Abstract
Introduction
On 4 February, 2020, the Secretary of the Department of Health and Human Services declared a public health emergency related to coronavirus disease 2019 (COVID-19), and on 27 March, 2020 declared circumstances existed to justify the authorization of the emergency use of drug and biological products (hereafter, “drugs”) for COVID-19. At the outset of the pandemic with uncertainty relating to the virus, many drugs were being used to treat or prevent COVID-19, resulting in the US Food and Drug Administration’s (FDA’s) need to initiate heightened surveillance across these drugs.
Objective
We aimed to describe the FDA’s approach to monitoring the safety of drugs to treat or prevent COVID-19 across multiple data sources and the subsequent actions taken by the FDA to protect public health.
Methods
The FDA conducted surveillance of adverse event and medication error data using the FDA Adverse Event Reporting System, biomedical literature, FDA-American College of Medical Toxicology COVID-19 Toxicology Investigators Consortium Pharmacovigilance Project Sub-registry, and the American Association of Poison Control Centers National Poison Data System.
Results
From 4 February, 2020, through 31 January, 2022, we identified 22,944 unique adverse event cases worldwide and 1052 unique medication error cases domestically with drugs to treat or prevent COVID-19. These were from the FDA Adverse Event Reporting System (22,219), biomedical literature (1107), FDA-American College of Medical Toxicology COVID-19 Toxicology Investigator’s Consortium Sub-registry (638), and the National Poison Data System (32), resulting in the detection of several important safety issues.
Conclusions
Safety surveillance using near real-time data was critical during the COVID-19 pandemic because the FDA monitored an unprecedented number of drugs to treat or prevent COVID-19. Additionally, the pandemic prompted the FDA to accelerate innovation, forging new collaborations and leveraging data sources to conduct safety surveillance to respond to the pandemic.
Key Points
| The emergence of a variety of drugs to treat or prevent coronavirus disease 2019 highlighted the need for the US Food and Drug Administration to move beyond routine pharmacovigilance practices to rapidly identify emerging safety concerns. |
| Establishing collaborations and leveraging new data sources including near real-time data were essential in the Food and Drug Administration’s response to the pandemic. |
| The Food and Drug Administration evaluated over 20,000 adverse event cases from multiple data sources with drugs used to treat or prevent coronavirus disease 2019 during the first 2 years of the pandemic, resulting in the identification of safety issues and subsequent actions to protect the health of Americans during the pandemic. |
Introduction
In December 2019, a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) was first detected in Wuhan City, Hubei Province, China. On 4 February, 2020, the Secretary of the Department of Health and Human Services declared a national public health emergency related to coronavirus disease 2019 (COVID-19) [1]. On 27 March, 2020, pursuant to section 564 of the Federal Food, Drug, and Cosmetic Act, the Secretary of the Department of Health and Human Services declared that circumstances existed justifying the authorization of the emergency use of drug and biological products during the COVID-19 pandemic [2]. This declaration allowed the FDA to authorize unapproved drug or biological products or unapproved uses of approved drug or biological products for emergency use during the COVID-19 public health emergency upon an Agency determination for a particular product that the criteria for issuance of an Emergency Use Authorization (EUA) are met. In addition to using drugs authorized under an EUA, some members of the public and medical community turned to other drugs to treat or prevent COVID-19 without any available data to support their efficacy.
Therefore, we quickly identified the need to adapt our existing surveillance practices into a system that could accomplish heightened surveillance based on the urgency related to the rapidly evolving nature of the virus, the illness it caused, and the therapies used to treat or prevent it. In 2009, during the H1N1 pandemic, our surveillance was limited to adverse event (AE) reports in the FDA Adverse Event Reporting System (FAERS) [3]. During the COVID-19 pandemic, the FAERS remains the FDA’s principal repository for drug safety information. Over the course of the pandemic, we established collaborations and leveraged new sources that enabled us to conduct safety surveillance using near real-time data across all drug and biological products (hereafter, “drugs”) regulated by the FDA’s Center for Drug Evaluation and Research that were used to treat or prevent COVID-19. The FDA was able to rapidly identify new safety issues, revise Fact Sheets (FSs) for drugs authorized under EUAs (“EUA drugs”), and issue public communications. Herein, we provide a descriptive analysis of the safety data from four sources: FAERS, biomedical literature, FDA-American College of Medical Toxicology (ACMT) COVID-19 Toxicology Investigator’s Consortium [FACT] Pharmacovigilance Project Sub-registry, and American Association of Poison Control Centers (AAPCC) National Poison Data System (NPDS). Our use of administrative claims data and electronic health records has been previously described elsewhere [4].
Methods
This analysis covers a 2-year period starting with the Department of Health and Human Services Secretary’s declaration of the public health emergency on 4 February, 2020 through 31 January, 2022. To conduct heightened safety surveillance of drugs used during the pandemic, the FDA assembled a team of safety experts from the Center for Drug Evaluation and Research to monitor AEs and medication errors (MEs) across all available sources reporting data related to any drug used to treat or prevent COVID-19. These drugs included both new therapies and off-label uses of drugs with proven efficacy for their approved indications. Because of differences among the data sources, including the coding of AE terms and the method of case retrieval or submission, we used a customized approach to search for and identify relevant cases of interest from each source. We defined a “relevant” case as a case that reported an AE or death with a drug used for the treatment or prevention of COVID-19. We systematically collected and evaluated all the data received and excluded duplicate cases using a stepwise approach to identify duplicate cases by looking for exact matches of various data elements, such as age, sex, country, drug product, and description of the AE, and comparing them with cases already documented. Regardless of source, the overall assessment of the cases focused on demographic information, reported AEs and MEs, concomitant medications, time to onset of events, clinical outcome, reporter source, and dechallenge and rechallenge information. We evaluated each case to identify emerging safety issues. Once we identified a safety signal through our surveillance, we performed a stepwise approach to building a case series for a pharmacovigilance evaluation. This stepwise approach consisted of creating and applying a case definition and assessing the causal association between the reported AE and product using the World Health Organization-Uppsala Monitoring Center Causality Assessment System [5].
