Summary
Objective
Fibroblast growth factor 18 (FGF18) is involved in chondrogenesis and articular cartilage repair. We investigated tissue distribution and pharmacokinetics of radioactive [3H]sprifermin, a recombinant human FGF18, in rats after a single intravenous (i.v.) or intra-articular (i.a.) injection.
Design
In two studies (48–96-h [n = 23] and 28-day [n = 12]), 35 male albino (Sprague Dawley) rats received single i.v. or i.a. dose [3H]sprifermin (0.24 mg/kg). Radioactivity was measured in blood, serum, and (in animals receiving i.a. administration) in the knee joint by liquid scintillation counting. Radioactivity in organs, tissues, and distribution in the whole body were measured with whole-body autoradiography.
Results
After i.v. injection, radioactivity peaked in serum and whole blood after 4 and 24 h, respectively, with greater total radioactivity in serum. After i.a. injection, radioactivity peaked in serum and whole blood after 24 and 48 h, respectively; intact [3H]sprifermin was not detected in vena caval serum and systemic exposure was low, approximately 20% of that with i.v. injection. Following i.v. injection, radioactivity was mainly found in the liver, adrenal glands, kidney, and spleen; following i.a. injection, radioactivity was preferentially concentrated in articular cartilage after initial distribution in the joint capsule, and still evident in the joint after 28 days.
Conclusions
After i.a. injection of [3H]sprifermin in rats, radioactivity was concentrated in the knee joint, particularly articular cartilage, with low levels in other investigated tissues. Systemic exposure to sprifermin was greater with i.v. than i.a. injection. Subsequent clinical investigation in patients with osteoarthritis has reported consistent results.
Keywords: Distribution, FGF18, Intra-articular, Pharmacokinetics, Rat model
1. Introduction
Fibroblast growth factor 18 (FGF18) is an anabolic growth factor involved in chondrogenesis and articular cartilage repair, specifically stimulating chondrocyte proliferation, osteoblast differentiation, and cartilage matrix production [[1], [2], [3], [4]]. Endogenous human FGF18 is a 19.8 KDa protein expressed by chondrocytes in articular cartilage. Sprifermin is a recombinant, truncated, form of human FGF18 that is currently being investigated as a potential disease-modifying treatment in osteoarthritis (OA) of the knee [5]. Preclinical data have shown that sprifermin stimulates chondrocyte proliferation, cartilage matrix formation, and cartilage repair in in vitro and in vivo models [3,4,6,7]. A first-in-human trial [8] found no systemic sprifermin exposure in patients with OA of the knee after intra-articular (i.a.) sprifermin injections. In addition, a subsequent proof-of-concept trial in humans [9] showed serum sprifermin levels to remain below the lower limit of quantification (100 pg/ml) after a single i.a. injection.
To further describe the safety profile of sprifermin, this article reports results from two studies investigating tissue distribution and pharmacokinetics of radioactive drug-related material after a single intravenous (i.v.; evaluating maximum exposure to drug) or i.a. (preferred route of therapeutic administration) injection of [3H]sprifermin in rats. The first study was a short-term investigation, studying time points up to 48 or 96 h after injection; the second extended to 28 days after injection. Each study had two parts: in the first part, liquid scintillation counting (LSC) was used to assess tissue distribution and blood/serum levels of total radioactivity to derive pharmacokinetic parameters, and to perform whole-body autoradiography in the sacrificed animals; in the second part, high performance liquid chromatography (HPLC) was used to assess levels of intact and aggregated [3H]sprifermin, as well as [3H]sprifermin degradation fragments, in serum and i.a. fluid obtained at the investigation time points from animals used in the first part of each study.
2. Methods
2.1. Preparation of radiolabelled sprifermin
[3H]Sprifermin was supplied by the study sponsor as a liquid formulation dissolved in buffer solution (pH 7.3) at a concentration of 3.73 mg/ml, specific activity 907–1300 μCi/mg; the material was stored in dark conditions at −80 °C. The formulation was used as supplied for i.a. injection and diluted with bi-distilled water to a concentration of 0.24 mg/ml for i.v. injection. Homogeneity and concentration of radioactivity of solutions were determined before dosing by LSC of weighed aliquots mixed with 18 ml Ultima Gold scintillation cocktail (Perkin Elmer, Groningen, The Netherlands) using a Tri-Carb 1900 TR liquid scintillation analyzer (Packard [now Perkin Elmer], Meriden, CT, USA). In the HPLC analyses of [3H]sprifermin distribution, radiochemical [3H]sprifermin purity of reference samples was assessed by reverse-phase HPLC (see below for details of HPLC methodology).
2.2. Experimental animals
All procedures were carried out in male albino (Sprague Dawley) rats (48–96-h study: age 7 weeks, body weight 233–258 g; 28-day study: age 9–10 weeks, body weight 324–364 g), supplied by Charles River Laboratories Italia Srl, Calco, Italy. All animals were visually inspected for signs of illness and were deemed fit for use. Before treatment, the rats were housed in polypropylene and stainless-steel cages with wood shavings as bedding. During the experimental period, the animals were individually housed in polypropylene and stainless-steel cages with raised wire mesh floors. Lighting was controlled in a 12-h light-dark cycle. A complete dry diet (Mucedola 4RF21) and domestic mains quality water was available ad libitum throughout. The animals were observed during treatment and over the course of the experiments to evaluate any evidence of reaction to treatment, change in general appearance, overt signs of suffering, or evidence of toxicity.
Studies were carried out in compliance with: the Organisation for Economic Co-operation and Development Principles of Good Laboratory Practice (as revised in 1997); the Decreto legislativo 27 Gennaio 1992, No. 120, and the Decreto 5 Agosto 1999: ‘Disposizioni relative all'ispezione e verifica della buona prassi di laboratorio in recepimento delle direttive 1999/11/CE e 1999/12/CE’; and the Directive 2004/10/EC of the European Parliament and of the Council of 11 February 2004.
