Abstract
Yinqiao powder, with significant anti‐inflammatory and antiviral effects, is a classical formula for the treatment of febrile diseases in China. During the SARS period in 2003, Yinqiao powder showed a good antipyretic effect. It also plays a major role in the treatment for COVID-19 in China. Although there are many studies on the chemical compositions and pharmacological effects of Yinqiao powder, there are few studies on the quality standard system of it. In our study, a systematic quality evaluation method of Yinqiao powder combining HPLC fingerprint with quantitative analysis of multi-components by single marker (QAMS) based on network pharmacology and UPLC-Q-Exactive-Orbitrap-MS was established for the first time. In the UPLC-Q-Exactive-Orbitrap-MS experiment, a total of 53 compounds were identified in the extract solution of Yinqiao powder. In addition, 33 blood components were characterized, 23 of which were prototypes. The results of network pharmacology analysis showed that Yinqiao powder may inhibit inflammatory responses by suppressing IL-6, CXCL2, TNFα, NF-κB, etc., in the treatment of COVID-19. The HPLC fingerprint analysis of Yinqiao powder was conducted at 237 nm and 29 characteristic peaks were matched, 11 of which were identified. Forsythoside A was selected as the internal standard reference and double-wavelength (237 nm and 327 nm) was established in QAMS experiment. The repeatability was well under different conditions, and the results measured by QAMS were consisted with that of the external standard method (ESM), indicating that the QAMS method was reliable and accurate. The quality evaluation method of Yinqiao powder would be helpful to evaluate the intrinsic quality of Yinqiao powder more comprehensively, which is conducive to improve the quality standard of Yinqiao powder and provide a beneficial guarantee for the clinical treatment of COVID-19.
1. Introduction
Yinqiao powder is a well-known traditional Chinese medicine (TCM) formula in China. With significant anti-inflammatory and antiviral effects, it is clinically used for the treatment of influenza, infantile pneumonia, hand-foot-mouth disease, etc. [1–3]. In particular, during the SARS period in 2003, Yinqiao powder showed good antipyretic effect. Nowadays, coronavirus disease 2019 (COVID-19) is spreading around the world and causing severe respiratory illnesses and even death. Yinqiao powder is one of the heat-clearing and detoxification prescriptions recommended by the traditional Chinese medicine prevention and treatment plan for COVID-19 in Shaanxi, Jiangsu, Guangdong and Hubei provinces in China [4, 5]. However, the quality standard of Yinqiao powder is not perfect at present, so it is necessary to improve the quality control standard of Yinqiao powder.
Yinqiao powder is composed of ten herbal slices with complex ingredients [6]. The theory of serum pharmacochemistry of TCM believes that only components absorbed into bloodstream are likely to be virtually effective components [7]. Therefore, only by analyzing the serum components after oral administration and determining the direct acting components in the body of Yinqiao powder can fundamentally control the quality of the Yinqiao powder. The chemical components in TCM and biological samples could be accurately and quickly identified by UPLC-Q-Exactive-Orbitrap-MS technology, which provides a new method for improving the quality standard of Yinqiao powder [8]. So, UPLC-Q-Exactive-Orbitrap-MS was employed to detect the blood components of Yinqiao powder. In addition, HPLC fingerprint and quantitative analysis of multi-components by single marker (QAMS) are internationally recognized methods. The comprehensive information of Yinqiao powder could be obtained by HPLC fingerprints [9, 10] and QAMS can simultaneously determine the content of various components in Yinqiao powder through one reference substance [11–13].
Yinqiao powder is a complex system with multi-components, multi-targets, and multi-action mechanisms through the joint action of various chemical components. The quality evaluation of it can not only use a single index. In this study, a qualitative and quantitative quality standard evaluation method of Yinqiao powder combining HPLC fingerprint with QAMS was established based on network pharmacology and UPLC-Q-Exactive-Orbitrap-MS technology. Firstly, UPLC-Q-Exactive-Orbitrap-MS was employed to detect the components in Yinqiao powder extract and rat blood. Meanwhile, network pharmacology was used to predict the possible pathways and components of Yinqiao powder in treating for COVID-19. Then, the common peaks of ten batches of Yinqiao powder were identified with the existing reference materials and the source of the common peaks was assigned through the establishment of HPLC fingerprint. Finally, the content of the potential active components screened in the first step was determined by the QAMS method with forsythoside A as the internal standard. The flowchart of the established analytical strategy is shown in Figure 1.
Figure 1.
Flowchart of the established analytical strategy.
2. Materials and Methods
2.1. Chemicals and Reagents
Caffeic acid, hesperidin, rutin, liquiritin, cynaroside, and forsythoside A were provided by National Institutes for Food and Drug Control (Beijing, China). Arctiin, neochlorogenic acid, chlorogenic acid, isochlorogenic acid A, and isochlorogenic acid C were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). As for phillyrin, it was provided by Chengdu Chroma-Biotechnology Co., Ltd. (Chengdu, Sichuan Province, China). The detailed information of the above mentioned standard materials is listed in Table S1. All the herbal slices of Yinqiao powder were purchased from Beijing Tongrentang drugstore (Beijing, China), and information of these herbal slices is shown in Table 1.
Table 1.
