Abstract
We report here the characterization of a novel Leishmania infantum protein termed papLe22 (22-kDa potentially aggravating protein of Leishmania). A positive clone from a cDNA library was identified by serum of a visceral leishmaniasis (VL) patient. Full-length cDNA obtained using rapid amplification of cDNA ends-PCR codes for a 22-kDa protein. In L. infantum promastigotes an endogenous nuclear protein of 14-kDa electrophoretic mobility was found by using an antiserum prepared against the fusion protein glutathione S-transferase–papLe22. Its expression was also shown in L. infantum amastigotes and in Leishmania major and Leishmania guyanensis promastigotes. VL patients' sera showed anti-papLe22 immunoglobulin M (IgM) and IgG reactivities, indicating that a primary response against the leishmanial protein papLe22 accompanied acute VL manifestations. Specific IgG levels were correlated with patients' clinical status. The presence of IgG1, IgG2, and IgG3 subclasses suggested a mixed Th1- and Th2-type response; there was no correlation between subclass reactivity and the disease course. The recombinant papLe22 specifically activated interleukin-10 production by VL patients' peripheral blood mononuclear cells collected at diagnosis and after treatment-induced cure, indicating its contribution to VL pathogenesis and concomitant immunosuppression and its potential role in the reactivation of latent parasites. As a dominant immunogen, papLe22 might be used as a vaccine component, provided that the vaccination protocol directs the response toward the Th1 pattern.
Visceral leishmaniasis (VL) due to viscerotropic species Leishmania donovani and Leishmania infantum (Leishmania chagasi) is fatal if untreated (41), and when VL is associated with other diseases, such as AIDS (2, 32), the existing anti-Leishmania therapies are often ineffective. Each year, 500,000 new VL cases are reported (15).
When it is met with an appropriate immune response, L. infantum, endemic in Brazil and around the Mediterranean, may remain relatively harmless, surviving in macrophages of its human host. The existence of individuals without history of VL but presenting a positive leishmanin skin test (LST), a specific indicator of the delayed-type antileishmanial hypersensitivity (DTH), has been reported (4, 34, 40). However, an inappropriate immune response to L. infantum infection leads to patent VL, clinically characterized by fever, pancytopenia, and hepatosplenomegaly, and patients do not spontaneously heal. VL patients present high levels of antileishmanial antibodies (18, 36, 44), likely to be T cell dependent, low-level or absent Leishmania-induced T-cell responses such as proliferation and interleukin-2 (IL-2) and gamma interferon (IFN-γ) production, and negative DTH tests (10, 11, 48).
Several (at least partially) protective, i.e., generating a predominant Th1 response, antigens of Leishmania have been identified and cloned: Lcr1 (60), TSA (58), gp63 (61), GP46/M-2 (35), PSA-2 (52), hsp70 (53), LeIF (54), Ldp23 (8), LmSTI1 (59), Lt-1, and Lt-2 (17). But the search for and characterization of well-defined parasite antigens with a potential to aggravate the disease are of critical importance. Indeed, an efficient vaccine should not only induce an enhanced secondary response to protective antigens but also, maybe more imperatively, should be able to reorient the response to naturally aggravating antigens toward protection. At present only one such protein antigen, from a mouse model, has been described (29).
Antigen-induced production of IL-10 is of particular interest because of its antagonistic effects on IFN-γ (3, 6, 28, 57), a potent activator of macrophages for intracellular Leishmania killing (37). In mice, IL-4 and IL-10 have been shown to be associated with susceptibility to visceralization of Leishmania major (24, 25), and the production of IL-10 has been determined to be a crucial factor in susceptibility to Trypanosoma cruzi (51). In human Leishmania infections, IL-10 production correlates with the degree of pathology, and IL-10 appears to be the major cytokine involved in the progression to visceral disease (5, 9, 22, 27, 30, 45).
In this paper we report the cloning and characterization of a novel L. infantum immunogen termed papLe22 (for 22-kDa potentially aggravating protein of Leishmania). The analyses of antibody and cellular responses manifested by VL patients toward papLe22 show that it is one of the parasite antigens that have a potential to aggravate the disease.
MATERIALS AND METHODS
Patients, blood, and PBMC.
Patients presenting parasitologically documented VL due to L. infantum (all strains typed as zymodeme MON-1) and clinically cured by meglumine antimonate treatment (20 mg of Sb5+/kg of body weight/day for 28 days) were regularly monitored at 1, 3, 6, and 12 months after the diagnosis. Peripheral blood was obtained from VL patients at diagnosis and at follow-up examinations, from asymptomatic subjects with positive LST and detectable antibodies against 14- and/or 18-kDa leishmanial fractions (34), and from LST-negative, volunteer control donors. Peripheral blood mononuclear cells (PBMC) were isolated as described previously (46, 56) and stored (in 90% heat-inactivated fetal calf serum, 10% dimethyl sulfoxide) in liquid nitrogen until use. For cytokine assays PBMC were seeded at 106 cells/ml in complete culture medium (46, 56), activated as described below, and cultured at 37°C in a 5% CO2 humidified atmosphere in 24-well plates (0.7 ml/well) for 2 to 6 days (as indicated in legends to figures).
Parasites.
L. infantum MON-1 (MHOM/FR/94/LPN101), isolated from a patient with VL, was maintained by serial passages in Syrian hamsters. The promastigote form was cultured under the usual conditions (56), and 5- to 7-day-old stationary-phase cells (2 × 107 to 2.5 × 107 promastigotes/ml) were used. The amastigote form was purified from hamster spleen (50).
Screening of cDNA libraries.
Two libraries of L. infantum promastigote cDNA (synthesized with an oligo(dT) primer or random hexaprimers) in λgt11 were kindly provided by Carlos Alonso (Madrid, Spain). Approximately 105 λgt11 plaques were screened for each library, using an acute-phase patient serum (previously absorbed for 2 h at room temperature with Escherichia coli Y 1090r− lysate [49]), by classical procedures (49). The positive plaques were purified by two more rounds of screening.
cDNA synthesis.
