Summary
Background
The clinical presentation and outcomes of non-alcoholic fatty liver disease (NAFLD)-related hepatocellular carcinoma are unclear when compared with hepatocellular carcinoma due to other causes. We aimed to establish the prevalence, clinical features, surveillance rates, treatment allocation, and outcomes of NAFLD-related hepatocellular carcinoma.
Methods
In this systematic review and meta-analysis, we searched MEDLINE and Embase from inception until Jan 17, 2022, for articles in English that compared clinical features, and outcomes of NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma due to other causes. We included cross-sectional and longitudinal observational studies and excluded paediatric studies. Study-level data were extracted from the published reports. The primary outcomes were (1) the proportion of hepatocellular carcinoma secondary to NAFLD, (2) comparison of patient and tumour characteristics of NAFLD-related hepatocellular carcinoma versus other causes, and (3) comparison of surveillance, treatment allocation, and overall and disease-free survival outcomes of NAFLD-related versus non-NAFLD-related hepatocellular carcinoma. We analysed proportional data using a generalised linear mixed model. Pairwise meta-analysis was done to obtain odds ratio (OR) or mean difference, comparing NAFLD-related with non-NAFLD-related hepatocellular carcinoma. We evaluated survival outcomes using pooled analysis of hazard ratios.
Findings
Of 3631 records identified, 61 studies (done between January, 1980, and May, 2021; 94 636 patients) met inclusion criteria. Overall, the proportion of hepatocellular carcinoma cases secondary to NAFLD was 15·1% (95% CI 11·9–18·9). Patients with NAFLD-related hepatocellular carcinoma were older (p<0·0001), had higher BMI (p<0·0001), and were more likely to present with metabolic comorbidities (diabetes [p<0·0001], hypertension [p<0·0001], and hyperlipidaemia [p<0·0001]) or cardiovascular disease at presentation (p=0·0055) than patients with hepatocellular carcinoma due to other causes. They were also more likely to be non-cirrhotic (38·5%, 27·9–50·2 vs 14·6%, 8·7–23·4 for hepatocellular carcinoma due to other causes; p<0·0001). Patients with NAFLD-related hepatocellular carcinoma had larger tumour diameters (p=0·0087), were more likely to have uninodular lesions (p=0·0003), and had similar odds of Barcelona Clinic Liver Cancer stages, TNM stages, alpha fetoprotein concentration, and Eastern Cooperative Oncology Group (ECOG) performance status to patients with non-NAFLD-related hepatocellular carcinoma. A lower proportion of patients with NAFLD-related hepatocellular carcinoma underwent surveillance (32·8%, 12·0–63·7) than did patients with hepatocellular carcinoma due to other causes (55·7%, 24·0–83·3; p<0·0001). There were no significant differences in treatment allocation (curative therapy, palliative therapy, and best supportive care) between patients with NAFLD-related hepatocellular carcinoma and those with hepatocellular carcinoma due to other causes. Overall survival did not differ between the two groups (hazard ratio 1·05, 95% CI 0·92–1·20, p=0·43), but disease-free survival was longer for patients with NAFLD-related hepatocellular carcinoma (0·79, 0·63–0·99; p=0·044). There was substantial heterogeneity in most analyses (I2>75%), and all articles had low-to-moderate risk of bias.
Interpretation
NAFLD-related hepatocellular carcinoma is associated with a higher proportion of patients without cirrhosis and lower surveillance rates than hepatocellular carcinoma due to other causes. Surveillance strategies should be developed for patients with NAFLD without cirrhosis who are at high risk of developing hepatocellular carcinoma.
Funding
None.
Editorial note:
the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Introduction
Hepatocellular carcinoma is the third leading cause of cancer-related deaths worldwide.1,2 With recent advances in the treatment of hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, the burden of hepatocellular carcinoma due to viral hepatitis is declining; however, the prevalence of non-alcoholic fatty liver disease (NAFLD)-related hepatocellular carcinoma is rising rapidly.3,4 Nearly a third of the global population has NAFLD, of whom around 20% harbour non-alcoholic steatohepatitis, which can progress to cirrhosis and hepatocellular carcinoma.5–8 NAFLD is the fastest rising cause of hepatocellular carcinoma in the USA and parts of Europe, and is expected to rise exponentially in parallel with the global obesity epidemic.9–12
However, data regarding the clinical presentation and outcomes for NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma due to other causes are conflicted. Several studies reported more advanced disease at presentation and poorer survival among patients with NAFLD-related hepatocellular carcinoma compared with those with hepatocellular carcinoma due to other causes, whereas other studies have reported similar clinical presentation and improved survival.13–15 In addition, it is unclear what proportion of patients with NAFLD-related hepatocellular carcinoma do not have cirrhosis or receive surveillance before a hepatocellular carcinoma diagnosis. Previous studies on this topic have been specific to a country, region, or treatment. To our knowledge, a comprehensive meta-analysis evaluating the clinical presentation, proportion without cirrhosis, surveillance rates, and overall outcomes between NAFLD-related and non-NAFLD-related hepatocellular carcinoma has not been reported. Therefore, through a systematic review and meta-analytic approach, we aimed to identify the clinical features, surveillance rates, treatment allocation, and survival outcomes of NAFLD-related hepatocellular carcinoma compared with hepatocellular carcinoma due to other causes.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for its synthesis.16 MEDLINE and Embase were searched for articles describing prevalence, risk factors, and outcomes of NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma due to other causes from inception to Jan 17, 2022. Key search terms included “non-alcoholic fatty liver” and “hepatocellular carcinoma”. The full search strategy can be found in the appendix (p 1). To ensure a comprehensive search, we screened for grey literature by reviewing the bibliographies of included articles and previous meta-analyses.
Four authors (DJHT, SYL, PT, and XHP) independently filtered the title abstracts, followed by full-text review. Discrepancies were resolved by consensus or in consultation with a senior author (DQH). Original articles, including prospective and retrospective cohort studies and randomised control trials, were considered for inclusion whereas we excluded reviews, commentaries, and editorials. We included studies written or translated into English. Studies were included if they reported clinical characteristics, risk factors, screening, treatment allocation, or survival outcomes of patients diagnosed with hepatocellular carcinoma, and compared these characteristics between NAFLD-related versus non-NAFLD-related hepatocellular carcinoma. Studies done in the paediatric population, and studies focusing on hepatocellular carcinoma secondary to cryptogenic liver disease were excluded, unless the authors of the study specified that the cryptogenic liver disease cases were probably due to NAFLD. We did not include data from unpublished studies and trial registries up to Jan 17, 2022, in the pooled analysis. For multiple studies inferring results from the same databases, we removed overlapping studies and only included the most updated studies. The protocol is available in the appendix (pp 29–31).
