Abstract
Objective
While targeting obesity is central to osteoarthritis management, recent meta-analyses demonstrate only modest effects of weight loss on symptoms, and little on structure. The World Health Organisation recommends that effective management of obesity include prevention of weight gain, weight maintenance and weight loss. Therefore, we systematically reviewed the recommendations and approaches for management of obesity in clinical practice guidelines (CPGs) for osteoarthritis.
Design
Nine databases were searched (01.01.2010–15.03.2022) to identify guidelines informing the non-pharmacological management of osteoarthritis. Three reviewers appraised guidelines according to the AGREE II instrument, and independently extracted data on their characteristics. One author extracted and summarised guideline recommendations on weight management. This systematic review is registered on PROSPERO (CRD42021274195).
Results
Of the included fifteen CPGs (median AGREE II domain score 78.7%), weight loss was recommended for knee (12 of 13) and hip (10 of 11) but not hand (0 of 4) osteoarthritis. Combination approaches of diet and/or exercise were recommended for overweight or obese individuals in knee (8 of 12) and hip (4 of 10) osteoarthritis. Two guidelines specified ≥5% weight loss. One guideline specified strategies for maintenance of lost weight; none specifically recommended preventing weight gain. There was discordance between strength of recommendation for weight loss and level of evidence (3 of 15).
Conclusion
Most CPGs for knee and hip osteoarthritis recommend weight loss to manage obesity in osteoarthritis. As steady weight accumulation is common in adults, preventing weight gain should also be considered as it is a missed opportunity to improve outcomes in osteoarthritis.
Keywords: Systematic review, Recommendation, Weight management, Osteoarthritis, Clinical practice guidelines
1. Introduction
Osteoarthritis is a progressive whole joint disorder that causes significant pain and disability [1], resulting in substantial healthcare burden. There are no approved disease-modifying drugs for osteoarthritis, so a major focus is on tackling modifiable risk factors to improve symptoms and slow disease progression. Obesity is one of the most significant risk factors for osteoarthritis symptoms and disease progression, especially for the knee involvement [1,2]. As such, addressing obesity is one of the central messages in the management of osteoarthritis [[2], [3], [4]].
Weight loss is a common approach to addressing obesity. However recent meta-analysis have shown that weight loss of 5–10% of total body weight had only a modest effect on pain improvement in knee osteoarthritis [standardised mean difference 0.33 [95% confidence interval (CI) 0.17, 0.48] [5], comparable to that of paracetamol (effect size 0.21, 95% CI 0.02, 0.41) [6] and no significant effect on structural outcome in knee and hip osteoarthritis [5,[7], [8], [9]] even with substantial weight loss (up to 20% of total body weight) [8]. A meta-analysis showed that osteoarthritis pain, function and stiffness scores only improved by 2% for every 1% weight loss [10]. As established by the Outcome Measures in Rheumatology-Osteoarthritis Research Society International (OMERACT-OARSI) [11], a 20% improvement from baseline pain level is required to achieve a clinically important improvement in pain and function [12], which means a 10% weight loss is necessary; and for a patient with osteoarthritis to experience a 50% reduction in pain, as much as 25% weight loss is necessary [10]. Consistently, in a recent randomised clinical trial that utilised video-based telehealth exercise and ketogenic very-low-calorie diet intervention, which successfully achieved an average of 10% body weight loss over 12 months, compared with controls, it only showed a mean reduction of pain by 1.3 points on a 10-point numeric rating scale (mean −1.3, 95% CI -2.0, −0.7, p < 0.01) [13].
While addressing obesity in osteoarthritis, it is important to consider the trajectory of weight gain that results in an increasing proportion of the population shifting from healthy weight to overweight and obesity [[14], [15], [16]]. The weight trajectory which is similar across different communities [[14], [15], [16], [17]] tends to show that weight gain is typically accelerated during early adulthood [15] and at certain transitional stages in life (e.g. pregnancy [18]), with progressive weight increase at a rate of 0.5–1 kg per year from early to middle adulthood [15,17]. For example, recent analysis of The National Health and Nutrition Examination Survey (NHANES) data found that over a 10-year period, women gained 5.4 kg and men 2.7 kg [19]. The greatest weight gains were in young and middle-aged adults with less weight gain as age increased: 20–30 years, 8.0 kg; 30–40 years, 6.5 kg; 40–50 years, 4.3 kg and 50–60 years, 2.1 kg [19]. The World Health Organisation (WHO) has recommended that effective management of obesity should encompass a whole range of strategies ranging from prevention of weight gain, weight maintenance and the management of obesity related comorbidities, in addition to promotion of weight loss [20]. Targeting the gradual accumulation of weight may offer another opportunity to address obesity. Given that obesity and its management is important in osteoarthritis, we systematically reviewed the recommendations regarding weight management and the related strategies advised in all current osteoarthritis clinical practice guidelines.
2. Methods
This systematic review was reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [21]. This review is prospectively registered on PROSPERO (CRD42021274195).
2.1. Search strategy
Nine databases (Ovid MEDLINE, Ovid Embase, Cochrane Library, CINAHL Plus, PsycINFO, Scopus, PEDro, ScienceDirect and Google Scholar) were searched from January 1, 2010 to March 15, 2022 using MeSH terms, Boolean operators and key words to identify guidelines for the non-pharmacological management of osteoarthritis. The following search strategies were used: (i) MEDLINE [Osteoarthritis AND (Guideline∗ OR Evidence∗ OR Best∗ OR Recommend∗ OR Protocol∗) AND (Weight OR BMI OR Overweight OR Obes∗ OR Body weight OR Body composition OR Weight reduction programs)] and (ii) other databases [(Osteoarthriti∗ Guideline∗ OR Osteoarthriti∗ Protocol OR Osteoarthriti∗ Evidence OR Osteoarthriti∗ Recommend∗ OR Osteoarthriti∗ Best∗) AND (Weight∗ OR Body Mass Index (BMI) OR BMI OR Overweight OR Obes∗ OR Waist circumference)]. Searches were limited to English language. Websites of individual international renowned arthritis societies and organisations (Appendix A) and the Guidelines International Network (GIN) International Guidelines Library were browsed to further identify potentially relevant guidelines.
2.2. Guideline selection
JW and SMH independently assessed the eligibility of available guidelines using a 3-stage determination method: title then abstract screening, followed by full text screening, with disagreement between the two authors resolved by adjudication from a senior author, FMC.
The inclusion criteria were all latest versions (up to March 15, 2022) of international or national clinical practice guidelines on non-pharmacological management of osteoarthritis, especially related to recommendation on weight management in osteoarthritis, without limitation on the site of osteoarthritis. Guidelines were excluded if: (1) guidelines only targeted on a single component of non-pharmacological management and/or pharmacological management and/or surgical management of osteoarthritis; (2) guidelines involved patients with joint replacements; (3) recommendations were derived without a systematic literature search or critical appraisal of studies.
2.3. Data extraction
Two authors (JW and SMH) independently extracted data from each of the included guidelines. Extracted data were cross-checked for consistency by YZL. The following data were extracted and tabulated: guideline characteristics (guideline organisation affiliation, year of publication), target group, guideline development group, evidence base, grading system, site of osteoarthritis, recommendation and approaches for weight management (target group, weight loss strategies, magnitude of weight loss) and its priority in relation to other recommendations in the guidelines.
2.4. Guideline appraisal
JW and MME independently appraised each included guideline using the appraisal of guidelines for research and evaluation (AGREE) II instrument [22] after completion of the appropriate training [23]. Any significant discrepancies in the scores (where assigned scores differed by more than two points) were resolved and independently reassessed by a third author (SMH). AGREE II includes 23 items across 6 domains: scope and purpose, stakeholder involvement, rigour of development, clarity and presentation, applicability, and editorial independence (each rated from 1 [strongly disagree] to 7 [strongly agree]) and users rate the overall quality of each guideline (1–7) and recommend for or against its use. Scaled domain scores (0%–100%) are based on the sum of ratings across all appraisers and the difference between the maximum and minimum possible scores [22]. The domain score is calculated by summing all the scores of the individual items in a domain for each of the appraisal, and subsequently scaling and standardising as a percentage of the maximum possible score for that domain. Although the AGREE II instrument does not provide a cut-off to distinguish between high- and low-quality guidelines, we considered guidelines with domain and overall scores of <50% to be low quality [[24], [25], [26]].
2.5. Synthesis of guideline recommendations
Each guideline was initially analysed to gain an overall understanding of the content and followed by textual descriptive synthesis to analyse the scope, context and consistency of guideline recommendations on weight management in osteoarthritis. JW independently coded the guidelines to identify and extract weight recommendations in each guideline, which were cross-checked by YZL. Recommendations from guidelines were tabulated and summarised to provide an overview of all recommendations. In this systematic review, key recommendation refers to either “core recommendation”, “key priorities”, “good clinical practice” or when the recommendation is positioned as the top three recommendations within the osteoarthritis clinical practice guideline.