FAERS
Established AE reporting requirements for applicants, manufacturers, packers, and distributors are detailed under 21CFR314.80 [6], 21CFR600.80 [7], 21CFR314.98 [8], and 21CFR310.305 [9]. For EUA drugs, under the conditions of authorization, the FDA requires that EUA sponsors and healthcare professionals (HCPs) report all serious AEs and MEs. The FDA determined that more stringent reporting requirements for EUA drugs are necessary to protect the public health.
MedWatch is the FDA’s medical product safety reporting program for HCPs, patients, and consumers. Cases submitted to MedWatch are incorporated into the FAERS, a large database of spontaneously reported AEs used to support the FDA’s post-marketing surveillance program for drugs [10].
The FAERS data are useful for identifying unknown, rare, and serious AEs reported with the use of FDA-approved or authorized drugs, which is especially important throughout the pandemic. Because FAERS is an established AE database, it is our primary data source for conducting surveillance, especially early in the pandemic when there were many drugs being used to treat or prevent COVID-19. We adapted our strategies throughout the pandemic, from broadly searching for any case mentioning the novel coronavirus or COVID-19-related terms (e.g., coronavirus infection, coronavirus test positive, SARS-CoV-2), to searching more narrowly for cases involving drugs authorized or approved to treat or prevent COVID-19. We reviewed all cases identified from the search strategies and tracked all the relevant cases. As the pandemic evolved, we began utilizing additional data sources.
Biomedical Literature
We screened recurring weekly search queries of PubMed and Embase and reviewed article titles and abstracts to identify cases describing an AE occurring after administration of any drug to treat or prevent COVID-19.
FACT Pharmacovigilance Project Sub-registry [11]
In 2010, the ACMT established the Toxicology Investigators Consortium as a multicenter toxico-surveillance and research network of medical toxicologists and has continued expanding the capabilities of the registry to serve as a database for public health surveillance and emerging concerns within the field of toxicology, including AEs [12, 13]. In response to the COVID-19 pandemic, the FDA contracted with ACMT’s Toxicology Investigators Consortium to establish the FACT Pharmacovigilance Project Sub-registry to leverage the existing capabilities of the Toxicology Investigators Consortium registry as a surveillance tool to identify emerging safety issues associated with drugs to treat or prevent COVID-19 [14]. The FACT Sub-registry includes medical toxicology site investigators and research assistants from 17 medical centers across the USA. FACT enables direct outreach by the FDA to site investigators when additional information on reported cases is needed. In conjunction with the FDA, ACMT and site investigators developed enhanced data collection forms for some AEs of special interest to the FDA in an effort to obtain additional clinical details that could be used in FDA evaluations of safety concerns.
The FDA received, and continues to receive, real-time e-mail alerts as new and follow-up cases are entered into the Sub-registry providing an opportunity to review cases in near real-time. All cases identified from the real-time e-mail alerts were reviewed for relevance and tracked.
NPDS [15]
The NPDS, a database managed by AAPCC and derived from a nationwide network of US Poison Control Centers (PCCs), captures near real-time data from calls from individuals, HCPs, and others regarding exposures to prescription drugs, over-the-counter medications, and unapproved drugs. Healthcare professionals at PCCs who respond to calls about exposures have specialized training in toxicology needed to assess, triage to the most appropriate level of care, provide recommendations, and follow up on toxic emergencies. Within the NPDS, calls for exposures may result in documentation of an event, provision of information, or advice regarding medical management; PCC staff managing these cases undergo training in the effort to standardize documentation across centers.
Documentation of cases includes details on the drug(s), patient characteristics, route of exposure, reasons for exposure, level of care received (e.g., admitted to critical care unit vs treated and released), medical outcomes (e.g., death, no effect), and other more curated variables, such as “relatedness” of the reported exposure to the outcomes of interest. Reasons for use are categorized into groups by the AAPCC and include such categories as “intentional” and “unintentional,” the former encompassing the subgroups of intentional misuse, abuse, suspected suicide, or unknown intent.
The PCC case data do not represent the national-level incidence of exposures or cases of misuse/abuse related to any substance. These data only capture events if the exposure resulted in a call to a PCC. The PCC data rely on information voluntarily shared by patients and healthcare personnel, and most substance classification is based on history alone and does not involve any laboratory confirmation. Exposures may be unconfirmed ingestions (i.e., the product may not have been ingested at all by the patient). Drug exposures resulting in an unattended or out-of-hospital death are unlikely to generate a call to a PCC, and therefore, fatal poisonings are expected to be substantially under-documented in PCC case data.
Throughout the pandemic, we used the NPDS to identify exposure cases reporting an AE across a variety of prescription (e.g., hydroxychloroquine, ivermectin [16]), over-the-counter (e.g., hand sanitizers [17, 18], hydrogen peroxide), and unapproved products (e.g., Miracle Mineral Solution [19], oleander extract) used in an effort to treat or prevent COVID-19. Daily surveillance queries were generated to identify exposure calls related to these products. All cases identified from the queries were reviewed for relevance and tracked. The NPDS served as a valuable data source in the surveillance efforts for many of these products, especially hand sanitizers, including those contaminated with methanol [20].
