Abstract
Objective
The objective of this study was to evaluate the effectiveness of an online patient decision aid with individualised potential outcomes of surgery, on the quality of decisions for knee replacement surgery in routine clinical care.
Design
A pragmatic Randomized Controlled Trial (RCT) in patients considering total knee replacement at a high-volume orthopedic clinic. Patients were randomized at their routine online pre-surgical assessment to either complete a decision aid or not. At their consultation, those in the intervention arm had a surgeon report summarizing the decision aid results. The primary outcome was decision quality, defined as being knowledgeable and choosing the option that matched informed treatment preferences. Multivariate logistic and linear regression analysis was conducted to consider surgeon level clustering and baseline differences between study arms.
Results
Of 163 patients randomized, 155 completed post-surgical surveys and were included in the analysis. The average patient was aged 65 years, obese and had moderate to severe osteoarthritis symptoms at baseline. Patients in the intervention arm had a higher odds of making a quality decision (Odds Ratio = 2.08, 95% CI: 1.08 to 4.02), predominantly through increased knowledge.
Conclusions
This study supports the benefit of a decision aid in combination with a surgeon report to significantly improve decision quality in routine care. While the independent contribution of tailoring the decision aid to patient baseline characteristics and including a surgeon report remains unclear, we demonstrated the feasibility of integrating the decision aid into an online pre-surgical assessment in routine clinical care.
Keywords: Shared decision-making, Joint arthroplasty, Decision aids
1. Introduction
Total knee arthroplasty (TKA) is the most common elective surgery which is effective for most patients with knee osteoarthritis (OA). While TKA reduces pain and disability in a majority of patients, 15–30% experience little or unsatisfactory symptom relief [1,2], 3% require revision within 5 years [3], and a small number experience serious side-effects [4]. With a growing demand for TKA, there is a need to prioritize those most appropriate for surgery [5]. A set of both clinically-relevant and patient focussed ‘appropriateness’ criteria have been defined [6], which include helping set patient's expectations about the possible outcomes of surgery so they can consider whether the potential benefits outweigh the potential harms [6,7]. Patients with unmet expectations following TKA are more likely to be dissatisfied with their surgical results [8].
Shared decision making (SDM) has been widely advocated as a way to better set these expectations [9], by informing patients about the benefits and harms of TKA and non-surgical conservative management options which are effective [10], but underutilized [11]. Previous studies have found that using a patient decision aid to promote SDM can improve knowledge and the quality of decisions [12], overcoming common biases where individuals often overestimated an intervention's benefit and underestimated its harms [13]. However, these studies have typically not been conducted in routine care and so the results are potentially less generalizable [12]. The decision aids studied have also used evidence for the average population on the potential outcomes of surgery, such as “if 100 people had TKA, about 75 would have less pain post surgery.” [14] The potential challenge of providing a 50 year old with moderate pain and an 80 year old with severe pain the same information to help set expectations is twofold. First, the evidence may be inaccurate and so expectations not properly informed as various risk factors such as pre-surgical pain are known to be associated with outcomes following TKA [15]. Second, there can be a lack of trust in average data, with some believing they would be better or worse than an average. While age, sex and other sociodemographic variables have not been found to be associated with different outcomes following TKA surgery, they do influence patients' expectations for surgery [8], and the salience of tailored evidence to people has been shown to increase belief and trust in evidence [16].
The aim of this study was to evaluate the effectiveness of an online patient decision aid with individualised potential outcomes of surgery in combination with a surgeon report, on the quality of decisions for knee replacement in routine clinical care.
2. Methods
The reporting of this Randomized Controlled Trial (RCT) follows Consolidated Standards of Reporting Trials (CONSORT) guidelines [17] for RCTs and the Standards for UNiversal reporting of patient Decision Aid Evaluation (SUNDAE) guidelines for reporting the results of patient decision aid studies [18]. This study was approved by the University of Calgary and University of British Columbia research ethics boards and is registered on Clinicaltrials.gov (#NCT03240913). A detailed trial protocol was published previously and served as the basis for this analysis [19].
2.1. Study design
This prospective, two-arm RCT was embedded into routine care at the Edmonton Bone and Joint Centre, a high-volume orthopedic clinic in Edmonton, Alberta, Canada between July 2017 and December 2019. Approximately 6000 patients have knee replacement surgery each year, and are routinely asked to answer an online survey prior to their surgical consultation.
2.2. Study population
Eligible participants included adults over the age of 30 years who were considering total knee replacement, scheduled for a consultation at the clinic, could understand, speak, read English, and provide informed consent. Patients were excluded if they had previously received a total knee replacement, were having bilateral knee replacement, or had physician-diagnosed rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, fibromyalgia or gout.
