Summary
Objectives
To clarify the effects of mechanical unloading on joint components, including the articular cartilage by knee compartments, synovial membrane, and the infrapatellar fat pad (IFP), using a hindlimb suspension rat model.
Design
Twenty-five male rats were divided into the three following experimental groups: the baseline, control (CON), and hindlimb unloading (HU) groups. Rats in the HU group were subjected to periods of hindlimb unloading by tail suspension. The joint components were evaluated by histopathological and histomorphometric analyses.
Results
The unloading condition caused articular cartilage thinning and decreased matrix staining. The tendency of these histological changes in articular cartilage was similar between the knee compartments. In general, the unloading environment resulted in no critical histological changes to the synovial membrane and IFP. At 4 weeks, thickening of the synovial membrane and synovial-like tissue invasion were observed in the HU group, but there was no significant change in inflammation. There was no obvious histological change to the IFP caused by unloading.
Conclusion
The unloading environment caused disuse histological changes in the articular cartilage; however, the tendency of the changes was similar between compartments. Furthermore, the unloading condition caused no critical histological changes to the synovial membrane or IFP. Clinically, the unloading environment without joint immobilization over a short period may not have caused critical histological changes to the joint components.
Keywords: Joint unloading, Articular cartilage, Synovial membrane, Infrapatellar fat pad, Hindlimb suspension, Histology
1. Introduction
Moderate mechanical stress, including joint loading, is essential for histological and functional maintenance of the musculoskeletal system. Immobility and underactivity cause disuse histological changes in skeletal muscle and bone. Similarly, in 2019, Vincent and Wann reported that disuse histological changes occurred in the articular cartilage due to the unloading condition and proposed this event as disuse atrophy in the articular cartilage [1]. These histological changes mainly include cartilage thinning and matrix loss; they are unaccompanied by chondrocyte necrosis or surface irregularities, such as osteoarthritis (OA) [1]. Several studies have investigated the histological effects of the unloading condition on articular cartilage, and these studies have reported chondrocyte hypertrophy and clustering, tidemark advancement, and subchondral vascular encroachment into the cartilage [[2], [3], [4], [5], [6], [7]].
The knee joint consists of the following three compartments: the patellofemoral, lateral, and medial tibiofemoral compartments (PFC, LTFC, and MTFC, respectively) [8,9]. These compartments exhibit different structural, pathomechanical, and clinical characteristics [10]. The structure and style of joint movements differ kinematically among these compartments [9,11,12]. Therefore, considering these differences, it is possible that the histological influence induced by the unloading condition may differ among compartments. However, the histological changes in the articular cartilage induced by the unloading condition on each knee compartment remain unclear.
In addition to the articular cartilage, the important joint structures surrounding the knee joint include the synovial membrane [13,14] and infrapatellar fat pad (IFP) [[15], [16], [17]]. The influence of histological changes on joint immobilization of these two joint structures has been well studied with the use of a joint contracture model. Many studies conducted with the use of a joint contracture model have reported that joint immobilization induces degeneration, fibrosis, and decreased matrix staining in articular cartilage, adhesion to the synovial membrane, and adipocyte atrophy and fibroblast hyperplasia of the IFP [[18], [19], [20]]. Recent studies have focused on the roles of these joint structures in the pathogenesis of OA [[21], [22], [23]]. However, the histological changes in the synovial membrane and IPF induced by the unloading condition remain unclear.
Considering the above information, the purpose of this study was to histologically clarify the effect of unloading on the articular cartilage separately for the PFC, LTFC, and MTFC as well as on the synovial membrane and IFP in the knee using a hindlimb unloading (HU) rat model by tail suspension.
2. Method
2.1. Experimental animals and animal care
This study protocol was approved by the Animal Research Committee of the Graduate School of Medicine of Kanazawa University (Kanazawa, Japan; approval no. 183933) and was conducted in accordance with the ARRIVE guidelines [24] and the guidelines for the care and use of laboratory animals of Kanazawa University.
Twenty-five male Wistar rats (8 weeks old) were purchased from Japan SLC (Shizuoka, Japan) and housed under normal conditions for 1 week before the start of the experiments to acclimatize the animals to their new environment. One or two rats were housed per cage in a sanitary ventilated room under controlled temperature and humidity conditions, and a 12-h light-dark cycle with ad libitum access to food and water.
Five randomly selected rats were killed at 9 weeks old for baseline measurements, while the remaining 20 were randomly allocated to one of the two following experimental groups: the control (CON) group or the HU group. The animals in each experimental group were assigned to two subgroups according to the experimental period as follows: 2 and 4 weeks (n = 5 per subgroup). After the start of the experiment, other than tail suspension in the HU group, no further interventions were performed during the experimental period. No analgesics or anti-inflammatory drugs were administered.
2.2. Hindlimb suspension
Rats in the HU group were subjected to tail suspension throughout the experiment. In the present study, hindlimb suspension was performed according to the modified tail suspension method described by Ferreira et al. [[25], [26], [27]]. Briefly, under inhalation anesthesia with isoflurane, the tail of each rat was disinfected. A sterile steel wire was then used to drill into the proximal coccyx, in which the wire remained and was shaped into a ring. The tail ring was then connected to a track hung above the cage using another wire, thereby enabling the animals to move freely on their forelimbs in the cage.
