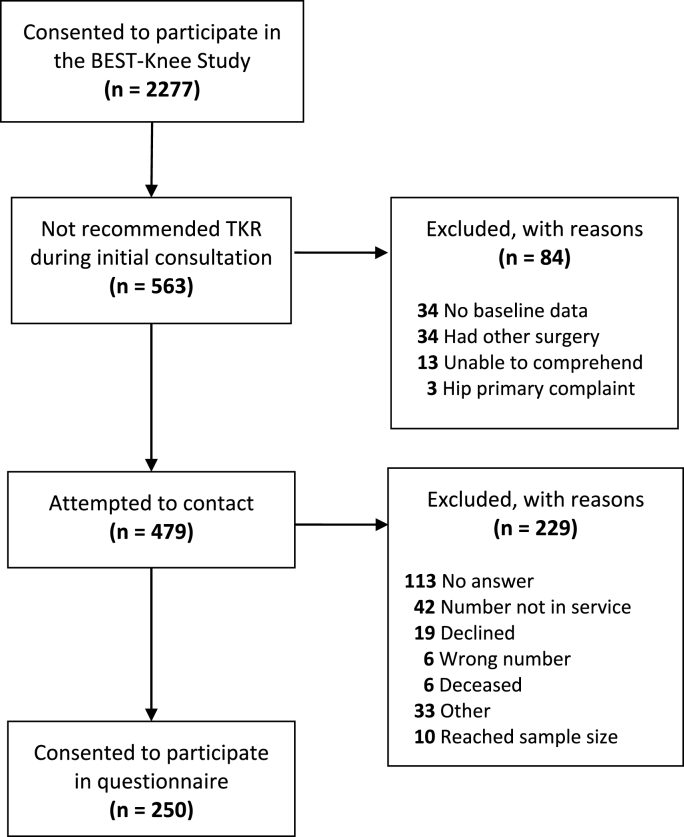
Of 563 people who were not candidates for surgical intervention during the initial orthopaedic consultation, we attempted to contact 479, and 250 agreed to participate (52% response rate) (Fig. 1). All participant characteristics in Table 1 were similar between respondents (58% female, mean age 66.2 year, 95% CI, 65.1 to 67.2) and non-respondents (61% female, mean age 64.1 year, 95% CI, 62.7 to 65.4) except the orthopaedic surgeon selected “other” as the reason for not recommending TKR in significantly less respondents than non-respondents (n = 11 vs n = 36, p = 0.001). We could not identify a pattern in the written responses that accompanied the “other” classification.
Fig. 1.
Participant flow diagram.
Table 1.
Participant characteristics.
| Overall n = 250 |
Use of recommended nonsurgical treatments |
|||
|---|---|---|---|---|
| Used All n = 46 | Did Not Use All n = 191 | P-value | ||
| Demographics | ||||
| Female | 146 (58.4) | 34 (73.9) | 112 (58.0) | 0.047∗ |
| Age, years, mean (SD)a | 66.2 (8.3) | 63.3 (7.8) | 66.9 (8.3) | 0.008∗ |
| Working | 48 (19.2) | 13 (28.3) | 34 (17.6) | 0.103 |
| Retired | 178 (71.2) | 29 (63.0) | 140 (72.5) | 0.297 |
| Post-secondary | 137(54.8) | 33(71.7) | 104(54.5) | 0.033∗ |
| Annual income > $60,000 | 157 (62.8) | 27(58.7) | 124(64.3) | 0.265 |
| Married | 172 (68.8) | 28 (60.9) | 136 (70.5) | 0.171 |
| Living w/spouse | 166(66.4) | 32(69.6) | 126(65.3) | 0.426 |
| Proceeded to surgery at later date | 85 (34.0) | 18 (39.1) | 64 (33.2) | 0.443 |
| Co-Morbidities | ||||
| BMI, kg/m2, mean (SD)a | 33.5 (6.7) | 34.7 (7.3) | 33.2 (6.6) | 0.2527 |
| BMI ≥25 kg/m2 (overweight or obese)a | 221 (88.4) | 37 (80.4) | 184 (95.4) | 0.213 |
| Heart disease | 43 (17.2) | 5 (10.9) | 37 (19.2) | 0.280 |
| Hypertension | 136 (54.4) | 21 (45.7) | 109 (56.5) | 0.192 |
| High cholesterol | 87 (34.8) | 11 (23.9) | 73 (37.8) | 0.087 |
| Stroke | 5 (2.0) | – | 5 (2.6) | 0.586 |
| Asthma | 18 (7.2) | 8 (16.7) | 10 (5.2) | 0.001∗ |
| Lung disease | 18 (7.2) | 2 (4.2) | 16 (8.4) | 0.538 |
| Diabetes | 55 (22.0) | 12 (26.1) | 42 (21.8) | 0.558 |
| Kidney disease | 11 (4.4) | – | 10 (5.2) | 0.216 |
| Liver disease | 4 (1.6) | – | 4 (2.1) | 1.0 |
| Gastrointestinal disease | 11 (4.4) | 1 (2.2) | 8 (4.2) | 1.0 |
| Rheumatoid arthritis | 4 (1.6) | 1 (2.1) | 3 (1.6) | 0.577 |
| Depression | 35 (14.0) | 9 (19.6) | 23 (11.9) | 0.226 |
| Low back pain | 123 (49.2) | 17 (35.4) | 100 (52.4) | 0.032∗ |
| Other physical impairment | 67 (26.8) | 12 (26.1) | 51 (26.4) | 0.963 |
| Number of Co-Morbidities | ||||
| 0 | 34 (13.6) | 9 (19.6) | 24 (12.4) | |
| 1 | 61 (24.4) | 14 (30.4) | 43 (22.3) | 0.246 |
| 2 | 50 (20.0) | 9 (19.6) | 40 (20.7) | 0.861 |
| 3a | 105 (42.0) | 14 (30.4) | 86 (44.6) | 0.081 |
| Missing | 11(4.4) | |||
| Reason for Non-Surgical Diagnosis | ||||
| Symptoms are not severe enough | 140 (56.0) | 27(58.7) | 110 (57.0) | 0.834 |
| Another treatment should be tried first | 50 (20.0) | 8 (17.4) | 40 (20.7) | 0.612 |
| Co-morbidity | 35 (14.0) | 6 (13.0) | 26 (13.5) | 1.00 |
| Patient declined surgery | 14 (5.6) | 2 (4.3) | 10 (5.2) | 0.816 |
| Other | 11 (4.4) | 3 (6.5) | 7 (3.6) | |
| Missing | 11(4.4) | |||
Note: values represent n (%) unless otherwise stated. Thirty-four different univariate analyses with p-value of 0.05 suggests there is an 83% chance that a statistically significant finding is a false positive.