Information of the herbal slices in Yinqiao powder.
| Herbal slice name | Original plant name | Collection location | Lot number |
|---|---|---|---|
| Lonicerae Japonicae Flos | Lonicera japonica Thunb. | Shandong | 20200108 |
| Forsythiae Fructus | Forsythia suspensa (Thunb.) | Shanxi | 20200101 |
| Platycodonis Radix | Platycodon grandiflorum (Jacq.) A.DC. | Guizhou | 191201 |
| Menthae Haplocalycis Herba | Mentha haplocalyx Briq. | Jiangsu | 20190822 |
| Sojae Semen Praeparatum | Glycine max (L.) Merr. | Hebei | 20200525 |
| Lophatheri Herba | Lophatherum gracile Brongn. | Zhejiang | 20191127 |
| Arctii Fructus | Arctium lappa L. | Liaoning | 180901 |
| Schizonepetae Spica | Schizonepeta tenuifolia Briq. | Hebei | 20190304 |
| Phragmitis Rhizoma | Phragmites communis Trin. | Hebei | 349190801 |
| Glycyrrhizae Radix et Rhizoma | Glycyrrhiza uralensis Fisch. | Gansu | 20200501 |
The deionized water was obtained from Watsons (Watsons Food and Beverage Co., Ltd., Guangzhou, China). Acetonitrile (HPLC grade), formic acid (HPLC grade), and methanol (HPLC grade) used in UPLC analysis were provided by Fisher Scientific (Thermo Fisher, CA, USA). In quantitative analysis, acetonitrile (HPLC grade) and methanol (HPLC grade) were purchased from BCL International Trading Co., Ltd. (USA), and methanol (AR) was obtained from Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjin, China).
2.2. Network Pharmacology Analysis
2.2.1. Prediction of Chemical Components and Related Targets of Yinqiao Powder
The chemical components and related targets of Yinqiao powder were predicted using TCMSP (https://tcmspw.com/tcmsp.php) and Swiss Target Prediction platform (https://www.swisstargetprediction.ch). Oral bioavailability (OB) ≥30% and drug-likeness (DL) ≥0.18 were used as the screening criteria. The active ingredients of Yinqiao powder that are not included in TCMSP or do not meet the screening standards were added according to the Pharmacopoeia of the People's Republic of China (Ch. P) and literature. The herbal slices-chemical composition-gene data sheet of Yinqiao powder was established based on above information.
2.2.2. Prediction of Related Targets of COVID-19
The targets related to COVID-19 were obtained by searching in DisGeNET (https://www.disgenet.org/), DrugBank (https://go.drugbank.com/), GeneCards (https://www.genecards.org/), and OMIM (https://www.omim.org/) databases and screened with the keywords of “COVID-19,” “Coronavirus disease 2019,” “novel coronavirus pneumonia,” “coronavirus,” “SARS-coronavirus,” “Severe Acute Respiratory Syndrome” and “Coronavirus Infections.” In GeneCards database, score ≥10 was used as the screening criteria.
Then, the intersection targets of Yinqiao powder and COVID-19 were obtained using the online platform of Bioinformatics (https://www.bioinformatics.com.cn/) through Venn diagram analysis.
2.2.3. Construction and Analysis of the PPI Network
The intersection targets of Yinqiao powder and COVID-19 were submitted to STRING database (https://cn.string-db.org/, Version 11.5) for the construction and analysis of the protein-protein interaction (PPI) network. The organism was restricted to Homo sapiens and the minimum required interaction score was set as medium confidence (0.400). Then, the result of TSV format was exported and imported into Cytoscape 3.8.2 software for further analysis.
2.2.4. GO and KEGG Enrichment Analysis
The potential genes of Yinqiao powder for the treatment of COVID-19 were imported into Metascape database (https://metascape.org) for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. Species of Homo sapiens and p < 0.01 were set as the filter criteria. The visual analysis of GO and KEGG enrichment results was performed using Bioinformatics online platform (https://www.bioinformatics.com.cn/).
2.3. Instruments and Conditions
2.3.1. Instruments
Ultimate 3000 ultra-high performance liquid chromatography system (Thermo Fisher, CA, USA) was connected to a Q-Exactive Orbitrap tandem mass spectrometer (Thermo Fisher, CA, USA) via an ESI source. Oil-Free Air Compressors (AC-1Y) was purchased from Beijing Purkinje General Instrument Co., Ltd. (Beijing, China). High-speed refrigerated centrifuge was acquired from Heraeus Holding GmbH (GER). MX-F vortex Mixers was provided by Wuhan Servicebio Technology Co., Ltd. (Wuhan, China). Both CNC ultrasonic cleaner (Q-250D, Kun Shan Ultrasonic Instruments Co., Ltd.) and 1/10000 electronic scale (XS205DU, Mettler Toledo International Trading (Shanghai) Co., Ltd.) were also used in quantitative analysis.
2.3.2. Conditions
The chemical identification analysis was performed on UPLC-Q-Exactive-Orbitrap-MS. Samples were firstly separated on HALO-C18 Column (2.1 mm × 100 mm, 2.7 μm, AMT Company). The column temperature was set at 30°C. The flow rate was 0.3 mL·min−1 and the sample injection volume was 5 μL. The mobile phase consisted of 0.1% formic acid-water (A) and acetonitrile (B). The optimized gradient elution was as follows: 0–2 min, 2% B; 2–32 min, 2%–75% B; 32–33 min, 75%–2% B; 33–35 min, 2% B.