2.3. Histological preparation
Within the first few minutes after the discontinuation of the inhalation-induced anesthesia with isoflurane, all animals regained consciousness and were mobile. None of the rats exhibited any signs of tail infection or died during the experimental period. Decalcified paraffin sections were prepared for histology as described previously [27]. The right and left knees were excised via the sagittal approach into the three following specimens: lateral, central, and medial. Thereafter, the specimens were cut into 3-μm-thick sections and stained separately with hematoxylin and eosin and 0.05% toluidine blue [28]. Finally, the sections were viewed under a light microscope and imaged using a digital camera (BX-51 and DP-50; Olympus Corporation, Tokyo, Japan) to evaluate the histopathological features of the articular cartilage, synovial membrane, and IFP.
2.4. Selection of the regions for assessment
Based on the results of previous studies and our preliminary experiment, we decided to evaluate the following regions: the articular cartilage, synovial membrane, and IFP [7,[29], [30], [31], [32]].
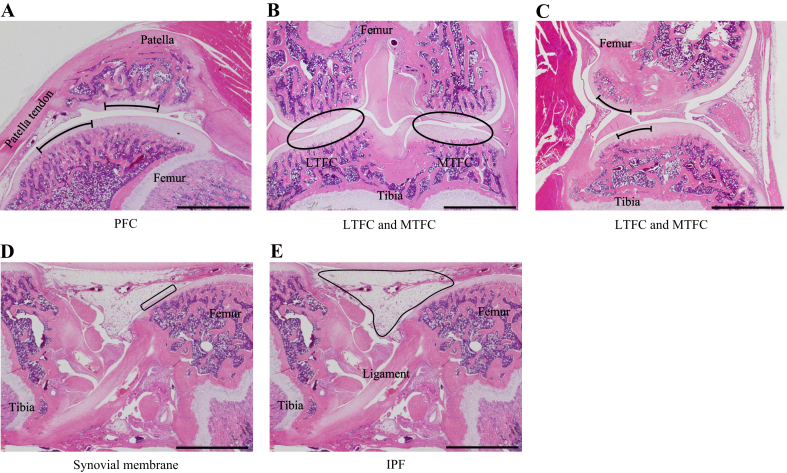
The lateral, central, and medial specimens from both knees were used to analyze the articular cartilage in the PFC, LTFC, and MTFC. The central specimens were serially sliced at the center of the patellar groove to observe the cruciate ligaments at the center of the medial and lateral condyles. The central regions of the patella and femoral trochlea were determined as the evaluation regions of the articular cartilage in the PFC (Fig. 1-A). The lateral and medial specimens were serially sliced at the center of the LTFC and MTFC to observe the region where the articular cartilage of the femur and tibia could be in direct contact without involvement of the meniscus. The posterior region of the femur condyle and tibia were determined as the evaluation regions of the articular cartilage in the LTFC and MTFC (Fig. 1-B and C).
Fig. 1.
Regions of the knee joint used for histopathological and histomorphometric analyses. (A) Articular cartilage at the center of the patella and the femoral groove in the center of the PFC as the central specimens. (B and C) Articular cartilage at the posterior region of the femur condyle and tibia in the center of the LTFC and MTFC as the lateral and medial specimens, respectively. (D) Synovial membrane in front of the femur in the center of the PF joint as the central specimens. (E) IFP in the center of the PF joint as the central specimens. (C) Synovial plica was observed in the joint space between the femur and tibia in the MTFC. Scale bar = 2 mm.
We determined the evaluation region of the synovial membrane and IFP of the central specimens. The specimens of both knees were used to histopathologically and histomorphometrically analyze the synovial membrane and IFP. The region located in front of the femur was determined to be the evaluation region of the synovial membrane (Fig. 1-D and E).
2.5. Histological and histomorphometrical analyses
Histological and histomorphometrical analyses for articular cartilage were performed to evaluate the following four parameters: cartilage thickness, intensity of matrix staining with toluidine blue, chondrocyte density, and modified Mankin score (MMS, Supplementary Method 1; for details, see Supplementary Method 2). We evaluated these parameters at six regions of three specimens of each knee. For four parameters, the data obtained from the regions of the patella and femur in the PF joint were considered as the data of the PFC. In the same way, the data from the tibia and femur in the lateral and medial TF joints were considered as the data of the LTFC and MTFC, respectively. Then, all data of all three compartments were considered as the data of the whole knee joint.
Histological and histomorphometrical analyses for the synovial membrane and IFP were performed to evaluate the following four parameters: the scoring system for synovitis [33] (SSS, Supplementary Method 1), thickness and length of the synovial membrane that invaded the joint cavity for the synovial membrane, and the entire area and one fat cell of the IFP (Supplementary Method 2).