2.3. Study design
Experimental design is shown in Table 1. A total of 35 rats was used in the two studies, 23 in the 48–96-h study, receiving i.v. or i.a. administration of [3H]sprifermin, and 12 in the 28-day study, receiving i.a. administration of [3H]sprifermin. The 23 in the first study were divided into five groups. Groups 1–4 each included five animals, which were sacrificed at 0.25, 1, 4, 24 and 48 h; Group 5 included three animals, all sacrificed at 96 h. Rats in Group 1 received i.v. 3[H]sprifermin; those in all other groups received i.a. 3[H]sprifermin. The following samples were collected across the groups: whole body sections, blood and serum samples (Groups 1 and 2); joint articulation, blood and serum (Groups 3 and 4); IA fluid (Group 4); and serum (Group 5). The 12 rats in the second study were divided into two groups of six animals, all receiving i.a. 3[H]sprifermin and sacrificed at 1, 2, 4, 7, 14 and 28 days. Whole body sections, blood and serum was collected from animals in Group 1; knee joint, IA fluid, blood and serum from those in Group 2. Dosing was carried out on the same day for all animals within a single group.
Table 1.
Experimental design.
| Group | Route | n | Sacrifice time | Samples collected/analyzed | |
|---|---|---|---|---|---|
| 48–96-h study | 1 | i.v. | 5 | 0.25, 1, 4, 24, 48 h | Whole body sections, whole blood, serum |
| 2 | i.a. | 5 | 0.25, 1, 4, 24, 48 h | Whole body sections, whole blood, serum | |
| 3 | i.a. | 5 | 0.25, 1, 4, 24, 48 h | Knee joint (with i.a. fluid), whole blood, serum | |
| 4 | i.a. | 5 | 0.25, 1, 4, 24, 48 h | Knee joint (without i.a. fluida), whole blood, serum | |
| 5 | i.a. | 3 | 96 h | Serum (serial samples via SVC cannula)b | |
| 28-day study | 1 | i.a. | 6 | 1, 2, 4, 7, 14, 28 days | Whole body sections, whole blood, serum |
| 2 | i.a. | 6 | 1, 2, 4, 7, 14, 28 days | Knee joint (without i.a. fluida), whole blood, serum |
i.a.: intra-articular, HPLC: high performance liquid chromatography, i.v.: intravenous, SVC: superior vena cava.
Intra-articular fluid was taken before removal and solubilization of the knee joint.
Samples at each time point were pooled before HPLC analysis.
2.4. Administration of [3H]sprifermin
[3H]sprifermin was administered by i.v. or i.a. injection at a target dose of 0.24 mg/kg. I.v. administration was via a single bolus (target volume 1 ml/kg) injected into the tail vein without anaesthesia. After dosing, the syringe was washed with methanol:water 1:1 (vol/vol). The dose dispensed was determined by subtraction of the radioactivity in the washing from the radioactivity contained in the syringe before administration (determined from the weight of dose formulation administered and the concentration of radioactivity in the dose formulation). Before i.a. administration, animals were lightly anaesthetized (aqueous mixture of ketamine, xylazine, and acepromazine given s.c.); [3H]sprifermin was injected into the articular capsule of the right hind knee joint. The dose dispensed was determined from the difference between the weights of the syringe before and after administration, and from the concentration of radioactivity in the dose formulation and the specific activity of the [3H]sprifermin used.
2.5. Sample collection and preparation
In the 48–96-h study, a subset of rats selected on the basis of satisfactory clinical examinations and acceptable blood flow through a cannula (Group 5; n = 3; see Table 1) were fitted with a cannula to enable serial blood sampling; the cannula was implanted in the superior vena cava via the jugular vein under general anaesthesia (aqueous mixture of ketamine, xylazine, and acepromazine) approximately 5 days before administration of [3H]sprifermin. Serial blood samples were taken from these animals at 0.25, 0.5, 1, 2, 4, 8, 24, 48, 72, and 96 h after administration of [3H]sprifermin; the animals were then sacrificed by cardiac puncture (i.e. exsanguination) under deep anaesthesia induced by diethyl ether inhalation; in addition, the final blood sample was collected from the puncture site. Using this procedure, all remaining animals in both studies were sacrificed, and terminal blood samples obtained at the scheduled times (i.e. 0.25–48 h or 1–28 days; see Table 1). Animals to be analyzed by whole-body autoradiography were immediately frozen in hexane and dry ice, and stored at −20 °C.
For analysis of [3H]sprifermin in (treated) knee joints, the knee joint was surgically exposed and excised, then solubilized by stirring in 3 M sodium hydroxide solution containing 30% v/v methanol and 10% v/v Triton X (all Carlo Erba Reagenti SPA, Rodano, Italy) for 48 h at 60 °C. I.a. fluid was removed before solubilization from some knee joints (see Table 1) by i.a. fluid washings, flushed from the knee joint capsule using 10 μl saline.
2.6. Whole-body autoradiography
Frozen carcasses, after partial shaving and removal of ears, paws, and tail, were embedded in 2.0% weight/volume aqueous carboxymethylcellulose gel (Sigma-Aldrich) and stored at −20 °C. Sagittal sections (30 μm thick) of embedded frozen carcasses were prepared using a Bright 9400 cryomicrotome (Bright Instruments Co., Huntingdon, UK), mounted on wooden frames and dried in a cryochamber at −20 °C for 48 h before treatment with talcum powder. Prepared sections were placed in Fuji BAS 2040 exposition cassettes (Fujifilm Imaging and Information, Tokyo, Japan) and exposed to a 20 × 40 cm IP BAS-IIITR phosphor imaging plate (Fujifilm Imaging and Information, Tokyo, Japan) at room temperature for 144–192 h in a shielding box. The photostimulated luminescence of each section was recorded electronically (as an autoradioluminogram) using a CD-Writer Plus 7570i (Hewlett Packard Co., Palo Alto, CA, USA). Organs examined included the salivary glands, adrenal glands, lungs, liver, kidney, urinary bladder, and spleen; other evaluated regions included the knee joint and bone marrow.
