Abstract
Objective
To test whether combined patellofemoral and tibiofemoral osteoarthritis (OA), in addition to symptoms, is associated with greater changes in quality of life and objective physical function measures when compared with asymptomatic isolated tibiofemoral osteoarthritis.
Design
Of the 4796 participants in the Osteoarthritis Initiative, 577 were categorized into four groups based on the presence of symptoms (asymptomatic and symptomatic) and the structural involvement within the knee, where tibiofemoral OA was graded with the Kellgren and Lawrence scale, while patellofemoral OA was based on the Magnetic Resonance Imaging Osteoarthritis Knee Scoring cartilage loss feature. Knee-related quality of life was examined using the Knee Injury and Osteoarthritis Outcome Scale quality of life subscale, and objective physical function was examined by the 20 m Walk Test, 30-s Chair Stand Test, and isometric knee strength. These outcomes were measured at Baseline, Year 2, and Year 4. Mixed effects models were fit to test whether the change in outcome, and the Baseline scores, differed based on group.
Results
Quality of life worsened for the asymptomatic combined group but improved for the symptomatic combined group. However, these quality of life changes and changes in other outcomes were all within measurement error. Large between-group differences were found at Baseline, whereby individuals with symptoms had worse quality of life and physical function test scores.
Conclusions
Quality of life and physical function are largely stable over four years. However, having symptoms is strongly associated with worse quality of life and physical function, regardless of structural disease distribution within the knee.
Keywords: Knee osteoarthritis, Symptoms, Quality of life, Physical function
1. Introduction
Knee osteoarthritis (OA) is a globally prevalent and serious disease [1], which accounts for most of the OA-related disease burden [2]. In North American adults, knee OA prevalence has significantly increased in the last century [3] with current estimates ranging from 12 to 19% [4,5]; although, recent data suggests these values are conservative [6]. This increase is, in part, a result of the growing prevalence of risk factors like obesity, physical inactivity, and joint injuries [7]. While the economic and societal impact is large, the disease can have substantial and long lasting effects on the individual [8].
Knee OA presents with multifaceted symptoms and disruptions to daily life. Individuals with symptomatic knee OA report significantly worse function and overall health-related quality of life compared to age-matched healthy adults [9]. Knee pain is the primary complaint of those with knee OA [10] and is associated with worse physical function [11], quality of life [12], and periarticular knee muscle function [13]. Moreover, quality of life, physical function, and muscle function are interrelated [11,14], where worse scores in one of these variables is associated with worse scores in another. This points to the detrimental effect of pain on the individual.
The influence of pain on quality of life and physical function is not equivocal across knee-OA subgroups. While data from the Osteoarthritis Initiative (OAI) show that most people with knee OA report stable quality of life and physical function over 7–8 years of follow up, more than 1 in 4 people significantly worsen over this timeframe [15,16], with the presence of knee pain being a primary predictor of who was more likely to exhibit worsening quality of life, though structural distribution was not assessed [16]. Accordingly, an important research objective is to identify subgroups that are more likely to experience this worsening. Doing so can assist with allocating resources and researcher priorities to treatments specifically targeting the subgroups with increased risk of worsening. Unfortunately, much of this earlier work focused on radiographic or clinically defined tibiofemoral OA (TFOA), while the involvement of the patellofemoral joint is often not considered. As a result, the implications of patellofemoral OA (PFOA) in addition to TFOA, and how pain influences these subgroups, remain unclear.
The patellofemoral joint is frequently affected by OA and has largely gone underrecognized in the literature [17]. One half of those with knee pain or symptomatic knee OA present with structural signs in the patellofemoral joint [18]. Indeed, symptomatic knees with combined PFOA and TFOA on magnetic resonance imaging may actually be more prevalent than compartmentally-isolated OA, while this difference may not be true for asymptomatic knees [19]. Moreover, people with isolated PFOA are 5.8 and 2.1 times more likely to develop structural signs of OA in multiple compartments seven years later, compared to those without any cartilage degeneration or isolated TFOA at baseline, respectively [20]. Isolated PFOA negatively affects quality of life [21] and may increase the risk of knee pain over and above isolated TFOA [22]. However, individuals with combined PFOA and TFOA report higher pain severity, worse physical function [23,24], and lower health-related quality of life [21] compared to those with compartmentally-isolated signs. Symptoms, physical function, and knee-related quality of life were also worse in a group with radiographically confirmed combined-compartment OA when compared to isolated OA, 15–22 years after meniscectomy [25]. Taken together, the presence of radiographic PFOA may have a more substantial impact on the individual when compared to TFOA alone, the addition of symptoms may magnify this effect, and radiographically combined compartment OA could be worse yet.
Given the potential influence pain has on different radiographic subgroups of knee OA over and above radiographic findings alone, and the need to understand the additive effect of radiographic signs of OA in the PF compartment compared to isolated TFOA, an analysis of these subgroups combined and apart is necessary. Therefore, the objectives of this study were to examine baseline differences in quality of life and physical function, as well as changes over the course of 4 years, between four groups with TFOA. The groups were defined by the combination of the presence or absence of symptoms (asymptomatic versus symptomatic) and the presence or absence of radiographic PFOA (isolated TFOA or combined PFOA and TFOA). Using asymptomatic TFOA as the reference group, we hypothesized that the presence of symptoms and/or combined PFOA and TFOA group would show lower overall quality of life and physical function, and greater worsening in these outcomes over time.
2. Methods
2.1. Study design
Data were retrieved from the OAI publicly available database (n = 4796). This multicenter, longitudinal observational project was approved by the Committee on Human Research at the University of California, San Francisco (approval 10–00532) and all participants provided informed consent. Study details and the database itself are publicly available at https://nda.nih.gov/oai.
