Abstract
Objective
Total knee arthroplasty (TKA) is the gold-standard treatment for end-stage knee osteoarthritis, and the primary expectations are reduced pain and improved function. However, there is conflicting evidence regarding functional changes post-TKA. Commonly, functional changes are measured using Oxford Knee Score (OKS). No previous study has investigated physical behaviour (PB) changes in terms of volume and patterns post-TKA. The aims of this study were to explore volume and pattern changes in PB following TKA using an objective tool and to assess the correlation between this and OKS.
Design
An activPAL measured the PB of individuals on a waiting list for TKA for a period of 7–8 days pre-TKA, and for the same length of time at 12 months post-TKA. OKS was completed at similar follow-up time points.
Results
Thirty-three individuals completed the study, where stepping time, the number of steps and the time spent on moderate to vigorous physical activity (MVPA) (>100 steps/minute) improved significantly post-TKA p = 0.0001. Steps at 12 months post-TKA improved by 45.6% (from 4240 to 6174) and stepping time increased by 38.8% (from 0.98 to 1.36 h). MVPA improved by 35 min at 12 months (from 6.6 to 41.7 min). There were no significant correlations between PB and OKS.
Conclusion
This is the first study to explore PB volumes and event-based patterns post-TKA. Activity improved in terms of volume and patterns. No correlation was found between OKS and ActivPAL, which emphasises the need to use objective methods in addition to patient-reported outcome measures.
Keywords: Physical activity, Knee arthroplasty, Accelerometery, activPAL, Osteoarthritis
1. Introduction
With an ageing population and increasing obesity, osteoarthritis (OA) has become a leading cause of global disability [1]. End-stage knee osteoarthritis (KOA) pain and a limited range of motion are major sources limiting physical activity (PA), increased sedentary behaviour and subsequent chronic disability. OA is associated with increasing all-cause mortality and serious cardiovascular disease events [[1], [2], [3]]. Total knee arthroplasty (TKA) is an effective gold-standard treatment for end-stage KOA [4,5].
The main expected outcomes of TKA are reduced pain and improved PA. Pain reduction and functional changes are commonly measured using Oxford Knee Score (OKS), where under/overestimation and recall bias cannot be excluded [6]. Agreement between Knee Injury and Osteoarthritis Outcome Score (KOOS) and a sedentary time questionnaire with activity monitor outcomes has been shown to range between weak and no correlation [[7], [8], [9], [10]]. No previous study has correlated the time spent in physical behaviour (PB) before and after TKA with OKS score, as it is patient-reported outcome measures (PROMs) that are most commonly used to assess outcomes post-TKA. Correlation assessment may enhance the understanding of functional recovery using different assessment tools.
However, there has been limited research on free-living PB outcomes post-TKA using reliable assessment methods and good methodology [[11], [12], [13], [14]]; and no study has explored daily PB, in terms of the time spent in sedentary, standing, upright and stepping states, number of steps per day and event-based patterns post-TKA using an objective reliable method. Most studies have found that PB remained the same as pre-TKA or reduced, based on an average taken over 48 h or shorter measurement periods using either unfeasible postural or energy expenditure accelerometers [11,[13], [14], [15], [16]]. The postural accelerometer's considerable weight and multiple-sensor design (one sensor along the lateral aspect of the thigh, a second one on the lateral aspect of the calf with connecting cables to a processor monitor at hip level) render it unsuitable for wearing round the clock, and previous studies' records cover fewer than the recommended number of days, which makes their results questionable [13,[16], [17], [18], [19]]. Differences in methodology in terms of monitors' sensitivity and accuracy, the numbers of days worn and the times of day when they were worn have crucial effects on the results and may explain these variations.
ActivPAL is a feasible postural classification accelerometer that provides objective quantification of free-living PB without any modification; it is small, lightweight, waterproof and offers simple application to patients. ActivPAL has been shown to be superior to other accelerometers, given its ability to measure volume and pattern of free-living PB [[20], [21], [22], [23]]. It assesses low-energy positions (sitting and lying) to estimate sedentary behaviour. It also accurately assesses the start time for each position and the duration spent in it. In a similar manner, it estimates upright event (standing, stepping) times, in addition to the number of steps and cadence [20,21]. ActivPAL showed good inter-device reliability, ranging from 0.79 to 0.99. The mean percentage differences between activPAL and direct observation for the total time spent sitting were 0.19% (limit of agreement from −0.68% to 1.06%) and for standing 1.4% (limit of agreement from −6.2% to 9.1%) [24]. The only study that utilised an activPAL post-TKA [12] attached the device over the anterolateral of the tibia instead of the anterior aspect of the upper thigh, and therefore was not validated for this position for stepping and in this position would be unable to differentiate standing from sitting [20].
No previous study has compared PA post-TKA with global PA guideline recommendations to promote and maintain health. Therefore, the current study aimed to determine any changes in free-living PB at 12 months post-TKA in order to determine whether there was any correlation between PB and OKS and to compare PA post-TKA to global PA recommendations. The hypotheses of the current study are: there are significant changes in PB volume and pattern post-TKA, there is a significant correlation between PB and OKS score, and PA post-TKA meets global PA guideline recommendations.