Data analysis
NAFLD was defined based on either imaging, histology, or International Classification of Diseases codes in the absence of significant alcohol consumption and coexisting causes of chronic liver disease. Hepatocellular carcinoma was diagnosed either on the basis of histology, regardless of subtypes, or by imaging (including CT, MRI, and contrast-enhanced ultrasound). Surveillance for hepatocellular carcinoma was defined as bi-annual imaging of the liver via ultrasound scan, CT or MRI, with or without measurement of serum alpha fetoprotein.17 Treatment methods were classified into curative therapies, palliative therapies, and best supportive care. Curative therapies included liver transplantation, liver resection, and ablation. Palliative therapies included transarterial chemoembolisation, radioembolisation, radiotherapy, and systemic therapy. Six authors (DJHT, SYL, CHN, XHP, PT, and WHL) independently extracted study-level aggregated data including study characteristics (author, country, and study design), patient characteristics (age, sex, presence of cirrhosis, diagnostic criteria, body-mass index [BMI], presence of metabolic conditions, and Eastern Cooperative Oncology Group [ECOG] status), tumour characteristics (Barcelona Clinic Liver Cancer [BCLC] staging, TNM staging, tumour diameter, and serum alpha fetoprotein), treatment methods, whether patients underwent surveillance before diagnosis of hepatocellular carcinoma, and survival outcomes (overall survival and disease-free survival). Overall survival was defined as the duration from the date of hepatocellular carcinoma diagnosis to date of death by any cause. Disease-free survival was defined as the duration from date of definitive curative treatment to date of disease recurrence or death. We extracted survival data via reconstruction of published Kaplan-Meier curves using the method by Guyot and colleagues,18 which remains the gold standard in survival analysis.19–21 During the data extraction process, for articles that only provided data in the form of median (IQR) and not mean (SD), we transformed values using pre-existing formulae, where mean and SDs were estimated from median and IQR with the widely adopted formulas by Wan and colleagues.22
Outcomes
The primary outcomes of the study were (1) the proportion of hepatocellular carcinoma secondary to NAFLD globally, with prespecified subgroup analysis by WHO region (European Region, Region of the Americas, South-East Asian Region, African region, Eastern Mediterranean region, and the Western Pacific Region) and over time (before 2000; 2000–04; 2005–09; 2010, and beyond); (2) comparison of patient and tumour characteristics of NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma due to other causes; and (3) comparison of surveillance, treatment allocation (curative treatment, palliative treatments, or best supportive care), and survival outcomes of NAFLD-related hepatocellular carcinoma versus other causes. We performed prespecified subgroup analyses by individual causes of liver disease (HBV, HCV, and alcohol-associated liver disease) for comparison of patient and tumour characteristics, surveillance, treatment allocation, and survival outcomes. Subgroup analysis was also done for the proportion of patients with NAFLD-related hepatocellular carcinoma receiving each treatment type (curative vs palliative treatment), and for the specific type of curative treatment (liver transplantation, liver resection, or ablation). For analysis of survival outcomes, prespecified sensitivity analyses were conducted in patients with cirrhosis, in patients receiving all types of curative treatment, and by type of curative treatment (liver transplantation, liver resection, and ablation).
Statistical analysis
Statistical heterogeneity for the pooled estimates was assessed via I2 and Cochran’s Q test values, where an I2 value of 25% represented a low degree of heterogeneity, 50% represented a moderate degree, and 75% represented a high degree.23,24
We used a random effects model in all analyses regardless of heterogeneity measures as evidence has shown more robust effect estimates compared with fixed effect models.25,26 Meta-analysis of proportions was done with a generalised linear mixed model with Clopper-Pearson intervals to estimate the overall proportion and corresponding 95% CI of hepatocellular carcinoma secondary to NAFLD and for the prespecified subgroup analysis by WHO region and time periods.27,28 Post-hoc subgroup analysis for proportion of hepatocellular carcinoma secondary to NAFLD was performed by clinical cohort versus administrative database studies with similar methods to the primary analysis.
Differences in patient and tumour characteristics, surveillance, and treatment allocation between NAFLD-related hepatocellular carcinoma and hepatocellular carcinoma due to other causes for both prespecified subgroup analyses and post-hoc analyses were evaluated via a comparative meta-analysis in odds ratios (OR) for binary variables, and mean difference for continuous variables with the DerSimonian and Laird random effects model.24 Additional post-hoc subgroup analyses of patient and tumour characteristics in NAFLD-related hepatocellular carcinoma versus other causes were further stratified by cause of hepatocellular carcinoma (HBV, HCV, and alcohol) and WHO region, and analysed with the DerSimonian and Laird random effects model.
For survival outcomes (overall survival and disease-free survival) and in prespecified sensitivity analysis, pairwise analysis was conducted with hazard ratios (HR) with the DerSimonian and Laird random effects model to compare between NAFLD-related and non-NAFLD-related hepatocellular carcinoma. Prespecified meta-regression was considered for the adjustment of baseline characteristics on treatment allocation and survival outcomes.29,30
We used RStudio (version 1.3.1093) for all analyses. A two-tailed p value of less than 0·05 was considered as the threshold for statistical significance.
We used the Joanna Briggs Institute (JBI) Critical Appraisal Tool for quality assessment of included articles.31 The JBI assessment rates the risk of bias of cohort studies on the basis of appropriateness of sample frame, sampling method, adequacy of sample size, data analysis, methods for identification and measurement of relevant conditions, statistical analysis, and response rate adequacy. We assessed publication bias with Egger’s test for continuous variables and Harbord’s test for dichotomous variables. Funnel plots were generated for analyses involving more than ten studies, and were visually inspected for asymmetrical distribution of data points across the vertical treatment effect axis.32,33
Role of the funding source
There was no funding source for this study.
Results
The initial search from MEDLINE and Embase yielded 3631 articles. After screening, 61 studies (done between January, 1980, and May, 2021) were included in the meta-analysis (figure 1). The included studies were done in Argentina, Austria, Belgium, Canada, mainland China, France, Germany, India, Italy, Japan, Latin America, the Netherlands, Singapore, South Korea, Slovakia, Sweden, Taiwan, the UK, and the USA. Additionally, one study was a European multicentre study, and another was a multicentre study involving centres from Germany, Italy, Japan, and South Korea. A total of 94 636 patients were included: 15 377 patients with NAFLD-related hepatocellular carcinoma and 79 259 patients with hepatocellular carcinoma due to other causes. The median age of included patients was 67·8 years (IQR 60·2– 74·5). The quality of included studies, based on the JBI Checklist, was generally moderate to good (appendix pp 2–9).