2.6. Statistical analysis
All statistical analyses were performed using JMP 14 software (SAS Institute, Cary, NC, USA). The sample size was five for each group, which included two knees per rat, corresponding to 10 knees per group. All continuous and categorical data were assessed for normality using the Shapiro–Wilk test and for homoscedasticity using the Levene test. To evaluate the differences among the five groups (baseline, CON, and HU at 2 and 4 weeks) in the parametric continuous data, an analysis of variance with a subsequent post hoc Tukey's honestly significant difference test was used. For the nonparametric continuous data or categorical data, the Kruskal–Wallis test with subsequent post hoc Steel–Dwass test was used. P-values of <0.05 were considered statistically significant for all analyses. Exact P-values are shown in the figures.
3. Results
Inflammation was macroscopically and microscopically well-controlled. The body weights of the rats throughout the experimental period are shown in Supplementary Result 1. In all groups, significant increases in the body weight were observed over time. However, the body weight of the HU group decreased significantly compared with that of the SE group at 2 and 4 weeks. In the MTFC, synovial plica was frequently observed in the joint space between the femur and tibia (baseline group, 8/10; CON group, 8/10 and 5/10 at 2 and 4 weeks; HU group, 8/10 and 7/10 at 2 and 4 weeks; Fig. 1-C). No synovial plica was observed in the PFC or LTFC.
3.1. Articular cartilage thickness
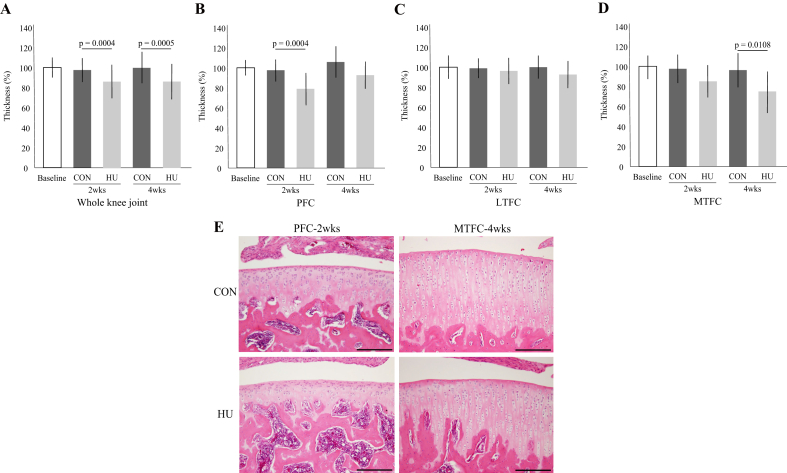
In the whole knee joint and PFC, the cartilage thickness of the HU group was significantly thinner at 2 weeks than that of the CON group (Fig. 2-A, B, and E). Furthermore, in the whole knee joint and MTFC, the cartilage thickness of the HU group was significantly thinner at 4 weeks than that of the CON group (Fig. 2-A, D, and E). However, there was no significant difference in the LTFC between the two groups (Fig. 2-C). For details, see Supplementary Result 2.
Fig. 2.
Changes to articular cartilage thickness in the whole knee joint (A), PFC (B), LTFC (C), and MTFC (D). (E) Histological changes to articular cartilage thickness. Scale bar = 200 μm.
3.2. Toluidine blue staining intensity of the matrix
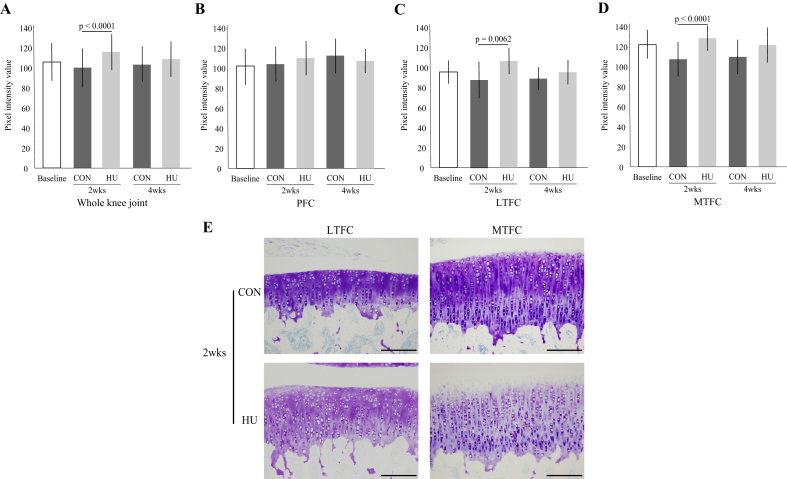
In the whole knee joint (Fig. 3-A), LTFC (Fig. 3-C, E), and MTFC (Fig. 3-D, E), the intensity of the matrix staining of the HU group at 2 weeks decreased significantly, as compared with that of the CON group, but there was no significant difference at 4 weeks. In the PFC, there was no significant difference in the intensity of the matrix staining between the two groups at 2 and 4 weeks (Fig. 3-B). For details, see Supplementary Result 3.
Fig. 3.
Changes to the intensity of the matrix staining by toluidine blue in the whole knee joint (A), PFC (B), LTFC (C), and MTFC (D). (E) Representative histological image of the intensity of the matrix staining. Scale bar = 200 μm.