Results
AEs
Through our surveillance, we monitored the safety of COVID-19 drugs and evaluated potential AEs of interest across the four data sources. From 4 February, 2020, through 31 January, 2022, we identified 22,944 unique cases for inclusion in this analysis reporting an AE with a drug to treat or prevent COVID-19. Of these, we identified 21,167 in the FAERS database, 1107 in the biomedical literature, 638 in the FACT Sub-registry, and 32 in the NPDS database. A majority of these cases were reported from US sources. Variation in reporting between other countries and the USA is likely multifactorial and may include reasons such as authorization/approval status of drugs in other countries, the standard of care for treating COVID-19, and the number of literature publications varying from each country. In addition, in the USA, there are different regulatory requirements for domestic and foreign case reports. Adverse events that are both serious and unexpected (i.e., not in the drug product label), whether domestic or foreign, from all sources must be submitted to the FDA within 15 days of initial receipt of the information by the applicant. For AEs that are serious and expected or non-serious, only domestic reports must be submitted to the FDA by the applicant. Additionally, the NPDS database and the FACT Sub-registry are US-based data sources; therefore, foreign cases from these sources are not anticipated. Table 1 summarizes the descriptive characteristics of the patients described in the 22,944 cases.
Table 1.
Patient characteristics and drug product data from 4 February, 2020 through 31 January, 2022
| Case demographics | All casesa (n = 22,944) | HCQ or CQ (n = 3649) | RDV (n = 6747) | BAM (n = 3377) | BCT (n = 685) | CASI and IMDE (n = 3464) | BAM and ETE (n = 1497) | SOT (n = 409) | TCZ (n = 2913) | TIX and CIL (n = 21) | NIRM and RTV (n = 148) | MOV (n = 50) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date of authorization | – | 28 March, 2020b | 1 May, 2020c | 9 November, 2020 | 19 November, 2020d | 21 November, 2020 | 9 February, 2021 | 26 May, 2021 | 24 June , 2021 | 8 December, 2021 | 22 December, 2021 | 23 December, 2021 |
| Age, median (IQR) |
n = 20,594 62 (48–73) |
n = 3340 60 (48–71) |
n = 6331 60 (50–74) |
n = 3006 68 (59–77) |
n = 627 61 (51–71) |
n = 3356 55 (40–68) |
n = 1391 52 (37.5–66) |
n = 336 61 (45–73) |
n = 2295 62 (50–71) |
n = 18 55 (44–63) |
n = 108 62 (47–72) |
n = 47 67 (20–79) |
| Sex | n = 21,529 | n = 3429 | n = 6628 | n = 3279 | n = 645 | n = 3414 | n = 1440 | n = 361 | n = 2353 | n = 20 | n = 128 | n = 50 |
| Male, n (%) | 12,205 (56) | 2299 (67) | 4,131 (62) | 1,714 (52) | 427 (66) | 1469 (43) | 541 (38) | 166 (46) | 1,681 (71) | 13 (65) | 45 (35) | 21 (42) |
| Female, n (%) | 9319 (43) | 1130 (33) | 2495 (37) | 1565 (48) | 218 (34) | 1943 (57) | 899 (62) | 194 (54) | 672 (29) | 7 (35) | 83 (65) | 29 (58) |
| Transgender, n (%) | 4 (<1) | 0 | 2 (<1) | 0 | 0 | 2 (<1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Intersex, n (%) | 1 (<1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<1) | 0 | 0 | 0 | 0 |
| Country | ||||||||||||
| USA | 16,036 | 1112 | 5412 | 3330 | 586 | 3450 | 1370 | 284 | 1058 | 20 | 147 | 32 |
| Other countries | 6908 | 2537 | 1335 | 47 | 99 | 14 | 127 | 125 | 1855 | 1 | 1 | 18 |
| Reported deaths,e n (%) | 5061 (22) | 1217 (33) | 1932 (29) | 201 (6) | 186 (27) | 148 (4) | 64 (4) | 28 (7) | 1386 (48) | 1 (5) | 2 (1) | 7 (14) |
BAM bamlanivimab, BCT baricitinib, CASI casirivimab, CIL cilgavimab, COVID-19 coronavirus disease 2019, CQ chloroquine, ECMO extracorporeal membrane oxygenation, ETE etesevimab, EUA emergency use authorization, FDA Food and Drug Administration, HCQ hydroxychloroquine, IMDE imdevimab, IQR interquartile range, MOV molnupiravir, NIRM nirmatrelvir, RDV remdesivir, RTV ritonavir, SOT sotrovimab, TCZ tocilizumab, TIX tixagevimab
aThis total includes all drugs, EUA and non-EUA, identified during surveillance. Other drugs used to treat or prevent COVID-19 that were included in this total and reported in 50 or more cases are: azithromycin, lopinavir/ritonavir, convalescent plasma, oseltamivir, favipiravir, sarilumab, interferon (i.e., alpha, alfa-2b, beta-1a, 2b, 1b), anakinra, nitric oxide, ivermectin, umifenovir, ribavirin, ritonavir, canakinumab, darunavir/cobicistat, darunavir, and tofacitinib. More than one treatment may have been reported in each case; therefore, the total number for “all cases” is lower than the sum across all products
bThe FDA revoked the HCQ/CQ EUA on 15 June, 2020
cOn 22 October, 2020, the FDA approved remdesivir for use in adults and pediatric patients (12 years of age and older and weighing at least 40 kg) for the treatment of COVID-19 requiring hospitalization. On April 25, 2022, the FDA approved remdesivir for the remaining populations (pediatric patients aged 28 days and older and weighing at least 3 kg) removing the need for the EUA. Corresponding revisions to the EUA with respect to its authorized uses and populations were similarly made during this time frame