Figure 1:
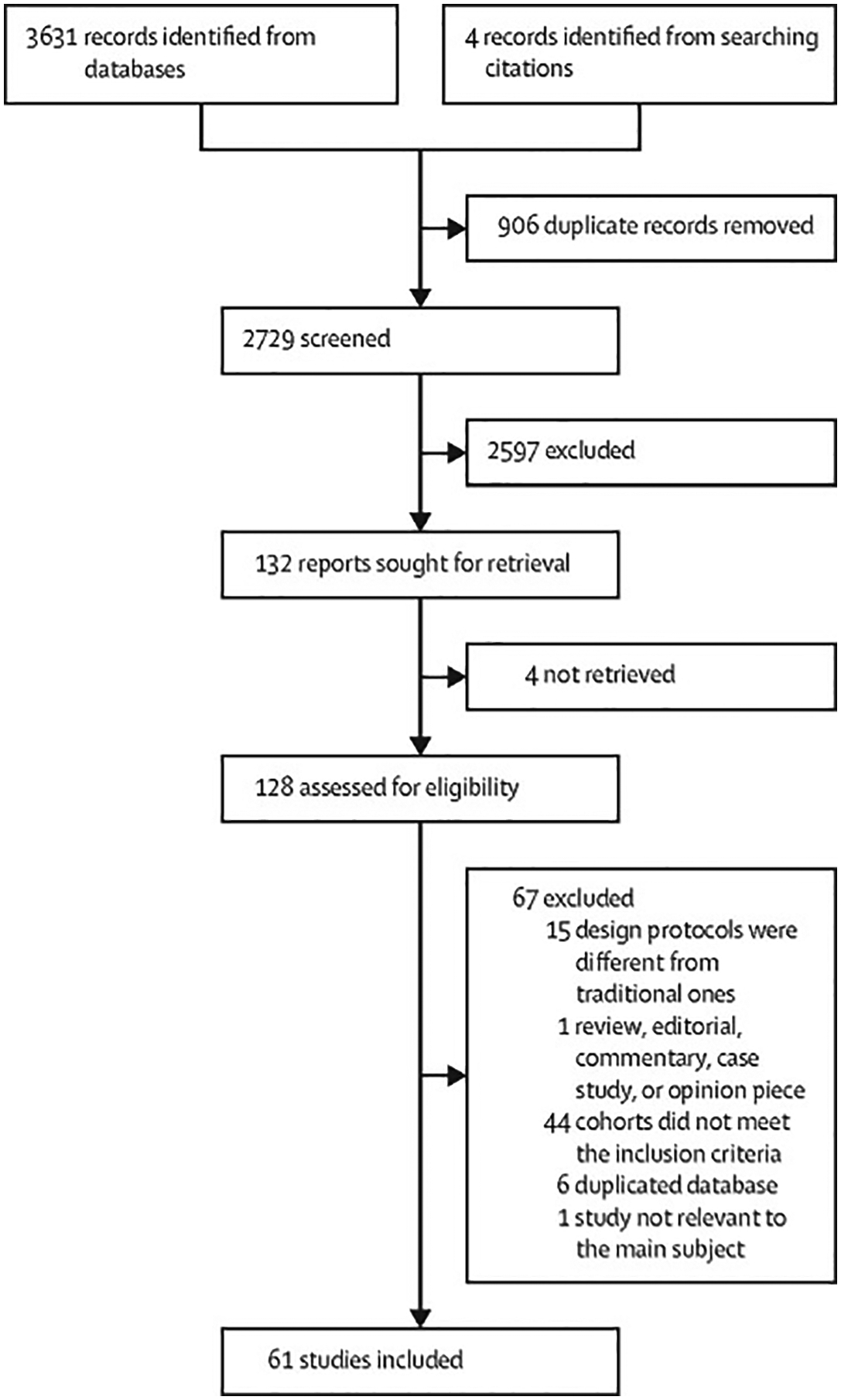
Study selection
The overall proportion of patients with hepatocellular carcinoma secondary to NAFLD was 15·1% (95% CI 11·9–18·9). The proportion of patients with NAFLD-related hepatocellular carcinoma was highest in the South-East Asia region, followed by the Western Pacific region, European region, and the region of the Americas (figure 2; appendix p 10–11). There was an increase in the global proportion of hepatocellular carcinoma secondary to NAFLD over time periods (9·77%, 95% CI 7·33–12·92 for before 2000 vs 16·97%, 12·17–23·16 for 2010 and beyond; p=0·045; appendix pp 10–11). NAFLD-related hepatocellular carcinoma did not differ between clinical cohort studies versus administrative database studies (post-hoc analysis; appendix p 11).
Figure 2:
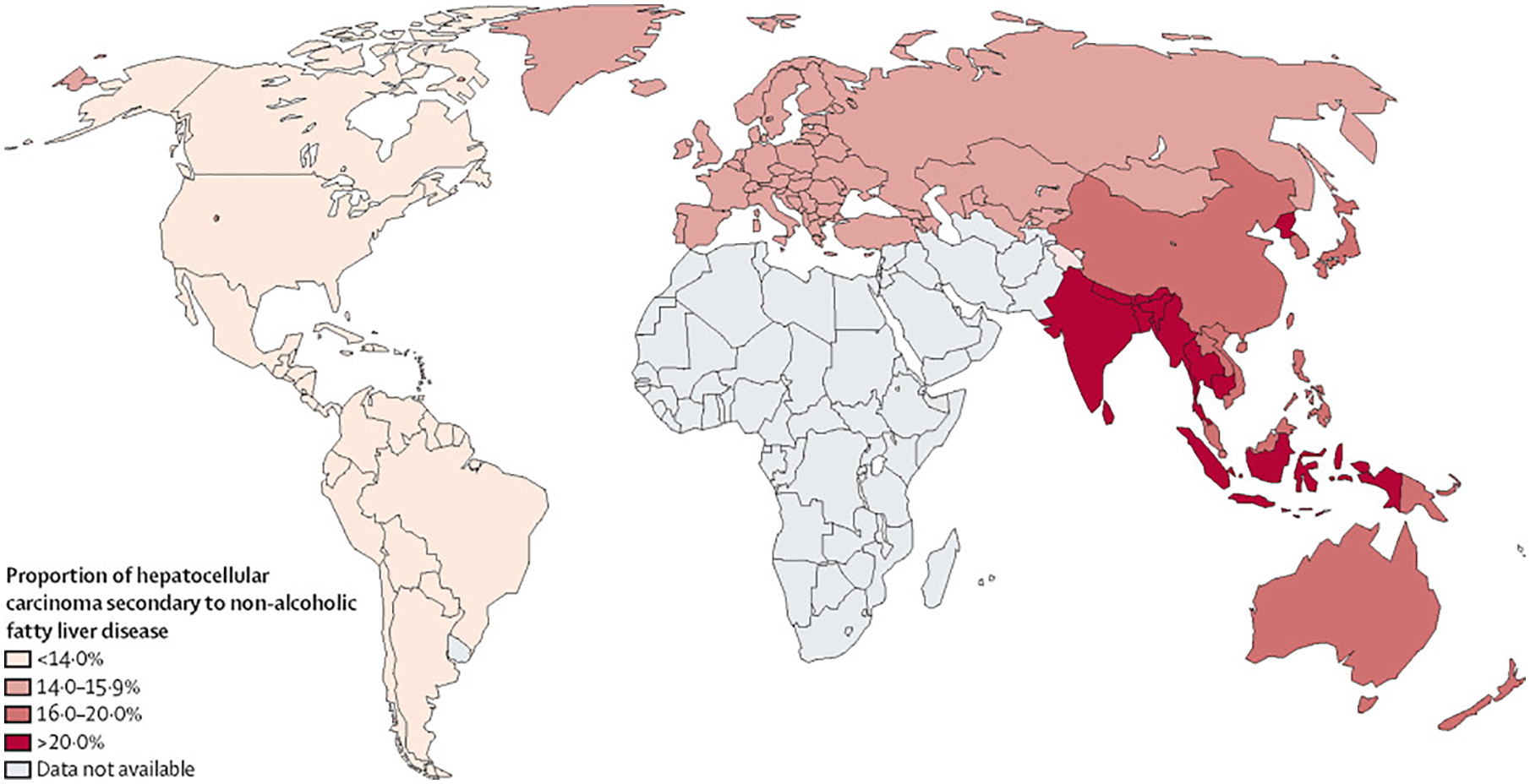
Proportion of hepatocellular carcinoma secondary to non-alcoholic fatty liver disease worldwide, by WHO region
Patients with NAFLD-related hepatocellular carcinoma were older (mean difference 5·62 years; 95% CI 4·63–6·61; p<0·0001), and had higher BMI (mean difference 2·99 kg/m2, 2·20–3·78; p<0·0001) compared with patients with hepatocellular carcinoma due to other causes (table 1). There was no significant difference in terms of sex between the two groups. Patients with NAFLD-related hepatocellular carcinoma were also more likely to present with metabolic complications, including diabetes (OR 4·31, 95% CI 3·19–5·80; p<0·0001), hypertension (2·84, 2·09–3·86; p<0·0001), and hyperlipidaemia (3·43, 2·39–4·95; p<0·0001) than were patients with hepatocellular carcinoma due to other causes (table 1). Patients with NAFLD-related hepatocellular carcinoma were also more likely to have cardiovascular disease at presentation than were patients with hepatocellular carcinoma due to other causes (2·23, 1·43–3·48; p=0·0055). Additional subgroup analyses comparing the clinical characteristics of NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma secondary to HBV, HCV, and alcohol consumption are summarised in the appendix (pp 12, 16).