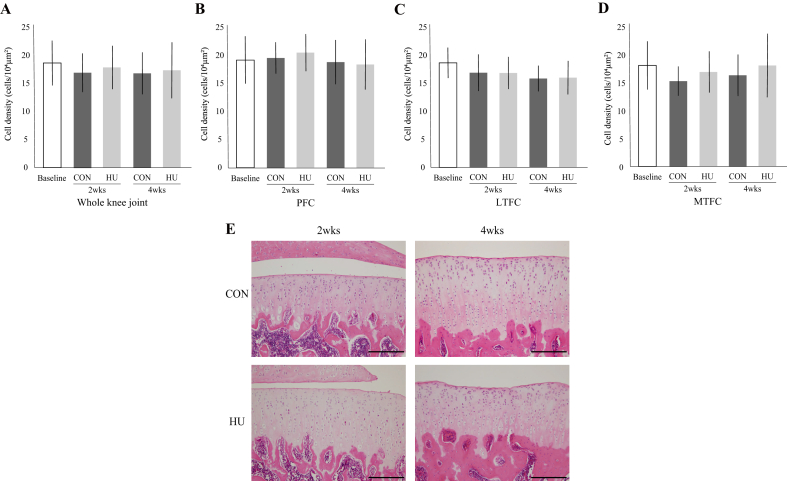
3.3. Chondrocyte density
In the whole knee joint (Fig. 4-A) and all compartments (Fig. 4-B, C, D, and E), there was no significant change in the chondrocyte density between the two groups throughout the experimental period. At 2 weeks, the staining intensity of the chondrocyte nuclei of the HU group had decreased, but at 4 weeks, the staining intensity had recovered (Fig. 4-E). For details, see Supplementary Result 4.
Fig. 4.
Changes to chondrocyte density in the whole knee joint (A), PFC (B), LTFC (C), and MTFC (D). (E) Representative histological image of the chondrocyte density at the tibia in the LTFC. Scale bar = 200 μm.
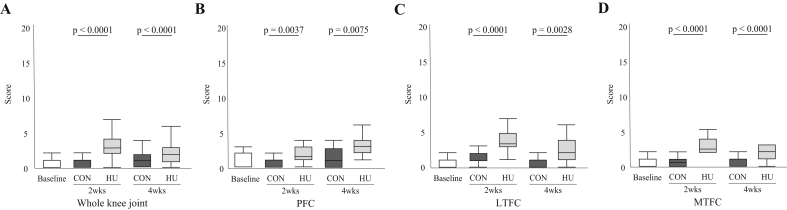
3.4. Modified mankin score
In the whole knee joint (Fig. 5-A) and all compartments (Fig. 5-B, C, and D), the MMS was significantly higher in the HU group, as compared with the CON group, at 2 and 4 weeks. For details, see Supplementary Result 5.
Fig. 5.
Changes to the MMS in the whole knee joint (A), PFC (B), LTFC (C), and MTFC (D).
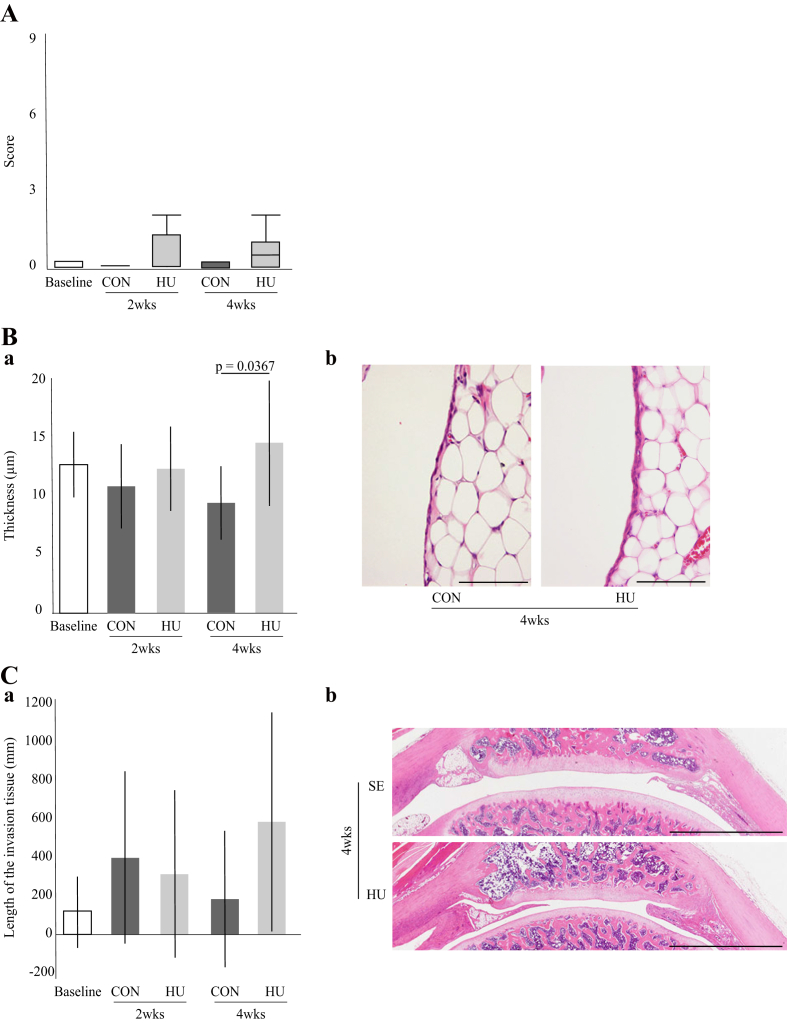
3.5. Synovial membrane and IFP
Throughout the experimental period, the synovitis scores remained low, with no significant difference between the two groups at any time point (Fig. 6-A). Furthermore, there was no significant difference in synovial membrane thickness between the two groups at 2 weeks. However, at 4 weeks, the synovial membrane thickness was significantly greater in the HU group (Fig. 6-Ba). Although thickening of the synovial membrane and multiple layers of synovial cells were observed, there was no obvious inflammatory cell infiltration (Fig. 6-Bb). There was no significant difference in the length of invading tissue between the two groups at 2 and 4 weeks (Fig. 6-Ca). Invading tissue was observed on the peripheral and/or central side of the patella. The presence/absence, region, and length of the invading tissue varied among the samples (Fig. 6-Cb). For details, see Supplementary Result 6.