Total RNA from 5 × 108 parasites was extracted with 1 ml of RNA-B (Bioprobe) by following the manufacturer's instructions and quantitated by spectrophotometry analysis. RNA (2.5 μg) was reverse transcribed as previously described (46).
PCR amplifications.
PCRs were carried out using 0.2 mM deoxynucleoside triphosphate, a 1 μM concentration of each primer (Eurogentec), and 0.014 U of thermostable DNA polymerase/μl, in a final volume of 25 μl. First, λgt11 inserts corresponding to the positive clones were amplified by Taq polymerase (Appligene) using 1 μl of recombinant phage stock (49) and the following phage primers: 5′-GGTGGCGACGACTCCTGGAGCCCG-3′ and 5′-TTGACACCAGACCAACTGGTAATG-3′. An initial denaturation (94°C, 5 min) was followed by 35 amplification cycles (denaturation at 94°C for 1 min, annealing at 51°C for 1 min, and elongation at 72°C for 2 min) and a final 10-min elongation at 72°C. After sequencing (see below), the specific primers were chosen and the total sequence of the coding region was obtained by rapid amplification of cDNA ends (RACE)-PCR using high-fidelity Pwo polymerase (Boehringer Mannheim) and 5 μl of L. infantum cDNA. The cDNA insert corresponding to a clone termed 9C contained a stop codon. The amplification of the 5′ end of its cDNA was obtained with the specific primer R1 and the “miniexon” primer (19) with a BamHI site (underlined) 5′-TAGGGATCCAACTAAGCGTATATAAGTATCAGTTT-3′. A nested RACE-PCR was carried out using an aliquot of RACE-PCR product with the specific primer R2. Finally, the coding region corresponding to the clone 9C was amplified from L. infantum cDNA with Pwo polymerase and the specific primers R and F. Locations of primers R1, R2, R, and F are indicated in Fig. 1b.
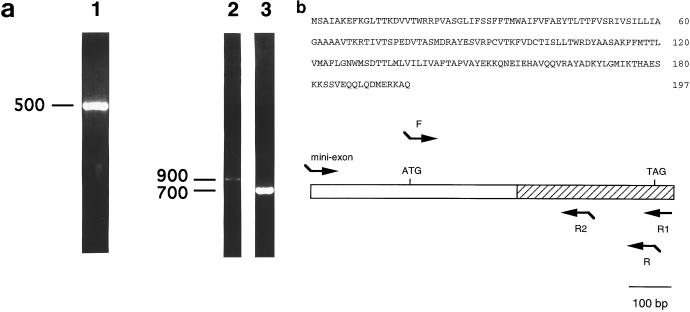
FIG. 1.
Cloning of papLe22 cDNA. (a) PCR product of the λgt11 clone 9C amplified with λgt11 primers (lane 1) and RACE-PCR products of L. infantum cDNA amplified with miniexon and R1 primers (lane 2) or miniexon and R2 primers (lane 3) were electrophoresed on 1.5% (lane 1) or 1% (lanes 2 and 3) agarose gels containing 0.5 μg of ethidium bromide/ml. DNA length markers are indicated in base pairs. (b) Amino acid sequence of papLe22 (top) and diagrammatic representation of the 876-bp papLe22 5′ cDNA (bottom). The 594-bp ORF is between the ATG initiation and TAG stop codons. Hatched box, sequence of the λgt11 clone 9C (5′ end at nucleotide 258 of the ORF); arrows, PCR primers; segments, 5′ restriction enzyme sites.
Cloning of PCR products and DNA sequencing.
Purified PCR products (Geneclean kit; Bio 101), digested overnight with 50 μg of proteinase K/ml as described previously (12), were cloned into pCR-Blunt vector, and competent E. coli TOP10 cells were transformed in accordance with the manufacturer's instructions (Zero Blunt PCR cloning kit; Invitrogen). pCR-Blunt inserts, isolated from three recombinant colonies, were sequenced by automated DNA sequencing (ABI Prism 100) with M13 Reverse and M13 (−20) Forward primers (Eurogentec). Nucleotide and protein sequences were compared to data available by the BLAST search method (1).
Expression and purification of recombinant antigen.
The PCR-amplified coding region of clone 9C (9C open reading frame [ORF]) and pGEX-6P-1 vector (Pharmacia) were digested at 37°C for 4 h with an excess of BamHI (Biolabs) and EcoRI (Biolabs) restriction enzymes and ligated. The product of the ligation was first propagated in TOP10 competent bacteria and then expressed as a fusion protein with glutathione S-transferase (GST) in E. coli BL21; all steps were performed in accordance with the supplier's protocols (Pharmacia). A glycerol stock of one recombinant BL21 colony was induced (49) for 2 h with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to express the recombinant protein, termed GST-papLe22. The purification of GST-papLe22 was done essentially as recommended by the supplier (bulk GST purification module; Pharmacia). Briefly, the bacteria were washed, resuspended in phosphate-buffered saline (1:20 [vol/vol]) containing protease inhibitors (EDTA and phenylmethysulfonyl fluoride [both 2 mM] and aprotinin [1,000 U/ml]) and lysed by five cycles of freezing and thawing. After solubilization with 1% Triton X-100, the fusion protein was adsorbed to a glutathione-Sepharose column and, after three washes, was eluted with 3 volumes of elution buffer (10 mM reduced glutathione in 50 mM Tris, pH 8.0). Purified material was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 12% polyacrylamide gel) after being stained with 0.003% Coomassie blue in 40% methanol–10% acetic acid. BL21 cells transformed with pGEX-6P-1 vector without the 9C ORF were treated similarly, and the resulting recombinant GST was purified in parallel.
Rabbit antiserum.
A New Zealand white female rabbit was immunized subcutaneously three times at 1-month intervals with 50 μg of purified GST-papLe22 administered as an emulsion of 1 volume of homogenized SDS-polyacrylamide gel and 1 volume of incomplete Freund's adjuvant. After the last injection the animal was bled once per week for 3 weeks.
Western blot analyses.