∗= p-value < 0.05 when evaluating participant characteristic between those who used and did not use guideline-consistent nonsurgical treatments.
during initial orthopaedic consultation.
Participants were 58% female, mean age of 66.2 years (95% CI, 65.1 to 67.2) and 55% had attended post-secondary education (Table 1). Orthopaedic surgeons did not recommend TKR during the initial consultation because: symptoms were not severe enough (56%), recommended trying another treatment first (20%), co-morbidities made surgical risk outweigh the benefits (14%), patient declined surgery (6%), and other reason (4%). Over 6 years, 34% of participants proceeded to TKR (these participants reported use of nonsurgical treatments between the initial and second orthopaedic consultation).
3.2. Primary outcome
Guideline-consistent nonsurgical treatments were used by 19% of participants following their initial orthopaedic consultation (Table 2). Participants received education from at least one health professional (60%, n = 150), exercised regularly (74%, n = 185), used weight management techniques (37%, n = 89), and used guideline-consistent pain medications (91%, n = 228). Almost everyone (99%) reported using at least one guideline-consistent nonsurgical treatment. Participants received education from an orthopaedic surgeon (40%, n = 101), family doctor (31%, n = 77), physiotherapist (8%, n = 20), chiropractor (<2%, n=<5), naturopath (<2%, n=<5), education class (6%, n = 15), friends (10%, n = 26), internet (10%, n = 27), other source (9%, n = 23) or have not learned anything about OA (20%, n = 50). Participants reported regularly exercising by walking (52%, n = 129), biking (18%, n = 44), strength training (12%, n = 31), take the GLA:D program [33] (4%, n = 9), swimming (6%, n = 14), aquacise (7%, n = 18), deep water workouts (<2%, n=<5), or other (9%, n = 23). The average active person reported exercising for 343 min per week (95% CI, 299.5 to 386.8) over 3.7 days per week (95% CI 3.0 to 4.3), but 15% did not meet the minimum dosage to maintain their functional status (n = 27) and 33% did not meet the minimum dosage to maintain overall health (n = 61). Participants reported taking acetaminophen (46%, non-prescription and 8% prescription, n = 116 and n = 19 respectively), topical non-steroidal autoinflammatory (NSAIDs) (8%, non-prescription and 18% prescription, n = 19 and n = 46 respectively), oral NSAIDs (23%, n = 55), opioids (10%, n = 26), anti-depressant (7%, n = 18), chondroitin (3%, n = 7), risedronate (<2%, n=<5), or other medication (12%, n = 29). Most participants received at least one corticosteroid injection (65%, n = 163). No participants reported using disease modifying anti-rheumatic drugs. Capsaicin, diacerein, and rosehip powder were recommended in the 2014 guidelines, but not used by any participants. Medications were taken as needed (48%, n = 96), daily (44%, n = 88), weekly (6%, n = 12), monthly (<3%, n=<6), or other (<3%, n=<6). Participants spoke with their family doctor about weight management (4%, n = 9), saw a dietician (11%, n = 36), followed the Canada Food Guide [34] (<2%, n=<5), attended a weight loss program (4%, n = 11), ate less (16%, n = 40), or other (10%, n = 24). People were 3% less likely to use guideline-consistent treatments for each additional year of age (RR 0.97, 95% CI 0.94 to 0.99) (Table 3).
