Abstract
Objective
To elucidate the possible role of MRI-detected osteophytes as a predictive imaging biomarker for knee osteoarthritis (KOA).
Design
Subjects (n = 303) were selected according to the following inclusion criteria from the Osteoarthritis Initiative (OAI) data set: (1) < 55 years old; (2) Western Ontario and McMaster Universities Arthritis Index pain score of 0; (3) Kellgren-Lawrence (KL) system grade 0 or 1; and (4) Complete MRI data set of the right knee. A pre-OA group (POA) consisted of subjects who developed KL grade 2 or more within 96 months, and a non-OA group (NOA) that remained KL 0 or 1 during that period. Baseline MRIs were assessed for osteophyte formation. Twenty-five locations were examined according to the MOAKS osteophyte score. Osteophytes at each location were assessed in terms of their predictive value for OA development.
Results
Thirty-two subjects were POA and 271 were NOA. Age, BMI, and sex did not differ between the two groups. In the POA group, the number of subjects with osteophytes tended to be higher at all 25 sites. Forward stepwise regression analysis revealed five locations - medial patella, lateral intra-condylar notch of the femur, lateral femoral condyle, tibial spine, and lateral posterior condyle - were important for the prediction of KOA development. Having more than two osteophytes at these five locations predicted KOA development with a sensitivity of 0.75 and specificity of 0.79.
Conclusions
MRI-detected osteophytes could serve as a predictive biomarker of KOA development within 96 months after detection.
Keywords: Osteoarthritis of the knee, Osteophytes, Osteoarthritis initiative, Imaging biomarker, Predictive biomarker, MRI
1. Introduction
Osteoarthritis of the knee joint (KOA) is one of the most commonly encountered diseases that impairs the quality of life (QOL) in the elderly population [1]. It also affects the quality-adjusted life-year [2]. Thus the prevention of disease development or slowing of disease progression is important but has not been achieved yet, in part because of the difficulty in predicting who will progress to KOA. One solution for this would be to establish effective biomarkers to predict KOA. MRI could be used for this as it can depict pathological changes in every tissue of the knee joint without being affected by OA changes in other joints, as are systemic biomarkers. The quality and quantity of hyaline cartilage, bone marrow lesions (BMLs), and meniscal extrusion have been reported to be potential parameters detected with MRI that predict KOA development [3]. Osteophyte formation is a typical imaging feature of OA. An increase in the number and size of osteophytes over time might be a reasonable biomarker to predict the development and progression of KOA. In our previous study we demonstrated that osteophyte formation at several sites could serve as a predictive biomarker for OA development within four years using MRI data from the Osteoarthritis Initiative (OAI) [4]. In that study eight specific sites were assessed for osteophyte formation but there are other potential locations. In this study we again employed OAI data to more thoroughly examine osteophyte formation by increasing the number of locations examined in the knee joint to 25 and determined whether detecting osteophytes at specific sites could serve as a better predictive biomarker of KOA development in individuals. For this purpose, we thought younger and asymptomatic participants were ideal as a baseline cohort because the incidence of OA increases with aging. Population based studies reveal that one out of four people have already developed radiographic knee OA at age 60 or older [5]. Jordan et al. reported from the Johnston County osteoarthritis project that radiographic knee OA almost doubled when compared between ages 45–54 (13.3 %) and 55–64 (24.2 %), including a 2.5-fold increase of symptomatic OA (7.3 % and 16.3 %) [6]. The value of establishing a predictive biomarker is to find knees at higher risk of developing knee OA in the near future. As participants in the OAI were ≥45 years old, we set an age less than 55 years as the inclusion criterion to create a baseline cohort composed of 45–54 years old. Then the relationship of osteophytes at baseline and KOA development within 96 months was examined. Middle-aged asymptomatic individuals who might be nearing KOA development are a proper target in which to study the risk of developing KOA.
2. Subjects and methods
2.1. Cohort
Data used in the present study were obtained from the Osteoarthritis Initiative (OAI). The OAI was developed to allow researchers to prospectively study the development and progression of knee osteoarthritis (KOA). It consists of several sets of data including clinical data, demographic characteristics of the participants, and imaging data related to the development of OA. The data from OAI version 0.E.1 (Entire cohort version 1), which includes 2110 subjects, was used for this study. We established the study cohort utilizing Kellgren-Lawrence (KL) grading provided by the OAI found in the “Xray outcome” data and the Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain score. As KL grading of OAI was determined by an AP x-ray projection, the characteristics of the cohort were determined by the status of the tibiofemoral joint and not by that of the patellofemoral joint.