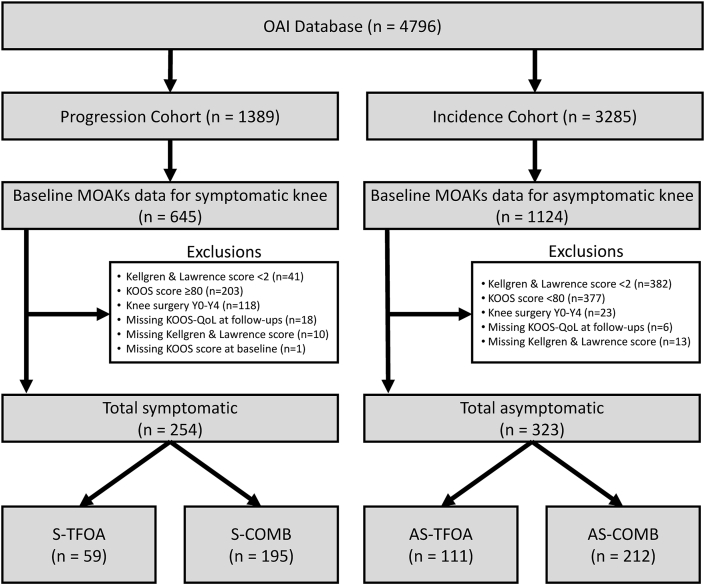
Participant data were selected from the overall OAI dataset (Fig. 1) for the current study based on several criteria which provided well-defined groups based on isolated TFOA or combined PFOA and TFOA, plus the presence or absence of symptoms. Symptom and radiographic assessment data were included in the OAI dataset and used in our study to arrive at the final sub sample. First, all participants were required to have at least one knee with radiographic evidence of OA in the tibiofemoral compartment at the Baseline assessment, based on a Kellgren and Lawrence (KL) scale grade ≥2 [26] (semi-quantitative assessment of osteoarthritis features on radiographs from 0 = no signs to 4 = severe). Within the sample of radiographically-confirmed TFOA knees, we only included those with patellofemoral grading outcomes at Baseline, which allowed us to determine if the knee had isolated TFOA or combined PFOA and TFOA. Features of PFOA were graded using the Magnetic Resonance Imaging Osteoarthritis Knee Scoring (MOAKS), where a score 2+ for the cartilage volume loss feature in either medial or lateral patellofemoral compartment was used to define the presence of PFOA [27,28], in addition to the previous confirmed TFOA. Scoring of the patellofemoral compartment has shown high reliability coefficients (kappa = 0.85–0.92) [29]. Finally, the separation of the symptomatic vs. asymptomatic knees was first determined from the Baseline self-reported answer to the binary (yes/no) question of “having pain, aching or stiffness on most days of a month, within the past twelve months” [30]. However, there were instances of conflicting data in the incidence sub-cohort of the OAI, such that some knees were reported as having no pain, aching or stiffness on most days of a month within the past twelve months but were also scored low on the Knee Injury and Osteoarthritis Outcome Score (KOOS) pain subscale (suggesting they were not asymptomatic). Thus, we opted to remove any knees that were designated as asymptomatic on the binary (yes/no) question but scored <80 on the KOOS pain subscale due to this conflicting data. No symptomatic knees scored 80 or above. Only a single study knee was included per participant. Therefore, if symptoms (and structural signs) were present in both knees, the knee with more severe symptoms (based on KOOS questionnaires completed for each knee independently) was selected as the study limb. If neither knee was symptomatic, the right was selected as the study limb. Additional exclusions are shown in Fig. 1, including if participants reported any knee surgery between the Baseline and Year 4 assessment. This exclusion of surgery was made to avoid potential influence on the outcome measures.
Figure 1.
Flow chart illustrating the inclusion and exclusion of participants from the entire Osteoarthritis Initiative dataset.
Therefore, the classification of participants from this subset of the larger dataset was based on the presence of isolated TFOA, or combined PFOA and TFOA (COMB) in the study knee; and whether this knee was asymptomatic (AS) or symptomatic (S). From this, four subgroups were defined: (1) asymptomatic isolated TFOA (AS-TFOA; n = 111), (2) symptomatic isolated TFOA (S-TFOA; n = 59), (3) asymptomatic combined TFOA and PFOA (AS-COMB; n = 212), and (4) symptomatic combined PFOA and TFOA (S-COMB; n = 195). Response variables were measured at Baseline, Year 2, and Year 4 timepoints.
2.2. Variables of interest
All variables of interest were collected as part of the OAI protocol [30]. The primary outcome of this study was the KOOS quality of life subscale (KOOS-QOL). The KOOS-QOL subscale consists of 4 questions which are scored on a Likert scale (0–4), summed and converted to percent total score. The questions address domains related to awareness of, and lifestyle changes due to, knee OA [31]. A higher score indicates better quality of life. Previous psychometric testing has demonstrated moderate to excellent test-retest reliability of the KOOS-QOL (intraclass correlation coefficients: 0.60–0.91)32. The minimum detectable change (smallest amount of change that can be detected by a measure) for the KOOS-QOL is 21.1% [32].
Secondary outcomes of interest pertained to objective measurements of physical function. The 20 m walk test (m/s) measured walking speed. Previous reports show reliability coefficients of 0.93, and 90% minimum detectable change of 1.7s (equivalent to 0.09 m/s) for this test [33]. The average walking speed of two trials was calculated. The chair stand test (repetitions per second) represents a functional measure of leg strength. Previous studies show reliability coefficients of 0.81–0.98 and standard errors of the measurement up to 1.27 stands (equivalent to a 0.04 stands/second) [34]. The number of stands performed over 30 s were recorded and divided by the time. We also examined knee strength due to its potential influence on physical function [35]. Knee strength (expressed in N) was tested via the Good Strength Chair (Metitur Oy, Jyvaskyla, Finland). After two warm-up trials, three maximum effort isometric bouts of knee flexion and extension were performed with the knee flexed 60° while in a seated position and the hips and thigh stabilized with straps. Dynamometer testing of knee strength exhibits test-retest reliability coefficients >0.90 [36] and 90% minimum detectable changes of 25 Nm [37]. The largest magnitude of force from the three trials for each movement was taken. Taken together, these previously reported data suggest the secondary measures included in the present study are reliable.
2.3. Statistical analysis
Statistical analyses were performed in R stats (v. 3.5.1) [38]. As the groups from the OAI are defined by naturally occurring criteria, not intentionally randomized groups, these outcomes were not entered in the subsequent models as covariates according to previous recommendations [39].
Linear mixed effects models were constructed for each response variable using restricted maximum likelihood estimation [40]. The group (AS-TFOA, S-TFOA, AS-COMB, and S-COMB) and time (Baseline, Year 2, and Year 4) factors were entered as interacting categorical fixed effects, to estimate the group average change with respect to time. Random intercepts were specified for the participant factor, with an unstructured random effects variance-covariance matrix. Model residuals were assessed for normality using quantile-quantile plots, and the random intercepts were further checked for centering. Homogeneity of variance across the effects was assessed using fitted versus residual plots [41]. Overall fixed effect terms (group, time, group by time) were significance tested using Wald's chi-squared test with a type-two sum of squares [38]. Model beta estimates were tested for significance [42] with the Satterthwaite approximation [43]. These results were corroborated by performing a percentile bootstrap to generate beta estimates and 95% confidence intervals (95% CI) [40,44] to address any heterogeneity. Random effects variance estimates were reported via the standard deviation (SD), and significance tested using likelihood ratio test (LRT) [42]. Contrasts were calculated using AS-TFOA and Baseline as the reference points. Finally, to complement the mixed effects model results, we calculated the odds with 95% confidence intervals of a worsening response variable over time compared to no change or improvement over time, with the AS-TFOA group as the reference. . Alpha was set at 0.05.
3. Results
Based on the inclusion criteria described above, a subset (n = 577) from the total OAI was included in the present study. Demographic variables are included in Table 1.
Table 1.