The mass spectrometer was operated in both positive and negative ion modes. The spray voltage was maintained at 3.5 kV in the positive ion mode and 3 kV in the negative ion mode. Full MS spectra were acquired with resolution at 70,000 and AGC target at 1e6. MS/MS fragments (spectra) were performed with resolution at 17,500 and AGC target at 1e5. The stepped normalized collision energy (NCE) was set at 20, 40, and 60. The following parameter settings were used: capillary temperature of 320°C, heater temperature of 300°C, sheath gas velocity of 35 arb, auxiliary gas flow rate of 10 arb, and mass range of m/z 80–1200.
In the fingerprint analysis and QAMS experiments, chromatographic separation was performed on a Thermo Syncronis Column (4.6 mm × 250 mm, 5 μm, Thermo Fisher, CA, USA). Water with 0.1% phosphoric acid (A) and acetonitrile (B) were used as mobile phase. The flowing gradient was applied at 30°C and set as follows: 0–5 min, 7%-8% B; 5–15 min, 8%–10% B; 15–20 min, 10%–11% B; 20–35 min, 11%–16% B; 35–70 min, 16%–18% B; 70–90 min, 18%–20% B; 90–100 min, 20%–23% B; 100–130 min, 23%–60% B. The flow rate was 1 mL·min−1 and the sample injection volume was 5 μL. Under the above mentioned chromatographic conditions, peak area of each component at 237 nm was measured and recorded for fingerprint spectrum, while the peak area of each component at 237 nm and 327 nm was measured and recorded for QAMS. The relative correction factors (RCFs) of parallel experiments were recorded and calculated using two high-performance liquid chromatographs (Thermo UltiMate3000 (1), Thermo UltiMate3000 (2)) and two chromatographic columns (Hypersil ODS2 (250 mm × 4.6 mm, 5 μm), Thermo Syncronis C18 (250 mm × 4.6 mm, 5 μm)).
2.4. Animal Experiments
Healthy male and female Wistar rats (SPF grade, 240 ± 10 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (License No.: SCXK (Jing) 2016-0006) and housed for 4 days of acclimation before the experiment. The animal research was conducted in accordance with international rules for animal experimentation and internationally recognized ethical principles for the use and care of laboratory animals. The animal study protocol was approved by the Ethics Committee of Shandong Academy of Chinese Medicine.
Serum sample preparation: twelve Wistar rats were divided into an experimental group and a blank group (half of the rats in each group were female). Eight hours before the administration, rats were fasted with free access to water. Rats in the experimental group were given 4 mL of extract of Yinqiao powder, while rats in the blank group were given the same volume of normal saline (0.9% NaCl). 0.5 mL blood samples were collected from the jugular vein of rats at 30 min, 60 min, 90 min, 120 min, and 180 min, respectively after administration. After standing for 1 h, each blood sample was centrifuged at 3,000 rpm for 15 min to obtain the serum. The serum samples were stored at −80°C until further pre-treatment.
The freeze-thaw of all serum samples was carried out at 4°C. Each serum sample (200 μL) was mixed with acetonitrile (600 μL), and then centrifuged at 13,000 rpm at 4°C for 15 min. Afterward, the supernatant was dried with a stream of nitrogen at room temperature to obtain the residue. Then, it was redissolved in 70% methanol‐water (100 μL) and centrifuged at 15,000 rpm at 4°C for 15 min. The supernatant was used for further analysis.
2.5. Preparation of Sample Solutions and Negative Sample Solutions
2.5.1. Sample for Animals
100 g of Yinqiao powder was prepared according to the Ch. P of the 2020 edition [6]. In a volumetric flask (2000 mL), Yinqiao powder was soaked in 10 times of water for 1 h and filtered after reflux extraction for 1 h. The filtrate was collected, and then the water with residues (1 : 8, w/v) was boiled for an additional 1 h. Two batches of filtrate were mixed. After collecting the volatile oil, the concentration of the filtrate was concentrated to 2 g·mL−1. Afterward, the filtrate cooled to room temperature was mixed with the volatile oil and the mixture was used in intragastric administration of rats.
2.5.2. Sample for UPLC-Q-Exactive-Orbitrap-MS
Yinqiao powder (10 g) was extracted according to the above method. The filtrate was collected and then concentrated to 100 mL. The mixture of the abovementioned solution (1 mL) and methanol (1 mL) was processed by an ultrasonic assisted extraction (UAE) method for 15 min and then centrifuged at 15,000 rpm at 4°C for 15 min. The supernatant was isolated for further analysis.
2.5.3. Sample for Fingerprint and QAMS Analysis
Sample of Yinqiao powder: 0.5 g of Yinqiao powder was accurately weighed and placed in a volumetric flask (25 mL). The volumetric flask was filled with 70% methanol-water. After being processed by the UAE method for 30 min, the solution of Yinqiao powder was cooled to room temperature and then filtered to obtain the filtrate. Afterward, the filtrate was filtered through a 0.45 μm millipore filter membrane (organic-system) for further analysis.
Preparation of negative control samples: the negative control samples of Lonicerae Japonicae Flos, or Forsythiae Fructus, or Arctii Fructus, or Lonicerae Japonicae Flos and Arctii Fructus were respectively produced as same as “Sample of Yinqiao powder” for further analysis.