2.7. Quantification of total radioactivity by liquid scintillation counting
Total radioactivity in blood, serum, and knee joint samples was quantified by LSC using a Tri-Carb 1900 TR liquid scintillation analyzer. Aliquots of serum (~50 μl/100 mg) were weighed in polypropylene vials, then mixed with 18 ml Ultima Gold scintillation cocktail. Radioactivity in whole blood was assessed by LSC of [3H]H2O generated by combustion of whole blood samples (~50 mg in the 48–96-h study, and 100–150 mg in the 28-day study); combusted whole blood samples were prepared using Oximate 80 and Oxidizer 307 (Hewlett Packard) and the steam generated was collected into counting vials containing 18 ml tritium scintillator Monophase-S (Perkin Elmer) using an air-cooled condenser. Duplicate aliquots of solubilized knee joint samples were mixed with 18 ml Hioic Fluor scintillation cocktail (Perkin Elmer).
2.8. Quantification of radioactivity in autoradioluminograms
Quantitative evaluation of autoradioluminograms was carried out using the Advanced Image Data Analyser (AIDA) applicative software, version 2.43 (Raytest GmbH, Straubenhardt, Germany). The signal, recorded as photostimulated luminescence units, was evaluated in areas corresponding to regions of interest (tissues and organs identifiable by visual observation of the latent image). Image densities were converted, using precalibrated values, to radioactivity concentration (nCi/mg tissue equivalent) and to ng equivalent/g tissue (ngeq/g). The limit of quantification was defined as 10 times the standard deviation of background values measured in each analysis.
2.9. Pharmacokinetic parameters
Pharmacokinetic parameters were determined from the 28-day study using a non-compartmental approach, with the aid of WinNonLin® version 3.1 (Pharsight Corp., Sunnyville, CA, USA) and Excel spreadsheet (Microsoft Inc., Seattle, WA, USA). The following pharmacokinetic parameters were determined: maximum concentration of total radioactivity (Cmax); time to maximal total radioactivity concentration (tmax); time when last detectable concentration of radioactivity was measured (tlast); area under the total radioactivity concentration versus time curve up until the last detectable concentration (AUC0–t(last)); area under the total radioactivity concentration-versus-time curve to infinity (AUC0–∞); and apparent terminal half-life of total radioactivity (t½,z). Total radioactivity in bone marrow, liver, kidney, and spleen was used to determine Cmax, tmax, tlast and AUC0–t(last). AUC0–t(last) was calculated by the linear trapezoid rule as linear combination concentrations. t1/2,z was determined by linear regression of the natural log (ln) concentration-versus-time curve using the equation:
| t½,z = ln(2)/slope of the regression line |
AUC0–∞ was determined by the linear trapezoidal rule up to Ct(last) (the concentration of total radioactivity at tlast), with extrapolation beyond tlast to infinity, assuming monolinear exponential decay, using the following equation:
The fraction of AUC represented by the extrapolated portion (EXT) was calculated as follows:
| %EXT AUCt(last)–∞ = 100% (AUCt(last)–∞ – AUC0–∞) |
2.10. Quantification of serum and i.a. levels of unchanged sprifermin, aggregates, and fragments by HPLC
Samples were analyzed by HPLC (1100 Series quaternary pump, Agilent Technologies, Inc., Santa Clara, CA, USA) and an MPS3 autosampler (Gerstel GmbH, Mülheim an der Ruhr, Germany) under either reverse-phase or size-exclusion analytical conditions. Serum samples (50–100 μl) were injected directly into the HPLC system. I.a. articular fluid samples (9 μl) were added to 500 μl size-exclusion mobile phase (50 mM sodium phosphate/0.5 M ammonium sulphate, pH 7.2 with 10% isopropyl alcohol); 100 μl samples were then injected into the HPLC system. Radioactivity was detected using either an online flow radiodetector (Flo One Beta; Perkin Elmer, Waltham, MA, USA) or an offline liquid scintillation analyzer (1600CA or 2900 TR, Perkin Elmer), after fraction collection (FC 203B collector; Gilson Italia, Middleton, WI, USA). Samples of serum and i.a. fluid (10 μl) were added to 10 ml Ultima Gold for LSC; values obtained were compared with results obtained by standard HPLC. Calibration curves were obtained using 5–100 ng/ml [3H]sprifermin (48.1 MBq/mg).
For reverse-phase analysis, the following solvents were used at a flow rate of 1.2 ml/min: mobile phase A, 0.1% (v/v) trifluoroacetic acid (TFA) in Milli-Q water; mobile phase B, 0.1% TFA in acetonitrile; and mobile phase C, 0.1% TFA in isopropanol (all Sigma-Aldrich, St Louis, MO, USA). The column was 150 × 4.6 mm Jupiter C4 5 μm (Phenomenex) or Zorbax 3000SB-C18 3.5 μm (Agilent Technologies, Inc.). The security guard cartridges were Widepore C18 4 × 2 mm or C5 4 × 2 mm (both Phenomenex). Injection volumes were 100, 50, and 80 μl, respectively, for i.a. washings and i.v. and vena caval serum samples. Acquisition time was 65 min, and column and sample temperatures were 40 °C and 4 °C, respectively. The scintillation cocktail for flow counting was Ultima Flo M 25% plus Hionic Fluor 75% in 3% triethanolamine (all Perkin Elmer). Size-exclusion analysis used a mobile phase of 50 mM sodium phosphate/0.5 M ammonium sulphate (pH 7.2) with 10% isopropanol. Acquisition time was 25 min, and column and sample temperatures were room temperature and 4 °C, respectively.
Molecular weights (MWs) of sprifermin fragments obtained by size-exclusion analysis were determined using a calibration curve generated by an MW-GF-1000 Kit (Sigma-Aldrich). The MWs of fragments of sprifermin were evaluated by comparing the ratio of elution volume:void volume (Ve:V0) against the protein standards, where V0 was the volume of eluent required to elute Blue Dextran.
3. Results
3.1. Animals
All 35 rats were treated and included in the analysis and grouped as described in the Methods. Weights and doses received by each animal are shown in Supplementary Table I. No overt adverse signs were observed in the test animals during the conduction of either study.