Participant demographics measured at baseline and presented as median (interquartile range) or n (%).
| AS-TFOA (n = 111) | S-TFOA (n = 59) | AS-COMB (n = 212) | S-COMB (n = 195) | |
|---|---|---|---|---|
| Sex (Male, Female) | 38 (34%), 73 (66%) | 42 (71%), 17 (29%) | 82 (39%), 130 (61%) | 64 (33%), 131 (67%) |
| KL Grade (n) | ||||
| KL2 | 65 (59%) | 16 (27%) | 126 (59%) | 77 (40%) |
| KL3 | 40 (36%) | 27 (46%) | 67 (32%) | 83 (43%) |
| KL4 | 6 (5%) | 16 (27%) | 19 (9%) | 35 (18%) |
| Age (years) | 61.0 (55.0, 68.5) | 59.0 (52.0, 66.0)d | 61.0 (56.0, 69.3) | 65.0 (57.0, 71.0)b |
| BMI (kg/m2) | 27.6 (24.9, 31.0)bd | 29.7 (27.3, 33.8)c | 27.9 (25.3, 31.3)bd | 30.0 (26.6, 33.2)ac |
| KOOS-Pain | 97.2 (91.7, 100.0)bd | 63.9 (52.8, 75.0)ac | 97.2 (91.7, 100.0)bd | 63.9 (52.8, 70.7)ac |
| KOOS-Symptoms | 92.9 (89.3, 100.0)bd | 71.4 (57.7, 82.1)ac | 92.9 (89.3, 100.0)bd | 71.4 (57.1, 82.1)ac |
| WOMAC-PF | 1.0 (0.0, 3.0)bd | 19.0 (11.3, 26.3)ac | 1.0 (0.0, 4.0)bd | 20.0 (13.8, 27.0)ac |
Abbreviations: AS-TFOA, asymptomatic tibiofemoral osteoarthritis group; S-TFOA, symptomatic tibiofemoral osteoarthritis group; AS-COMB, asymptomatic combined tibiofemoral and patellofemoral osteoarthritis group; S-COMB, symptomatic combined tibiofemoral and patellofemoral osteoarthritis group; BMI, body mass index; KOOS, Knee Injury and Osteoarthritis Index; WOMAC-PF, Western Ontario and McMaster Osteoarthritis Index – Physical Function subscale.
3.1. KOOS quality of life
Quality of life scores based on group and time variables are illustrated in Fig. 2. Modeling of the KOOS-QOL (Table 2) found that both the individual and interaction effects were significant (group χ2 = 633.5, p < 0.001; time χ2 = 12.4, p < 0.001; interaction χ2 = 27.6, p < 0.001), accounting for 43% of the variance. The addition of a random intercept increased the explained variance to 74%. The AS-TFOA group scores significantly improved at Year 2 (mean change [95%CI] = 3.74 [0.56, 6.92]; p = 0.022) and regressed at Year 4, such that they were no longer significantly different from Baseline (2.16 [-1.02, 5.33]; p = 0.185). The AS-COMB showed significant worsening compared to the AS-TFOA group at Year 2 and Year 4 (-4.7 [-8.62, -0.78]; p = 0.019 and -4.03 [-7.96, -0.11]; p = 0.045). Conversely, the S-TFOA group significantly improved from Baseline to Year 4 (7.23 [1.84, 12.63]; p = 0.009). The S-COMB group did not differ compared to the AS-TFOA group at Year 2 or Year 4. At Baseline, symptomatic groups had significantly lower quality of life compared to the AS-TFOA group (S-TFOA: -32.92 [-38.50, -27.33] and S-COMB: -35.43 [-39.56, -31.31]; p < 0.001). While the symptomatic groups had significantly worse quality of life compared to asymptomatic groups, the variability between participants was evident when examining Fig. 2. Moreover, there was 70% increased odds of worsening quality of life (Table 3) over the four years based on having asymptomatic combined OA, though these changes over time are well within error (Table 2). The between-participant variability was formally tested by the random intercept standard deviation, which was significant (SD [95% CI] = 12.96 [11.97, 13.93], LRT = 456.5, p < 0.001).
Figure 2.
Response variables at each time point with group averages (black rimmed circles) and 95% confidence intervals (colored bands).
Table 2.
Fixed effects derived from linear mixed effects models accounting for random variability of the intercept (participant factor).
| Group Factor |
Time Factor |
Interaction |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | S-TFOA | AS-COMB | S-COMB | Year 2 | Year 4 | S-TFOA Y0–Y2 |
AS-COMB Y0–Y2 | S-COMB Y0–Y2 | S-TFOA Y0–Y4 |
AS-COMB Y0–Y4 | S-COMB Y0–Y4 | |
| KOOS QOL | ||||||||||||
| Model Parameter | 82.10 | -32.92 | -1.36 | -35.43 | 3.74 | 2.16 | 1.77 | -4.70 | 0.38 | 7.23 | -4.03 | 1.72 |
| 95% CI | [78.81, 85.39] | [-38.50, -27.33] | [-5.42, 2.71] | [-39.56, -31.31] | [0.56, 6.92] | [-1.02, 5.33] | [-3.63, 7.16] | [-8.62, -0.78] | [-3.60, 4.37] | [1.84, 12.63] | [-7.96, -0.11] | [-2.26, 5.70] |
| p-value | <0.001 | <0.001 | 0.514 | <0.001 | 0.022 | 0.185 | 0.522 | 0.019 | 0.851 | 0.009 | 0.045 | 0.398 |
| Model Parametera | 82.07 | -32.94 | -1.34 | -35.39 | 3.71 | 2.25 | 1.88 | -4.67 | 0.37 | 7.13 | -4.13 | 1.64 |
| 95% CIa | [78.45, 85.49] | [-38.30, -27.37] | [-5.65, 2.78] | [-39.62, -31.46] | [0.38, 7.27] | [-0.80, 5.73] | [-3.80, 7.60] | [-8.52, -0.67] | [-3.96, 4.44] | [1.98, 12.51] | [-8.35, -0.28] | [-2.47, 5.34] |
| 20m Walk Pace | ||||||||||||
| Model Parameter | 1.39 | -0.14 | 0.00 | -0.15 | -0.02 | -0.04 | 0.03 | 0.01 | 0.01 | 0.04 | 0.00 | 0.02 |
| 95% CI | [1.35, 1.42] | [-0.20, -0.07] | [-0.05, 0.04] | [-0.20, -0.10] | [-0.05, 0.01] | [-0.07, -0.01] | [-0.02, 0.08] | [-0.04, 0.03] | [-0.02, 0.05] | [-0.01, 0.09] | [-0.03, 0.04] | [-0.02, 0.05] |
| p-value | <0.001 | <0.001 | 0.89 | <0.001 | 0.188 | 0.007 | 0.244 | 0.783 | 0.425 | 0.108 | 0.929 | 0.379 |
| Model Parametera | 1.39 | -0.14 | 0.00 | -0.15 | -0.02 | -0.04 | 0.03 | -0.01 | 0.01 | 0.04 | 0.00 | 0.02 |
| 95% CIa | [1.35, 1.42] | [-0.20, -0.07] | [-0.05, 0.05] | [-0.20, -0.10] | [-0.05, 0.01] | [-0.07, -0.01] | [-0.01, 0.08] | [-0.04, 0.03] | [-0.02, 0.05] | [0.00, 0.09] | [-0.03, 0.04] | [-0.02, 0.05] |
| Chair Stand Pace | ||||||||||||
| Model Parameter |
0.53 | -0.06 | -0.01 | -0.11 | 0.00 | 0.00 | -0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 |