2.6. Preparation of the Standard Solutions
Neochlorogenic acid, chlorogenic acid, forsythoside A, isochlorogenic acid A, isochlorogenic acid C, phillyrin, and arctiin were accurately weighed and dissolved in 50% methanol. The finally concentration of them was 7.7 μg·mL−1, 96 μg·mL−1, 296 μg·mL−1, 65 μg·mL−1, 15.2 μg·mL−1, 27.5 μg·mL−1, and 113 μg·mL−1, respectively. All standard solutions were stored at 4°C.
2.7. Data Processing and Analysis
The UPLC-MS data were handled by Compound Discoverer 3.2 software, and compared with ChemSpider, Thermo's Chinese Medicine database and mzCloud database. The HPLC fingerprint analysis was performed using the “Similarity Evaluation System of Traditional Chinese Medicine Chromatographic Fingerprint” software (2004, edition). SPSS17.0 software was used for principal component analysis (PCA) of the key components affecting the quality of Yinqiao powder.
3. Results and Discussion
3.1. Method Development
3.1.1. Optimization of Chromatographic Conditions for Fingerprint and QAMS
We examined different mobile phases according to related literatures [14, 15]. The results showed that the baseline was more stable when acetonitrile-0.1% phosphoric acid water was used as mobile phase compared with methanol-0.1% phosphoric acid water. Therefore, acetonitrile-0.1% phosphoric acid water was chosen as mobile phase. The elution gradient was based on the conditions in Section 2.3.2. Under this gradient, there were more chromatographic peaks of Yinqiao powder with better resolution and larger peak area.
Furthermore, in order to select detection wavelengths, the chromatograms at 237 nm, 279 nm, 327 nm, and 365 nm were compared. The results suggested that information collected at 237 nm was the most abundant, and peak area and resolution were better, which could well reflect the characteristics of Yinqiao powder in fingerprint analysis. Furthermore, in QAMS experiment, forsythoside A, phillyrin and arctiin had better asymmetry and resolution at 237 nm. The resolution of neochlorogenic acid, chlorogenic acid, forsythoside A, isochlorogenic acid A, and isochlorogenic acid C were greater than 1.5 without interference of impurity peaks at 327 nm. However, there was no absorption for phillyrin and arctiin at 327 nm. Therefore, the content detection was performed at 237 nm and 327 nm, while 237 nm was selected as the detection wavelength in the fingerprint analysis.
3.1.2. Optimization of Experimental Conditions for Fingerprint Analysis
The Yinqiao powder was extracted by the ultrasonic extraction method according to Ch. P [6] and related literatures [16, 17]. In our study, 50% methanol, 80% methanol, and 100% methanol were investigated. The results showed that there was no significant difference on the number of components extracted by the three solvents and on the resolution of chromatographic peaks. However, the chromatographic peak area of the samples extracted with 50% methanol was largest under the same chromatographic conditions. Thus, 50% methanol was chosen as the extraction solvent.
In addition, the influence of solvent peaks on the results must be taken into account [18]. So, 50% methanol was injected according to the above chromatographic conditions. The solvent peaks of 0–5 min was sheared during peak matching based on the results of the chromatogram. Then, the chromatograms of 10 batches of Yinqiao powder were matched with common peaks. As a result, there were 112 common peaks under the unrestricted conditions. However, the peak area of most of the common peaks was small and the signal-to-noise ratio (SNR) did not meet the requirements. When the peak area was greater than 0.3, there were 38 common peaks and 11 chromatographic peaks could be identified. However, some peaks with small area had low resolution and asymmetry. For the further filter, the condition that peak area was greater than 0.4 was carried out. A total of 29 common peaks were obtained, 11 of which could be identified and the resolution and asymmetry of each common peak were good. In the end, peak area greater than 0.4 was determined as the screening condition.
3.1.3. Optimization of Experimental Conditions for QAMS
Forsythoside A showed absorption at 237 nm and 327 nm, and the peak shape and resolution were good. Besides, it had high content and stable property in Yinqiao powder. Therefore, forsythoside A was selected as the internal standard reference.
For the location of chromatographic peaks for each component, relative retention value and retention time differences are the common qualitative parameters [19]. In our study, the two parameters of each component relative to the internal standard reference were calculated. Finally, neochlorogenic acid and chlorogenic acid were located by retention time differences while isochlorogenic acid A, isochlorogenic acid C, phillyrin, and arctiin were located by a relative retention value.
3.2. UPLC-Q-Exactive-Orbitrap-MS Technology
3.2.1. Analysis of Reference Substances
The total ion chromatograms (TICs) in positive and negative ion modes of reference substances were analyzed. The results indicated that the information of each component responded better in negative ion mode. The detailed information of reference substances is listed in Table 2.
Table 2.