Recombinant proteins, purified from 5-ml bacterial cultures (approximately 1 μg of GST-papLe22), were loaded on each centimeter of a 1.5-mm-thick gel. After SDS-PAGE (33), proteins were electrotransferred to a nitrocellulose membrane at 60 V for 1 h (Mini-Protean II; Bio-Rad); the membrane was further treated as described previously (56). Patient and control sera were used at 1:50 and 1:20 dilutions, respectively. Peroxidase conjugates directed against human immunoglobulin G (IgG) subclasses (Binding Site) and against IgM (Sigma) were used at 1:100 dilution. Preimmune rabbit serum and sera of LST-negative individuals were used as negative controls.
ELISA.
The time course of specific anti-papLe IgG levels in VL patient sera was determined by a classical enzyme-linked immunosorbent assay (ELISA) procedure analogous to that described previously for antileishmanial antibody determination (47). GST-papLe22 or control GST was coated overnight at 1 μg/ml (50 μl), and the sera were tested at a 1:100 dilution and revealed with anti-human IgG peroxidase conjugate used at a 1:2,000 dilution. Incubation steps were performed with 0.1 M phosphate buffer, pH 7.2, containing 1% (wt/vol) skimmed dry milk, 0.12% (vol/vol) Triton X-100, 0.2% (vol/vol) chloroform, 0.02% thimerosal, and 100 μg of phenol red/ml.
Cell activation and cytokine assays.
PBMC were stimulated by incubation with GST-papLe22 (0.1 and 0.3 μg/ml), GST (1 and 3 μg/ml), unfractionated promastigote antigens (30 μg/ml) prepared as described previously (56), phytohemagglutinin (PHA) (50 μg/ml), or lipopolysaccharide (LPS) (10 μg/ml) or were left unstimulated. After 2 and 6 days of culture, supernatants were collected and stored at −20°C until IL-10 and IFN-γ concentrations were measured. The cytokine levels were determined in duplicate using indirect sandwich ELISAs (Genzyme) in accordance with the manufacturer's instructions (the detection threshold was 50 pg/ml).
Indirect immunofluorescence.
Stationary-phase promastigotes in 18-well immunofluorescence slides (5 × 103 cells/well) were fixed for 10 min in acetone at −20°C and either stored at −20°C (up to 1 month) or used immediately. Preimmune or anti-papLe22 rabbit sera were used at a 1:5 dilution. Antibody fixation was revealed using fluorescein isothiocyanate-conjugated anti-rabbit Ig (Dako), which was diluted at 1:20 in PBS containing 0.01% (wt/vol) Evans blue for counterstaining (56).
Nucleotide sequence accession number.
The papLe22 cDNA sequence obtained in this study has been assigned GenBank accession no. AF123892.
RESULTS
Cloning of a novel L. infantum antigen.
Fifteen positive clones were identified by screening two L. infantum cDNA libraries with an acute-phase VL patient serum. Among the 12 clones sequenced, four inserts belonged to cDNAs coding for L. infantum histone H2A and heat shock protein 70 (hsp70) already described (42, 55). Clone 9C, corresponding to a 378-bp insert (Fig. 1a, lane 1) with a stop codon at position 335, was further characterized. The missing 5′ end of the coding region was amplified by 5′ RACE-PCR from L. infantum cDNA using a specific primer, R1, that was designed from the insert sequence (Fig. 1b) and the miniexon primer common to all Leishmania cDNA, described in Materials and Methods. Figure 1a (lane 2) shows that a unique 900-bp PCR product was detected. Its specificity was confirmed by a secondary RACE-PCR with nested primer R2 (Fig. 1b), which produced an amplicon 200 bp shorter (Fig. 1a, lane 3). The 900-bp PCR product was cloned in pCR-Blunt vector for sequencing, and Fig. 1b shows the amino acid sequence deduced from a 594-nucleotide ORF. The corresponding 22.1-kDa protein (papLe22), with a predicted pI of 8.2, did not exhibit homologies with sequences of Leishmania proteins reported to date.
A nuclear L. infantum protein of 14-kDa electrophoretic mobility is recognized by the rabbit antiserum.
To generate a recombinant GST-papLe22 protein, the strict ORF of 9C cDNA was amplified by PCR with two specific primers (Fig. 1b) and directionally cloned in phase in pGEX-6P-1 vector. Figure 2a shows fusion protein GST-papLe22 purified in parallel with GST (lanes 1 and 2, respectively). The lower band in lane 1 was recognized by an anti-GST monoclonal antibody (not shown) and thus corresponds to a proteolytic degradation product of the recombinant protein. A yield of approximately 2 μg of GST-papLe22 per 10-ml culture was determined after electrophoresis gel staining.
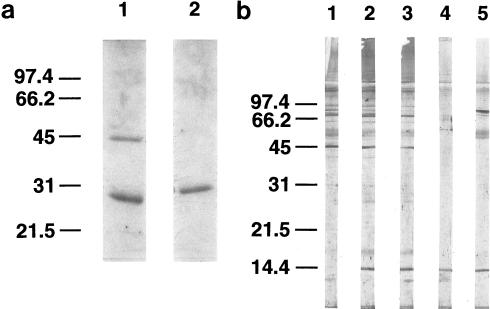
FIG. 2.