| 95% CI | [0.50, 0.56] | [-0.10, -0.02] | [-0.04, 0.02] | [-0.14, -0.07] | [-0.02, 0.02] | [-0.02, 0.02] | [-0.05, 0.03] | [-0.01, 0.04] | [-0.01, 0.05] | [-0.02, 0.05] | [0.00, 0.05] | [0.00, 0.06] |
| p-value | <0.001 | 0.009 | 0.624 | <0.001 | 0.967 | 0.856 | 0.682 | 0.235 | 0.147 | 0.41 | 0.096 | 0.035 |
| Model Parametera |
0.53 | -0.06 | -0.01 | -0.11 | 0.00 | 0.00 | -0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 |
| 95% CIa | [0.50, 0.56] | [-0.10, -0.01] | [-0.04, 0.02] | [-0.14, -0.07] | [-0.02, 0.02] | [-0.02, 0.02] | [-0.04, 0.03] | [-0.01, 0.04] | [-0.01, 0.05] | [-0.02, 0.05] | [0.00, 0.05] | [0.00, 0.06] |
| Max Knee Flexion Strength | ||||||||||||
| Model Parameter |
142.28 | 14.28 | 4.46 | -16.23 | -24.83 | -23.16 | 11.02 | 6.23 | 11.06 | -1.41 | 7.05 | 10.19 |
| 95% CI | [130.66, 153.88] | [-5.65, 34.21] | [-9.88, 18.79] | [-30.80, -1.65] | [-33.90, -15.77] | [-32.35, -13.96] | [-4.83, 26.87] | [-5.02, 17.49] | [-0.41, 22.53] | [-17.85, 15.02] | [-4.39, 18.50] | [-1.56, 21.93] |
| p-value | <0.001 | 0.162 | 0.544 | 0.03 | <0.001 | <0.001 | 0.175 | 0.28 | 0.06 | 0.867 | 0.229 | 0.091 |
| Model Parametera | 142.31 | 14.57 | 4.31 | -16.3 | -24.89 | -23.28 | 10.66 | 6.27 | 11.09 | -1.44 | 7.22 | 10.27 |
| 95% CIa | [130.30, 154.15] | [-5.52, 36.75] | [-10.04, 17.97] | [-31.30, -1.67] | [-34.01, -15.71] | [-32.96, -14.17] | [-6.19, 27.20] | [-4.41, 17.36] | [0.22, 22.62] | [-18.37, 16.15] | [-3.88, 19.14] | [-1.41, 22.49] |
| Max Knee Extension Strength | ||||||||||||
| Model Parameter | 350.63 | 6.44 | 6.75 | -51.14 | -21.36 | -25.52 | 5.82 | 2.35 | 10.67 | 5.48 | 12.21 | 12.34 |
| 95% CI | [327.71, 373.55] | [-32.84, 45.71] | [-21.55, 35.05] | [-79.90, -22.38] | [-36.14, -6.59] | [-40.51, -10.54] | [-20.04, 31.68] | [-15.99, 20.70] | [-8.01, 29.35] | [-21.35, 32.31] | [-6.45, 30.87] | [-32.84, 45.71] |
| p-value | <0.001 | 0.749 | 0.641 | <0.001 | 0.005 | <0.001 | 0.66 | 0.803 | 0.265 | 0.69 | 0.201 | 0.208 |
| Model Parametera | 350.5 | 7.39 | 6.95 | -51.06 | -21.65 | -25.94 | 5.79 | 2.51 | 11.11 | 6.21 | 12.46 | 12.82 |
| 95% CIa | [327.83, 374.52] | [-32.59, 45.68] | [-21.39, 34.88] | [-79.21, -23.18] | [-37.17, -7.02] | [-40.04, -10.68] | [-20.08, 30.96] | [-14.59, 21.09] | [-6.52, 29.77] | [-20.67, 31.50] | [-5.62, 30.26] | [-5.16, 31.14] |
Abbreviations: S-TFOA, symptomatic tibiofemoral osteoarthritis; AS-COMB, asymptomatic combined tibiofemoral and patellofemoral osteoarthritis; S-COMB, symptomatic combined tibiofemoral and patellofemoral osteoarthritis; Y0, Baseline; Y2, Year 2 follow up; Y4, Year 4 follow up; 95% CI, 95% confidence interval.
Parametric bootstraps parameter estimates and percentile-based 95% confidence intervals based on 1000 iterations.
Table 3.
Response variable estimated marginal means and odds ratios (reference group: AS-TFOA) for Baseline, Year 2, and Year 4.
| Baseline |
Year 2 |
Year 4 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Odds Ratio | 95% CI | Mean | 95% CI | Odds Ratio | 95% CI | |
| KOOS QOL | ||||||||||
| AS-TFOA (reference) | 82.1 | [78.8, 85.4] | 85.8 | [82.5, 89.1] | – | – | 84.3 | [81.0, 87.6] | – | – |
| S-TFOA | 49.2 | [44.7, 53.7] | 54.7 | [50.2, 59.2] | 0.71 | [0.34, 1.45] | 58.6 | [54.0, 63.1] | 0.70 | [0.36,1.52] |
| AS-COMB | 80.7 | [78.4, 83.1] | 79.8 | [77.4, 82.2] | 1.46 | [0.89, 2.38] | 78.9 | [76.5, 81.3] | 1.70 | [1.05, 2.79] |
| S-COMB | 46.7 | [44.2, 49.2] | 50.8 | [48.3, 53.3] | 1.25 | [0.76, 2.06] | 50.5 | [48.1, 53.0] | 1.20 | [0.73, 2.00] |
| 20m Walk Pace (m/s) | ||||||||||
| AS-TFOA (reference) | 1.39 | [1.35, 1.42] | 1.37 | [1.33, 1.41] | – | – | 1.35 | [1.31, 1.39] | – | – |
| S-TFOA | 1.25 | [1.19, 1.30] | 1.26 | [1.20, 1.31] | 1.18 | [0.61, 2.28] | 1.25 | [1.19, 1.30] | 0.58 | [0.30, 1.14] |
| AS-COMB | 1.38 | [1.36, 1.41] | 1.41 | [1.33, 1.39] | 1.37 | [0.86, 2.20] | 1.35 | [1.32, 1.37] | 0.98 | [0.61, 1.60] |
| S-COMB | 1.23 | [1.23, 1.26] | 1.26 | [1.20, 1.26] | 1.09 | [0.68, 1.75] | 1.21 | [1.18, 1.24] | 0.8 | [0.49, 1.31] |
| Chair Stand Pace (repetitions/second) | ||||||||||
| AS-TFOA (reference) | 0.53 | [0.50, 0.56] | 0.53 | [0.50, 0.56] | – | – | 0.53 | [0.51, 0.56] | – | – |
| S-TFOA | 0.47 | [0.43, 0.51] | 0.46 | [0.43, 0.50] | 0.80 | [0.40, 1.58] | 0.49 | [0.45, 0.53] | 0.48 | [0.24, 0.99] |
| AS-COMB | 0.52 | [0.50, 0.54] | 0.54 | [0.52, 0.56] | 0.72 | [0.44, 1.16] | 0.55 | [0.53, 0.57] | 0.64 | [0.39, 1.04] |
| S-COMB | 0.42 | [0.40, 0.44] | 0.45 | [0.42, 0.47] | 0.75 | [0.45, 1.24] | 0.46 | [0.44, 0.48] | 0.47 | [0.28, 0.79] |
| Max Knee Flexion Strength (N) | ||||||||||
| AS-TFOA (reference) | 142.28 | [130.63, 153.92] | 117.44 | [105.81, 129.07] | – | – | 119.12 | [107.39, 130.84] | – | – |
| S-TFOA | 156.56 | [140.31, 172.81] | 142.74 | [126.46, 159.03] | 1.05 | [0.49, 2.26] | 131.99 | [115.16, 148.81] | 0.56 | [0.27, 1.13] |
| AS-COMB | 146.73 | [138.29, 155.17] | 128.13 | [119.64, 136.61] | 1.03 | [0.61, 1.74] | 130.63 | [122.01, 139.25] | 0.85 | [0.51, 1.43] |
| S-COMB | 126.05 | [117.21, 134.89] | 112.27 | [103.37, 121.17] | 0.85 | [0.50, 1.44] | 113.07 | [103.91, 122.23] | 1.00 | [0.59, 1.71] |
| Max Knee Extension Strength (N) | ||||||||||
| AS-TFOA (reference) | 350.63 | [327.64, 373.63] | 329.27 | [306.31, 352.24] | – | – | 325.11 | [302.01, 348.21] | – | – |
| S-TFOA | 357.07 | [325.08, 389.07] | 341.53 | [309.48, 373.59] | 0.79 | [0.38, 1.65] | 337.04 | [304.24, 369.84] | 1.54 | [0.76, 3.11] |
| AS-COMB | 357.38 | [340.73, 374.03] | 338.37 | [321.65, 355.09] | 0.81 | [0.49, 1.34] | 344.07 | [327.17, 360.97] | 1.36 | [0.83, 2.22] |