Table 1:
Clinical and tumour characteristics of NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma due to other causes
| Studies, n | Patients, n | Effect size (95% CI) | p value | Cochran’s Q | I 2 | |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age, years | 35 | 73 028 | MD 5·62 (4·63 to 6·61) | <0·0001 | <0·0001 | 94·6% |
| Body-mass index, kg/m2 | 21 | 45 560 | MD 2·99 (2·20 to 3·78) | <0·0001 | <0·0001 | 96·1% |
| Men | 35 | 72 021 | OR 0·71 (0·39 to 1·29) | 0·25 | <0·0001 | 98·2% |
| Women | 35 | 72 021 | OR 1·41 (0·77 to 2·57) | 0·25 | <0·0001 | 98·1% |
| Diabetes | 26 | 43 406 | OR 4·31 (3·19 to 5·80) | <0·0001 | <0·0001 | 91·8% |
| Hypertension | 18 | 6147 | OR 2·84 (2·09 to 3·86) | <0·0001 | <0·0001 | 90·3% |
| Hyperlipidaemia | 8 | 4240 | OR 3·43 (2·39 to 4·95) | <0·0001 | 0·083 | 44·4% |
| Cardiovascular disease | 6 | 21 223 | OR 2·23 (1·43 to 3·48) | 0·0055 | <0·0001 | 94·2% |
| Cirrhosis | 27 | 21 791 | OR 0·27 (0·18 to 0·41) | <0·0001 | <0·0001 | 89·2% |
| No cirrhosis | 27 | 21 791 | OR 3·71 (2·46 to 5·59) | <0·0001 | <0·0001 | 89·1% |
| Tumour characteristics | ||||||
| Tumour diameter, cm | 18 | 11 639 | MD 0·67 (0·35 to 0·98) | 0·0087 | <0·0001 | 79·0% |
| Uninodular hepatocellular carcinoma | 13 | 9345 | OR 1·36 (1·19 to 1·56) | 0·0003 | 0·51 | 0 |
| Alpha fetoprotein | 17 | 18 637 | MD –0·04 (–0·16 to 0·07) | 0·47 | <0·0001 | 75·6% |
| BCLC O or A | 9 | 7506 | OR 1·14 (0·72 to 1·80) | 0·53 | 0·0009 | 69·2% |
| BCLC B | 11 | 8925 | OR 1·27 (0·86 to 1·90) | 0·21 | <0·0001 | 79·9% |
| BCLC C or D | 11 | 8925 | OR 1·28 (0·70 to 2·36) | 0·38 | <0·0001 | 91·9% |
| TNM 1 or 2 | 3 | 250 | OR 1·82 (0·30 to 11·16) | 0·29 | 0·30 | 16·0% |
| TNM 3 or 4 | 3 | 250 | OR 0·81 (0·23 to 2·87) | 0·54 | 0·54 | 0 |
| ECOG performance status of 2 or worse | 3 | 3142 | OR 1·17 (0·71 to 1·92) | 0·32 | 0·52 | 0 |
NAFLD=non-alcoholic fatty liver disease. MD=mean difference. OR=odds ratio. BCLC=Barcelona Clinic Liver Cancer. ECOG=Eastern Cooperative Oncology Group.
A higher proportion of patients with NAFLD-related hepatocellular carcinoma did not have cirrhosis (38·5%, 95% CI 27·9–50·2) compared with patients with hepatocellular carcinoma secondary to other causes (14·6%, 8·7–23·4; p<0·0001). The proportion of patients without cirrhosis and with hepatocellular carcinoma related to HBV was 21·7% (14·0–31·9), to HCV was 6·4% (2·1–17·6), and to alcohol was 9·1% (4·0–19·0; figure 3A). The odds of not having cirrhosis in NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma of other causes was increased (OR 3·71, 95% CI 2·46–5·59; p<0·0001). Patients with NAFLD-related hepatocellular carcinoma were more likely to be non-cirrhotic than were patients with hepatocellular carcinoma secondary to HBV (3·28, 1·68–6·43; p=0·0040), HCV (6·41, 2·30–17·91; p=0·0037), and alcohol (3·13, 1·66–5·90; p=0·0034).
Figure 3: Proportion of hepatocellular carcinoma.