3. Results
3.1. Search results
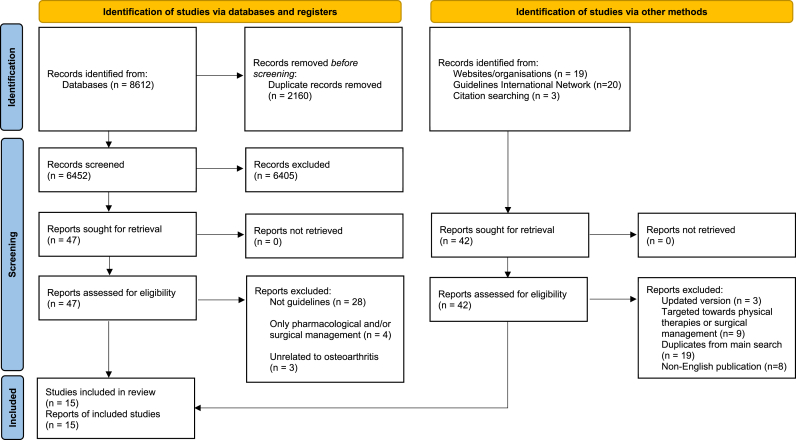
A total of 8612 records were identified through electronic database searching, with 6452 records remained (2160 duplicates) for title and abstract screening (Fig. 1). Forty-seven records proceeded to full text screening (35 records were excluded with reasons) while an additional 42 records were identified through other sources (websites of individual internationally renowned arthritis societies and organisations, GIN library and citation search), of which 39 were excluded with reasons (Fig. 1). In total, 15 guidelines were included in this systematic review.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of search algorithm.
3.2. Guideline characteristics
Table 1 shows the characteristics and development processes of all the included guidelines. Guideline development groups were affiliated with either a professional organisation or a government department: American College of Rheumatology (ACR) [3]; European League Against Rheumatism (EULAR) [27,28]; Italian Society for Rheumatology (ISR) [29]; Turkish League Against Rheumatism (TLAR) [30]; Pan American League of Associations for Rheumatology (PANLAR) [31]; Rheumatology and Immunology Specialised Committee [32]; Malaysian Society of Rheumatology (MSR) [33]; Osteoarthritis Research Society International (OARSI) [4]; European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) [34]; American Academy of Orthopaedic Surgeons (AAOS) [35,36]; the Royal Australasian College of General Practitioners (RACGP) [37]; Department of Veterans Affairs and Department of Defence (VaDoD) [38] and National Institute for Health and Care Excellence (NICE) [39]. Development of all guidelines involved a multidisciplinary team, most (n = 9) comprised of a working group of medical experts, literature review team, allied health and patient representatives [3,4,[27], [28], [29],31,34,38,39]. There were no patient representatives in the RACGP, AAOS, TLAR, MSR and the Chinese guidelines [30,32,33,[35], [36], [37]]. The target groups included mostly clinicians, health professionals and allied health managing patients with osteoarthritis [3,4,[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]] while 4 guidelines also targeted other stakeholders (e.g. patients, policy makers and health insurance agencies) [[27], [28], [29],33].
Table 1.
Characteristics of the included guidelines (n = 15).
| Author/ Year/Country |
Organisational affiliation | Funding body | Target group | Guideline development group |
Guideline review/journal publication |
Guideline update | Evidence base methods | LoE | SoR |
|---|---|---|---|---|---|---|---|---|---|
| AAOS 2021 US [36] |
AAOS | AAOS | Orthopaedic surgeons, other healthcare providers, medical practitioners | Multidisciplinary | Internal and external N |
5 years | SLR GRADE |
Y | Y |
| VADoD 2020 US [38] |
Department of Veterans Affairs (VA) and Department of Defence (DoD) | VADoD | Primary care providers or specialists | Multidisciplinary | Internal and external N |
NR | SLR GRADE |
N | Y |
| Zhang 2020 China [32] |
Rheumatology and Immunology Specialized Committee, Cross-Straits Medicine Exchange Association | Rheumatology and Immunology Expert Committee of the Cross-Strait medical and Health Exchange Association | Chinese clinicians, specialists, professionals involved in management of OA | Multidisciplinary | Internal and external Y |
2022 | SLR GRADE RIGHT checklist |
Y | Y |
| Kolasinski 2019 US [3] |
ACR | ACR and the Arthritis Foundation | Patients and clinicians | Multidisciplinary | External Y |
NR | SLR GRADE |
Y | Y |
| Bannuru 2019 US [4] |
OARSI | OARSI | Clinicians | Multidisciplinary | External Y |
NR | SLR GRADE |
Y | Y |
| Bruyere 2019 Belgium [34] |
ESCEO | ESCEO | Clinicians | Multidisciplinary | Internal and external Y |
NR | SLR GRADE |
N | Y |
| Ariani 2019 Italy [29] |
ISR | ISR | Physicians, health professionals, patients and policy makers | Multidisciplinary | External Y |
Stated (no planned date) | SLR AGREEII |
Y | N |
| RACGP 2018 Australia [37] |
RACGP | Funded in part by Medibank Better Health Foundation | General practitioners, health professionals | Multidisciplinary | External (approved by Chief Executive Officer of NHMRC) N |
5 years | SLR GRADE |
Y | Y |
| Kloppenburg 2018 The Netherlnds [28] |
EULAR | EULAR | All health professionals, patients and relevant stakeholders (e.g. policy makers, health insurance companies) | Multidisciplinary | Internal Y |
NR | SLR AGREEII |
Y | Y |
| AAOS 2017 US [35] |
AAOS | AAOS | Clinicians, surgeons, specialists, allied health | Multidisciplinary | Internal and External N |
5 years | SLR GRADE |
Y | Y |
| Tuncer 2017 Turkey [30] |
TLAR | NR | Clinicians | Multidisciplinary | Internal Y |
NR | SLR Oxman-Guyatt index and Jadad Scale |
Y | Y |
| Rillo 2016 Venezuela [31] |
PANLAR | PANLAR | NR | Multidisciplinary | Internal and External Y |
NR | SLR Jadad scale |
Y | Y |
| NICE 2014 UK [39] |
NICE | NICE | Clinicians, patients | Multidisciplinary | External N |
Stated (No planned date) | SLR GRADE |
Y | N |
| Fernandes 2013 Norway [27] |
EULAR | EULAR | Clinicians, healthcare providers, researchers in OA, policy makers | Multidisciplinary | Internal Y |
NR | SLR EULAR Standard operating procedure |
Y | Ya |
| MOH 2013 Malaysia [33] |
MSR | Ministry of Health Malaysia (MOH) and MSR | Healthcare professionals, relevant stakeholders in all healthcare setting | Multidisciplinary | External N |
2017 | SLR Scottish Intercollegiate Guidelines Network |
Y | Y |
Abbreviation.
AAOS: American Academy of Orthopaedic Surgeons.
ACR: American College of Rheumatology.
AGREE II: Appraisal of Guidelines Research and Evaluation II.
ESCEO: European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases.
EULAR: European League Against Rheumatism.
GRADE: Grading of Recommendations Assessment, Development and Evaluation.
ISR: Italian Society for Rheumatology.
LoE: Level of evidence.
N: No.
NHMRC: National Health and Medical Research Council.
NICE: National Institute for Health and Care Excellence.
NR: not reported.
MSR: Malaysian Society of Rheumatology.
OA: osteoarthritis.
OARSI: Osteoarthritis Research Society International.
PANLAR: Pan American League of Associations for Rheumatology.
RACGP: Royal Australasian College of General Practitioners.
RIGHT: Reporting Items for Practice Guidelines in Healthcare.
SLR: systematic literature review.
SoR: strength of recommendation.
TLAR: Turkish League Against Rheumatism.
VADoD: Department of Veterans Affairs and Department of Defence.
Y: Yes.
Presented as Level of agreement.
In all the included guidelines, the evidence to support recommendations was derived from a systematic literature review (SLR) [3,4,[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]], with detailed methodology outlined, except for the PANLAR guideline (Appendix B). The method used to grade the quality/certainty of the evidence differed among guidelines: majority (8 of 15) of the guidelines used Grading of Recommendations Assessment, Development and Evaluation (GRADE) [3,4,32,34,[36], [37], [38], [39]]; others are detailed in Table 1 and Appendix B. Thirteen guidelines described the strength of their recommendations [3,4,[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]] using different criteria, as described in Appendix B. The 2 guidelines (EULAR and NICE) that did not provide strength of recommendations (SoR) had graded the level of evidence [27,39]. In 8 of 13 guidelines, the SoR was concordant with the quality of evidence from the SLR [3,4,32,[34], [35], [36], [37], [38]]. The EULAR guidelines (knee/hip [25] and hand [28]) provided level of agreement among all task force members [27,28] with additional grading of their recommendations [28]. Economic considerations were taken into account in the NICE guideline [39].
All guidelines were peer-reviewed, either internally from experts within the affiliated organisation group (n = 9) [27,28,[30], [31], [32],[34], [35], [36],38] and/or externally by experts within the relevant field (n = 12) [3,4,29,[31], [32], [33], [34], [35], [36], [37], [38], [39]]. Nine guidelines were also subjected to a peer-review process required for journal publication [3,4,[27], [28], [29], [30], [31], [32],34].
The site of osteoarthritis in the included guidelines varies (Table 2, Table 3, Table 4): knee osteoarthritis (n = 13) [3,4,27,[29], [30], [31], [32], [33], [34],[36], [37], [38], [39]], followed by hip osteoarthritis (n = 11) [3,4,27,29,[31], [32], [33],35,[37], [38], [39]] and hand osteoarthritis (n = 4) [3,28,29,31]. The OARSI provided guideline for polyarticular osteoarthritis [4]. The NICE and osteoarthritis guideline in China did not specify the type of osteoarthritis, but encompassed knee, hip and hand osteoarthritis [32,39].
Table 2.
