Abstract
Objective
Osteophytes, also small ones, are an important imaging feature of OA. However, due to their high prevalence on MR, the question has arisen whether these are truly pathophysiologic features of early OA, a result of physiologic aging, or rather a merely transient phenomenon. The aim of this study was to explore the prevalence of osteophytes on MR in various locations of the knee, with special emphasis on small osteophytes, across multiple large studies conducted in our institution comprising a wide range of subjects at different ages.
Method
Retrospective explorative study of the prevalence of osteophytes, particularly grade 1 according to MOAKS, among four studies with a wide variety in age and OA risk factors.
Results
A large number of grade 1 osteophytes were found in all four studies. The largest number of osteophytes were present in the youngest age group of <30 years (69.6%) compared to 36.8% in the age group of ≥30 < 50 years and 54,3% when aged ≥50 years, of which most were grade 1 osteophytes.
Conclusion
Small osteophytes are highly prevalent among populations with varying age and OA risk factors, in particular among young subjects without other OA features. This might suggest that these “osteophytes” do not necessarily represent early OA, but rather indicate a transient physiologic phenomenon.
Keywords: Osteophytes, Osteoarthritis, MRI, Knee, Grading
1. Introduction
Bony outgrowths, also known as osteophytes, are one of the main features of osteoarthritis next to joints space narrowing, subchondral sclerosis and cysts. Osteophytes develop early in the OA disease process and are therefore an important early OA imaging feature [1]. Identification of early OA features has recently gained interest, since new disease modifying drugs (DMOADS) are targeted particularly at the early stage [2]. This has led to an increased utility of magnetic resonance imaging (MRI), a far more sensitive technique compared to radiographs, for detecting early OA features [3]. For MRI, the most widely used scoring method for knee OA is the MRI Osteoarthritis Knee Score (MOAKS) in which osteophytes are graded as absent (grade 0), small (grade 1), moderate (grade 2), or large (grade 3) [4]. Following this surge in MR, it has been reported, though, that a large number of early OA features, including osteophytes, are detected, even in young populations without OA risk factors or other OA features [5]. Given this fact and the tendency in research and clinical practice to regard small osteophytes as early OA feature, it is important to determine if these are truly pathophysiologic or represent normal aging in older adults or transient bone-cartilage transitions that undergo remodelling later in life in younger populations. Until now, osteophyte development and function is not well understood, yet biomechanical stimuli are thought to be critical [6]. They might serve merely as joint stabilizers by dividing stresses across a bigger surface [6] or might be the result of an altered internal joint milieu resulting in chondrogenesis of precursor cells in the periosteum and synovial lining [7].
There is a lack of longitudinal studies of these small osteophytes, given the long follow-up needed. Albeit on radiography, Hart et al. did study the natural history of small osteophytes over 10 years and stipulated the fact that so called ‘doubtful’ osteophytes appear to be ‘real’ and significantly related to OA knee and therefore cannot be ignored or classified as normal [8]. Moreover, following a Delphi exercise for the definition of knee OA on MRI, osteophyte formation plays a major role in the definition of osteoarthritis [9]. However, the characteristics of a ‘definite’ OA osteophyte were not specified yet in this process and need further investigation [9]. The absence of a clear definition does render the assessment of small bony outgrowths rather subjective.
Therefore, the aim of this study was to pinpoint possible directions for future research into small osteophytes by exploring the prevalence of osteophytes on MR in various locations of the knee, with special emphasis on small osteophytes, across multiple large studies conducted in our institution comprising a wide range of subjects at different ages with various OA risk factors.
2. Method
2.1. Study population
This retrospective study comprised study subjects of four different studies: the Penetrating Patellofemoral Pain (Triple P) study, KNee osteoArthritis with an anterior cruciate Ligament Lesion (KNALL) study, PRevention of knee Osteoarthritis in Overweight Females (PROOF) study, and a subgroup of the population-based Rotterdam Study [[10], [11], [12], [13]]. For all studies, baseline MRI's with complete osteophyte scores were included. For the Triple P and KNALL studies, MRI scans of one knee were available, whereas for PROOF and Rotterdam Study they were available of both knees.
