Abstract
Objective
Test the feasibility of conducting an individually randomised controlled trial recruiting people with knee osteoarthritis (OA) in community pharmacies and evaluate the impacts of a novel information booklet.
Design
People with knee OA were identified by pharmacy staff using clinical criteria and randomised to receive a novel information booklet (intervention) or the currently available written OA resource (active control). Mixed-methods process evaluation assessed participant recruitment, retention, and experience. Participant-reported outcome measures, assessing OA illness perceptions, OA knowledge, fear of movement, and pain when walking at baseline and 4-weeks, were analysed using linear regression models (adjusted for baseline).
Results
Of 72 eligible people, 64 were randomised to intervention (n = 33) or control (n = 31). The randomisation sequence was followed correctly and no protocol deviations identified. Mean recruitment rate was 2.7 participants per pharmacy per week. One-in-five participants had no educational qualifications and one-in-four had not received a knee OA diagnosis prior to the trial. Three meta-themes emerged from pharmacist and participant qualitative analysis: ‘pleased to be asked’; ‘easy process’; and ‘successful process’. Three participants were lost to follow-up. At 4 weeks, intervention arm Knee Osteoarthritis Knowledge Scale scores improved (mean difference = 3.6, 95%CI 0.7 to 6.5). Brief Illness Perceptions Questionnaire scores were similar between groups (mean difference 0.4, 95%CI -3.7 to 4.5).
Conclusion
It is feasible to conduct an individually randomised trial in community pharmacy, a potentially effective setting to initiate accessible OA care. A novel information booklet improved OA knowledge, but is unlikely to affect illness perceptions on its own.
Keywords: Knee osteoarthritis, Patient education, Randomised controlled trial, Feasibility study, Community pharmacy services, Community pharmacists
1. Introduction
Core recommended treatments for knee osteoarthritis (OA) are education, structured land-based exercise, and weight loss for people who are overweight [[1], [2], [3], [4]]. Providing these relatively low-cost but high-value treatments early in the disease course could considerably reduce the impact of knee OA [[5], [6], [7], [8], [9]]. Effectively delivering these interventions in accessible and equitable ways remains a challenge for health systems, with barriers identified at consumer, clinician, health service and health system levels [10,11].
People with OA report that reliable information about OA is hard to find [[12], [13], [14]]. Beliefs that OA is an inevitable age-related condition that can only be treated with medication and/or joint replacement result in OA often not being identified or managed during early- and mid-stage disease, when much of the knee OA morbidity burden accrues [15,16].
There is little evidence available to inform the selection of educational resources to improve OA knowledge or health outcomes. De Rezende et al. [17] found small short-term improvements in pain and function for people who attended face-to-face multi-disciplinary educational classes compared to receiving video recordings and printed summaries of these classes, however, these improvements were not sustained at one-year. Losina et al. [18] found a personalized OA risk calculator may increase the willingness of young adults who do not have OA to change exercise behaviours more than receiving general OA risk information. Furthermore, until recently, there have been no appropriate tools to measure knowledge about OA. The development of the Knee Osteoarthritis Knowledge Scale (KOAKS) has enabled this important construct to be measured [19].
We have developed a novel evidence-based OA information resource (booklet and website) that aims to target and modify common unhelpful beliefs about knee OA. This co-designed resource was positively evaluated by key stakeholders including people with knee OA, health professionals and arthritis advocates [20]. This resource will form part of a complex knee OA intervention to be delivered through community pharmacy (Care for Osteoarthritis through Pharmacy Education and Referral [COPER]).
Pharmacists are trusted and accessible community-based health professionals [21,22]. Community pharmacies are a largely unexplored opportunity to identify OA and initiate effective care [23,24]. A previous trial in Canada found that identifying people with knee OA through community pharmacy and providing multi-disciplinary care was effective and cost-effective [25,26]. We plan to test the COPER intervention through a large-scale randomised controlled trial (RCT) based in community pharmacies.
The aim of this study was to assess the feasibility of conducting an individually randomised RCT recruiting people with knee OA in a community pharmacy setting, and to evaluate the likely impact of the novel information booklet on OA illness beliefs, knowledge, fear of movement, and pain compared to the currently available written OA resource.
2. Method
This feasibility trial was registered with the Australian New Zealand Clinical Trial Registry (ACTRN12620000020987). It is reported as per the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Supplementary 1). Intervention and control conditions are described according to the Template for Intervention Description and Replication (TIDieR) guidelines (Supplementary 2 and 3).
Study design. We conducted an RCT in community pharmacies. Mixed-methods process evaluation assessed fidelity of randomisation and intervention delivery, and explored perceptions of trial processes.
Patient and public involvement. People with lived experience of knee OA contributed to the development and refinement of the novel information resource, through participation in qualitative interviews that informed the initial design and focus groups that further developed this material [15,20]. Stakeholder consultation with the Canterbury Community Pharmacist Group informed the trial design, pharmacist training, liaison with participating pharmacists, data collection, and dissemination of findings. A person with lived experience of knee OA (JC) contributed to study design, study management, carrying out the research, and dissemination of findings as part of the research team. JC was paid for their time.
Participants. People with knee OA were recruited from six community pharmacies in Christchurch, New Zealand. These pharmacies were purposively chosen to represent diverse socioeconomic and ethnic communities, and a range of pharmacy business models (e.g., mall-based, medical centre based, suburban). Twelve pharmacists (two per pharmacy) took part in the study.