2. Method
2.1. Study design and participants
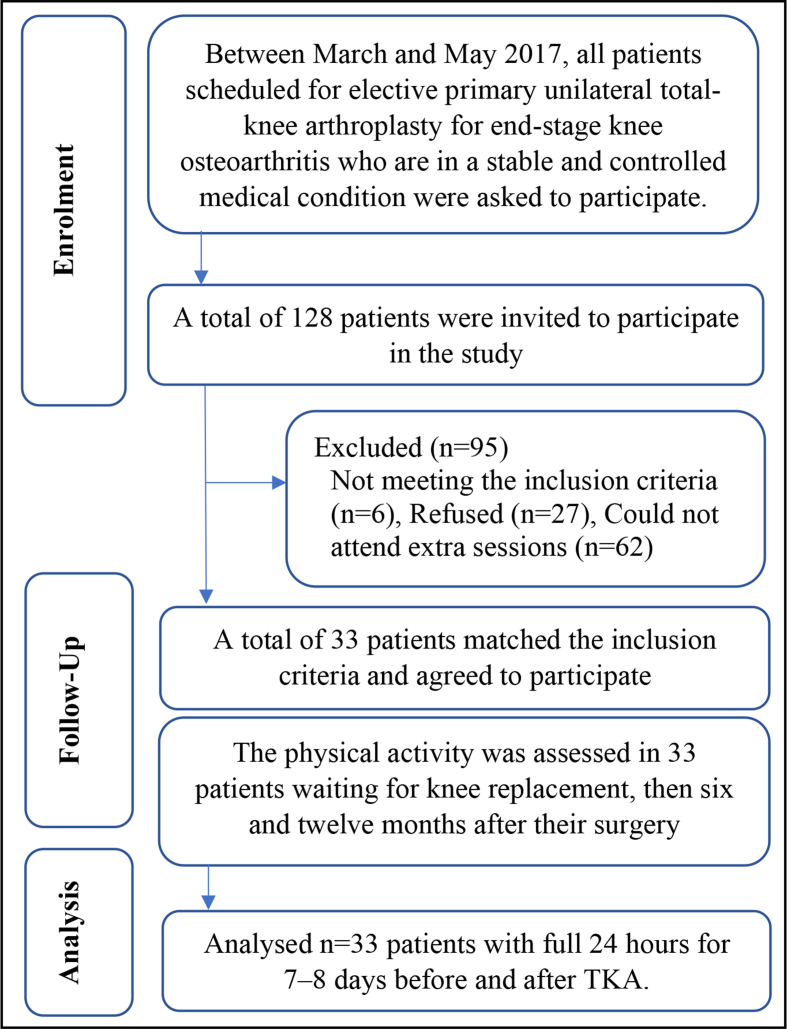
The study was a prospective six- and twelve-month follow-up trial to explore outcomes post-TKA (ClinicalTrials.gov number NCT02998125). Ethical approval was obtained from both Salford University (HSR1617-39) and King Khaled University Hospital ethical committees (E−17-2395), and each individual signed a consent form. All patients who were scheduled for elective primary unilateral total-knee arthroplasty, for end-stage knee osteoarthritis, were asked to participate during preadmission orthopaedic clinic visits between March and May 2017 (Fig. 1). End-stage KOA, according to the Kellgren-Lawrence scale for Grade 4, occurs when large osteophytes are present and joint space narrows, with severe sclerosis and definite bone-contour deformity [25]. Age, gender and body-mass-index (BMI) were not significantly different for the included participants and those who could not volunteer, p = 0.55, p = 0.39 and p = 0.79, respectively. Potential participants were excluded from the study if they: were scheduled for bilateral knee surgery, unicompartmental replacement or revision surgery; had limited function due to musculoskeletal conditions other than unilateral knee osteoarthritis; had been diagnosed with uncontrolled diabetes mellitus or blood pressure, any neurologic disorders, such as stroke, Parkinson's disease, multiple sclerosis, or psychological pathologies; had advanced osteoporosis or some other unstable chronic disease; had been diagnosed with a peripheral vascular or uncontrolled cardiac disease.
Fig. 1.
Patient flowchart showing exclusions.
The required sample-size estimation was based on step number changes post-TKA in a previous study using an ActivPAL, although that study was conducted without a power calculation [12]. The standard deviation of difference (σ) = 0.5, effect size (standardized mean difference) = 0.3 with a significance level of α 0.05 and a power of (1-β) 0.90 indicated that a minimum of 30 patients was required. A possible 10% drop-out rate was expected and therfore a corrected sample size of 33 patients was required [26].
2.2. Surgical intervention and rehabilitation
All patients underwent a midline incision with a medial parapatellar approach to surgery performed by one of five consultant surgeons. No intra-operative complications were reported. Three different prosthesis designs were used: NexGen® LPS Flex cemented knee-replacement (Zimmer Inc.) or Persona knee-replacement system (Zimmer Inc), and Attune® knee-replacement system (DePuy Synthes, A Johnson & Johnson Company). The hospital stay after TKA was 5–6 days. Physiotherapy was standardised according to hospital protocols to minimise confounding factors for both in-patient and out-patient periods. The aim of in-patient physiotherapy is to mobilize patients on the first day after surgery, employing either weight-bearing or partial weight-bearing, as tolerated. In-patient exercises include bed exercise, lower limb strengthening, a range of motion exercises, gait training and stair training, all designed to get patients back to an acceptable level of functional independence. Out-patient physiotherapy continues for not less than one month, with an average of three physiotherapy sessions per week. The exercise programme includes progressive lower-limb range-of-motion exercises, strengthening exercises and gait-and-balance training.
2.3. Testing procedures
During a preadmission session (7–8 days before surgery), patients were instructed to complete an Arabic Oxford knee-score (OKS) form [27]; then, an ActivPAL was attached to the patient's mid-thigh, secured by non-allergic waterproof adhesive tape under their clothes, for 7–8 days before surgery. The ActivPAL was collected on admission day, with the same measures repeated at six and twelve months post-TKA.
PB in the current study was measured for a minimum of 7 consecutive days to adhere to free-living PB recommendations to include variation on weekdays and at the weekend [21]. In addition, measuring PB each week enhanced the comparison with PA global recommendations as these mention per week not per day [28].
2.4. Physical activity outcome measures
PB measurements were taken using an ActivPAL activity monitor (PAL Technologies, Glasgow, UK), which provides objective quantification of free-living PB. The device is light-weight (20g), small (53 x 35 × 7 mm, Fig. 2) and has a battery life of up to 14 days; it contains a microprocessor, sensing element, recording element, associated electronics and power supply. The microprocessor controls the processing and recording of the sensor signal and communication with a host PC [23,29]. Before application, the monitor was charged and programmed (ActivPAL3™, version 7.2.32). The device was worn by the patient for 7–8 days at each measurement period. Clear written and verbal instructions were given to patients, advising that they had to wear it all day and all night, except when bathing or swimming (they could shower with it attached). After the test period, the monitor was removed, and data were downloaded for analysis [11,12].
Fig. 2.
ActivPAL monitor.
2.5. Patient reported outcome measure
Arabic OKS, which is a validated PROM commonly used in the assessment of pain and function post-TKA, was calculated during testing sessions [27].
2.6. Data processing
2.6.1. Patient-reported outcomes
OKS data entry was undertaken according to OKS guidelines 2015, with scores for each question (item) from 0 to 4, with 0 being the worst outcome and 4 the best. The scores were then summed to produce an overall score between 0 (worst possible) to 48 (best possible) [30].
2.6.2. Physical-activity measurements
ActivPAL is a postural classification device that has the ability to determine the inclination of one or more body segments and drive the body position, with the position being recorded every 20th of a second over 24 h. These raw acceleration data are stored in memory [20]. Data were extracted from the ActivPAL monitor using proprietary software; then, a CSV format file was imported into Excel for further statistical analysis using Excel and SPSS (SPSS Inc., Chicago, IL, USA, release 21 for Windows).