Subjects selected met four inclusion criteria: (1) subjects < 55 years old; (2) Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain score of 0: i.e., all items on the pain questionnaire were zero; (3) KL system grade 0 or 1; and (4) Complete MRI data set of the right knee. A pre-OA group (POA) consisted of subjects who developed KL grade 2 or more radiographically in the right knee within 96 months, and a non-OA group (NOA) that remained KL 0 or 1 during that period.
2.2. MRI sequence
MRI images were obtained with a 3T MRI scanner (Siemens MAGNETOM Trio, Erlangen, Germany) and a quadrature transmit-receive knee coil (USA Instruments, Aurora, OH). The protocol used for MRI acquisition was reported in detail in the previous study [7]. Axial multiplanar reconstruction (MPR), coronal MPR, and sagittal T2 mapping were used for the assessment of osteophytes.
MPR was reformatted from SAG 3D DESS WE. The SAG 3D DESS WE series utilizes near anisotropic voxels (0.7 mm slice thickness × 0.37 mm × 0.46 mm) to maximize in-plane sagittal spatial resolution in a reasonable acquisition time (10.5 min). The resultant in-plane coronal MPR spatial resolution is thus 0.7 mm × 0.37 mm7. In addition, T2 mapping was generated from SAG 2D MESE with a slice thickness of 0.7 mm and 0.31 mm × 0.45 mm in-plane resolution (acquisition time 10.6 min) [7].
2.3. Image analysis
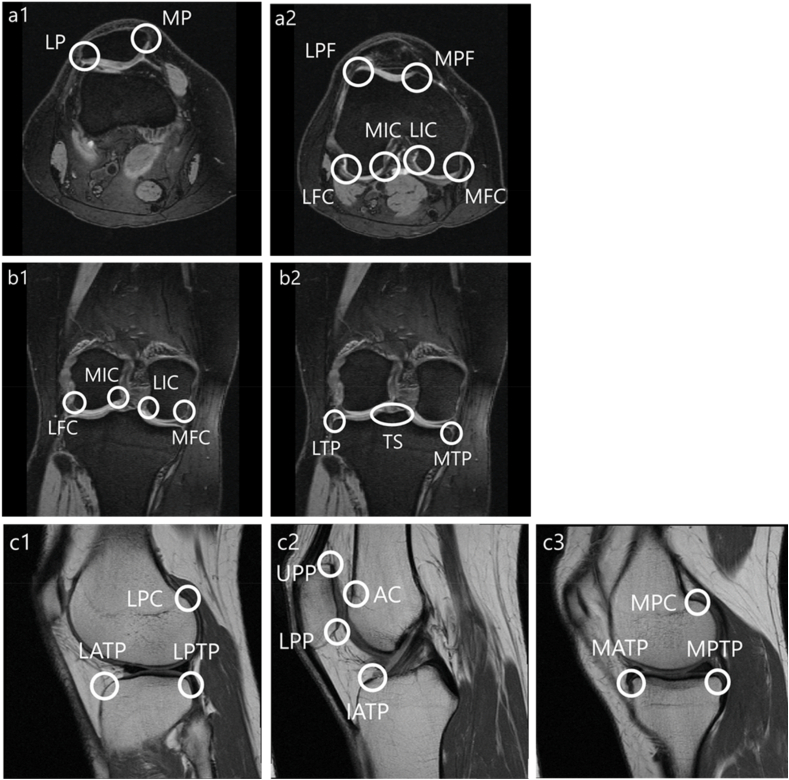
Osteophyte size was assessed employing a 0–3 scale from the MRI Osteoarthritis Knee Score (MOAKS) system. In MOAKS, the scoring of osteophytes reflects how far the osteophyte extends from the joint rather than the total volume of the osteophyte. The score was determined by the largest osteophyte within a given location and was defined by four grades (grade 0 = none, grade 1 = small, grade 2 = medium and grade 3 = large). Contrary to the original MOAKS, in which 12 locations were assessed, 25 sites were assessed in a slice specific manner (Fig. 1): eight sites from axial images (medial patella [MP], lateral patella [LP], medial facet of the patellofemoral joint [MPF], lateral facet of the patellofemoral joint [LPF], medial femoral condyle [MFC], lateral femoral condyle [LFC], medial intra-condylar notch of the femur [MIC], and lateral intra-condylar notch of the femur [LIC]); seven sites from coronal images (MFC, LFC, MIC, LIC, medial tibial plateau [MTP], lateral tibial plateau [LTP], and tibial spine [TS]); and eight sites from sagittal images (upper pole of the patella [UPP], lower pole of the patella [LPP], anterior femoral condyle [AC], lateral and medial posterior femoral condyle [LPC and MPC], lateral, medial and inter-condylar anterior tibial plateau [LTP and MTP, IATP], and the lateral and medial posterior tibial plateau [LPTP and MPTP]). A single examiner (RT) who had been trained in MRI readings of knee joints solely for the purpose of the present study determined the MOAKS grading of osteophytes. The examiner received 2 h of education weekly for six months with the experienced musculoskeletal radiologist (SW) until achieving reliable scoring. Another reader (OY) who has been engaged in musculoskeletal imaging analysis for 11 years served to examine inter-observer reliability.