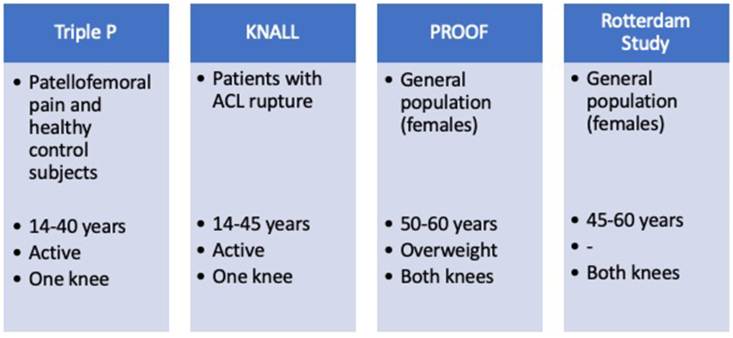
Details of these studies have been published elsewhere, but, in short (see also Fig. 1), the Triple P study is a cross-sectional study focusing on structural abnormalities on MRI in patients with patellofemoral pain and healthy control subjects, consisting of a young active population aged between 14 and 40 years. The KNALL study is a prospective multicentre cohort study of patients aged 18–45 years with a recent anterior cruciate ligament (ACL) rupture, aimed to detect early OA features on MRI. The PROOF study is a preventive randomized controlled trial on knee OA within a group of overweight (BMI ≥27 kg/m2) females between 50 and 60 years without knee OA at baseline. The Rotterdam Study is an open population-based cohort study in which the incidence and risk factors for chronic disabling diseases are investigated, of which a subgroup of middle aged (45–60 years) women underwent knee MRI to study OA.
Fig. 1.
Overview of the included studies.
All studies were granted permission by the Medical Ethical commission of our institution and informed consent was obtained from all participants.
2.2. Magnetic resonance imaging (MRI)
Varying MRI hardware and scan protocols were used in the different studies, but all MRI scan protocols included sagittal, coronal, and axial pulse sequences with and without fat saturation, as recommended for the application of MOAKS.
An experienced musculoskeletal radiologist (EO; 11 years of experience with musculoskeletal MRI in clinical and research settings) trained all readers (JR, BvM, DS, JK, DvE, PvdP) in the use of MOAKS. All readers were tested on a training set of 50 images of the four included studies after a training period of several months before scoring started.
According to MOAKS, osteophytes were scored at 12 locations: patella (superior, medial, inferior, and lateral), trochlear, central and posterior femur (medial and lateral) and central tibia (medial and lateral). Osteophytes were graded as none (0), small (1), medium (2) or large (3) on non-fat saturated, intermediate weighted sequences [4].
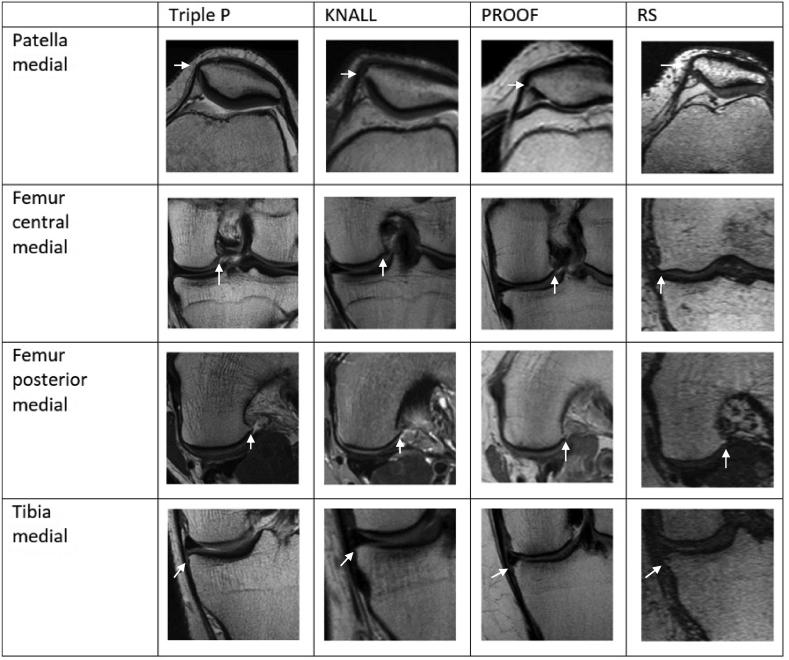
A consensus meeting with all of the readers across all studies was held with the purpose to assess whether osteophytes had been scored in a similar fashion across studies, by visually comparing images of osteophytes scored at four different locations (patella medial, femur posterior medial, femur central medial, and tibia central medial) in ten randomly selected patients from all studies for all grades (see Fig. 2).