Potentially eligible pharmacy customers self-identified as having knee pain by approaching pharmacy staff after seeing publicity material in the pharmacy (posters, shelf labels, bag stickers, booklet stands), or were identified by pharmacy staff after making product enquiries (such as seeking joint health supplements or over-the-counter pain relief) or receiving prescriptions that may have been for knee OA (such as analgesia or anti-inflammatories). Potentially eligible participants were screened by pharmacists using a written form. Participants were eligible if they were 18 years of age or over, had knee pain, and had knee OA.
Knee OA was diagnosed by one of two methods:
-
1.
Self-reported (by participant) as diagnosed by a specified health professional (e.g. general practitioner)
-
2.Fulfilled National Institute for Health and Care Excellence (NICE) knee OA diagnostic criteria [4] assessed by questionnaire:
-
a.45 years of age or older
-
b.Activity-related knee pain of any duration
-
c.No signs of other (non-OA) explanations for knee pain:
-
i.Acute knee injury in the last 6 months
-
ii.Stiffness in the morning that takes longer than 30 min to ease
-
iii.Knee is hot and swollen or rapidly deteriorating
-
i.
-
a.
Customers were excluded if they had received any type of knee surgery within the last 12 months, had received a joint replacement in either knee at any time, or were unable to read and write in English.
All participants gave written informed consent to participate in the RCT, with optional additional consent for an invitation to take part in a brief audio-recorded and transcribed telephone interview as part of the qualitative process evaluation. The trial was approved by the New Zealand Health and Disability Ethics Committee (19/NTA/146).
Randomisation and blinding. An independent statistician at a central administration site created a computer-generated randomisation schedule, stratified by pharmacy with random block sizes (2–4 allocations per block), with 1:1 allocation to either the intervention or control arm. Allocation was conducted by using sealed envelopes (opaque, identical size and appearance) containing either the intervention or the control information, which were ordered according to the randomisation schedule. Study identifiers on each envelope had no reference to group allocation. Each pharmacy was provided with a pre-ordered set of envelopes, and pharmacists gave these envelopes to participants in the order in which they presented.
Pharmacists were blinded to group allocation. They broadly knew that each package contained one of two types of written information about OA, but were not aware of the content of either type of information. Participants were blinded to information received by the other group, and were not told whether they had the newly developed or existing information. Participants did not open their envelopes until after they had left the pharmacy. Participants were asked not to share any of the information they received with the pharmacist, researchers, or anyone who had knee pain and might take part in the study. The research fellow who entered study data and the trial statistician who cleaned and analysed the study data were both blinded to group allocation.
Procedures. Pharmacists were trained in study procedures (screening and recruitment, consent, baseline data collection, and adhering to randomisation) through a 2-h face-to-face group training that was facilitated by BD and LV. Following training, pharmacists had ongoing access to a study manual to ensure standardised delivery of the study protocols across pharmacies.
During participant recruitment, pharmacists administered screening questionnaires and obtained written consent. After the participant had self-completed the paper baseline questionnaire, the pharmacist checked for data completeness and then handed the participant an envelope containing either the intervention or the control information, with instructions to open the envelope after leaving the pharmacy. Pharmacists answered questions about the study, but did not provide further information about OA.
Participants received a 4-week follow-up survey online or by post or telephone (according to participant preference). Non-responders to the 4-week survey were followed-up by email, telephone or text message.
Some participants were invited to take part in telephone interviews after submission of their 4-week survey. Pharmacists took part in focus groups. Examples of questions related to the fidelity assessment in the interview schedules are found in Supplementary 4.
Intervention participants received a novel information booklet to take home, supported by an accompanying website (www.freefromkneepain.org) with further information, patient-experience quotations, video explanations, and links to resources. No further advice or information on OA was given. The brightly coloured, co-designed 20-page booklet consists of evidence-based messages integrated with consumer voices. It aims to: 1) shift focus away from inaccurate and unhelpful aetiologic models of inevitable progressive joint damage caused by age and by wear and tear; 2) address myths about knee OA; and 3) empower effective self-care and self-management to support living well with knee OA (Table 1). The booklet was developed by an interdisciplinary research team in collaboration with Arthritis New Zealand. The process involved 1:1 face-to-face interviews with people who have knee OA [15], followed by development of draft information resources and pilot testing of the resources in stakeholder focus groups [20]. Stakeholders were people who have knee OA, Arthritis New Zealand educators and corporate staff, general practitioners, and primary health care nurses. Participants were very positive about the draft resources but also made suggestions for improvement that were integrated into the final resources.
Table 1.
Intervention and control characteristics.
| Intervention (novel booklet) | Control (PSNZ Fact Card) | |
|---|---|---|
| Topics covered |
|
|
| Website | http://www.freefromkneepain.org | No supporting information |
| Resource URL | http://www.freefromkneepain.org/resources/print | Not available online |
Abbreviations: PSNZ, Pharmaceutical Society of New Zealand; OA, osteoarthritis.
Control participants received the Pharmaceutical Society of New Zealand (PSNZ) Arthritis Fact Card to take home. No further advice or information on OA was given. The double-sided self-care sheet provides OA education using traditional biomedical models and management advice consistent with OA guidelines (Table 1). This free resource is available only in print version within pharmacies (not available to consumers online). It was chosen as the comparator because it is the information currently available in New Zealand community pharmacy outside of the study. This is in contrast to ‘usual care’, which would typically be no provision of written resources, as knee OA generally goes unrecognised.