3.2. Dosing
In the 48–96-h study, the dose of [3H]sprifermin administered by i.v. injection was 0.20–0.23 mg/kg (mean 0.21 mg/kg). The dose of [3H]sprifermin administered by i.a. injection in the 48–96-h study was 0.11–0.18 mg/kg (mean 0.14 mg/kg); these values exclude two rats, 1 and 48 h, who received inappropriately high (0.31 mg/kg) and low (0.06 mg/kg) doses. In the 28-day study, the dose administered (by i.a. injection) was 0.14–0.25 mg/kg (mean 0.18 mg/kg).
3.3. Tissue distribution of total radioactivity in rats
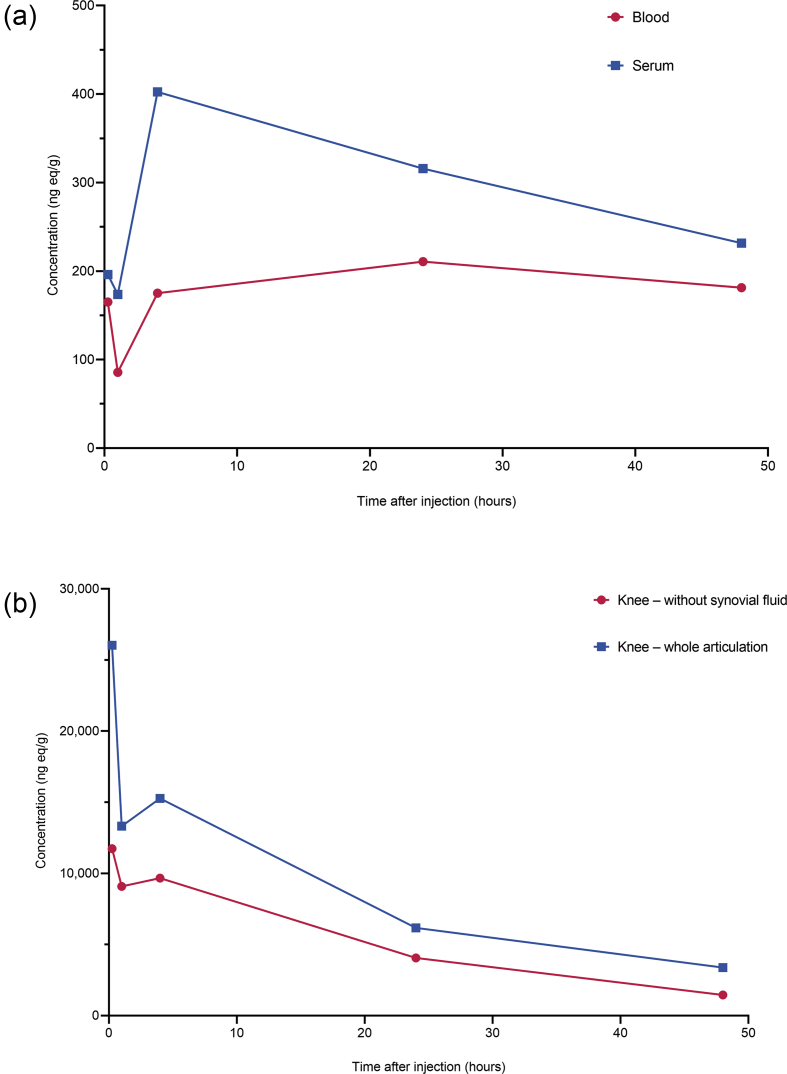
Following i.v. administration of [3H]sprifermin, maximal radioactivity in serum and whole blood was detected after 4 and 24 h, respectively (48–96-h study; Fig. 1a, Supplementary Table II). Total radioactivity was higher in serum than in whole blood. The half-life in serum was approximately 55 h, as measured by total radioactivity. Following i.a. administration, radioactivity peaked in serum after 24 h and in whole blood after 48 h; systemic exposure was about 20% of that obtained following i.v. administration. Radioactivity was high in the knee joint; the decline in radioactivity in the knee joint was biphasic, with a terminal t½ of 20 h (Fig. 1b).
Fig. 1.
Concentration of radioactivity in (a) whole blood and serum following a single intravenous (i.v.) injection of [3H]sprifermin (target dose 0.24 mg/kg) and (b) knee joint following a single intra-articular (i.a.) injection of [3H]sprifermin (target dose 0.24 mg/kg) to male rats. Data in (b) are five rats from which the knee joint was removed intact, i.e. including i.a. fluid, and from another five rats from which i.a. fluid was removed from the knee joint before solubilization (see Table 1).
