Abstract
Streptococcus suis serotype 2 is a worldwide causative agent of many forms of swine infection and is also recognized as a zoonotic agent causing human disease, including meningitis. The pathogenesis of S. suis infections is poorly understood. Bacteria circulate in the bloodstream in the nonimmune host until they come in contact with brain microvascular endothelial cells (BMEC) forming the blood-brain barrier. The bacterial polysaccharide capsule confers antiphagocytic properties. It is known that group B streptococci (GBS) invade and damage BMEC, which may be a primary step in the pathogenesis of neonatal meningitis. Interactions between S. suis and human endothelial cells were studied to determine if they differ from those between GBS and endothelial cells. Invasion assays performed with BMEC and human umbilical vein endothelial cells demonstrated that unlike GBS, S. suis serotype 2 could not invade either type of cell. Adherence assays showed that S. suis adhered only to BMEC, whereas GBS adhered to both types of cell. These interactions were not affected by the presence of a capsule, since acapsular mutants from both bacterial species adhered similarly compared to the wild-type strains. Lactate dehydrogenase release measurements indicated that some S. suis strains were highly cytotoxic for BMEC, even more than GBS, whereas others were not toxic at all. Cell damage was related to suilysin (S. suis hemolysin) production, since only suilysin-producing strains were cytotoxic and cytotoxicity could be inhibited by cholesterol and antisuilysin antibodies. It is possible that hemolysin-positive S. suis strains use adherence and suilysin-induced BMEC injury, as opposed to direct cellular invasion, to proceed from the circulation to the central nervous system.
Streptococcus suis is a worldwide causative agent of many different swine diseases, such as meningitis, endocarditis, septicemia, and arthritis (21). Of the 35 official serotypes described to date for S. suis, serotype 2 is the most frequently isolated from diseased animals (21). This serotype is also recognized as a zoonotic agent since it has been identified as a cause of meningitis (2, 27), septicemia (15), and endocarditis (31, 49) in humans, particularly those occupationally exposed to pigs or pig products (2, 6, 31). Human survivors of S. suis infection often suffer sequelae such as arthritis or deafness (53).
The pathogenesis of S. suis infections is poorly understood. It has been recently demonstrated that the polysaccharide capsule, which is rich in sialic acid (8), confers antiphagocytic properties on S. suis. Acapsular mutants were readily phagocytosed by murine and porcine macrophages, unlike the wild-type strain (7, 38, 39). Furthermore, S. suis capsule is a virulence factor in the murine and porcine infection models since acapsular mutants were nonpathogenic and more rapidly cleared from the bloodstream than the wild-type strain (7). One hypothesis to explain S. suis-associated meningitis suggests that virulent strains are phagocytosed, survive in macrophages, and cross the blood-brain barrier (BBB) inside the phagocytes, whereas nonvirulent strains are destroyed once phagocytosed (56). Among the proposed extracellular factors is a hemolysin (named suilysin) belonging to the family of toxins known as antigenically related cholesterol-binding cytolytic toxins (20, 23). Antibodies against this hemolysin are protective (22). However, like those of the other proposed virulence factors, its role in pathogenesis remains to be determined.
Meningitis caused by S. suis is often preceded by a phase of bacteremia (2). Since the presence of the capsule inhibits phagocytosis, S. suis circulating in the bloodstream would come in contact with brain microvascular endothelial cells (BMEC), a single layer of specialized cells forming the BBB. This barrier, responsible for maintaining biochemical homeostasis within the central nervous system, is characterized by the presence of tight junctions and regulates fluid, macromolecule, and cell trafficking on both sides of the layer (4, 50). In order to cause meningitis, bacteria would have to pass between or through these cells to circumvent the BBB. Many meningeal pathogens are known to interact with BMEC, such as Streptococcus pneumoniae (34), Escherichia coli K1 (32) and group B Streptococcus (GBS) (30).
We studied the interactions between S. suis and endothelial cells to determine if they differ from GBS-endothelial cell interactions. Using immortalized BMEC and human umbilical vein endothelial cells (HUVEC), we tested the ability of S. suis to invade, adhere to, and damage these cells, compared to that of GBS.
MATERIALS AND METHODS
Bacterial strains and mutants.
Wild-type S. suis type 2 reference strain S735-SM and two isogenic derivatives, an acapsular mutant (2A [7]) and a weakly hemolytic mutant (C3P2E5 [M. Gottschalk, S. Lacouture, M. Lalonde, L. Odierno, M. Segura, and N. Charland, Proc. Int. Pig Vet. Soc., 15:86, 1998]) (each containing a single Tn916 insertion into the chromosome), were used. In addition, selected North American (89-1591 and 89-999) and European (S735-SM and 31533) type 2 isolates (24, 33) were studied. GBS type III strain COH1 and its isogenic acapsular mutant (COH1-13 [35]), were used for comparison. Streptococcus gordonii strain Challis was used as a noninvasive control (30). Bacteria were grown on blood agar plates, and single colonies were used as inocula in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) for growth to mid-log phase to an optical density at 600 nm (OD600) of 0.4 (∼108 CFU/ml).
Cell cultures. (i) BMEC.
BMEC originated from a brain biopsy of an adult human female with epilepsy. The cells, immortalized by transfection with simian virus 40 large T antigen (41, 42), maintained their morphologic and functional characteristics (43) and were grown as previously described (30). Briefly, cells from passage 19 were grown in RPMI 1640 medium (Gibco, Burlington, Ontario, Canada) supplemented with 10% (vol/vol) fetal bovine serum (Gibco), 10% (vol/vol) Nu-Serum IV supplement (Becton Dickinson, Bedford, Mass.), l-glutamine, and penicillin-streptomycin. Falcon flasks and 24- or 96-well tissue culture plates (Becton Dickinson) were precoated with rat tail collagen to support the cell monolayers.
(ii) HUVEC.