Feasibility assessment. Key feasibility variables were assessed by recording recruitment rates, participant characteristics (including ethnicity, educational attainment, duration of knee pain, and being diagnosed with OA as part of the study), data completeness, randomisation adherence and contamination, follow-up rates, and participant perceptions. Fidelity to the randomisation sequence was assessed by cross-referencing the recorded date and time of recruitment with study code allocation to check whether information packs were distributed in the order dictated by the randomisation sequence. A member of the research team who has OA (JC) also visited pharmacies unannounced as a ‘Secret Shopper’ to report on their experiences of recruitment and observations of fidelity to randomisation and blinding processes from the perspective of being a customer with knee OA; pharmacies were not aware when and if this would happen.
Following completion of recruitment, pharmacists took part in face-to-face focus groups. Following 4-week data collection, a sub-set of participants took part in 1:1 telephone interviews. A member of the research team, who was not involved in data collection or analysis, had access to the randomisation schedule and selected participants for interviews, ensuring equal balance between both arms of study. To ensure diversity of views, the interview participants were purposively selected according to recruitment site (per pharmacy), age (<50 years/>50 years), ethnicity (Māori/non-Māori), duration of knee pain (<2 years/>2 years), prior OA diagnosis (yes/no), and how they found out about the study. Interviews and focus groups were conducted by an experienced qualitative researcher or Māori pharmacist who were both blind to participant group allocation. Semi-structured interview guides were used to explore perceptions of the trial processes such as recruitment, data collection, blinding and randomisation procedures (Supplementary 4). Audio-recorders were used to enable verbatim transcription, and field notes were kept.
Participant reported outcome measures. Illness perceptions, OA knowledge, fear of movement and pain while walking were measured at baseline and 4-weeks (considered sufficient to assess participant follow-up rates and belief changes). Illness perceptions were measured with the Brief Illness Perceptions Questionnaire (B-IPQ) [27]. The B-IPQ consists of eight items scored on a Likert scale from 0 to 10 and one open-ended causal question. Higher scores reflect a more threatening view of the illness. The B-IPQ was selected because illness perceptions are associated with disability due to OA [28]. Knowledge was measured with the KOAKS [19]. The KOAKS was originally developed with 15-items and reduced to 11-items following Rasch analysis to create a unidimensional scale with acceptable internal consistency (Cronbach's alpha 0.74). Each item is scored on a scale of 1–5, with total scores ranging from 11 to 55; higher scores reflect greater knowledge about OA. Fear of movement was measured with the 6-item Brief Fear of Movement Scale for Osteoarthritis (FOMOA) [29]. Each item is scored on a scale of 1–4, with total scores ranging from 6 to 24; higher scores indicate greater fear of movement. Average knee pain when walking over the last week was measured with an 11-point ordinal numeric pain rating scale (NPRS) [30].
There was active surveillance of potential harms. Participants reported all unexpected or serious health events in the 4-week follow-up survey, using an Adverse Event Reporting Form adapted from the World Health Organization template (Supplementary 5). Pharmacists and participants’ general practitioners were also asked to communicate any potential harms. An academic general practitioner was appointed to review Adverse Event reports. No data monitoring committee was appointed given the low risk of harm.
All data were entered and managed using Research Electronic Data Capture (REDCap) [31], hosted at the University of Otago. Qualitative process evaluation data were managed using NVivo 12 software (QSR International Pty Ltd).
Analysis. The recruitment target of 12 participants per pharmacy (72 in total) was primarily designed to enable adequate testing of study processes, but also gave reasonable estimation precision for belief change. A total sample of n = 56 completing participants (28 per arm, assuming 80% participant retention) was à priori considered to provide 80% power (two-tailed α of 0.05) to detect an 8-point between-group difference at 4 weeks on the B-IPQ (assuming SD of 10.5), representing a 20% improvement on the baseline score [32].
Analyses were conducted on an intention-to-treat basis, with all participants analysed in the group to which they were assigned. Continuous outcomes (B-IPQ, KOAKS, FOMOA, NPRS) were analysed using linear regression models, adjusted for baseline level of that outcome (e.g. baseline B-IPQ included as a covariate for analysis of follow-up B-IPQ) along with important baseline covariates (age, gender and duration of knee pain). Individuals lost to follow-up were excluded from analysis; individuals who were missing a specific measure(s) at follow-up were excluded from those analyses. Analysis was conducted in R 4.0 (R Institute, Vienna, Austria). Outcomes data are presented as mean estimates with 95% confidence intervals (95%CI).
Qualitative process evaluation data were analysed using Thematic Analysis [33]. Pharmacist focus groups and participant interviews were analysed separately. Analysis was inductive and iterative. Initial transcript coding was undertaken independently by MB on a line-by-line basis, using ‘open coding’ to allow multiple codes to be applied to single segments of data. MB and BD subsequently discussed and agreed on codes and categories within each transcript. The relationships between and within categories emerging from this process were explored with increasingly higher levels of conceptualisation. Negative case analysis was used to broaden understanding. Theme commonalities and divergences across the two independently analysed datasets (participants and pharmacists) were compared to produce meta-themes. A summary document was discussed with the wider research team.