Table 2.
Use of nonsurgical treatments over three to six years post consult.
| Overall n(%) n = 250 |
|
|---|---|
| All guideline-consistent treatmentsawith any volume of self-reported exercise | 46 (19.3) |
| All guideline-consistent treatments with ≥55 min/wk exercise threshold | 39 (16.3) |
| All guideline-consistent treatments with ≥150 min/wk exercise threshold | 31 (13.0) |
| First-line treatmentsb | 48(19.2) |
| Guideline-inconsistent treatmentsc | 105 (42.0) |
| Educationd | 150 (60.0) |
| All self-reported exercisee | 185 (74.0) |
| ≥55 min/wk exercise threshold | 158 (63.2) |
| ≥150 min/wk exercise threshold | 124 (49.6) |
| Weight managementf | 89 (37.2) |
| Medicationsg | 228 (91.2) |
| Used at least 1 guideline-consistent therapy | 247 (98.8) |
used education, exercise, weight management (if body mass index ≥25 kg/m2), and at least 1 recommended medication (oral or topical anti-inflammatory, acetaminophen, or corticosteroid injection) unless gastrointestinal or cardiovascular contraindications are reported.
education, exercise, and weight management (if body mass index ≥25 kg/m2).
joint protection, opioid use, and injections (hyaluronic acid, platelet rich plasma, and stem cell therapy).
reported that a registered health professional (orthopaedic surgeon, family doctor, physiotherapist, chiropractor, massage therapist, naturopath, or other registered health professional) provided formal instruction about OA and self-management techniques.
participants self-reported use of any amount of any exercise requiring muscular contraction for the purpose of health benefits or managing knee OA symptoms.
attempted weight management by people with body mass index ≥25 kg/m2. All people <25 kg/m2 were defined as receiving guideline-consistent weight management if they did or did not report attempting weight management.
used any dose of oral or topical anti-inflammatory, acetaminophen, or corticosteroid injection.
Table 3.
Relationship between participant characteristics and use of nonsurgical treatments.
| Use of Recommended Nonsurgical Treatmentsa Adjusted RR (95% CI) n = 237 |
|
|---|---|
| Age, per yr increase | 0.97 (0.94–0.99)∗ |
| Low back pain | 0.59 (0.31–1.11) |
| Did not attend post-secondary education (attended post-secondary reference) | 0.58 (0.33–1.04) |
| Female sex (male reference) | 1.50 (0.81–2.77) |
| 3 or more co-morbidities | 0.78 (0.39–1.59) |
| Not married (married reference) | 1.37 (0.83–2.28) |
| Obeseb (non-obese reference) | 0.85 (0.45–1.59) |
| Depression | 1.29 (0.67–2.42) |
| Working (not working reference) | 1.05 (0.77–1.44) |
| Had surgery later (did not have surgery reference) | 1.48 (0.74–2.06) |
| Reference: age, no reported low back pain, attended post-secondary education, male, < 3 co-morbidities, married, not obese, no reported depression, not working, and did not have surgery at a later date |
1.00 |
Log likelihood = −112.50, AIC = 1.04, BIC = −1102.77.
Note: values are derived from a Poisson regression model.
RR = Risk Ratio, the risk of a health event in one group divided by the risk of a health event in another group [22]; adjusted for age, low back pain, post-secondary education, sex, three or more comorbidities, marital status, obesity, depression, work status and proceeded to surgery after a subsequent orthopaedic consultation.
∗= p-value < 0.05.
used education, exercise, weight management (if body mass index ≥25 kg/m2), and at least 1 recommended medication (oral or topical anti-inflammatory, acetaminophen, or corticosteroid injection) unless gastrointestinal or cardiovascular contraindications are reported.
Obese defined as ≥ 30.0 kg/m.2.
3.3. Secondary outcomes
First-line treatments were used by 19% of participants after their initial orthopaedic consultation (Table 2). People were 3% less likely to use first-line treatments for each additional year of age (RR 0.97, 95% CI 0.94 to 0.99) and 45% less likely to use first-line treatments if they did not attend post-secondary education (RR 0.54, 95% CI 0.30 to 0.96) (Table 4).
Table 4.