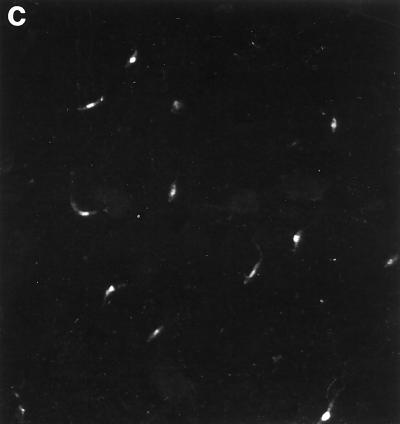
Purification of recombinant proteins and detection of endogenous papLe22 of 14-kDa electrophoretic mobility in L. infantum nuclear fraction. (a) The recombinant proteins GST-papLe22 (43 kDa; lane 1) and GST (29 kDa; lane 2) were purified from 2.5 ml and 12.5 μl, respectively, of E. coli BL21-induced cultures (0.1 mM IPTG for 2 h). Bacteria were extracted with 1% Triton X-100, and fusion proteins were purified on a glutathione-Sepharose column. Eluted material was analyzed by SDS-PAGE (12% polyacrylamide) and Coomassie blue staining. (b) L. infantum promastigotes harvested from a 7-day-old culture were washed three times with saline, resuspended at 4 × 108/ml, and incubated at 25 (lanes 1 and 2) or 37°C (lane 3) for 3 h. The amastigote form (lane 4) was purified from infected hamster spleen as described previously (50), and the nuclear fraction of the promastigote form (lane 5) was obtained as described previously (56). In each case the equivalent of 5 × 106 cells was separated on an SDS–14% polyacrylamide gel. Western blotting was performed with an anti-GST-papLe22 rabbit antiserum (lanes 2 to 5) or the preimmune serum (lane 1), both revealed by peroxidase-labeled anti-rabbit IgG. Molecular mass markers in kilodaltons are indicated. (c) Stationary-phase promastigotes were air dried on slides and fixed in acetone at −20°C. Anti-GST-papLe22 rabbit antiserum and fluorescein isothiocyanate-conjugated anti-rabbit Ig were diluted 1:5 in PBS and 1:20 in PBS containing 0.01% Evans blue, respectively. Incubations were performed sequentially at 37°C for 30 min, and slides were washed three times in PBS for 5 min. Magnification, ×400. No fluorescent labeling was detected with preimmune serum.
Characterization of the native L. infantum protein by Western blotting using a rabbit antiserum raised against fusion protein GST-papLe22 revealed an immunoreactive band at 14 kDa (Fig. 2b, lane 2) not detected by preimmune serum (Fig. 2b, lane 1). This apparent molecular mass coincided with the difference between the electrophoretic mobility of GST-papLe22 and that of GST (Fig. 2a). Therefore, the band reactive with the rabbit antiserum corresponds to a 14-kDa native papLe22 in L. infantum rather than a proteolytic fragment. Figure 2b shows that both promastigotes (lane 2) and amastigotes (lane 4) of L. infantum express the antigen. In promastigotes, the antigen expression remained unchanged after a thermic shock of 37°C for 3 h (lane 3). The antigen papLe22 was also detected in promastigotes of L. major and Leishmania guyanensis (not shown). The Western blot analysis of subcellular fractions showed that the native protein was localized in the promastigote nuclear fraction (Fig. 2b, lane 5). All subcellular fractions obtained in the process of nucleus preparations (56) were analyzed together with the nuclear fraction and were found to be papLe22 negative (not shown). The nuclear localization in promastigotes was further corroborated by indirect immunofluorescence staining (Fig. 2c). No anti-GST reactivity was detected in the rabbit antiserum.
VL patients exhibit a marked anti-papLe22 antibody response, correlated with their clinical condition.
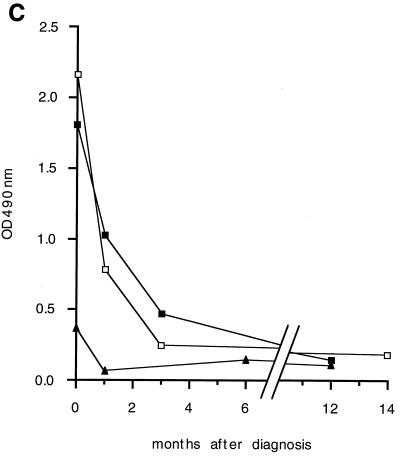
Sera obtained from patients with clinically patent VL were strongly reactive to GST-papLe22. No GST reactivity was detectable in these sera. Figure 3a shows examples for two patients of anti-GST-papLe22 IgMs (lanes 1 and 2) and IgGs (lanes 3 and 5) at VL diagnosis and of IgGs 1 year later (lanes 4 and 6), as detected by Western blotting. Specific IgMs were found in four patients of the seven who were tested. Interestingly, the same sera revealed IgMs at 14 kDa when examined on a total leishmania protein preparation (not shown). IgG reactivity declined after cure (Fig. 3a, lane 4 versus 3 and 6 versus 5). All sera tested at diagnosis contained anti-GST-papLe22 antibodies of IgG1, IgG2, and IgG3 subclasses; for most, but not all, patients, IgG1 and IgG2 reactivities were stronger than that of IgG3. Typical patterns for 2 patients are shown in Fig. 3b (IgG1, lanes 1 and 4; IgG2, lanes 2 and 5; IgG3, lanes 3 and 6). IgG4 reactivities were not assessed. The time course of anti-papLe22 IgG in patient sera was determined by ELISA. Figure 3c demonstrates that the antibodies had strongly decreased already at clinical cure due to successful treatment (1 month after the diagnosis) for all examined patients but one, who exhibited only a moderate decrease. In this patient the weak reactivity to papLe22 at diagnosis was associated with mild clinical symptoms of VL. Control sera from LST-negative donors (n = 4) and sera from LST-positive subjects (n = 4) living in the area of endemicity but without a history of VL (34) were papLe22 negative. Sera from patients with pathologies other than VL (toxoplasmosis [n = 3], malaria [n = 3], amebiasis [n = 3], and schistosomiasis [n = 3]) were also examined by Western blotting, and none reacted with papLe22.
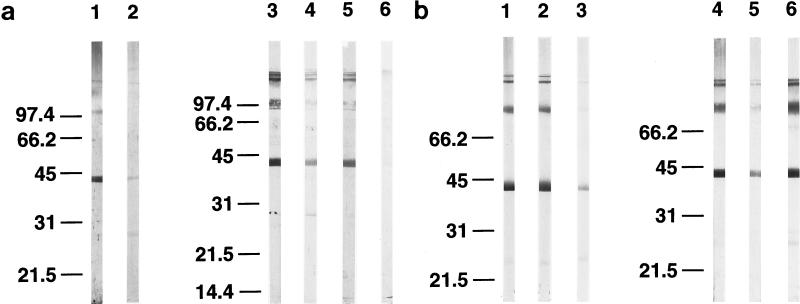
FIG. 3.