Mass spectrometry information of reference substances.
| Reference substance | t R (min) | Ion mode | Theoretical (m/z) | Measured (m/z) | Fragment ions (m/z) |
|---|---|---|---|---|---|
| Neochlorogenic acid | 5.80 | [M − H]− | 353.0867 | 353.0876 | 191.0554, 179.0341, 135.0439 |
| Chlorogenic acid | 6.93 | [M − H]− | 353.0867 | 353.0876 | 191.0554, 179.0341, 135.0439 |
| Caffeic acid | 7.34 | [M − H]− | 179.0339 | 179.0341 | 135.0439 |
| Liquiritin | 10.00 | [M − H]− | 417.1180 | 417.1196 | 255.0662, 135.0074, 119.0488 |
| Rutin | 10.02 | [M − H]− | 609.1450 | 609.1464 | 300.0279, 271.0250, 255.0299 |
| Cynaroside | 10.41 | [M − H]− | 447.0922 | 447.0936 | 285.0407 |
| Forsythoside A | 10.93 | [M − H]− | 623.1970 | 623.1980 | 461.1674, 161.0232 |
| Isochlorogenic acid A | 11.80 | [M − H]− | 515.1184 | 515.1195 | 353.0882, 191.0554, 179.0446, 135.0439 |
| Isochlorogenic acid C | 11.83 | [M − H]− | 515.1184 | 515.1195 | 353.0882, 191.0554, 179.0446, 135.0439 |
| Hesperidin | 11.54 | [M − H]− | 609.1814 | 609.1813 | 301.0721 |
| Phillyrin | 13.05 | [M + HCOO]− | 579.2072 | 579.2091 | 371.1507 |
| Arctiin | 13.34 | [M + HCOO]− | 579.2072 | 579.2091 | 371.1504 |
Neochlorogenic acid and chlorogenic acid are isomers of each other with the same theoretical [M − H]− ion and fragment ions. Chlorogenic acid is a phenolic acid compound produced by the reaction of caffeic acid and quinic acid, and generated the [M − H]− ion at m/z 353.0876. The fragment ions at m/z 191.0554 and m/z 179.0341 were detected in ESI-MS/MS spectrum, which were [M − H]− ions of quinic acid and caffeic acid, respectively. Isochlorogenic acid A and isochlorogenic acid C, with the same [M − H]− ion at m/z 515.1195, belong to the dicaffeoylquinic acid. The positions of their functional group were different. In their ESI-MS/MS spectrums, characteristic fragment ions were detected, including m/z 353.0882, m/z 191.0554, and m/z 179.0446. Arctiin and phillyrin are also isomers of each other. The [M + HCOO]− ion at m/z 579.2091 was formed by the combination of arctiin with a formate according to published literatures [20, 21] and spectra.
3.2.2. Analysis of Active Ingredients in Yinqiao Powder
According to the above analysis methods and related literatures, a total of 53 compounds were identified in the extract solution of Yinqiao powder, including 14 flavonoids, 8 organic acids, 8 amino acids, 7 phenylethanoid glycosides, 6 isoflavones and isoflavone glycosides, 4 iridoids, 2 lignans, 1 coumarin, and 3 other compounds (Table 3).
Table 3.
Information of identified compounds in Yinqiao powder extract.
| No | t R (min) | Name | Formula | Ion mode | Theoretical (m/z) | Measured (m/z) | ppm | Fragments ion (m/z) | Peak area | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.64 | Arginine | C6H14N4O2 | [M + H]+ | 175.1190 | 175.1191 | 0.615 | 175.1191, 116.0709, 70.0659 | 9.26E + 08 | [22] |
| 2 | 0.72 | Histidine | C6H9N3O2 | [M + H]+ | 156.0768 | 156.0768 | 0.557 | 138.0549, 110.0717 | 3.29E + 07 | – |
| 3 | 0.72 | Quinic acid | C7H12O6 | [M − H]− | 191.0550 | 191.0552 | 1.180 | 127.0338, 85.0279 | 4.62E + 09 | [23] |
| 4 | 0.73 | Proline | C5H9NO2 | [M + H]+ | 116.0706 | 116.0710 | 2.971 | 70.0656 | 5.20E + 09 | [24] |
| 5 | 0.73 | Valine | C5H11NO2 | [M + H]+ | 118.0863 | 118.0866 | 3.090 | 100.1125, 72.0816 | 1.41E + 09 | [22] |
| 6 | 0.77 | Glutamic acid | C5H9NO4 | [M + H]+ | 148.0604 | 148.0605 | 0.241 | 148.0605, 130.0500, 102.0554, 84.0451 | 8.99E + 07 | [25] |
| 7 | 1.02 | Adenine | C5H5N5 | [M + H]+ | 136.0618 | 136.0619 | 0.869 | 119.0495, 91.0549 | 2.17E + 08 | [26] |