The quantity of data was rounded to 24-h data sets and incomplete data sets for the first and last days were removed. Full 24-h data sets for a minimum of 7 consecutive days at all three time points were analysed. Data from the ActivPAL were activities classified into sedentary, standing and stride events, with consecutive stride events being able to be combined to give stepping events. Using a Matlab script, stepping events were extracted, and these events had associated start times, durations, numbers of steps and average cadences recorded against them [20]. For each PB position (sedentary, standing, stepping and upright), the average time spent in each position during the testing period of 7–8 days at all assessment time points was calculated for each participant to detect changes in PB volume post-TKA. Similarly, the average number of steps during the testing period (7–8 days) at all time points was calculated. Then, the calculated average for the time spent in each position and the number of steps per day at the three assessment time points for each participant were imported into SPSS for further analysis.
To explore overall changes in cadence post-TKA, the total time spent in cadence bands of less than 60 steps/minute, slow to medium physical activity (SMPA) (≥60 and ≤100 steps/minute) and moderate to vigorous physical activity (MVPA) (>100 steps/minute) each week prior to TKA, and 6 and 12 months post-TKA were explored in all stepping event lengths for each participant [31]. In addition, total MVPA time was explored for events ≥10 continuous minutes, events ≥5 continuous minutes and events ≥ one continuous minute to assess sedentary time interruption [20,32].
2.6.3. Data analysis
A one-way repeated-measures analysis of variance (ANOVA) was used to assess the changes in time spent in sedentary, standing, upright and stepping states, the number of steps and overall cadence before and 6 and 12 months post-TKA, as the data were normally distributed, as assessed by a Kolmogorov-Smirnov test, and the ANOVA assumptions were met. As no prior hypothesis existed regarding which post-TKA time might affect PA, post hoc testing was used to explore potential significances [33].
A Friedman test was additionally used as a non-parametric alternative test to the one-way repeated-measures ANOVA, because the assumption of normality distribution was violated for MVPA and OKS score, as assessed by a Kolmogorov-Smirnov test (p = 0.001 and p = 0.007 respectively). Pairwise comparisons were performed with a Bonferroni correction for multiple comparisons to minimise the risk of Type I error, with the original alpha in the current study set at 0.05, and for three comparisons, the adjusted p-value required for significance would be 0.05/3 = 0.0167 [33]. Before and 12 months post-TKA, OKS score correlation was explored with PB before and after TKA (sedentary, standing, stepping, upright time and steps number), which resulted in 20 correlations.
PA at 12 months post-TKA was compared to PA adult guidelines that recommend a minimum of 150 min of MVPA per week in bouts lasting for 10 continuous minutes or more to maintain health [34]. The accumulated time spent in MVPA was assessed in bout lengths of less than 10 continuous minutes to explore sedentary time interruption changes post-TKA.
3. Results
Twenty-seven females and six male patients (mean ages of 59 ± 6 years (range, 49–76) and 76 ± 7 years (range, 63–85) years, respectively) completed the study. The BMI means were 37.21 ± 7.65 for females and 32.38 ± 2.01 for males. All patients wore an activPAL during each assessment period for a full 24 h for a minimum of 7 consecutive days without difficulty or any adverse reactions.
3.1. Physical activity volume changes
The mean times spent for all outcomes per day at each time point are shown in Table 1. The stepping times and step numbers were significantly different at 6 months post-TKA (F (2,64) = 57.78, p = 0.0001) and 12 months post-TKA (F (1.47,47.08) = 260.63, p = 0.0001). The effect sizes for stepping time and steps (partial η2) were large, at 0.7 and 0.9, respectively (Table 1).
Table 1.
Physical behaviour volume descriptive statistics and between-subject effects for one-way repeated ANOVA.
| Activity | Time Points | Mean | Standard Deviation | Standard Error of the Mean | 95% Confidence Interval |
F | P | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Sedentary Time (Hour) | Pre-Total Knee Arthroplasty | 19.48 | 1.51 | 0.26 | 18.94 | 20.01 | .940 | .396 |
| 6 months Post-Total Knee Arthroplasty | 19.27 | 1.66 | 0.29 | 18.68 | 19.86 | |||
| 12 months Post-Total Knee Arthroplasty | 19.08 | 1.54 | 0.27 | 18.53 | 19.62 | |||
| Standing Time (Hour) | Pre-Total Knee Arthroplasty | 3.47 | 1.27 | 0.22 | 3.02 | 3.92 | .216 | .807 |
| 6 months Post-Total Knee Arthroplasty | 3.64 | 1.44 | 0.25 | 3.13 | 4.15 | |||
| 12 months Post-Total Knee Arthroplasty | 3.54 | 0.97 | 0.17 | 3.20 | 3.89 | |||
| Upright Time (Hour) | Pre-Total Knee Arthroplasty | 4.48 | 1.45 | 0.25 | 3.96 | 4.99 | 1.08 | .343 |
| 6 months Post-Total Knee Arthroplasty | 4.73 | 1.67 | 0.29 | 4.14 | 5.32 | |||
| 12 months Post-Total Knee Arthroplasty | 4.88 | 1.47 | 0.26 | 4.36 | 5.41 | |||
| Stepping Time (Hour) | Pre-Total Knee Arthroplasty | .98 | .43 | .075 | .33 | 1.95 | 57.78 | .0001 |
| 6 months Post-Total Knee Arthroplasty | 1.17 | .42 | .074 | .56 | 2.11 | |||
| 12 months Post-Total Knee Arthroplasty | 1.36 | .38 | .67 | .68 | 2.18 | |||
| Steps Number | Pre-Total Knee Arthroplasty | 4240 | 2268 | 394 | 3435 | 5044 | 260.6 | .0001 |
| 6 months Post-Total Knee Arthroplasty | 4853 | 2108 | 367 | 4105 | 5601 | |||
| 12 months Post-Total Knee Arthroplasty | 6174 | 2287 | 398 | 5363 | 6985 | |||
The mean of daily stepping time increased from pre-TKA by 11.48 ± 2.05 (95% CI, 6 to 16) minutes/day at 6 months post-TKA, by 22.66 ± 2.24 (95% CI, 16 to 28) minutes/day at 12 months post-TKA, and by 11.2 ± 2.03 (95% CI, 6 to 16) minutes/day from 6 months post-TKA to 12 months post-TKA (Table 2).
Table 2.