Immortalized HUVEC were purchased from the American Type Culture Collection (ATCC CRL-1730). Cells from passage 13 were grown in F-12K medium (Sigma, Oakville, Ontario, Canada) supplemented with 12% (vol/vol) fetal bovine serum, 50 ng of Endothelial Cell Growth Supplement (Becton Dickinson) per ml, and penicillin-streptomycin in Falcon flasks and tissue culture plates.
Both types of endothelial cells were incubated at 37°C in 5% CO2 in a humid atmosphere and split twice a week with trypsin-EDTA at a ratio of 1/8 (BMEC) or 1/3 (HUVEC).
Cells were used before passage 35 for all experiments. Prior to each assay, the monolayers were washed twice with Hanks balanced saline solution (Gibco) and fresh medium without antibiotics (adherence and invasion assays) or RPMI 1640 medium alone (BMEC cytotoxicity assay) was added.
Cell invasion assay.
The cell invasion assay was performed as previously described (30), with some modifications. Log-phase bacteria (∼108 CFU/ml) were pelleted, washed once with phosphate-buffered saline (PBS; 140 mM NaCl, 3 mM KCl, 10 mM NaH2PO4, 1.5 mM KH2PO4, pH 7.3), and resuspended in fresh cell culture medium without antibiotics. Dilutions in cell culture medium were performed such that inocula between 107 and 103 CFU were added to wells of a 24-well tissue culture plate containing a monolayer (∼106 cells) of endothelial cells (BMEC or HUVEC) in 0.5 ml of medium (multiplicity of infection of 10 to 0.001 bacteria/cell). The plates were centrifuged at 800 × g for 10 min to enhance the contact of bacteria with the surface of the monolayer. The plates were incubated for 2 h at 37°C in 5% CO2 to allow cell invasion by the bacteria. The monolayers were then washed three times with PBS, 1 ml of cell culture medium containing 100 μg of gentamicin per ml and 5 μg of penicillin G per ml was added to each well, and the plates were incubated for 2 h at 37°C in 5% CO2 to kill extracellular bacteria. The monolayers were washed three times with PBS, and cells were disrupted by the addition of 0.5 ml of sterile deionized water and repeated pipetting to liberate intracellular bacteria. One hundred microliters from each well was plated onto Todd-Hewitt agar and incubated overnight at 37°C. The percent invasion of endothelial cells was calculated as [5 × (CFU on plate count/CFU in original inoculum)] × 100%. Assays were performed in duplicate and repeated at least three times.
Adherence assay.
Percent adherence was determined by subtracting intracellular bacteria from total cell-associated (intracellular plus surface-adherent) bacteria. Total cell-associated bacteria were quantified as for the cellular invasion assay, only without the antibiotic exposure step. Cells were washed five times with PBS, lysis was performed as described above, and 100 μl-aliquots diluted 1:10 or 1:100 in PBS were used for quantitative plating. All assays were performed in duplicate and repeated at least three times.
Hemolysin purification.
In order to construct an affinity column to purify the hemolysin, sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed using the culture supernatant of strain 31533 (which produces large amounts of hemolysin) grown for 16 h in Todd-Hewitt broth produced by Oxoid (Unipath, Nepean; Ontario, Canada), a medium favoring hemolysin production. A 52-kDa band representing the hemolysin was excised, emulsified with Freund complete adjuvant, and injected into a New Zealand rabbit intramuscularly. The injection was repeated twice with Freund incomplete adjuvant. The rabbit was bled 2 weeks after the last inoculation, and the immunoglobulin G was purified with a protein A affinity column. The activity and specificity of the anti-hemolysin immunoglobulin G were confirmed by inhibition of the hemolytic activity and immunoblotting with supernatant of a hemolysin-positive strain (results not shown). An affinity column was constructed with the monospecific antibodies using the Pierce CarboLink Coupling Gel (Pierce, Rockford, Ill.), and the hemolysin was purified from culture supernatant of strain 31533 grown as described above. Hemolysin and monospecific antibody concentrations were determined by the method of Markwell et al. (26). The purity of the hemolysin preparation was verified by silver staining.
Cellular injury assays.
A microtiter plate assay to determine the cytolytic activity of S. suis was performed as previously described, with some modifications (30). Briefly, 108 CFU of log-phase bacteria were centrifuged, washed in PBS, and resuspended in 1 ml of RPMI 1640 medium without serum. A 100-μl aliquot of bacteria was added to the first well of a 96-well tissue culture plate containing a BMEC monolayer (∼5 × 104 cells; multiplicity of infection of 200 bacteria/cell), and serial twofold dilutions in RPMI 1640 medium were performed across the plate. Noninfected cells and bacteria in RPMI 1640 medium without a BMEC monolayer were used as negative controls, whereas cells lysed with 100 μl of sterile deionized water were used as a positive control. The plate was incubated at 37°C in 5% CO2 for 4 h in most experiments. At the end of the incubation period, a 75-μl aliquot of each supernatant was transferred to a replica plate which was centrifuged at 3,000 × g for 20 min to pellet bacteria. Lactate dehydrogenase (LDH) measurement was performed on 20-μl aliquots of each centrifuged supernatant using a miniaturized version of the Sigma colorimetric assay as previously described (29). Percent cytotoxicity was calculated as [(OD0% − OD100%) − (ODbacteria − OD100%)/(OD0% − OD100%)] × 100, where OD0% represents the OD414 of noninfected cells and OD100% represents the OD414 of lysed cells (adapted from reference 14). To determine if cytolytic components were produced extracellularly, culture supernatants of late-log-phase bacteria were recovered by centrifugation at 3,000 × g for 10 min and filtration through 0.22-μm-pore-size filters. Supernatants were kept at −80°C until use for LDH measurement. The assay was also performed with purified hemolysin. All of the assays were performed in duplicate and repeated at least three times.
Inhibition of cytolytic activity.