dOn 10 May, 2022, the FDA approved baricitinib for the treatment of COVID-19 in hospitalized adults requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or ECMO. This is the first immunosuppressant to be approved for this condition. The FDA also revised the EUA to authorize baricitinib for emergency use to treat COVID-19 in hospitalized pediatric patients aged 2 to less than 18 years
eIncludes mortality from all causes and does not imply a causal relationship to the drug
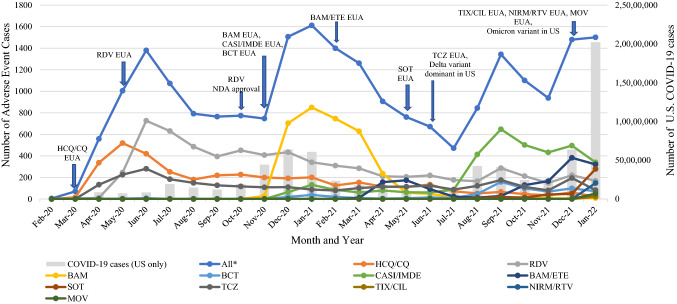
Figure 1 illustrates the trend in cases reporting an AE with a drug to treat or prevent COVID-19 from 4 February, 2020 through 31 January, 2022. There were several upward trends in AE reporting during this period that appeared to correlate with the COVID-19 pandemic trajectory [21]. Early in the pandemic, prior to the issuance of any EUAs, certain drugs were used off-label to combat COVID-19, including antivirals and antimicrobials, such as lopinavir/ritonavir, azithromycin, ceftriaxone, and interferon. In addition to the overall reporting trends during this period, there were several trends observed with EUA drugs. As illustrated in Fig. 1, peaks and valleys in AE reporting for EUA drugs related to the issuance of new EUAs, shifts in variants, or revocations of EUAs. For example, the FDA issued an EUA for bamlanivimab on 9 November, 2020, which was followed by an increase in AE reporting for this product shortly thereafter in December 2020 and January 2021, followed by a sharp decline in April 2021 after revocation of the EUA of bamlanivimab because of a lack of susceptibility [22]. In addition, there are mandatory reporting requirements of all serious AEs and MEs for EUA drug products, which could be one explanation for an increase in AE reporting trends seen in Fig. 1. The trends depicted in the graph are unlikely related to changes in search strategies or the addition of new data sources, as these changes occurred early in the surveillance process prior to granting EUAs for COVID-19 products. As the pandemic evolved, changes in therapy to treat or prevent COVID-19 are reflected in the AE reporting trends.
Fig. 1.
Cases from the FDA Adverse Event Reporting System, biomedical literature, FDA-American College of Medical Toxicology COVID-19 Toxicology Investigators Consortium Pharmacovigilance Sub-registry, and the National Poison Data System reporting an adverse event with a drug to treat or prevent COVID-19 from 4 February, 2020 through 31 January, 2022. *All products, EUA and non-EUA, identified during surveillance. Monthly COVID-19 cases were calculated from the CDC COVID Data Tracker: https://covid.cdc.gov/covid-data-tracker/#trends_dailycases. BAM bamlanivimab, BCT baricitinib, CASI casirivimab, CDC Centers for Disease Control and Prevention, CIL cilgavimab, COVID coronavirus disease, CQ chloroquine, ETE etesevimab, EUA Emergency Use Authorization, HCQ hydroxychloroquine, IMDE imdevimab, MOV molnupiravir, NDA New Drug Application, NIRM nirmatrelvir, RDV remdesivir, RTV ritonavir, SOT sotrovimab, TCZ tocilizumab, TIX tixagevimab, US United States
Actions Resulting from Surveillance Activities
Here forward, we focus on data collected through our heightened surveillance efforts to support actions related to certain COVID-19 EUA drugs. These actions included FDA public communications and safety-related updates to EUA FSs. Reporting frequencies, summarized in Tables 2, 3 and 4, were used to identify potential AEs of interest, which the FDA further investigated and assessed the need for regulatory action. An overview of these actions and the evidence supporting them follows.
Table 2.
Most frequently reported adverse events with a reporting frequency ≥ 2% with hydroxychloroquine or chloroquine (n = 3649)
| Adverse eventsa | Reporting frequencyb, n (%) |
|---|---|
| Electrocardiogram QT prolongedc | 424 (11.6) |
| Respiratory disordersd | 368 (10.1) |
| Liver impairmente | 353 (9.7) |
| Renal impairmentf | 229 (6.3) |
| Gastrointestinal disordersg | 153 (4.2) |
| Cardiac arrythmiah | 138 (3.8) |
| Cardiac arrest and cardio-respiratory arrest | 94 (2.6) |
| Pulmonary embolism | 78 (2.1) |
Data sources include the FDA’s Adverse Event Reporting System, literature, National Poison Data System, and FDA-American College of Medical Toxicology COVID-19 Toxicology Investigator’s Consortium Sub-registry
aA single case could describe more than one adverse event
bReporting frequency is the number of reported cases of a particular adverse event for a product used to treat or prevent coronavirus disease 2019 divided by the total number of reported cases for that product × 100
cIncludes long QT syndrome
dIncludes respiratory failure, acute respiratory distress syndrome, hypoxia, dyspnea, and acute respiratory failure
eIncludes hepatitis, increased alanine aminotransferase, increased aspartate aminotransferase, hepatic cytolysis, increased transaminases, liver injury, and hypertransaminasemia
fIncludes acute kidney injury, increased serum creatinine, and renal failure
gIncludes diarrhea, nausea, and vomiting
hIncludes atrial fibrillation, ventricular tachycardia, bradycardia, and tachycardia
Table 4.