| S-COMB | 299.49 | [282.07, 316.91] | 288.8 | [271.28, 306.32] | 0.71 | [0.42, 1.19] | 286.31 | [268.43, 304.19] | 1.22 | [0.74, 2.00] |
Odds ratios that did not span 1.00 are in bold. Abbreviations: 95% CI, 95% confidence interval; KOOS-QOL, Knee Injury and Osteoarthritis Outcome Scale – quality of life subscale; AS-TFOA, asymptomatic tibiofemoral OA, S-TFOA, symptomatic tibiofemoral osteoarthritis; AS-COMB, asymptomatic combined tibiofemoral and patellofemoral osteoarthritis; S-COMB, symptomatic combined tibiofemoral and patellofemoral osteoarthritis.
3.2. Objective physical function
Group and Time fixed effects, but not interaction effects, were significant for the 20 m walk test (χ2 = 72.8, p < 0.001; χ2 = 20.7, p < 0.001; χ2 = 4.7, p = 0.578, respectively) and chair stand test (χ2 = 67.4, p < 0.001; χ2 = 18.5, p < 0.001; χ2 = 6.9, p = 0.328, respectively). The fixed effects accounted for 10% and 9% of the variance for the 20 m walk test and chair stand test, while adding the random intercept increased this to 77% and 70% respectively. The AS-TFOA group walking speed significantly slowed (-0.04 m/s [-0.07, -0.01]; p = 0.007) from Baseline to Year 4, but no other group significantly differed in their trajectory compared to the AS-TFOA group. No groups significantly changed over time for the chair stand test. The symptomatic groups walked slower (S-TFOA: -0.14 m/s [-0.2, -0.07]; p < 0.001 and S-COMB: (-0.15 m/s [-0.20, -0.10]; p < 0.001) and performed fewer chair stands (S-TFOA: -0.06 repetitions/second [-0.10, -0.02]; p < 0.009 and S-COMB: -0.11 repetitions/second [-0.14, -0.07]; p < 0.001) compared to the AS-TFOA group. This was also reflected in the 52% and 53% odds of worsening chair stand performance for the S-TFOA and S-COMB groups, respectively. Like the KOOS-QOL model, significant group differences were found, although the 20 m walk test (SD [95% CI] = 0.18 m/s [0.17, 0.19], LRT = 964.8, p < 0.001) and chair stand test (SD [95% CI] = 0.12 repetitions/second [0.11, 0.12], LRT = 655.0, p < 0.001) scores still significantly varied between participants.
3.3. Leg strength
As with the 20 m walk and chair stand tests, Group and Time, but not the interaction, effects were significant for flexion (χ2 = 15.7, p < 0.001; χ2 = 93.6, p < 0.001; χ2 = 7.4, p = 0.282, respectively) and extension strength (χ2 = 26.5, p < 0.001; χ2 = 30.7, p < 0.001; χ2 = 3.6, p = 0.738, respectively). The fixed effects only accounted for 4% of the variance and 72% with the random intercept included. The AS-TFOA group exhibited a significant reduction from Baseline to Year 2 and Year 4 for knee flexion (-24.83 N [-33.9, -15.77]; p < 0.001 and -23.16 N [-32.35, -13.96]; p < 0.001) and extension (-21.36 N [-36.14, -6.59]; p = 0.004 and -25.52 N [-40.51, -10.54]; p < 0.001). All other groups did not significantly differ from the AS-TFOA group trajectory, suggesting all groups exhibited significant worsening of muscle strength. The only significant between-group difference was the lower knee flexion and extension strength scores for those in the S-COMB group compared to the AS-TFOA group (-16.23 N [-30.80, -1.65]; p = 0.03 and -51.14 N [-79.9, -22.38]; p < 0.001, respectively). Between participant variability for knee flexion (SD [95% CI] = 52.1 N [48.6, 55.6], LRT = 769.1, p < 0.001) and extension strength (SD [95% CI] = 109.9 N [102.9, 116.7], LRT = 1123.8, p < 0.001) was significant and very large relative to group differences, likely explaining the largely non-significant between group differences.
4. Discussion
The most notable finding in this study is the overall stability of all groups over time, across all outcomes examined. While there were significant changes in outcomes over the four year follow up, and in several cases these changes differed between groups, the changes were almost entirely within known error, and likely not clinically significant. These results did not support our hypothesis regarding greater worsening in the combined OA groups. However, the results do suggest that quality of life and the secondary physical function measures are significantly worse in individuals with knee OA symptoms, regardless of the distribution of structural signs within the knee, supporting our second hypothesis. Overall, we found that the presence of symptoms is more important than the distribution of OA within the knee compartments, when determining quality of life and physical function.
Structural signs of OA generally progress with time, but patient-reported outcomes, like quality of life, seem to remain stable for most patients. Törmälehto et al. [16] found that two thirds of people with mild knee OA or those at risk of developing knee OA (n = 3053 from the OAI dataset) did not report a minimally important change in quality of life over eight years. Also using data from the OAI from people with symptomatic knee OA, Han and Gellhorn [45] found that both low and moderate quality of life groups (68% of the sample) did not significantly change over eight years. Our results add to this, by including those with PFOA in addition to TFOA. Although three of the four groups in our study (AS-TFOA, S-TFOA, AS-COMB) statistically improved, the magnitude of the differences were less than minimum clinically important changes [31] and minimum detectable changes [32]. Statistical significance was likely a product of the relatively large sample size, not due to the presence of a clinically meaningful effect.