Inhibition of cytotoxicity by heat was performed by heat killing log-phase bacteria (∼108 CFU) by placing them in a 60°C waterbath for 45 min (minimal killing time and temperature as determined in our laboratory) before adding them to BMEC monolayers for LDH measurement. Inhibition of cytotoxicity by cholesterol was measured by incubating log-phase bacteria in increasing concentrations of ethanol-soluble cholesterol (Sigma) for 1 h at 37°C before adding them to BMEC monolayers. Noninfected cells with cholesterol in culture medium were used as a negative control. Inhibition of cytotoxicity by monospecific antibodies (specificity of antibodies was demonstrated by culture supernatant immunoblotting) was performed by mixing increasing concentrations of antihemolysin antibodies with log-phase bacteria and incubating them for 1 h at 37°C before adding them to BMEC monolayers. An anti-Actinobacillus pleuropneumoniae lipopolysaccharide monospecific antibody was used as a negative control.
Electron microscopic studies.
To monolayers of BMEC in 24-well tissue culture plates, 107 CFU of log-phase S. suis were added in culture medium, centrifuged at 800 × g for 10 min, and then incubated at 37°C in 5% CO2 for 30 min, 2 h, and 4 h. The supernatants were removed by gentle aspiration, the cells were washed once with Hanks balanced saline solution, and the monolayers were fixed for 1 h at room temperature with 3% glutaraldehyde in 0.1 M phosphate buffer (0.1 M Na2HPO4, 0.1 M NaH2PO4, pH 7.3) and then postfixed for 1 h at room temperature in potassium ferrocyanide-reduced osmium tetroxide (28). Specimens were dehydrated in graded alcohols and processed for embedding in Epon. Ultrathin sections were placed on Formvar-coated nickel grids, stained with uranyl acetate and lead citrate, and then examined by transmission electron microscopy using a JEM1200 EX II operated at 60 kV.
Statistics.
All data are expressed as means ± standard deviations (error bars). Data were analyzed by two-tailed, unpaired t test. A P value of <0.05 was considered significant.
RESULTS
S. suis does not invade endothelial cells.
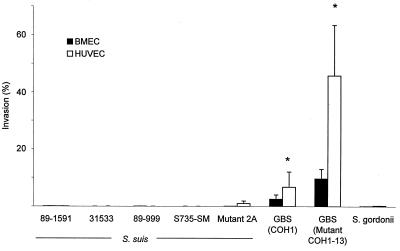
Before using the standard conditions for comparative assays (105 CFU, 2-h invasion time), we tested many different variables in order to find experimental conditions that promote S. suis invasion. Different invasion times, cell confluences, inocula, stationary and logarithmic growth phases, and serum concentrations were tested, but none induced S. suis invasion. S. suis did not invade confluent cells from the apical surface, nor did it invade subconfluent cells from the basolateral surface (results not shown). Results were comparable to those obtained with the noninvasive S. gordonii control regardless of the cell type (Fig. 1). The S. suis acapsular mutant was also noninvasive. As a comparison, GBS invaded both types of cells. The acapsular GBS mutant COH1-13 exhibited significantly greater invasiveness than wild-type strain COH1 (P < 0.05; Fig. 1).
FIG. 1.
Invasion of BMEC and HUVEC by S. suis 89-1591, 31533, 89-999, and S735-SM and acapsular mutant 2A and GBS strain COH1 and acapsular mutant COH1-13. S. gordonii was used as a noninvasive control. ∗, P < 0.05 versus wild-type strain COH1. Results were determined after a 2-h exposure with 100-μl aliquots of 106 CFU/ml, followed by an additional 2-h incubation in the presence of penicillin-gentamicin to kill extracellular bacteria, and BMEC lysis to retrieve 100-μl aliquots of intracellular bacteria for viable plate counts.
S. suis adheres to BMEC.
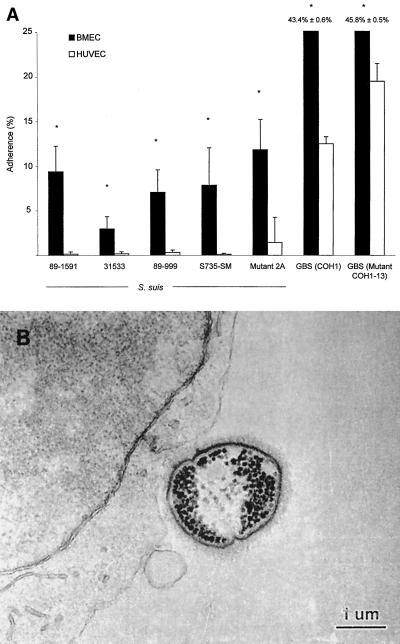
Although S. suis does not invade endothelial cells, it appears that this bacterium has a tropism for BMEC. Figure 2A shows a significantly greater adherence of all S. suis strains to BMEC than to HUVEC (P < 0.05). Adherence of S. suis type 2 to BMEC is illustrated in Fig. 2B. GBS adherence was similar for both the encapsulated strain and the nonencapsulated mutant (Fig. 2A). Since no invasion was detected for S. suis, total cell-associated bacteria represent adherent organisms. Adherence levels were similar when assays were performed at 4°C, indicating that de novo protein synthesis was not required for active BMEC binding by S. suis (data not shown).
FIG. 2.
(A) Adherence to BMEC and HUVEC by S. suis 89-1591, 31533, 89-999, and S735-SM and acapsular mutant 2A and GBS strain COH1 and acapsular mutant COH1-13. ∗, P < 0.05 versus adherence to HUVEC. Results were determined after a 2-h exposure with 100-μl aliquots of 106 CFU/ml, followed by extensive washing of nonadherent bacteria and BMEC lysis to retrieve 100-μl aliquots of total cell-associated bacteria for viable plate counts. (B) Transmission electron micrograph showing S. suis adherent to BMEC.
Effect of S. suis polysaccharide capsule on adherence.
The presence of the capsule did not seem to influence S. suis or GBS adherence to BMEC. The adherence levels of the acapsular S. suis mutant 2A and GBS COH1-13 were similar to those of wild-type strains S735-SM and GBS COH1, respectively (Fig. 2; P > 0.05).
Some S. suis strains can damage BMEC.