| 8 | 1.19 | Isoleucine | C6H13NO2 | [M + H]+ | 132.1019 | 132.1021 | 1.096 | 132.1021, 86.0971 | 4.87E + 08 | [25] |
| 9 | 1.24 | Tyrosine | C9H11NO3 | [M + H]+ | 182.0812 | 182.0813 | 0.935 | 165.0546, 136.0757, 123.0443 | 2.02E + 08 | [24] |
| 10 | 2.32 | Phenylalanine | C9H11NO2 | [M + H]+ | 166.0863 | 166.0864 | 0.872 | 120.0810, 107.0496 | 7.23E + 07 | [26] |
| 11 | 5.51 | Loganic acid | C16H24O10 | [M − H]− | 375.1286 | 375.1299 | 3.403 | 213.0763, 151.0752, 125.0594 | 3.35E + 08 | [23] |
| 12∗ | 5.61 | Neochlorogenic acid | C16H18O9 | [M − H]− | 353.0867 | 353.0882 | 4.139 | 191.0553, 179.0340, 135.0438 | 8.78E + 08 | [24] |
| 13 | 5.69 | Loganin | C17H26O10 | [M − H]− | 389.1442 | 389.1458 | 3.923 | 345.1195, 227.0565, 183.0655, 139.0388 | 6.16E + 04 | [27] |
| 14 | 5.84 | Forsythoside E | C20H30O12 | [M + H]+ | 463.1810 | 463.1809 | −0.265 | 155.0704, 137.0596 | 1.72E + 07 | [28] |
| 15 | 5.92 | Methyl cinnamate | C10H10O2 | [M + H]+ | 163.0754 | 163.0754 | 0.392 | 135.0442, 131.0493, 103.0547 | 1.69E + 07 | [25] |
| 16 | 7.10 | Sweroside | C16H22O9 | [M − H]− | 357.1180 | 357.1195 | 4.288 | 195.0661, 177.0545 | 5.87E + 05 | [29] |
| 17∗ | 7.31 | Chlorogenic acid | C16H18O9 | [M − H]− | 353.0867 | 353.0880 | 1.331 | 191.0553, 179.0431, 135.0438 | 1.38E + 09 | [30] |
| 18 | 7.80 | Secoxyloganin | C17H24O11 | [M + H]+ | 405.1391 | 405.1392 | 0.054 | 243.0862, 225.0757, 165.0547, 151.0390 | 1.72E + 08 | [23] |
| 19 | 7.80 | Scopoletin | C10H8O4 | [M + H]+ | 193.0495 | 193.0497 | 0.698 | 151.0396, 95.0497 | 8.68E + 07 | [31] |
| 20 | 7.97 | Glycitin | C22H22O10 | [M + H]+ | 447.1286 | 447.1285 | −0.142 | 285.0756, 270.0522, 242.0572 | 5.36E + 07 | [32] |
| 21 | 8.55 | Rutin | C27H30O16 | [M + H]+ | 611.1607 | 611.1605 | −0.329 | 465.1021, 303.0498 | 2.11E + 08 | [23] |
| 22 | 8.60 | Liquiritigenin | C15H12O4 | [M + H]+ | 257.0808 | 257.0806 | −1.032 | 147.0441, 137.0235, 119.0495 | 1.57E + 09 | [33] |
| 23 | 8.80 | Isoquercitrin | C21H20O12 | [M + H]+ | 465.1028 | 465.1029 | 0.403 | 303.1497 | 4.34E + 07 | [34] |
| 24∗ | 8.80 | Quercetin | C15H10O7 | [M + H]+ | 303.0499 | 303.0498 | −0.426 | 153.0183, 137.0234 | 2.02E + 07 | [34] |
| 25 | 8.85 | Cynaroside | C21H20O11 | [M + H]+ | 449.1078 | 449.1078 | −0.040 | 287.0549, 241.0501 | 9.26E + 07 | [23] |
| 26 | 8.93 | Genistin | C21H20O10 | [M + H]+ | 433.1129 | 433.1129 | 0.062 | 271.0598, 243.0652, 215.0702 | 8.08E + 07 | [32] |
| 27 | 9.13 | Isoschaftoside | C26H28O14 | [M − H]− | 563.1395 | 563.1411 | 2.802 | 383.0769, 353.0667, 297.0754 | 3.46E + 07 | — |
| 28 | 9.22 | (+)-Pinoresinol | C20H22O6 | [M + H]+ | 359.1489 | 359.1487 | −0.654 | 175.0755, 137.0598 | 2.20E + 07 | [35] |
| 29 | 9.23 | Pµlegone | C10H16O | [M + H]+ | 153.1274 | 153.1274 | 0.119 | 109.1016, 81.0706 | 2.98E + 07 | — |
| 30 | 9.43 | Daidzin | C21H20O9 | [M − H]− | 415.1024 | 415.1041 | 1.741 | 253.0506, 135.0074 | 3.76E + 06 | [32] |
| 31 | 9.70 | Hesperidin | C28H34O15 | [M + H]+ | 611.1970 | 611.1971 | 0.153 | 449.1436, 303.0860, 195.0288 | 1.64E + 08 | [24] |
| 32 | 9.83 | Forsythoside I | C29H36O15 | [M − H]− | 623.1970 | 623.1985 | 2.316 | 461.1655, 161.0233 | 2.53E + 09 | [28] |
| 33∗ | 10.00 | Liquiritin | C21H22O9 | [M − H]− | 417.1180 | 417.1195 | 3.527 | 255.0663, 135.0074, 119.0488 | 1.29E + 09 | [33] |
| 34∗ | 10.26 | Forsythoside A | C29H36O15 | [M − H]− | 623.1970 | 623.1985 | 2.316 | 461.1674, 161.0232 | 3.38E + 09 | [28] |
| 35 | 10.45 | Lonicerin | C27H30O15 | [M − H]− | 593.1501 | 593.1520 | 3.175 | 447.0936, 285.0406 | 3.51E + 07 | [23] |
| 36 | 10.51 | Ononin | C22H22O9 | [M + H]+ | 431.1337 | 431.1335 | −0.345 | 269.0806, 254.0572 | 2.98E + 08 | [36] |
