Abstract
The present study was performed to evaluate the role of matrix metalloproteinases (MMP) in the pathogenesis of the inflammatory reaction and the development of neuronal injury in a rat model of bacterial meningitis. mRNA encoding specific MMPs (MMP-3, MMP-7, MMP-8, and MMP-9) and the inflammatory cytokine tumor necrosis factor alpha (TNF-α) were significantly (P < 0.04) upregulated, compared to the β-actin housekeeping gene, in cortical homogenates at 20 h after infection. In parallel, concentrations of MMP-9 and TNF-α in cerebrospinal fluid (CSF) were significantly increased in rats with bacterial meningitis compared to uninfected animals (P = 0.002) and showed a close correlation (r = 0.76; P < 0.001). Treatment with a hydroxamic acid-type MMP inhibitor (GM6001; 65 mg/kg intraperitoneally every 12 h) beginning at the time of infection significantly lowered the MMP-9 (P < 0.02) and TNF-α (P < 0.02) levels in CSF. Histopathology at 25.5 ± 5.7 h after infection showed neuronal injury (median [range], 3.5% [0 to 17.5%] of the cortex), which was significantly (P < 0.01) reduced to 0% (0 to 10.8%) by GM6001. This is the first report to demonstrate that MMPs contribute to the development of neuronal injury in bacterial meningitis and that inhibition of MMPs may be an effective approach to prevent brain damage as a consequence of the disease.
Bacterial meningitis continues to be an important clinical problem and is characterized by an intense inflammatory reaction of the subarachnoid and ventricular space, breakdown of the blood-brain barrier (BBB), and subsequent brain edema and vasculitis of the brain vessels (43, 45). Long-term neurological sequelae result from neuronal destruction due to an intense inflammatory response rather than from the infectious agent per se (28, 46). Accordingly, the glucocorticoid dexamethasone is currently used in some centers to protect the brain from the harmful effects of inflammation (29). However, the efficacy of dexamethasone as adjunctive therapy to antibiotics is modest, and increasing evidence suggests that therapeutic doses of steroids have no or even adverse effects (9, 20, 49). It appears, therefore, that therapies which target harmful processes more selectively than do steroids may be more effective. Understanding the processes that lead to brain damage in bacterial meningitis is crucial for the development of new drugs that can preserve neuronal function.
Matrix metalloproteinases (MMPs) are a family of closely related Zn2+-dependent endopeptidases that degrade virtually all components of the extracellular matrix. Neutrophils, neurons, glial cells, vascular smooth muscle cells, and endothelial cells produce MMP upon stimulation (10, 11, 17, 33). MMPs can be subdivided according to their substrate specificity into collagenases (MMP-1, MMP-8, MMP-13, and MMP-18, gelatinases (MMP-2 and MMP-9), stromelysins (MMP-3, MMP-10, and MMP-11), and other MMPs such as matrilysin (MMP-7), macrophage elastase (MMP-12), and membrane-type MMPs (MMP-14 through MMP-17). The ability to lyse the subendothelial basement membrane, which forms the BBB around cerebral capillaries, makes MMPs likely candidates as effector molecules of leukocyte extravasation and BBB breakdown in bacterial meningitis (16, 31, 35). Furthermore, substrates of MMP also include pro-forms of MMP and precursors of cytokines and their receptors (6, 15). We and others have previously shown that MMPs are present at high concentration in the cerebrospinal fluid (CSF) of patients with bacterial meningitis and MMP inhibition in a rat model of early meningitis reduced subarachnoid space inflammation, brain edema, and BBB permeability (5, 21, 26, 35).
With regard to the pathogenesis of bacterial meningitis, the potential of MMPs to activate cytokines is intriguing. A pivotal element in the meningeal inflammatory process is tumor necrosis factor alpha (TNF-α), which exists in two biologically active forms, a 17-kDa soluble form and a 26-kDa membrane-bound form. TNF-α-converting enzyme (TACE), a metalloproteinase closely related to MMPs, cleaves cell-associated TNF-α to its soluble form (44). TNF-α is a strong stimulus for the release and activation of MMPs in the brain (42). In bacterial meningitis, metalloproteinases, including MMPs, may therefore contribute to the development of brain injury by both their proteolytic activity on the extracellular matrix and their ability to increase the levels of soluble TNF-α.
The hydroxamic acid-type MMP inhibitor GM6001 is a broad-spectrum MMP inhibitor (14). GM6001 acts by chelating the zinc cation in the active site of MMPs and has previously been reported to block leukocyte migration, to suppress autoimmune encephalomyelitis, to prevent brain edema after intracerebral hemorrhage, and to abrogate endotoxin-induced death (14, 16, 27, 44, 47).
The aim of the present study was to use an infant-rat model of bacterial meningitis caused by Streptococcus pneumoniae to investigate the induction and expression of specific MMPs in relation to TNF-α and to assess the role of MMP in the pathogenesis of neuronal injury.
MATERIALS AND METHODS
Infecting organism.