Fig. 1.
Sites for osteophyte scoring.
2.4. Statistical analysis
The data were expressed as case numbers or means ± standard deviation (SD). Statistical comparisons of demographic data between NOA and POA groups were performed using a Chi-square test for categorical variables, and a Student's t-test for continuous variables. First, we evaluated the intra- and inter-observer reliability of osteophyte assessment. Two examiners (RT and OY) assessed osteophytes in 14 subjects at 25 sites using MOAKS osteophyte scoring. One of two examiners (RT) conducted a re-evaluation after four days. The weighted kappa coefficients with a 95 % confidence interval (CI) and percent agreement were calculated for intra- and inter-observer reliability. Second, we analyzed the results of osteophyte scoring of 25 sites in 303 subjects. The Youden index was used to determine the optimal cut-off values of each site for identification of knee osteoarthritis. The forward stepwise regression model analysis was performed to identify the independent variables associated with knee osteoarthritis (p ≤ 0.05 included and p > 0.05 removed). Sensitivity and specificity with 95%CI were calculated for each identified variable in the multivariate regression. In addition, the area under the curve (AUC) was calculated to assess the ability of each variable to discriminate selected locations. An AUC >0.9 was considered excellent; 0.8–0.9, very good; 0.7–0.8, good; 0.6–0.7, average; and <0.6, poor [8,9]. In this study, a single outcome that compares POA with OA was assessed. A two-sided test result of p < 0.05 was considered statistically significant, and all results were calculated using SAS version 9.4 for Windows (SAS Institute, Cary, NC, USA).
3. Results
3.1. Subjects
Among the 303 subjects who met the inclusion criteria, 32 subjects progressed to radiographic KOA (K/L grade ≧ 2) within 96 months (POA group). The other 271 subjects remained at K/L grades 0 or 1 (NOA group). There were no significant demographic differences between the groups at baseline (Table 1). In addition, many POA subjects developed KL grade 2 or more radiographically within 48–96 months (Table 2).
Table 1.
Subject characteristics.
| Variables | NOA (n = 271) | POA (n = 32) | P-value | ||||
|---|---|---|---|---|---|---|---|
| Age | 49.92 | ± | 2.72 | 50.13 | ± | 2.50 | 0.69∗ |
| Height, cm | 171.21 | ± | 10.14 | 169.70 | ± | 8.41 | 0.31∗ |
| Weight, kg | 77.43 | ± | 15.83 | 79.77 | ± | 14.57 | 0.43∗ |
| BMI, kg/m2 | 26.28 | ± | 4.30 | 27.68 | ± | 4.50 | 0.09∗ |
| Male:Female, number | 144 : 127 | 13 : 19 | 0.18† | ||||
Mean ± SD or number of subjects.∗ :P-values determined via Student's t-test.
†: P-values determined via Chi-square test.
Table 2.
Number of knees that developed KL grade ≧ 2 in each 12-month period.
| Duration (months) | 0–12 | 12–24 | 24–36 | 36–48 | 48–60 | 60–72 | 72–84 | 84–96 | 0–96 |
|---|---|---|---|---|---|---|---|---|---|
| Number of knees | 2 | 0 | 3 | 22 | 0 | 1 | 0 | 4 | 32 (total) |
3.2. Reliability of osteophyte assessment
The weighted kappa coefficients representing intra- and inter-rater reliability for MOAKS osteophyte scoring are presented in Supplement 1. The intra-rater reliabilities were 0.756–0.936 in the axial plane, 0.781 to 0.935 in the coronal plane, and 0.689 to 0.913 in the sagittal plane. The inter-rater reliability was 0.604–0.936 in the axial plane, 0.523 to 0.935 in the coronal plane, and 0.689 to 0.857 in the sagittal plane.
3.3. Predictive value of osteophytes at each location
The number of knees with osteophytes is shown in Table 3. In the POA group, the proportion of knees with osteophytes tended to be higher at all sites. The total WOMAC pain score at 96 months was 0.7 ± 1.7 for the NOA group and 17.0 ± 3.2 for the POA group.
Table 3.