2.3. Study interventions
-
•
Decision aid and surgeon report: The online, individualised, decision aid and a one-page summary report for their surgeon has been described previously [19]. The patient decision aid was developed following International Patient Decision Aid Standards criteria (IPDAS) [20,21] and included information on osteoarthritis and two treatment options: 1) total knee replacement and 2) non-surgical treatment. As described previously, the decision aid describes and compares treatment options based on the available evidence for chance of repeat surgery, need for physiotherapy, recovery period, and changes 6 months after surgery across the five dimensions of health-related quality-of-life as defined by the EuroQol-5D (EQ-5D) 5-level instrument: mobility, self-care, usual activities, pain/discomfort, anxiety/depression. [22] Evidence for each factor came from previous RCT evidence for average effects [23] supplemented for the five dimensions of the EQ-5D data from the Alberta Bone & Joint Health database which included over 50,000 pre- and post-responses to the EQ-5D. Where evidence allowed, estimates of the potential outcomes from knee replacement surgery were individualised based on clinical (baseline EQ-5D responses) and demographic characteristics (age, sex and body mass index (BMI)) which are important proxies for expectations for surgery [8]. The one-page surgeon report was developed based on the previously published Canadian appropriateness checklist and includes information on the patient's knowledge and preferences. A pdf version of the report was placed in the patient's file prior to meeting with the surgeon at the clinic, and the surgeon completed the appropriateness checklist for the patient [6].
-
•
Routine care: This arm followed standard clinic procedures [19]. This included completing routine data collection prior to consult with a surgeon, some study-specific outcomes (e.g. knowledge), and the appropriateness checklist, but did not include any information on the potential outcomes of surgery or non-surgical treatment options.
2.4. Study endpoints
2.4.1. Study outcomes
The primary outcome of this study was the difference in decision quality, which requires that the person is both sufficiently knowledgeable and chooses a treatment option that is concordant with their treatment preference (defined as 3 out of 5 knowledge questions answered correctly AND surgical preference equaling actual treatment decision) [24]. Secondary outcomes included patient-reported decisional conflict, perceived shared decision-making and preference for involvement in decision-making.
2.4.2. Instruments
Decision quality was measured using a modified version of the 5 item knowledge questionnaire from the validated hip and knee decision quality instrument (HK-DQI) [25]. The existing question about improvement in pain was modified to fit the language of the EQ-5D which was used to describe the pain experience in the decision aid. Knowledge was assessed within the decision aid, or in a separate survey for routine care patients prior to consult visit. Concordance required that the patient's treatment preference matched the treatment decision. Treatment preference was assessed at baseline, and measured using the following question: “Do you feel the potential benefits of knee replacement surgery outweigh the potential surgical risks?” from the appropriateness checklist [6]. Treatment decision was ascertained after the surgical consult through chart review.
Secondary outcomes included the individual components of knowledge and value concordance from the decision quality measure. We also assessed the 4-item SURE Test to measure decisional conflict [26], the 3-item CollaboRATE instrument following the surgical consult [27], a single question on willingness to have surgery and the single item Control Preference Scale to measure preferred role in decision making [28].
2.4.3. Sample size
We estimated a required sample size of 280 (140 per arm) based on an absolute difference in decision quality of 17.5% (from 44.5% to 62%) based on previous research [29], and using a Normal approximation of the Binomial distribution at the 5% significance level with 80% power, and assuming 10% loss to follow-up [19]. However, recruitment was closed before reaching the estimated sample size outlined in the protocol, since recruitment was slower than expected given long waiting times for surgery.
2.4.4. Randomisation
We randomized patients to the decision aid and surgeon report arm or routine care in a 1:1 allocation ratio using computer-generated randomisation schedule using permuted blocks (generated in SAS). Randomisation was implemented with the Research Electronic Data Capture (RED-Cap) software platform assigned as patients consented to participate.
2.4.5. Blinding
Patients were blinded to treatment allocation, but due to the nature of the decision aid intervention, which included a preference report attached to the patients file prior to the surgical consultation, surgeons and some staff were not blinded to treatment allocation.
2.4.6. Statistical methods
As per the protocol, the primary analysis followed patients as assigned by treatment [19]. Due to low levels of missing data, only complete cases were analysed. While randomisation occurred at the individual patient level, patients were clustered by surgeon. It is widely known that ignoring clustering in RCTs will give an unbiased estimate of the treatment effect but can lead to biased estimates of the standard error [30,31]. To determine the impact of clustering, intraclass correlation coefficients (ICC) were estimated using the Analysis of Variance (ANOVA) method [18]. The primary outcome (decision quality) was dichotomous. It was analysed using a logistic regression model which does not allow for clustering (termed the ‘independence’ model), and one that does (termed the ‘random intercept’ model). [32] Models were compared using the likelihood ratio test. The use of regression models allowed for the inclusion of additional covariates that could control for imbalances between treatment arms that persist despite randomisation. Results for dichotomous primary and secondary outcomes were expressed using the Odds Ratio (OR) and 95% confidence interval (CI). In addition, the predicted probability of making a good quality decision and associated 95% CI were estimated for each treatment arm using coefficients of the regression model. Continuous secondary outcomes (patient knowledge) were modelled using linear regression. Ordered categorical secondary outcomes (willingness to have surgery; preference for involvement in decision-making) were modelled using ordinal logistic regression (if the parallel trends assumption held) and multinomial logistic regression if not. All data were analysed using R version 3.5.2.