Fig. 2.
Appearance of grade 1 osteophytes across studies.
2.3. Data analysis
We explored the frequency of osteophytes at the 12 locations separately and overall using cross-tabulations. Differences in age, and body mass index (BMI) categories were tested using Chi square tests. Age was divided into three groups: <30, ≥30 < 50 and ≥50 years. BMI was divided into normal (<25 kg/m2), overweight (25–30 kg/m2) and obesity (≥30 kg/m2) according to international standards. Additional analyses to study the distribution of grade 1 osteophytes in different populations were done, in which locations were summarized into patellofemoral (patella and femoral trochlea) and tibiofemoral (tibia and femur central and posterior). All analyses were performed with IBM SPSS statistics version 22.
3. Results
For this study only subjects with complete MRI osteophyte scores were included. For Triple P, MRI's of 64 patients with PFP and 70 healthy control subjects were included, with a mean population age of 23.2 years and mean BMI of 22.9 kg/m2. For KNALL, 149 baseline MRI's were included, with a mean population age of 27.1 years and mean BMI of 24.5 kg/m2. For PROOF, 782 baseline MRI's were included, with a mean population age of 55.7 years and mean BMI of 32.4 kg/m2. For the Rotterdam Study, 1360 baseline MRI's were included, with a mean population age of 54.6 years and mean BMI of 26.8 kg/m2. In Triple P and KNALL, 57% and 34% of subjects were female, respectively, while this was 100% in PROOF and the Rotterdam Study, consistent with the inclusion criteria. See Table 1 for an overview of the patient characteristics of the available MRI scorings.
Table 1.
Patient characteristics of available MRIs per study.
| Triple P | KNALL | PROOF | ROTTERDAM STUDY | |
|---|---|---|---|---|
| Number of subjects | 136 | 154 | 407 | 888 |
| Knees | 136 | 149 | 782 | 1360 |
| Age, mean | 23.2 | 27.1 | 55.7 | 54.6 |
| Age, range | 14–40 | 18–45 | 50–60 | 45–60 |
| Gender (% ♀) | 56.7 | 34 | 100 | 100 |
| BMI (kg/m2) | 22.9 | 24.5 | 32.4 | 26.8 |
Osteophyte grading appeared similar across studies in the consensus meeting (Fig. 1).
Overall, the frequency of osteophytes was the highest in Triple P (91.0%) followed by PROOF (69.2%), KNALL (42.9%) and Rotterdam Study (38.2%). In general, grade 1 osteophytes had the highest prevalence across all four studies (see Table 2), in which the highest prevalence's appeared in the young population of the Triple P study, reaching a frequency of 72.1% in the posteromedial femur. It was apparent that this high prevalence of osteophytes occurred both in patients with patellofemoral pain and healthy control subjects [10]. The fewest grade 1 osteophytes were scored in the KNALL study.
Table 2.
Prevalence of osteophytes across studies split into locations (%).