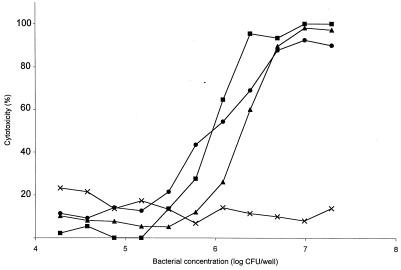
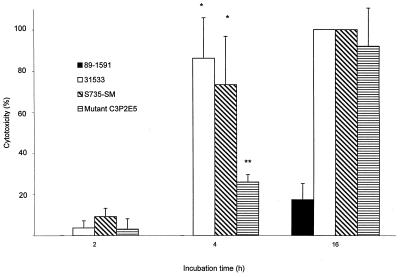
LDH release measurements were performed to determine if S. suis could be cytotoxic to BMEC. Figure 3 shows that while some S. suis isolates did not injure BMEC at all, others were highly cytotoxic, even more than GBS (P < 0.05). Almost 100% cell lysis was noted for strains S735-SM and 31533, whereas 55% cell lysis was obtained for GBS at the same bacterial concentration (107 CFU per well). Cytotoxicity was directly proportional to bacterial concentration. The quantity of bacteria used for invasion and adherence assays (105 CFU per well) was not toxic to BMEC. Indeed, little cytotoxicity was recorded for isolates after a 2-h incubation period, the incubation time used for invasion-adherence assays (Fig. 4). Cell lysis by cytotoxic S. suis strains increased over time and always remained higher than that due to nontoxic isolates (Fig. 4; P < 0.05). Interestingly, all of the cytotoxic strains produce suilysin. Injury assays with a weakly hemolytic S. suis mutant demonstrated that the mutant was less toxic than the wild-type strain until the bacterial concentrations reached about 107 CFU per well, at which point their cytotoxicities became comparable (Fig. 3). Similar results were obtained over time, as cytotoxicity of the mutant was lower than that of the wild-type strain until the incubation time was extended to 16 h (Fig. 4).
FIG. 3.
Effect of S. suis concentration on BMEC injury. Symbols: ●, hemolytic strain 31533; ■, hemolytic strain S735-SM (wild-type strain); ▴, weakly hemolytic mutant C3P2E5; ×: nonhemolytic strain 89-1591. BMEC cytotoxicity was determined by measuring LDH release in the presence of different concentrations of S. suis strains after a 4-h incubation. Results of a representative experiment are shown.
FIG. 4.
Effect of time of incubation with S. suis hemolytic strains 31533 and S735-SM (wild type strain), weakly hemolytic mutant C3P2E5, and nonhemolytic strain 89-1591 on BMEC injury. ∗, P < 0.05 versus 2-h incubation; ∗∗, P < 0.05 versus S735-SM (4 h). BMEC cytotoxicity was determined by measuring LDH release in the presence of 100-μl aliquots of 108 CFU/ml after different incubation times.
Suilysin-associated injury to BMEC.
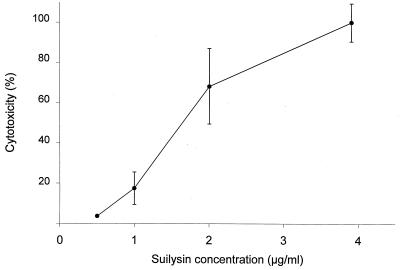
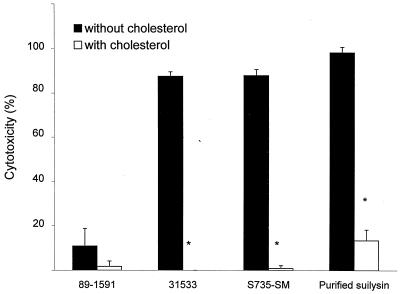
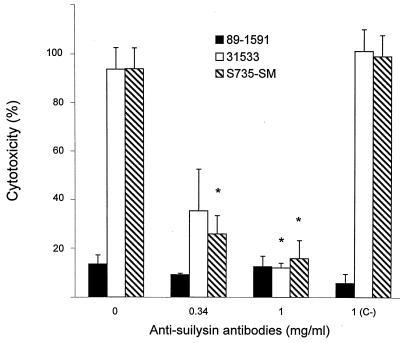
It is known that suilysin is excreted in vitro in culture supernatants (20). Addition of the culture supernatant from suilysin-producing strain 31533 to BMEC monolayers induced cell injury, whereas the culture supernatant of non-suilysin-producing strain 89-1591 did not affect the cells (Table 1). Moreover, purified suilysin injured BMEC in a concentration-dependent manner, reaching a peak at ∼4 μg of purified toxin per ml (Fig. 5). Experiments were performed to determine which treatment(s) could inhibit cytolytic activity and to confirm the involvement of suilysin in cell injury. First, to determine if cytotoxicity requires live bacteria, strain 31533 was heat killed and the suspension was added to a BMEC monolayer. Results showed that only live bacteria induced BMEC injury (Table 1). Cholesterol can inhibit suilysin activity (20). Thus, bacteria were mixed with increasing cholesterol concentrations and added to BMEC monolayers. Figure 6 shows that at 2 mg/ml, cholesterol completely inhibited the cytolytic activity of suilysin-producing strains, while 100 μg/ml was sufficient to significantly inhibit purified suilysin toxicity (P < 0.05). It is known that antihemolysin antibodies can inhibit suilysin hemolytic activity (20). Figure 7 shows that increasing concentrations of antisuilysin antibodies, but not a nonrelated control antibody, inhibited suilysin cytotoxicity from a hemolysin-producing strain.
TABLE 1.
Cytotoxicity of viable S. suis or of its culture supernatant to BMEC as determined by measurement of LDH release
| Treatment | Hemolysin productiona | % Cytotoxicityb |
|---|---|---|
| Strain 31533 overnight culture supernatant | Yes | 73.0 ± 9.5 |
| Strain 89-1591 overnight culture supernatant | No | 0.0 ± 0.0c |
| Live strain 31533 | Yes | 98.5 ± 2.6 |
| Heat-killed strain 31533 | No | 0.0 ± 0.0c |
| Live strain 89-1591 | No | 0.0 ± 0.0c |
Determined by red-cell lysis assay (22).