Relationship between participant characteristics and use of first-line treatments.
| Use of Recommended First-Line Treatmentsa Adjusted RR (95% CI) n = 237 |
|
|---|---|
| Age, per yr increase | 0.97 (0.94–0.99)∗ |
| Did not attend post-secondary education (attended post-secondary reference) | 0.54 (0.30–0.96)∗ |
| Low back pain | 0.60 (0.32–1.11) |
| 3 or more co-morbidities | 0.72 (0.36–1.44) |
| Female sex (male reference) | 1.44 (0.80,–2.60) |
| Working (not working reference) | 1.14 (0.89–1.47) |
| Depression | 1.23 (0.65–2.33) |
| Obeseb (non-obese reference) | 0.87 (0.48–1.57) |
| Not married (married reference) | 1.27 (.78–2.07) |
| Reference: age, attended post-secondary education, no reported low back pain, <3 co-morbidities, male, not working, no reported depression, non-obese, and married |
1.00 |
Log likelihood = −115.65, AIC = 1.06, BIC = −1105.95.
Note: values are derived from a Poisson regression model.
RR = Risk Ratio, the risk of a health event in one group divided by the risk of a health event in another group [22]; adjusted for age, post-secondary education, low back pain, three or more comorbidities, sex, work status, depression, obesity and marital status.
∗= p-value < 0.05.
education, exercise, and weight management (if body mass index ≥25 kg/m2).
Obese defined as ≥ 30.0 kg/m2.
Guideline-inconsistent treatments were used by 42% of participants after their initial orthopaedic consultation (Table 2). Participants reported using knee braces (31%, n=77), foot orthotics (<2%, n=<5), other orthoses (<2%, n=<5), opioid use (10%, n=26), hyaluronic acid (9%, n=24), platelet rich plasma (<2%, n=<5), and stem cell therapy (<2%, n=<5) over the study period. People were 3% less likely to use guideline-inconsistent treatments for each additional year of age (RR 0.97, 95% CI 0.96 to 0.99) and 39% less likely to use guideline-inconsistent treatments if they were female (RR 0.62, 95% CI 0.47 to 0.81) (Table 5).
Table 5.
Relationship between participant characteristics and use of guideline-inconsistent treatments.
| Use of Recommended Guideline-Inconsistenta Treatments Adjusted RR (95% CI) n = 237 |
|
|---|---|
| Age, per yr increase | 0.97 (0.96–0.99)∗ |
| Female sex (male reference) | 0.62 (0.47–0.81)∗ |
| Working (not working reference) | 0.97 (0.81–1.17) |
| Did not attend post-secondary education (attended post-secondary reference) | 0.86 (0.64–1.14) |
| Had surgery later (did not have surgery reference) | 0.88 (0.65–1.20) |
| Reference: age, male sex, not working, attended post-secondary education, and did not proceed to surgery at a later date | 1.00 |
Log likelihood = −181.99, AIC = 1.59, BIC = −1105.14.
Note: values are derived from a Poisson regression model.
∗= p-value < 0.05.
RR = Risk Ratio, the risk of a health event in one group divided by the risk of a health event in another group [22]; adjusted for age, sex, work status, post-secondary education, and proceeded to surgery after a subsequent orthopaedic consultation.
joint protection, opioid use, and injections (hyaluronic acid, platelet rich plasma, and stem cell therapy).
4. Discussion
Only one in five people reported using all guideline-consistent nonsurgical treatments and first-line treatments after an orthopaedic surgeon suggested nonsurgical treatment. However, two in five people reported using treatments which do not align with current clinical guidelines. Older participants were 3% less likely to report using guideline-consistent, first-line and guideline-inconsistent treatments per year of age which is equivalent to 26% less likely over a 10-year age span. Meanwhile, people who did not attend post-secondary education were 45% less likely to use guideline-consistent treatments and females were 38% less likely to use guideline-inconsistent treatments. We assume these results are best interpreted as gender differences, rather than sex, because sociocultural factors (i.e., family, caregiving, or care-receiving roles) are more likely to impact use of treatments than biology. Our results show a wide gap between what guidelines recommend and what treatments people use to manage their knee OA before surgery is indicated.
Clinical guidelines have recommended education, exercise, and weight management for 25 years [35], but use remains low. Our results showed that 80% of participants have not used all the guideline-consistent treatments after an orthopaedic consultation. These findings are lower than two systematic reviews [16,18] that showed 60% of community-based participants have not received appropriate first-line OA care in the USA, UK, Norway, Canada, and Australia. Our results may have been different because our aggregate measure and sample were more specific than the cumulative quality indicators and broad sample of community-based participants in both systematic reviews [16,18]. Our results were also lower than King et al. [20] who observed that 40% of people with knee OA have not attempted first-line treatments before proceeding to TKR. Our study and King et al. both evaluated people with knee OA referred to the same clinic, but King et al. evaluated nonsurgical treatments before consultation in people who proceeded to surgery while we evaluated nonsurgical treatments after consultation in people who did not proceed to surgery. These two populations may have different treatment preferences, but our results combined with King et al. suggests people are not using optimal nonsurgical OA care before or after an orthopaedic consultation. Our results can be generalized to people with symptomatic knee OA who are not currently eligible for TKR, but our sample was younger, had higher income and were more highly educated than a population-level cohort in Ontario Canada [36]. Low uptake of first-line treatments aligns with global trends showing these safe, effective, and appropriate treatments are underused in clinical practice [[15], [16], [17], [18], [19]]. Barriers to optimize use of first-line treatments include service availability, time, cost, referral patterns, and beliefs held by patients and health care professionals which may not align with current evidence [37,38]. We also observed that age, education, and gender-related differences might be associated with barriers to access care. In a forthcoming publication, we report on our qualitative study which explored access barriers in a subset of participants from this study.