We used a clinical isolate of S. pneumoniae (serogroup 3) from a patient with bacterial meningitis. The organism was grown on blood agar plates, cultured overnight in 10 ml of brain heart infusion medium, diluted in fresh medium, and grown for 6 h to logarithmic phase. The culture broth was centrifuged for 10 min at 5,000 × g, pelleted, resuspended in sterile saline to the desired density, and used for intracisternal injection. The accuracy of the inoculum size was routinely confirmed by quantitative cultures.
Model of meningitis.
The animal studies were approved by the Animal Care and Experimentation Committee of the Kanton of Berne, Switzerland, and followed National Institutes of Health guidelines for the performance of animal experiments. A total of 56 Sprague-Dawley rats were used throughout the experiments. Nursing Sprague-Dawley rats with their dams were purchased (RCC Biotechnology & Animal Breeding, Füllinsdorf, Switzerland), and pups were infected on postnatal day 11, when they weighed 25.6 ± 1.4 g. Infection was induced by direct intracisternal injection of 10 μl of saline containing log10 7.0 ± 0.3 CFU of S. pneumoniae per ml via a 32-gauge needle as published previously (22, 24, 25). Uninfected control animals were injected with 10 μl of sterile saline. At 18 h later, the animals were weighed and assessed clinically by applying the following score: 1, coma; 2, does not turn upright; 3, turns upright within 30 s; 4, minimal ambulatory activity, turns upright in <5 s; and 5, normal. Cerebrospinal fluid (CSF) (10 to 30 μl) was obtained by puncture of the cisterna magna, and 5 μl was cultured quantitatively to document meningitis. The remaining CSF was immediately centrifuged, the pellet was resuspended in lysis buffer (Qiagen AG, Basel, Switzerland), and the supernatant and pellet were frozen separately at −80°C until used for analysis. The animals then received antibiotic therapy (ceftriaxone [Roche Pharma, Reinach, Switzerland] at 100 mg/kg subcutaneously twice daily). The animals were sacrificed with an overdose of pentobarbital (100 mg/kg intraperitoneally [i.p.]) and perfused via the left cardiac ventricle with 30 ml of ice-cold phosphate-buffered saline (PBS) for assessment of mRNA in brain homogenates or with 4% paraformaldehyde in PBS for histopathologic evaluation. Because brain injury evolves over time, the sacrificed animals and the animals that were observed to die spontaneously were randomly matched with littermates from the comparison group sacrificed at the same time point in order to obtain groups with comparable survival times. Thus, there were no significant differences in survival times (25.1 ± 4.7 h for GM6001 treatment and 26.1 ± 6.62 h for controls). Animals dying unobserved were excluded from the evaluation.
MMP inhibition.
Treatment with GM6001 (Glycomed Inc., Alameda, Calif.) at 65 mg/kg i.p. every 12 h [n = 19]) or vehicle (250 μl of 10% 1,2-propandiol–2% methylcellulose i.p. every 12 h [n = 17]) was initiated at the time of infection.
Reverse transcription-PCR (RT-PCR).
Cortices from animals with bacterial meningitis at 20 h after infection (n = 3) and from uninfected controls (n = 3) were dissected in ice-cold PBS. The cerebral hemispheres of each brain were individually rolled on filter paper to remove the meninges and brain vessels. mRNA was isolated by using the MicroFastTrack kit (Invitrogen BV, Groningen, The Netherlands) and converted to cDNA by using the Universal RiboClone System (Promega Corp., Madison, Wis.). The levels of individual mRNAs encoding MMPs with likely relevance to the pathophysiology of bacterial meningitis (i.e., MMP-2, MMP-3, MMP-7 through MMP-13, MMP-15, and MMP-16), as well as the level of TNF-α mRNA, were measured by comparison to the mRNA level of the β-actin housekeeping gene by using a PCR based method for the quantitation of mRNA as described in detail previously (3, 8).
MMP-2 and MMP-9 in CSF.
The presence of the gelatinases MMP-2 and MMP-9 in CSF was assessed by measuring their size and zymographic activity in a gelatin-containing electrophoresis gel, since they are widely used in the study of inflammatory diseases of the central nervous system (CNS) and can be readily detected. Samples of CSF (3 μl) were diluted into sample buffer (0.4 M Tris-HCl [pH 6.8], 5% sodium dodecyl sulfate [SDS], 20% glycerol, 1% bromphenol blue) to a loading volume of 10 μl and electrophoresed under nonreducing conditions in 10% polyacrylamide–SDS gels containing type A gelatin (1% [vol/vol]; Sigma, Buchs, Switzerland) as the proteinase substrate (23). After electrophoresis for 2 h at 95 V, MMPs were renatured by removal of SDS by immersing the gel for 1 h in Triton X-100 (2% [vol/vol]). The gels were then incubated in Ca2Cl-Tris-NaCl buffer for 18 h at 37°C to allow proteolysis of the gelatin substrate, fixed, and stained with Coomassie blue. The gelatinolytic activities of MMP-9 and MMP-2 were determined by quantitation of gelatin lysis zones. Gels were scanned at 1,200 by 1,200 dpi on a transparency scanner (Power Look III; UMAX Technologies Inc., Fremont, Calif.), and densitometry of substrate lysis zones at 92 (MMP-9) and 72 (MMP-2) kDa was measured by using the public domain NIH Image program (version 1.61; National Institutes of Health, Bethesda, Md.). The lysis zone of the induced MMP-9 protein was expressed as a percentage of the lysis zone of the constitutively expressed MMP-2 for each sample.