Multiple comparisons of post hoc tests for stepping time and steps.
| Physical activity | Time (I) | Time (J) | Mean Difference (I-J) | Standard Error | Significant | 95% Confidence Interval for Difference |
|
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Stepping time | Pre-TKA | 6 months post-TKA | −11.48 | 2.04 | .0001 | −16.64 | −6.31 |
| 12 months post-TKA | −22.66 | 2.24 | .0001 | −28.32 | −16.99 | ||
| 6 months post-TKA | Pre-TKA | 11.48 | 2.04 | .0001 | 6.31 | 16.64 | |
| 12 months post-TKA | −11.18 | 2.03 | .0001 | −16.32 | −6.05 | ||
| 12 months post-TKA | Pre-TKA | 22.66 | 2.24 | .0001 | 16.99 | 28.32 | |
| 6 months post-TKA | 11.18 | 2.03 | .0001 | 6.05 | 16.32 | ||
| Steps Number | Pre-TKA | 6 months post-TKA | −613 | 89.63 | .0001 | −839 | −386 |
| 12 months post-TKA | −1934 | 105.35 | .0001 | −2200 | −1668 | ||
| 6 months post-TKA | Pre-TKA | 613 | 89.63 | .0001 | 386 | 839 | |
| 12 months post-TKA | −1321 | 58.02 | .0001 | −1467 | −1174 | ||
| 12 months post-TKA | Pre-TKA | 1934 | 105.35 | .00011 | 1668 | 2200 | |
| 6 months post-TKA | 1321 | 58.02 | .000 | 1174 | 1467 | ||
The daily steps number mean increased at 6 months post-TKA by 613 ± 89 (95% CI, 386 to 839) steps/day, at 12 months post-TKA by 1934 ± 105 (95% CI, 1668 to 2200) steps/day, and between 6 months post-TKA and 12 months post-TKA by 1321 ± 58 (95% CI, 1174 to 1467) steps/day (Table 2).
There were no significant changes in mean time for sedentary (F (2,64) = 0.940, p = 0.396), standing (F (2,64) = 0.216, p = 0.807) and upright times (F (2,64) = 1.088, p = 0.343) at all time points post-TKA (Table 1).
3.2. Physical activity pattern changes
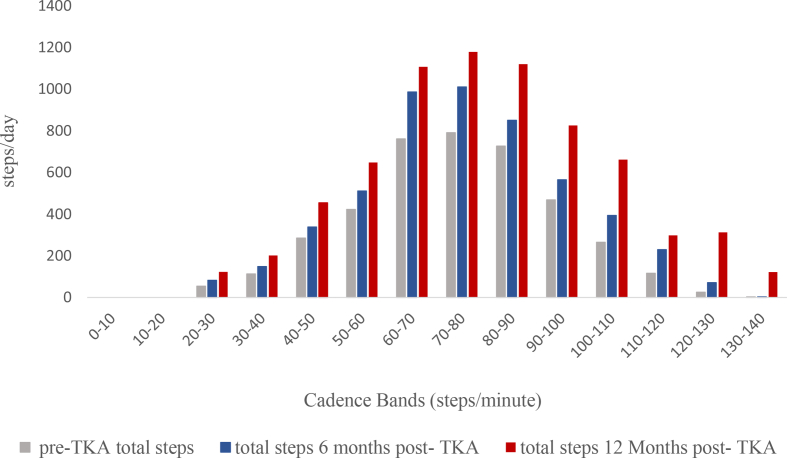
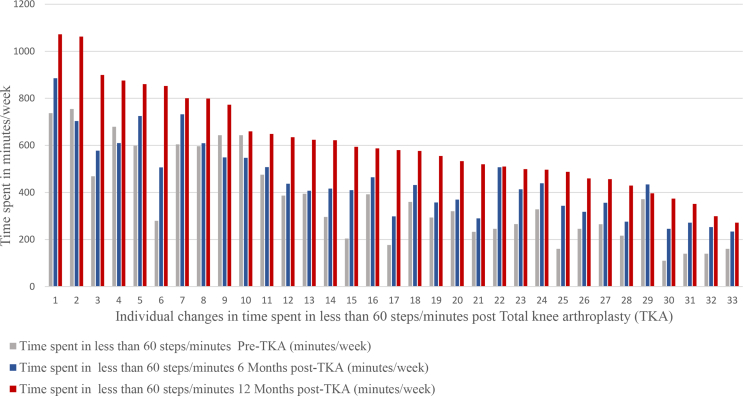
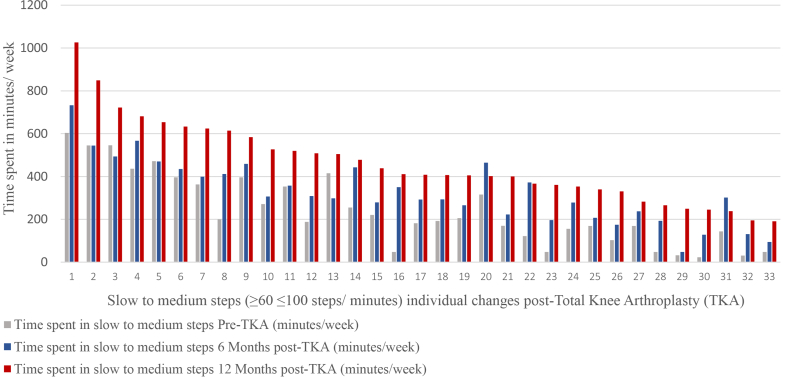
The averages of times spent in all cadence bands post-TKA are shown in Fig. 3. The time spent in less than 60 steps/minute per week significantly increased at all time points post-TKA (F (2,64) = 117.8, p = 0.0001) with a large effect size (partial η2), 0.8. The mean time spent in a cadence of less than 60 steps/minute increased from pre-TKA by: 89 ± 15 (95% CI, 49 to 129) minutes/week at 6 months post-TKA; by 224 ± 21 (95% CI, 169 to 279) minutes/week at 12 months post-TKA; and by 135 ± 20 (95% CI, 81 to 187) minutes/week from 6 months post-TKA to 12 months post-TKA (Table 3). Fig. 4 shows the individual changes in time spent in cadence bands less than 60 steps/minute per week at all assessment time points.
Fig. 3.
Cadence Band changes post-Total knee arthroplasty.
Table 3.