We found a significant gap between “usual care” and clinical guidelines. People might use a combination of treatments that manage their symptoms and fit their preferences, but using all treatments could provide significant health benefits [39,40]. We could not separate therapeutic exercise for OA from general exercise. However, 32% of participants reported seeing a physiotherapist so we can assume that only a small subset of our active participants were actually prescribed therapeutic exercise. Half our sample did not meet the Canadian Physical Activity Guidelines and 37% were not physically active enough to maintain their mobility. This level of sedentarism was surprising since exercise improves OA-related health outcomes [41] and prevents 35 chronic conditions [42,43]. Also, 87% of participants had at least one co-morbidity where exercise was recommended as standard treatment [42] which means increasing physical activity in the OA population is an opportunity to produce multi-system health benefits. Almost 90% of participants were overweight or obese (≥ 25 kg/m2), but only 37% of participants attempted to manage or reduce their weight. Reducing body weight by 5–10% can improve OA-related health outcomes [39,40,44], but people may not believe weight management will have a meaningful impact on their OA symptoms and disease progression [45]. Increasing use of weight management programs is critical because the combination of diet and exercise produces better clinical improvements than exercise or diet alone [39,40]. Almost every participant used guideline-consistent pain medications which is similar to previous findings [19], suggesting pharmaceuticals are people's primary method of managing knee OA instead of lifestyle interventions. Low opioid use is noteworthy. Only 10% of participants reported using opioids which is lower than King et al.’s finding where 30% reported currently using opioids [46]. However, participants in King et al. were taking opioids before consultation and proceeded to surgery so they might have had more severe disease than our sample who reported after consultation use of opioids and did not proceed to surgery following the initial orthopedic consultation.
First-line treatments are safe [30], appropriate [30], effective [41], and efficient [47], but these proven treatments continue to be underused. Increasing use of first-line treatments could improve health outcomes for people living with OA and lead to better health system performance. A randomized controlled trial evaluating knee replacement observed that 68% of surgical candidates randomized to an education and exercise program have not proceeded to surgery two years after the intervention [48]. This program would pay for itself if 8% of people avoided TKR [49]. An efficient health insurance provider would offer coverage for low-cost services before committing to high-cost care. For example, US Centers for Medicare and Medicaid Services provides coverage for lifestyle change programs [50] shown to reduce diabetes incidence by 58% [51]. Integrating structured education, exercise, and weight management programs into standardized clinical pathways could ensure first-line treatments are exhausted before surgical referral. Customizing interventions to address sociocultural factors related to age, education, and gender may help improve use of proven therapies in these subpopulations. Future research should implement and evaluate referral pathways and structured first-line therapy programs in health systems and health insurance plans. Developing implementation guidelines, health professional training programs, resources, models of care and frameworks for quality monitoring have been identified as global priorities [52].
Our study has limitations. Long duration between initial orthopaedic consultation and telephone interview could lead to recall bias, although the three to six year time period after orthopaedic consultation allowed us to capture treatments for multiple years as this population manages their chronic disease. Self-reported data can also be over or under-reported, potentially leading to systematic bias [53,54]. However, alternative data sources such as administrative data were not possible because most education programs, exercise therapy, physiotherapy, and dietician consultations are paid privately in the Canadian health system. Second wave data collection, which was used in our study, is known to produce lower response rates than the initial data collection [55]. Our response rate (52%) could bias results, but similar respondent and nonrespondent characteristics would suggest non-response bias is unlikely. The nonsurgical treatment suggestions made by the orthopaedic surgeon were not recorded during the initial consultation. A measure of disease severity was not collected over the telephone. We estimated a priori that 40% of our sample would use guideline-consistent nonsurgical treatments, but only 20% met the case definition. Fewer participants meeting the case definition meant our regression analyses were underpowered and limited our ability to evaluate associations between participant characteristics and use of nonsurgical treatments. Lastly, our analysis was unable to separate the influence of sex (i.e., biological factors) and gender (i.e., sociocultural factors).