The number of knees with osteophytes.
| Sites | NOA (n = 271) | POA (n = 32) | ||||||||||||
| None | Grade≧1 | Grade 1 | Grade 2 | Grade 3 | None | Grade≧1 | Grade 1 | Grade 2 | Grade 3 | |||||
| Axial | Patella | MP | 119 | 152 | (56.0 %) | 138 | 14 | 0 | 5 | 27 | (84.3 %) | 22 | 3 | 2 |
| LP | 58 | 213 | (78.5 %) | 162 | 51 | 0 | 2 | 30 | (93.7 %) | 17 | 13 | 0 | ||
| Femoral | MPF | 58 | 213 | (78.5 %) | 183 | 30 | 0 | 4 | 28 | (87.5 %) | 19 | 8 | 1 | |
| LPF | 110 | 161 | (59.4 %) | 152 | 9 | 0 | 9 | 23 | (71.8 %) | 18 | 4 | 1 | ||
| MFC | 132 | 139 | (51.2 %) | 130 | 9 | 0 | 12 | 20 | (62.5 %) | 11 | 7 | 2 | ||
| LIC | 31 | 240 | (88.5 %) | 205 | 35 | 0 | 1 | 31 | (96.8 %) | 15 | 15 | 1 | ||
| MIC | 108 | 163 | (60.1 %) | 155 | 8 | 0 | 5 | 27 | (84.3 %) | 18 | 9 | 0 | ||
| LFC | 116 | 155 | (57.1 %) | 144 | 11 | 0 | 12 | 20 | (62.5 %) | 15 | 4 | 1 | ||
| Coronal | Femoral | MFC | 107 | 164 | (60.5 %) | 146 | 18 | 0 | 7 | 25 | (78.1 %) | 16 | 7 | 2 |
| LIC | 32 | 239 | (88.1 %) | 199 | 40 | 0 | 1 | 31 | (96.8 %) | 15 | 14 | 2 | ||
| MIC | 81 | 190 | (70.1 %) | 173 | 17 | 0 | 5 | 27 | (84.3 %) | 19 | 7 | 1 | ||
| LFC | 70 | 201 | (74.1 %) | 182 | 19 | 0 | 4 | 28 | (87.5 %) | 14 | 12 | 2 | ||
| Tibial | TS | 117 | 154 | (56.8 %) | 126 | 28 | 0 | 9 | 23 | (71.8 %) | 14 | 9 | 0 | |
| MTP | 99 | 172 | (63.4 %) | 158 | 14 | 0 | 4 | 28 | (87.5 %) | 18 | 10 | 0 | ||
| LTP | 129 | 142 | (52.3 %) | 127 | 15 | 0 | 12 | 20 | (62.5 %) | 13 | 6 | 1 | ||
| Sagittal | Patella | UPP | 227 | 44 | (16.2 %) | 41 | 3 | 0 | 16 | 16 | (50.0 %) | 15 | 1 | 0 |
| LPP | 176 | 95 | (35.0 %) | 85 | 10 | 0 | 16 | 16 | (50.0 %) | 14 | 2 | 0 | ||
| Femoral | AC | 88 | 183 | (67.5 %) | 153 | 30 | 0 | 3 | 29 | (90.6 %) | 20 | 9 | 0 | |
| LPC | 147 | 124 | (45.7 %) | 111 | 13 | 0 | 11 | 21 | (65.6 %) | 11 | 10 | 0 | ||
| MPC | 206 | 65 | (23.9 %) | 60 | 5 | 0 | 14 | 18 | (56.2 %) | 10 | 7 | 1 | ||
| Tibial | LATP | 176 | 95 | (35.0 %) | 92 | 3 | 0 | 11 | 21 | (65.6 %) | 17 | 4 | 0 | |
| MATP | 205 | 66 | (24.3 %) | 57 | 8 | 1 | 16 | 16 | (50.0 %) | 12 | 4 | 0 | ||
| IATP | 172 | 99 | (36.5 %) | 88 | 11 | 0 | 17 | 15 | (46.8 %) | 11 | 3 | 1 | ||
| LPTP | 187 | 84 | (30.9 %) | 72 | 12 | 0 | 16 | 16 | (50.0 %) | 14 | 2 | 0 | ||
| MPTP | 177 | 94 | (34.6 %) | 89 | 5 | 0 | 15 | 17 | (53.1 %) | 14 | 3 | 0 | ||
Unit; person, grade = osteophytes score using MOAKS.
MP = medial patella; LP = lateral patella; MPF = medial facet of the patellofemoral joint; LPF = lateral facet of the patellofemoral joint; MFC = medial femoral condyle; LFC = lateral femoral condyle; MIC = medial intra-condylar notch of the femur; LIC = lateral intra-condylar notch of the femur; MTP = medial tibial plateau; LTP = lateral tibial plateau; TS = tibial spine; UPP = upper pole of patella; LPP = lower pole of patella; AC = anterior condyle; LPC = lateral posterior condyle; MPC = medial posterior condyle; LATP = lateral anterior tibial plateau; MATP = medial anterior tibial plateau; IATP = intercondylar anterior tibial plateau; LPTP = lateral posterior tibial plateau; MPTP = medial posterior tibial plateau.