The 20 m walk test and 30 s chair stand test, which are assessments of knee function, also remained stable. These results mirror previous six-year 20 m walk test performance stability from the Multicenter Osteoarthritis Study and the OAI [15]. Similarly, data from the Mechanical Factors in Arthritis of the Knee study showed that chair stand test (rate of 5 successive stands) performance didn't change in 44% of participants with knee OA (only TFOA was confirmed) over three years [46]. Moreover, a synthesis of 45 studies involving people with knee OA showed that physical function did not significantly change over 0.5–8 years [47]. Unlike the movement-based functional assessments, knee strength significantly worsened in the current study's AS-TFOA group. Meanwhile, the interaction effects were not significant, which suggests that the other three groups experienced a similar decrease in strength, though these changes were again within minimum detectable change [37].
The measures of quality of life and physical function included in this study largely remained stable over the four years. This lack of progression in the OAI cohort may be, in part, a result of concurrently stable knee pain [48], a tendency for outcomes from participants of large cohort studies to differ from the general population (e.g. volunteer bias) [49], or that people living with knee OA find strategies to cope with their disease over time (e.g. response shift). Moreover, treatment can improve quality of life in those with PFOA [21], and it is unclear if participants in our dataset received treatment over the four year timeframe. The small changes, as seen in the present study and others [[15], [45]] may also reflect regression to the mean. These results are certainly encouraging and should be an important point to communicate to patients when discussing typical knee OA prognosis.
While the present study did not find meaningful changes over time in our outcomes of interest, groups did significantly differ in their scores at Baseline. The observed differences were driven by the presence of symptoms not structural distribution, where people with symptomatic knees reported worse outcomes compared to those with asymptomatic knees. With respect to KOOS-QOL, symptomatic and asymptomatic groups differed by over 30 points. These findings are supported by previous work examining quality of life, which showed that symptom status is most influential [12] while structural distributions of OA within the knee are not [50]. Although recent work did find that combined PFOA and TFOA was related to worse quality of life compared to TFOA alone [23]. We observed a similarly lower quality of life in the S-COMB compared to S-TFOA, but we did not test this directly nor was the difference clinically meaningful. Taken together, our results and other previous work [51], shows that the presence of pain alone is a good indicator of quality of life in patients with knee OA, regardless of compartment-specific imaging findings.
Physical function is also sensitive to the presence of knee pain. Previous research using the OAI dataset showed that higher pain severity, was related to worse chair stand and 400 m walk test performance [52]. Additionally, those with radiographic knee OA and pain were found to have slower 20 m walk test speeds and were more likely to decline in speed over time compared to healthy controls [53]. Our data further confirm that the presence of pain can independently and negatively influence physical function test performance. This relationship is likely influenced by the person's perceptions of pain [54] or kinesiophobia behaviour due to knee pain [55]. In our data, the addition of PFOA to a knee with TFOA was not a significant factor in the models, again suggesting that symptoms are more important than structural distribution of OA, in determining physical function. Consequently, rehabilitation strategies should focus on improving knee OA-related symptoms regardless of whether the patellofemoral joint is involved or not.
Lower knee muscle strength is associated with the presence of pain [56]. The structural consequences of OA are also thought to play a role, particularly in quadriceps weakness, due to abnormal afferent sensory information [57]. From previous reports of the OAI dataset, individuals with symptomatic knee OA presented with 11–13% lower knee extension strength and 7–16% lower knee flexion strength compared to asymptomatic participants [13]. The Beijing Osteoarthritis Study examined knee strength across groups with different compartmental involvement and reported that the strongest odds of having weak quadriceps was for the group with combined PFOA and TFOA [58]. Our data also showed the lowest knee extension force in the S-COMB group. Knee flexion strength exhibited a similar pattern, though the differences were far smaller, partly a product of the smaller overall values. These results point to the additive effect of pain and combined knee compartment involvement on knee strength, and that knee strength may be more sensitive to compartmental involvement compared to the KOOS-QOL and measures of physical function. Moreover, those in the S-COMB group are uniquely positioned to benefit from quadriceps strengthening given its protective effect on disease progression and pain [59].
Our study has limitations that must be considered when interpreting the results. The variables of interest in our study were limited to the follow-up timepoints available, thereby limiting any conclusions we can make beyond four years. We also separated groups based on the presence of TFOA or PFOA. However, the structural signs of OA and the distribution within one compartment of the knee (e.g. medial versus lateral patellofemoral joint) may affect pain and quality of life [60]. Examining subgroups based on the location and severity of structural and symptomatic disease characteristics may better inform quality of life outcomes [61], given that structural disease severity relates to knee pain in later-staged disease [62]. Defining TFOA and PFOA by MRI features may allow for inclusion of those with earlier knee OA and broaden the sample. As our data show, pain is an important factor in estimating quality of life and physical function. Readers must keep in mind that we only examined unilateral pain, while previous data from the OAI shows that bilateral pain may have a greater effect on quality of life [51]. While we focused on factors related to pain and structural signs of OA, psychological factors certainly play an important role in quality of life [55]. Lastly, we categorized participants based on MRI readings performed by OAI project groups. These projects selected participants either randomly or based on additional criteria to address their study question (e.g., presence vs. absence of KL grade progression, medial TFOA vs. lateral TFOA), meaning our dataset is not a truly random sample. Nevertheless, none of these projects assessed the presence or absence of PFOA, and as such, we are confident that the data do not contain any biases that relate to the specific purpose of our study.
Osteoarthritis does not always affect the tibiofemoral joint alone, and the influence of concurrent PFOA is becoming clearer. For clinicians, treating knee OA can be complex and identifying the likely prognosis may be difficult. The present study's results suggest that, for the most part, knee-related quality of life, physical function, and knee strength remain stable in the short to medium term (out to four years). This is important to convey when consulting with patients. However, it may be helpful to identify who is likely to have lower quality of life and physical function overall, as these individuals are in greater need of focused rehabilitation to improve these outcomes. Our findings show that individuals with lower quality of life and physical function can largely be identified based solely on the presence of symptoms, regardless of the distribution of structural OA within the knee.
Role of the funding source
Data used in the preparation of this manuscript were obtained and analyzed from the controlled access datasets distributed from the Osteoarthritis Initiative (OAI). The OAI is a collaborative informatics system resulting from a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, USA (Institute of Mental Health and Institute of Arthritis, Musculoskeletal and Skin Diseases), a branch of the Department of Health and Human Services, USA and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partner.
The authors of this work were supported by a Natural Sciences and Engineering Research Council of Canada, Canada Discovery Grant (MAH). Salary support was provided by the Canadian Institutes of Health Research, Canada (JMC, JFE, DK, MAH), and the Michael Smith Foundation for Health Research, Canada (MAH). Funding sources played no role in any aspect of this study.
Author contributions
Conception and design: JMC, JFE, DK, DT, MAH.
Analysis and interpretation of the data: JMC, JFE, MAH.
Drafting of the article: JMC, MAH.
Critical revision of the article for important intellectual content: JMC, JFE, DK, DT, MAH.
Final approval of the article: JMC, JFE, DK, DT, MAH.
Statistical expertise: JMC, MAH.
Collection and assembly of data: JFE, DK, DT, OAI team.
Declaration of competing interest
None to report.