Physical activity pattern descriptive statistics and between-subject effects for one-way repeated ANOVA (less than 100 steps/minutes) and a Friedman test (>100 steps/minutes).
| Pattern | Time Points | Mean/median | Standard Deviation | Standard Error of the Mean | 95% Confidence Interval |
F | P | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| <60 steps/min | Pre-Total Knee Arthroplasty | 369 | 189 | 33.07 | 301 | 436 | 117.8 | .0001 |
| 6 months Post-Total Knee Arthroplasty | 458 | 161 | 28.13 | 401 | 516 | |||
| 12 months Post-Total Knee Arthroplasty | 593 | 188 | 32.76 | 526 | 660 | |||
| Slow to medium steps (≥60 and ≤100 steps/min) | Pre-Total Knee Arthroplasty | 232 | 148 | 25.79 | 180 | 285 | 65.6 | .0001 |
| 6 months Post-Total Knee Arthroplasty | 326 | 147 | 25.62 | 274 | 379 | |||
| 12 months Post-Total Knee Arthroplasty | 457 | 179 | 31.26 | 393 | 520 | |||
| Moderate to vigorous (>100 steps/min) | Pre-Total Knee Arthroplasty | 6.6 | ∗ | ∗ | .21 | 49 | 18.72 | .0001 |
| 6 months Post-Total Knee Arthroplasty | 10.5 | .15 | 114 | |||||
| 12 months Post-Total Knee Arthroplasty | 41.7 | .28 | 379 | |||||
∗A Friedman test was used as a non-parametric test alternative to the one-way repeated measure ANOVA because the assumption of normality distribution was violated for moderate to vigorous physical activity (MVPA).
Fig. 4.
Time spent in less than 60 steps/minute individual changes post-Total knee arthroplasty.
The time spent in SMPA per week significantly increased at all time points post-TKA (F (2,64) = 65.6, p = 0.0001), with a large effect size (partial η2), 0.9. The time spent in SMPA increased by 94 ± 14 (95% CI, 58 to 129) minutes/week at 6 months post-TKA; by 225 ± 15 (95% CI, 184 to 263) minutes/week at 12 months post-TKA; and by 131 ± 14 (95% CI, 94 to 166) minutes/week from 6 months post-TKA to 12 months post-TKA (Table 3). Fig. 5 shows individual changes in time spent in SMPA per week at all assessment time points.
Fig. 5.
Slow to medium steps (≥60 < 100 steps/minute) individual changes post-Total knee arthroplasty.
The time spent in MVPA (>100 steps/minute) per week was significantly different at 6 months post-TKA and 12 months post-TKA (χ2 (2) = 18.727. p = 0.0001). The MVPA median time increased by 3.9 min/week at 6 months post-TKA, and by 35 min/week at 12 months post-TKA (Table 3). MVPA values were significantly different between pre-TKA and 12 months post-TKA (p = 0.0001) and between 6 months and 12 months post-TKA (p = 0.003). No significant differences were detected between baseline and 6 months post-TKA (p = 0.793).
3.3. Physical activity correlation with OKS
OKS significantly improved at different time points post-TKA: (χ2 (2) = 232), (χ2 (2) = 209.6) and (χ2 (2) = 221.49), with p = 0.0005, respectively. OKS median changes were from 14 (pre-TKA) to 34 (6 months post-TKA) and 40 (12 months post-TKA). However, there was no significant correlation between OKS and PB (Table 4).
Table 4.
Oxford knee score (OKS) correlation with physical behaviour before and after Total knee arthroplasty (TKA).
| Correlation pairs | Oxford knee score before Total knee arthroplasty (TKA) |
Oxford knee score 12 months post - Total knee arthroplasty (TKA) |
||
|---|---|---|---|---|
| Pearson Correlation | Significant | Pearson Correlation | Significant | |
| Pre-TKA sedentary time | .030 | .867 | .235 | .189 |
| Pre-TKA standing time | .063 | .727 | .228 | .203 |
| Pre-TKA stepping time | .292 | .099 | .183 | .309 |
| Pre-TKA upright time | .046 | .797 | .220 | .219 |
| Pre-TKA steps number | .221 | .216 | .138 | .443 |
| Post-TKA sedentary time | .028 | .875 | .231 | .195 |
| Post-TKA standing time | .118 | .515 | .240 | .179 |
| Post-TKA stepping time | .232 | .193 | .211 | .239 |
| Post-TKA upright time | .001 | .993 | .236 | .186 |
| Post-TKA 12 steps number | .191 | .286 | .210 | .241 |
3.4. Physical activity post-TKA compared to global PA guidelines
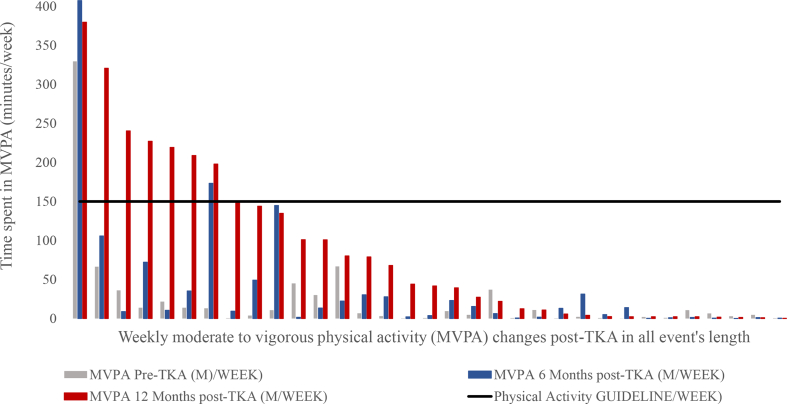
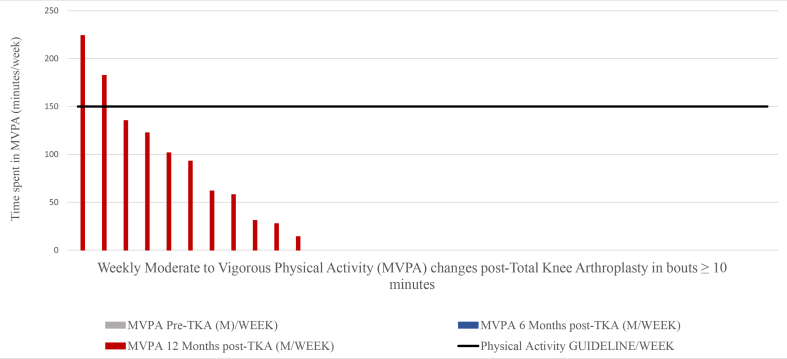
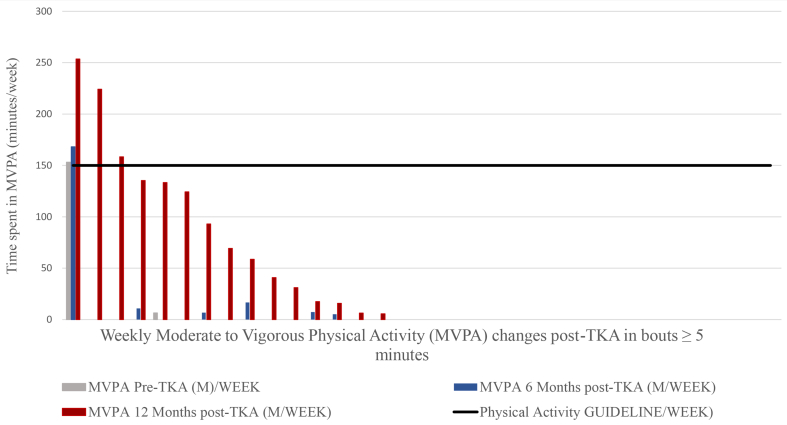
MVPA performance was compared to health-enhancing PA guideline recommendations and the majority of patients did not meet the recommendations 12 months post-TKA. Only 24% of the participants (8 patients) met the recommendation of 150 min of MVPA per week for all stepping event lengths (Fig. 6); 6% of the participants (2 patients) met the recommendation of 150 min of MVPA per week for walking events greater than10 min (Fig. 7); 9% of the participants (3 patients) met the recommendation of 150 min of MVPA per week for events greater than 5 min (Fig. 8); 12% of the participants (4 patients) met the recommendation of 150 min of MVPA per week for events greater than 1 min.