Values are means ± standard deviations (at least three samples per group).
Significantly different (P < 0.05) from the value for nontreated suilysin-producing strain 31533 or for its culture supernatant, as calculated by Student's t test.
FIG. 5.
Effect of increasing concentrations of purified suilysin on BMEC injury. BMEC cytotoxicity was determined by measuring LDH release in the presence of different concentrations of purified suilysin after a 4-h incubation.
FIG. 6.
Inhibition by cholesterol of S. suis cytotoxicity for BMEC. ∗, P < 0.05 versus no inhibitor. We incubated 108 CFU/ml or purified suilysin at 4.6 μg/ml in the absence and presence of 2 mg (bacteria) or 100 μg (suilysin) of cholesterol per ml for 1 h at 37°C before incubation of 100-μl aliquots with BMEC for 4 h. BMEC cytotoxicity was determined by measuring LDH release.
FIG. 7.
Inhibition by antisuilysin antibodies of S. suis cytotoxicity for BMEC. ∗, P < 0.05 versus the no-antibody or nonspecific antilipopolysaccharide antibody (C−) control. We incubated 108 CFU/ml in the presence of increasing antibody concentrations for 1 h at 37°C before incubation of 100-μl aliquots with BMEC for 4 h. BMEC cytotoxicity was determined by measuring LDH release.
Electron microscopy.
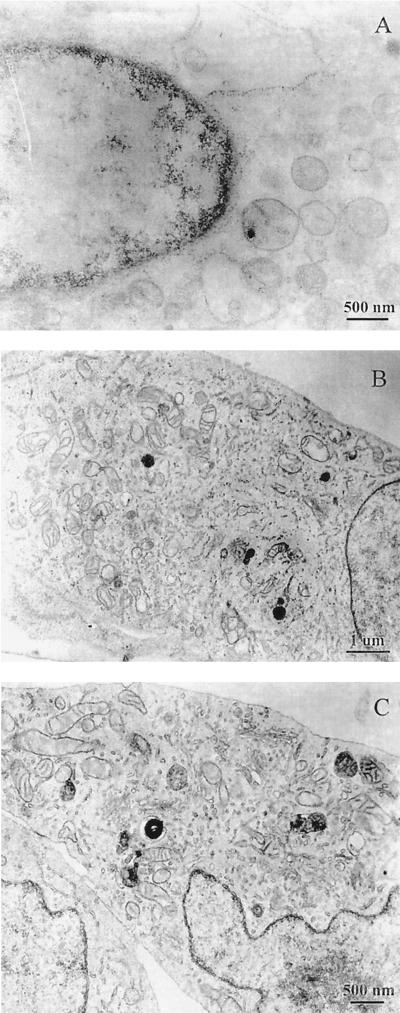
Figure 8 confirms the cytotoxicity data obtained with LDH measurements. The cytotoxicity of suilysin-producing strain 31533 increased with time, reaching a peak at 4 h (data not shown), whereas non-suilysin-producing strain 89-1591 did not affect cell integrity. After a 2-h exposure, normal cell characteristics (similar to those seen in uninfected cells; Fig. 8C), such as dense regular cytoplasmic contents, few pinocytic vesicles, and evenly distributed nuclear chromatin, were seen with strain 89-1591 (Fig. 8B). On the other hand, exposure of BMEC to strain 31533 resulted in cellular injury demonstrated by loss of cytoplasmic density, discontinuity of cytoplasmic membranes, and clumping of nuclear chromatin (Fig. 8A).
FIG. 8.
Transmission electron micrographs demonstrating BMEC injury caused by S. suis at 108 CFU/ml. Panels: A, suilysin-producing strain 31533; B, non-suilysin-producing strain 89-1591; C, no-bacterium control. BMEC integrity after a 2-h incubation with strain 89-1591 (B) was comparable to that of the no-bacterium control (C).
DISCUSSION
S. suis is an important swine pathogen that causes significant economic losses to producers. In addition, these bacteria represent a health risk for those involved in the pig industry. Comparatively, GBS is an important human pathogen associated with meningitis. Serotype III strains are implicated in most cases of meningitis (3). GBS and S. suis share many characteristics, and since they produce similar diseases, their pathogeneses have often been compared. Like S. suis, GBS possesses a sialic acid-containing capsule and produces a hemolysin, and different virulence potentials are observed among strains (3, 25, 54). However, although the two species present similar characteristics, the pathogeneses of the infections caused by the two bacteria may be different (38). It is known that GBS can invade BMEC and that GBS hemolysin can injure lung and brain endothelial cells (18, 30). These events may be primary steps in the pathogenesis of GBS meningitis (30). GBS capsule itself attenuates endothelial cell invasion (17, 30). In contrast, the means by which S. suis may proceed from the circulation to the central nervous system are unknown. Understanding these mechanisms is important in unraveling S. suis pathogenesis in order to develop strategies for prevention or therapy.
S. suis did not invade endothelial cells under the conditions tested in this study, whereas GBS easily entered the cells. Many different variables were tested in order to find combinations that could favor invasion by S. suis; however, none induced invasion. Our results strongly suggest that entry of S. suis into the central nervous system follows a pattern different from pathogens such as GBS, E. coli K1, and S. pneumoniae, which invade from the apical surface (30, 32, 34), or shigellae, which invade from the basolateral surface (10). It remains possible that S. suis could cross the BBB by invading intercellular junctions of BMEC monolayers. This mechanism of invasion, which was described for the spirochetes Treponema pallidum and Borrelia burgdorferi (44, 47), remains to be studied for S. suis.