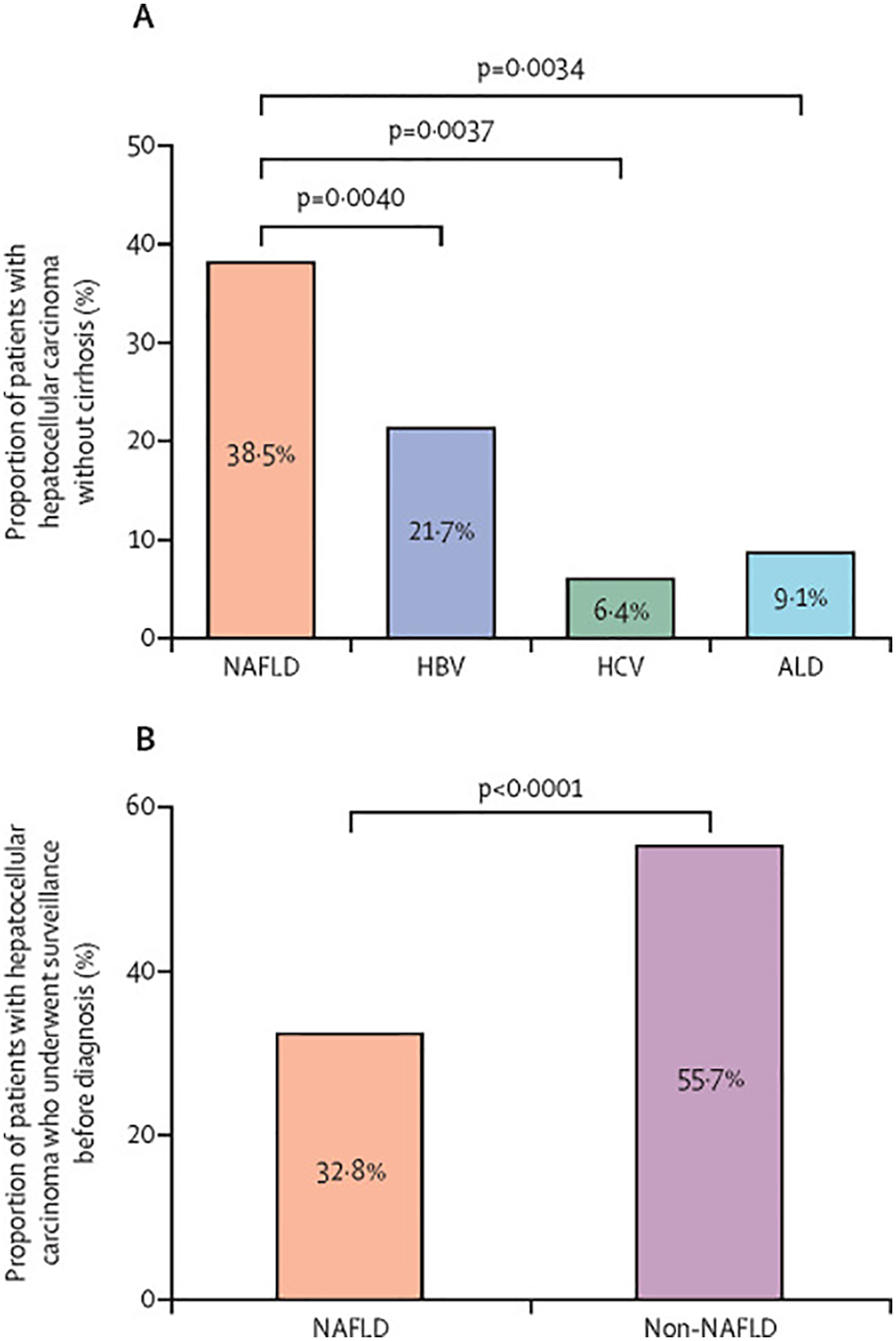
Patients without cirrhosis (A) and patients who underwent surveillance before hepatocellular carcinoma diagnosis (B). HBV=hepatitis B virus. HCV=hepatitis C virus. NAFLD=non-alcoholic fatty liver disease. ALD=alcohol-associated liver disease.
Patients with NAFLD-related hepatocellular carcinoma had a larger tumour diameter (mean difference 0·67 cm, 95% CI 0·35–0·98; p=0·0087) and greater odds of having uninodular lesions (OR 1·36, 95% CI 1·19–1·56; p=0·0003) than had patients with hepatocellular carcinoma due to other causes (table 1). However, the odds of patients with BCLC stage 0 or A, BCLC stage B, BCLC stage C or D, TNM stage 1 or 2, TNM stage 3 or 4, or ECOG of 2 or worse at presentation did not differ (table 1); alpha fetoprotein concentration was also not significantly different between groups. Subgroup analyses comparing tumour characteristics of NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma secondary to HBV, HCV, and alcohol are summarised in the appendix (pp 13–15).
Only 32·8% (95% CI 12·0–63·7; four studies, 393 patients) of patients with NAFLD-related hepatocellular carcinoma had surveillance for hepatocellular carcinoma before cancer diagnosis versus 55·7% (24·0–83·3; eight studies, 3583 patients) of patients with hepatocellular carcinoma secondary to other causes (OR 0·36, 95% CI 0·28–0·48; p<0·0001; four studies, 2826 patients; figure 3B).
Overall, 65·0% (95% CI 30·4–88·8) of patients with NAFLD-related hepatocellular carcinoma were assigned to curative therapy and 42·1% (24·8–61·6) of patients to palliative therapy. Best supportive care could not be assessed due to insufficient studies. A prespecified subgroup analysis identified that the proportion of patients with NAFLD-related hepatocellular carcinoma who received liver transplant was 3·9% (1·4–12·3), who received resection was 33·6% (11·1–67·1), and who received ablation was 12·0% (6·9–20·1). The odds of receiving curative therapy did not differ between NAFLD-related versus non-NAFLD-related hepatocellular carcinoma (OR 1·05, 95% CI 0·52–2·09; p=0·90; table 2). However, patients with NAFLD-related hepatocellular carcinoma were less likely to undergo liver transplant (p=0·017) but more likely to undergo liver resection (p=0·0090) than patients with hepatocellular carcinoma due to other causes (table 2). Patients with NAFLD-related hepatocellular carcinoma had similar odds to patients with hepatocellular carcinoma due to other causes (p=0·18) of receiving ablation (table 2). The odds of receiving palliative therapy (1·01, 0·72–1·42; p=0·76) or best supportive care (1·46, 0·77–2·77; p=0·20) did not differ between NAFLD-related versus non-NAFLD-related hepatocellular carcinoma (table 2). Subgroup analysis for treatment allocation by cause of liver disease could not be done due to a lack of data in available studies.
Table 2:
Treatment allocation and survival outcomes between NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma due to other causes
| Studies, n | Events, n | Patients, n | Effect size (95% CI) | p value | Cochran’s Q | I 2 | |
|---|---|---|---|---|---|---|---|
| Overall treatment allocation | |||||||
| Curative therapy* | 18 | 23 601 | 30 082 | OR 1·05 (0·52–2·09) | 0·90 | <0·0001 | 88·8% |
| Palliative therapy† | 13 | 3026 | 8100 | OR 1·01 (0·72–1·42) | 0·76 | <0·0001 | 95·2% |
| Best supportive care | 7 | 1948 | 4799 | OR 1·46 (0·77–2·77) | 0·20 | <0·0001 | 80·4% |
| Method of curative treatment | |||||||
| Liver transplantation | 8 | 457 | 5177 | OR 0·49 (0·29–0·84) | 0·017 | 0·028 | 55·3% |
| Liver resection | 12 | 1229 | 7171 | OR 1·59 (1·16–2·18) | 0·0090 | 0·026 | 52·4% |
| Ablation | 10 | 1188 | 6495 | OR 0·88 (0·71–1·08) | 0·18 | 0·57 | 0 |
| Survival outcomes | |||||||
| Overall survival | |||||||
| Overall | 28 | ·· | 39 585 | HR 1·05 (0·92–1·20) | 0·43 | <0·0001 | 99·5% |
| All curative therapies*‡ | 13 | ·· | 7168 | HR 1·00 (0·85–1·16) | 0·94 | 0·073 | 40·4% |
| Liver transplantation | 3 | ·· | 1811 | HR 0·74 (0·50–1·10) | 0·13 | 0·61 | 0 |
| Liver resection | 6 | ·· | 3325 | HR 1·13 (0·72–1·79) | 0·58 | <0·0001 | 75·0% |
| Disease-free survival | |||||||
| Overall | 16 | ·· | 5922 | HR 0·79 (0·63–0·99) | 0·044 | 0·018 | 47·3% |
| All curative therapies*‡ | 10 | ·· | 4984 | HR 0·76 (0·64–0·89) | 0·0011 | 0·28 | 17·5% |
| Liver transplantation | 3 | ·· | 1445 | HR 0·56 (0·27–1·15) | 0·12 | 0·12 | 53·0% |
| Liver resection | 6 | ·· | 3325 | HR 0·82 (0·71–0·95) | 0·0043 | 0·90 | 0 |
NAFLD=non-alcoholic fatty liver disease. OR=odds ratio. HR=hazard ratio.