| 37 | 10.60 | Calceolarioside B | C23H26O11 | [M − H]− | 477.1391 | 477.1407 | 3.253 | 161.0232, 133.0281 | 3.76E + 08 | [36] |
| 38 | 10.83 | Kaempferol-3-O-rutinoside | C27H30O15 | [M − H]− | 593.1501 | 593.1517 | 2.754 | 285.0407, 255.0298, 227.0345 | 3.70E + 07 | [23] |
| 39 | 10.91 | Daidzein | C15H10O4 | [M + H]+ | 255.0652 | 255.0650 | −0.766 | 227.0701, 199.0754, 137.0234 | 4.22E + 08 | [32] |
| 40 | 11.03 | Isochlorogenic acid B | C25H24O12 | [M − H]− | 515.1184 | 515.1180 | −0.704 | 353.0877, 191.0552, 179.0340, 173.0445, 135.0437 | 6.27E + 08 | [23] |
| 41 | 11.09 | Arctigenin | C21H24O6 | [M + H]+ | 373.1646 | 373.1642 | −0.924 | 237.1121, 137.0597 | 2.93E + 09 | [21] |
| 42 | 11.17 | Linarin | C28H32O14 | [M + H]+ | 593.1865 | 593.1863 | −0.341 | 447.1284, 285.0756, 270.0523, 242.0571 | 7.65E + 07 | — |
| 43 | 11.22 | Glycitein | C16H12O5 | [M + H]+ | 285.0758 | 285.0757 | −0.351 | 270.0522, 242.0573 | 1.10E + 08 | [32] |
| 44∗ | 11.23 | Isochlorogenic acid A | C25H24O12 | [M − H]− | 515.1184 | 515.1189 | 0.966 | 353.0881, 191.0554, 179.0341, 173.0445, 135.0438 | 4.17E + 08 | [23] |
| 45 | 11.46 | Azelaic acid | C9H16O4 | [M − H]− | 187.0965 | 187.0968 | 1.681 | 143.1061, 125.0958, 97.0643 | 2.03E + 08 | — |
| 46∗ | 11.76 | Isochlorogenic acid C | C25H24O12 | [M − H]− | 515.1184 | 515.1171 | −2.470 | 353.0869, 191.0557, 179.0340, 135.0439 | 8.75E + 08 | [23] |
| 47 | 11.79 | Ferµlic acid | C10H10O4 | [M − H]− | 193.0495 | 193.0499 | 2.045 | 178.0261, 161.0232, 133.0281 | 6.04E + 07 | [37] |
| 48 | 12.52 | Isoliquiritin | C21H22O9 | [M − H]− | 417.1180 | 417.1197 | 3.959 | 255.0661, 180.0054, 135.0074, 119.0487 | 2.29E + 08 | [33] |
| 49 | 12.67 | Genistein | C15H10O5 | [M + H]+ | 271.0601 | 271.0600 | −0.258 | 243.0649, 215.0704, 153.0183 | 7.06E + 07 | [32] |
| 50∗ | 13.04 | Phillyrin | C27H34O11 | [M + HCOO]− | 579.2072 | 579.2088 | 2.732 | 371.1496, 356.1265 | 1.03E + 09 | [21] |
| 51∗ | 13.32 | Arctiin | C27H34O11 | [M + HCOO]− | 579.2072 | 579.2087 | 2.525 | 371.1500, 356.1268 | 5.10E + 09 | [21] |
| 52∗ | 13.69 | Luteolin | C15H10O6 | [M − H]− | 285.0394 | 285.0406 | 4.194 | 151.0025, 133.0282, 107.0124 | 6.19E + 04 | [33] |
| 53 | 15.37 | Matairesinol | C20H22O6 | [M − H]− | 357.1332 | 357.1347 | 3.935 | 83.0122 | 2.13E + 08 | — |
∗ : components with potential pharmacological effects; —: compared with Chinese medicine database.
3.2.3. Analysis of Blood Components in Yinqiao Powder
A total of 33 blood components were identified by comparing the chemical components in Yinqiao powder extract, blank serum, and drug serum, 23 components of which were found in both Yinqiao powder extract and drug serum and other 10 components were only present in the drug serum (Table 4). According to related literatures [38–41], chlorogenic acid, isochlorogenic acid A, isochlorogenic acid C, phillyrin, forsythoside A, arctiin, liquiritin, neochlorogenic acid, cynaroside, rutin, and hesperidin can be absorbed into blood in the form of prototype. It has been reported that phillyrin and forsythoside A have good antiviral and immune regulation effects [42–44], neochlorogenic acid, isochlorogenic acid A, and isochlorogenic acid C have antiviral and anti-inflammatory activities [45, 46], while cynaroside has the effect of inhibiting influenza virus [47]. Liquiritin, hesperidin, and rutin have obvious anti-inflammatory effects [48–50]. The content of the abovementioned components in the Yinqiao powder extract is relatively high, and all of them can be absorbed into the blood with potent anti-inflammatory and antiviral effects. Therefore, these components can be used as indicators to further improve the quality standard of Yinqiao powder.
Table 4.