Concentration of TNF-α in CSF.
The concentration of rat-specific TNF-α in CSF was measured by a commercial sandwich enzyme-linked immunosorbent assay ELISA kit (Cytoscreen, Rat Tumor Necrosis Factor-Alpha Ultra Sensitive ELISA [no. KRC3010-UB and KRC 3013]; BioSource International, Camarillo, Calif.). CSF supernatant (4.4 μl) was diluted 1:50, and the assays were performed in duplicate as specified by the manufacturer and expressed as the range of change at an absorption of 450 nm. The detection limit of the assay was <0.7 pg/ml.
Histopathology.
For quantitative evaluation, coronal sections were examined for cortical neuronal injury, defined as areas of decreased density of neurons or frank cortical necrosis. Brains (n = 45) were postfixed overnight in 5 ml of 4% paraformaldehyde in PBS (pH 7.4) at 4°C, placed in 30% sucrose in PBS for 24 h, and cut at 40-μm intervals on a cryostat (Cryocut 1800; Leica Instruments, Nussloch, Germany). Twelve coronal sections, four each from the frontal, middle, and dorsal brain regions (corresponding to the dorso-ventral coordinates bregma 1.70 to 1.00 mm, bregma −0.80 to −1.40 mm, and bregma −3.30 to −5.20 mm in adult rats) were mounted on polylysine-coated slides for Nissl staining with cresyl violet, and coverslips were fixed with Entellan (Merck, Darmstadt, Germany).
Cortical sections were scanned at 2,700 by 2,700 dpi with a film scanner (SprintScan 35 Plus; Polaroid Europe Ltd., Uxbridge, United Kingdom), and the digitized images were analyzed by using the NIH Image program (version 1.61). Neuronal injury to the cortex was confirmed by simultaneous bright-field microscopy, and the area of cortical brain damage was expressed as a percentage of the total cortex in each section. The mean value per brain was calculated and used for statistical evaluation. All histopathological evaluations were performed by an investigator blinded to the clinical, microbiological, and treatment data for the respective animal.
Statistics.
Normally distributed variables are presented as mean ± standard deviation, and differences between groups were analyzed by an unpaired t test. Variables that were not normally distributed were compared by the Kruskal-Wallis test. When the latter yielded a statistically significant value (P < 0.05), pairwise comparison was done by the two-tailed nonparametric Mann-Whitney U test. The association between continuous variables was assessed by using the Pearson correlation coefficient. Proportions between different groups were compared by Fisher's exact test.
RESULTS
Expression of MMPs and TNF-α.
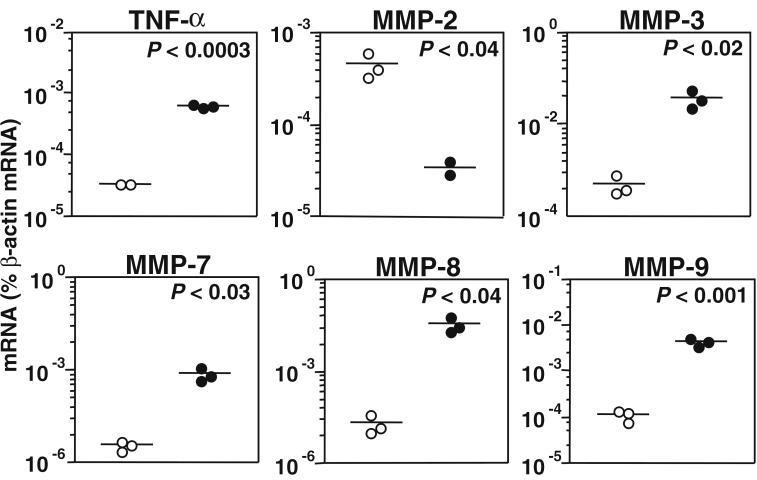
mRNA encoding TNF-α, MMP-2, MMP-3, MMP-7 through MMP-13, MMP-15, and MMP-16 was quantitated by competitive RT-PCR in cortical homogenates and expressed as a percentage of the mRNA encoding the β-actin housekeeping gene. Compared to uninfected controls, bacterial meningitis at 20 h after infection led to an upregulation of mRNA encoding TNF-α (P < 0.0003), MMP-3 (P < 0.02), MMP-7 (P < 0.03), MMP-8 (P < 0.04), and MMP-9 (P < 0.001) but to a downregulation of mRNA encoding MMP-2 (P < 0.04) (Fig. 1). MMP-10, MMP-11, MMP-12, MMP-13, MMP-15, and MMP-16 exhibited no statistically significant difference between infected animals and controls (data not shown).
FIG. 1.
mRNA encoding TNF-α, MMP-2, MMP-3, MMP-7, MMP-8, and MMP-9, assessed by RT-PCR in cortical homogenates. In animals with bacterial meningitis at 20 h after infection (●) compared to uninfected controls (○), the mRNA levels encoding TNF-α, MMP-3, MMP-7, MMP-8, and MMP-9 were increased while MMP-2-mRNA was downregulated.
Clinical parameters of meningitis.