In agreement with previous reports (17, 30), the GBS capsule attenuated invasion. However, in this study, the presence of a capsule did not interfere with GBS and S. suis endothelial cell adherence, since both the wild-type and acapsular mutant strains bound similarly. Similar results have been obtained with pneumococci (16). St. Geme and Cutter suggested that encapsulation may be modulated depending on the infectious stage (40). Encapsulation may be down-regulated during colonization of epithelial cells (lungs, nasopharynx), and once the bacteria are in the bloodstream, up-regulation of capsule production protects them against the immune system. It was shown that S. suis capsule expression may vary with growth conditions (19). In addition, the presence of the capsule seems to restrict access to S. suis putative adhesins, limiting hemagglutination activity (48). Interestingly, capsule-attenuated adherence to lung epithelial cells has been reported for GBS (45) and the capsule also seems to significantly interfere with S. suis adhesion to epithelial cells (unpublished observations; 55). To date however, there is no direct evidence of such encapsulation modulation for either GBS or S. suis.
Despite the observation that S. suis does not invade endothelial cells, it does adhere to these cells, especially BMEC. As mentioned before, the presence of the capsule does not seem to interfere with adhesion. Interestingly, E. coli K1 also interacts with BMEC preferentially to HUVEC (32). It is possible that the preferential adherence of S. suis to BMEC has a role in the pathogenesis of infection. For example, it may be hypothesized that, after adherence of S. suis to BMEC, bacteria can secrete toxic factors which would affect the endothelial cells. Such factors could increase BBB permeability, which could lead to the development of cerebral edema, increased intracranial pressure, and cerebral blood flow blockage characteristic of bacterial meningitis (46). It was recently proposed that damage to BMEC by GBS hemolysin could contribute to increased BBB permeability (30). In the case of S. suis, many lines of evidence implicate suilysin as the bacterial component responsible for in vitro BMEC cytotoxicity. First, only suilysin-producing strains are toxic for BMEC and a weakly hemolytic S. suis mutant exhibited decreased toxicity. The observed toxicity of this mutant with longer exposures and increasing bacterial concentrations correlates with the proposed mode of action of suilysin, namely, a multihit activity (1, 20). Hence, the accumulation of suilysin molecules at the BMEC surface may have resulted in toxicity equal to that of the wild-type strain as the incubation time and bacterial concentrations increased. Second, the BMEC-cytotoxic component is present in culture supernatants. It is known that suilysin is excreted during bacterial growth (20, 23). Third, the purified suilysin is toxic to BMEC. Fourth, cholesterol inhibits BMEC cytotoxicity as it inhibits suilysin lysis of erythrocytes (20, 23). Cholesterol is required for the binding of the toxin to cell membranes, and free cholesterol acts as a competitive inhibitor (5). Finally, antisuilysin antibodies also inhibit the BMEC cytotoxicity of suilysin-producing strains, providing further evidence that suilysin was responsible for the cellular injury. Pneumolysin, a toxin with strong sequence homology to suilysin (37), is also known to damage endothelial cells (36).
While suilysin is implicated as an important virulence factor in European S. suis type 2 strains, the same does not seem to be the case for North American strains. In fact, unlike European strains, most virulent field strains isolated from diseased pigs or humans in the United States and Canada do not produce suilysin (9, 37); Gottschalk et al., Proc. Int. Pig Vet. Soc.; 1998). In this study, the hemolysin-negative virulent strain 89-1591 showed significant adherence to BMEC but did not exhibit any detectable toxicity to these cells. It is possible that the pathogenesis of the infection caused by suilysin-positive strains and that of the infection caused by suilysin-negative strains are different and that different virulence factors are involved in each case. It has been reported that adherence of Neisseria meningitidis and pneumococci to endothelial cells results in widening or disruption of intercellular junctions, effects not related to the production of cytotoxins (16, 52). However, no BMEC morphological changes were observed after adherence of the hemolysin-negative strain of S. suis.
It is possible that the tropism of hemolysin-negative strains for BMEC has consequences other than direct damage to the cells. One mechanism may be the stimulation of cytokine production through bacterial adherence with resultant recruitment of inflammatory cells and/or alteration of BBB permeability. It has been shown that E. coli adherence is required for interleukin-6 induction from epithelial cells and that oral viridans group streptococcal adhesins and pneumococcal attachment initiate the release of various cytokines from endothelial and epithelial cells (12, 16, 51). S. pneumoniae invasion of unstimulated endothelial cells is low (less than 0.1%), whereas cytokine activation increased invasion levels to 2 to 3% (11). In addition, the release of cytokines from BMEC could lead to increased BBB permeability. In fact, it was recently demonstrated that tumor necrosis factor alpha opens a paracellular route for human immunodeficiency virus type 1 through the BBB (13). Preliminary studies in our laboratory indicate that BMEC stimulated with S. suis release more than 200 pg of interleukin-6 per ml, as measured by immunoassays (unpublished results). However, the roles of this and other cytokines in the pathogenesis of S. suis infection remain to be studied.
S. suis and GBS host cell interactions appear to differ in important ways. For example, S. suis and GBS interact differently with macrophages, and these interactions imply different virulence roles for capsular sialic acid and the whole capsule (8, 38). Whereas S. suis capsule prevents nonopsonic phagocytosis, in contrast to an acapsular mutant, the presence of capsular polysaccharides on GBS did not block phagocytosis under the same experimental conditions (38). Instead, intracellular survival of encapsulated GBS and of its isogenic acapsular mutant was higher than that of S. suis strains (38). The results described herein give additional evidence that pathogenesis of the infection differs between S. suis and GBS. In particular, it is possible that hemolysin-positive S. suis strains use adherence and suilysin-induced BMEC injury, as opposed to direct cellular invasion, to proceed from the circulation to the central nervous system. Since part of the toxic effect of any toxic molecule on the luminal side of the endothelial cells may be partially washed away by circulation through the cerebral vascular bed, in vivo investigation is necessary to further elucidate the pathogenesis of S. suis meningitis.
ACKNOWLEDGMENTS
We thank A. Nanci and D. Montpetit for the transmission electron micrographs and M. Lalonde and D. Nguyen for invaluable technical assistance.