Table 4 shows the results of calculating the cut-off values using Youden's Index, the odds ratios (OR), the 95 % CIs, and p-values for each site. In addition, five locations were selected using stepwise logistic regression analysis as having a large impact on KOA. The selected locations were axial MP and LIC, coronal LFC and TS, and sagittal LPC. The cut-off value for axial MP on the MOAKS osteophyte scale was 1, and was 2 for the other sites (Table 5). The AUC of five sites individually showed a good fit (axial MP [AUC = 0.64; 95%CI: 0.04–0.57], axial LIC [AUC = 0.64; 95%CI: 0.05–0.60], coronal LFC [AUC = 0.68; 95%CI: 0.05–0.60], coronal TS [AUC = 0.68; 95%CI: 0.04–0.51], and sagittal LPC [AUC = 0.63; 95%CI: 0.04–0.55]). In addition, the AUC of the model of the five sites showed an excellent fit (AUC = 0.84; 95%CI: 0.04–0.76). Higher percentage of knees in POA had osteophytes in all the 5 sites than those in NOA with the use of cut-off value. When comparing distribution of number of osteophytes in both groups, about 80 % of knees showed none or one osteophyte in NOA group whereas 70% of knees had 2 to 5 osteophytes in POA group (Supplement 2).
Table 4.
Univariate analysis of the cut-off value of osteophytes at each site.
| Sites | The scoring of osteophytes with MOAKS Cutoff value∗ | Non-adjusted OR | 95%CI | p-value | ||
|---|---|---|---|---|---|---|
| Axial | Patella | MP | 1 | 4.23 | 1.58–11.31 | 0.004 |
| LP | 2 | 2.95 | 1.37–6.37 | 0.006 | ||
| Femoral | MPF | 2 | 3.14 | 1.33–7.42 | 0.009 | |
| LPF | 1 | 1.75 | 0.78–3.92 | 0.176 | ||
| MFC | 2 | 11.39 | 4.12–31.51 | <.0001 | ||
| LIC | 2 | 6.74 | 3.1–14.69 | <.0001 | ||
| MIC | 2 | 12.86 | 4.53–36.51 | <.0001 | ||
| LFC | 2 | 4.38 | 1.42–13.54 | 0.01 | ||
| Coronal | Femoral | MFC | 2 | 5.5 | 2.22–13.62 | 0.000 |
| LIC | 2 | 5.78 | 2.67–12.47 | <.0001 | ||
| MIC | 2 | 4.98 | 1.95–12.74 | 0.001 | ||
| LFC | 2 | 10.32 | 4.46–23.89 | <.0001 | ||
| Tibial | TS | 2 | 3.4 | 1.43–8.06 | 0.006 | |
| MTP | 2 | 8.35 | 3.32–20.96 | <.0001 | ||
| LTP | 2 | 4.78 | 1.78–12.82 | 0.002 | ||
| Sagittal | Patella | UPP | 1 | 5.16 | 2.4–11.08 | <.0001 |
| LPP | 1 | 1.85 | 0.89–3.87 | 0.101 | ||
| Femoral | AC | 1 | 4.65 | 1.38–15.68 | 0.013 | |
| LPC | 2 | 9.02 | 3.55–22.92 | <.0001 | ||
| MPC | 1 | 4.08 | 1.92–8.65 | 0.000 | ||
| Tibial | LATP | 1 | 3.54 | 1.64–7.65 | 0.001 | |
| MATP | 1 | 3.106 | 1.47–6.55 | 0.0029 | ||
| IATP | 1 | 1.533 | 0.73–3.20 | 0.2557 | ||
| LPTP | 1 | 2.226 | 1.06–4.66 | 0.0338 | ||
| MPTP | 1 | 2.134 | 1.02–4.46 | 0.0441 | ||
∗Cutoff values were determined by Yuden's index.MP = medial patella; LP = lateral patella; MPF = medial facet of the patellofemoral joint; LPF = lateral facet of the patellofemoral joint; MFC = medial femoral condyle; LFC = lateral femoral condyle; MIC = medial intra-condylar notch of the femur; LIC = lateral intra-condylar notch of the femur; MTP = medial tibial plateau; LTP = lateral tibial plateau; TS = tibial spine; UPP = upper pole of patella; LPP = lower pole of patella; AC = anterior condyle; LPC = lateral posterior condyle; MPC = medial posterior condyle; LATP = lateral anterior tibial plateau; MATP = medial anterior tibial plateau; IATP = intercondylar anterior tibial plateau; LPTP = lateral posterior tibial plateau; MPTP = medial posterior tibial plateau.