All infected animals had meningitis, as evidenced by lethargy to obtundation and positive bacterial titers in CSF at 18 h after infection. Treatment with GM6001 (n = 19) compared to vehicle (n = 17) had no effect on CSF bacterial titers (7.6 ± 0.7 versus 7.7 ± 0.6 log10 CFU/ml), clinical score (median [range], 4 [3 to 4] versus 4 [3 to 4], or weight loss after 18 h of infection (0.7 ± 0.5 g versus 0.7 ± 0.5 g).
MMP-9 activity and TNF-α in CSF.
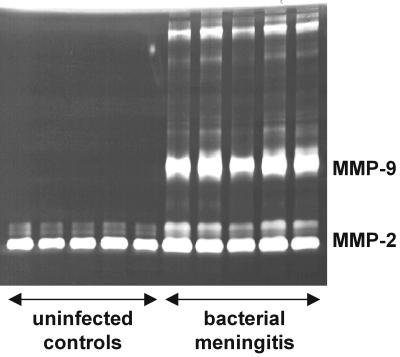
We measured the concentrations of TNF-α and semiquantitatively assessed the amount of MMP-2 and MMP-9 in CSF samples from animals with bacterial meningitis and uninfected controls at 18 h after infection. MMP-2, as measured by zymographic densitometry, was constitutively expressed in CSF of controls, and its levels did not show significant changes in the animals with bacterial meningitis. MMP-9, in contrast, was virtually absent in uninfected controls but was strongly upregulated in the animals with bacterial meningitis (Fig. 2).
FIG. 2.
Zymography of CSF samples from uninfected controls and animals with pneumococcal meningitis 18 h after infection. MMP-2 was present in all samples, and there were no significant differences between uninfected and infected animals, indicating a constitutive release. Animals with bacterial meningitis showed a significant increase in the zymographic activity of MMP-9.
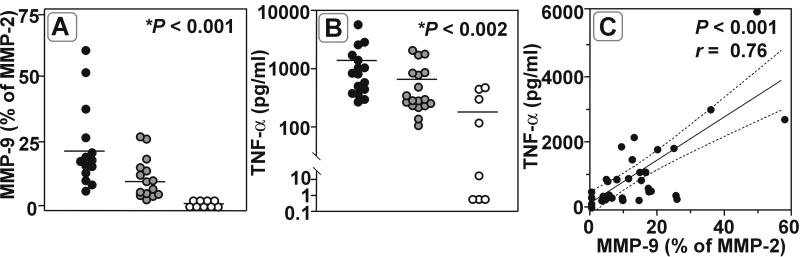
Treatment with GM6001 significantly reduced MMP-9 zymographic activity in CSF of infected animals compared to vehicle-treated controls (percentage of MMP-2 activity; 21.56% ± 15.0% [n = 15] versus 10.12% ± 7.2% [n = 14]; P < 0.02), while MMP-9 was barely detectable in CSF from uninfected animals (0.02% ± 0.04% [n = 9]; P < 0.001 [Kruskal-Wallis test for all groups]) (Fig. 3A). In parallel, bacterial meningitis led to a marked increase in TNF-α levels in CSF (1,382 ± 1,458 pg/ml in vehicle-treated animals [n = 16] versus 288 ± 190 pg/ml in uninfected controls; P < 0.0001). This increase was significantly attenuated in animals treated with GM6001 (658 ± 635 pg/ml [n = 18] [P < 0.02] versus vehicle-treated animals [P < 0.002] [Kruskal Wallis test for all groups]) (Fig. 3B). MMP-9 zymographic activity and TNF-α concentration in CSF of all animals were significantly correlated (P < 0.001; Pearson r = 0.76, n = 36) (Fig. 3C).
FIG. 3.
(A and B) Bacterial meningitis (●) leads to a significant increase in the concentrations of MMP-9 (P < 0.001) (A) and TNF-α (P < 0.002) (B) in CSF compared to those in uninfected controls (○). MMP inhibition by GM6001 ( ) significantly lowered MMP-9 (P < 0.02) and TNF-α (P < 0.02) levels compared to vehicle treatment (∗, Kruskal Wallis test for all groups). (C) The close correlation of TNF-α and MMP-9 in CSF from GM6001-treated and control animals suggests an interdependence between TNF-α and MMP-9.
) significantly lowered MMP-9 (P < 0.02) and TNF-α (P < 0.02) levels compared to vehicle treatment (∗, Kruskal Wallis test for all groups). (C) The close correlation of TNF-α and MMP-9 in CSF from GM6001-treated and control animals suggests an interdependence between TNF-α and MMP-9.
Effect of MMP inhibition on neuropathologic outcome.
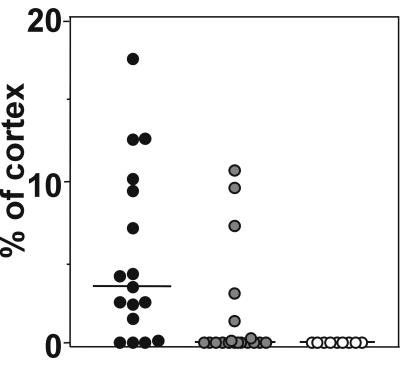
Control animals sham infected with sterile saline had no cortical damage detected by histopathology. Bacterial meningitis led to substantial brain damage (>1% of the cortex was injured) in 73% of infected control animals (median [range], 3.5% [0 to 17.5%] of the cortex). This proportion was reduced to 26% in the animals that received GM6001 at the time of infection (median [range], [0 to 10.8%]; P < 0.01) (Fig. 4). GM6001 treatment in uninfected control animals had no clinical or histopathological effects.