Reactivity of VL patient sera against recombinant papLe22. (a) GST-papLe22 was purified and electrophoresed as described in the legend for Fig. 2a. One microgram of protein was loaded per centimeter of gel. Western blotting was performed with sera, diluted 1:50, from patient 1 at diagnosis (lanes 1 and 3) and 14 months later (lane 4) and from patient 2 at diagnosis (lanes 2 and 5) and 12 months later (lane 6). Anti-IgM (lanes 1 and 2) and anti-IgG (lanes 3 to 6) peroxidase-conjugated secondary antibodies were diluted 1:100 and 1:1,000, respectively. (b) Diagnostic sera from patient 1 (lanes 1 to 3) and patient 3 (lanes 4 to 6) were incubated as described for panel a. Anti-IgG1 (lanes 1 and 4), anti-IgG2 (lanes 2 and 5), and anti-IgG3 (lanes 3 and 6) peroxidase-conjugated secondary antibodies were diluted 1:100. Molecular mass markers in kilodaltons are indicated. (c) GST-papLe22 and GST were purified as described in the legend for Fig. 2a and were eluted from glutathione-Sepharose with 0.1% SDS in PBS. Recombinant proteins at 0.05 μg per well were used to coat microtiter plates and were sequentially incubated with patient serum diluted 1:100 and peroxidase-labeled anti-human IgG. Absorbances obtained with GST-papLe22 were corrected for absorbances measured with GST alone. Data for diagnosis and follow-up sera from patient 1 (open squares), patient 2 (triangles), and patient 4 (solid squares) are shown. Sera from LST-negative controls and positive asymptomatic subjects gave values for optical density at 490 nm (OD490 nm) below 0.12.
papLe22 stimulates in vitro IL-10 production by PBMC of VL patients.
The cellular responses to papLe22 were next analyzed in terms of proliferation and cytokine production by in vitro-stimulated PBMC. The presence of GST-papLe22 inhibited, slightly but systematically, the proliferation of PBMC from VL patients but not from control subjects, without inducing cell death (not shown). This observation suggested that a factor inhibiting T-cell proliferation, such as IL-10, could be produced in response to the recombinant antigen. Figure 4 shows the typical patterns of IL-10 and IFN-γ synthesis by cells obtained from one VL patient at diagnosis and at 1-, 6-, and 12-month follow-up examinations. Similar results were obtained using the entire PBMC series from a second VL patient and PBMC obtained at 1 month from a third patient.
FIG. 4.
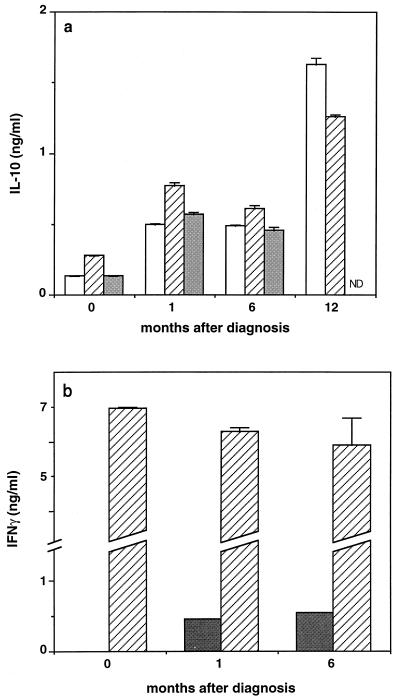
PapLe22 induces IL-10 production by PBMC of VL patients. (a) IL-10 production quantitated by ELISA in culture supernatant of PBMC (7 × 105 in 0.7 ml of culture medium) obtained at diagnosis and follow-up examinations from patient 4 and activated in vitro for 2 days with 0.3 μg of GST-papLe22/ml (open bars), 50 μg of PHA/ml (hatched bars), or 10 μg of LPS/ml (grey bars) is shown. Under control conditions (3 μg of GST/ml) Il-10 production was 0.07, 0.20, 0.21, and 0.41 ng/ml at 0, 1, 6, and 12 month after diagnosis, respectively. (b) IFN-γ production measured by ELISA of the supernatants of the cells from panel a activated with 30 μg of unfractionated promastigote antigens/ml (grey bars) or 50 μg of PHA/ml (hatched bars). Neither cytokine was detected (<50 pg/ml) in unstimulated cultures. Results represent means ± standard errors of the means of duplicate measurements. ND, not determined.
To ascertain that GST-papLe22-stimulated IL-10 production (Fig. 4a) was ascribable specifically to papLe22, two types of controls were included in each experiment. First, cells were concurrently stimulated by the fusion protein GST-papLe22 and by recombinant GST, prepared, and purified in parallel; second, in each experiment PBMC of LST-negative subjects were stimulated together with patient PBMC. In the experiment shown in Fig. 4, the stimulation index, defined as GST-papLe22-induced IL-10 production divided by GST-induced IL-10 production, reached 3.6 ± 0.15 and 5.3 ± 0.12 in supernatants collected after 2 and 6 days, respectively, of stimulation of patient PBMC, while it never exceeded 1.5 for control cells (not shown). These results indicate that in PBMC of VL patients IL-10 production was specifically induced by the immunogen papLe22. The level of GST-papLe22-induced IL-10 was comparable to (or even exceeded) those elicited by PHA or LPS (Fig. 4a). At all times, PBMC activation by total leishmania antigens resulted in low IL-10 production, in the range of 50 to 90 pg/ml (not shown).
No detectable IFN-γ production was induced by papLe22 in patient PBMC at any time (not shown). Figure 4b shows that total leishmania antigens were ineffective to stimulate IFN-γ in PBMC obtained at VL diagnosis but that the cells recovered their capacity for antigen-induced IFN-γ production after a successful therapy and that very large amounts of IFN-γ were stimulated by PHA at all times.
DISCUSSION
In this paper we report the cloning and characterization of a novel L. infantum immunogen. The 594-nucleotide ORF was identified after a classical screening of an L. infantum cDNA library with an acute-phase VL patient serum and an original application of RACE-PCR, which takes advantage of the 5′ sequence, called the miniexon, common to all Leishmania mRNA (19). The first 5′ ATG was chosen as the initiation codon, since no consensus Kozak sequence was found (31). The deduced amino acid sequence corresponded to a 22-kDa protein which did not exhibit homologies with sequences of known Leishmania proteins. Sequence homologies with a neuroendocrine-specific family of proteins from mammals (humans and rats) and chickens were found, mainly in the regions of amino acids 15 to 35 and 101 to 197 (63 conserved residues). A 120-base terminal region of the ORF (comprising nucleotides between 468 and 587) showed 58% identity with common sequences (but not at the protein level) of the hsp83 genes of Leishmania amazonensis and L. infantum and of the hsp90 gene of L. donovani.