Most frequently reported adverse events with a reporting frequency ≥5% with monoclonal antibodies (n = 8737)
| Adverse eventsa | Reporting frequencyb, n (%) |
|---|---|
| Infusion-related reactionsc | 3923 (45) |
| Respiratory disordersd | 2537 (29) |
| Nausea/vomiting | 966 (11) |
| Fever | 912 (10) |
| Chest pain/discomfort | 918 (10) |
| Chills | 697 (8) |
| Dizziness | 651 (7) |
| Rash/erythema | 581 (7) |
| Cough | 546 (6) |
| Flushing | 507 (6) |
| Hypotension | 538 (6) |
| Headache | 446 (5) |
| Tachycardia | 446 (5) |
Data sources include the FDA Adverse Event Reporting System, literature, and FACT Sub-registry
aA single case could describe more than one adverse event
bReporting frequency is the number of reported cases of a particular adverse event for a product used to treat or prevent coronavirus disease 2019 divided by the total number of reported cases for that product × 100
cInfusion-related reactions was defined as those adverse events that occurred within 2 hours of monoclonal antibody administration
dIncludes dyspnea, hypoxia, oxygen saturation decreased, respiratory distress, respiratory failure, and respiratory depression
Hydroxychloroquine and Chloroquine
On 28 March, 2020, the FDA issued an EUA for the antimalarial drugs, hydroxychloroquine and chloroquine, for adults and adolescents weighing ≥ 50 kg hospitalized with COVID-19 for whom a clinical trial was not available, or participation was not feasible. The FDA revoked the EUA on 15 June, 2020, after emerging scientific data, including a randomized trial, failed to show evidence of a benefit [23], prompting the National Institutes of Health to recommend against using these drugs to treat or prevent COVID-19 [24].
Cardiotoxicity, particularly when associated with the cardiac conduction system, is a known risk of hydroxychloroquine and chloroquine. Notably, prolongation of the QT interval was the most common AE reported with hydroxychloroquine and chloroquine (Table 2). Related life-threatening arrhythmias, torsades de pointes (26 cases), and ventricular fibrillation (16 cases), were also reported. An FDA evaluation of cases, which included four cases of torsades de pointes, prompted the FDA to issue a Drug Safety Communication on 24 April, 2020 (during the EUA period), reminding the public of the risk of arrhythmia and cautioning against hydroxychloroquine and chloroquine use for COVID-19 outside of the hospital setting or a clinical trial [25]. The FDA further evaluated cardiotoxicity in patients receiving these drugs for COVID-19 in a safety assessment during the EUA period, and concluded that the current hydroxychloroquine and chloroquine labeling, which describes cardiotoxicity in the Warnings and Precautions section, adequately conveyed these risks for approved indications, and further recommended an update to the chloroquine labeling to better describe cardiac electrophysiology study findings. The non-cardiac AEs were more likely related to COVID-19 infection than the use of hydroxychloroquine and chloroquine. For example, a recent study described the use of the FDA’s Sentinel System to measure the 90-day risk of arterial thromboembolism and venous thromboembolism in patients hospitalized with COVID-19 before or during COVID-19 vaccine availability versus patients hospitalized with influenza. The authors concluded that there was a higher risk of venous thromboembolism in hospitalized patients with COVID-19 compared with hospitalized patients with influenza [26].
Remdesivir
Remdesivir, a SARS-CoV-2 nucleotide analog RNA polymerase inhibitor, became the first novel antiviral product issued an EUA on 1 May, 2020, and on 22 October, 2020 became the first approved treatment for COVID-19 in the USA. Hypersensitivity reactions, including infusion-related reactions (IRRs), were recognized as an important safety issue for remdesivir at the time of EUA issuance and were included in the Warnings and Precautions section of the FS for HCPs. Post-authorization AE cases received through the FAERS for remdesivir identified signs and symptoms of hypersensitivity reactions that were not included in the initial FS, including tachycardia, bradycardia, dyspnea, wheezing, angioedema, rash, and anaphylaxis. On 15 June, 2020, shortly after identification of these additional events, the FDA updated the FS to inform the medical community and public of these emerging safety issues.
Hepatotoxicity was another important safety issue for remdesivir, with liver impairment being the most frequently reported AE (Table 3). Transaminase elevations were observed across the remdesivir development program. Upon EUA issuance, the FS for HCPs included the risk of transaminase elevations in the Warnings and Precautions section with advice to discontinue or not initiate remdesivir in patients whose alanine aminotransferase (ALT) was greater than five times the upper limit of normal or who had an ALT elevation with signs or symptoms of liver inflammation. Although additional cases of transaminase elevations were identified post-authorization, all cases reporting hepatic failure had information suggesting alternative explanations or did not have sufficient information to attribute this AE to remdesivir. Labeling upon FDA approval retained transaminase elevations in the Warnings and Precautions with risk mitigation strategies including discontinuing remdesivir if ALT levels exceeded ten times the upper limit of normal or if the ALT elevation was accompanied by signs or symptoms of liver inflammation.
Table 3.
Most frequently reported adverse events with a reporting frequency ≥ 2% with remdesivir (n = 6746)
| Adverse eventsa | Reporting frequencyb, N (%) |
|---|---|
| Liver impairmentc | 1750 (26) |
| Renal impairmentd | 1016 (15) |
| Respiratory disorderse | 748 (11) |
| Bradycardiaf | 698 (10) |
| Hypotensiong | 222 (3) |
| Cardiac arrest | 163 (2) |
| Infusion site extravasation | 130 (2) |
Data sources include the FDAs Adverse Event Reporting System, literature, and FACT Sub-registry
aA single case could describe more than one adverse event
bReporting frequency is the number of reported cases of a particular adverse event for a product used to treat or prevent coronavirus disease 2019 divided by the total number of reported cases for that product × 100
cIncludes increased alanine aminotransferase, increased aspartate aminotransferase, increased hepatic enzyme, increased transaminases, increased liver function tests, increased blood alkaline phosphatase, increased blood bilirubin, acute hepatic failure, liver injury, drug-induced liver injury
dIncludes increased blood creatinine, acute kidney injury, decreased glomerular filtration, renal failure
eIncludes dyspnea, hypoxia, decreased oxygen saturation, respiratory distress, respiratory failure, acute respiratory failure
fIncludes decreased heart rate
gIncludes decreased blood pressure
Renal impairment was also a frequently reported AE for remdesivir. Review of the cases did not identify a causal relationship between remdesivir and renal impairment; therefore, no FS changes were warranted.