Contributor Information
Jesse M. Charlton, Email: jesse.charlton@ubc.ca.
Jean-Francois Esculier, Email: jfesculier@therunningclinic.com.
Dylan Kobsar, Email: kobsard@mcmaster.ca.
Daniel Thatcher, Email: d.thatcher100@gmail.com.
Michael A. Hunt, Email: michael.hunt@ubc.ca.
References
- 1.Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2013;2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace I.J., Worthington S., Felson D.T., Jurmain R.D., Wren K.T., Maijanen H., et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. U.S.A. 2017;114:9332–9336. doi: 10.1073/pnas.1703856114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence R.C., Felson D.T., Helmick C.G., Arnold L.M., Choi H., Deyo R.A., et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bombardier C., Hawker G., Mosher D. Arthritis Alliance of Canada; Ottawa, ON: 2011. The Impact of Arthritis in Canada: Today and over the Next 30 Years; p. 52. [Google Scholar]
- 6.Jafarzadeh S.R., Felson D.T. Updated estimates suggest a much higher prevalence of arthritis in United States adults than previous ones. Arthritis & Rheumatology. 2018;70:185–192. doi: 10.1002/art.40355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray C.J.L., Lopez A.D., World Health O., World B., Harvard School of Public H. World Health Organization; Geneva: 1996. The Global Burden of Disease : a Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. [Google Scholar]
- 8.Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014;10:437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 9.Salaffi F., Carotti M., Stancati A., Grassi W. Health-related quality of life in older adults with symptomatic hip and knee osteoarthritis: a comparison with matched healthy controls. Aging Clin. Exp. Res. 2005;17:255–263. doi: 10.1007/BF03324607. [DOI] [PubMed] [Google Scholar]
- 10.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAlindon T.E., Cooper C., Kirwan J.R., Dieppe P.A. Determinants of disability in osteoarthritis of the knee. Ann. Rheum. Dis. 1993;52:258–262. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiadaliri A.A., Lamm C.J., de Verdier M.G., Engstrom G., Turkiewicz A., Lohmander L.S., et al. Association of knee pain and different definitions of knee osteoarthritis with health-related quality of life: a population-based cohort study in southern Sweden. Health Qual. Life Outcome. 2016;14:1–7. doi: 10.1186/s12955-016-0525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruhdorfer A., Wirth W., Hitzl W., Nevitt M., Eckstein F., for the Osteoarthritis Initiative I. Association of thigh muscle strength with knee symptoms and radiographic disease stage of osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res. 2014;66:1344–1353. doi: 10.1002/acr.22317. [DOI] [PubMed] [Google Scholar]
- 14.Gonçalves R.S., Pinheiro J.P., Cabri J. Evaluation of potentially modifiable physical factors as predictors of health status in knee osteoarthritis patients referred for physical therapy. Knee. 2012;19:373–379. doi: 10.1016/j.knee.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Øiestad B.E., White D.K., Booton R., Niu J., Zhang Y., Torner J., et al. Longitudinal course of physical function in people with symptomatic knee osteoarthritis: data from the Multicenter Osteoarthritis Study and the Osteoarthritis Initiative. Arthritis Care Res. 2016;68:325–331. doi: 10.1002/acr.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Törmälehto S., Aarnio E., Mononen M.E., Arokoski J.P.A., Korhonen R.K., Martikainen J.A. Eight-year trajectories of changes in health-related quality of life in knee osteoarthritis: data from the Osteoarthritis Initiative (OAI) PloS One. 2019;14 doi: 10.1371/journal.pone.0219902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinman R.S., Crossley K.M. Patellofemoral joint osteoarthritis: an important subgroup of knee osteoarthritis. Rheumatology. 2007;46:1057–1062. doi: 10.1093/rheumatology/kem114. [DOI] [PubMed] [Google Scholar]
- 18.Hart H.F., Stefanik J.J., Wyndow N., Machotka Z., Crossley K.M. The prevalence of radiographic and MRI-defined patellofemoral osteoarthritis and structural pathology: a systematic review and meta-analysis. Br. J. Sports Med. 2017;51:1195–1208. doi: 10.1136/bjsports-2017-097515. [DOI] [PubMed] [Google Scholar]
- 19.Stefanik J.J., Niu J., Gross K.D., Roemer F.W., Guermazi A., Felson D.T. Using magnetic resonance imaging to determine the compartmental prevalence of knee joint structural damage. Osteoarthritis Cartilage. 2013;21:695–699. doi: 10.1016/j.joca.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefanik J.J., Guermazi A., Roemer F.W., Peat G., Niu J., Segal N.A., et al. Changes in patellofemoral and tibiofemoral joint cartilage damage and bone marrow lesions over 7 years: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2016;24:1160–1166. doi: 10.1016/j.joca.2016.01.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart H.F., Filbay S.R., Coburn S., Charlton J.M., Sritharan P., Crossley K.M. Is quality of life reduced in people with patellofemoral osteoarthritis and does it improve with treatment? A systematic review, meta-analysis and regression. Disabil. Rehabil. 2018:1–15. doi: 10.1080/09638288.2018.1482504. [DOI] [PubMed] [Google Scholar]
- 22.Hunter D.J., March L., Sambrook P.N. The association of cartilage volume with knee pain. Osteoarthritis Cartilage. 2003;11:725–729. doi: 10.1016/s1063-4584(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 23.Hart H.F., Crossley K.M., Hunt M.A. Gait patterns, symptoms, and function in patients with isolated tibiofemoral osteoarthritis and combined tibiofemoral and patellofemoral osteoarthritis. J. Orthop. Res. 2018;36:1666–1672. doi: 10.1002/jor.23805. [DOI] [PubMed] [Google Scholar]
- 24.Iijima H., Fukutani N., Aoyama T., Fukumoto T., Uritani D., Kaneda E., et al. Clinical impact of coexisting patellofemoral osteoarthritis in Japanese patients with medial knee osteoarthritis. Arthritis Care Res. 2016;68:493–501. doi: 10.1002/acr.22691. [DOI] [PubMed] [Google Scholar]
- 25.Englund M., Lohmander L.S. Patellofemoral osteoarthritis coexistent with tibiofemoral osteoarthritis in a meniscectomy population. Ann. Rheum. Dis. 2005;64:1721–1726. doi: 10.1136/ard.2005.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter D.J., Arden N., Conaghan P.G., Eckstein F., Gold G., Grainger A., et al. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage. 2011;19:963–969. doi: 10.1016/j.joca.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter D.J., Guermazi A., Lo G.H., Grainger A.J., Conaghan P.G., Boudreau R.M., et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roemer F.W., Guermazi A., Collins J.E., Losina E., Nevitt M.C., Lynch J.A., et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort - methodologic aspects and definition of change. BMC Muscoskel. Disord. 2016;17:466. doi: 10.1186/s12891-016-1310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nevitt M., Felson D., Lester G. Vol. 1.1. NIHM Data Archive; 2003. (The Osteoarthritis Initiative: Protocol for the Cohort Study). [Google Scholar]