The fusion protein with GST, termed GST-papLe22, was generated and was used to carry out a characterization of the parasite native protein and of immune responses elicited in patients with VL. A rabbit antiserum raised against GST-papLe22, in conjunction with SDS-PAGE, revealed a nuclear protein of 14-kDa apparent electrophoretic mobility, compatible with the percentage of hydrophobic residues in the recombinant papLe22. The antigen was found to be expressed in both amastigote and promastigote stages and was found to be present in promastigotes of at least three Leishmania species (L. infantum, L. major, and L. guyanensis). In promastigote preparations a weak reactivity of the rabbit anti-GST-papLe22 antiserum to an 18-kDa leishmanial fraction was observed, compatible with the cross-reactivity between 14- and 18-kDa leishmanial fractions that we described previously (56). PapLe22 cDNA could be amplified by PCR from promastigotes and amastigotes (not shown).
Sera obtained at diagnosis from several VL patients showed an IgM reactivity to the recombinant protein, indicating that the clinical manifestations of patent disease coincided with the primary response against the L. infantum protein corresponding to papLe22. This was the first observation suggesting that this parasite protein might be implicated in VL pathogenesis. Next, the IgG titers in the sera were correlated with the patients' clinical status: the high anti-papLe22 antibody response measured at the diagnosis decreased two- to threefold 1 month later (after a successful antimony therapy), and a low antibody response at the diagnosis in one patient was associated with mild VL symptoms. These features should now be confirmed with a larger number of patients, and in particular it has to be established if the antibody response to papLe22 might represent a tool for predicting relapses in patients coinfected with human immunodeficiency virus and Leishmania (2, 32). The presence of anti-papLe22 of IgG1, IgG2, and IgG3 subclasses suggested that the protein induced a mixed Th1- and Th2-type-specific immune response. However, there was no manifest correlation between the relative isotypes and the disease course for different patients.
In preliminary experiments exploring in vitro cellular responses of patients' PBMC we found that cell proliferation was slightly but consistently decreased in the presence of GST-papLe22. No detectable GST-papLe22-induced IL-4 production occurred (not shown), in line with the reported lack of elevation of IL-4 transcripts in bone marrow of acute VL patients (30). We then checked, by trypan blue exclusion, that the fusion protein did not induce an enhanced cell death, and next we turned our attention to IL-10. Indeed, IL-10 is a potent inhibitor of T cells (14) and deactivator of all known functions of macrophages. It suppresses the ability of LPS- and/or IFN-γ-activated macrophages to produce inflammatory cytokines (IL-1, IL-6, IL-8, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and granulocyte/macrophage colony-stimulating factor) and inhibits their microbicidal effectors (6, 13, 16, 20, 21, 38, 39, 43). Its contribution to the pathogenesis of VL has been well documented (5, 22, 27, 30, 45). Thus, elevated expression of IL-10 mRNA was evidenced in lymph nodes (22) and in bone marrow (30) of VL patients during acute stages of infection and was shown to be decreased after therapy. Similarly, high levels of IL-10 transcripts were found in lesions of diffuse cutaneous leishmaniasis; these levels decreased after treatment (7), again indicating that the presence of IL-10 might represent an indicator of an uncontrolled disease.
The recombinant protein papLe22 activated in vitro IL-10 production by VL patient PBMC, which were collected at the diagnosis and after the treatment-induced cure, again indicating that this parasite protein might be involved in Leishmania-induced pathogenesis. By its IL-10-inducing ability papLe22 could contribute to the poor proliferation and impaired IFN-γ production in VL patient PBMC in vitro (10, 26, 48), since it has been shown that IL-10 inhibits basal and antigen-induced proliferative responses (22) and that the addition of an anti-IL-10 monoclonal antibody restores proliferation and IFN-γ production (9, 22, 27). The ability of papLe22 to stimulate IL-10 was greater in postacute stages of the disease, possibly reflecting a deactivation of PBMC which were collected during the patent VL and/or a progressive development of papLe22-specific, IL-10-producing memory T cells. The latter could be related to a possible papLe22 contribution to the persistence of Leishmania within host cells, in agreement with the coincidence of persistent parasites and high levels of IL-10 transcripts, already reported in postkala-azar dermal leishmaniasis (22).
The correlation of patient anti-papLe22 antibodies with the clinical manifestations of the disease and the ability of papLe22 to stimulate IL-10, together with the inability to find anti-papLe22 antibodies in asymptomatic L. infantum carriers, show the aggravating potential of this new leishmanial antigen.
Finally, in agreement with previously published reports (10), PHA-induced production of IFN-γ was conserved in PBMC of VL patients, even at acute stages of the disease, and the IFN-γ response to leishmanial antigens, absent at diagnosis, was progressively recovered. In contrast to other cloned antigens of Leishmania, papLe22 did not generate a detectable IFN-γ production.
By its ability to generate an inappropriate T-cell response, papLe22 might contribute to the pathogenesis of the disease and to the parasite persistence after clinical recovery. In particular, in immunosuppressed conditions, its capacity to stimulate IL-10 could be one of the mechanisms leading to a reactivation of latent parasites (2, 32, 47). As one of the dominant immunogens of L. infantum, papLe22 could be used as a vaccine constituent, provided that the immune response is oriented toward the IFN-γ-producing Th1 cells, using an appropriate vaccination protocol. Such a successful cDNA vaccination against L. major was demonstrated in mice (23), using the antigen LACK, which naturally induces a polarized Th2 response (29). Experiments using papLe22 cDNA in an animal model are in progress in our laboratory.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Education and Research (EA2675), the Ministry of Defense (97-2511A), and by gifts from le Groupe d'Action Contre la Leishmaniose (GACL).