Neutralizing Monoclonal Antibodies
The FDA issued EUAs for six SARS-CoV-2 neutralizing IgG1 monoclonal antibody (mAb) therapies to treat or prevent COVID-19, including bamlanivimab, bamlanivimab/etesevimab, casirivimab/imdevimab, sotrovimab, tixagevimab/cilgavimab, and bebtelovimab, with the first, bamlanivimab, authorized on 9 November, 2020. Through heightened surveillance, the FDA identified AEs of interest, resulting in several updates to the mAb FS for HCPs.
Based on clinical trial data for mAbs administered via an infusion, the FSs for HCPs included a warning for hypersensitivity reactions including anaphylaxis and IRRs, which included bronchospasm, hypotension, angioedema, rash including urticaria, myalgia, and dizziness. Most IRRs and hypersensitivity reactions in the clinical trials were reported as mild to moderate in severity. To mitigate the potential risks of severe IRRs, the FDA requires the administration of bamlanivimab, bamlanivimab/etesevimab, casirivimab/imdevimab, and sotrovimab, in settings in which HCPs have immediate access to medications to treat a severe reaction, such as anaphylaxis, and the ability to activate the emergency medical system as necessary. Patients should be monitored during infusion/injection(s) and observed for at least 1 hour after the infusion is complete.
A review of the post-authorization data identified difficulty breathing, reduced oxygen saturation, fatigue, arrhythmia, altered mental status, hypertension, and diaphoresis as the most frequently reported AEs occurring within 2 hours of mAb administration (i.e., IRRs) that were not included in the FSs at the time of initial authorization. In February 2021, the FDA updated the FS for HCP for the authorized mAbs at the time based on post-authorization cases identified in the FAERS database and FACT Sub-registry. The updates included the addition of new signs and symptoms under hypersensitivity including IRRs in the Warnings and Precautions section of the FSs. Additionally, the FDA included a new warning communicating the potential for clinical worsening after mAb administration based on cases identified of rapid respiratory decompensation during or shortly after mAb administration, some of which required hospitalization. In June 2021, the FDA updated the Warnings and Precautions section of the mAb FS for HCPs to include hypersensitivity reactions occurring more than 24 hours after infusion and vasovagal reactions (i.e., pre-syncope and syncope) based on post-authorization cases identified in the FAERS database and FACT Sub-registry describing pre-syncopal and syncopal episodes occurring during or shortly after administration of mAbs that were identified in the context of IRRs and hypersensitivity reactions, many of which required treatment in the emergency department or hospital admission. Additionally, the FDA identified cases of non-immediate (>2 hours after infusion) hypersensitivity reactions, which included cutaneous reactions and angioedema. The most frequently reported AEs (Table 4) with a reporting frequency of ≥5% with mAbs, including IRRs, are all labeled AEs in the mAb FS for HCPs, except for cough.
MEs
From 4 February, 2020, through 31 January, 2022, we identified 1052 unique US ME cases submitted to the FAERS. Healthcare professionals reported 90% of the cases. Approximately 20% (227/1,052) of the cases reported a serious AE; however, based on individual case reviews, the serious AEs were more likely related to drug therapy or underlying medical conditions (including COVID-19) than a ME.
As shown in Table 5, the number of reported ME cases varied by product, from one case (molnupiravir) to 484 cases (casirivimab/imdevimab). The number of cases was influenced by the length of time the product was authorized for use, individual definitions of an ME, utilization patterns, and product characteristics (e.g., route of administration, packaging, and procedures for use).
Table 5.
EUA drug product characteristics and number of medication error cases received by the US FDA between 4 February, 2020 through 31 January, 2022 (n = 1052)
| Producta | Case count | Route of administration | Dosage forms | Packaging |
|---|---|---|---|---|
|
Bamlanivimab or etesevimab |
175 | IV |
Injection, 700 mg/20 mL (bamlanivimab) Injection, 700 mg/20 mL (etesevimab) |
Single-dose vials in individual cartons |
| Olumiant (baricitinib) | 17 | Oral | Tablets, 1 mg, 2 mg, and 4 mg | Bottles |
|
REGEN-COV (casirivimab/imdevimab or casirivimab or imdevimab) |
484 | IV or SC |
Injection, 600 mg of casirivimab and 600 mg of imdevimab per 10 mL (co-formulated) Injection, 300 mg/2.5 mL, 1332 mg/11.1 mL (casirivimab) Injection, 300 mg/2.5 mL, 1332 mg/11.1 mL (imdevimab) |
Single-dose vials (co-formulated) in individual cartons Single-dose vials in individual cartons Single-dose vials, co-packaged in one carton Dose packs providing 1200 mg casirivimab and 1200 mg imdevimab (containing 2, 5, or 8 single-dose vials in individual cartons per dose pack) |
| Evusheld (cilgavimab; tixagevimab) | 4 | IM |
Injection, 150 mg/1.5 mL (cilgavimab) Injection, 150 mg/1.5 mL (tixagevimab) |
Single-dose vials, co-packaged in one carton |
|
Plaquenil (hydroxychloroquine) or Aralen (chloroquine) |
3 | Oral |
200 mg (hydroxychloroquine sulfate) 500 mg (chloroquine phosphate) |
Bottles |
| Veklury (remdesivir) | 246 | IV |
Injection, 100 mg/20 mLb For injection, 100 mg of lyophilized powder for reconstitution |
Single-dose vials in individual cartons |
| Sotrovimab | 96 | IV | Injection, 500 mg/8 mL | Single-dose vial in individual cartons |
|
Actemra (tocilizumab) |
12 | IV | Injection, 80 mg/4 mL, 200 mg/10 mL, or 400 mg/20 mL | Single-dose vials in individual cartonsc |
|
Lagevrio (molnupiravir) |
1 | Oral | Capsules, 200 mg | Bottles |
|
Paxlovid (nirmatrelvir; ritonavir) |
17 | Oral |
Nirmatrelvir tablets, 150 mg Ritonavir tablets, 100 mg |
Blister packs of tablets, co-packaged |
COVID-19 coronavirus disease 2019, EUA Emergency Use Authorization, FDA Food and Drug Administration, IM intramuscular, IV intravenous, SC subcutaneous
aLimited to EUA drugs authorized to treat or prevent COVID-19. See Table 1 for the date of first EUA issuance