FIG. 4.
Effect of MMP inhibition by GM6001 on neuronal injury in infant rats with pneumococcal meningitis. Histopathology at 25.5 ± 5.7 h after infection showed extensive neuronal injury (median [range], 3.5% [0 to 17.5%] of the cortex [●]), which was significantly (P < 0.01) reduced to 0% (0 to 10.8%) in animals treated with GM6001 ( ). Uninfected control animals (○) showed no cortical injury at 24 h after sham infection (P < 0.001; Kruskal Wallis test for all groups).
). Uninfected control animals (○) showed no cortical injury at 24 h after sham infection (P < 0.001; Kruskal Wallis test for all groups).
DISCUSSION
MMPs have been implicated as mediators of brain injury in a wide variety of disease processes, including multiple sclerosis, Alzheimer's disease, stroke, tumor invasion, and other inflammatory diseases of the brain (2, 7, 23, 34, 41, 48). Pathophysiologic processes characteristic of bacterial meningitis, such as neutrophil extravasation, subarachnoid space inflammation, BBB disruption, and brain edema, have been attributed to the action of MMPs (5, 23, 35, 36). The present study provides strong evidence that MMP-mediated processes are critically involved in the pathogenesis of brain injury in bacterial meningitis. Furthermore, the study demonstrates for the first time that inactivation of MMPs by hydroxamic acid is an effective strategy to reduce neuronal damage in this disease model.
Meningeal inflammation is the hallmark of pathophysiologic alterations during bacterial meningitis and is initiated by the release of bacterial cell wall components during growth and lysis of bacterial pathogens in the subarachnoid and ventricular space. These bacterial products induce the release of proinflammatory cytokines, such as TNF-α and interleukins, in the initial phase of the inflammatory cascade (45). TNF-α can be detected in the CSF within minutes after intracisternal injection of bacterial cell wall components and is a key trigger of the inflammatory response, produced within the CSF predominantly by inflammatory cells, glial cells, and endothelial cells (32). Upon activation, the membrane-bound form of TNF-α is converted to its active soluble form by TACE, a metalloproteinase closely related to the MMP family (6, 15). Intracisternal administration of TNF-α resulted in CSF leukocytosis and an increase in cerebral blood flow (1, 40), while intracerebral injection of TNF-α produced a dose-dependent increase in BBB permeability and MMP activity (42). Thus, in bacterial meningitis, TNF-α participates in the inflammatory response of the subarachnoid space, mediates pathophysiologic changes characteristic of bacterial meningitis, and is likely to perpetuate its own release through the induction of MMP.
The acute inflammatory involvement of the subarachnoid and ventricular space and the contained vasculature is characteristic of bacterial meningitis (24). The extravasation of leukocytes requires an MMP-dependent transmigration of the inflammatory cells through the vessel wall (19, 27, 30). These processes are located at the level of the brain microvasculature and might explain why brain vessels are a primary site of injury in bacterial meningitis (12, 37). Vasculitis caused by bacterial meningitis results in disruption of vascular integrity, breakdown of the BBB, and subsequent brain edema and a decrease in global cerebral blood perfusion (13, 38). The occurrence of thrombosis then leads to focal ischemia/reperfusion injury (38, 45). Converging evidence indicates that MMPs are involved in all these steps: MMPs mediate cell migration across the BBB and can degrade collagens IV and V, major components of the subendothelial basal lamina forming the BBB around cerebral capillaries, and intracerebral injection of TNF-α causes MMP-dependent breakdown of the BBB (31, 35). Accordingly, an increase in the level of MMP-9 in CSF during experimental pneumococcal meningitis was correlated not only with the amount of protein as an index of BBB permeability but also with the extent of CSF pleocytosis (5).
In the present model, bacterial meningitis led to a marked transcriptional upregulation of TNF-α, MMP-3, MMP-7, MMP-8, and MMP-9 in cortical homogenates. On the protein level, CSF of animals with disease demonstrated an increase in concentrations of TNF-α and MMP-9. The close positive correlation between TNF-α and MMP-9 in CSF suggests a role of TNF-α in the induction of MMP and emphasizes the importance of TNF-α as an initiator of inflammation, acting on secondary effectors such as MMP (31). Moreover, MMP inhibition not only resulted in a significant attenuation of MMP-9 activity in zymography but also lowered TNF-α levels in CSF. This finding points to an additional beneficial effect of broad MMP inhibition in bacterial meningitis. A combined decrease in TACE and MMP activity has the potential to block a possibly self-perpetuating circle of mutual activation between TNF-α and MMP, attenuating the overshooting immune response at the origin of neuronal damage in bacterial meningitis.