We thank Yves Le Fichoux for helpful discussions and critical reading of the manuscript, Carlos Alonso for the generous gift of cDNA libraries, Claude Minghelli and Aurore Grima for performing illustration work, and Georgette Pagliardini for administrative assistance.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Canavate C, Gutierrez-Solar B, Jimenez M, Laguna F, Lopez-Velez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacellar O, Brodskyn C, Guerreiro J, Barral-Netto M, Costa C H, Coffman R L, Johnson W D, Carvalho E M. Interleukin-12 restores interferon-γ production and cytotoxic responses in visceral leishmaniasis. J Infect Dis. 1996;173:1515–1518. doi: 10.1093/infdis/173.6.1515. [DOI] [PubMed] [Google Scholar]
- 4.Badaro R, Jones T C, Carvalho J M, Sampaio D, Reed S G, Barral A, Teixeira R, Johnson W D. New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 5.Barral-Netto M, Brodskyn C, Carvalho E M, Barral A. Human_leishmaniasis@cytokines.bahia.br. Braz J Med Biol Res. 1998;31:149–155. doi: 10.1590/s0100-879x1998000100021. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomfim G, Nascimiento C, Costa J, Carvalho E M, Barral-Netto M, Barral A. Variation of cytokine patterns related to therapeutic response in diffuse cutaneous leishmaniasis. Exp Parasitol. 1996;88:188–194. doi: 10.1006/expr.1996.0104. [DOI] [PubMed] [Google Scholar]
- 8.Campos-Neto A, Soong L, Cordova J L, Sant'Angelo D, Skeiky Y A W, Ruddle N H, Reed S G, Janeway C, McMahon-Pratt D. Cloning and expression of a Leishmania donovani gene instructed by a peptide isolated from major histocompatibility complex class II molecules of infected macrophages. J Exp Med. 1995;182:1423–1433. doi: 10.1084/jem.182.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho E M, Bacellar O, Brownell C, Regis T, Coffman R L, Reed S G. Restoration of IFN-γ production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–5956. [PubMed] [Google Scholar]
- 10.Carvalho E M, Badaro R, Reed S G, Jones T C, Johnson W D. Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Investig. 1985;76:2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho E M, Teixeira R S, Johnson W D. Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981;33:498–502. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe J S, Cooper H J, Smith M A, Sims M J, Parker D, Gewert D. Improved cloning efficiency of polymerase chain reaction (PCR) products after proteinase K digestion. Nucleic Acids Res. 1991;19:184. doi: 10.1093/nar/19.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunha F Q, Moncada S, Liew F W. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-γ in murine macrophages. Biochem Biophys Res Commun. 1992;182:1155–1159. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- 14.Del Prete G, De Carli M, Almerigogna F, Giudizi M G, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–360. [PubMed] [Google Scholar]
- 15.Desjeux P UNAIDS. Leishmania and HIV in gridlock. Division of Control of Tropical Diseases. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 16.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by human monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon D C, Day G H, Whittle J A, Magill A J, Reed S G. Characterization of a Leishmania tropica antigen that detects immune responses in Desert Storm viscerotropic leishmaniasis patients. Proc Natl Acad Sci USA. 1995;92:7981–7985. doi: 10.1073/pnas.92.17.7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elassad A M S, Younis S A, Siddig M, Grayson J, Petersen E, Ghalib H W. The significance of blood levels of IgM, IgA, IgG and IgG subclasses in Sudanese visceral leishmaniasis patients. Clin Exp Immunol. 1994;95:294–299. doi: 10.1111/j.1365-2249.1994.tb06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes O, Murthy V K, Kurath U, Degrave W M, Campbell D A. Mini-exon gene variation in human pathogenic Leishmania species. Mol Biochem Parasitol. 1994;66:261–271. doi: 10.1016/0166-6851(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 20.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 21.Gazzinelli R T, Oswald I P, James S L, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ-activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 22.Ghalib H W, Piuvezam M R, Skeiky Y A W, Siddig M, Hashim F A, El-Hassan A M, Russo D M, Reed S G. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Investig. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurunathan S, Sacks D L, Brown D R, Reiner S L, Charest H, Glaichenhaus N, Seder R A. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon-γ or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzel F P, Sadick M D, Mutha S S, Locksley R M. Production of interferon-γ, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holaday B J, de Lima Pompeu M M, Evans T, de Melo Braga D N, Texeira M J, de Queiroz Sousa A, Sadick M D, Vasconcelos A W, Abrams J S, Pearson R D, Locksley R M. Correlates of Leishmania-specific immunity in the clinical spectrum of infection with Leishmania chagasi. J Infect Dis. 1993;167:411–417. doi: 10.1093/infdis/167.2.411. [DOI] [PubMed] [Google Scholar]
- 27.Holaday B J, de Lima Pompeu M M, Jeronimo S, Texeira M J, de Queiroz Sousa A, Vasconcelos A W, Pearson R D, Abrams J S, Locksley R M. Potential role for interleukin-10 in the immunosuppression associated with kalar azar. J Clin Investig. 1993;92:2626–2632. doi: 10.1172/JCI116878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard M, O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 29.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 30.Karp C L, El-Safi S H, Wynn T A, Satti M M H, Kordofani A M, Hashim F A, Hag-Ali M, Neva F A, Nutman T B, Sacks D L. In vivo cytokine profiles in patients with kalar-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Investig. 1993;91:1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubar J, Marty P, Lelièvre A, Quaranta J F, Staccini P, Caroli-Bosc C, Le Fichoux Y. Visceral leishmaniasis in HIV-positive patients: primary infection, reactivation and latent infection. Impact of the CD4+ T-lymphocyte counts. AIDS. 1998;12:2147–2153. doi: 10.1097/00002030-199816000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Marty P, Lelièvre A, Quaranta J F, Rahal A, Gari-Toussaint M, Le Fichoux Y. Use of the leishmanin skin test and Western blot analysis for epidemiological studies in visceral leishmaniasis areas: experience in a highly endemic focus in Alpes-Maritimes (France) Trans R Soc Trop Med Hyg. 1994;88:658–659. doi: 10.1016/0035-9203(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 35.McMahon-Pratt D, Rodriguez D, Rodriguez J-R, Zhang Y, Manson K, Bergman C, Rivas L, Rodriguez J F, Lohman K L, Ruddle N H, Esteban M. Recombinant vaccinia viruses expressing GP46/M-2 protect against Leishmania infection. Infect Immun. 1993;61:3351–3359. doi: 10.1128/iai.61.8.3351-3359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millesimo M, Zucca M, Caramello P, Savoia D. Evaluation of the immune response in visceral leishmaniasis. Diagn Microbiol Infect Dis. 1996;26:7–11. doi: 10.1016/s0732-8893(96)00168-x. [DOI] [PubMed] [Google Scholar]