bBoth remdesivir injection and remdesivir for injection formulations were authorized for emergency use in the management of COVID-19; both formulations are now FDA approved
cTocilizumab is also approved in the USA as an autoinjector (Actemra Actpen); however, only the single-dose vial was authorized for emergency use in the management of COVID-19
The most frequently reported MEs across all products were improper dose, product prepared incorrectly, product dispensing error (e.g., wrong strength or formulation dispensed), transcription error (e.g., incorrect order entry), wrong drug product administered (e.g., casirivimab administered instead of the intended COVID-19 vaccine), and wrong route of administration. Depending on the product and type of ME, multiple contributing factors for the errors were reported, including inconsistent or unfamiliar formatting and content on container labels (e.g., use of investigational drug nomenclature [27], lack of barcodes for product verification), multiple packaging presentations for the same product, limitations of electronic health systems, miscommunication, and changes to dosage regimens. Fast-paced stressful work environments with staffing and supply shortages and deviations from usual workflow practices also contributed to the errors.
As part of the EUA process, the FDA reviewed proposed FS language and packaging to minimize potential MEs. Post-authorization, the FDA monitored ME cases and subsequently updated FSs and requested manufacturers revise container labels, carton labeling, and packaging to help mitigate reported errors.
Discussion
Although many drugs have been used throughout the pandemic to treat or prevent COVID-19, this analysis primarily focused on the FDA’s heightened surveillance efforts that were integral to identifying emerging safety issues across many EUA drugs leading to rapid actions to alert the public of these risks. The FDA was uniquely positioned as an agency because of our access to both internal and external AE and ME data sources providing robust safety data. This was possible through existing and new partnerships and leveraging and building onto our infrastructure through data sources providing near real-time data. Additionally, to improve transparency and expand data access to the public, the FDA launched the COVID-19 EUA FAERS Public Dashboard [28].
The COVID-19 pandemic provided the opportunity to learn key lessons that contributed to the success of our surveillance efforts. During the COVID-19 pandemic, the FDA requires stringent AE/ME reporting for FDA EUA drugs and has had access to valuable data through multiple sources, which allowed us to rapidly assess and identify trends/signals to take prompt action. One example of a new collaboration between the FDA and ACMT resulted in the FACT Sub-registry, which was developed to capture AEs associated with COVID-19 drugs in near real-time. At the outset, it was unknown what type of AEs would be captured given the setting of data collection, but it proved to be a very useful source that complemented other data sources. We were also able to contribute to developing targeted data collection forms and, as a result, were better positioned to assess these cases given the additional details on AEs of interest obtained. The FDA staff also collaborated with the AAPCC to develop recurring queries in the NPDS to capture potential toxic exposures related to hydroxychloroquine and chloroquine early in the pandemic. Because of these collaborations, the near real-time data sources diversified our sources of AE case reports, thereby enhancing our surveillance efforts during the pandemic.
Post-authorization surveillance of AEs and MEs for EUA drugs was critical to inform benefit-risk determinations to support continuation of an EUA. When safety concerns were identified, the FDA took actions, including FS revisions and communications. Data obtained through the FDA’s surveillance for hydroxychloroquine and chloroquine resulted in the release of a Drug Safety Communication alerting the public of the risk of arrhythmias and cautioning against its use for COVID-19 outside of the hospital setting or clinical trials. The FDA also used data identified with remdesivir to rapidly update the FS and during the New Drug Application review to ensure the product labeling on approval adequately described the known safety profile.
Additionally, we continue to identify emerging safety issues through ongoing surveillance. Most recently, the FDA identified hypersensitivity reactions related to nirmatrelvir/ritonavir and molnupiravir shortly after distribution began that resulted in updates to the FSs for each product within weeks of identification, which further highlights the importance of the near real-time access to data on AEs and MEs. The FDA’s safety assessments provide a valuable window into the safety of the drugs to treat or prevent COVID-19.
Conclusions
The emergence of a variety of drugs to treat or prevent COVID-19 highlighted the need for the FDA to move beyond routine pharmacovigilance practices to rapidly identify emerging safety concerns related to these drugs and communicate these risks to the public. Establishing collaborations and leveraging new data sources were essential in our response to the pandemic.
Declarations
Funding
No sources of funding were received for the preparation of this article.
Conflicts of Interest/Competing Interests
None of the authors has any conflicts of interest to disclose.
Ethics Approval
Ethics approval was not considered necessary for this study because FDA surveillance activity is deemed necessary for the protection of public health.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The FAERS data are available via the FAERS Public Dashboard and as Quarterly Data files. Additionally, individual case reports can be requested via a Freedom of Information Act request to the FDA. Additional details can be found here: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. The FDA-AAPCC NPDS data were obtained under contract with ACMT and AAPCC, respectively.
Code Availability
Not applicable.