3. Results
3.1. Trial implementation and study population
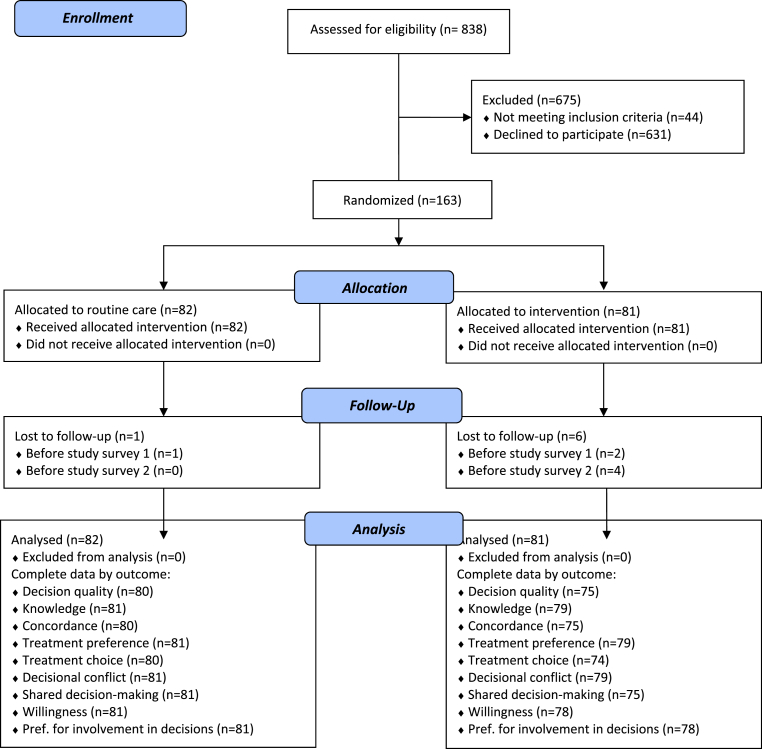
A total of 838 individuals were assessed for eligibility, with 666 declining to participate and 44 deemed ineligible. Of eligible participants, only 163 of 794 (21%) were recruited to the trial (see Fig. 1). Of the 163 individuals who were randomised, 160 completed the baseline survey, and 156 completed the second study survey, resulting in a survey completion rate of 97%. We had sufficient data to evaluate the primary outcome (decision quality) for 155 participants. Some baseline data were available for all 163 participants due to data coming from routine data collection (e.g., baseline EQ-5D-5L) rather than the study specific surveys.
Fig. 1.
Consort diagram.
Patient characteristics are outlined in Table 1. Data were nearly complete: two individuals were missing data on BMI, and one was missing data for the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Patient Health Questionnaire (PHQ-9) instruments. The average patient was about 65 years old and had a BMI classified as obese (BMI≥ 30 kg/m2). Despite randomisation, there was a higher proportion of females in the intervention arm and consequently all analyses included sex as a covariate. In terms of health status, the average patient was experiencing mild/moderate symptoms of depression (as measured by the PHQ-9), moderate to severe osteoarthritis symptoms (as measured by the WOMAC), and moderate to severe pain or discomfort as indicated by the EQ-5D. Around 40% of participants in each arm wanted to make the final decision on surgery after seriously considering their surgeons opinion while approximately 45% of participants want to share the responsibility of that decision with their surgeon.
Table 1.
Characteristics of participants in each arm.