Includes liver transplantation, resection, and ablation.
Includes transarterial chemoembolisation, radioembolisation, radiotherapy, and systemic therapy.
The remaining treatment methods for curative therapies (ablation) had insufficient studies for pooled analysis to be done.
The factors associated with receiving curative treatment among patients with NAFLD-related hepatocellular carcinoma are summarised in the appendix (p 17).
Overall survival did not differ between NAFLD-related versus non-NAFLD-related hepatocellular carcinoma (HR 1·05, 95% CI 0·92–1·20; p=0·43; table 2). Subgroup analyses also did not differ for overall survival between NAFLD-related hepatocellular carcinoma and hepatocellular carcinoma secondary to HBV, HCV, or alcohol (appendix pp 19–20). However, among patients with cirrhosis (sensitivity analysis), NAFLD-related hepatocellular carcinoma was associated with increased mortality (p=0·0041) compared with hepatocellular carcinoma due to other causes (1·74, 1·21–2·67; p=0·0041). A sensitivity analysis of patients who underwent curative therapy established that NAFLD-related hepatocellular carcinoma was associated with longer overall survival than HCV-related hepatocellular carcinoma for patients receiving curative treatment (p=0·041; appendix p 20), but not when compared with HBV-related hepatocellular carcinoma (p=0·78; appendix p 20) or all other causes combined (p=0·94; table 2). For the sensitivity analysis for type of curative treatment, overall survival did not differ between NAFLD-related hepatocellular carcinoma compared with other causes in patients receiving liver transplant (p=0·13) or resection (p=0·58; table 2). Meta-regression of study level data did not reveal any study-level factors to be associated with overall survival among patients with NAFLD-related hepatocellular carcinoma (appendix p 18).
NAFLD-related hepatocellular carcinoma had improved disease-free survival compared with non-NAFLD-related hepatocellular carcinoma (HR 0·79, 95% CI 0·63–0·99; p=0·044; table 2), although subgroup analysis by specific causes (HBV, HCV, and alcohol) did not differ (appendix p 20). Disease-free survival among patients with cirrhosis was not analysed due to insufficient data. In a sensitivity analysis, NAFLD-related hepatocellular carcinoma was associated with improved disease-free survival among patients who received curative therapy (p=0·0011). In sensitivity analysis for specific types of curative treatment, NALFD-related hepatocellular carcinoma was also associated with improved disease-free survival in patients who underwent liver resection (p=0·0043; table 2), but not for liver transplantation (p=0·12). Meta-regression of study-level data revealed that only increased alpha fetoprotein was associated with reduced disease-free survival, all other assessed risk factors were not associated with disease-free survival (p=0·029; appendix p 18).
There was substantial heterogeneity in most analyses (I2>75%). Additionally, there was publication bias in the analysis of baseline characteristics between NAFLD-related and non-NAFLD-related hepatocellular carcinoma, notably in the analysis of BMI (p=0·032), diabetes (p=0·0042), and hypertension (p=0·020), but not age (p=0·50; appendix pp 21–23) or male gender (p=0·50). No publication bias was noted in the analysis for cirrhosis, tumour diameter, uninodular cancer, BCLC stage, treatment allocation, overall survival, and disease-free survival between NAFLD-related and non-NAFLD-related hepatocellular carcinoma (appendix pp 24–28).
Discussion
In this large systematic review and meta-analysis of 61 studies and 94 636 individuals, globally, about 15% of hepatocellular carcinoma were secondary to NAFLD. The highest proportion of NAFLD-related hepatocellular carcinoma occurred in the South-East Asia region, and the lowest in the region of the Americas. The global proportion of hepatocellular carcinoma secondary to NAFLD increased over time. Patients with NAFLD-related hepatocellular carcinoma were older, had higher BMI, and had more metabolic comorbidities than patients with hepatocellular carcinoma due to other causes. Nearly 40% of patients with NAFLD-related hepatocellular carcinoma did not have cirrhosis, compared with about 15% of patients with hepatocellular carcinoma due to other causes. In addition, only 33% of patients with NAFLD-related hepatocellular carcinoma underwent surveillance before hepatocellular carcinoma diagnosis, versus 56% of patients with hepatocellular carcinoma due to other causes. These findings have important implications. Because of the absence of cirrhosis, nearly 40% of patients with NAFLD-related hepatocellular carcinoma did not have routine indication for hepatocellular carcinoma surveillance before hepatocellular carcinoma diagnosis based on current practice guidelines.34,35 This probably contributed to the dismal rate of hepatocellular carcinoma surveillance among patients with NAFLD-related hepatocellular carcinoma, in addition to poor disease awareness.36,37 Several studies have shown that NAFLD is severely underdiagnosed in clinical practice and associated with high mortality.38,39 In addition, patients with NAFLD are often overweight, which reduces the sensitivity of ultrasound to detect early hepatocellular carcinoma, and alternative methods for hepatocellular carcinoma screening might be required for patients with poor liver visualisation on ultrasound.3,40,41 Better strategies are required to identify non-cirrhotic patients with NAFLD at high risk of hepatocellular carcinoma who require surveillance.42
Patients with NAFLD-related hepatocellular carcinoma had larger tumours at diagnosis than patients with hepatocellular carcinoma due to other causes, but were more likely to have uninodular lesions. This occurrence might be related to the higher proportion of patients with NAFLD-related hepatocellular carcinoma without cirrhosis, as several studies have reported a larger tumour size in livers without cirrhosis and a higher proportion of uninodular lesions than for livers with cirrhosis.43–45 Larger tumour size might be related to the lower resistance to expansive tumour growth in non-cirrhotic livers, but more data are required to validate this hypothesis.43,44 The odds of BCLC stage 0 or A at diagnosis were similar between NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma of other causes despite the larger tumour size, which might be related to the higher odds of uninodular disease in NAFLD-related hepatocellular carcinoma. However, the similar odds of BCLC stage 0 or A between NAFLD-related and non-NAFLD-related hepatocellular carcinoma should be interpreted with caution. There were insufficient data to establish the effect of lower surveillance rates in NAFLD-related hepatocellular carcinoma on the proportion of BCLC stage 0 or A at presentation as only four of 11 cohorts that provided data for BCLC stage underwent hepatocellular carcinoma surveillance. More data are required to establish the effect of lower hepatocellular carcinoma surveillance rates in NAFLD-related hepatocellular carcinoma on tumour burden at diagnosis. Compared with hepatocellular carcinoma due to other causes, patients with NAFLD-related hepatocellular carcinoma were less likely to receive liver transplantation and more likely to receive liver resection, but had a similar likelihood of receiving ablation. The higher odds of receiving resection and lower odds of receiving liver transplantation might be related to the higher proportion of patients with NAFLD-related hepatocellular carcinoma without cirrhosis. In addition, the older age, higher BMI, and higher proportion of patients with cardiovascular disease probably contributed to the lower proportion of patients with NAFLD receiving liver transplant. Overall, patients with NAFLD-related hepatocellular carcinoma had similar odds of receiving curative therapy versus patients with hepatocellular carcinoma due to other causes. Meta-regression showed that older age and the presence of cirrhosis were associated with a lower likelihood of receiving curative therapy. However, among patients with cirrhosis, NAFLD-related hepatocellular carcinoma was associated with increased mortality versus hepatocellular carcinoma due to other causes.