Authors Contributions
Conceptualization: ILD, NG, RK, KS, KM, MB, JK, LW, LK, JW, MDB, GDP; data analysis: KS, KM, MB, JK, TS, MF, JW; writing: ILD, NG, KS, KM, MB, JK, TS, JW; review and editing: ILD, KS, KM, MB, JK, NG, RK, LW, ALB, PM, MDB, GDP; approval of the manuscript: all authors.
References
- 1.Determination of Public Health Emergency. https://www.federalregister.gov/documents/2020/02/07/2020-02496/determination-of-public-health-emergency. Accessed 25 Mar 2022.
- 2.Notice of Emergency Use Authorization Declaration. https://www.federalregister.gov/documents/2020/04/01/2020-06905/emergency-use-authorization-declaration. Accessed 25 Mar 2022.
- 3.Sorbello A, Jones SC, Carter W, et al. Emergency use authorization for intravenous peramivir: evaluation of safety in the treatment of hospitalized patients infected with 2009 H1N1 influenza A virus. Clin Infect Dis. 2012;55(1):1–7. doi: 10.1093/cid/cis351. [DOI] [PubMed] [Google Scholar]
- 4.Cocoros NM, Fuller CC, Adimadhyam S, et al. A COVID-19-ready public health surveillance system: the Food and Drug Administration’s Sentinel System. Pharmacoepidemiol Drug Saf. 2021;30(7):827–837. doi: 10.1002/pds.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO-UMC system for standardization case causality assessment. http://www.who-umc.org/. Accessed 14 Oct 2022.
- 6.CFR - Code of Federal Regulations Title 21 Sec. 314.80 Postmarketing reporting of adverse drug experiences. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=314.80. Accessed 22 May 2022.
- 7.CFR - Code of Federal Regulations Title 21 Sec. 600.80 Postmarketing reporting of adverse experiences. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=600.80. Accessed 22 May 2022.
- 8.CFR - Code of Federal Regulations Title 21 Sec. 314.98 Records and reports concerning adverse drug experiences on marketed prescription drugs for human use without approved new drug applications: abbreviated applications. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=314.98. Accessed 22 May 2022.
- 9.CFR - Code of Federal Regulations Title 21 Sec. 310.305 Records and reports concerning adverse drug experiences on marketed prescription drugs for human use without approved new drug applications. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=310.305. Accessed 22 May 2022].
- 10.Questions and answers on FDA’s Adverse Event Reporting System (FAERS). https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. Accessed 17 Nov 2022.
- 11.FDA ACMT COVID-19 ToxIC (FACT) Pharmacovigilance Project Sub-Registry description. https://www.toxicregistry.org/FACT.html. Accessed 17 Nov 2022.
- 12.Wax PM, Kleinschmidt KC, Brent J. The Toxicology Investigators Consortium (ToxIC) Registry. J Med Toxicol. 2011;7(4):259–265. doi: 10.1007/s13181-011-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spyres MB, Aldy K, Farrugia LA, et al. The toxicology investigators consortium 2020 annual report. J Med Toxicol. 2021;17(4):333–362. doi: 10.1007/s13181-021-00854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldy K, Wax P, Brent J, on behalf of the ToxIC FACT Study Group et al. Rapid development of the FDA ACMT COVID-19 ToxIC (FACT) pharmacovigilance pilot project to monitor adverse events reported in association with COVID-19 therapeutics. Clin Toxicol (Phila) 2021;59(11):1037–1093. [Google Scholar]
- 15.American Association of Poison Control Centers National Poison Data System Information. https://www.aapcc.org/national-poison-data-system. Accessed 22 Mar 2022.
- 16.FDA consumer update: why you should not use ivermectin to treat or prevent COVID-19. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19. Accessed 18 Mar 2022.
- 17.FDA updates on hand sanitizers consumers should not use. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-hand-sanitizers-consumers-should-not-use. Accessed 18 Mar 2022.
- 18.McCulley L, Cheng C, Mentari E, et al. Alcohol-based hand sanitizer exposures and effects on young children in the U.S. during the COVID-19 pandemic. Clin Toxicol (Phila) 2021;59(4):355–356. doi: 10.1080/15563650.2020.1811298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lardieri A, Cheng C, Jones SC, McCulley L. Harmful effects of chlorine dioxide exposure. Clin Toxicol (Phila) 2021;59(5):448–449. doi: 10.1080/15563650.2020.1818767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coronavirus (COVID-19) update: FDA takes action to warn, protect consumers from dangerous alcohol-based hand sanitizers containing methanol. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-action-warn-protect-consumers-dangerous-alcohol-based-hand. Accessed 18 Mar 2022.
- 21.Centers for Disease Control and Prevention. COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases. Accessed 31 Mar 2022.
- 22.US FDA. FDA revocation letter of the Emergency Use Authorization for bamlanivimab. https://www.fda.gov/media/147629/download. Accessed 3 Apr 2022.
- 23.US FDA. FDA revocation letter of the Emergency Use Authorization of oral formulations of chloroquine phosphate and hydroxychloroquine sulfate. https://www.fda.gov/media/138945/download. Accessed 3 Apr 2022.
- 24.COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Accessed 30 Mar 2022. [PubMed]
- 25.US FDA. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. Accessed 30 Mar 2022.
- 26.Lo Re V, Dutcher SK, Connolly JG, et al. Association of COVID-19 vs influenza with risk of arterial and venous thrombotic events among hospitalized patients. JAMA. 2022;328(7):637–651. doi: 10.1001/jama.2022.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Institute for Safe Medication Practices. COVID-19 related medication errors. 14 May 2020. ISMP Med Saf Alert Acute Care. 25(9):1–3. https://www.ismp.org/acute-care/special-edition-medication-safety-alert-may-14-2020/covid-19. Accessed 6 May 2022.
- 28.FDA Adverse Event Reporting System Public Dashboard. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard. Accessed 10 Apr 2022.