| Routine Care | Decision Aid | |
|---|---|---|
| N = 82 | N = 81 | |
| Age, mean (SD) | 64.95 (7.54) | 64.17 (8.34) |
| Females, number (%) | 38 (46.3) | 52 (64.2) |
| Body mass index, mean (SD) | 31.96 (5.32) | 32.20 (5.16) |
| WOMACa, mean (SD) | 57.77 (16.18) | 54.24 (15.82) |
| PHQ-9 Ϯ, mean (SD) | 10.46 (3.39) | 10.96 (2.93) |
| EQ-5D-5L - Utility, mean (SD) | 0.47 (0.26) | 0.48 (0.23) |
| EQ-5D -5L (Mobility), number (%) | ||
| No problems | 1 (1.2) | 3 (3.7) |
| Slight problems | 8 (9.8) | 12 (14.8) |
| Moderate problems | 38 (46.3) | 32 (39.5) |
| Severe problems | 34 (41.5) | 34 (42.0) |
| Extreme problems | 1 (1.2) | 0 (0.0) |
| EQ-5D-5L (Self-care), number (%) | ||
| No problems | 34 (41.5) | 34 (42.0) |
| Slight problems | 19 (23.2) | 27 (33.3) |
| Moderate problems | 23 (28.0) | 17 (21.0) |
| Severe problems | 6 (7.3) | 3 (3.7) |
| Extreme problems | 0 (0.0) | 0 (0.0) |
| EQ-5D-5L (Usual activities), number (%) | ||
| No problems | 1 (1.2) | 2 (2.5) |
| Slight problems | 9 (11.0) | 13 (16.0) |
| Moderate problems | 37 (45.1) | 32 (39.5) |
| Severe problems | 28 (34.1) | 31 (38.3) |
| Extreme problems | 7 (8.5) | 3 (3.7) |
| EQ-5D-5L (Pain/discomfort), number (%) | ||
| No problems | 0 (0.0) | 0 (0.0) |
| Slight problems | 7 (8.5) | 7 (8.6) |
| Moderate problems | 33 (40.2) | 31 (38.3) |
| Severe problems | 30 (36.6) | 35 (43.2) |
| Extreme problems | 12 (14.6) | 8 (9.9) |
| EQ-5D-5L (Anxiety/depression), number (%) | ||
| No problems | 34 (41.5) | 24 (29.6) |
| Slight problems | 14 (17.1) | 23 (28.4) |
| Moderate problems | 24 (29.3) | 26 (32.1) |
| Severe problems | 8 (9.8) | 6 (7.4) |
| Extreme problems | 2 (2.4) | 2 (2.5) |
| Control Preference Scale, n (%) | ||
| I prefer to make the final treatment decision. | 6 (7.4) | 3 (3.8) |
| I prefer to make the final treatment decision after seriously considering my doctor's opinion. | 32 (39.5) | 34 (43.6) |
| I prefer that my doctor and I share responsibility for deciding which treatment is best. | 35 (43.2) | 36 (46.2) |
| I prefer that my doctor makes the final decision about which treatment, but seriously considers my opinion. | 5 (6.2) | 5 (6.4) |
| I prefer to leave all treatment decisions to my doctor. | 3 (3.7) | 0 (0.0) |
WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).
PHQ-9 (Patient Health Questionnaire).
EQ-5D 5 L (EuroQol-5D 5-level instrument).
Range 0–96. Higher = more symptoms; Ϯ Range: 0–27, Higher = more symptoms (depression).
3.2. Primary outcome: decision quality
Of the 155 patients with complete outcomes (75 in the decision aid arm, 80 routine care), patients exposed to the decision aid and surgeon report had a higher odds of making a quality decision (OR = 2.08, 95% CI: 1.08 to 4.02) (Table 2). This was based on a model that included sex as a covariate, but did not include clustering by surgeon based on an ICC of 0.005, and a likelihood ratio test showing clustering did not improve model fit (p = 0.60). The predicted probability (adjusted) of making a quality decision in the decision aid arm was 61% (95% CI: 49%–72%) compared to 43% (95% CI: 30%–57%) in the routine care arm (unadjusted probabilities were 60% vs 41%).
Table 2.
Primary outcomes.
| Routine Care (Total n = 81) | Decision Aid (Total n = 79) | Adjusted Odds Ratioa (95% CI) | |
|---|---|---|---|
| Knowledge Questionnaire (5-item), n correct/N (%) | |||
| Over time, without surgery, what usually happens to the pain from hip (knee) osteoarthritis? | 75/81 (92.6) | 74/79 (93.7) | 1.18 (0.33–4.33) |
| After knee replacement surgery, about how many months does it take most people to get back to doing their usual activities? | 49/81 (60.5) | 69/79 (87.3) | 4.45 (2.04–10.43)∗ |
| About how many people who have hip (knee) replacement surgery will need to have the same hip (knee) replaced again in less than 15 years? | 28/81 (34.6) | 48/79 (60.8) | 3.00 (1.57–5.85)∗ |
| If 100 people like you have knee replacement surgery, how many people improve and have NO or SLIGHT pain or discomfort after surgery? | 22/81 (27.2) | 24/79 (30.4) | 1.11 (0.55–2.24) |
| Out of 100 people who have hip (knee) replacement surgery, about how many will have a serious complication (e.g., death, life-threatening blood clots, infection, heart attack) within the 3 months after surgery? | 33/81 (40.7) | 60/79 (75.9) | 5.35 (2.66–11.25)∗ |
| Knowledgeable (3 of 5 knowledge questions correct), n/N (%) | 43/80 (53.1) | 65/79 (82.3) | 4.01 (1.97–8.60)∗ |
| Treatment Preference, n/N (%) | |||
| Surgery | 59/81 (72.8) | 65/79 (82.3) | 0.59 (0.03–6.48) |
| Non-surgical | 1/81 (1.2) | 2/79 (2.5) | Reference |
| Unsure | 21 /81 (25.9) | 12/79 (15.2) | – |
| Decided (vs unsure) | 1.98 (0.90–4.52) | ||
| Treatment Decision, n/N (%) | |||
| Surgery | 69/80 (86.3) | 54/74 (73.0) | 0.41 (0.17–0.93) |
| Non-surgical | 11/80 (13.8) | 20/74 (27.0) | Reference |
| Concordant (treatment Preference equals Decision), n/N (%) | 53/80 (66.2) | 52/75 (69.3) | 1.15 (0.58–2.29) |
| Quality Decision (knowledgeable and concordant), n/N (%) | 33/80 (41.3) | 45/75 (60.0) | 2.08 (1.08–4.02)∗ |
Models do not account for clustering by surgeon given findings on ICC, but do adjust for sex; ∗p < 0.05.