Table 5.
Sensitivity and specificity of five sites.
| Sites | Cutoff value∗ | Sensitivity | 95%CI | Specificity | 95%CI | ||
|---|---|---|---|---|---|---|---|
| Axial | Patella | MP | 1 | 0.84 | 0.72–0.97 | 0.44 | 0.38–0.50 |
| Femoral | LIC | 2 | 0.50 | 0.34–0.66 | 0.87 | 0.83–0.91 | |
| Coronal | Femoral | LFC | 2 | 0.44 | 0.28–0.61 | 0.93 | 0.89–0.95 |
| Tibial | TS | 2 | 0.28 | 0.13–0.44 | 0.90 | 0.86–0.93 | |
| Sagittal | Femoral | LPC | 2 | 0.31 | 0.15–0.47 | 0.95 | 0.93–0.98 |
∗Cutoff values (scoring of osteophytes with MOAKS) were determined by Youden's index.
MP = medial patella; LIC = lateral intra-condylar notch of the femur; LFC = lateral femoral condyle; TS = tibial spine; LPC = lateral posterior condyle.
When the subjects had osteophytes at two or more of the five selected sites, the sensitivity for KOA progression was 0.75, and the specificity was 0.79. When three or more of the five selected sites had osteophytes, the sensitivity was 0.50, and the specificity was 0.97.
4. Discussion
In this study osteophyte formation at five specific sites was found to be a possible imaging biomarker to predict radiographic OA development (K/L grade 2 or more) within eight years.
Although the etiology and mechanisms to form osteophytes have not been fully determined, the importance of osteophytes in the diagnosis and estimation of OA severity is well-known. The presence of osteophytes on plain x-rays has been used as one key objective finding for the diagnosis of OA [10]. Zhu used MRI-defined osteophytes as an endpoint for early development of KOA [11]. The size and number of osteophytes correlated to the intensity of knee pain [12,13]. Because osteophytes increase in size and number in a unidirectional manner and often form prior to symptomatic OA [14,15], they can be used as a predictive biomarker for KOA beyond their diagnostic and assessment value. In 2015, we reported using OAI data that the presence of osteophytes at specific sites at baseline could discriminate between knees that developed radiographic OA and knees that did not progress to OA within four years [4]. In the paper we examined eight locations in the knee and reported that osteophytes at the intercondylar notch best predicted the future development of OA. Calculated sensitivity and accuracy depending on a single osteophyte for predicting OA development in 48 months were 0.85 and 0.58, respectively. In the present paper, sensitivity dropped to 0.75 but accuracy was improved to 0.79 by increasing the sites to be examined. Oudenaarde et al. used a multivariate analysis to show that among several MRI features osteophytes were the most predictive for OA development within five years [16]. Although they did not examine the whole knee joint, they reported that the intercondylar region of the femur was the most frequent site for osteophytes, which was in accordance with the present study. Zhu et al. also reported the possibility of MRI-detected osteophytes as a predictive biomarker following evaluation at 14 sites employing the Knee Osteoarthritis Scoring System (KOSS) [17]. They did not refer to the specific site of osteophyte formation but suggested the presence of osteophytes could predict OA-related changes early in the disease process. In the present study, we examined the value of osteophytes as an imaging biomarker at 25 locations of the knee joint to incorporate as many peripheral osteophytes as possible and found that five specific sites were most important to discriminate knees at risk of OA development. Interestingly, the five locations appeared independent of each other. They were distributed in the lateral compartment, patellofemoral compartment, and intercondylar area. The mechanical environment has been considered a key element of osteophyte formation and even a single impact load could induce osteophytes. Venne reported that a single impact on the periosteum of the MFC triggered osteophyte formation in a rat model [18]. However, our results might indicate the existence of a pathological state of the whole joint rather than in a single compartment prior to OA development. Synovial fluid that fills the entire joint could induce osteophyte formation. Several cytokines in the synovial fluid have been reported to have this effect. Van Beuningen reported that injection of TGF-beta caused osteophyte formation [19]. Studies using PET-MRI revealed that abnormal bone remodeling of the whole joint might account for this [20,21]. Another possibility is that osteophytes are not an indicator of pathology but a physiological aging phenomenon. The fact that the average number of knees with osteophytes per knee was 13.5 even in the NOA group supports this. (Note that this number was calculated from Table 3 and there is a possibility that the same osteophyte was counted twice from another slice). Further support for this as an aging phenomenon is that only one-third of radiographic OA in which osteophytes were considered a major factor were symptomatic [22]. Considering that OA changes increase with age and the rate of aging differs individually, the number of osteophytes might simply be correlated to the biological age of the individual. Structural changes of the knee are common irrespective of the presence of pain or other OA risk factors making it difficult to discriminate aging from pathological changes [23].