3. Results
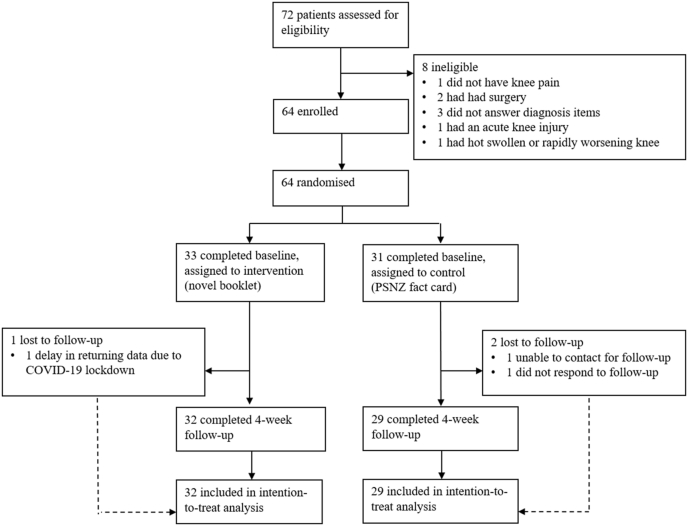
Participants. Of 72 people assessed for eligibility between February and July 2020, 64 participants were enrolled and randomised to the intervention (n = 33) or control (n = 31) (see trial profile in Fig. 1). The mean recruitment rate was 2.7 participants per pharmacy per week. All follow-up survey data were collected by August 30, 2020. Sufficient data were available to include 95% of participants (n = 61) in participant-reported outcome measure analysis. Three participants were lost to follow-up: one participant in the intervention group was not sent a 4-week follow-up survey because delivery of their baseline data was delayed due to Coronavirus disease 2019 (COVID-19) lockdown restrictions; two participants in the control group did not respond to follow-up invitations.
Fig. 1.
Trial profile.
Baseline demographic and clinical characteristics. Participant baseline characteristics were reasonably balanced between groups (Table 2). The intervention group were slightly older (mean (Standard Deviation; SD) 67.1 (8.5) years versus 64.1 (12.1) years) and therefore more likely to be not working (77% versus 48%), and had longer duration of knee pain (mean + - SD 10.4 (13.5) years versus 7.9 (8.3) years). The proportion of NZ Māori participants (14%) exceeded their proportion in the Canterbury population (9.8%) [34]. One-in-five participants had no educational qualifications. A quarter of participants had not received a knee OA diagnosis prior to the trial. B-IPQ, KOAKS, FOMOA and NPRS scores were similar in both groups at baseline.
Table 2.
Baseline characteristics.
| Characteristics | Control arm (PSNZ Fact Card) n = 31 Frequency (%) or mean (SD) |
Intervention arm (novel booklet) n = 33 Frequency (%) or mean (SD) |
|---|---|---|
| Age (yrs) | 67.1 (8.5) | 64.1 (12.1) |
| Gender | ||
| Female | 19 (61) | 21 (64) |
| Male | 12 (39) | 12 (36) |
| Ethnicitya | ||
| NZ European | 24 (77) | 29 (88) |
| Māorib | 5 (16) | 4 (12) |
| Pacific | 0 (0) | 0 (0) |
| Asian | 1 (3) | 0 (0) |
| Other | 1 (3) | 0 (0) |
| Education qualificationc | ||
| Nil | 7 (23) | 5 (17) |
| High school | 9 (29) | 9 (30) |
| Diploma or technical institute | 10 (32) | 13 (43) |
| University degree/postgraduate | 5 (16) | 3 (10) |
| Work Status | ||
| Not working | 24 (77) | 16 (48) |
| 1–20 h | 2 (6) | 2 (6) |
| 21–30 h | 0 (0) | 3 (9) |
| 31+ h | 5 (16) | 12 (36) |
| Duration of knee pain (yrs) | 10.4 (13.5) | 7.9 (8.3) |
| No previous diagnosis | 8 (25) | 8 (24) |
| B-IPQ | 49.7 (10.0) | 49.5 (9.0) |
| KOAKS | 33.2 (5.4) | 33.6 (5.7) |
| FOMOA | 15.0 (5.5) | 15.0 (3.7) |
| NPRS | 5.6 (2.5) | 6.0 (2.2) |
Abbreviations: PSNZ, Pharmaceutical Society of New Zealand; SD, standard deviation B-IPQ, Brief Illness Perceptions Questionnaire, scores range from 0 to 80, higher scores reflecting a more threatening view of the illness; KOAKS, Knee Osteoarthritis Knowledge Scale, scores range from 11 to 55, higher scores indicate greater knowledge about osteoarthritis; FOMOA, Fear of Movement for Osteoarthritis, scores range from 6 to 24, higher scores indicate greater fear of movement; NPRS, Numeric Pain Rating Scale while walking, scores range from 0 (no pain) to 10 (worst pain imaginable).
Participants could select more than one option.
Māori are the indigenous people of New Zealand.
Two participants did not provide these data.
Fidelity assessment. Recruitment targets were achieved by most pharmacies. Cross-referencing of the sequence of envelope distribution with the recorded date and time of recruitment showed that the randomisation sequence was followed correctly. The ‘Secret Shopper’ did not identify any protocol deviations.
Pharmacists (n = 11) participated in one of two focus groups (average duration 59 min). Twelve consumer participants were invited and participated (no participants declined) in telephone interviews (average duration 22 min, range 15–35 min). Three meta-themes emerged from pharmacist and participant qualitative analysis: ‘pleased to be asked’; ‘easy process’; ‘successful process’. An additional theme (‘occasional challenges’) emerged from the pharmacist focus groups. Themes and sub-themes are summarised in Table 3.
Table 3.
Qualitative meta-themes from pharmacists and participants with knee osteoarthritis.
| Theme | Sub-theme | Description |
|---|---|---|
| Pleased to be asked | Taking an interest |
|
| Build relationships |
|
|
| Easy process | Simple to follow |
|
| Acceptable time commitment |
|
|
| Successful process | Recruitment |
|
| Randomisation |
|
|
| Blinding |
|
|
| Occasional challenges | Discomfort |
|
| Competition |
|
Abbreviations: OA, osteoarthritis; COVID-19, Coronavirus disease 2019.