Fig. 6.
Moderate to vigorous physical activity (MVPA) per week in all stepping events length changes post-Total knee arthroplasty.
Fig. 7.
Moderate to vigorous physical activity (MVPA) per week in stepping events length ≥10 min changes post-Total knee arthroplasty.
Fig. 8.
Moderate to vigorous physical activity (MVPA) per week in stepping events length ≥ 5 min changes post-Total knee arthroplasty.
4. Discussion
This study is the first to explore PB changes post-TKA and it demonstrates that PB 12 months post-TKA improved significantly in terms of volume and pattern. The current study's steps performance improved by 1934 steps per day at 12 months post-TKA; this is an improvement of 45.6%, which is higher than in the only previous study by Lutzner Dipl-Pad et al. [12], who reported a 22.6% improvement. These daily step number improvements result in approximately 2.3 million steps annually (SD 834,755 steps).
The previous study assessed PA post-TKA [12], though this study did have a major methodological limitation, as the ActivPAL monitor was applied to the anterolateral aspect of the tibia (a position not in compliance with recommendations and a position not previously validated). In addition, they only recorded for 4–6 days, which may not be sufficient to capture actual PB performance, including weekends and working days. Other studies have measured the numbers of steps post-TKA, but their methods too did not meet the recommendations, such as measurements on one day; using a pedometer, which is considered inaccurate for detecting the number of steps; or not measuring steps before surgery to detect improvements [[35], [36], [37]]. In terms of stepping time, the current study found an increase of 22 min per day at 12 months post-TKA, whereas Lutzner Dipl-Pad et al. [12] showed no significant change in stepping time, which is surprising in light of their demonstrated increase in steps.
Many previous studies concluded that PB post-TKA remained at or below pre-surgery level, which may be due to many confounding factors that affected the accuracy of their conclusions. Those studies' conclusions were based on PB patients’ reported outcome measures (questionnaire) or using energy expenditure as a measure of PB. A few studies rely on questionnaires, so the risk of subjective under/overestimation and recall bias cannot be excluded [[38], [39], [40]]. In this study, OKS scores improved significantly at each time point post-TKA, although these did not correlate with PB changes. The recovery profiles vary for each outcome measure; such discrepancies between PB and OKS show the importance of using both subjective and objective methods to assess outcomes post-TKA. OKS average score was 40, which indicates satisfactory joint function; however, only 6% of the participants met the recommendation of 150 min of MPVA per week in bouts ≥10 continuous minutes to promote and maintain health. Further education may encourage and motivate patients to improve their PA post-TKA, as pain and functional ability improve post-TKA. A future study could explore the effectiveness of behavioural treatments to change sedentary behaviour post-TKA, achieve the global PA recommendations and minimise the risk of a sedentary lifestyle.
A major change detected by the present study was a 583% increase in the time spent on MVPA at 12 months post-TKA, it was 6 min per week at the baseline and this increased to 41 min at 12 months post-TKA. MVPA at 6 months post-TKA showed a 75% improvement, from 6 min to 10.5 min. In fact, 41 min of MVPA per week is less than for symptomatic KOA (1–24 min/day) and the general US population of a similar age (1–22 min/day) [37]. This may be due to a different measurement methodology being used, and their conclusion being based on an ActiGraph uniaxial accelerometer. ActiGraph is an energy expenditure accelerometer that inaccurately assesses energy expenditure based on cut-off points and thresholds to classify PA intensity based on many regression equations rather than accurate postural detection [11,13,14]. For example, an ActiGraph worn at waist level records minimal acceleration, while standing quietly in a similar way to sitting and overestimation of low-level activity and underestimation of vigorous activity cannot be excluded [20].
One novel feature of this study was the ability to look at how MVPA steps accumulated, considering both MVPA stepping time and sedentary time interruption according to new public health recommendations [32]. ActivPAL precisely estimated the time spent in each stepping intensity (start and end time in seconds), therefore stepping event lengths were assessed accurately. The current study found that 6% of the participants met the recommendation of 150 min of MVPA per week for events ≥10 continuous minutes. Furthermore, 24% of participants accumulated 150 min per week of MVPA for all event lengths, this indicates more interruptions to sedentary time. According to an Australian cohort study, regular interruptions to sedentary time have positive health benefits, such as: improving the profile of triglyceride and plasma glucose, in addition to reducing BMI and waist circumference [32]. Prolonged sedentary time is associated with reductions in blood flow, pulmonary oxygen uptake and fat metabolism, which increases the risk of chronic diseases such as diabetes mellitus and cardiovascular disease [41]. Therefore, to understand the full picture of PB changes post-TKA, it is essential to measure the time spent in MVPA, sedentary time and sedentary time interruption.
Our findings' implication for clinical practice is that arthroplasty significantly decreases pain, although, in isolation, it may not adequately improve patients’ PA, so further interventions are required. Effective strategies are essential to modify a sedentary lifestyle and increase PA performance post-TKA. Behavioural treatments and further education pre/post-TKA may improve motivation and minimise the risk of a sedentary lifestyle.