Patients with NAFLD-related hepatocellular carcinoma had similar overall survival versus patients with hepatocellular carcinoma due to other causes, although overall survival was longer among patients with NAFLD-related hepatocellular carcinoma who had undergone curative therapy than among those with HCV-related hepatocellular carcinoma who had undergone curative therapy. However, most of the included studies were conducted before the widespread availability of direct-acting antivirals for HCV and the comparative survival outcomes between NAFLD-related hepatocellular carcinoma and HCV-related hepatocellular carcinoma are likely to change as more patients with HCV are treated with direct-acting antivirals. Patients with NAFLD-related hepatocellular carcinoma had improved disease-free survival versus patients with hepatocellular carcinoma due to other causes in the overall analysis. This outcome remained true in subgroup analysis of patients who had undergone curative therapy, and those patients who underwent liver resection. However, in patients who received liver transplantation, disease-free survival was similar between NAFLD-related and non-NAFLD-related hepatocellular carcinoma. This outcome might be related to the higher proportion of patients with NAFLD-related hepatocellular carcinoma without cirrhosis, as cirrhosis increases the risk of hepatocellular carcinoma recurrence after resection.46,47
Several studies have reported improved outcomes in NAFLD-related compared with non-NAFLD-related hepatocellular carcinoma,48,49 although there have also been reports of more advanced disease at presentation and poorer outcomes for NAFLD-related hepatocellular carcinoma.14,50 These conflicting results could have arisen from previous studies being specific to a country, region, or treatment, hence limiting their generalisability. The current study provides a comprehensive global overview of the clinical presentation, treatment allocation, and outcomes of NAFLD-related hepatocellular carcinoma. In addition, we did multiple subgroup analyses to account for causes, treatment type, region, time period, and study setting to inform practice, with most of the included studies of at least moderate-to-high quality.
To our knowledge, the current study provides the most comprehensive analysis of the existing literature regarding clinical characteristics and outcomes of NAFLD-related hepatocellular carcinoma to date. However, our study is not without limitations, some of which are inherent to the nature of meta-analyses.51,52 There was substantial heterogeneity in certain comparisons, which could have been arisen from the large sample size involved in pooled analysis. We attempted to account for possible sources of heterogeneity by conducting subgroup analyses and meta-regression where appropriate. There were also insufficient studies that provided comparative data for the tumour characteristics of patients without cirrhosis. In addition, there were relatively few studies from the South-East Asia region and South America, and the number of included patients were modest, therefore, the analyses for the proportion of hepatocellular carcinoma secondary to NAFLD from these regions require cautious interpretation and more data are required. However, the high proportion of hepatocellular carcinoma secondary to NAFLD in the South-East Asia region is consistent with a large meta-analysis reporting an NAFLD prevalence of 42·04% in South-East Asia, which probably contributes to the high proportion in the region.5,53 However, data regarding the contribution of genetic variants to the risk of NAFLD-related hepatocellular carcinoma from the South-East Asia region are lacking. There was a paucity of data from the African region and the Eastern Mediterranean region. In addition, the various causes of liver disease are not well distributed geographically, therefore, it is possible that pooling data from different regions into a single estimate might result in subtle bias. However, we attempted to mitigate this effect by performing extensive subgroup analyses stratified by both cause and region where possible to provide granular data. Finally, as the included studies used a combination of clinical, radiological, and histological assessments to diagnose cirrhosis, the proportion of patients without cirrhosis might have been overestimated.
This meta-analysis provides a comprehensive global overview of the clinical presentation, surveillance rates, treatment allocation, and outcomes of NAFLD-related hepatocellular carcinoma. This study provides high-level evidence that a substantially higher proportion of patients with NAFLD-related hepatocellular carcinoma do not have cirrhosis and have lower surveillance rates than have patients with hepatocellular carcinoma due to other causes. The proportion of hepatocellular carcinoma secondary to NAFLD is rising globally, and urgent measures are required to tackle the metabolic risk factors associated with NAFLD-related hepatocellular carcinoma. Further studies are required to improve hepatocellular carcinoma surveillance strategies for patients with NAFLD who are at high-risk of hepatocellular carcinoma without cirrhosis.
Supplementary Material
Research in context.
Evidence before this study
Data regarding the clinical presentation and outcomes for non-alcoholic fatty liver disease (NAFLD)-related hepatocellular carcinoma versus hepatocellular carcinoma due to other causes are conflicting. We searched PubMed for systematic reviews published from database inception until Jan 17, 2022, using search terms “non-alcoholic fatty liver disease” and “hepatocellular carcinoma”, but did not identify any previous comprehensive meta-analysis evaluating the overall clinical presentation, surveillance rates, and survival outcomes between NAFLD-related and non-NAFLD-related hepatocellular carcinoma.
Added value of this study
Unlike previous meta-analyses on this topic that were specific to a treatment, country, or region, this study provides a comprehensive global overview of the proportion of hepatocellular carcinoma secondary to NAFLD, clinical presentation, treatment allocation, and outcomes of NAFLD-related hepatocellular carcinoma. This study determined that the global proportion of hepatocellular carcinoma secondary to NAFLD is rising. In addition, this meta-analysis provides high-level evidence that a higher proportion of patients with NAFLD-related hepatocellular carcinoma do not have cirrhosis and these patients have substantially lower surveillance rates than those with hepatocellular carcinoma due to other causes.
Implications of all the available evidence
Many patients with NAFLD-related hepatocellular carcinoma do not have cirrhosis and lack a routine indication for hepatocellular carcinoma surveillance based on current practice guidelines. This contributes to few patients with NAFLD-related hepatocellular carcinoma receiving surveillance before diagnosis. Further studies are required to improve hepatocellular carcinoma surveillance strategies for patients with NAFLD and without cirrhosis who are high risk. Urgent measures are required to tackle the underlying metabolic risk factors and reduce the growing burden of NAFLD-related hepatocellular carcinoma.
Acknowledgments
RL receives funding support from National Center for Advancing Translational Sciences (5UL1TR001442), National Institute of Diabetes and Digestive and Kidney Diseases (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, and P30DK120515), National Heart, Lung, and Blood Institute (P01HL147835), and National Institute on Alcohol Abuse and Alcoholism (U01AA029019). DQH receives funding support from Singapore Ministry of Health’s National Medical Research Council under its Research Training Fellowship (MOH-000595-01).