Summary of weight loss recommendation in knee osteoarthritis guidelines (n = 13).
| AAOS 2021 [36] | VADoD 2020 [38] | Zhanga 2020 [32] | ACR 2019 [3] | OARSIa 2019 [4] | ESCEO 2019 [34] | ISR 2019 [29] | RACGPa 2018 [37] | TLARa 2017 [30] | PANLAR 2016 [31] | NICE 2014 [39] | EULAR2013 [27] | MSR 2013 [33] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Is weight loss recommended? | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | EGY_press_logoY |
| LoE | Moderate | NR | Level A | Moderate (Overall level of certainty) | Knee: Strong | NR | Level 1-3 | Very low | Level Ia, IIa | NR | Knee: Ib | NR | |
| SoR | Moderate | Weak | 1 | Strongly recommended | Core but optional | Core: Strong | NR | Strongly recommended | 9.71 | NR | 8.7 | Grade A | |
| Target group | Overweight and obese | Overweight and obese |
|
Overweight or obese | Knee: NR | Overweight | Overweight |
|
BMI≥25 | overweight or obese who have associated functional limitations | Overweight or obese | Overweight | |
| Recommendation on magnitude of weight loss | NR | NR | NR | ≥5% of body weight. There is dose-response relationship. |
NR | NR | NR | Minimum weight loss target 5–7.5% for those with BMI≥25 | NR | NR | NR | NR | |
| Weight loss strategies | Diet and exercise combined | Self-management program including exercise and weight loss | NR | Concomitant exercise program | Exercise with or without dietary weight management, regardless of comorbidity | NR | Exercise (aerobic activities) | Diet and exercise combined | Lifestyle alteration. Diet and exercise programmes. |
Refer to NICE guideline for obesity on evidence of the most effective weight loss strategies. | Diet and exercise. Also included individualised strategies for weight loss maintenance |
NR | |
| Pharmacological weight management | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | |
| Surgical weight management | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Bariatric surgery for BMI≥40 | Not mentioned | |
| Priority in relation to other recommendations | No 10th of 27 recommendations | 3rd in the algorithm of management | No 4th of 16 recommendations | 3rd on the figure following exercise, self-efficacy and self-management programs. | Part of core recommendations | No 1 of 16 recommendations. Part of “core set” recommendations | No 13th of 16 recommendations | 1st point in the plain language summary. 3rd point in Core Long-term management |
No 2nd of 11 recommendations | In “Key priorities for implementation”, part of a “core treatment”. No 6th of 43 recommendations |
3rd and 8th of 11 recommendations. | 2nd in the algorithm on management of knee and hip osteoarthritis No 3rd of 16 recommendations. |
Abbreviation.
AAOS: American Academy of Orthopaedic Surgeons.
ACR: American College of Rheumatology.
BMI: body mass index.
ESCEO: European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases.
EULAR: European League Against Rheumatism.
ISR: Italian Society for Rheumatology.
LoE: level of evidence.
NICE: National Institute for Health and Care Excellence.
MSR: Malaysian Society of Rheumatology.
NR: not reported.
OA: osteoarthritis.
OARSI: Osteoarthritis Research Society International.
PANLAR: Pan American League of Associations for Rheumatology.
RACGP: Royal Australasian College of General Practitioners.
SoR: strength of recommendation.
TLAR: Turkish League Against Rheumatism.
VADoD: Department of Veterans Affairs and Department of Defence.
Guidelines also included weight management in addition to weight loss recommendation.
Table 3.
Summary of weight loss recommendation in hip osteoarthritis guidelines (n = 11).
| VADoD 2020 [38] | Zhanga 2020 [32] | ACR 2019 [3] |
OARSIa 2019 [4] |
ISR 2019 [29] |
RACGPa 2018 [37] |
AAOS 2017 [35] |
PANLAR 2016 [31] |
NICE 2014 [39] |
EULAR 2013 [27] |
MSR 2013 [33] |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Is weight loss recommended? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| LoE | NR | Level A | Moderate | Hip: Conditional | Level 1-3 | Very low | Level B | NR | Hip: Ib | NR | |
| SoR | Weak | 1 | Strongly recommended | Hip: Good clinical practice statement | NR | Strongly recommended | Level I | NR | 8.7 | NR | |
| Target group | Overweight and obese | Overweight or obese | Overweight or obese | Hip: BMI≥30 | Overweight | Overweight (BMI≥25) or obese (BMI≥30) | NR | Overweight or obese who have associated functional limitations | Overweight or obese | Overweight | |
| Recommendation on magnitude of weight loss | NR | NR | ≥5% of body weight. There is dose-response relationship. |
NR | NR | Minimum weight loss target 5–7.5% for those with BMI≥25 | NR | NR | NR | NR | |
| Weight loss strategies | Self-management program, exercise and weight loss Refer to the current VA/DoD CPG for the management of adult overweight and obesity |
NR | Concomitant exercise program | Dietary management may be considered as a part of a healthy lifestyle regimen. | Exercise (aerobic activities) | Diet and exercise combined | NR | Refer to NICE guideline for obesity on evidence of the most effective weight loss strategies. | Diet and exercise. Also included individualised strategies for weight loss maintenance |
NR | |
| Pharmacological weight management | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | |
| Surgical weight management | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Bariatric surgery for BMI≥40 | Not mentioned | |
| Priority in relation to other recommendations | 3rd in the algorithm of management | No 4th of 16 recommendations | 3rd on the figure following exercise, self-efficacy and self-management programs. | Part of good clinical practice statement | 13th of 16 recommendations | 1st point in the plain language summary. 3rd point in Core Long-term management |
1st of 8 in nonpharmacological treatment modalities recommendations | In “Key priorities for implementation”, part of a “core treatment”. No 6th of 43 recommendations |
3rd and 8th of 11 recommendations. | 2nd in the algorithm on management of knee and hip osteoarthritis |
Abbreviation:
AAOS: American Academy of Orthopaedic Surgeons.
ACR: American College of Rheumatology.
BMI: body mass index.
CPG: clinical practice guidelines.
EULAR: European League Against Rheumatism.
ISR: Italian Society for Rheumatology.
LoE: level of evidence.
OARSI: Osteoarthritis Research Society International.
MSR: Malaysian Society of Rheumatology.
N: no.
NICE: National Institute for Health and Care Excellence.
NR: not reported.
PANLAR: Pan American League of Associations for Rheumatology.
RACGP: Royal Australasian College of General Practitioners.
SoR: strength of recommendation.
VADoD: Department of Veterans Affairs and Department of Defence.
Y: yes.
Guidelines also included weight management in addition to weight loss recommendation.
Table 4.
Summary of weight loss recommendation in osteoarthritis (other than knee and hip or unspecified) guidelines.
| Zhang 2020 [32] | ACR 2019 [3] |
OARSI 2019 [4] |
ISR 2019 [29] |
EULAR 2018 [28] |
PANLAR 2016 [31] |
NICE 2014 [39] |
|
|---|---|---|---|---|---|---|---|
| Site of osteoarthritis | All types of OA | Hand | Polyarticular | Hand | Hand | Hand | All types of OA |
| Is weight loss recommended? | Y | N | Y | N | N | N | Y |
| LoE | Level A | Polyarticular: Level 1B | NR | ||||
| SoR | Strong | Polyarticular OA: Conditional | NR | ||||
| Target group | no comorbid conditions | overweight or obese who have associated functional limitations | |||||
| Recommendation on magnitude of weight loss | NR | NR | NR | ||||
| Weight loss strategies/other comments | Evidence for weight loss were mainly derived from knee osteoarthritis. | Weight management only recommended for knee and/or hip osteoarthritis | Dietary ± exercise in certain subgroup (no comorbid conditions; widespread pain and/or depression). | Weight loss recommended for patients with hip and knee osteoarthritis who are overweight. | NR |
Abbreviation:
ACR: American College of Rheumatology.
EULAR: European League Against Rheumatism.
ISR: Italian Society for Rheumatology.
LoE: level of evidence.
N: no.
NICE: National Institute for Health and Care Excellence.
NR: not reported.
OA: osteoarthritis.
OARSI: Osteoarthritis Research Society International.
PANLAR: Pan American League of Associations for Rheumatology.
SoR: strength of recommendation.
Y: yes.
4. Methodological quality
The mean scores for the AGREE-II domains were 35.5–92.5 (Table 5). Six guidelines (AAOS knee [36], ACR [3], OARSI [4], RACGP [37], EULAR hand [28], EULAR knee/hip [27]) had mean domain scores of >80%. Overall guideline assessment scores ranged from 3.00 to 6.50 out of 7 maximum possible score [median (%) 6.0, (interquartile range 4.6, 6.0)]. Of the guidelines (ISR [29] and MSR [33]) that scored low (<50% mean domain scores), shortcomings included limited or no descriptions of input from guideline end users or patients; criteria for selecting evidence, strengths and limitations of evidence, and methods for formulating recommendations; external reviews before publication; plans for updating; barriers to implementation, resource implications, and how to implement guideline recommendations; and measures taken to ensure editorial independence.
Table 5.