Parenchymal damage to the brain is the most important consequence of bacterial meningitis (4, 18, 39). Neurologic sequelae in patients surviving the disease include hearing impairment, obstructive hydrocephalus, and brain parenchymal damage, reflected by sensorimotor syndromes, cerebral palsy, mental retardation, learning deficits, cortical blindness, and seizure disorders (39). In experimental allergic encephalitis, a neuroinflammatory disease model with pathophysiologic similarities to bacterial meningitis, MMP inhibitors reduced disease severity and CNS invasion by inflammatory cells (8, 16, 33). Related to the present study are results from experimental meningitis in adult rats, where broad inhibition of MMPs reduced subarachnoid space inflammation and brain edema and partially prevented the breakdown of the BBB. Treatment with the MMP inhibitor GM6001 abrogates endotoxin-induced death by blocking the processing of soluble TNF-α (44). No beneficial effect of treatment with GM6001 was found on weight loss and clinical score in the present study. This is most probably because systemic symptoms were mild at the time of assessment and systemic disease is influenced by multiple factors, some of which are not affected by MMP inhibition.
In the present study, treatment with the MMP inhibitor GM6001 reduced MMP-9 levels in CSF and significantly attenuated brain damage. The decrease of MMP-9 levels in CSF probably reflects the phenomenon of decreased cellular invasion of the CNS across the BBB, as already observed in experimental meningitis and allergic encephalitis (8, 35). During inflammation, metalloproteinases may cause a prolonged inflammatory stimulation because of their ability to process TNF-α to its soluble form. In humans with bacterial meningitis, levels of MMP in the CSF are increased at the time of admission to the hospital and the high levels persist for up to 3 days after initiation of antibiotic therapy (26, 35). This prolonged activity profile of MMP in bacterial meningitis suggests a role of MMP not only in the initial inflammatory phase but also in the development and spreading of parenchymal damage after removal of the initial bacterial stimulus by antibiotic therapy.
In summary, this study documents a role for MMPs in the pathogenesis of neuronal injury in a model of neonatal pneumococcal meningitis. In the cerebral cortex of animals with established meningitis, we found an increase in the levels of mRNAs encoding TNF-α, MMP-3, MMP-7, MMP-8, and MMP-9. In CSF, concentrations of TNF-α and MMP-9 were upregulated and showed a close correlation. Inhibition of MMPs with GM6001 reduced the concentrations of TNF-α and MMP-9 in CSF and attenuated the extent of cortical brain damage. This is the first report to demonstrate a significant beneficial effect of an MMP inhibitor on the neuropathologic outcome in bacterial meningitis.
ACKNOWLEDGMENTS
We thank Caroline Grygar, Oliver Schütz, Philipp Joss, and Erwin Studer for excellent technical support.
This work was supported by grants from the Swiss National Science Foundation (NRP 4038-52841 and SNF 31-51084.97) and by the NIH (NS-32553 and NS-34028).
REFERENCES
- 1.Angstwurm K, Freyer D, Dirnagl U, Hanisch U K, Schumann R R, Einhaupl K M, Weber J R. Tumour necrosis factor alpha induces only minor inflammatory changes in the central nervous system, but augments experimental meningitis. Neuroscience. 1998;86:627–634. doi: 10.1016/s0306-4522(98)00032-3. [DOI] [PubMed] [Google Scholar]
- 2.Anthony D C, Ferguson B, Matyzak M K, Miller K M, Esiri M M, Perry V H. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. [PubMed] [Google Scholar]
- 3.Anthony D C, Miller K M, Fearn S, Townsend M J, Opdenakker G, Wells G M, Clements J M, Chandler S, Gearing A J, Perry V H. Matrix metalloproteinase expression in an experimentally-induced DTH model of multiple sclerosis in the rat CNS. J Neuroimmunol. 1998;87:62–72. doi: 10.1016/s0165-5728(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 4.Arditi M, Mason E O, Jr, Bradley J S, Tan T Q, Barson W J, Schutze G E, Wald E R, Givner L B, Kim K S, Yogev R, Kaplan S L. Three-year multicenter surveillance of pneumococcal meningitis in children: clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics. 1998;102:1087–1097. doi: 10.1542/peds.102.5.1087. [DOI] [PubMed] [Google Scholar]
- 5.Azeh I, Mader M, Smirnov A, Beuche W, Nau R, Weber F. Experimental pneumococcal meningitis in rabbits: the increase of matrix metalloproteinase-9 in cerebrospinal fluid correlates with leucocyte invasion. Neurosci Lett. 1998;256:127–30. doi: 10.1016/s0304-3940(98)00776-9. [DOI] [PubMed] [Google Scholar]
- 6.Chandler S, Cossins J, Lury J, Wells G. Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-alpha fusion protein. Biochem Biophys Res Commun. 1996;228:421–429. doi: 10.1006/bbrc.1996.1677. [DOI] [PubMed] [Google Scholar]