5. Conclusions
Only one in five participants used guideline-consistent nonsurgical treatments to manage their knee OA within six years of orthopaedic surgeon consultation, while two in five people used treatments not consistent with clinical guidelines over the same time period. Increasing use of education, exercise, and weight management could improve health outcomes for people living with OA, reduce wait times for joint replacement and increase value for money in the health system. Findings may help inform decision-makers planning future OA service delivery to optimize nonsurgical care.
Contributions
DRM, JW, PF, TW and DAM developed the study concept. DRM and DAM developed the study protocol with JW, AKR, PF, and TW providing critical input. Data analysis was conducted by DRM with input by PF and DAM. Results were interpreted by DRM, PF, DAM with critical feedback provided by all other authors. Manuscript draft preparation was performed by DRM. All authors provided input on final manuscript.
Funding
This project was funded by the Alberta Health Services Bone and Joint Health Strategic Clinical Network™. DRM receives funding from the Arthritis Society Training Graduate PhD Salary Award, Cumming School of Medicine Graduate Student Scholarship, and the Arthur J.E. Child Chair in Rheumatology Outcomes Research. JLW is supported by the Michael Smith Foundation for Health Research and the Arthritis Society. DAM is supported by the Arthur J.E. Child Chair in Rheumatology and a Canada Research Chair in Health Systems and Services Research (2008–2018).
Declaration of competing interest
We declare no competing interests.
Acknowledgements
Anne-Marie Adachi conducted telephone interviews for this study. Her experience, talents and work ethic are greatly admired. The authors would also like to thank the staff at the Edmonton Bone and Joint Centre because this project would not have been possible without their support.
Contributor Information
D.R. Mazzei, Email: darren.mazzei@ucalgary.ca.
J.L. Whittaker, Email: jackie.whittaker@ubc.ca.
A. Kania-Richmond, Email: anna.kania-richmond@albertahealthservices.ca.
P. Faris, Email: peter.faris@albertahealthservices.ca.
T. Wasylak, Email: tracy.wasylak@albertahealthservices.ca.
J. Robert, Email: Jill.Robert@albertahealthservices.ca.
G. Hawker, Email: g.hawker@utoronto.ca.
D.A. Marshall, Email: damarsha@ucalgary.ca.
References
- 1.Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014;10(7):437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 2.Safiri S., Kolahi A.A., Hoy D., Smith E., Bettampadi D., Mansournia M.A., et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann. Rheum. Dis. 2019;78(11):1463–1471. doi: 10.1136/annrheumdis-2019-215920. [DOI] [PubMed] [Google Scholar]
- 3.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Osteoarthritis: Care and Management in Adults. National Institute of Health Care Excellence; London, UK: 2014. https://www.nice.org.uk/guidance/cg177 Available from. [PubMed] [Google Scholar]
- 5.Fernandes L., Hagen K.B., Bijlsma J.W., Andreassen O., Christensen P., Conaghan P.G., et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013;72:1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 6.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72:149–162. doi: 10.1002/acr.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruyère O., Honvo G., Veronese N., Arden N.K., Branco J., Curtis E.M., et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO) Semin. Arthritis Rheum. 2019;49:337–350. doi: 10.1016/j.semarthrit.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Osteoarthritis tool: Canada. Arthritis Alliance of Canada: Centre for Effective Practice and the College of Family Physicians of Canada 2017.
- 9.First-line therapies. NCI dictionaries. Bathesda, Maryland: NIH National Cancer Institute. [cited 2020 July 12]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/first-line-therapy.
- 10.Kroon F.P., van der Burg L.R., Buchbinder R., Osborne R.H., Johnston R.V., Pitt V. Self-management education programmes for osteoarthritis. Cochrane Database Syst. Rev. 2014:Cd008963. doi: 10.1002/14651858.CD008963.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global Recommendations on Physical Activity for Health Geneva: World Health Organization. Copyright © World Health Organization 2010; 2010. WHO guidelines approved by the guidelines review committee. [Google Scholar]
- 12.Holden M.A., Button K., Collins N.J., Henrotin Y., Hinman R.S., Larsen J.B., et al. Guidance for implementing best practice therapeutic exercise for patients with knee and hip osteoarthritis: what does the current evidence base tell us? Arthritis Care Res. 2021;73:1746–1753. doi: 10.1002/acr.24434. [DOI] [PubMed] [Google Scholar]
- 13.Rausch Osthoff A.-K., Niedermann K., Braun J., Adams J., Brodin N., Dagfinrud H., et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018;77:1251. doi: 10.1136/annrheumdis-2018-213585. [DOI] [PubMed] [Google Scholar]