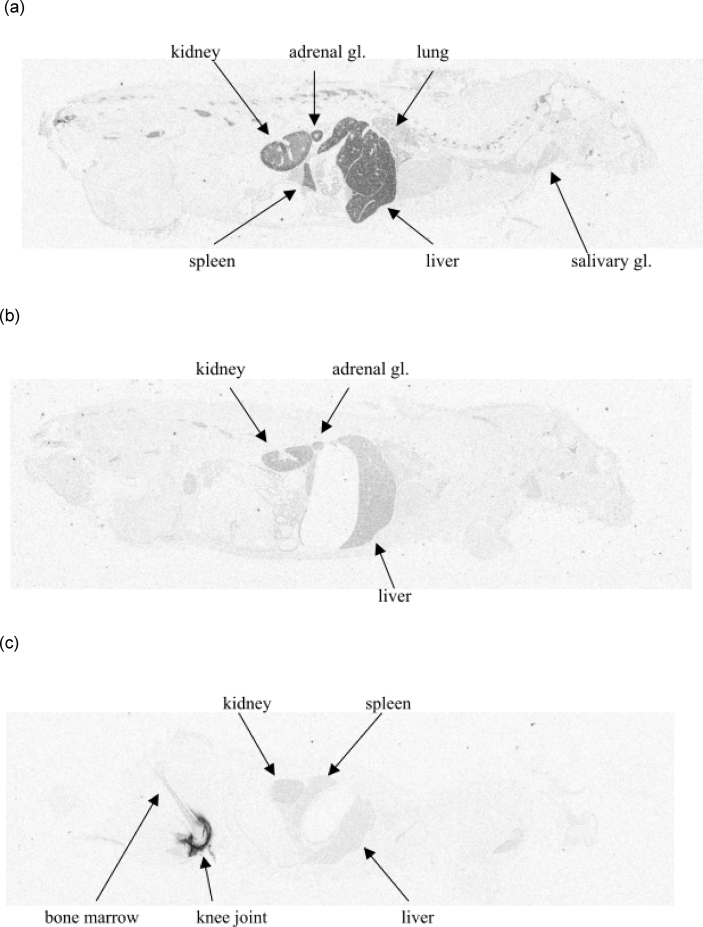
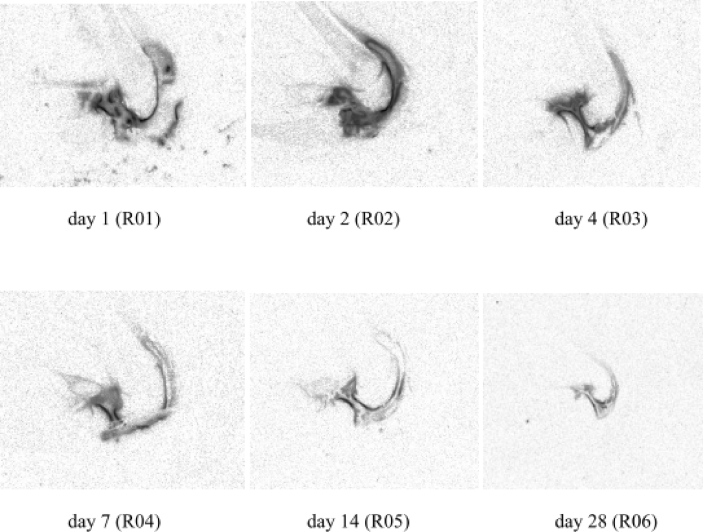
Whole-body autoradiography following i.v. administration showed that radioactivity was mainly present in the liver, adrenal glands, kidney, and spleen (Fig. 2a and b, Supplementary Table III). Radioactivity in these organs was highest at 0.25 h after i.v. injection, declining rapidly thereafter. Levels in lungs and salivary gland were relatively low. Whole-body autoradiography following i.a. injection revealed low levels in all tissues (mostly at background levels), although some radioactivity was detected in the kidney (Fig. 2c) and urinary bladder at the 1- and 4-h time points (Supplementary Table III). Autoradiography of the knee joint after i.a. injection showed that radioactivity was preferentially distributed from the synovial fluid into the articular cartilage, and that radioactivity was still evident in the joint 28 days after injection (Fig. 3).
Fig. 2.
Whole-body autoradiograph (a) 0.25 h after intravenous (i.v.) injection of [3H]sprifermin; (b) 24 h after i.v. injection of [3H]sprifermin; (c) 48 h after intra-articular (i.a.) injection of [3H]sprifermin (target dose 0.24 mg/kg).
Fig. 3.
Distribution of total radioactivity in the knee joint 1–28 days after a single intra-articular injection of [3H]sprifermin at a target dose of 0.24 mg/kg.
3.4. Pharmacokinetic parameters
Pharmacokinetic parameters (derived from 28-day study data, single i.a. injection) of the total radioactivity in whole blood, serum, and knee joint determined using LSC, and in bone marrow, liver, kidney, and spleen quantified using whole-body radiography, are shown in Table 2.
Table 2.
Pharmacokinetic parameters of total radioactivity in whole blood, serum, knee joint, bone marrow, liver, kidney, and spleen of rats after intra-articular injection of [3H]sprifermin at a target dose of 0.24 mg/kg.
| Pharmacokinetic parameter | Liquid scintillation counting |
Whole-body autoradiography |
|||||
|---|---|---|---|---|---|---|---|
| Whole blood | Serum | Knee joint | Bone marrow | Liver | Kidney | Spleen | |
| Cmax (ngeq/g) | 89.0 | 180 | 11,742 | 220 | 114 | 162 | 130 |
| tmax (hours) | 48 | 24 | 24 | 24 | 48 | 24 | 48 |
| tlast (hours) | 672 | 672 | 672 | 48 | 168 | 168 | 168 |
| AUC0-t(last) (ng/eq . h/g) | 31,766 | 33,013 | 1,109,388 | 7805 | 11,666 | 17,784 | 12,542 |
| t1/2,z (hours) | 359 | 134 | 231 | – | – | – | – |
| Regression range (hours) | 48–672 | 24–672 | 48–672 | – | – | – | – |
| AUC0–∞ (ng/eq . h/g) | 43,202 | 34,281 | 1,266,853 | – | – | – | – |
| % EXT AUC0–t(last) | 26 | 4 | 12 | – | – | – | – |
Cmax: maximum concentration of total radioactivity, tmax: time to maximal total radioactivity concentration, tlast: time when last detectable concentration of radioactivity was measured, AUC0-t(last): area under the total radioactivity concentration versus time curve up until the last detectable concentration, t1/2,z: apparent terminal half-life of total radioactivity, AUC0–∞: area under the total radioactivity concentration-versus-time curve to infinity, %EXT AUC0–t(last): the fraction of AUC represented by the extrapolated portion (EXT).
3.5. Serum and intra-articular levels of unchanged sprifermin, aggregates and fragments in rats
Following i.v. administration, levels of unchanged sprifermin in serum declined rapidly. The concentration profile for [3H]sprifermin was higher with reverse-phase than with size-exclusion HPLC; but with both methods, levels of intact sprifermin were below the lower limit of quantification (LLOQ; 4.3 and 5.0 ng/ml, respectively) after the 4-h time point. After i.a. administration, intact sprifermin was not detected in the vena caval samples from cannulated rats; in sacrificed rats, unchanged sprifermin in serum was below the LLOQ by size-exclusion HPLC at all time points in (i.e. up to 48 h) and was not detected by reverse-phase HPLC. Unchanged sprifermin levels in i.a. washings were maximal 1 h after i.a. injections (>350 ng/ml) by size-exclusion HPLC; with reverse-phase HPLC, the highest concentration, 152 ng/ml, was measured at 0.25 h, with the level falling to 16 ng/ml at 48 h.