Guideline assessment according to the AGREE-II instrument.
| Author/Guideline organisation or society/year | Domain scores (%) |
Mean overall quality (maximum possible score = 7) | ||||||
|---|---|---|---|---|---|---|---|---|
| Scope and purpose | Stakeholder involvement | Rigour of development | Clarity and presentation | Applicability | Editorial independence | Mean domain scores (%) | ||
| AAOS 2021 [36] (knee) | 100.0 | 88.1 | 96.4 | 100.0 | 82.1 | 82.1 | 91.5 | 6.0 |
| VADoD 2020 [38] | 95.2 | 61.9 | 93.8 | 95.2 | 73.2 | 53.6 | 78.8 | 6.0 |
| Zhang 2020 [32] | 90.5 | 81.0 | 87.5 | 88.1 | 30.4 | 82.1 | 76.6 | 6.0 |
| ACR 2019 [3] | 97.6 | 92.9 | 94.6 | 100.0 | 37.5 | 75.0 | 82.9 | 6.0 |
| OARSI 2019 [4] | 96.1 | 74.3 | 100.0 | 95.3 | 68.9 | 81.7 | 86.1 | 6.0 |
| ESCEO 2019 [34] | 90.5 | 69.0 | 76.8 | 100.0 | 50.0 | 85.7 | 78.7 | 6.0 |
| ISR 2019 [29] | 30.7 | 78.0 | 54.8 | 89.0 | 33.0 | 12.8 | 49.7 | 3.5 |
| RACGP 2018 [37] | 100.0 | 98.0 | 84.0 | 79.9 | 87.2 | 82.3 | 88.6 | 6.2 |
| EULAR 2018 [28] (hand) | 90.0 | 88.9 | 100.0 | 97.6 | 85.3 | 93.2 | 92.5 | 5.6 |
| AAOS 2017 [35] (hip) | 56.0 | 78.5 | 65.2 | 84.0 | 78.9 | 99.0 | 77.1 | 4.6 |
| TLAR 2017 [30] | 58.0 | 78.2 | 88.3 | 28.3 | 36.2 | 32.2 | 53.5 | 3.0 |
| PANLAR 2016 [31] | 81.0 | 69.0 | 58.9 | 92.6 | 41.1 | 85.7 | 71.4 | 5.0 |
| NICE 2014 [39] | 86.1 | 44.2 | 99.0 | 100.0 | 70.6 | 50.1 | 75.0 | 5.8 |
| EULAR 2013 [27] (knee/hip) | 94.4 | 90.6 | 89.4 | 53.1 | 100.0 | 98.0 | 87.9 | 6.5 |
| MSR 2013 [33] | 20.0 | 18.8 | 12.0 | 67.1 | 30.2 | 65.0 | 35.5 | 4.0 |
| Median score (%, IQR) | 90.5 (58.0,96.1) | 78.2 (69.0,88.9) | 88.3 (65.2,96.4) | 92.6 (79.9100.0) | 68.9 (36.2,82.1) | 82.1 (53.6,85.7) | 78.7 (71.4,87.9) | 6.0 (4.6,6.0)_ |
Abbreviation:
AAOS: American Academy of Orthopaedic Surgeons.
ACR: American College of Rheumatology.
AGREE II: Appraisal of Guidelines Research and Evaluation II.
ESCEO: European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases.
EULAR: European League Against Rheumatism.
ISR: Italian Society for Rheumatology.
IQR: interquartile range.
NICE: National Institute for Health and Care Excellence.
MSR: Malaysian Society of Rheumatology.
OARSI: Osteoarthritis Research Society International.
PANLAR: Pan American League of Associations for Rheumatology.
RACGP: Royal Australasian College of General Practitioners.
TLAR: Turkish League Against Rheumatism.
VADoD: Department of Veterans Affairs and Department of Defence.
4.1. Weight management recommendations
Of the total fifteen guidelines, 13 incorporated obesity management as part of weight management recommendations [3,4,27,[29], [30], [31], [32], [33], [34],[36], [37], [38], [39]]. There were no weight management recommendations in the EULAR hand osteoarthritis [28] and AAOS hip osteoarthritis guidelines [35]. Where weight management was included, it was one of the key recommendations in 9 [3,4,27,30,33,34,[37], [38], [39]] of the 12 [3,4,27,29,30,[32], [33], [34],[36], [37], [38], [39]] guidelines for knee osteoarthritis and 8 [3,4,27,31,33,[37], [38], [39]] of the 10 [3,4,27,29,[31], [32], [33],[37], [38], [39]] guidelines for hip osteoarthritis.
All 13 knee osteoarthritis guidelines, except PANLAR [31] had recommendations for management of obesity (Table 2) and weight management was one of the key recommendations in 9 [3,4,27,30,33,34,[37], [38], [39]] of them. All guidelines recommended “weight loss” while 4 guidelines [4,30,32,37] used the term “weight management” instead of “weight loss”. Of these 4 guidelines, 2 guidelines recommended controlling body weight for all patients [30,32] as part of management of obesity. No guidelines specifically mentioned preventing weight gain. Nine of 12 knee osteoarthritis guidelines had moderate to strong recommendations for weight loss [3,4,27,30,[32], [33], [34],36,37], but the level of evidence behind these recommendations varied (Appendix B). Notably, in 3 of 12 knee osteoarthritis guidelines, the SoR for weight loss was discordant to the level of evidence: ACR [3] had strong recommendation on moderate level of evidence; ESCEO [34] had strong recommendation while level of evidence not reported; RACGP [37] had strong recommendation on very low level of evidence. Although the level of evidence behind the weight loss recommendation was drawn from randomised clinical trials, there were several issues that resulted in the quality of evidence being rated moderate (ACR [3]) and very low (RACGP [37]): serious risk of bias from single-blind or unblinded study design; high attrition rates; wide confidence interval and short study period [3,40]. The strength of recommendation was justified by the general view that weight loss has low risk of harms, such that the overall benefits outweigh the risks [3,40].
Among the 11 hip osteoarthritis guidelines: 10 had weight loss recommendation for weight management [3,4,27,29,[31], [32], [33],[37], [38], [39]] (Table 3), of which 8 [3,4,27,31,33,[37], [38], [39]] guidelines had this recommendation as one of their key recommendations; 7 guidelines [3,27,29,31,33,38,39] recommended weight loss for hip osteoarthritis while 3 guidelines [4,32,37] used the term “weight management”. While the strength for weight loss recommendation was strong in 4 of 9 guidelines (ACR [3], RACGP [37], EULAR [27] and PANLAR [31]), the level of evidence behind these recommendations varied (Appendix B). Discordance were seen in ACR [3] (strong recommendation on moderate level of evidence) and RACGP [37] (strong recommendation on very low level of evidence).
None of the hand osteoarthritis guidelines recommended weight management [3,28,29,31] (Table 4). For polyarticular osteoarthritis guideline, weight management was conditionally recommended [4].
4.2. Target group for weight loss
Eleven of 12 recommendations for weight loss in knee osteoarthritis specifically targeted people who were overweight or obese [3,27,29,30,[32], [33], [34],[36], [37], [38], [39]] (Table 2). Table 3 shows 10 of 11 guidelines that recommended weight loss for hip osteoarthritis targeted people who are overweight or obese, with the OARSI [4] guideline specifically targeted those with BMI of ≥30 kg/m2. While the NICE [39] guideline recommended weight loss for those who are overweight or obese with associated functional limitations, the OARSI [4] guideline specifically targeted weight loss to those with no comorbid conditions, with gastrointestinal or cardiovascular conditions and with widespread pain and/or depression [4] (Table 4).
4.3. Weight loss strategies suggested by guidelines
Nine of 12 knee osteoarthritis guidelines provided a general, non-specific weight loss strategy [3,4,27,29,30,[36], [37], [38], [39]], mostly comprised of a combination approach of exercise and/or dietary weight loss (8 of 9 guidelines) [3,4,27,29,30,[36], [37], [38]] (Table 2). NICE [39] provided reference to its own obesity guideline (NICE guideline for obesity [41] on evidence of the most effective weight loss strategies) for strategies to lose weight. The EULAR [27] guideline further described examples of strategies that were recognised to effect successful weight loss and maintenance, such as increase physical activity, follow a structured meal plan, limit portion size, nutritional education etc.
For hip osteoarthritis, 7 of 10 guidelines described a general, non-specific weight loss strategy that comprised of a combination of dietary and/or concomitant exercise [3,4,27,29,[37], [38], [39]] (Table 3). Conversely, OARSI [4] guideline recommended against dietary weight loss for individuals with hip osteoarthritis with comorbidity such as gastrointestinal, cardiovascular, frailty or widespread pain and/or depression, but acknowledged that it may be recommended as part of a healthy lifestyle regimen to those with BMI ≥30 kg/m [24].
The OARSI [4] guideline for polyarticular osteoarthritis recommended weight loss using a combination of dietary weight management with or without an exercise component for those without comorbid conditions but recommended against dietary weight management for individuals with frailty [4]. The NICE [39] guideline specifically referred to the NICE obesity guideline [41] to provide recommendation for individuals who are overweight or obese with associated functional limitations.
Except for the EULAR [27] guideline, all other guidelines have not provided details on strategies to effective dietary or concomitant exercise interventions for weight loss, specifically no details regarding type, duration, frequency or intensity of the recommended approach. There were no guidelines that mentioned the role of pharmacological or surgical weight loss interventions for osteoarthritis except for EULAR knee/hip [27] guideline (Table 2, Table 3) that acknowledged the role of bariatric surgery as part of comprehensive weight management in people with knee or hip osteoarthritis who have a BMI≥40 kg/m2.
4.4. Magnitude of weight loss
Two [3,37] of 12 knee and 2 [3,37] of 10 hip osteoarthritis guidelines specified the magnitude of weight loss required for weight management: ACR guideline recommended ≥5% of body weight [3]; RACGP guideline recommended a minimum weight loss target of 5–7.5% for those with BMI≥25 kg/m237. The ACR guideline acknowledged a dose-response relationship in the degree of weight loss, such that clinically important benefits continue to increase with weight loss of 5–10%, 10–20% and >20% of body weight [3].