| Triple P | KNALL | PROOF | ROTTERDAM STUDY | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOAKS grade | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Patella | ||||||||||||||||
|
66.2 66.9 64.7 71.3 |
32.4 33.1 35.3 27.9 |
0 0 0 0.7 |
1.5 0 0 0 |
79.4 88.7 99 98.7 |
20 10 1 1.3 |
0.6 1.3 0 0 |
0 0 0 0 |
63.4 65.2 68 70.7 |
19.8 17.5 16 14.5 |
4.0 4.6 3.7 2.4 |
0.6 0.5 0.1 0.3 |
93.2 92.9 92.4 96.7 |
4.9 3.9 4.3 1.8 |
1.5 2.9 2.9 1.4 |
0.4 0.3 0.4 0.1 |
| Femur | ||||||||||||||||
|
86 94.9 51.5 67.6 19.9 40.4 |
13.2 5.1 47.8 30.9 72.1 57.4 |
0.7 0 0.7 1.5 8.8 2.2 |
0 0 0 0 0 0 |
97 99.4 95.5 97 82.8 88.7 |
3 0.6 4.5 3 14 10 |
0 0 0 0 3.2 1.3 |
0 0 0 0 0 0 |
71.6 78.0 58.7 63.1 47.8 66.8 |
10.6 5.6 16.2 15.4 28.5 18.1 |
4.2 3.2 9.4 6.1 8.9 2.6 |
1.1 0.8 3.2 2.8 2.7 0.4 |
81.3 91.9 85.3 89.2 78.9 92.9 |
12.1 4.3 6.7 5.0 14.0 4.7 |
5.4 2.5 6.4 4.1 5.4 1.5 |
1.2 1.1 1.6 1.7 1.6 0.8 |
| Tibia | ||||||||||||||||
|
94.9 97.1 |
5.1 2.9 |
0 0 |
0 0 |
100 100 |
0 0 |
0 0 |
0 0 |
52.9 68.8 |
25.4 14.8 |
7.3 2.8 |
2.0 1.3 |
88.4 92.0 |
6.5 5.4 |
4.0 1.9 |
1.0 0.6 |
The presence of osteophytes was significantly different between age categories, in which the highest frequency of osteophytes was present in the youngest age group of <30 years 69,6% compared to 36.8% when ≥30 < 50 years and 54,3% when ≥50 years (p<0.05). Within the youngest age category most osteophytes were grade 1 (60.3%). Almost 75% of young subjects aged <30 years had grade 1 osteophytes at multiple sites, even up to 10 locations in one subject. As expected, grade 2 and 3 osteophytes were found more frequently in the older study populations of PROOF and the Rotterdam Study, compared to the younger populations of KNALL and Triple P.
With regard to BMI, the frequency of osteophytes was higher in knees of subjects in a higher BMI category (39.3, 48,0 and 66.7%). This difference was only significant (p<0.05) between the normal weight and obese category. The amount of grade 2 and 3 osteophytes was higher in knees of subjects with a higher BMI. With regard to grade q osteophytes, knees of subjects with a higher BMI did also demonstrate a higher number of grade 1 osteophytes.
With regard to location, grade 1 osteophytes were prevalent at the patella in all groups and were equally distributed across patellar sites. At the femur, more grade 1 osteophytes were seen medially than laterally. Most frequent sites in the youngest age category were femur posterior medial (51,5%), femur central medial (23,4%), patella superior (23,0%), femur posterior lateral (22,2%), tibia central medial (20,3%), patella inferior and trochlea medial (18,0%), patella medial (15,4%), femur central lateral (15,3%). When having a closer look at the distribution of grade 1 osteophytes in the populations the frequency was the highest at the patellofemoral and the tibiofemoral joint of subjects in the Triple P study (Table 3). Further divided, femoral grade 1 osteophytes had the highest prevalence in Triple P, contrary to tibial grade 1 osteophytes, which were highly prevalent in PROOF.
Table 3.
Prevalence of small osteophytes across studies split into patellofemoral and tibiofemoral (%).
| Triple P | KNALL | PROOF | ROTTERDAM STUDY | ||
|---|---|---|---|---|---|
| Patellofemoral | 67.2 | 27.9 | 49.0 | 22.9 | |
| Tibiofemoral | 90.3 | 23.8 | 56.8 | 27.1 | |
4. Discussion
The aim of this study was to identify directions for future research into small osteophytes by exploring the prevalence of osteophytes of the knee on MR, with special focus on small osteophytes, across multiple large studies conducted in our institution comprising a wide range of subjects at different ages and risk factors for knee osteoarthritis.
Grade 1 osteophytes were highly prevalent in various populations, also in those without clinical or radiographic OA. The most prevalent location of these small osteophytes seems to vary based on possible OA risk factors given the fact that tibial osteophytes were highly prevalent in an overweight population (PROOF) and femoral osteophytes were observed most frequently in an active population of patients with patellofemoral pain and healthy control subjects (Triple P). It was also apparent that a larger number of grade 1 osteophytes was observed in subjects with higher BMI. Previous studies have suggested that osteophytes may signify an adaptive response to mechanical stimuli that accompany increased loading [7]. Future studies might focus on the influence of BMI and physical activity in more detail. Our finding that the frequency of osteophytes in general, and in particular grade 1 osteophytes, was largest in the youngest age group, suggests that a “small osteophyte” represents rather a transient physiologic bone-cartilage transition at a younger age that undergoes remodelling later in life. This suggestion is corroborated by the fact that, typically, no other features indicative of OA such as cartilage defects or symptoms were present in these subjects [10,14].