Fig. 6.
Histological features of the synovial membrane. (A) Change to the SSS over time. (B) (a) Changes to synovial membrane thickness over time. (b) Representative histological sections of synovial membrane stained with hematoxylin and eosin. Scale bar = 100 μm. (C) (a) Change in invading tissue over time. (b) Representative histological sections of invasion tissue stained with hematoxylin and eosin. Scale bar = 2 mm.
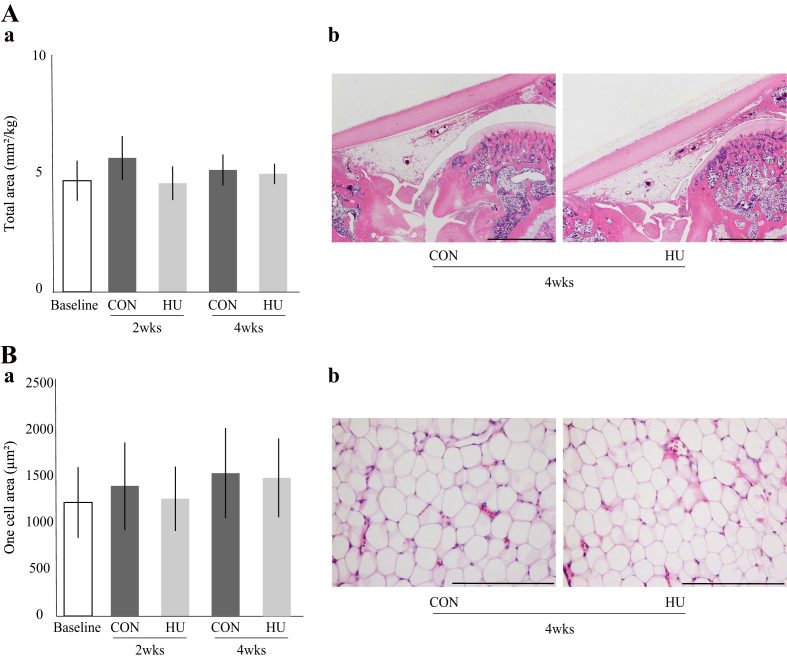
The total area and one cell area of the IFP were maintained throughout the experimental period, with no significant differences between the two groups at 2 and 4 weeks (Fig. 7-Aa and Ba). No obvious fibrous tissue proliferation or fibrosis was observed (Fig. 7-Ab and Bb). For details, see Supplementary Result 7.
Fig. 7.
Histological features of the IFP. (A) (a) Change in the total area over time. (b) Representative histological sections of IFP tissue stained with hematoxylin and eosin. Scale bar = 2 mm. (B) (a) Change in one cell area over time. (b) High magnified histological images of IFP stained with hematoxylin and eosin. Scale bar = 200 μm.
4. Discussion
The purpose of this study was to clarify the differences in histological changes to the articular cartilage among the PFC, LTFC, and MTFC and to clarify the histological changes to the synovial membrane and IFP of the knee using a HU rat model. As results, four major findings became clear. First, the results of the present study provided the physiological and histological data for the normal articular cartilage in each knee compartment (see Supplementary Results 2–4). Second, the unloading condition induced disuse histological changes of articular cartilage, which included decreased cartilage thickness and matrix staining intensity, but no change in chondrocyte density. Third, there were no obvious differences in the tendency of histological changes in the articular cartilage of each knee compartment. Fourth, the unloading condition caused no critical histological changes to the articular cartilage, synovial membrane, or IFP.