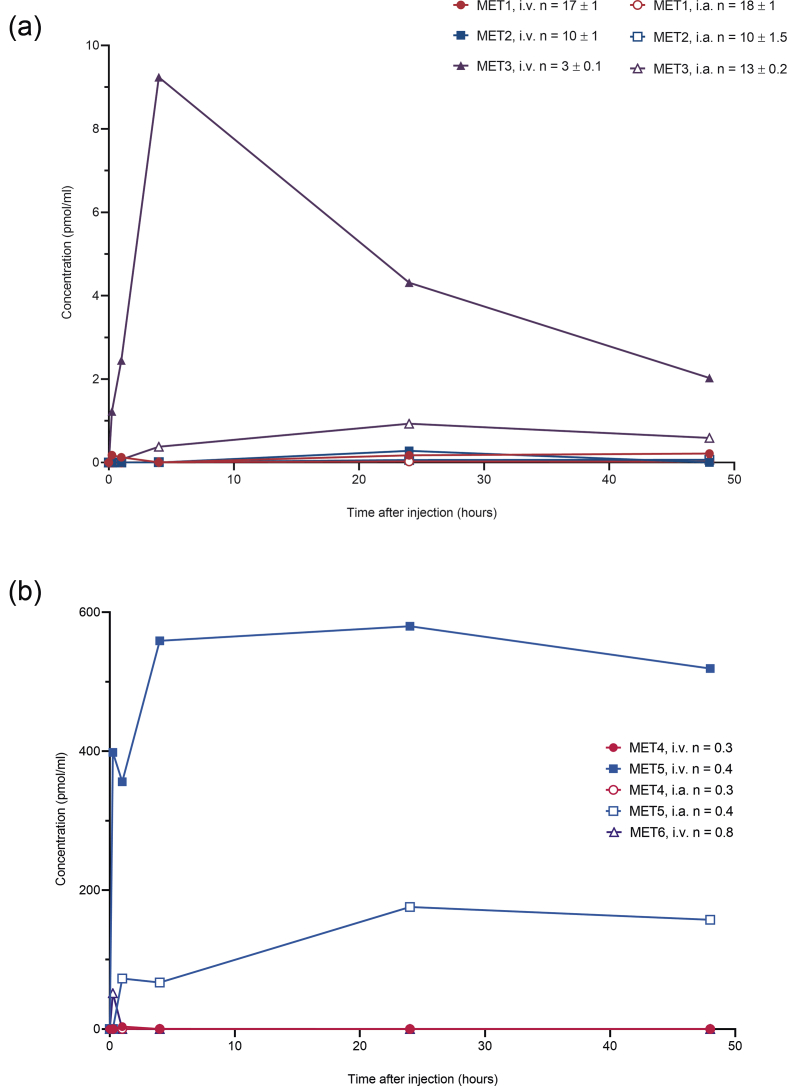
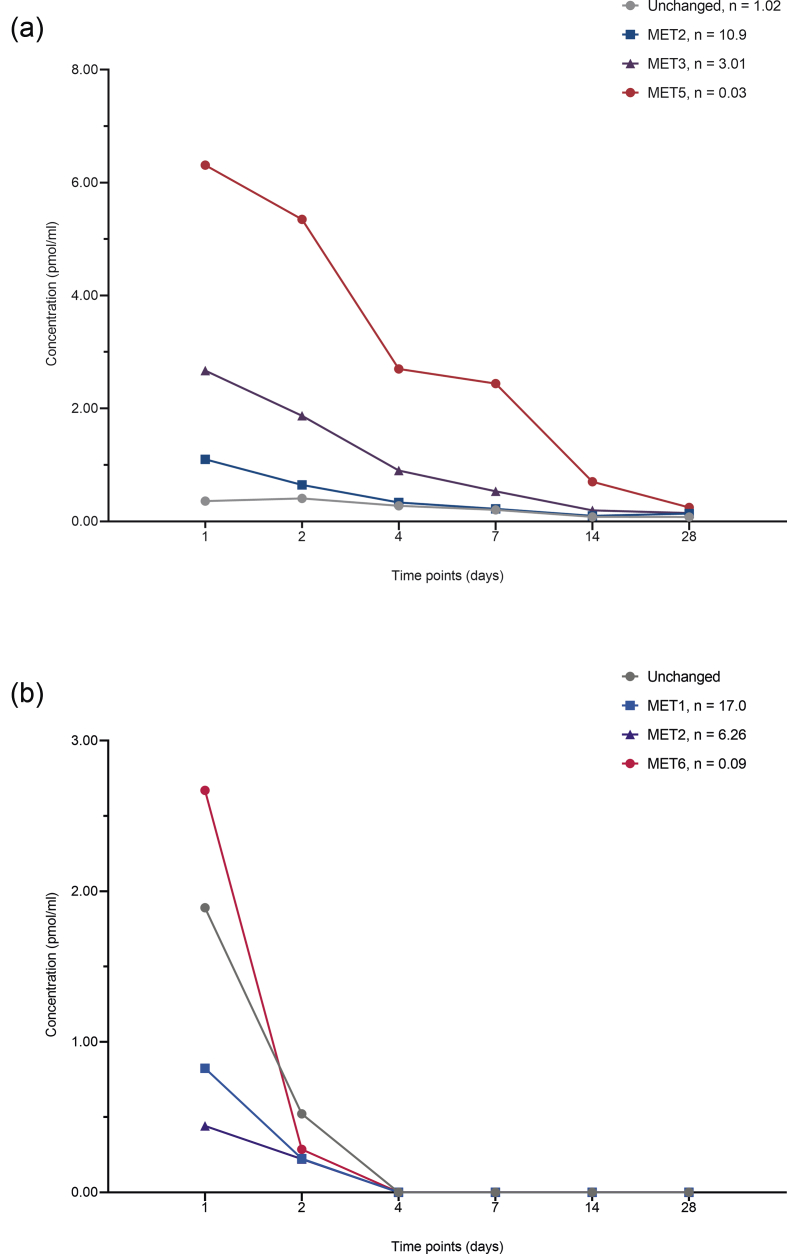
In the 48–96-h study, a total of three sprifermin aggregates (MET1, MET2, and MET3) and three metabolites (MET4, MET5, and MET6) were identified in serum. The predominant aggregate after either i.v. or i.a. [3H]sprifermin administration was MET3; serum levels were maximal at 4 h after i.v. administration and at 24 h after i.a. injection (Fig. 4a). After i.v. and after i.a. administration, MET5 (785 ± 79 Da) was the predominant fragment found in serum; levels of MET4 (MW 5049 ± 707 Da) were low after administration of sprifermin by either route (Fig. 4b). MET5 was also detected (>110 pmol/ml) in vena caval serum samples, at 0.25 h, only following i.a. injection. A single aggregate (MET1) and a single fragment (MET6; MW 1387 ± 109 Da) were detected in i.a. washings following i.a. injection; the metabolite MET6 was the most abundant i.a. species at all time points measured (0.25–48 h).