This work was supported by grant 0GP0154280 from the Natural Sciences and Engineering Research Council of Canada (M.G.), grant 98-NC-1037 from the Fonds pour la Formation des Chercheurs et l'Aide à la Recherche (M.G.), and grants RO-1 NS 26310 (K.S.K.) and HD37224 (V.N.) from the National Institutes of Health. N.C. is the recipient of an NSERC scholarship.
REFERENCES
- 1.Alouf J E, Geoffroy C. The family of the antigenically-related cholesterol-binding (“sulphydryl-activated”) cytolytic toxins. In: Alouf J E, editor. Sourcebook of bacterial protein toxins. New York, N.Y: Academic Press, Inc.; 1991. pp. 147–186. [Google Scholar]
- 2.Arends J P, Zanen H C. Meningitis caused by Streptococcus suis in humans. J Infect Dis. 1988;10:131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- 3.Baker C J, Edwards M S. Group B streptococcal infections. In: Klein J S, Klein J O, editors. Infectious diseases of the fetus and the newborn. Philadelphia, Pa: The W. B. Saunders Co.; 1990. pp. 742–811. [Google Scholar]
- 4.Betz A L, Goldstein G W. Specialized properties and solute transport in brain capillaries. Annu Rev Physiol. 1986;48:241–250. doi: 10.1146/annurev.ph.48.030186.001325. [DOI] [PubMed] [Google Scholar]
- 5.Boulnois G J, Paton J C, Mitchell T J, Andrew P W. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2611–2616. doi: 10.1111/j.1365-2958.1991.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 6.Büngener W, Bialek R. Fatal Streptococcus suis septicemia in an abattoir worker. Eur J Clin Microbiol Infect Dis. 1989;8:306–308. doi: 10.1007/BF01963457. [DOI] [PubMed] [Google Scholar]
- 7.Charland N, Harel J, Kobisch M, Lacasse S, Gottschalk M. Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology. 1998;144:325–332. doi: 10.1099/00221287-144-2-325. [DOI] [PubMed] [Google Scholar]
- 8.Charland N, Kobisch M, Martineau-Doizé B, Jacques M, Gottschalk M. Role of capsular sialic acid in virulence and resistance to phagocytosis of Streptococcus suis capsular type 2. FEMS Immunol Med Microbiol. 1996;14:195–203. doi: 10.1111/j.1574-695X.1996.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 9.Chatellier S, Gottschalk M, Higgins R, Brousseau R, Harel J. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J Clin Microbiol. 1999;37:362–366. doi: 10.1128/jcm.37.2.362-366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clerc P, Baudry B, Sansonetti P J. Molecular mechanisms of entry, intracellular multiplication and killing of host cells by shigellae. Curr Top Microbiol Immunol. 1988;138:3–13. [PubMed] [Google Scholar]
- 11.Cundell D R, Gerard N P, Gerard C, Idänpään-Heikkilä I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 12.de Man P, van Kooten C, Aarden L, Engberg I, Linder H, Svanborg-Eden C. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989;57:3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiala M, Looney D J, Stins M, Way D D, Zhang L, Gan X, Chiappelli F, Schweitzer E S, Shapshak P, Weinand M, Graves M C, Witte M, Kim K S. TNF-α opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- 14.Flick D A, Gifford G E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984;68:167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- 15.François B, Gissot V, Ploy M C, Vignon P. Recurrent septic shock due to Streptococcus suis. J Clin Microbiol. 1998;36:2395. doi: 10.1128/jcm.36.8.2395-2395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geelen S, Bhattacharyya C, Tuomanen E. The cell wall mediates pneumococcal attachment to and cytopathology in human endothelial cells. Infect Immun. 1993;61:1538–1543. doi: 10.1128/iai.61.4.1538-1543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson R L, Lee M K, Soderland C, Chi E Y, Rubens C E. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect Immun. 1993;61:478–485. doi: 10.1128/iai.61.2.478-485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson R L, Nizet V, Rubens C E. Group B streptococcal β-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr Res. 1999;45:626–634. doi: 10.1203/00006450-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Gottschalk M, Higgins R, Jacques M. Production of capsular material by Streptococcus suis serotype 2 under different growth conditions. Can J Vet Res. 1993;57:49–52. [PMC free article] [PubMed] [Google Scholar]
- 20.Gottschalk M, Lacouture S, Dubreuil J D. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology. 1995;141:189–195. doi: 10.1099/00221287-141-1-189. [DOI] [PubMed] [Google Scholar]
- 21.Higgins R, Gottschalk M. Streptococcal diseases. In: Leman A D, Straw B E, Mengeling W L, D'Allaire S, Taylor D J, editors. Diseases of swine. 8th ed. Ames: Iowa State University Press; 1999. pp. 563–570. [Google Scholar]
- 22.Jacobs A A, Van Den Berg A J, Loeffen P L. Protection of experimentally infected pigs by suilysin, the thiol-activated haemolysin of Streptococcus suis. Vet Rec. 1996;139:225–228. doi: 10.1136/vr.139.10.225. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs A A C, Loeffen P L W, van den Berg A J G, Storm P K. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect Immun. 1994;62:1742–1748. doi: 10.1093/benz/9780199773787.article.b00034458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobisch M, Gottschalk M, Morvan P, Cariolet R, Bénévent G, Joly J P. Experimental infection of piglets by Streptococcus suis serotype 2. J Rech Porc France. 1995;27:97–102. [Google Scholar]
- 25.Marchlewicz B A, Duncan J L. Properties of a hemolysin produced by group B streptococci. Infect Immun. 1980;30:805–813. doi: 10.1128/iai.30.3.805-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markwell M A K, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 27.Michaud S, Duperval R, Higgins R. Streptococcus suis meningitis: first case reported in Québec. Can J Infect Dis. 1996;7:329–331. doi: 10.1155/1996/354693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neiss W F. Electron staining of the cell surface coat by osmium-low ferrocyanide. Histochemistry. 1984;80:231–242. doi: 10.1007/BF00495771. [DOI] [PubMed] [Google Scholar]