- 7.Chandler S, Miller K M, Clements J M, Lury J, Corkill D, Anthony D C, Adams S E, Gearing A J. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72:155–161. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 8.Clements J M, Cossins J A, Wells G M, Corkill D J, Helfrich K, Wood L M, Pigott R, Stabler G, Ward G A, Gearing A J, Miller K M. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol. 1997;74:85–94. doi: 10.1016/s0165-5728(96)00210-x. [DOI] [PubMed] [Google Scholar]
- 9.Daoud A S, Batieha A, Al-Sheyyab M, Abuekteish F, Obeidat A, Mahafza T. Lack of effectiveness of dexamethasone in neonatal bacterial meningitis. Eur J Pediatr. 1999;158:230–233. doi: 10.1007/s004310051056. [DOI] [PubMed] [Google Scholar]
- 10.Deb S, Gottschall P E. Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with beta-amyloid peptides. J Neurochem. 1996;66:1641–1647. doi: 10.1046/j.1471-4159.1996.66041641.x. [DOI] [PubMed] [Google Scholar]
- 11.Dencoff J E, Rosenberg G A, Harry G J. Trimethyltin induces gelatinase B and urokinase in rat brain. Neurosci Lett. 1997;228:147–150. doi: 10.1016/s0304-3940(97)00380-7. [DOI] [PubMed] [Google Scholar]
- 12.Faustmann P M, Krause D, Dux R, Dermietzel R. Morphological study in the early stages of complement C5a fragment-induced experimental meningitis: activation of macrophages and astrocytes. Acta Neuropathol (Berlin) 1995;89:239–247. doi: 10.1007/BF00309339. [DOI] [PubMed] [Google Scholar]
- 13.Forderreuther S, Tatsch K, Einhaupl K M, Pfister H W. Abnormalities of cerebral blood flow in the acute phase of bacterial meningitis in adults. J Neurol. 1992;239:431–436. doi: 10.1007/BF00856807. [DOI] [PubMed] [Google Scholar]
- 14.Galardy R E, Cassabonne M E, Giese C, Gilbert J H, Lapierre F, Lopez H, Schaefer M E, Stack R, Sullivan M, Summers B, et al. Low molecular weight inhibitors in corneal ulceration. Ann N Y Acad Sci. 1994;732:315–323. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- 15.Gearing A J, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson A H, Drummond A H, Galloway W A, Gilbert R, Gordon J L, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 16.Gijbels K, Galardy R E, Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Investig. 1994;94:2177–2182. doi: 10.1172/JCI117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottschall P E, Deb S. Regulation of matrix metalloproteinase expressions in astrocytes, microglia and neurons. Neuroimmunomodulation. 1996;3:69–75. doi: 10.1159/000097229. [DOI] [PubMed] [Google Scholar]
- 18.Grimwood K, Nolan T M, Bond L, Anderson V A, Catroppa C, Keir E H. Risk factors for adverse outcomes of bacterial meningitis. J Paediatr Child Health. 1996;32:457–462. doi: 10.1111/j.1440-1754.1996.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 19.Hess D C, Thompson Y, Sprinkle A, Carroll J, Smith J. E-selectin expression on human brain microvascular endothelial cells. Neurosci Lett. 1996;213:37–40. doi: 10.1016/0304-3940(96)12837-8. [DOI] [PubMed] [Google Scholar]
- 20.Keenan P A, Jacobs M W, Solemani R W, Newcomer J W. Commonly used therapeutic doses of glucocorticoids impair explicit memory. Ann N Y Acad Sci. 1995;761:400–402. doi: 10.1111/j.1749-6632.1995.tb31402.x. [DOI] [PubMed] [Google Scholar]
- 21.Kieseier B C, Paul R, Koedel U, Seifert T, Clements J M, Gearing A J, Pfister H W, Hartung H P. Differential expression of matrix metalloproteinases in bacterial meningitis. Brain. 1999;122:1579–1587. doi: 10.1093/brain/122.8.1579. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y S, Sheldon R A, Elliott B R, Liu Q, Ferriero D M, Täuber M G. Brain injury in experimental neonatal meningitis due to group B streptococci. J Neuropathol Exp Neurol. 1995;54:531–539. doi: 10.1097/00005072-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kolb S A, Lahrtz F, Paul R, Leppert D, Nadal D, Pfister H W, Fontana A. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in viral meningitis: upregulation of MMP-9 and TIMP-1 in cerebrospinal fluid. J Neuroimmunol. 1998;84:143–150. doi: 10.1016/s0165-5728(97)00247-6. [DOI] [PubMed] [Google Scholar]
- 24.Leib S L, Kim Y S, Chow L L, Sheldon R A, Täuber M G. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Investig. 1996;98:2632–2639. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leib S L, Kim Y S, Ferriero D M, Täuber M G. Neuroprotective effect of excitatory amino acid antagonist kynurenic acid in experimental bacterial meningitis. J Infect Dis. 1996;173:166–171. doi: 10.1093/infdis/173.1.166. [DOI] [PubMed] [Google Scholar]
- 26.Leib S L, Leppert D, Clements J, Täuber M G. Keystone Symposia: effectors of inflammation in the CNS. Taos, N. Mex: Keystone Symposia on Molecular and Cellular Biology; 1999. Role of matrix metalloproteinases in the pathogenesis of bacterial meningitis; p. 57. [Google Scholar]
- 27.Leppert D, Waubant E, Galardy R, Bunnett N W, Hauser S L. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol. 1995;154:4379–4389. [PubMed] [Google Scholar]