In regard to the first finding, to the best of our knowledge, no physiological or histological characteristics have yet been reported for each compartment of the knee joint. Therefore, the basic data obtained in this study may be useful for the clinical treatment of various diseases of the knee joint, including OA and articular cartilage defects. In regard to the second and third findings, the histological changes observed in this study due to disuse have already been reported (i.e., articular cartilage thinning, decreased matrix staining intensity, SB expansion, and no change in chondrocyte density) [[1], [2], [3], [4], [5],7]. Therefore, the results of the present study support those of previous studies. As mentioned above, several researchers have reported differences in the structure, clinical characteristics, kinematics, and type of movement between the PF and TF joints [[9], [10], [11], [12],34]. However, the tendency and extent of the histological changes of articular cartilage caused by the unloading condition were similar for most parameters, and there were no notable differences between the compartments. Such differences among the three compartments might not affect the histological changes caused by the unloading condition. Therefore, the data obtained in this study provide new histological evidence regarding disuse histological changes in the articular cartilage. With regard to the fourth finding, although significant changes were observed in the articular cartilage, overall, the unloading condition caused only slight histological changes in the joint components. The experiments were performed in an unloading environment with unlimited joint movement. The joint immobilization model is often used because of the lack of models of mechanical stress. With this model, it has been reported that severe histological changes of these joint components occur early after joint immobilization [7,[18], [19], [20],31,[35], [36], [37], [38]]. Furthermore, Matsuzaki et al. examined the effects of unloading and joint immobilization on joint components and reported that more severe histological changes and joint contracture were observed in the joint immobilization group [39]. Based on these findings, joint motion may be more important than loading for histological maintenance of the joint components. Clinically, there are situations wherein the surgical lower limb must remain unloaded after surgery for fractures or cartilage defects [40,41]. In such situations, early or excessive loading can exert an adverse effect on tissue repair, such as irregularities, displacement, and subsidence [26,42,43]. Therefore, the use of continuous passive motion and exercise of the range of motion by physical therapists may be useful for histological maintenance and to inhibit disuse histopathological changes to the joint components.
There were four potential limitations to this study. First, although the unloading condition was achieved by tail suspension during the experimental period, joint motion (with or without muscle contraction) was not restricted. Mechanical stress including shear and compressive forces induced by joint motion may affect the histological changes. Second, the weight of the HU group was significantly lower than that of the CON group throughout the experimental period. Evaluating the nutritional status by measuring food and water consumption and serum albumin and total protein levels throughout the experiment may be required. Third, the sample size in this study was considerably small. With regard to the study design, using a larger sample size for the statistical analysis is recommended. Fourth, this HU model of rat and mice by the tail suspension test has been established to analyze body adaptations to unloading environments [25]; however, the model has been unreliable for modeling human joint unloading. Rodents are quadruped and their knee joint is in a flexed position under a physiological environment; conversely, humans are biped, and their knee is in an extension position. However, despite these limitations, the present results of the histological and histomorphometrical analyses enhance the understanding of the histological change of joint components induced by the unloading condition.
In conclusion, the results of the present study revealed that the unloading environment caused disuse histological changes in articular cartilage; however, the tendency of the changes was similar between compartments. Furthermore, the unloading condition caused no serious or critical histological changes to the synovial membrane or IFP. Clinically, the unloading environment without joint immobilization over a short period may not have caused critical histological changes to the joint components. Further studies are required to assess the effects of re-loading and exercise intervention on the disuse histological change to the articular cartilage.
Author contributions
All of the authors have made substantial contributions to the following: (1) conception and design of the study, data acquisition, or data analysis and interpretation; (2) drafting the article or critically revising it for important intellectual content; and (3) final approval of the submitted version of the manuscript.
The authors’ specific contributions were as follows:
-
(1)
Conception and design of the study: IT, TM, HK, and MH
-
(2)
Data analysis and interpretation: IT, TM, HK, and MH
-
(3)
Drafting of the article: IT, HK, and MH
-
(4)
Critical revision of the article for important intellectual content: IT, HK, and MH
-
(5)
Final approval of the article: IT, TM, HK, and MH
-
(6)
Obtaining funding: IT, TM, HK, and MH
-
(7)
Data collection and assembly: IT, TM, and MH
Ikufumi Takahashi (t_ikuhumi@med.kanazawa-u.ac.jp) is responsible for the integrity of the work as a whole, from inception to the finished article.
Funding
This study was supported by a JSPS KAKENHI grant-in-aid for Young Scientists B (number: 17K13051).
Competing interest statement
The authors have no conflicts of interest to declare. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors thank the members of the Department of Human Pathology at Kanazawa University Graduate School of Medicine for offering advice regarding the histopathological techniques.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2019.100008.
Contributor Information
Ikufumi Takahashi, Email: t_ikuhumi@med.kanazawa-u.ac.jp.
Taro Matsuzaki, Email: tarosan@mhs.mp.kanazawa-u.ac.jp.
Hiroshi Kuroki, Email: kuroki.hiroshi.6s@kyoto-u.ac.jp.