3.3. Secondary outcomes
Individuals who completed the decision aid had increased odds of being knowledgeable (OR: 4.01, 95% CI: 1.88 to 8.57), and reduced odds of decisional conflict on the feeling informed subscale (“Do you know the benefits and risks of knee replacement surgery and non-surgical treatment?“, OR = 6.48, 95% CI: 2.04–28.84) (Table 1). Fewer individuals who completed the decision aid were undecided about treatment (15% vs 26% not reporting being ‘unsure’) but this difference had wider confidence intervals (OR = 1.98, 95% CI: 0.90–4.52).
Despite no difference in preference for surgical (vs. non-surgical) treatment options, willingness to have surgery, or having preferences concordant with surgical decision, those exposed to the decision aid had a decreased odds of deciding to actually have surgery during the timeframe of the study (OR = 0.41, 95% CI: 0.17–0.93) (Table 3). Less than half of participants reported all aspects of shared decision making occurred, (47.5% vs 44.0%, OR = 0.86, 95% CI: 0.45–1.64).
Table 3.
Secondary outcomes.
| Routine Care (Total N = 81) | Decision Aid (Total N = 79) | Adjusted Odds Ratios (95% CI)b | |
|---|---|---|---|
| Decisional Conflict SURE Test Scale, n ‘yes’ /N | |||
| Do you feel sure about the best choice for you? | 67/81 (82.7) | 62/79 (78.5) | 0.75 (0.33–1.67) |
| Do you know the benefits and risks of knee replacement surgery and non-surgical treatment? | 64/81 (79.0) | 76/79 (96.2) | 6.48 (2.04–28.84) |
| Are you clear about which benefits and risks matter most to you? | 66/81 (81.5) | 71/79 (93.7) | 1.93 (0.77–5.13) |
| Do you have enough support and advice to make a choice? | 66/81 (81.5) | 62/79 (78.5) | 0.75 (0.34–1.67) |
| Combined (4 out of 4) | 57/81 (70.4) | 56/79 (70.9) | 0.96 (0.48–1.92) |
| Shared decision-making (top scorea), n (%) | 38/80 (47.5) | 33/75 (44.0) | 0.86 (0.45–1.64) |
| Willingness to have surgery, n (%) | |||
| I would definitely consider having knee joint replacement surgery now. | 60 (74.1) | 51 (65.4) | 1.64 (0.83–3.28) |
| I would probably consider having knee joint replacement surgery now. | 13 (16.0) | 13 (16.7) | |
| I am not sure. | 5 (6.2) | 12 (15.4) | |
| I would probably not consider having knee joint replacement surgery now. | 3 (3.7) | 2 (2.6) | |
| I would definitely not consider having knee joint replacement surgery now. | 0 (0.0) | 0 (0.0) | |
Top score is best outcome on all items for collaborate scale.
Models do not account for clustering by surgeon given ICC results, but do adjust for sex; ∗p < 0.05.
4. Discussion
The patient decision aid in combination with surgeon report resulted in better quality of decisions around TKA in routine clinical care, predominantly through better knowledge about benefits and harms of the respective interventions. This confirms results of previous studies, but is the first to determine this in a pragmatic design based in routine care with both high internal and external validity. On average, patients who completed the patient decision aid scored one point higher on the 5-item knowledge test than those in the routine care arm. Without the decision aid, many patients would have surgery with less informed expectations of what they might experience.
Patients with the decision aid and surgeon report were less likely to have surgery, despite no difference in reported shared decision-making. Trends in a reduction in surgery have been seen in previous studies of decision aids in TKA in similar contexts to this. Importantly, studies of decision aids in underserved populations have shown an increase in surgery, hence the focus of shared decision-making interventions should be on the quality of decisions rather than changes in uptake [33]. Whether the decision aid and surgeon report change clinical outcomes, and patient satisfaction post surgery, particularly in those who do not have expected outcomes will be assessed once patients have been followed up post surgery.