- 31.Roos E.M., Lohmander L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual. Life Outcome. 2003;1:1–8. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins N.J., Misra D., Felson D.T., Crossley K.M., Roos E.M. Measures of knee function: international knee documentation committee (IKDC) subjective knee evaluation form, knee injury and osteoarthritis outcome score (KOOS), knee injury and osteoarthritis outcome score physical function short form (KOOS-PS), knee outcome survey activities of daily living scale (KOS-ADL), lysholm knee scoring scale, oxford knee score (OKS), western Ontario and McMaster universities osteoarthritis index (WOMAC), activity rating scale (ARS), and tegner activity score (TAS) Arthritis Care Res. 2011;63(Suppl 11):S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villadsen A., Roos E.M., Overgaard S., Holsgaard-Larsen A. Agreement and reliability of functional performance and muscle power in patients with advanced osteoarthritis of the hip or knee. Am. J. Phys. Med. Rehabil. 2012;91:401–410. doi: 10.1097/PHM.0b013e3182465ed0. [DOI] [PubMed] [Google Scholar]
- 34.Dobson F., Hinman R.S., Hall M., Terwee C.B., Roos E.M., Bennell K.L. Measurement properties of performance-based measures to assess physical function in hip and knee osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2012;20:1548–1562. doi: 10.1016/j.joca.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald G.K., Piva S.R., Irrgang J.J., Bouzubar F., Starz T.W. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Care Res. 2004;51:40–48. doi: 10.1002/art.20084. [DOI] [PubMed] [Google Scholar]
- 36.Holm P.M., Nyberg M., Wernbom M., Schrøder H.M., Skou S.T. Intrarater reliability and agreement of recommended performance-based tests and common muscle function tests in knee osteoarthritis. J. Geriatr. Phys. Ther. 2020:1–9. doi: 10.1519/JPT.0000000000000266. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 37.Kean C.O., Birmingham T.B., Garland S.J., Bryant D.M., Giffin J.R. Minimal detectable change in quadriceps strength and voluntary muscle activation in patients with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2010;91:1447–1451. doi: 10.1016/j.apmr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Team R.C.R. R Foundation for Statistical Computing; Vienna, Austria: 2019. A Language and Environment for Statistical Computing. [Google Scholar]
- 39.Miller G.A., Chapman J.P. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 40.Bates D., Mächler M., Bolker B., Walker S. vol. 67. 2015. (Fitting Linear Mixed-Effects Models Using Lme 4). 2015-10-07 Edition. [Google Scholar]
- 41.Ludecke D, Makowski D, Waggoner P. Performance: Assessment of Regression Models Performance. vol. R package 0.4.42020.
- 42.Kuznetsova A., Brockhoff P.B., Christensen R.H.B. vol. 82. 2017. pp. 11–29. (lmerTest Package: Tests in Linear Mixed Effects Models). ed2017:26. [Google Scholar]
- 43.Luke S.G. Evaluating significance in linear mixed-effects models in R. Behav. Res. Methods. 2017;49:1494–1502. doi: 10.3758/s13428-016-0809-y. [DOI] [PubMed] [Google Scholar]
- 44.Canty A, Ripley B. Boot: Bootstrap R (S-Plus) Functions. vol. R package 1.3-222019.
- 45.Han A., Gellhorn A.C. Trajectories of quality of life and associated risk factors in patients with knee osteoarthritis: findings from the Osteoarthritis Initiative. Am. J. Phys. Med. Rehabil. 2018;97:620–627. doi: 10.1097/PHM.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 46.Sharma L., Cahue S., Song J., Hayes K., Pai Y.-C., Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359–3370. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- 47.de Rooij M., van der Leeden M., Heymans M.W., Holla J.F.M., Häkkinen A., Lems W.F., et al. Prognosis of pain and physical functioning in patients with knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res. 2016;68:481–492. doi: 10.1002/acr.22693. [DOI] [PubMed] [Google Scholar]
- 48.Collins J.E., Katz J.N., Dervan E.E., Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22:622–630. doi: 10.1016/j.joca.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernán M., Hernández-Díaz S., Robins J. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi S., Pappas E., Fransen M., Refshauge K., Simic M. The prevalence of patellofemoral osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2016;24:1697–1707. doi: 10.1016/j.joca.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Bindawas S.M., Vennu V., Al Snih S. Differences in health-related quality of life among subjects with frequent bilateral or unilateral knee pain: data from the Osteoarthritis Initiative study. J. Orthop. Sports Phys. Ther. 2015;45:128–136. doi: 10.2519/jospt.2015.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davison M.J., Ioannidis G., Maly M.R., Adachi J.D., Beattie K.A. Intermittent and constant pain and physical function or performance in men and women with knee osteoarthritis: data from the osteoarthritis initiative. Clin. Rheumatol. 2016;35:371–379. doi: 10.1007/s10067-014-2810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White D.K., Niu J., Zhang Y. Is symptomatic knee osteoarthritis a risk factor for a trajectory of fast decline in gait speed? Results from a longitudinal cohort study. Arthritis Care Res. 2013;65:187–194. doi: 10.1002/acr.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Somers T.J., Keefe F.J., Pells J.J., Dixon K.E., Waters S.J., Riordan P.A., et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J. Pain Symptom Manag. 2009;37:863–872. doi: 10.1016/j.jpainsymman.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helminen E.-E., Sinikallio S.H., Valjakka A.L., Väisänen-Rouvali R.H., Arokoski J.P.A. Determinants of pain and functioning in knee osteoarthritis: a one-year prospective study. Clin. Rehabil. 2016;30:890–900. doi: 10.1177/0269215515619660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Reilly S.C., Jones A., Muir K.R., Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann. Rheum. Dis. 1998;57:588–594. doi: 10.1136/ard.57.10.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurley M.V., Scott D.L., Rees J., Newham D.J. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann. Rheum. Dis. 1997;56:641–648. doi: 10.1136/ard.56.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker K.R., Xu L., Zhang Y., Nevitt M., Niu J., Aliabadi P., et al. Quadriceps weakness and its relationship to tibiofemoral and patellofemoral knee osteoarthritis in Chinese: the Beijing osteoarthritis study. Arthritis Rheum. 2004;50:1815–1821. doi: 10.1002/art.20261. [DOI] [PubMed] [Google Scholar]
- 59.Amin S., Baker K., Niu J., Clancy M., Goggins J., Guermazi A., et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60:189–198. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iijima H., Fukutani N., Isho T., Yamamoto Y., Hiraoka M., Miyanobu K., et al. Changes in clinical symptoms and functional disability in patients with coexisting patellofemoral and tibiofemoral osteoarthritis: a 1-year prospective cohort study. BMC Muscoskel. Disord. 2017;18:1–11. doi: 10.1186/s12891-017-1486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oak S.R., Ghodadra A., Winalski C.S., Miniaci A., Jones M.H. Radiographic joint space width is correlated with 4-year clinical outcomes in patients with knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2013;21:1185–1190. doi: 10.1016/j.joca.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 62.Øiestad B.E., Holm I., Engebretsen L., Risberg M.A. The association between radiographic knee osteoarthritis and knee symptoms, function and quality of life 10–15 years after anterior cruciate ligament reconstruction. Br. J. Sports Med. 2011;45:583–588. doi: 10.1136/bjsm.2010.073130. [DOI] [PubMed] [Google Scholar]