A strength of our study is that we were able to include four large clinical and population-based MRI knee studies with varying age and OA risk factors, all from our own institution and scored with the same semi-quantitative scoring system.
Limitations of this study include that, although our multidisciplinary team was trained together in the application of MOAKS, each of the four studies was scored by different readers and different expected prevalence of osteophytes in the individual studies could have influenced the way osteophytes were scored. Unexpectedly, the fewest grade 1 osteophytes were scored in the KNALL study, despite a consistent scoring method compared to the other studies. A slight underestimation might have occurred based on a comparison of the prevalence of OA features in KNALL to the prevalence in a comparable population [15]. It should be noted, though, that if KNALL would have demonstrated a higher prevalence of (grade 1) osteophytes, this would only have strengthened our results. Furthermore, for the two youngest cohorts (KNALL and Triple P) MRI of only one knee was available and we anticipate that this has also introduced an underestimation of our results. Due to this unequal availability of knee MRIs across populations and the cross-sectional nature of our data, we only present exploratory results regarding the influence of age and BMI and only suggest possible directions for future research instead of final claims.
The ultimate goal would be a clear definition or cut-off point for a truly pathologic osteophyte versus a physiologic bony transition. This could be facilitated by a more detailed osteophyte grading, similar to the recently introduced anterior cruciate ligament osteoarthritis score (ACLOAS), comprising the questionable (grade 1) and small beak like definite osteophyte (grade 2) [16]. The authors reported their osteophyte scoring to be slightly less reliable, though, compared to the other structural abnormalities. Another approach would be to further characterize small osteophytes, based on additional metabolic information obtained by positron emission tomography (PET). A recent feasibility study of PET-MRI in OA showed that many of the grade 1 osteophytes apparently did not show metabolic activity, which suggests that no active process is present [17].
Further research is needed to further characterize these small osteophytes. Meanwhile, care should be taken when considering small osteophytes as early OA features.
In conclusion, small osteophytes are highly prevalent among populations with varying age and OA risk factors, in particular among young subjects without other OA features. This suggests that these “osteophytes” do not necessarily represent early OA, but rather indicate a transient physiologic phenomenon.
Contributions
De Kanter JL: Conception and design, Analysis and interpretation of the data, Drafting of the article, Final approval of the article. Oei EHG: Conception and design, Analysis and interpretation of the data, Critical revision of the article for important intellectual content, Final approval of the article. Schiphof D: Critical revision of the article for important intellectual content, Data acquisition of the Rotterdam study, Final approval of the article. Van Meer BL: Critical revision of the article for important intellectual content, Data acquisition of the KNALL study, Final approval of the article. Van Middelkoop M: Conception and design, Critical revision of the article for important intellectual content, Final approval of the article. Reijman M: Conception and design, Critical revision of the article for important intellectual content, final approval of manuscript. Bierma-Zeinstra SMA: Conception and design, Critical revision of the article for important intellectual content, Final approval of the article. Runhaar J: Analysis and interpretation of the data, Critical revision of the article for important intellectual content, Data acquisition of the PROOF study, Final approval of the article. Van der Heijden RA: Conception and design, Analysis and interpretation of the data, Drafting of the article, Final approval of the article, data acquisition of the TRIPLE study.
Role of the funding source
The funding source had no involvement in the data processing and writing.
Declaration of competing interest
There are no conflicts of interest to disclosure.
Acknowledgments
We thank all participants for their participation and the caregivers for recruiting the participants. We would also like to acknowledge the Dutch Arthritis Society and the Erasmus University Rotterdam for their financial support for the original studies. We would like to acknowledge Diana van Emmerik and Peter van der Plas for their help in scoring the scans.