The study has several strengths points regarding sampling, such as: although the sample size was small, it may reflect the general population, as it included both genders and a wide age group and did not have significant differences from a large sample invited in terms of age, gender and BMI. In addition, recruitment was from a large teaching hospital with wider eligibility for different patient populations, other than hospital settings. In terms of measurements of strength, PA was estimated based on an average of a minimum of 7 days to include variations on weekdays and at weekends. The PA measurement timeline was standarised for all time points pre/postTKA to eliminate constant error.
The study has several limitations, such as: sleeping time was included in sedentary time, which may affect the accuracy of sedentary time changes, as sleeping may improve as pain reduces post-TKA. The 12 months follow-up may not reflect maximum functional recovery post-TKA, so a longer follow-up is recommended. The current study showed no correlation with OKS, a future study is recommended to assess the correlation with other activity scores, such as the University of California Los Angeles (UCLA) activity scale, Tegner score and the Physical Activity Scale for the Elderly (PASE).
5. Conclusion
PA improved in terms of stepping, step numbers and patterns. OKS did not reflect the magnitude of the functional improvements post-TKA and there were no significant correlations with ActivPAL as an objective method. This emphasises the need to use objective methods in addition to PROMs, which merely track subjective improvements and may be influenced by recall bias.
Contributions
BB, MG, and RJ contributed to the design of the study, the analysis and interpretation of data. BB wrote the manuscript. MG & BB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AW and DJ contributed to the interpretation of data and participated substantially in the reviewing and editing of the manuscript before submission. All the authors were involved in drafting the article or revising it critically for important intellectual content, and they all approved the final version to be published.
Declaration of Competing Interest
The author has no financial or personal relationships with other people or organisations that could inappropriately influence (bias) this work.
Acknowledgements
Many thanks to the University of Salford Manchester and Princess Nourah bint Abdul Rahman University for funding this study, and to all the orthopaedics department surgeons and nursing team at King Khaled University Hospital in Riyadh for their help in the recruitment process.
Contributor Information
Bodor Bin sheeha, Email: bhbinsheeha@pnu.edu.sa.
Malcolm Granat, Email: m.h.granat@salford.ac.uk.
Anita Williams, Email: a.e.williams1@salford.ac.uk.
David Sands Johnson, Email: david.johnson@stockport.nhs.uk.
Richard Jones, Email: r.k.jones@salford.ac.uk.
References
- 1.Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2.Hawker G.A., Croxford R., Bierman A.S., Harvey P.J., Ravi B., Stanaitis I., et al. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PloS One. 2014;9(3) doi: 10.1371/journal.pone.0091286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J Intern Med. 2011;2(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- 4.Carr A.J., Robertsson O., Graves S., Price A.J., Arden N.K., Judge A., et al. Knee replacement. Lancet. 2012;379(9823):1331–1340. doi: 10.1016/S0140-6736(11)60752-6. [DOI] [PubMed] [Google Scholar]
- 5.Tambascia R.A., Vasconcelos R.A., Mello W., Teixeira P.P., Grossi D.B. Pre-operative functional parameters of patients undergoing total knee arthroplasty. Physiother. Res. Int. 2016;21(2):77–83. doi: 10.1002/pri.1622. [DOI] [PubMed] [Google Scholar]
- 6.Mizner R.L., Petterson S.C., Clements K.E., Zeni J.A., Jr., Irrgang J.J., Snyder-Mackler L. Measuring functional improvement after total knee arthroplasty requires both performance-based and patient-report assessments: a longitudinal analysis of outcomes. J. Arthroplasty. 2011;26(5):728–737. doi: 10.1016/j.arth.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busschaert C., De Bourdeaudhuij I., Van Holle V., Chastin S.F., Cardon G., De Cocker K. Reliability and validity of three questionnaires measuring context-specific sedentary behaviour and associated correlates in adolescents, adults and older adults. Int. J. Behav. Nutr. Phys. Activ. 2015;12:117. doi: 10.1186/s12966-015-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chastin S.F., Culhane B., Dall P.M. Comparison of self-reported measure of sitting time (IPAQ) with objective measurement (activPAL) Physiol. Meas. 2014;35(11):2319–2328. doi: 10.1088/0967-3334/35/11/2319. [DOI] [PubMed] [Google Scholar]
- 9.Healy G.N., Clark B.K., Winkler E.A., Gardiner P.A., Brown W.J., Matthews C.E. Measurement of adults' sedentary time in population-based studies. Am. J. Prev. Med. 2011;41(2):216–227. doi: 10.1016/j.amepre.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luna I.E., Kehlet H., Peterson B., Wede H.R., Hoevsgaard S.J., Aasvang E.K. Early patient-reported outcomes versus objective function after total hip and knee arthroplasty: a prospective cohort study. Bone Joint Lett. J. 2017;99-b(9):1167–1175. doi: 10.1302/0301-620x.99b9.bjj-2016-1343.r1. [DOI] [PubMed] [Google Scholar]
- 11.Meiring R.M., Frimpong E., Mokete L., Pietrzak J., Van Der Jagt D., Tikly M., et al. Rationale, design and protocol of a longitudinal study assessing the effect of total knee arthroplasty on habitual physical activity and sedentary behavior in adults with osteoarthritis. BMC Muscoskel. Disord. 2016;17:281. doi: 10.1186/s12891-016-1141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutzner C., Kirschner S., Lutzner J. Patient activity after TKA depends on patient-specific parameters. Clin. Orthop. Relat. Res. 2014;472(12):3933–3940. doi: 10.1007/s11999-014-3813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding P., Holland A.E., Delany C., Hinman R.S. Do activity levels increase after total hip and knee arthroplasty? Clin. Orthop. Relat. Res. 2014;472(5):1502–1511. doi: 10.1007/s11999-013-3427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M., Osman K., Green G., Haddad F.S. The epidemiology of failure in total knee arthroplasty: avoiding your next revision. Bone Joint Lett. J. 2016;98-b(1 Suppl A):105–112. doi: 10.1302/0301-620x.98b1.36293. [DOI] [PubMed] [Google Scholar]