Fig. 4.
(a) Aggregate (MET1, MET2, MET3) and (b) fragment (MET4; MW5049 Da; MET5, MW 785 Da) profiles in serum of peripheral blood samples determined using size-exclusion high performance liquid chromatography in rats receiving a single injection (at a target dose of 0.24 mg/kg) of [3H]sprifermin into the tail vein (intravenous [i.v.], mean value from two analyses from one rat at each time point) or the articular capsule of the knee joint (intra-articular [i.a.]; mean value from two analyses from each of three rats at each time point). n, calculated number of intact molecules in the reported aggregate/fragment (molecular weights of aggregate/fragment divided by molecular weights of intact sprifermin).
In the 28-day study (i.a. injection), levels of unchanged [3H]sprifermin in serum were low, being slightly above the LLOQ by size-exclusion HPLC until Day 4, and below the LLOQ by reverse-phase HPLC at all time points after Day 1 (Fig. 5a); i.a. levels at Days 1 and 2 (size-exclusion HPLC) were comparable with those seen at the equivalent time points in the 48–96-h study (see Fig. 4), and fell below the LLOQ (0.25 pmol/ml) thereafter (Fig. 5b). Aggregates MET2 and MET3 were above the LLOQ in serum up to Days 4 and 7, respectively. As in the 48–96-h study, MET5 was the predominant modified form identified in serum; levels decreased with time but remained above the LLOQ at Day 28. In i.a. washings, aggregates MET1 and MET2 and fragment MET6 (the most abundant modified form) were above the LLOQ up to Day 2 (Fig. 5b).
Fig. 5.
Levels of unchanged sprifermin, aggregates (MET1, MET2, MET3) and fragments (MET5, MW 785 Da; MET6, MW 1387 Da) determined by size-exclusion high performance liquid chromatography in (a) serum (mean value from two analyses from each of two rats at each time point) and (b) intra-articular (i.a.) washings after i.a. injection of [3H]sprifermin at a target dose of 0.24 mg/kg (mean value from two analyses from one rat at each time point). n, number of intact molecules in the aggregate/fragment.
4. Discussion
Cartilage degradation, mediated by increased catabolic activity and inappropriate cartilage repair responses, is a hallmark of OA [6]. Consequently, a rational approach to targeting the pathophysiology and structural changes associated with OA is to develop a therapeutic agent with the capacity to repair or regenerate cartilage in affected joints. As a member of the FGF family of homeostatic factors involved in tissue repair and injury response [10], FGF18 can repair cartilage through stimulation of chondrogenesis [1,10,11]. Sprifermin is a recombinant human version of naturally occurring FGF18 that is currently being investigated as a potential disease-modifying treatment in OA of the knee [5].
Preclinical data show sprifermin to stimulate chondrocyte proliferation, cartilage matrix formation, and cartilage repair [3,4,6,7]. Initial results in humans, evaluating blood levels of FGF18 and anti-FGF antibody formation, did not find any evidence for systemic effects of sprifermin after i.a. injection [5,8,9], and serum levels of the drug in single and multiple dose cohorts remained below the lower limit of quantification [5,9]. The animal studies described herein evaluated the safety profile of sprifermin further. Using radioactive drug-related material, [3H]sprifermin, tissue distribution and pharmacokinetics were investigated in a rat model after i.a. administration, the preferred therapeutic route, as well as via i.v. delivery, to help determine drug exposure and tissue distribution.
After i.a. administration of [3H]sprifermin in rats, pharmacokinetic analysis confirmed that radioactivity was preferentially concentrated in the treated knee joint in comparison with blood, serum, and organs; Cmax was higher in the knee joint than in blood, serum, bone marrow, liver, kidney, and spleen, and AUC values showed that exposure to total radioactivity was greatest in the knee joint. The decline of radioactivity in the knee joint follows a well-known biphasic pattern seen with i.a. injections: the initial “drug burst” was followed by an initial distribution phase from synovial tissue into the cartilage and subsequently by a redistribution from the cartilage into the synovial fluid according to the concentration sink of the synovial fluid. The t1/2 was consistently above 24 h.
Whole-body autoradiography showed radioactivity distributed mainly around the i.a. injection site and the articular cavity, and particularly within the articular cartilage; radioactivity was preferentially concentrated at the injection site and articular cavity within 24 h of i.a. injection, and was still evident in the knee joint up to 28 days after i.a. injection. According to our unpublished data, the driving factor for this preferential distribution of sprifermin is its strong positive charge. With an isoelectric point (pI) of 10, sprifermin is attracted to the negatively charged hyaline cartilage and therefore to the cartilaginous parts of the ligaments (insertion points) and the hyaline parts of the menisci but not to the non-charged synovium.
Radioactivity was poorly distributed to other tissues, with the exception of some radioactivity in the kidney and urinary bladder. An important finding was that intact sprifermin was not detected in vena caval serum samples after i.a. injection; i.e. there was no demonstrable systemic exposure to intact sprifermin. This has important clinical safety implications, indicating that the compound was concentrated mostly at the i.a. injection site, with much less systemic exposure (below the detection limit at timepoints after 4 h), thus reducing the risk of systemic adverse effects.