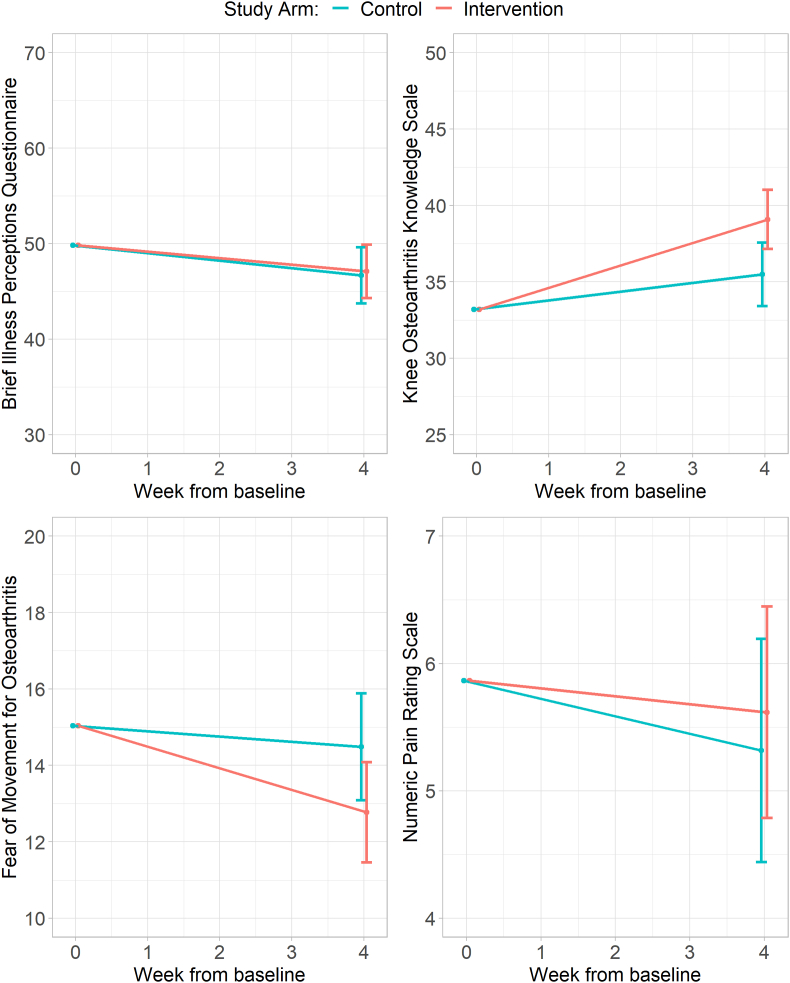
Participant reported outcome measures. Changes in participant outcomes are shown in Table 4 and Fig. 2. The novel booklet did not reduce B-IPQ scores compared to the control information at 4 weeks (mean difference 0.4, 95%CI -3.7 to 4.5; higher scores in the intervention arm) nor walking NPRS scores (mean difference 0.3, 95%CI -0.9 to 1.5; higher scores in the intervention arm).
Table 4.
Mean change scores from baseline at 4-weeks.
| Characteristica | Control (PSNZ Fact Card) (95% CI) | Intervention (novel booklet) (95% CI) | Mean difference (95% CI) |
|---|---|---|---|
| B-IPQ | −3.2 (−6.1 to −0.2) | −2.7 (−5.5 to 0.1) | 0.4 (−3.7 to 4.5) |
| KOAKS | 2.3 (0.2–4.4) | 5.9 (4.0–7.8) | 3.6 (0.7–6.5) |
| FOMOA | −0.5 (−2.0 to 0.9) | −2.3 (−3.6 to −1.0) | −1.7 (−3.7 to 0.2) |
| NPRS | −0.5 (−1.4 to 0.3) | −0.2 (−1.1 to 0.6) | 0.3 (−0.9 to 1.5) |
Abbreviations: PSNZ, Pharmaceutical Society of New Zealand; CI, confidence interval; B-IPQ, Brief Illness Perceptions Questionnaire, scores range from 0 to 80, higher scores reflecting a more threatening view of the illness; KOAKS, Knee Osteoarthritis Knowledge Scale, scores range from 11 to 55, higher scores indicate greater knowledge about osteoarthritis; FOMOA, Fear of Movement for Osteoarthritis, scores range from 6 to 24, higher scores indicate greater fear of movement; NPRS, Numeric Pain Rating Scale, scores range from 0 (no pain) to 10 (worst pain imaginable).
Adjusted for baseline score, Age, Gender, Duration of knee pain.
Fig. 2.
Mean patient reported outcome measure scores by study arm at baseline and follow-up.
The KOAKS scores for those who received the novel booklet improved compared to those who received the control information (mean difference = 3.6, 95%CI 0.7 to 6.5; higher scores in the intervention arm).
There was a trend toward reduction of FOMOA scores for those who received the novel booklet (mean difference −1.7, 95%CI -3.7 to 0.2; lower scores in the intervention arm) but the 95%CI crossed zero and is potentially consistent with no real difference between the two arms.
Potential harms. There were no reports of any adverse events or incidents during the trial from participants, their general practitioners or pharmacists.