- 15.Wimmer M.A., Nechtow W., Schwenke T., Moisio K.C. Knee flexion and daily activities in patients following total knee replacement: a comparison with ISO standard 14243. BioMed Res. Int. 2015;2015:157541. doi: 10.1155/2015/157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vissers M.M., Bussmann J.B., de Groot I.B., Verhaar J.A., Reijman M. Physical functioning four years after total hip and knee arthroplasty. Gait Posture. 2013;38(2):310–315. doi: 10.1016/j.gaitpost.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Arnold J.B., Walters J.L., Ferrar K.E. Does physical activity increase after total hip or knee arthroplasty for osteoarthritis? A systematic review. J. Orthop. Sports Phys. Ther. 2016;46(6):431–442. doi: 10.2519/jospt.2016.6449. [DOI] [PubMed] [Google Scholar]
- 18.Hammett T., Simonian A., Austin M., Butler R., Allen K.D., Ledbetter L., et al. Changes in physical activity after total hip or knee arthroplasty: a systematic review and meta-analysis of six- and twelve-month outcomes. Arthritis Care Res. 2018;70(6):892–901. doi: 10.1002/acr.23415. [DOI] [PubMed] [Google Scholar]
- 19.Paxton R.J., Melanson E.L., Stevens-Lapsley J.E., Christiansen C.L. Physical activity after total knee arthroplasty: a critical review. World J. Orthoped. 2015;6(8):614–622. doi: 10.5312/wjo.v6.i8.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granat M.H. Event-based analysis of free-living behaviour. Physiol. Meas. 2012;33(11):1785–1800. doi: 10.1088/0967-3334/33/11/1785. [DOI] [PubMed] [Google Scholar]
- 21.Edwardson C.L., Winkler E.A.H., Bodicoat D.H., Yates T., Davies M.J., Dunstan D.W., et al. Considerations when using the activPAL monitor in field-based research with adult populations. Journal of Sport and Health Science. 2016;May:1–17. doi: 10.1016/j.jshs.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyden K., Keadle S.K., Staudenmayer J., Freedson P.S. The activPALTM accurately classifies activity intensity categories in healthy adults. Med. Sci. Sports Exerc. 2017;49(5):1022–1028. doi: 10.1249/mss.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahlgren G., Carlsson D., Moorhead A., Hager-Ross C., McDonough S.M. Test-retest reliability of step counts with the ActivPAL device in common daily activities. Gait Posture. 2010;32(3):386–390. doi: 10.1016/j.gaitpost.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Grant P., Ryan C., Tigbe W., Granat M. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br. J. Sports Med. 2006;40(12):992–997. doi: 10.1136/bjsm.2006.030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerejo R., Dunlop D., Cahue S., Channin D., Song J., Sharma L. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46(10):2632–2636. doi: 10.1002/art.10530. [DOI] [PubMed] [Google Scholar]
- 26.Naing N.N. Determination of sample size. Malays. J. Med. Sci. 2003;10(2):84–86. [PMC free article] [PubMed] [Google Scholar]
- 27.Bin Sheeha B., Williams A., Johnson D., Bin Nasser A., Granat M., Jones R. A validation study of the Arabic version of the Oxford knee score for use in end stage knee osteoarthritis. Osteoarthritis Cartilage. 2018;26:S261. doi: 10.1016/j.joca.2018.02.533. [DOI] [Google Scholar]
- 28.World Health Organization Global strategy on diet, physical activity & health. https://www.who.int/dietphysicalactivity/factsheet_adults/en/
- 29.Schmalzried T.P., Szuszczewicz E.S., Northfield M.R., Akizuki K.H., Frankel R.E., Belcher G., et al. Quantitative assessment of walking activity after total hip or knee replacement. J Bone Joint Surg Am. 1998;80(1):54–59. [PubMed] [Google Scholar]
- 30.Tugay B.U., Tugay N., Guney H., Kinikli G.I., Yuksel I., Atilla B. Oxford knee score: cross-cultural adaptation and validation of the Turkish version in patients with osteoarthritis of the knee. Acta Orthop. Traumatol. Turcica. 2016;50(2):198–206. doi: 10.3944/aott.2015.15.0127. [DOI] [PubMed] [Google Scholar]
- 31.Tudor-Locke C., Han H., Aguiar E.J., Barreira T.V., Schuna J.M., Jr., Kang M., et al. How fast is fast enough? Walking cadence (steps/min) as a practical estimate of intensity in adults: a narrative review. Br. J. Sports Med. 2018;52(12):776–788. doi: 10.1136/bjsports-2017-097628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Healy G.N., Dunstan D.W., Salmon J., Cerin E., Shaw J.E., Zimmet P.Z., et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 33.Field A. fifth ed. SAGE Publications Ltd; 2018. Discovering Statistics Using SPSS. [Google Scholar]
- 34.Haskell W.L., Lee I.M., Pate R.R., Powell K.E., Blair S.N., Franklin B.A., et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 35.Tsonga T., Kapetanakis S., Papadopoulos C., Papathanasiou J., Mourgias N., Georgiou N., et al. Evaluation of improvement in quality of life and physical activity after total knee arthroplasty in Greek elderly women. Open Orthop. J. 2011;5:343–347. doi: 10.2174/1874325001105010343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker D.J., Heslop P.S., Chandler C., Pinder I.M. Measured ambulation and self-reported health status following total joint replacement for the osteoarthritic knee. Rheumatology. 2002;41(7):755–758. doi: 10.1093/rheumatology/41.7.755. [DOI] [PubMed] [Google Scholar]
- 37.Thoma L.M., Dunlop D., Song J., Lee J., Tudor-Locke C., Aguiar E.J., et al. Are older adults with symptomatic knee osteoarthritis less active than the general population? Analysis from the osteoarthritis initiative and the national health and nutrition examination survey. Arthritis Care Res. 2018;70(10):1448–1454. doi: 10.1002/acr.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang M.J., Kim S.H., Kang Y.G., Chang C.B., Kim T.K. Activity levels and participation in physical activities by Korean patients following total knee arthroplasty. BMC Muscoskel. Disord. 2014;15:240. doi: 10.1186/1471-2474-15-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kersten R.F., Stevens M., van Raay J.J., Bulstra S.K., van den Akker-Scheek I. Habitual physical activity after total knee replacement. Phys. Ther. 2012;92(9):1109–1116. doi: 10.2522/ptj.20110273. [DOI] [PubMed] [Google Scholar]
- 40.Smith T.O., Mansfield M., Dainty J., Hilton G., Mann C.J.V., Sackley C.M. Does physical activity change following hip and knee replacement? Matched case-control study evaluating Physical Activity Scale for the Elderly data from the Osteoarthritis Initiative. Physiotherapy. 2018;104(1):80–90. doi: 10.1016/j.physio.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Vasankari V., Husu P., Vaha-Ypya H., Suni J., Tokola K., Halonen J., et al. Association of objectively measured sedentary behaviour and physical activity with cardiovascular disease risk. Eur J Prev Cardiol. 2017;24(12):1311–1318. doi: 10.1177/2047487317711048. [DOI] [PubMed] [Google Scholar]