Masahiro Hoso, Email: hoso@mhs.mp.kanazawa-u.ac.jp.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Vincent T.L., Wann A.K.T. Mechanoadaptation: articular cartilage through thick and thin. J. Physiol. 2019;597:1271–1281. doi: 10.1113/JP275451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basso N., Heersche J.N. Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone. 2006;39:807–814. doi: 10.1016/j.bone.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Tomiya M., Fujikawa K., Ichimura S., Kikuchi T., Yoshihara Y., Nemoto K. Skeletal unloading induces a full-thickness patellar cartilage defect with increase of urinary collagen II CTx degradation marker in growing rats. Bone. 2009;44:295–305. doi: 10.1016/j.bone.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Luan H.Q., Sun L.W., Huang Y.F., Wu X.T., Niu H., Liu H., et al. Use of micro-computed tomography to evaluate the effects of exercise on preventing the degeneration of articular cartilage in tail-suspended rats. Life Sci. Space Res. 2015;6:15–20. doi: 10.1016/j.lssr.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor K.M. Unweighting accelerates tidemark advancement in articular cartilage at the knee joint of rats. J. Bone Miner. Res.: The Official Journal of the American Society for Bone and Mineral Research. 1997;12:580–589. doi: 10.1359/jbmr.1997.12.4.580. [DOI] [PubMed] [Google Scholar]
- 6.Palmoski M.J., Colyer R.A., Brandt K.D. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 1980;23:325–334. doi: 10.1002/art.1780230310. [DOI] [PubMed] [Google Scholar]
- 7.Nomura M., Sakitani N., Iwasawa H., Kohara Y., Takano S., Wakimoto Y., et al. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthr. Cartil. 2017;25:727–736. doi: 10.1016/j.joca.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Lankhorst N.E., Damen J., Oei E.H., Verhaar J.A., Kloppenburg M., Bierma-Zeinstra S.M., et al. Incidence, prevalence, natural course and prognosis of patellofemoral osteoarthritis: the Cohort Hip and Cohort Knee study. Osteoarthr. Cartil. 2017;25:647–653. doi: 10.1016/j.joca.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Smith P.N., Refshauge K.M., Scarvell J.M. Development of the concepts of knee kinematics11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated. Arch. Phys. Med. Rehabil. 2003;84:1895–1902. doi: 10.1016/s0003-9993(03)00281-8. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S., Pappas E., Fransen M., Refshauge K., Simic M. The prevalence of patellofemoral osteoarthritis: a systematic review and meta-analysis. Osteoarthr. Cartil. 2016;24:1697–1707. doi: 10.1016/j.joca.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Loudon J.K. Biomechanics and pathomechanics of the patellofemoral joint. International Journal of Sports Physical Therapy. 2016;11:820–830. [PMC free article] [PubMed] [Google Scholar]
- 12.Masouros S.D., Bull A.M.J., Amis A.A. (i) Biomechanics of the knee joint. Orthop. Traumatol. 2010;24:84–91. [Google Scholar]
- 13.Kim H.K., Zbojniewicz A.M., Merrow A.C., Cheon J.E., Kim I.O., Emery K.H. MR findings of synovial disease in children and young adults: Part 1. Pediatr. Radiol. 2011;41:495–511. doi: 10.1007/s00247-011-1971-0. quiz 45-6. [DOI] [PubMed] [Google Scholar]
- 14.Favero M., El-Hadi H., Belluzzi E., Granzotto M., Porzionato A., Sarasin G., et al. Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology. 2017;56:1784–1793. doi: 10.1093/rheumatology/kex287. [DOI] [PubMed] [Google Scholar]
- 15.Stephen J.M., Sopher R., Tullie S., Amis A.A., Ball S., Williams A. The infrapatellar fat pad is a dynamic and mobile structure, which deforms during knee motion, and has proximal extensions which wrap around the patella. Knee Surg. Sport. Traumatol. Arthrosc. 2018;26:3515–3524. doi: 10.1007/s00167-018-4943-1. [DOI] [PubMed] [Google Scholar]
- 16.Steidle-Kloc E., Culvenor A.G., Dorrenberg J., Wirth W., Ruhdorfer A., Eckstein F. Relationship between knee pain and infrapatellar fat pad morphology: a within- and between-person analysis from the osteoarthritis initiative. Arthritis Care Res. 2018;70:550–557. doi: 10.1002/acr.23326. [DOI] [PubMed] [Google Scholar]
- 17.Eymard F., Chevalier X. Inflammation of the infrapatellar fat pad. Jt. Bone Spine. 2016;83:389–393. doi: 10.1016/j.jbspin.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Kojima S., Hoso M., Watanabe M., Matsuzaki T., Hibino I., Sasaki K. Experimental joint immobilization and remobilization in the rats. J. Phys. Ther. Sci. 2014;26:865–871. doi: 10.1589/jpts.26.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuzaki T., Yoshida S., Kojima S., Watanabe M., Hoso M. Influence of ROM exercise on the joint components during immobilization. J. Phys. Ther. Sci. 2013;25:1547–1551. doi: 10.1589/jpts.25.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe M., Kojima S., Hoso M. Effect of low-intensity pulsed ultrasound therapy on a rat knee joint contracture model. J. Phys. Ther. Sci. 2017;29:1567–1572. doi: 10.1589/jpts.29.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clockaerts S., Bastiaansen-Jenniskens Y.M., Runhaar J., Van Osch G.J., Van Offel J.F., Verhaar J.A., et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthr. Cartil. 2010;18:876–882. doi: 10.1016/j.joca.