Declaration of interests
AJS is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect, and Galmed; has served as a consultant to AstraZeneca, Nitto Denko, Enyo, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer-Ingelheim, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz, and Genfit; and has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, Surrozen, and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers Squibb, Shire, Intercept, Merck, AstraZeneca, Malinckrodt, Cumberland, and Novartis. He receives royalties from Elsevier and UptoDate. RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics. His institutions received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. RL is also the co-founder of LipoNexus. DQH has served as an advisory board member for Eisai. All other authors declare no competing interests.
Footnotes
See Online for appendix
Data sharing
All articles in this Article are available from MEDLINE and Embase.
References
- 1.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019; 16: 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–49. [DOI] [PubMed] [Google Scholar]
- 3.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021; 18: 223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021; 7: 6. [DOI] [PubMed] [Google Scholar]
- 5.Muthiah MD, Sanyal AJ. Burden of disease due to nonalcoholic fatty liver disease. Gastroenterol Clin North Am 2020; 49: 1–23. [DOI] [PubMed] [Google Scholar]
- 6.Le MH, Yeo YH, Li X, et al. 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021; published online Dec 7. 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol 2018; 69: 896–904. [DOI] [PubMed] [Google Scholar]
- 8.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021; 184: 2537–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younossi Z, Stepanova M, Ong JP, et al. nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019; 17: 748–55.e3. [DOI] [PubMed] [Google Scholar]
- 10.Dyson J, Jaques B, Chattopadyhay D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol 2014; 60: 110–17. [DOI] [PubMed] [Google Scholar]
- 11.Estes C, Chan HLY, Chien RN, et al. Modelling NAFLD disease burden in four Asian regions-2019–2030. Aliment Pharmacol Ther 2020; 51: 801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018; 67: 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong CR, Nguyen MH, Lim JK. Hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. World J Gastroenterol 2016; 22: 8294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015; 62: 1723–30. [DOI] [PubMed] [Google Scholar]
- 15.Benhammou JN, Aby ES, Shirvanian G, Manansala K, Hussain SK, Tong MJ. Improved survival after treatments of patients with nonalcoholic fatty liver disease associated hepatocellular carcinoma. Sci Rep 2020; 10: 9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang DQ, Yeo YH, Nguyen MH. Letter to the editor: hepatocellular carcinoma surveillance in cirrhosis patients: is the real-world situation even worse than reported? Hepatology 2021; 74: 1714–15. [DOI] [PubMed] [Google Scholar]
- 18.Guyot P, Ades AE, Ouwens MJNM, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017, Ahmad N, Ahuja SD, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018; 392: 821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudry S, Hajage D, Benichou N, et al. Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet 2020; 395: 1506–15. [DOI] [PubMed] [Google Scholar]
- 21.Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic–bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet 2021; 397: 1830–41. [DOI] [PubMed] [Google Scholar]
- 22.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher J What is heterogeneity and is it important? BMJ 2007; 334: 94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 25.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc 2015; 13: 196–207. [DOI] [PubMed] [Google Scholar]
- 26.Bell A, Fairbrother M, Jones K. Fixed and random effects models: making an informed choice. Qual Quant 2019; 53: 1051–74. [Google Scholar]
- 27.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods 2019; 10: 476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26: 404–13. [Google Scholar]
- 29.Yong JNLW, Lim WH, Ng CH, et al. Outcomes of nonalcoholic steatohepatitis after liver transplantation: an updated meta-analysis and systematic review. Clin Gastroenterol Hepatol 2021; published online Nov 18. 10.1016/j.cgh.2021.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Xiao J, Yong JN, Ng CH, et al. A meta-analysis and systematic review on the global prevalence, risk factors, and outcomes of coronary artery disease in liver transplantation recipients. Liver Transpl 2021; published online Oct 8. 10.1002/lt.26331. [DOI] [PubMed] [Google Scholar]
- 31.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014; 3: 123–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006; 25: 3443–57. [DOI] [PubMed] [Google Scholar]
- 34.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67: 358–80. [DOI] [PubMed] [Google Scholar]
- 35.Galle PR, Forner A, Llovet JM, et al. Management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 36.Cleveland ER, Ning H, Vos MB, et al. Low awareness of nonalcoholic fatty liver disease in a population-based cohort sample: the CARDIA Study. J Gen Intern Med 2019; 34: 2772–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alqahtani SA, Paik JM, Biswas R, Arshad T, Henry L, Younossi ZM. Poor awareness of liver disease among adults with NAFLD in the United States. Hepatol Commun 2021; 5: 1833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canbay A, Kachru N, Haas JS, Sowa JP, Meise D, Ozbay AB. Patterns and predictors of mortality and disease progression among patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2020; 52: 1185–94. [DOI] [PubMed] [Google Scholar]
- 39.Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med 2018; 16: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Best J, Bechmann LP, Sowa JP, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2020; 18: 728–35.e4. [DOI] [PubMed] [Google Scholar]
- 41.Huang DQ, Fowler KJ, Liau J, et al. Comparative efficacy of an optimal exam between ultrasound versus abbreviated MRI for HCC screening in NAFLD cirrhosis: a prospective study. Aliment Pharmacol Ther 2022; published online Feb 28. 10.1111/apt.16844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singal AG, El-Serag HB. Rational HCC screening approaches for patients with NAFLD. J Hepatol 2021; 76: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trevisani F, Caraceni P, Bernardi M, et al. Gross pathologic types of hepatocellular carcinoma in Italian patients. Relationship with demographic, environmental, and clinical factors. Cancer 1993; 72: 1557–63. [DOI] [PubMed] [Google Scholar]
- 44.Sarpel U, Ayo D, Lobach I, Xu R, Newman E. Inverse relationship between cirrhosis and massive tumours in hepatocellular carcinoma. HPB (Oxford) 2012; 14: 741–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamad B, Shah V, Onyshchenko M, et al. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int 2016; 10: 632–39. [DOI] [PubMed] [Google Scholar]
- 46.Huang DQ, Muthiah MD, Zhou L, et al. Predicting HCC response to multikinase inhibitors with in vivo cirrhotic mouse model for personalized therapy. Cell Mol Gastroenterol Hepatol 2021; 11: 1313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh JH, Tan DJH, Ong Y, et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: a meta-analysis of 18,421 patients. Hepatobiliary Surg Nutr 2021; published online Dec 11. 10.21037/hbsn-21-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molinari M, Kaltenmeier C, Samra P-B, et al. Hepatic resection for hepatocellular carcinoma in nonalcoholic fatty liver disease: a systematic review and meta-analysis of 7226 patients. Ann Surg 2021; 2: e065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakai T, Shirai Y, Sakata J, Korita PV, Ajioka Y, Hatakeyama K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg 2011; 15: 1450–58. [DOI] [PubMed] [Google Scholar]
- 50.Weinmann A, Alt Y, Koch S, et al. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer 2015; 15: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailar JC 3rd. The promise and problems of meta-analysis. N Engl J Med 1997; 337: 559–61. [DOI] [PubMed] [Google Scholar]
- 52.Greenland S Can meta-analysis be salvaged? Am J Epidemiol 1994; 140: 783–87. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019; 4: 389–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All articles in this Article are available from MEDLINE and Embase.