- 14.Hawker G., Bohm E.R., Conner-Spady B., De Coster C., Dunbar M., Hennigar A., et al. Perspectives of Canadian stakeholders on criteria for appropriateness for total joint arthroplasty in patients with hip and knee osteoarthritis. Arthritis Rheumatol. 2015;67(7):1806–1815. doi: 10.1002/art.39124. [DOI] [PubMed] [Google Scholar]
- 15.Runciman W.B., Hunt T.D., Hannaford N.A., Hibbert P.D., Westbrook J.I., Coiera E.W., et al. CareTrack: assessing the appropriateness of health care delivery in Australia. Med. J. Aust. 2012;197:100–105. doi: 10.5694/mja12.10510. [DOI] [PubMed] [Google Scholar]
- 16.Basedow M., Esterman A. Assessing appropriateness of osteoarthritis care using quality indicators: a systematic review. J. Eval. Clin. Pract. 2015;21:782–789. doi: 10.1111/jep.12402. [DOI] [PubMed] [Google Scholar]
- 17.Hinman R.S., Nicolson P.J.A., Dobson F.L., Bennell K.L. Use of nondrug, nonoperative interventions by community-dwelling people with hip and knee osteoarthritis. Arthritis Care Res. 2015;67:305–309. doi: 10.1002/acr.22395. [DOI] [PubMed] [Google Scholar]
- 18.Hagen K.B., Smedslund G., Østerås N., Jamtvedt G. Quality of community-based osteoarthritis care: a systematic review and meta-analysis. Arthritis Care Res. 2016;68(10):1443–1452. doi: 10.1002/acr.22891. [DOI] [PubMed] [Google Scholar]
- 19.Khoja S.S., Almeida G.J., Freburger J.K. Recommendation rates for physical therapy, lifestyle counseling, and pain medications for managing knee osteoarthritis in ambulatory care settings: a cross-sectional analysis of the National Ambulatory Care Survey (2007–2015) Arthritis Care Res. 2020;72(2):184–192. doi: 10.1002/acr.24064. [DOI] [PubMed] [Google Scholar]
- 20.King L.K., Marshall D.A., Faris P., Woodhouse L., Jones C.A., Noseworthy T., et al. Use of recommended non-surgical knee osteoarthritis management in patients prior to total knee arthroplasty: a cross-sectional study. J. Rheumatol. 2019;47(8):1–8. doi: 10.3899/jrheum.190467. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Hawker G.A., Conner-Spady B.L., Bohm E., Dunbar M.J., Jones C.A., Ravi B., et al. Patients' preoperative expectations of total knee arthroplasty and satisfaction with outcomes at one year: a prospective cohort study. Arthritis Rheumatol. 2021;73:223–231. doi: 10.1002/art.41510. [DOI] [PubMed] [Google Scholar]
- 23.Hawker G.A., Conner-Spady B.L., Bohm E., Dunbar M.J., Jones C.A., Ravi B., et al. The Relationship between Patient-Reported Readiness for Total Knee Arthroplasty and Likelihood of a Good Outcome at One Year. Arthritis Care & Research. 2022 doi: 10.1002/acr.24562. In press. [DOI] [PubMed] [Google Scholar]
- 24.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunlop D.D., Song J., Hootman J.M., Nevitt M.C., Semanik P.A., Lee J., et al. One hour a week: moving to prevent disability in adults with lower extremity joint symptoms. Am. J. Prev. Med. 2019;56:664–672. doi: 10.1016/j.amepre.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canadian 24-hour Movement Guidelines for Adults 65 Years or Older: an Integration of Physical Activity, Sedentary Behaviour, and Sleep. Canadian Society of Exercise Physiologists; Ottawa, Ontario: 2020. https://csepguidelines.ca/wp-content/uploads/2020/10/24HMovementGuidelines-Adults-65-2020-ENG.pdf Available from. [Google Scholar]
- 28.Canadian 24-hour Movement Guidelines for Adults Aged 18-64 Years: an Integration of Physical Activity, Sedentary Behaviour, and Sleep. Canadian Society of Exercise Physiologists; Ottawa, Ontario: 2020. https://csepguidelines.ca/wp-content/uploads/2020/10/24HMovementGuidelines-Adults18-64-2020-ENG.pdf Available from. [Google Scholar]
- 29.Gallagher D., Heymsfield S.B., Heo M., Jebb S.A., Murgatroyd P.R., Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 30.McAlindon T.E., Bannuru R.R., Sullivan M.C., Arden N.K., Berenbaum F., Bierma-Zeinstra S.M., et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 32.Risk Ratio. Lesson 3: Measures of Risk . Centers for Disease Control and Prevention; Washington, DC: 2012. Deputy Director for Public Health Science and Surveillance, Center for Surveillance, Epidemiology, and Laboratory Services, Division of Scientific Education and Professional Development.https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section5.html Available from. [Google Scholar]
- 33.Skou S.T., Roos E.M. Good Life with osteoArthritis in Denmark (GLA:D™): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Muscoskel. Disord. 2017;18(1):72. doi: 10.1186/s12891-017-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Health Canada . Government of Canada; Ottawa, Ontario: 2019. Canada's Food Guide.https://food-guide.canada.ca/en/ Available from. [Google Scholar]