The differences in knowledge and decision quality were larger than those seen in a previous decision aid trials in Canada [29]. This may be due to the patient group since the study was implemented in routine clinical care, or due to the use of a different decision aid. While there was an increased odds of correctly answering three of the five knowledge questions, there was no better odds of correctly answering the one modified question based on individualised estimates of potential improvement in pain or discomfort. Further investigation finds that individualization did change people's expectations towards improved knowledge, but not sufficiently to meet our pre-defined definition of being sufficiently ‘knowledgeable’. Given fewer people in the decision aid arm had surgery, it is possible the individualization led to discussions between the surgeon and patient that are not captured in the study outcomes. Nevertheless, further research is required to better describe what to expect post surgery – not just whether there is an improvement but how much of an improvement might be seen. Furthermore, studies should examine if patients with less pain improvement are less dissatisfied due to being better informed prior to surgery.
The primary limitation of our study was the inability to recruit our intended sample size, and the subsequent threat to the generalizability of patients included in our study. Follow-up with potential patients suggested a lack of comfort with an electronic tool, and the length of time required (the decision aid was given after patients had already completed a lengthy pre-surgical online survey) were important factors. This low uptake likely led to an imbalance in the sex between study arms. Biological sex is a proxy for many other variables that may influence TKA decision making e.g. employment status, expectations of surgery, OA severity at presentation. While we included sex as a covariate in the analysis, this may not explain this complex association with the outcomes. Another limitation in our study design was our inability to compare our individualised decision aid to a conventional decision aid that uses average information that has been used in previous studies. In part this was due to the lack of availability of the conventional decision aid, and issues with the evidence it contains for the Canadian context, but also a reflection that no decision aid is routinely used in Canadian clinical practice. However, while the decision aid developed for this study followed IPDAS guidelines for development, including user testing with patients, feedback from patients suggests that it can be further improved. Describing numerical risks was challenging, and even though we used graphical displays, describing improvements to different levels of an outcome is complicated. Importantly, there is a dearth of information on the outcomes of patients who choose to not have surgery - and so our decision aid was unable to provide individualised results for this option. In Canada, like other countries, new reporting of Patient Reported Outcome Measures (PROMs) for hip and knee replacement is being promoted, [34] and if it could include the outcomes for those who consider, but choose to delay or not have surgery, this information could then be provided to patients. While our study asked patients to rate their SDM experience post consultation, it is unclear if and how the decision aid and surgeon report influenced the consultation. Finally, due to many patients choosing not to participate in the study, the generalizability of results needs to be considered with caution. Included patients were likely to be more open-minded about being educated about surgery and other treatment options.
Future studies should continue to explore how to better inform patients of what they might expect from both surgical and non-surgical options. The results of this study suggest tailoring evidence with individualised potential outcomes of surgery may not be sufficient for this goal – though it could be argued on ethical grounds that if it is known that evidence varies for individuals, then this should be used rather than the average. How this evidence is described and framed, and integrated into the clinical consultation needs further research. Importantly, introducing the decision aid earlier in the patient journey, when expectations towards surgery and non-surgical treatment are less defined may be prudent, and could increase uptake of decision aid use.
In conclusion, this study suggests that using a patient decision aid, which individualizes information based on the characteristics of the patient, in combination with a surgeon report, can significantly improve decision quality in routine care. While the independent contribution of tailoring the decision aid to patient baseline characteristics and including a surgeon report remains unclear, we demonstrated the feasibility of integrating the decision aid into an online pre-surgical assessment in routine clinical care. This approach holds promise as a way to begin prioritizing surgery, and can be further improved as evidence accumulates on both surgical and non-surgical outcomes.
Author statement
Conceptualization; NB, LT, DM, Data curation; DM, DD, CS, Formal analysis; NB, LT, DM, Funding acquisition; NB, LT, DM, KM, GH, DS, JJ, Project administration; KM, LT, Roles/Writing – original draft; NB, LT, DM, Writing – review & editing. NB, LT, DM, KM, DD, GH, DS, CS, JJ.
This work was supported by an unrestricted grant from the EuroQol Research Foundation. The funding body is not involved in the design of the study and collection, analysis, and interpretation of data or writing the manuscript.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
We acknowledge the staff, surgeons and patients at the Edmonton Bone and Joint Centre. LT is supported by a Canadian Institutes for Health Research (CIHR) Postdoctoral Fellowship. DM received salary support from a Canada Research Chair (Health Services and Systems Research) and is the Arthur J.E. Child Chair in Rheumatology Outcomes Research. Dr. Hawker receives support as the Sir John and Lady Eaton Professor and Chair of Medicine, University of Toronto.
Contributor Information
Nick Bansback, Email: nick.bansback@ubc.ca.
Logan Trenaman, Email: logan.trenaman@ubc.ca.
Karen V. MacDonald, Email: karenv.macdonald@ucalgary.ca.
D'Arcy Durand, Email: ddurand@albertaboneandjoint.com.