5. Discussion
We systematically reviewed the recommendations and approaches for weight management in 15 current osteoarthritis clinical practice guidelines. Most clinical practice guidelines recommended weight loss for knee (12 of 13 guidelines) [3,4,27,29,30,[32], [33], [34],[36], [37], [38], [39]] and hip osteoarthritis (10 of 11 guidelines) [3,4,27,29,[31], [32], [33],[37], [38], [39]] but not hand osteoarthritis (0 of 4 guidelines) [3,28,29,31]. In guidelines recommending weight loss, it was often highlighted as one of the key recommendations for management of osteoarthritis, targeting individuals with overweight and obesity. However, the details varied with respect to recommendation for the degree of weight loss required and strategies suggested to achieve target weight loss, such that most guidelines do not provide much advice as to how to lose weight effectively or maintain weight once weight loss is achieved. Two guidelines recommended ≥5% loss of body weight for management of knee and hip osteoarthritis [3,37]. While the main strategies recommended included combination approaches such as diet and exercise or a concomitant exercise program, the advice was general and non-specific. Only 1 guideline included strategies for weight maintenance of the lost weight [27]. Importantly, there is a discordance between SoR and the level of evidence in some guidelines, with strong recommendation for weight loss being justified by overall lack of harms in weight loss, such that the benefits of weight loss outweigh the risks, despite limitation in the available evidence in osteoarthritis. Notably, no guidelines recommended prevention of weight gain as management of obesity in osteoarthritis.
Overall, osteoarthritis clinical practice guidelines of the knee and hip place significant emphasis on weight loss to manage obesity, despite the discordance between quality of evidence, SoR and evidence of modest effect of weight loss in knee and hip osteoarthritis symptoms and joint structure [5,[7], [8], [9],[42], [43], [44], [45], [46]]. This most likely relates to our limited therapeutic options in osteoarthritis management in the absence of disease-modifying therapies. However, osteoarthritis is disease continuum from a healthy joint to one with early osteoarthritis and then disease progression to end-stage disease [2,47], with obesity related structural changes detected even prior to the development of clinical symptoms in early adulthood [48,49]. The data from clinical trials of weight loss suggest that once symptomatic, radiographic osteoarthritis is present there is limited reversibility [47]. Successful weight loss is difficult to achieve and maintain for most patients even when recommended for other chronic diseases such as diabetes and cardiovascular disease [50,51]. With such barriers, there is the potential for setting unrealistic goals for patients which also need to be considered in a context where the likelihood of benefit is overstated.
Whilst some guidelines acknowledged the inconsistency of evidence underpinning the recommendation for weight loss in osteoarthritis [4,36,38], it is considered that weight loss is likely to have overall health benefits, with the notion of no anticipated harms, hence justifying their strength of recommendation. However it is important to consider recommendation of weight loss in the context of potential harm. In studies of the perspectives of people with osteoarthritis regarding weight loss, participants reported awareness about potential health benefits of weight loss, dissatisfaction with their weight, and emotions of anxiety and disempowerment about achieving weight loss [[52], [53], [54]]. Repeated failures to achieve idealistic weight loss outcome have the potential to demoralise and perpetuate negative thoughts and self-blame that further deter weight loss success [55,56]. More than 50% of people with overweight and obesity experienced internalised weight stigma [57], such that they were ‘blamed’ for not getting better, and can be associated with negative consequences, including maladaptive coping mechanisms such as unhealthy eating behaviours and exercise avoidance, that eventually leads to increased obesity and weight gain over time [58].
In line with the WHO recommendation, prevention of weight gain is as important as promotion of weight loss in addressing growing rates of obesity as the slow accumulation of weight results in people transitioning from normal to overweight and obesity [20]. Tackling weight gain provides another opportunity to address obesity but to date, preventing weight gain has received little attention [20]. In a recent Australian study, it was estimated that total knee replacement (TKR) in males and females could be prevented by 36.55% and 34.92% respectively if overweight and obesity were prevented [59]. This could be achieved with weight loss, but it needs to be early in the osteoarthritis disease course, since the evidence for weight loss in established symptomatic osteoarthritis has been modest [5,[7], [8], [9]], most likely due to limited reversibility in established knee osteoarthritis. Additionally, this weight loss would also need to be maintained, which for many patients is difficult to achieve [60,61]. The other option is to halt or slow the weight gain trajectory, to prevent the population from moving up one BMI category, which could theoretically reduce 20% of the proportion of TKRs attributed to overweight and obesity [59]. A recent meta-analysis demonstrated that low intensity weight related behaviour interventions including diet and physical activity which resulted in small energy deficits were effective at prevention of weight gain [62], where interventions were most effective in non-obese populations [62]. Hence, minor lifestyle changes targeting the estimated small cumulative energy imbalance of around 30kj per day may be considered more pragmatic, achievable and sustainable in the daily life through adulthood to prevent the insidious development of obesity over time [63,64]. We highlighted in this systematic review that no guidelines recommended prevention of weight gain in osteoarthritis. As such, weight gain prevention interventions should be considered when an individual presents with joint pain, which has been shown to be effective irrespective of weight, gender or BMI [62] and relevant to all individuals of a healthy weight or above [62,65].
This systematic review has several strengths. A methodologically comprehensive and rigorous search was conducted systematically in nine databases with additional browsing of citations and international arthritis organisations to limit the potential for missing guidelines. Pairs of independent reviewers screened, critically appraised, and extracted data from the guidelines, reflecting the high methodological rigour in this review. The limitation of this review is that it includes only guidelines published in English which limits the external validity of this review to users from English-speaking jurisdictions, particularly when osteoarthritis and obesity are a global issue.
In conclusion, most clinical practice guidelines for management of knee and hip osteoarthritis consistently recommend weight loss, generally targeted to people who are overweight or obese, despite evidence of modest at best effect of weight loss on symptoms [5,[7], [8], [9]] and no effect on joint structure [8]. Given obesity is a major risk factor for osteoarthritis, preventing weight gain may be a missed opportunity to improving clinical outcomes for osteoarthritis, and hence should be considered as part of the key management in osteoarthritis.
Author contributions
YZL: analysis and interpretation of the data; drafting of the article; final approval of the article. JW: acquisition of data; analysis and interpretation of the data; final approval of the article. SMH: conception and design; acquisition of data; analysis and interpretation of data; critical revision of the article for important intellectual content; final approval of the article. MME: analysis and interpretation of the data; final approval of the article. LZ: critical revision of the article for important intellectual content; final approval of the article. MJP: critical revision of the article for important intellectual content; final approval of the article. CLH: critical revision of the article for important intellectual content; final approval of the article. AEW: critical revision of the article for important intellectual content; final approval of the article. YW: analysis and interpretation of the data; critical revision of the article for important intellectual content; final approval of the article. FMC: conception and design; analysis and interpretation of the data; critical revision of the article for important intellectual content; final approval of the article.
Declaration of competing interest
All authors declare that they have no conflict of interest.
Acknowledgments
YZL is the recipient of National Health and Medical Research Council (NHMRC) Clinical Postgraduate Scholarship (#1133903) and Royal Australasian College of Physicians Woolcock Scholarship. SMH is the recipient of NHMRC Early Career Fellowship (APP1142198). MME is the recipient of Bangabandhu Science and Technology Fellowship from Ministry of Science and Technology, Government of the People's Republic of Bangladesh. MJP is the recipient of Australian Research Council Discovery Early Career Researcher Award (DE200101618). CLH is the recipient of a Senior Postdoctoral Fellowship from the NHMRC Centre for Research Excellence for Health in Preconception and Pregnancy (CRE-HiPP; APP1171142). AEW is the recipient of the Royal Australian College of Physicians Fellows Career Development Fellowship. YW is the recipient of NHMRC Translating Research into Practice Fellowship (APP1168185). FMC is the recipient of NHMRC Investigator Grant (APP1194829). The funders of the study had no role in the study design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Yuan Z. Lim, Email: yuan.lim@monash.edu.
Jeffrey Wong, Email: jeffreywongcs1897@gmail.com.
Sultana Monira Hussain, Email: monira.hussain@monash.edu.
Mahnuma Mahfuz Estee, Email: Mahnuma.Estee1@monash.edu.
Luigi Zolio, Email: drluigizolio@gmail.com.
Matthew J. Page, Email: matthew.page@monash.edu.
Cheryce L. Harrison, Email: cheryce.harrison@monash.edu.
Anita E. Wluka, Email: anita.wluka@monash.edu.
Yuanyuan Wang, Email: yuanyuan.wang@monash.edu.
Flavia M. Cicuttini, Email: flavia.cicuttini@monash.edu.
Appendix A. List of arthritis societies and organisations searched
3e Initiative in Rheumatology.
African League of Associations for Rheumatology.
American Academy of Orthopaedic Surgeons.
American College of Rheumatology.
American Geriatrics Society.
American Pain Society.
Arthritis.com.
Arthritis New Zealand.
Arthritis Research UK.
Arthritis Society Canada.
Asia Pacific League of Associations for Rheumatology.
Assessment of SpondyloArthritis International Society.
Brazilian Society of Rheumatology.
British Paediatric Rheumatology Group.
British Society for Rheumatology.
Canadian Medical Association Clinical Guidelines.
Canadian Rheumatology Association.
Centers for Disease Control and Prevention Guidelines.
Cochrane Reviewed Osteoarthritis.
Department of Veterans Affairs Department of Defense.