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Han W., Cai S., Liu Z., Jin X., Wang X., Antony B., et al. Infrapatellar fat pad in the knee: is local fat good or bad for knee osteoarthritis? Arthritis Res. Ther. 2014;16:R145. doi: 10.1186/ar4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lange-Brokaar B.J., Ioan-Facsinay A., van Osch G.J., Zuurmond A.M., Schoones J., Toes R.E., et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthr. Cartil. 2012;20:1484–1499. doi: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012;20:256–260. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira J.A., Crissey J.M., Brown M. An alternant method to the traditional NASA hindlimb unloading model in mice. J. Vis. Exp. 2011;(49) doi: 10.3791/2467. e2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi I., Matsuzaki T., Yoshida S., Kitade I., Hoso M. Differences in cartilage repair between loading and unloading environments in the rat knee. Journal of the Japanese Physical Therapy Association = Rigaku ryoho. 2014;17:22–30. doi: 10.1298/jjpta.Vol17_004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi I., Matsuzaki T., Kuroki H., Hoso M. Joint unloading inhibits articular cartilage degeneration in knee joints of a monosodium iodoacetate-induced rat model of osteoarthritis. Osteoarthr. Cartil. 2019;27:1084–1093. doi: 10.1016/j.joca.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz N., Laverty S., Kraus V.B., Aigner T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartil. 2010;18:S113–S116. doi: 10.1016/j.joca.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi I., Matsuzaki T., Kuroki H., Hoso M. Induction of osteoarthritis by injecting monosodium iodoacetate into the patellofemoral joint of an experimental rat model. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196625. e0196625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udo M., Muneta T., Tsuji K., Ozeki N., Nakagawa Y., Ohara T., et al. Monoiodoacetic acid induces arthritis and synovitis in rats in a dose- and time-dependent manner: proposed model-specific scoring systems. Osteoarthr. Cartil. 2016;24:1284–1291. doi: 10.1016/j.joca.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Hagiwara Y., Ando A., Chimoto E., Saijo Y., Ohmori-Matsuda K., Itoi E. Changes of articular cartilage after immobilization in a rat knee contracture model. J. Orthop. Res. 2009;27:236–242. doi: 10.1002/jor.20724. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe M., Hoso M., Kojima S., Matsuzaki T., Hibino I. Histopathological changes in joint components in a rat knee joint contracture model following mobilization. J. Phys. Ther. Sci. 2012;24:1199–1203. [Google Scholar]
- 33.Krenn V., Morawietz L., Haupl T., Neidel J., Petersen I., Konig A. Grading of chronic synovitis--a histopathological grading system for molecular and diagnostic pathology. Pathol. Res. Pract. 2002;198:317–325. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 34.Hirschmann M.T., Muller W. Complex function of the knee joint: the current understanding of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2015;23:2780–2788. doi: 10.1007/s00167-015-3619-3. [DOI] [PubMed] [Google Scholar]
- 35.Nagai M., Ito A., Tajino J., Iijima H., Yamaguchi S., Zhang X., et al. Remobilization causes site-specific cyst formation in immobilization-induced knee cartilage degeneration in an immobilized rat model. J. Anat. 2016;228:929–939. doi: 10.1111/joa.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ando A., Hagiwara Y., Onoda Y., Hatori K., Suda H., Chimoto E., et al. Distribution of Type A and B synoviocytes in the adhesive and shortened synovial membrane during immobilization of the knee joint in rats. Tohoku J. Exp. Med. 2010;221:161–168. doi: 10.1620/tjem.221.161. [DOI] [PubMed] [Google Scholar]
- 37.Kaneguchi A., Ozawa J., Kawamata S., Yamaoka K. Development of arthrogenic joint contracture as a result of pathological changes in remobilized rat knees. J. Orthop. Res. 2017;35:1414–1423. doi: 10.1002/jor.23419. [DOI] [PubMed] [Google Scholar]
- 38.Palmoski M., Perricone E., Brandt K.D. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 1979;22:508–517. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- 39.Matsuzaki T., Yoshida S., Ikeda A., Hoso M. Changes in joint components after knee immobilization associated with hindlimb unweighting in rats. Journal of Wellness and Health Care. 2018;42:33–40. [Google Scholar]
- 40.Hambly K., Bobic V., Wondrasch B., Van Assche D., Marlovits S. Autologous chondrocyte implantation postoperative care and rehabilitation: science and practice. Am. J. Sports Med. 2006;34(6):1020–1038. doi: 10.1177/0363546505281918. [DOI] [PubMed] [Google Scholar]
- 41.Hirschmuller A., Baur H., Braun S., Kreuz P.C., Sudkamp N.P., Niemeyer P. Rehabilitation after autologous chondrocyte implantation for isolated cartilage defects of the knee. Am. J. Sports Med. 2011;39(12):2686–2696. doi: 10.1177/0363546511404204. [DOI] [PubMed] [Google Scholar]
- 42.Kuroki H., Nakagawa Y., Mori K., et al. Ultrasound properties of articular cartilage immediately after osteochondral grafting surgery: in cases of traumatic cartilage lesions and osteonecrosis. Knee Surg. Sport. Traumatol. Arthrosc. 2009;17(1):11–18. doi: 10.1007/s00167-008-0586-y. [DOI] [PubMed] [Google Scholar]
- 43.Kuroki H., Nakagawa Y., Mori K., et al. Acoustic stiffness and change in plug cartilage over time after autologous osteochondral grafting: correlation between ultrasound signal intensity and histological score in a rabbit model. Arthritis Res. Ther. 2004;6(6):R492–R504. doi: 10.1186/ar1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.