Upon further investigation, after i.v. administration of [3H]sprifermin, radioactivity was mainly distributed in the liver, adrenal glands, kidney, and spleen. The detection of some radioactivity in the urinary bladder suggests that sprifermin and/or metabolites of the protein may be excreted by this route. Given the molecular weight of sprifermin (<20 KDa), it is feasible that both unchanged and fragmented sprifermin could pass through the glomerulus into urine and be excreted. Total radioactivity was higher in serum than in whole blood following i.v. injection, consistent with the parent compound and its metabolites having a low affinity for blood cells.
Three sprifermin aggregates (MET1, MET2 and MET3) and three metabolites (MET4, MET5 and MET6) were identified in our analyses. Currently, no data are available showing the processes involved in the formation of aggregate and degraded forms of sprifermin in vivo. Therefore, we can only speculate on the potential mechanisms involved. One conceivable such mechanism could be the formation of larger complexes by heparan sulphate binding of FGFs [12,13].
An important limitation of our study is the use of a small animal model. The small size of the rat joint may have allowed for systemic leakage following i.a. injection. Thus, some of the systemic exposure observed may have been specific to the animal model.
Our results demonstrate a preferential concentration of radioactivity in the knee joint, particularly in articular cartilage, after i.a. injection of [3H]sprifermin in rats, and higher systemic exposure to sprifermin with i.v. than with i.a. injection, supporting use of an i.a. route of administration in clinical investigations. The findings are particularly valuable in the context of the recently published results from the multicenter, randomized FORWARD study [5]. In this phase 2 clinical trial, patients with symptomatic radiographic knee OA received i.a. injections of sprifermin 30 μg or 100 μg every 6 or 12 months. At the 2-year follow-up, i.a. injections with the 100 μg dose led to an increase in total femorotibial joint cartilage thickness, confirming the highly local action of sprifermin within the joint [5]. As with earlier clinical results [9], all serum sprifermin levels were below the lower limit of quantification (100 pg/ml), and no safety concerns were identified [5]. Additional studies with sprifermin in targeted clinical populations are needed to clarify the clinical significance of the findings with this agent to date.
Author contributions
C.H. Ladel was involved in the conception and design of the study, analysis and interpretation of data, drafting the article, and approval of the final version to be submitted. L. Barbero was involved in the provision of study materials, as well as the collection, assembly, analysis, and interpretation of data, critically revising the article for important intellectual content, and approval of the final version to be submitted. S. Riva was involved in the provision of study materials, as well as analysis and interpretation of the data, critically revising the article for important intellectual content, and approval of the final version to be submitted. H. Guehring was involved in the analysis and interpretation of the study data, providing statistical expertise, as well as drafting the article, and approval of the final version to be submitted.
Role of the funding source
Both studies were funded by Merck Serono International S.A., an affiliate of Merck KGaA, Darmstadt, Germany. The study sponsor was involved in the study design, the collection, analysis, interpretation of data, and the decision to submit this manuscript for publication.
Declaration of Competing Interest
C.H. Ladel and H. Guehring are employees of, and shareholders in, Merck KGaA Darmstadt, Germany. L. Barbero is an employee of Merck Serono Ivrea, Colleretto, Italy (an affiliate of Merck KGaA, Darmstadt, Germany). S. Riva is an employee of Gentium s.r.l Villa Guardia, Italy (a company of Jazz Pharmaceuticals).
Acknowledgments
Editorial assistance was provided by Syntropy Medica Ltd, Worthing, UK and Bioscript Group, Macclesfield, UK, both supported by Merck Serono International S.A. (Geneva, Switzerland).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2020.100068.
Contributor Information
C.H. Ladel, Email: christoph.ladel@merckgroup.com.
L. Barbero, Email: luca.barbero@merckgroup.com.
S. Riva, Email: simonarivas@libero.it.
H. Guehring, Email: hans.guehring@merckgroup.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Davidson D., Blanc A., Filion D., Wang H., Plut P., Pfeffer G., et al. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J. Biol. Chem. 2005;280:20509–20515. doi: 10.1074/jbc.M410148200. [DOI] [PubMed] [Google Scholar]
- 2.Ellman M.B., An H.S., Muddasani P., Im H.J. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420:82–89. doi: 10.1016/j.gene.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellsworth J.L., Berry J., Bukowski T., Claus J., Feldhaus A., Holderman S., et al. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthritis Cartilage. 2002;10:308–320. doi: 10.1053/joca.2002.0514. [DOI] [PubMed] [Google Scholar]
- 4.Moore E.E., Bendele A.M., Thompson D.L., Littau A., Waggie K.S., Reardon B., et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005;13:623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg M.C., Guermazi A., Guehring H., Aydemir A., Wax S., Fleuranceau-Morel P., et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. Jama. 2019;322:1360–1370. doi: 10.1001/jama.2019.14735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gigout A., Guehring H., Froemel D., Meurer A., Ladel C., Reker D., et al. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthritis Cartilage. 2017;25:1858–1867. doi: 10.1016/j.joca.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Gigout A., Werkmann D., Lindemann S., Kleinschmidt K., Guehring H. Recombinant human fibroblast growth factor-18 (rhFG18) promotes bovine articular chondrocyte proliferation and cartilage matrix production in vitro. Osteoarthritis Cartilage. 2012;20 [Google Scholar]
- 8.Dahlberg L.E., Aydemir A., Muurahainen N., Guhring H., Fredberg Edebo H., Krarup-Jensen N., et al. A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin. Exp. Rheumatol. 2016;34:445–450. [PubMed] [Google Scholar]
- 9.Lohmander L.S., Hellot S., Dreher D., Krantz E.F., Kruger D.S., Guermazi A., et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2014;66:1820–1831. doi: 10.1002/art.38614. [DOI] [PubMed] [Google Scholar]
- 10.Ornitz D.M., Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. 3005.1–3005.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun Y.R., Won J.E., Jeon E., Lee S., Kang W., Jo H., et al. Fibroblast growth factors: biology, function, and application for tissue regeneration. J. Tissue Eng. 2010;2010:218142. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown A., Adam L.E., Blundell T.L. The crystal structure of fibroblast growth factor 18 (FGF18) Protein Cell. 2014;5:343–347. doi: 10.1007/s13238-014-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetz R., Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.