European League Against Rheumatism.
Group for Research in Psoriasis and Psoriatic Arthritis.
Hong Kong Society of Rheumatology.
Institute for Clinical Systems Improvement.
International League of Association for Rheumatology.
Italian Society of Rheumatology.
Japanese Journal of Joint Diseases.
Journal of Korean Knee Society.
Malaysian Society of Rheumatology.
Medical Journal of Australia Clinical Guidelines.
National Guidelines Clearinghouse.
National Health and Medical Research Council.
National Institute for Health and Care Excellence.
New South Wales Therapeutic Assessment Group.
New Zealand Guidelines Group.
Orthopaedic Research Society.
Osteoarthritis Research Society International.
Ottawa Panel Evidence.
Philippines Rheumatologic Association.
Pan-American League of Associations for Rheumatology.
Queensland University Clinical Practice Guidelines.
Royal Dutch Society for Physical Therapy.
Scottish Intercollegiate Guidelines Network (SIGN).
Singapore MOH Guidelines.
South Africa Arthritis Foundation.
Therapeutic Goods Administration.
Therapeutic Guidelines.
Turkish League Against Rheumatism.
U.S. Agency for Healthcare Research & Quality.
International Society of Orthopaedic Surgery and Traumatology.
Appendix B. Characteristics of methodology, quality of evidence and strength of recommendation of included guidelines
| Author/Year/Country | Methods | Clear inclusion/exclusion criteria | Quality of evidence/LoE | SoR | |
|---|---|---|---|---|---|
| AAOS 2021 [36] |
SLR Delphi consensus Formulation of workgroup, formulation of PICO questions, systematic literature search and review, recommendation development, review, revision and approval. |
✓ | GRADE |
Strong: Evidence from two or more “High” quality studies with consistent findings for recommending for or against the intervention. Moderate: Evidence from two or more “Moderate” quality studies with consistent findings, or evidence from a single “High” quality study for recommending for or against the intervention Limited: Evidence from one or more “Low” quality studies with consistent findings or evidence from a single “Moderate” quality study recommending for or against the intervention Consensus: There is no supporting evidence, or higher quality evidence was downgraded due to major concerns |
Strong Moderate Limited Consensus |
| VADoD 2020 [38] |
SLR Delphi consensus Formulation and prioritization of key questions and definition of critical outcomes, systematic literature review, patient focus group, development of recommendation and grading, review and submission for approval. |
✓ | GRADE | Not described |
Strong: high confidence in the quality of the available scientific evidence, a clear difference in magnitude between the benefits and harms of an intervention, similar patient or provider values and preferences, and understood influence of other implications Weak: work group has less confidence after assessment and believe additional evidence may change the recommendation. |
| Zhang 2020 [32] | SLR Delphi consensus Formulation of clinical questions (Delphi techniques), PICO formulation, systematic literature search, GRADE process, recommendation formation |
✓ | GRADE |
High: Level A Moderate: Level B Low: Level C Very low: Level D |
Strong: Class 1 Weak: Class 2 |
| ACR 2019 [3] |
SLR Formulation of PICO questions, systematic literature search, scoping and clinical question development, interprofessional voting for recommendation formation. |
✓ | GRADE | SLR of RCTs. Systematic reviews of observational studies were only included if judged by Voting Panel would add critical information for the formulation of recommendation. |
Strong: compelling evidence of efficacy and that benefits clearly outweighed harms and burdens Conditional: quality of the evidence proved low or very low and/or the balance of benefits versus harms and burdens was sufficiently close that warrant a shared decision-making between the patient and the clinician. |
| OARSI 2019 [4] |
SLR Formulation of clinical questions, systematic literature search, voting and formulation of recommendation |
✓ | GRADE | Recommendation level Level 1A, 1B: ≥75% vote in favour Level 2: 60–74% vote in favour Level 3: 41–59% vote in favour Level 4B: 26–40% vote in favour Level 4A, 5: ≤25% vote in favour |
Strong: Voting Panel members feel confident that the benefits of a particular intervention outweigh the harms, or that the harms outweigh the benefits. Conditional: recommendation that carries risks that could potentially outweigh the benefit. Quality of evidence and uncertainty in values and preference are also taken into consideration. Good clinical practice statement: recommendations made based on expert experience in the absence of direct, supportive RCT evidence |
| ESCEO 2019 [34] |
SLR Systematic literature search, review of summary of evidence, voting of recommendation. |
✓ | GRADE | Not described |
Strong: ≥75% vote in favour Weak |
| ISR 2019 [29] |
SLR Delphi consensus Based on framework of the Guidelines International Network Adaptation Working Group to identify, appraise, synthesize and customize the existing international guidelines to the needs of the Italian healthcare context Defining scope of the guideline and formulation of clinical questions. Systematic review of all guidelines endorsed by international scientific societies, development of recommendation in accordance to AGREE reporting checklist, external peer review and rating. |
✓ | AGREE II | Oxford Levels of Evidence: 1: From meta-analysis of randomised controlled trials or from at least one RCT 2: From ≥1 controlled study without randomisation or from ≥1 cohort study 3: From ≥1 case-control study 4: From case-series or poor-quality cohort and case-control studies 5: From expert committee reports or opinions and/or clinical experience of respected authorities. |
Not described |
| RACGP 2018 [37] |
SLR Systematic literature search build upon the literature in the first edition of the guideline, grading of recommendation, formulation of recommendation, voting, endorsement by NHMRC. Searches: systematic reviews and RCTs |
✓ | GRADE | Quality of evidence High Moderate Low Very low |
Strong: The working group is very confident that the benefits of an intervention clearly outweigh the harms (or vice versa) Conditional: Denotes uncertainty over the balance of benefits or harms, such as when the evidence quality is low or very low, or when personal preferences or costs are expected to impact the decision, and as such refer to decisions where consideration of personal preferences is essential for decision making |
| EULAR 2018 [28] |
SLR EULAR standard Operating Procedure (SOP) (according to AGREEII) Performed according to AGREEII. Formulation of research questions, systematic literature review, formulation of overarching principles, presentation of evidence from SLR and voting. |
✓ | AGREEII | Oxford Centre for Evidence-Based Medicine 1a: systematic review of RCTs 1b: individual RCT 2a: systematic review of cohort studies 2b: individual cohort study (including low-quality RCT; eg,<80% follow-up) 3a: systematic review of case-control studies 3b: individual case-control study 4: case-series (and poor quality cohort and case-control studies) 5: expert opinion without explicit critical appraisal, or based on physiology, bench research or ‘first principles’ |
Grade of recommendation A: based on consistent level 1 evidence B: based on consistent level 2 or 3 evidence or extrapolations from level 1 evidence C: based on level 4 evidence or extrapolations from level 2 or 3 evidence D: based on level 5 evidence or on troublingly inconsistent or inconclusive studies of any level Level of Agreement: anonymous votes for LOA, on a numeric rating scale from 0 (total disagreement) to 10 (total agreement) for each recommendation. Mean and 95% CI of scores were presented. |
| AAOS 2017 [35] |
SLR Delphi consensus Formulation of PICO questions, systematic literature search, review of evidence and integration of evidence to formulate recommendations and voting Searches: full peer-reviewed published report of a clinical study. |
✓ | GRADE | Prognostic Study Design Quality Key: High quality study: <2 flaws Moderate quality study: ≥2 and < 4 flaws Low quality study: ≥4 and < 6 flaws Very low quality study: ≥6 flaws |
Strong: Evidence from two or more “High” quality studies with consistent findings for recommending for or against the intervention. Moderate: Evidence from two or more “Moderate” quality studies with consistent findings, or evidence from a single “High” quality study for recommending for or against the intervention Limited: Evidence from two or more “Low” quality studies with consistent findings or evidence from a single “Moderate” quality study recommending for against the intervention or diagnostic or the evidence is insufficient or conflicting and does not allow a recommendation for or against the intervention Consensus: There is no supporting evidence. Recommendation is based on clinical opinion |
| TLAR 2017 [30] |
SLR Delphi consensus Systematic literature search, development of recommendation according to evidence, voting, reviewed and finalised draft. Search: preference for meta-analysis, systematic reviews and RCTs. |
✓ | Oxman-Guyatt index and Jadad Scale |
Ia: meta-analysi of RCTs Ib: ≥ 1 RCT IIa: ≥ well-designed IIIb: ≥ 1 well-designed quasi-IV: expert committee |
Oxman-Guyatt index (or metanalysis and systematic reviews); Jadad scale: RCTs |
| PANLAR 2016 [31] |
SLR Delphi consensus SLR performed by literature search team, expert consensus through Delphi technique, approval by members of working groups. |
Not available in manuscript | Oxford Centre for Evidence-Based Medicine |
Level A: Information from various randomised clinical trials or meta-analyses. Level B: Information from a randomised clinical trial or nonrandomized studies. Level C: Experts' consensus, case studies, or care standards |
Jadad scale I: There is evidence and/or general agreement that a procedure or treatment is beneficial, useful, or effective. II: Conflicting evidence and/or differing opinions about the efficacy of a procedure or treatment. IIa: Evidence and/or agreement favour usefulness or efficacy. IIb: Usefulness or efficacy is not established by evidence or opinion. III: Conditions for which there is evidence, general agreement, or both that the procedure treatment is not useful/effective and in some cases may be harmful. |
| NICE 2014 [39] |
SLR Expert consensus if absence of proof. Economic considerations Formulation of PICO questions by guideline development group, generation of summaries of evidence according to GRADE profiles, quality appraisal and grading of clinical evidence, review of evidence of cost-effectiveness, development of recommendations, peer review. |
✓ | GRADE | Overall quality of outcome evidence in GRADE: High: Future research is very unlikely to change the estimate of effect Moderate: Future research is likely to have an important impact in the estimate of effect and may change the estimate Low: Future research is very likely to have an important impact in the estimate of effect and is likely to change the estimate Very low: Any estimate of effect is very uncertain. |
Not described |
| EULAR 2013 [27] |
SLR Delphi consensus Delphi consensus. Systematic literature reviews, extensive discussion on recommendations with data from SLR, voting for level of agreement. |
✓ | EULAR standard Operating Procedure (SOP) |
Ia: meta-analysis of RCTs Ib: ≥ 1 RCT IIa: ≥ 1 controlled trial without randomisation IIIb: ≥ 1 well-designed quasi- experiment study IV: expert committee reports or opinions and/or clinical experience of respected authorities. |
Level of agreement: anonymous votes for LOA, on a numeric rating scale from 0 (total disagreement) to 10 (total agreement) for each recommendation. Mean and 95% CI of scores were presented. |
| MSR 2013 [33] |
SLR Development of clinical questions, systematic literature review, grading of evidence, external review of drafted guideline. |
✓ | US/Canadian Preventive Services Task Force |
I: evidence from ≥1 properly RCT II-1: Evidence from well-designed controlled trials without randomisation II-2: Evidence from well-designed cohort or case-control analytic studies, preferably from >1 centre or group. II-3: Evidence from multiple time series with or without intervention III: Opinions of respected authorities based on clinical experience; descriptive studies and case reports; or reports of expert committees |
Modified from the Scottish Intercollegiate Guidelines Network A: ≥ 1 meta analysis, systematic review or RCT or evidence rated as good and directly applicable to the target population B: Evidence from well conducted clinical trials, directly applicable to the target population, and demonstrating overall consistency of results, or evidence extrapolated from meta-analysis, systematic review or RCT C: Evidence from expert committee reports or opinions and/or clinical experience of respected authorities; indicates absence of directly applicable clinical studies of good quality |
Abbreviation:
AAOS: American Academy of Orthopaedic Surgeons.