References
- 1.Kellgren J.H., Moore R. Generalized osteoarthritis and Heberden's nodes. Br. Med. J. 1952;1(4751):181–187. doi: 10.1136/bmj.1.4751.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oo W.M., Yu S.P., Daniel M.S., Hunter D.J. Disease-modifying drugs in osteoarthritis: current understanding and future therapeutics. Expet Opin. Emerg. Drugs. 2018;23(4):331–347. doi: 10.1080/14728214.2018.1547706. [DOI] [PubMed] [Google Scholar]
- 3.Roemer F.W., Crema M.D., Trattnig S., Guermazi A. Advances in imaging of osteoarthritis and cartilage. Radiology. 2011;260(2):332–354. doi: 10.1148/radiol.11101359. [DOI] [PubMed] [Google Scholar]
- 4.Hunter D.J., Guermazi A., Lo G.H., Grainger A.J., Conaghan P.G., Boudreau R.M., et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19(8):990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culvenor A.G., Oiestad B.E., Hart H.F., Stefanik J.J., Guermazi A., Crossley K.M. Prevalence of knee osteoarthritis features on magnetic resonance imaging in asymptomatic uninjured adults: a systematic review and meta-analysis. Br. J. Sports Med. 2019;53(20):1268–1278. doi: 10.1136/bjsports-2018-099257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabelo G.D., Vom Scheidt A., Klebig F., Hemmatian H., Citak M., Amling M., et al. Multiscale bone quality analysis in osteoarthritic knee joints reveal a role of the mechanosensory osteocyte network in osteophytes. Sci. Rep. 2020;10(1):673. doi: 10.1038/s41598-019-57303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Kraan P.M., van den Berg W.B. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15(3):237–244. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Hart D.J., Spector T.D. Kellgren & Lawrence grade 1 osteophytes in the knee--doubtful or definite? Osteoarthritis Cartilage. 2003;11(2):149–150. doi: 10.1053/joca.2002.0853. [DOI] [PubMed] [Google Scholar]
- 9.Hunter D.J., Arden N., Conaghan P.G., Eckstein F., Gold G., Grainger A., et al. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage. 2011;19(8):963–969. doi: 10.1016/j.joca.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Heijden R.A., de Kanter J.L., Bierma-Zeinstra S.M., Verhaar J.A., van Veldhoven P.L., Krestin G.P., et al. Structural abnormalities on magnetic resonance imaging in patients with patellofemoral pain: a cross-sectional case-control study. Am. J. Sports Med. 2016;44(9):2339–2346. doi: 10.1177/0363546516646107. [DOI] [PubMed] [Google Scholar]
- 11.van Meer B.L., Oei E.H., Bierma-Zeinstra S.M., van Arkel E.R., Verhaar J.A., Reijman M., et al. Are magnetic resonance imaging recovery and laxity improvement possible after anterior cruciate ligament rupture in nonoperative treatment? Arthroscopy. 2014;30(9):1092–1099. doi: 10.1016/j.arthro.2014.04.098. [DOI] [PubMed] [Google Scholar]
- 12.Runhaar J., van Middelkoop M., Reijman M., Willemsen S., Oei E.H., Vroegindeweij D., et al. Prevention of knee osteoarthritis in overweight females: the first preventive randomized controlled trial in osteoarthritis. Am. J. Med. 2015;128(8):888–895 e4. doi: 10.1016/j.amjmed.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Schiphof D., Oei E.H., Hofman A., Waarsing J.H., Weinans H., Bierma-Zeinstra S.M. Sensitivity and associations with pain and body weight of an MRI definition of knee osteoarthritis compared with radiographic Kellgren and Lawrence criteria: a population-based study in middle-aged females. Osteoarthritis Cartilage. 2014;22(3):440–446. doi: 10.1016/j.joca.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 14.van Meer B.L., Oei E.H., Meuffels D.E., van Arkel E.R., Verhaar J.A., Bierma-Zeinstra S.M., et al. Degenerative changes in the knee 2 Years after anterior cruciate ligament rupture and related risk factors: a prospective observational follow-up study. Am. J. Sports Med. 2016;44(6):1524–1533. doi: 10.1177/0363546516631936. [DOI] [PubMed] [Google Scholar]
- 15.Culvenor A.G., Collins N.J., Guermazi A., Cook J.L., Vicenzino B., Khan K.M., et al. Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheum. 2015;67(4):946–955. doi: 10.1002/art.39005. [DOI] [PubMed] [Google Scholar]
- 16.Roemer F.W., Frobell R., Lohmander L.S., Niu J., Guermazi A. Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthritis Cartilage. 2014;22(5):668–682. doi: 10.1016/j.joca.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Kogan F., Fan A.P., McWalter E.J., Oei E.H.G., Quon A., Gold G.E. PET/MRI of metabolic activity in osteoarthritis: a feasibility study. J. Magn. Reson. Imag. 2017;45(6):1736–1745. doi: 10.1002/jmri.25529. [DOI] [PMC free article] [PubMed] [Google Scholar]