4. Discussion
This trial demonstrated that it is feasible to conduct a trial that individually randomises participants with knee OA in a community pharmacy setting. This setting enabled identification of people with previously undiagnosed knee OA, alongside recruiting participants who are often under-represented in health research [35] and have inequitable OA health outcomes [36]. These findings highlight the feasibility for a definitive RCT and the potential of community pharmacy and community pharmacists to initiate accessible and equitable OA care.
This trial provided evidence that a novel information booklet delivered by a pharmacist improved the knowledge of people who have knee OA more than an existing biomedical arthritis information resource. The trial also gave some evidence for reduced fear of movement for those who received the novel booklet, however, the confidence interval included the potential for a strong effect or no difference. The improvement in knowledge for those who received the novel information booklet is consistent with our previous qualitative study in which people with knee OA reported radical changes in their understanding of OA after reading the novel booklet [20]. While educational interventions for knee OA have not previously been found to be cost-effective [9], education is a process fundamental to behaviour change [37,38]. The novel information tested in this trial may prepare people to effectively engage in cost-effective and clinically-effective care. Public health initiatives that can be delivered at scale with minimal direct cost to engage, empower, and educate communities are a fundamental action needed to address the rising burden of knee OA [11].
Results were indeterminate in relation to effect on illness perceptions and pain, with wide confidence intervals for effects. However, the range of potential changes from these confidence intervals did not include clinically meaningful differences, and so these seem unlikely to have promise as outcomes that could be modified by this intervention. Improving knowledge alone may be insufficient to change illness perceptions without aligned individualised therapy and experiential learning. It is not surprising that pain while walking did not change, given the brief follow-up duration of the trial combined with the encouragement in the booklet to exercise despite pain. In our previous study, many people with knee OA reported an intention to increase their physical activity after reading the novel booklet and gaining new knowledge or reinforcing existing helpful beliefs [20].
Strengths and limitations. This trial was designed to test the feasibility of conducting a planned large-scale RCT of a complex intervention in a community pharmacy setting. As such, it did not aim to investigate impacts on health outcomes; nor was it anticipated that health outcomes would change through an educational intervention alone. Notwithstanding this, the trial provided meaningful evidence in relation to key processes integral to influencing health outcomes. It showed that it is feasible to individually randomise participants in community pharmacy, rather than a cluster randomised trial design [25]. To our knowledge, this is the first study to compare two types of written OA information.
The trial used a rigorous methodology with blinding of all participants, pharmacists, researchers involved with data collection, and analysts. There were high rates of conversion from screening to enrolment (64/72 recruited; 89%), suggesting that the findings will be applicable to those in the target population of people with knee pain due to OA who visit the pharmacy. Randomisation using opaque envelopes may generally be considered inferior to a centralised randomisation service, however, this was much simpler to implement in a community pharmacy setting and less prone to error in delivering material to participants. Pharmacists were unaware of the differences between the two information resources and multiple approaches to fidelity checking did not identify any attempt to bypass the randomisation sequence. The choice of control allowed for comparisons between two written resources, with the control group receiving the currently available resource from outside of the study, rather than comparing to ‘usual care’ of receiving no information at all. No negative or nocebo effects of the control information were observed, indicating that this functioned effectively as a comparator. Although the initial recruitment target was not quite met, excellent follow-up rates (97% in the novel information arm and 94% in the control arm) enabled the planned number of participants to be entered in analyses (number of followed-up respondents). The exclusion of three participants (due to loss-to-follow-up) means the analysis does not meet the strictest definition of intention-to-treat analysis [39]: a full RCT with a focus on clinical outcome measurement could address this with more sophisticated methods (which make their own assumptions), but given the small amount of missing data we believe the analysis outcomes from this study will be unbiased.
The study had an indeterminate answer to the question of whether the novel information reduced fear of movement, with a confidence interval that included potentially useful effects, and which may have been more clear-cut with a larger sample size. This is important to explore in future full-scale trials given the relationship between fear of movement and disability [40,41].
Finally, the key aim of the information booklet was to improve OA knowledge, but no validated psychometrically robust tools existed to test OA knowledge. The KOAKS is a new scale and this is the first study to report its use as an outcome measure [19]. There are no available data to indicate whether a mean difference of 3.6 points is clinically important on a scale range of 11–55.
5. Conclusion
It is feasible to conduct a trial that individually randomises participants with knee OA in a community pharmacy setting. This trial demonstrates that a co-designed information resource delivered by a pharmacist can improve core OA knowledge and may reduce fear of movement. Identifying people with knee OA through community pharmacy may enable care to be provided in early-to mid-stages of the OA disease course and to people whom traditionally have unequal access to care.
Credit author statement
Ben Darlow: Conceptualisation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – original draft; Writing – review & editing. Melanie Brown: Conceptualisation; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing. Ben Hudson: Conceptualisation; Funding acquisition; Investigation; Methodology; Writing – review & editing. Gareth Frew: Conceptualisation; Investigation; Methodology; Writing – review & editing. Jane Clark: Conceptualisation; Investigation; Methodology; Writing – review & editing. Loren Vincent: Conceptualisation; Investigation; Methodology; Writing – review & editing. J Haxby Abbott: Conceptualisation; Funding acquisition; Methodology; Writing – review & editing. Andrew M Briggs: Conceptualisation; Methodology; Writing – review & editing. Rebecca Grainger: Conceptualisation; Methodology; Writing – review & editing. Carlo Marra: Conceptualisation; Methodology; Writing – review & editing. Eileen McKinlay: Conceptualisation; Methodology; Writing – review & editing. James Stanley: Conceptualisation; Data curation; Formal analysis; Funding acquisition; Methodology;; Visualisation; Writing – review & editing.