- 28.McCracken G H J, Saez-Llorens X. Contribution of cytokines to meningeal inflammation in bacterial meningitis. In: Peterson P K, Remington J S, editors. In defense of the brain. Malden, Mass: Blackwell Science; 1997. pp. 107–123. [Google Scholar]
- 29.McIntyre P B, Berkey C S, King S M, Schaad U B, Kilpi T, Kanra G Y, Perez C M. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:925–931. doi: 10.1001/jama.278.11.925. [DOI] [PubMed] [Google Scholar]
- 30.Mollinedo F, Nakajima M, Llorens A, Barbosa E, Callejo S, Gajate C, Fabra A. Major co-localization of the extracellular-matrix degradative enzymes heparanase and gelatinase in tertiary granules of human neutrophils. Biochem J. 1997;327:917–923. doi: 10.1042/bj3270917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mun-Bryce S, Rosenberg G A. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol. 1998;274:R1203–R1211. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- 32.Mustafa M M, Ramilo O, Olsen K D, Franklin P S, Hansen E J, Beutler B, McCracken G H., Jr Tumor necrosis factor in mediating experimental Haemophilus influenzae type B meningitis. J Clin Investig. 1989;84:1253–1259. doi: 10.1172/JCI114292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pagenstecher A, Stalder A K, Kincaid C L, Shapiro S D, Campbell I L. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- 34.Parvathy S, Hussain I, Karran E H, Turner A J, Hooper N M. Alzheimer's amyloid precursor protein alpha-secretase is inhibited by hydroxamic acid-based zinc metalloprotease inhibitors: similarities to the angiotensin converting enzyme secretase. Biochemistry. 1998;37:1680–1685. doi: 10.1021/bi972034y. [DOI] [PubMed] [Google Scholar]
- 35.Paul R, Lorenzl S, Koedel U, Sporer B, Vogel U, Frosch M, Pfister H W. Matrix metalloproteinases contribute to the blood-brain barrier disruption during bacterial meningitis. Ann Neurol. 1998;44:592–600. doi: 10.1002/ana.410440404. [DOI] [PubMed] [Google Scholar]
- 36.Perides G, Charness M E, Tanner L M, Peter O, Satz N, Steere A C, Klempner M S. Matrix metalloproteinases in the cerebrospinal fluid of patients with Lyme neuroborreliosis. J Infect Dis. 1998;177:401–408. doi: 10.1086/514198. [DOI] [PubMed] [Google Scholar]
- 37.Perry J R, Bilbao J M, Gray T. Fatal basilar vasculopathy complicating bacterial meningitis. Stroke. 1992;23:1175–1178. doi: 10.1161/01.str.23.8.1175. [DOI] [PubMed] [Google Scholar]
- 38.Pfister H W, Borasio G D, Dirnagl U, Bauer M, Einhaupl K M. Cerebrovascular complications of bacterial meningitis in adults. Neurology. 1992;42:1497–1504. doi: 10.1212/wnl.42.8.1497. [DOI] [PubMed] [Google Scholar]
- 39.Pikis A, Kavaliotis J, Tsikoulas J, Andrianopoulos P, Venzon D, Manios S. Long-term sequelae of pneumococcal meningitis in children. Clin Pediatr. 1996;35:72–78. doi: 10.1177/000992289603500204. [DOI] [PubMed] [Google Scholar]
- 40.Ramilo O, Saez-Llorens X, Mertsola J, Jafari H, Olsen K D, Hansen E J, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken G H., Jr Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romanic A M, White R F, Arleth A J, Ohlstein E H, Barone F C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg G A, Estrada E Y, Dencoff J E, Stetler-Stevenson W G. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res. 1995;703:151–155. doi: 10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- 43.Schuchat A, Robinson K, Wenger J D, Harrison L H, Farley M, Reingold A L, Lefkowitz L, Perkins B A. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 44.Solorzano C C, Ksontini R, Pruitt J H, Auffenberg T, Tannahill C, Galardy R E, Schultz G P, MacKay S L, Copeland III E M, Moldawer L L. A matrix metalloproteinase inhibitor prevents processing of tumor necrosis factor alpha (TNF alpha) and abrogates endotoxin-induced lethality. Shock. 1997;7:427–431. doi: 10.1097/00024382-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Täuber M G, Kim Y S, Leib S L. Neuronal injury in meningitis. In: Peterson P K, Remington J S, editors. In defense of the brain. Malden, Mass: Blackwell Science; 1997. pp. 124–143. [Google Scholar]
- 46.Tunkel A R, Scheld W M. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev. 1993;6:118–136. doi: 10.1128/cmr.6.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P, Ba Z F, Galardy R E, Chaudry I H. Administration of a matrix metalloproteinase inhibitor after hemorrhage improves cardiovascular and hepatocellular function. Shock. 1996;6:377–382. doi: 10.1097/00024382-199611000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Yong V W, Krekoski C A, Forsyth P A, Bell R, Edwards D R. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- 49.Zysk G, Bruck W, Gerber J, Bruck Y, Prange H W, Nau R. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol. 1996;55:722–728. doi: 10.1097/00005072-199606000-00006. [DOI] [PubMed] [Google Scholar]