- 37.Murray H W, Rubin B Y, Rothermel C D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. J Clin Investig. 1983;72:1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oswald I P, Gazzinelli R T, Sher A, James S L. IL-10 synergizes with IL-4 and transforming growth factor-β to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 39.Oswald I P, Wynn T A, Sher A, James S L. IL-10 inhibits macrophage microbicidal activity by blocking the endogenous production of TNF-α required as a costimulatory factor for IFN-γ induced activation. Proc Natl Acad Sci USA. 1992;89:8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pampiglione S, Manson-Bahr P E C, La Placa M, Borgatti M A, Musumeci S. Studies in Mediterranean leishmaniasis. 3. The leishmanin skin test in kala-azar. Trans R Soc Trop Med Hyg. 1975;69:60–68. doi: 10.1016/0035-9203(75)90012-7. [DOI] [PubMed] [Google Scholar]
- 41.Pearson R D, de Queiroz Sousa A. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1995;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Quijada L, Requena J M, Soto M, Alonso C. During canine viscero-cutaneous leishmaniasis the anti-Hsp70 antibodies are specifically elicited by the parasite protein. Parasitologia. 1996;112:277–284. doi: 10.1017/s0031182000065793. [DOI] [PubMed] [Google Scholar]
- 43.Ralph P, Nakoinz I, Sampson-Johannes A, Fong S, Lowe D, Min H, Lin L. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992;148:808–814. [PubMed] [Google Scholar]
- 44.Reed S G, Shreffler W G, Burns J M, Scott J M, Orge M G, Ghalib H W, Siddig M, Badaro R. An improved serodiagnostic procedure for visceral leishmaniasis. Am J Trop Med Hyg. 1990;43:632–639. doi: 10.4269/ajtmh.1990.43.632. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro-de-Jesus A, Almeida R P, Lessa H, Bacellar O, Carvalho E M. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–148. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 46.Rousseau D, Le Fichoux Y, Stien X, Suffia I, Ferrua B, Kubar J. Progression of visceral lesihmaniasis due to Leishmania infantum in BALB/c mice is markedly slowed by prior infection with Trichinella spiralis. Infect Immun. 1997;65:4978–4983. doi: 10.1128/iai.65.12.4978-4983.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rousseau D, Suffia I, Ferrua B, Philip P, Le Fichoux Y, Kubar J. Prolonged administration of dexamethasone induces limited reactivation of visceral leishmaniasis in chronically infected BALB/c mice. Eur Cytokine Netw. 1998;9:655–661. [PubMed] [Google Scholar]
- 48.Sacks D L, Lal S L, Shrivastava S N, Blackwell J, Neva F A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987;138:908–913. [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Schnur L F, Jacobson R L. Parasitological techniques. In: Peters W, Killick-Kendrick R, editors. The leishmaniasis in biology and medicine. London, United Kingdom: Academic Press Ltd.; 1987. pp. 516–518. [Google Scholar]
- 51.Silva J S, Morrissey P J, Grabstein K H, Mohler K M, Anderson D, Reed S G. IL-10 and IFN-γ regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sjölander A, Baldwin T M, Curtis J M, Handman E. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J Immunol. 1998;160:3949–3957. [PubMed] [Google Scholar]
- 53.Skeiky Y A W, Benson D R, Guderian J A, Whittle J A, Bacelar O, Carvalho E M, Reed S G. Immune responses of leishmaniasis patients to heat shock proteins of Leishmania species and humans. Infect Immun. 1995;63:4105–4114. doi: 10.1128/iai.63.10.4105-4114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skeiky Y A W, Guderian J A, Benson D R, Bacelar O, Carvalho E M, Kubin M, Badaro R, Trinchieri G, Reed S G. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soto M, Requena J M, Gomez L C, Navarrete I, Alonso C. Molecular characterization of a Leishmania donovani infantum antigen identified as histone H2A. Eur J Biochem. 1992;205:211–216. doi: 10.1111/j.1432-1033.1992.tb16770.x. [DOI] [PubMed] [Google Scholar]
- 56.Suffia I, Quaranta J F, Eulalio M C M, Ferrua B, Marty P, Le Fichoux Y, Kubar J. Human T-cell activation by 14- and 18-kilodalton nuclear proteins of Leishmania infantum. Infect Immun. 1995;63:3765–3771. doi: 10.1128/iai.63.10.3765-3771.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vouldoukis I, Bécherel P A, Riveros-Moreno V, Arock M, da Silva O, Debré P, Mazier D, Mossalayi M D. Interleukin-10 and interleukin-4 inhibit intracellular killing of Leishmania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur J Immunol. 1997;27:860–865. doi: 10.1002/eji.1830270409. [DOI] [PubMed] [Google Scholar]
- 58.Webb J R, Campos-Neto A, Ovendale P J, Martin T I, Stromberg E J, Badaro R, Reed S G. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun. 1998;66:3279–3289. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webb J R, Kaufmann D, Campos-Neto A, Reed S G. Molecular cloning of a novel protein antigen of Leishmania major that elicits a potent immune response in experimental murine leishmaniasis. J Immunol. 1996;157:5034–5041. [PubMed] [Google Scholar]
- 60.Wilson M E, Young B M, Paetz Andersen K, Weinstock J V, Metwali A, Ali K M, Donelson J E. A recombinant Leishmania chagasi antigen that stimulates cellular immune responses in infected mice. Infect Immun. 1995;63:2062–2069. doi: 10.1128/iai.63.5.2062-2069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu D, Liew F Y. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology. 1995;84:173–176. [PMC free article] [PubMed] [Google Scholar]