Contributions
Study conception and design: all authors. BD, MB, JS, BH, and JHA obtained funding. BD, MB, BH, GF, and LV contributed to study conduct and data collection. All authors contributed to data analysis and interpretation. JS conducted statistical analyses and produced data visualisation. MB conducted qualitative analysis with support from BD. BD and MB drafted the article, all authors critically revised the article for important intellectual content. All authors approved the final version of the article. BD had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the funding source
This study was funded by the Health Research Council of New Zealand (HRC 19/675). The funder played no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Declaration of competing interest
BD, MB, BH, RG, JHA, and EM are co-authors of the novel information resource tested in this trial. All authors declare that there are no other conflicts of interest.
Acknowledgements
The authors gratefully acknowledge the assistance of the Canterbury Community Pharmacy Group (especially Dr Aarti Patel and Robyn Harris) with pharmacist recruitment, training, and study conduct, the Pharmaceutical Society of New Zealand who provided the Arthritis Fact Cards, and the pharmacists who recruited participants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2022.100254.
Contributor Information
Ben Darlow, Email: ben.darlow@otago.ac.nz.
Melanie Brown, Email: melanie.brown@otago.ac.nz.
Ben Hudson, Email: ben.hudson@otago.ac.nz.
Gareth Frew, Email: gareth.frew@ccpg.org.nz.
Jane Clark, Email: jclark_73@yahoo.com.au.
Loren Vincent, Email: lorenjcollett@gmail.com.
J.Haxby Abbott, Email: haxby.abbott@otago.ac.nz.
Andrew M. Briggs, Email: A.Briggs@curtin.edu.au.
Rebecca Grainger, Email: rebecca.grainger@otago.ac.nz.
Carlo Marra, Email: carlo.marra@otago.ac.nz.
Eileen McKinlay, Email: eileen.mckinlay@otago.ac.nz.
James Stanley, Email: james.stanley@otago.ac.nz.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes L., Hagen K.B., Bijlsma J.W., Andreassen O., Christensen P., Conaghan P.G., et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013;72:1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 3.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., et al. American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2019;72(2020):220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Clinical Guideline Centre (UK) National Institute for Health and Care Excellence (UK); London: 2014. Osteoarthritis: Care and Management in Adults (CG177) [PubMed] [Google Scholar]
- 5.French S.D., Bennell K.L., Nicolson P.J., Hodges P.W., Dobson F.L., Hinman R.S. What do people with knee or hip osteoarthritis need to know? An international consensus list of essential statements for osteoarthritis. Arthritis Care Res. 2015;67:809–816. doi: 10.1002/acr.22518. [DOI] [PubMed] [Google Scholar]
- 6.Nelson A.E., Allen K.D., Golightly Y.M., Goode A.P., Jordan J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin. Arthritis Rheum. 2014;43:701–712. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Skou S.T., Roos E.M. Good Life with osteoArthritis in Denmark (GLA:D™): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Muscoskel. Disord. 2017;18:72. doi: 10.1186/s12891-017-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin J., Briggs A., Bagg W., Larmer P. An osteoarthritis model of care should be a national priority for New Zealand. N. Z.Med. J. 2017;130:78–86. [PubMed] [Google Scholar]
- 9.Mazzei D.R., Ademola A., Abbott J.H., Sajobi T., Hildebrand K., Marshall D.A. Are education, exercise and diet interventions a cost-effective treatment to manage hip and knee osteoarthritis? A systematic review. Osteoarthritis Cartilage. 2021;29:456–470. doi: 10.1016/j.joca.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Briggs A.M., Houlding E., Hinman R.S., Desmond L.A., Bennell K.L., Darlow B., et al. Health professionals and students encounter multi-level barriers to implementing high-value osteoarthritis care: a multi-national study. Osteoarthritis Cartilage. 2019;27:788–804. doi: 10.1016/j.joca.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Briggs A.M., Huckel Schneider C., Slater H., Jordan J.E., Parambath S., Young J.J., et al. Health systems strengthening to arrest the global disability burden: empirical development of prioritised components for a global strategy for improving musculoskeletal health. BMJ Global Health. 2021;6 doi: 10.1136/bmjgh-2021-006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurley M., Dickson K., Hallett R., Grant R., Hauari H., Walsh N., et al. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst. Rev. 2018 doi: 10.1002/14651858.CD010842.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paskins Z., Sanders T., Croft P.R., Hassell A.B. The identity crisis of osteoarthritis in general practice: a qualitative study using video-stimulated recall. Ann. Fam. Med. 2015;13:537–544. doi: 10.1370/afm.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis J.A., Taylor N.F., Bunzli S., Shields N. Experience of living with knee osteoarthritis: a systematic review of qualitative studies. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darlow B., Brown M., Lennox Thompson B., Hudson B., Grainger R., McKinlay E., et al. Living with osteoarthritis is a balancing act: an exploration of patients' beliefs about knee pain. BMC Rheumatology. 2018;2 doi: 10.1186/s41927-018-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunzli S., O'Brien P., Ayton D., Dowsey M., Gunn J., Choong P., et al. Misconceptions and the acceptance of evidence-based nonsurgical interventions for knee osteoarthritis. A qualitative study. Clin. Orthop. Relat. Res. 2019;477:1975–1983. doi: 10.1097/corr.0000000000000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Rezende M.U., Hissadomi M.I., de Campos G.C., Frucchi R., Pailo A.F., Pasqualin T., et al. One-Year results of an educational program on osteoarthritis:A prospective randomized controlled trial in Brazil. Geriatr Orthop Surg Rehabil. 