A future study could mass screen knees of middle-aged people to determine their risk of OA development. As osteophytes are detectable with simple x-ray examination, osteophytes should be detectable at the five sites using multiple X-ray views. Axial MP osteophytes would be detected by the skyline view, coronal LFC and TS by the AP view, sagittal PC by the lateral view, and axial LIC by the tunnel view. Ogawa reported that the tunnel view x-ray can detect intercondylar osteophytes [24]. However, x-ray detection of osteophytes is less sensitive than MRI [25]. The main reason for this was overlapping of the femur or tibia. This seems reasonable because Hayashi et al. [26] reported tomosynthesis can detect osteophytes as effectively as MRI. As osteophytes are bony projections it might be possible to increase efficacy by modifying the direction of the x-ray beam.
The current study has several limitations. First, most subjects in the OAI database had risk factors for OA development so the results of this study may not be applicable to the general population. Second, the constructed model in the present study might be overfitted, thus a future study to confirm the result might be necessary using another data set. We established <55 years old as an inclusion criterion at baseline expecting more participants would develop radiographic OA in 96 months. However, only 32/303 developed OA even employing the incident cohort of OAI composed of participants with risk factors for OA development. This creates a limitation in the statistical analysis.
In conclusion, MRI-detected osteophytes may serve as a predictive biomarker for subsequent KOA development. Five locations within the knee joint were particularly important for discrimination of those knees that developed OA within eight years from those that did not.
Contributions
RT, SK, ST, RA, TS contributed to the conception and design of the study. RT, YO, SW contributed to the acquisition, collection, assembly of data and provision of study materials. YM, TN, YS, YK, TS contributed to the analysis and interpretation of data, technical support, statistical expertise. RT, YS, TS contributed to drafting the article. TO, SK, RA contributed to critical revisions for important intellectual content. All authors contributed to final approval of the final version. TS and RT take responsibility for the integrity of the work. Role of the funding source. No specific funding was used for this study.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Fig. 1 a1, a2: Axial (8 sites), b1, b2: Coronal (7 sites), c1 (lateral), c2 (intercondylar), c3 (medial): Sagittal (10 sites).
MP = medial patella; LP = lateral patella; MPF = medial facet of the patellofemoral joint; LPF = lateral facet of the patellofemoral joint; MFC = medial femoral condyle; LFC = lateral femoral condyle; MIC = medial intra-condylar notch of the femur; LIC = lateral intra-condylar notch of the femur; MTP = medial tibia plateau; LTP = lateral tibia plateau; TS = tibial spine; UPP = upper pole of patella; LPP = lower pole of patella; AC = anterior condyle; PC = posterior condyle; ATP = anterior tibia plateau; PTP = posterior tibia plateau; LPC = lateral posterior condyle; MPC = medial posterior condyle; LATP = lateral anterior tibial plateau; MATP = medial anterior tibial plateau; IATP = intercondylar anterior tibial plateau; LPTP = lateral posterior tibial plateau; MPTP = medial posterior tibial plateau.
Acknowledgments
The OAI dataset is for public use; this manuscript does not necessarily reflect the opinions or views of the OAI investigators, the National Institutes of Health (NIH), or the private funding partners.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2021.100200.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vitaloni M., Botto-van Bemden A., Sciortino Contreras R.M., Scotton D., Bibas M., Quintero M., et al. Global management of patients with knee osteoarthritis begins with quality of life assessment: a systematic review. BMC Muscoskel. Disord. 2019;20:493. doi: 10.1186/s12891-019-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losina E., Walensky R.P., Reichmann W.M., Holt H.L., Gerlovin H., Solomon D.H., et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann. Intern. Med. 2011;154:217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins J.E., Losina E., Nevitt M.C., Roemer F.W., Guermazi A., Lynch J.A., et al. Semi-quantitative imaging biomarkers of knee osteoarthritis progression: data from the FNIH OA biomarkers consortium. Arthritis Rheum. 2016;68:2422–2431. doi: 10.1002/art.39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsuragi J., Sasho T., Yamaguchi S., Sato Y., Watanabe A., Akagi R., et al. Hidden osteophyte formation on plain X-ray is the predictive factor for development of knee osteoarthritis after 48 months--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23:383–390. doi: 10.1016/j.joca.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Dillon C.F., Rasch E.K., Gu Q., Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the third national Health and nutrition examination survey 1991-94. J. Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 6.Jordan J.M., Helmick C.G., Renner J.B., Luta G., Dragomir A.D., Woodard J., et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in african Americans and caucasians: the Johnston county osteoarthritis project. J. Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 7.Peterfy C.G., Schneider E., Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou K.H., O'Malley A.J., Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 9.Choi B.C. Slopes of a receiver operating characteristic curve and likelihood ratios for a diagnostic test. Am. J. Epidemiol. 1998;148:1127–1132. doi: 10.1093/oxfordjournals.aje.a009592. [DOI] [PubMed] [Google Scholar]