ACR: American College of Rheumatology.
AGREE II: Appraisal of Guidelines Research and Evaluation II.
ESCEO: European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases.
EULAR: European League Against Rheumatism.
GRADE: Grading of Recommendations Assessment, Development and Evaluation.
ISR: Italian Society for Rheumatology.
LoE: Level of evidence.
NHMRC: National Health and Medical Research Council.
NICE: National Institute for Health and Care Excellence.
MSR: Malaysian Society of Rheumatology.
OARSI: Osteoarthritis Research Society International.
PANLAR: Pan American League of Associations for Rheumatology.
RACGP: Royal Australasian College of General Practitioners.
RCT: randomised controlled trial.
SLR: systematic literature review.
SoR: strength of recommendation.
TLAR: Turkish League Against Rheumatism.
VADoD: Department of Veterans Affairs and Department of Defence.
References
- 1.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2016;2(1) doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Katz J.N., Arant K.R., Loeser R.F. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. doi: 10.1001/jama.2020.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., et al. American College of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2019;72(2):149–162. doi: 10.1002/acr.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Chu I.J.H., Lim A.Y.T., Ng C.L.W. Effects of meaningful weight loss beyond symptomatic relief in adults with knee osteoarthritis and obesity: a systematic review and meta-analysis. Obes. Rev. 2018;19(11):1597–1607. doi: 10.1111/obr.12726. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W., Jones A., Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis?: a meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2004;63(8):901. doi: 10.1136/ard.2003.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen R., Bartels E.M., Astrup A., Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 2007;66(4):433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daugaard C.L., Hangaard S., Bartels E.M., Gudbergsen H., Christensen R., Bliddal H., et al. The effects of weight loss on imaging outcomes in osteoarthritis of the hip or knee in people who are overweight or obese: a systematic review. Osteoarthritis Cartilage. 2020;28(1):10–21. doi: 10.1016/j.joca.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Robson E.K., Hodder R.K., Kamper S.J., O'Brien K.M., Williams A., Lee H., et al. Effectiveness of weight-loss interventions for reducing pain and disability in people with common musculoskeletal disorders: a systematic review with meta-analysis. J. Orthop. Sports Phys. Ther. 2020;50(6):319–333. doi: 10.2519/jospt.2020.9041. [DOI] [PubMed] [Google Scholar]
- 10.Panunzi S., Maltese S., De Gaetano A., Capristo E., Bornstein S.R., Mingrone G. Comparative efficacy of different weight loss treatments on knee osteoarthritis: a network meta-analysis. Obes. Rev. 2021;22(8) doi: 10.1111/obr.13230. [DOI] [PubMed] [Google Scholar]
- 11.Pham T., van der Heijde D., Altman R.D., Anderson J.J., Bellamy N., Hochberg M., et al. OMERACT-OARSI initiative: osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12(5):389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Tubach F., Ravaud P., Martin-Mola E., Awada H., Bellamy N., Bombardier C., et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res (Hoboken) 2012;64(11):1699–1707. doi: 10.1002/acr.21747. [DOI] [PubMed] [Google Scholar]
- 13.Bennell K.L., Lawford B.J., Keating C., Brown C., Kasza J., Mackenzie D., et al. Comparing video-based, telehealth-delivered exercise and weight loss programs with online education on outcomes of knee osteoarthritis : a randomized trial. Ann. Intern. Med. 2022;175(2):198–209. doi: 10.7326/M21-2388. [DOI] [PubMed] [Google Scholar]
- 14.Stenholm S., Vahtera J., Kawachi I., Pentti J., Halonen J.I., Westerlund H., et al. Patterns of weight gain in middle-aged and older US adults. Epidemiology. 1992-2010;26(2):165–168. doi: 10.1097/EDE.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y., Manson J.E., Yuan C., Liang M.H., Grodstein F., Stampfer M.J., et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318(3):255–269. doi: 10.1001/jama.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brebal K.M.M., Silveira J., Menezes R.C.E., Epifânio S.B.O., Marinho P.M., Longo-Silva G. Weight gain and changes in nutritional status of Brazilian adults after 20 years of age: a time-trend analysis (2006-2012) Rev. Bras. Epidemiol. 2020;23 doi: 10.1590/1980-549720200045. [DOI] [PubMed] [Google Scholar]
- 17.Peeters A., Magliano D.J., Backholer K., Zimmet P., Shaw J.E. Changes in the rates of weight and waist circumference gain in Australian adults over time: a longitudinal cohort study. BMJ Open. 2014;4(1) doi: 10.1136/bmjopen-2013-003667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown W.J., Hockey R., Dobson A.J. Effects of having a baby on weight gain. Am. J. Prev. Med. 2010;38(2):163–170. doi: 10.1016/j.amepre.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 19.Tucker L.A., Parker K. 10-Year weight gain in 13,802 US adults: the role of age, sex, and race. J Obes. 2022;2022 doi: 10.1155/2022/7652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) 2000. Obesity : Preventing and Managing the Global Epidemic : Report of a WHO Consultation.https://apps.who.int/iris/handle/10665/42330 [PubMed] [Google Scholar]
- 21.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwers M.C., Kho M.E., Browman G.P., Burgers J.S., Cluzeau F., Feder G., et al. Agree II: advancing guideline development, reporting and evaluation in health care. CMAJ (Can. Med. Assoc. J.) 2010;182(18):E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appraisal of Guidelines Research & Evaluation (Agree) 2021. AGREE II Training Tools.https://www.agreetrust.org/resource-centre/agree-ii/agree-ii-training-tools/ [Google Scholar]
- 24.Hoffmann-Eßer W., Siering U., Neugebauer E.A.M., Lampert U., Eikermann M. Systematic review of current guideline appraisals performed with the Appraisal of Guidelines for Research & Evaluation II instrument-a third of AGREE II users apply a cut-off for guideline quality. J. Clin. Epidemiol. 2018;95:120–127. doi: 10.1016/j.jclinepi.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Cicchetti D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994;6(4):284–290. [Google Scholar]
- 26.Andrade R., Pereira R., van Cingel R., Staal J.B., Espregueira-Mendes J. How should clinicians rehabilitate patients after ACL reconstruction? A systematic review of clinical practice guidelines (CPGs) with a focus on quality appraisal (AGREE II) Br. J. Sports Med. 2020;54(9):512–519. doi: 10.1136/bjsports-2018-100310. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes L., Hagen K.B., Bijlsma J.W.J., Andreassen O., Christensen P., Conaghan P.G., et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013;72(7):1125. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 28.Kloppenburg M., Kroon F.P.B., Blanco F.J., Doherty M., Dziedzic K.S., Greibrokk E., et al. Update of the EULAR recommendations for the management of hand osteoarthritis. Ann. Rheum. Dis. 2018;78(1):16. doi: 10.1136/annrheumdis-2018-213826. [DOI] [PubMed] [Google Scholar]
- 29.Ariani A., Manara M., Fioravanti A., Iannone F., Salaffi F., Ughi N., et al. The Italian Society for Rheumatology clinical practice guidelines for the diagnosis and management of knee, hip and hand osteoarthritis. Reumatismo. 2019;71(S1):5–21. doi: 10.4081/reumatismo.2019.1188. [DOI] [PubMed] [Google Scholar]
- 30.Tuncer T., Cay F.H., Altan L., Gurer G., Kacar C., Ozcakir S., et al. 2017 update of the Turkish League against Rheumatism (TLAR) evidence-based recommendations for the management of knee osteoarthritis. Rheumatol. Int. 2018;38(8):1315–1331. doi: 10.1007/s00296-018-4044-y. [DOI] [PubMed] [Google Scholar]
- 31.Rillo O., Riera H., Acosta C., Liendo V., Bolaños J., Monterola L., et al. PANLAR consensus recommendations for the management in osteoarthritis of hand, hip, and knee. JCR. J. Clin. Rheumatol. 2016;22(7) doi: 10.1097/RHU.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z., Huang C., Jiang Q., Zheng Y., Liu Y., Liu S., et al. Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition) Ann. Transl. Med. 2020;8(19):1213. doi: 10.21037/atm-20-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ministry of Health Malaysia, Malaysian Society of Rheumatology, Academy of Medicine Malaysia . 2013. Clinical Practice Guidelines: Management of Osteoarthritis.https://www.moh.gov.my/moh/attachments/8933.pdf [Google Scholar]