Gillian Hawker, Email: g.hawker@utoronto.ca.
Jeffrey A. Johnson, Email: jeff.johnson@ualberta.ca.
Christopher Smith, Email: csmith@albertaboneandjoint.com.
Dawn Stacey, Email: Dawn.Stacey@uOttawa.ca.
Deborah A. Marshall, Email: damarsha@ucalgary.ca.
References
- 1.Hawker G.A., Wright J.G., Coyte P.C., Williams J.I., Harvey B., Glazier R., et al. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients' preferences. Med. Care. 2001;39(3):206. doi: 10.1097/00005650-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bourne R.B., Chesworth B.M., Davis A.M., Mahomed N.N., Charron K.D. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin. Orthop. Relat. Res. 2010;468(1):57–63. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong K.L., Lau E., Suggs J., Kurtz S.M., Manley M.T. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin. Orthop. 2010;468(11):3070–3076. doi: 10.1007/s11999-010-1399-0. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh J.A., Kundukulam J., Riddle D.L., Strand V., Tugwell P. Early postoperative mortality following joint arthroplasty: a systematic review. J. Rheumatol. 2011;38(7):1507–1513. doi: 10.3899/jrheum.110280. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipriano L.E., Chesworth B.M., Anderson C.K., Zaric G.S. An evaluation of strategies to reduce waiting times for total joint replacement in Ontario. Med. Care. 2008;46(11):1177–1183. doi: 10.1097/MLR.0b013e31817925e8. Nov. [DOI] [PubMed] [Google Scholar]
- 6.Hawker G., Bohm E.R., Conner-Spady B., De Coster C., Dunbar M., Hennigar A., et al. Perspectives of Canadian stakeholders on criteria for appropriateness for total joint arthroplasty in patients with hip and knee osteoarthritis. Arthritis Rheumatol. 2015;67(7):1806–1815. doi: 10.1002/art.39124. Jul. [DOI] [PubMed] [Google Scholar]
- 7.Scott C.E., Bugler K.E., Clement N.D., MacDonald D., Howie C.R., Biant L.C. Patient expectations of arthroplasty of the hip and knee. J. Bone Jt. Surg. Br. Vol. 2012;94(7):974–981. doi: 10.1302/0301-620X.94B7.28219. Jul. [DOI] [PubMed] [Google Scholar]
- 8.Hawker G.A., Conner-Spady B.L., Bohm E., Dunbar M.J., Jones C.A., Ravi B., Noseworthy T., Dick D., Powell J., Paul P., Marshall D.A. Patients' preoperative expectations of total knee arthroplasty and satisfaction with outcomes at one year: a prospective cohort study. Arthritis Rheumatol. 2021 Feb;73(2):223–231. doi: 10.1002/art.41510. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Orthopaedic Surgeons . 2011. Shared Physician-Patient Responsibilities, Position Statement.https://www.aaos.org/globalassets/about/position-statements/1182-shared-physician-patient-communication.pdf Available from: [Google Scholar]
- 10.Skou S.T., Roos E.M., Laursen M.B., Rathleff M.S., Arendt-Nielsen L., Simonsen O., et al. A randomized, controlled trial of total knee replacement. N. Engl. J. Med. 2015;373(17):1597–1606. doi: 10.1056/NEJMoa1505467. Oct 22. [DOI] [PubMed] [Google Scholar]
- 11.Klett M.-J., Frankovich R., Dervin G.F., Stacey D. Impact of a surgical screening clinic for patients with knee osteoarthritis: a descriptive study. Clin. J. Sport Med. 2012;22(3):274–277. doi: 10.1097/JSM.0b013e318248ed24. May. [DOI] [PubMed] [Google Scholar]
- 12.Riddle D.L., Sando T., Tarver T., Slover J., Sierra R.J., Brito J.P., Montori V.M. Shared decision-making applied to knee arthroplasty: a systematic review of randomized trials. Arthritis Care Res. 2021;73(8):1125–1133. doi: 10.1002/acr.24240. Aug. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann T.C., Del Mar C. Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern. Med. 2015;175(2):274–286. doi: 10.1001/jamainternmed.2014.6016. Feb. [DOI] [PubMed] [Google Scholar]
- 14.Arthritis Healthwise. Should I have knee replacement surgery? https://decisionaids.healthwise.net/knee-surgery/ [Internet]. Available from:
- 15.Jiang Y., Sanchez-Santos M.T., Judge A.D., Murray D.W., Arden N.K. Predictors of patient-reported pain and functional outcomes over 10 years after primary total knee arthroplasty: a prospective cohort study. J. Arthroplasty. 2017;32(1):92–100. doi: 10.1016/j.arth.2016.06.009. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimer B.K., Kreuter M.W. Advancing tailored health communication: a persuasion and message effects perspective. J. Commun. 2006;56:S184–S201. [Google Scholar]