- 10.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z., Aitken D., Cicuttini F., Jones G., Ding C. Ambulatory activity interacts with common risk factors for osteoarthritis to modify increases in MRI-detected osteophytes. Osteoarthritis Cartilage. 2019;27:650–658. doi: 10.1016/j.joca.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Hayes C.W., Jamadar D.A., Welch G.W., Jannausch M.L., Lachance L.L., Capul D.C., et al. Osteoarthritis of the knee: comparison of MR imaging findings with radiographic severity measurements and pain in middle-aged women. Radiology. 2005;237:998–1007. doi: 10.1148/radiol.2373041989. [DOI] [PubMed] [Google Scholar]
- 13.Magnusson K, Turkiewicz A, Kumm J, Zhang F, Englund M. The relationship between MRI features and knee pain over 6 years in knees without radiographic osteoarthritis at baseline. Arthritis Care Res. Published online August 2020. doi:10.1002/acr.24394. [DOI] [PMC free article] [PubMed]
- 14.Hakky M., Jarraya M., Ratzlaff C., Guermazi A., Duryea J. Validity and responsiveness of a new measure of knee osteophytes for osteoarthritis studies: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2015;23:2199–2205. doi: 10.1016/j.joca.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Z., Ding C., Han W., Zheng S., Winzenberg T., Cicuttini F., et al. MRI-detected osteophytes of the knee: natural history and structural correlates of change. Arthritis Res. Ther. 2018;20:237. doi: 10.1186/s13075-018-1734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Oudenaarde K., Jobke B., Oostveen A.C.M., Marijnissen A.C.A., Wolterbeek R., Wesseling J., et al. Predictive value of MRI features for development of radiographic osteoarthritis in a cohort of participants with pre-radiographic knee osteoarthritis-the CHECK study. Rheumatology. 2017;56:113–120. doi: 10.1093/rheumatology/kew368. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Z., Laslett L.L., Jin X., Han W., Antony B., Wang X., et al. Association between MRI-detected osteophytes and changes in knee structures and pain in older adults: a cohort study. Osteoarthritis Cartilage. 2017;25:1084–1092. doi: 10.1016/j.joca.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Venne G., Tse M.Y., Pang S.C., Ellis R.E. Mechanically-induced osteophyte in the rat knee. Osteoarthritis Cartilage. 2020;28:853–864. doi: 10.1016/j.joca.2020.02.834. [DOI] [PubMed] [Google Scholar]
- 19.van Beuningen H.M., Glansbeek H.L., van der Kraan P.M., van den Berg W.B. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998;6:306–317. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- 20.Tibrewala R., Pedoia V., Bucknor M., Majumdar S. Principal component analysis of simultaneous PET-MRI reveals patterns of bone-cartilage interactions in osteoarthritis. J. Magn. Reson. Imag. 2020;52:1462–1474. doi: 10.1002/jmri.27146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watkins L., MacKay J., Haddock B., Mazzoli V., Uhlrich S., Gold G., et al. Assessment of quantitative [18F] sodium fluoride PET measures of knee subchondral bone perfusion and mineralization in osteoarthritic and healthy subjects. Osteoarthritis Cartilage. 2021;29:849–858. doi: 10.1016/j.joca.2021.02.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muraki S., Oka H., Akune T., Mabuchi A., En-yo Y., Yoshida M., et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage. 2009;17:1137–1143. doi: 10.1016/j.joca.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson K., Kumm J., Turkiewicz A., Englund M. A naturally aging knee, or development of early knee osteoarthritis? Osteoarthritis Cartilage. 2018;26:1447–1452. doi: 10.1016/j.joca.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y., Akagi R., Nakagawa R., Kimura S., Ono Y., Watanabe S., et al. Osteophyte formation at the posterior notch of the femur serves as an early sign of osteoarthritic change of the knee joint. Chiba Med. J. 2020;96E:21–26. [Google Scholar]
- 25.Guermazi A., Niu J., Hayashi D., Roemer F., Englund M., Neogi T., et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (framingham osteoarthritis study) BMJ. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi D., Xu L., Roemer F.W., Hunter D.J., Li L., Katur A.M., et al. Detection of osteophytes and subchondral cysts in the knee with use of tomosynthesis. Radiology. 2012;263:206–215. doi: 10.1148/radiol.12111649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.