- 29.Nizet V, Gibson R L, Chi E Y, Framson P E, Hulse M, Rubens C E. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun. 1996;64:3818–3826. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nizet V, Kim K S, Stins M, Jonas M, Chi E Y, Nguyen D, Rubens C E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peetermans W E C, Moffie B G, Thompson J. Bacterial endocarditis caused by Streptococcus suis type 2. J Infect Dis. 1989;159:595–596. doi: 10.1093/infdis/159.3.595. [DOI] [PubMed] [Google Scholar]
- 32.Prasadarao N V, Wass C A, Kim K S. Endothelial cell GlcNAcβ1-4GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood-brain barrier. Infect Immun. 1996;64:154–160. doi: 10.1128/iai.64.1.154-160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quessy S, Dubreuil D, Caya M, Higgins R. Discrimination of virulent and avirulent Streptococcus suis capsular type 2 isolates from different geographical origins. Infect Immun. 1995;63:1975–1979. doi: 10.1128/iai.63.5.1975-1979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ring A, Weiser J N, Tuomanen E I. Pneumococcal trafficking across the blood-brain barrier. J Clin Investig. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubens C E, Heggen L M, Haft R F, Wessels M R. Identification of cspD, a gene essential for type III capsule expression in group B streptococci. Mol Microbiol. 1993;8:843–855. doi: 10.1111/j.1365-2958.1993.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 36.Rubins J B, Duane P G, Charboneau D, Janoff E N. Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect Immun. 1992;60:1740–1746. doi: 10.1128/iai.60.5.1740-1746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segers R P, Kenter T, de Haan L A, Jacobs A A. Characterization of the gene encoding suilysin from Streptococcus suis and expression in field strains. FEMS Microbiol Lett. 1998;167:255–261. doi: 10.1111/j.1574-6968.1998.tb13236.x. [DOI] [PubMed] [Google Scholar]
- 38.Segura M A, Cléroux P, Gottschalk M. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol Med Microbiol. 1998;21:189–195. doi: 10.1111/j.1574-695X.1998.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 39.Smith H E, Damman M, van der Velde J, Wagenaar F, Wisselink H J, Stockhofe-Zurwieden N, Smits M A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–1756. doi: 10.1128/iai.67.4.1750-1756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St. Geme J W, III, Cutter D. Influence of pili, fibrils, and capsule on in vitro adherence by Haemophilus influenzae type b. Mol Microbiol. 1996;21:21–31. doi: 10.1046/j.1365-2958.1996.6241331.x. [DOI] [PubMed] [Google Scholar]
- 41.Stins M F, Gilles F, Kim K S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 42.Stins M F, Prasadarao N V, Ibric I, Wass C A, Kim K S. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am J Pathol. 1994;145:1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 43.Stins M F, Prasadarao N V, Zhou J, Arditi M, Kim K S. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell Dev Biol. 1997;33:243–247. doi: 10.1007/s11626-997-0042-1. [DOI] [PubMed] [Google Scholar]
- 44.Szczepanski A, Furie M, Benach J, Lane B, Fleit H. Interactions between Borrelia burgdorferi and endothelium in vitro. J Clin Investig. 1990;85:1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura G S, Kuypers J M, Smith S, Raff H, Rubens C E. Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect Immun. 1994;62:2450–2458. doi: 10.1128/iai.62.6.2450-2458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tauber M G. Brain edema, intracranial pressure and cerebral blood flow in bacterial meningitis. Pediatr Infect Dis. 1989;8:915–917. doi: 10.1097/00006454-198912000-00042. [DOI] [PubMed] [Google Scholar]
- 47.Thomas D D, Navab M, Haake D A, Fogelman A M, Miller J N, Lovett M A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci USA. 1988;85:3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tikkanen K, Haataja S, Finne J. The galactosyl-(α1-4)-galactose-binding adhesin of Streptococcus suis: occurrence in strains of different hemagglutination activities and induction of opsonic antibodies. Infect Immun. 1996;64:3659–3665. doi: 10.1128/iai.64.9.3659-3665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trottier S, Higgins R, Brochu G, Gottschalk M. A case of human endocarditis due to Streptococcus suis in North America. Rev Infect Dis. 1991;13:1251–1252. doi: 10.1093/clinids/13.6.1251. [DOI] [PubMed] [Google Scholar]
- 50.Tuomanen E. Entry of pathogens into the central nervous system. FEMS Microbiol Rev. 1996;18:289–299. doi: 10.1111/j.1574-6976.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 51.Vernier A, Diab M, Soell M, Haan-Archipoff G, Beretz A, Wachsmann D, Klein J-P. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect Immun. 1996;64:3016–3022. doi: 10.1128/iai.64.8.3016-3022.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virji M, Alexandrescu C, Ferguson D J P, Saunders J R, Moxon E R. Variations in the expression of pili: the effect on adherence of Neisseria meningitidis with cultured human endothelial cells. Mol Microbiol. 1992;6:1271–1279. doi: 10.1111/j.1365-2958.1992.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 53.Walsh B, Williams A E, Satsangi J. Streptococcus suis type 2: pathogenesis and clinical disease. Rev Med Microbiol. 1992;3:65–71. [Google Scholar]
- 54.Wessels M R, Pozsgay V, Kasper D L, Jennings H J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. J Biol Chem. 1987;262:8262–8267. [PubMed] [Google Scholar]
- 55.Wibawan I W T, Lämmler C. Relationship between encapsulation and various properties of Streptococcus suis. J Vet Med B. 1994;41:453–459. doi: 10.1111/j.1439-0450.1994.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 56.Williams A E. Relationship between intracellular survival in macrophages and pathogenicity of Streptococcus suis type 2 isolates. Microb Pathog. 1990;8:189–196. doi: 10.1016/0882-4010(90)90046-s. [DOI] [PubMed] [Google Scholar]