2016;7:86–94. doi: 10.1177/2151458516645634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Losina E., Michl G.L., Smith K.C., Katz J.N. Randomized controlled trial of an educational intervention using an online risk calculator for knee osteoarthritis: effect on risk perception. Arthritis Care Res. 2017;69:1164–1170. doi: 10.1002/acr.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darlow B., Abbott J., Bennell K., Briggs A.M., Brown M., Clark J., et al. 2021. Consumers' Knowledge about Hip and Knee Osteoarthritis: Protocol for Developing and Testing the Hip and Knee Osteoarthritis Knowledge Scales Osteoarthritis and Cartilage Open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darlow B., Brown M., Grainger R., Hudson B., Briggs A.M., Abbott J., et al. Stakeholder views about a novel consumer health resource for knee osteoarthritis. Osteoarthritis and Cartilage Open. 2020 doi: 10.1016/j.ocarto.2020.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chukwu O.A. Beyond medicines provision: community pharmacists roles in meeting patient needs through value-added pharmacy services. J. Pharmaceut. Health Serv. Res. 2020;11:299–301. doi: 10.1111/jphs.12346. [DOI] [Google Scholar]
- 22.Gregory P.A.M., Austin Z. Understanding the psychology of trust between patients and their community pharmacists. Can. Pharm. J. 2021;154:120–128. doi: 10.1177/1715163521989760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Osteoarthritis Strategy Project Group . University of Sydney; Sydney: 2018. National Osteoarthritis Strategy. [Google Scholar]
- 24.Victorian Musculoskeletal Clinical Leadership Group . 2018. Victorian Model of Care for Osteoarthritis of the Hip and Knee. Melbourne: MOVE Muscle, Bone, and Joint Health. [Google Scholar]
- 25.Marra C.A., Cibere J., Grubisic M., Grindrod K.A., Gastonguay L., Thomas J.M., et al. Pharmacist-initiated intervention trial in osteoarthritis: a multidisciplinary intervention for knee osteoarthritis. Arthritis Care Res. 2012;64:1837–1845. doi: 10.1002/acr.21763. [DOI] [PubMed] [Google Scholar]
- 26.Marra C.A., Grubisic M., Cibere J., Grindrod K.A., Woolcott J.C., Gastonguay L., et al. Cost-utility analysis of a multidisciplinary strategy to manage osteoarthritis of the knee: economic evaluation of a cluster randomized controlled trial study. Arthritis Care Res. 2014;66:810–816. doi: 10.1002/acr.22232. [DOI] [PubMed] [Google Scholar]
- 27.Broadbent E., Petrie K., Main J., Weinman J. The brief illness perception questionnaire. J. Psychosom. Res. 2006;60:631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Bijsterbosch J., Scharloo M., Visser A.W., Watt I., Meulenbelt I., Huizinga T.W., et al. Illness perceptions in patients with osteoarthritis: change over time and association with disability. Arthritis Rheumatol. 2009;15:8. doi: 10.1002/art.24674. [DOI] [PubMed] [Google Scholar]
- 29.Shelby R.A., Somers T.J., Keefe F.J., DeVellis B.M., Patterson C., Renner J.B., et al. Brief fear of movement scale for osteoarthritis. Arthritis Care Res. 2012;64:862–871. doi: 10.1002/acr.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrar J.T., Young J.P., LaMoreaux L., Werth J.L., Poole R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 31.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claassen A.A.O.M., Schers H.J., Koëter S., van der Laan W.H., Kremers-van de Hei K.C.A.L.C., Botman J., et al. Preliminary effects of a regional approached multidisciplinary educational program on healthcare utilization in patients with hip or knee osteoarthritis: an observational study. BMC Fam. Pract. 2018;19:82. doi: 10.1186/s12875-018-0769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun V., Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 34.Ministry of Health . vol. 2021. Ministry of Health; Wellington, NZ: 2021. (Population of Canterbury DHB). [Google Scholar]
- 35.Smith T.O., Kamper S.J., Williams C.M., Lee H. Reporting of social deprivation in musculoskeletal trials: an analysis of 402 randomised controlled trials. Muscoskel. Care. 2021;19:180–185. doi: 10.1002/msc.1520. [DOI] [PubMed] [Google Scholar]
- 36.Singleton N., Buddicom E., Vane A., Poutawera V. Are there differences between Māori and non-Māori patients undergoing primary total hip and knee arthroplasty surgery in New Zealand? A registry-based cohort study. NZ Med J. 2013;126:23–30. [PubMed] [Google Scholar]
- 37.Fisher J.D., Fisher W.A. Changing AIDS-risk behavior. Psychol. Bull. 1992;111:455. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 38.Michie S., van Stralen M.M., West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement. Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moher D., Hopewell S., Schulz K.F., Montori V., Gøtzsche P.C., Devereaux P.J., et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;10:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunn A.H., Schwartz T.A., Arbeeva L.S., Callahan L.F., Golightly Y., Goode A., et al. Fear of movement and associated factors among adults with symptomatic knee osteoarthritis. Arthritis Care Res. 2017;69:1826–1833. doi: 10.1002/acr.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helminen E.E., Arokoski J.P., Selander T.A., Sinikallio S.H. Multiple psychological factors predict pain and disability among community-dwelling knee osteoarthritis patients: a five-year prospective study. Clin. Rehabil. 2020;34:404–415. doi: 10.1177/0269215519900533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.