- 17.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):1–8. doi: 10.4103/0976-500X.72352. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sepucha K.R., Abhyankar P., Hoffman A.S., Bekker H.L., LeBlanc A., Levin C.A., Ropka M., Shaffer V.A., Sheridan S.L., Stacey D., Stalmeier P. Standards for UNiversal reporting of patient decision aid evaluation studies: the development of SUNDAE checklist. BMJ Qual. Saf. 2018;27(5):380–388. doi: 10.1136/bmjqs-2017-006986. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bansback N., Trenaman L., MacDonald K.V., Hawker G., Johnson J.A., Stacey D., Marshall D.A. An individualized patient-reported outcome measure (PROM) based patient decision aid and surgeon report for patients considering total knee arthroplasty: protocol for a pragmatic randomized controlled trial. BMC Muscoskel. Disord. 2019;20(1):1. doi: 10.1186/s12891-019-2434-2. Dec 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elwyn G., O'Connor A., Stacey D., Volk R., Edwards A., Coulter A., Thomson R., Barratt A., Barry M., Bernstein S., Butow P. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. Bmj. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volk R.J., Llewellyn-Thomas H., Stacey D., Elwyn G. Ten years of the International Patient Decision Aid Standards Collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med. Inf. Decis. Making. 2013;13(2):1–7. doi: 10.1186/1472-6947-13-S2-S1. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herdman M., Gudex C., Lloyd A., Janssen M.F., Kind P., Parkin D., Bonsel G., Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual. Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skou S.T., Roos E.M., Laursen M.B., Rathleff M.S., Arendt-Nielsen L., Simonsen O., Rasmussen S. A randomized, controlled trial of total knee replacement. N. Engl. J. Med. 2015;373(17):1597–1606. doi: 10.1056/NEJMoa1505467. Oct 22. [DOI] [PubMed] [Google Scholar]
- 24.Sepucha K.R., Borkhoff C.M., Lally J., Levin C.A., Matlock D.D., Ng C.J., Ropka M.E., Stacey D., Joseph-Williams N., Wills C.E., Thomson R. Establishing the effectiveness of patient decision aids: key constructs and measurement instruments. BMC Med. Inf. Decis. Making. 2013;13(2):1. doi: 10.1186/1472-6947-13-S2-S12. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepucha K.R., Stacey D., Clay C.F., Chang Y., Cosenza C., Dervin G., et al. Decision quality instrument for treatment of hip and knee osteoarthritis: a psychometric evaluation. BMC Muscoskel. Disord. 2011;12:149. doi: 10.1186/1471-2474-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Légaré F., Kearing S., Clay K., Gagnon S., D'Amours D., Rousseau M., et al. Are you SURE?: assessing patient decisional conflict with a 4-item screening test. Can Fam Physician Médecin Fam Can. 2010;56(8):e308–314. Aug. [PMC free article] [PubMed] [Google Scholar]
- 27.Elwyn G., Barr P.J., Grande S.W., Thompson R., Walsh T., Ozanne E.M. Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ. Counsel. 2013;93(1):102–107. doi: 10.1016/j.pec.2013.05.009. Oct 1. [DOI] [PubMed] [Google Scholar]
- 28.Degner L.F., Sloan J.A., Venkatesh P. The control preferences scale. Can J Nurs Res Rev Can Rech En Sci Infirm. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 29.Stacey D., Taljaard M., Dervin G., Tugwell P., O'Connor A.M., Pomey M.P., et al. Impact of patient decision aids on appropriate and timely access to hip or knee arthroplasty for osteoarthritis: a randomized controlled trial. Osteoarthritis Cartilage. 2016;24(1):99–107. doi: 10.1016/j.joca.2015.07.024. Jan. [DOI] [PubMed] [Google Scholar]
- 30.Lee K.J., Thompson S.G. Clustering by health professional in individually randomised trials. Bmj. 2005;330(7483):142–144. doi: 10.1136/bmj.330.7483.142. Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahan B.C., Morris T.P. Assessing potential sources of clustering in individually randomised trials. BMC Med. Res. Methodol. 2013;13(1):1–9. doi: 10.1186/1471-2288-13-58. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleiss J.L., Levin B., Paik M.C. John Wiley & Sons; 2013. Statistical Methods for Rates and Proportions; p. 716. [Google Scholar]
- 33.Ibrahim S.A., Blum M., Lee G.-C., Mooar P., Medvedeva E., Collier A., et al. Effect of a decision aid on access to total knee replacement for black patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Surg. 2017;152(1) doi: 10.1001/jamasurg.2016.4225. Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canadian Institute for Health Information . 2019. Patient-Reported Outcome Measures Data Collection Manual: Hip and Knee Arthroplasty.https://www.cihi.ca/sites/default/files/document/proms-data-collection-manual-may2019-en-web.pdf Available from: [Google Scholar]