- 35.Hochberg M.C., Altman R.D., Brandt K.D., Clark B.M., Dieppe P.A., Griffin M.R., et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995;38:1541–1546. doi: 10.1002/art.1780381104. [DOI] [PubMed] [Google Scholar]
- 36.Hawker G.A., Wright J.G., Coyte P.C., Williams J.I., Harvey B., Glazier R., et al. Differences between men and women in the rate of use of hip and knee arthroplasty. N. Engl. J. Med. 2000;342:1016–1022. doi: 10.1056/NEJM200004063421405. [DOI] [PubMed] [Google Scholar]
- 37.Egerton T., Diamond L.E., Buchbinder R., Bennell K.L., Slade S.C. A systematic review and evidence synthesis of qualitative studies to identify primary care clinicians' barriers and enablers to the management of osteoarthritis. Osteoarthritis Cartilage. 2017;25(5):625–638. doi: 10.1016/j.joca.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 38.MacKay C., Hawker G.A., Jaglal S.B. Qualitative study exploring the factors influencing physical therapy management of early knee osteoarthritis in Canada. BMJ Open. 2018;8(11) doi: 10.1136/bmjopen-2018-023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messier S.P., Mihalko S.L., Legault C., Miller G.D., Nicklas B.J., DeVita P., et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messier S.P., Loeser R.F., Miller G.D., Morgan T.M., Rejeski W.J., Sevick M.A., et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 41.Fransen M., McConnell S., Harmer A.R., Van der Esch M., Simic M., Bennell K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2015;1 doi: 10.1002/14651858.CD004376.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen B.K., Saltin B. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015;25:1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 43.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. In: Comprehensive Physiology:1143-1211. [DOI] [PMC free article] [PubMed]
- 44.Christensen R., Bartels E.M., Astrup A., Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 2007;66:433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunzli S., O'Brien P., Ayton D., Dowsey M., Gunn J., Choong P., et al. Misconceptions and the acceptance of evidence-based nonsurgical interventions for knee osteoarthritis. A qualitative study. Clin. Orthop. Relat. Res. 2019;477:1975–1983. doi: 10.1097/CORR.0000000000000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King LK, Marshall DA, Jones CA, Woodhouse LJ, Ravi B, Faris PD, et al. Are Medical Comorbidities Contributing to the Use of Opioid Analgesics in Patients with Knee Osteoarthritis? Osteoarthritis Cartilage. [DOI] [PubMed]
- 47.Mazzei D.R., Ademola A., Abbott J.H., Sajobi T., Hildebrand K., Marshall D.A. Are education, exercise and diet interventions a cost-effective treatment to manage hip and knee osteoarthritis? A systematic review. Osteoarthritis Cartilage. 2021;29:456–470. doi: 10.1016/j.joca.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Skou S.T., Roos E., Laursen M., Arendt-Nielsen L., Rasmussen S., Simonsen O., et al. Cost-effectiveness of total knee replacement in addition to non-surgical treatment: a 2-year outcome from a randomised trial in secondary care in Denmark. BMJ Open. 2020;10(1) doi: 10.1136/bmjopen-2019-033495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackerman I.N., Skou S.T., Roos E.M., Barton C.J., Kemp J.L., Crossley K.M., et al. Implementing a national first-line management program for moderate-severe knee osteoarthritis in Australia: a budget impact analysis focusing on knee replacement avoidance. Osteoarthritis Cartilage Open. 2020;2(3):100070. doi: 10.1016/j.ocarto.2020.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.About the National DPP. Centers for Disease Control and Prevention; Washington DC: 2021. [Internet ] Available from: CDC - About the Program - National Diabetes Prevention Program - Diabetes DDT. [Google Scholar]
- 51.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eyles J.P., Hunter D.J., Bennell K.L., Dziedzic K.S., Hinman R.S., van der Esch M., et al. Priorities for the effective implementation of osteoarthritis management programs: an OARSI international consensus exercise. Osteoarthritis Cartilage. 2019;27(9):1270–1279. doi: 10.1016/j.joca.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Petrou S., Murray L., Cooper P., Davidson L.L. The accuracy or self-reported healthcare resource utilization in health economic studies. Int. J. Technol. Assess. Health Care. 2002;18(3):705–710. doi: 10.1017/s026646230200051x. [DOI] [PubMed] [Google Scholar]
- 54.Longobardi T., Walker J.R., Graff L.A., Bernstein C.N. Health service utilization in IBD: comparison of self-report and administrative data. BMC Health Serv. Res. 2011;11:137. doi: 10.1186/1472-6963-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halbesleben J.R.B., Whitman M.V. Evaluating survey quality in health services research: a decision framework for assessing nonresponse bias. Health Serv. Res. 2013;48:913–930. doi: 10.1111/1475-6773.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]