- 34.Bruyère O., Honvo G., Veronese N., Arden N.K., Branco J., Curtis E.M., et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European society for clinical and economic Aspects of Osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO) Semin. Arthritis Rheum. 2019;49(3):337–350. doi: 10.1016/j.semarthrit.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 35.American Academy of Orthopaedic Surgeons . 2017. Management of Osteoarthritis of the Hip Evidence-Based Clinical Practice Guideline.https://www.aaos.org/globalassets/quality-and-practice-resources/osteoarthritis-of-the-hip/oa-hip-cpg_6-11-19.pdf [Google Scholar]
- 36.American Academy of Orthopaedic Surgeons . 2021. Management of Osteoarthritis of the Knee (Non-arthroplasty). Evidence-Based Clinical Practice Guideline.https://www.aaos.org/globalassets/quality-and-practice-resources/osteoarthritis-of-the-knee/oak3cpg.pdf [Google Scholar]
- 37.The Royal Australian College of General Practitioners . East Melbourne; 2018. Guideline for the Management of Knee and Hip Osteoarthriti. [Google Scholar]
- 38.U.S. Department of Veterans Affairs . 2020. VA/DoD Clinical Practice Guidelines: the Non-surgical Management of Hip & Knee Osteoarthritis.https://www.healthquality.va.gov/guidelines/CD/OA/ [Google Scholar]
- 39.National Clinical Guideline Centre . Osteoarthritis: Care and Management in Adults. National Institute for Health and Care Excellence; London: 2014. National Institute for health and clinical excellence: guidance. (UK) Copyright © National Clinical Guideline Centre, 2014. [Google Scholar]
- 40.The Royal Australian College of General Practitioners . 2018. Guideline for the Management of Knee and Hip Osteoarthritis: Technical Document.https://www.racgp.org.au/getattachment/3d0c3153-fcaf-4eaf-be21-859a0c6b8c6d/Technical-document.pdf.aspx [Google Scholar]
- 41.National Clinical Guideline Centre . National Institute for Health and Care Excellence; (UK: 2014. Obesity: Identification, Assessment and Management.https://www.nice.org.uk/guidance/cg189 Copyright © National Clinical Guideline Centre, [PubMed] [Google Scholar]
- 42.Messier S.P., Mihalko S.L., Legault C., Miller G.D., Nicklas B.J., DeVita P., et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teichtahl A.J., Wluka A.E., Tanamas S.K., Wang Y., Strauss B.J., Proietto J., et al. Weight change and change in tibial cartilage volume and symptoms in obese adults. Ann. Rheum. Dis. 2015;74(6):1024–1029. doi: 10.1136/annrheumdis-2013-204488. [DOI] [PubMed] [Google Scholar]
- 44.Bliddal H., Leeds A.R., Stigsgaard L., Astrup A., Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann. Rheum. Dis. 2011;70(10):1798. doi: 10.1136/ard.2010.142018. [DOI] [PubMed] [Google Scholar]
- 45.Henriksen M., Christensen R., Hunter D.J., Gudbergsen H., Boesen M., Lohmander L.S., et al. Structural changes in the knee during weight loss maintenance after a significant weight loss in obese patients with osteoarthritis: a report of secondary outcome analyses from a randomized controlled trial. Osteoarthritis Cartilage. 2014;22(5):639–646. doi: 10.1016/j.joca.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Hunter D.J., Beavers D.P., Eckstein F., Guermazi A., Loeser R.F., Nicklas B.J., et al. The intensive diet and exercise for arthritis (IDEA) trial: 18-month radiographic and MRI outcomes. Osteoarthritis Cartilage. 2015;23(7):1090–1098. doi: 10.1016/j.joca.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahmoudian A., Lohmander L.S., Mobasheri A., Englund M., Luyten F.P. Early-stage symptomatic osteoarthritis of the knee — time for action. Nat. Rev. Rheumatol. 2021;17(10):621–632. doi: 10.1038/s41584-021-00673-4. [DOI] [PubMed] [Google Scholar]
- 48.Ding C., Jones G., Wluka A.E., Cicuttini F. What can we learn about osteoarthritis by studying a healthy person against a person with early onset of disease? Curr. Opin. Rheumatol. 2010;22(5):520–527. doi: 10.1097/BOR.0b013e32833b90e9. [DOI] [PubMed] [Google Scholar]
- 49.Magnusson K., Kumm J., Turkiewicz A., Englund M. A naturally aging knee, or development of early knee osteoarthritis? Osteoarthritis Cartilage. 2018;26(11):1447–1452. doi: 10.1016/j.joca.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Bray G.A., Frühbeck G., Ryan D.H., Wilding J.P. Management of obesity. Lancet. 2016;387(10031):1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 51.Puenpatom R.A., Victor T.W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad. Med. 2009;121(6):9–20. doi: 10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 52.Ekram A.R., Cicuttini F.M., Teichtahl A.J., Crammond B.R., Lombard C.B., Liew S.M., et al. Weight satisfaction, management strategies and health beliefs in knee osteoarthritis patients attending an outpatient clinic. Intern. Med. J. 2016;46(4):435–442. doi: 10.1111/imj.13007. [DOI] [PubMed] [Google Scholar]
- 53.Carmona-Terés V., Moix-Queraltó J., Pujol-Ribera E., Lumillo-Gutiérrez I., Mas X., Batlle-Gualda E., et al. Understanding knee osteoarthritis from the patients' perspective: a qualitative study. BMC Muscoskel. Disord. 2017;18(1):225. doi: 10.1186/s12891-017-1584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isla Pera P., Ferrér M.C., Nuñez Juarez M., Nuñez Juarez E., Maciá Soler L., López Matheu C., et al. Obesity, knee osteoarthritis, and polypathology: factors favoring weight loss in older people. Patient Prefer. Adherence. 2016;10:957–965. doi: 10.2147/PPA.S92183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall K.D., Kahan S. Maintenance of lost weight and long-term management of obesity. Med. Clin. 2018;102(1):183–197. doi: 10.1016/j.mcna.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kahan S., Puhl R.M. The damaging effects of weight bias internalization. Obesity. 2017;25(2):280–281. doi: 10.1002/oby.21772. [DOI] [PubMed] [Google Scholar]
- 57.Puhl R.M., Lessard L.M., Pearl R.L., Himmelstein M.S., Foster G.D. International comparisons of weight stigma: addressing a void in the field. Int. J. Obes. 2021;45(9):1976–1985. doi: 10.1038/s41366-021-00860-z. [DOI] [PubMed] [Google Scholar]
- 58.Rubino F., Puhl R.M., Cummings D.E., Eckel R.H., Ryan D.H., Mechanick J.I., et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 2020;26(4):485–497. doi: 10.1038/s41591-020-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen L., Zheng M., Chen Z., Peng Y., Jones C., Graves S., et al. Osteoarthritis Cartilage; 2021. The Burden of End-Stage Osteoarthritis in Australia: a Population-Based Study on the Incidence of Total Knee Replacement Attributable to Overweight/obesity. [DOI] [PubMed] [Google Scholar]
- 60.Messier S.P., Newman J.J., Scarlett M.J., Mihalko S.L., Miller G.D., Nicklas B.J., et al. Changes in body weight and knee pain in adults With knee osteoarthritis three-and-a-half years after completing diet and exercise interventions: follow-up study for a single-blind, single-center, randomized controlled trial. Arthritis Care Res. (Hoboken) 2022;74(4):607–616. doi: 10.1002/acr.24765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sumithran P., Prendergast L.A., Delbridge E., Purcell K., Shulkes A., Kriketos A., et al. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 62.Martin J.C., Awoke M.A., Misso M.L., Moran L.J., Harrison C.L. Preventing weight gain in adults: a systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2021;22(10) doi: 10.1111/obr.13280. [DOI] [PubMed] [Google Scholar]
- 63.Vandevijvere S., Chow C.C., Hall K.D., Umali E., Swinburn B.A. Increased food energy supply as a major driver of the obesity epidemic: a global analysis. Bull. World Health Organ. 2015;93(7):446–456. doi: 10.2471/BLT.14.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heymsfield S.B., Wadden T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017;376(3):254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 65.Doucet E., Hall K., Miller A., Taylor V.H., Ricupero M., Haines J., et al. Emerging insights in weight management and prevention: implications for practice and research. Appl. Physiol. Nutr. Metabol. 2021;46(3):288–293. doi: 10.1139/apnm-2020-0585. [DOI] [PubMed] [Google Scholar]