Information of blood components in Yinqiao powder.
| Number | Name | Extract | Drug serum | Blank serum |
|---|---|---|---|---|
| 1 | Ferulic acid | √ | √ | — |
| 2 | Daidzein | √ | √ | — |
| 3 | Genistein | √ | √ | — |
| 4 | Liquiritigenin | √ | √ | — |
| 5 | Sweroside | √ | √ | — |
| 6 | Loganic acid | √ | √ | — |
| 7 | Secoxyloganin | √ | √ | — |
| 8 | Loganin | √ | √ | — |
| 9∗ | Liquiritin | √ | √ | — |
| 10 | Ononin | √ | √ | — |
| 11∗ | Chlorogenic acid | √ | √ | — |
| 12 | Forsythoside E | √ | √ | — |
| 13∗ | Isochlorogenic acid C | √ | √ | — |
| 14∗ | Isochlorogenic acid A | √ | √ | — |
| 15∗ | Neochlorogenic acid | √ | √ | — |
| 16 | Glycitein | √ | √ | — |
| 17 | Genistin | √ | √ | — |
| 18 | Arctigenin | √ | √ | — |
| 19∗ | Arctiin | √ | √ | — |
| 20 | Pµlegone | √ | √ | — |
| 21∗ | Forsythoside A | √ | √ | — |
| 22 | Daidzein | √ | √ | — |
| 23∗ | Phillyrin | √ | √ | — |
| 24 | Apigenin 7-O-glucuronide | — | √ | — |
| 25 | Geniposidic acid | — | √ | — |
| 26 | Hexadecanedioic acid | — | √ | — |
| 27 | OroxylinA-7-O-β-D-glucuronide | — | √ | — |
| 28 | Naringin | — | √ | — |
| 29 | Glutathione | — | √ | — |
| 30 | Eucalyptol | — | √ | — |
| 31 | Formononetin | — | √ | — |
| 32 | Sinapine | — | √ | — |
| 33 | 18-β-Glycyrrhetinic acid | — | √ | — |
∗ : components with potential pharmacological effects; √: existence, —: nonexistence.
3.3. Network Pharmacology Analysis of Yinqiao Powder
3.3.1. Target Predication of Yinqiao Powder and COVID-19
A total of 136 components and 294 related targets of Yinqiao powder were obtained according to Ch. P, related literature combined with the TCMSP database. At the same time, GeneCards, OMIM, DisGeNET, and DrugBank databases were used to screen the targets of COVID-19. As a result, 945 targets were finally retained after removing duplicates. Then, Venn diagram analysis was performed on the targets of the two and it was found that there were 80 intersection targets (Figure 2).
Figure 2.
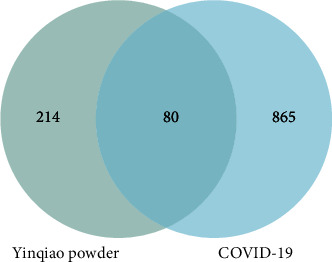
Venn diagram analysis of Yinqiao powder and COVID-19.
3.3.2. Construction and Analysis of the PPI Network
The 80 intersecting targets of Yinqiao powder and COVID-19 were submitted to STRING database to construct the PPI network. Then, the result was imported into Cytoscape 3.8.2 software for further analysis. In the end, a PPI network of Yinqiao powder intreating for COVID-19 with 80 nodes and 2824 edges was acquired, as shown in Figure 3. The area of the node in the figure is proportional to the degree value. So, it can be seen intuitively that ALB, IL6, VEGFA, AKT1, TNF, TP53, CASP3, and STAT3 may play important roles in the process of Yinqiao powder in treating for COVID-19.
Figure 3.
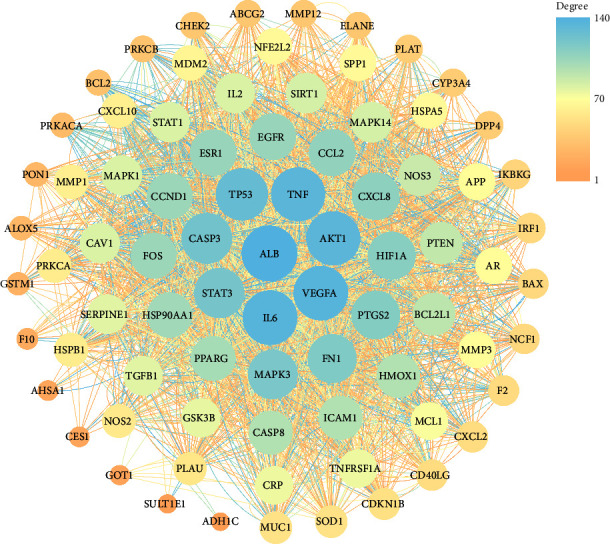
PPI network of intersection targets. The area of the node in Figure 3 is proportional to the degree value.
The chemical components corresponding to the intersection targets were initially identified as the effective active components of Yinqiao powder. A total of 126 components were found in our study, including quercetin, luteolin, kaempferol, chlorogenic acid, isochlorogenic acid A, isochlorogenic acid C, arctiin, neochlorogenic acid, forsythoside A, liquiritin, and so on. Studies have shown that chlorogenic acid and phillyrin could inhibit the production of TNF-α, IL-1β, IL-6 and alleviate lung infection in mice [43, 51]. Neochlorogenic acid could reduce the production of TNF-α, IL-6, and NO, further inhibit the protein expression of iNOS, COX2, TNF-α, IL-6, and attenuate the inflammatory response by activating the AMPK/Nrf2 signaling pathway [52]. Arctiin, together with daidzein, glycyrrhizic acid and liquiritin, can inhibit pneumonia by enhancing necroptosis and partial autophagy associated with plc γ1 phosphorylation in natural killer cells [53]. Forsythoside A could ameliorate lipopolysaccharide-induced pathological damage, decreased serum levels of TNF-α and IL-6, and inhibited macrophage infiltration in the lungs of acute lung injury mice [54].
3.3.3. GO and KEGG Enrichment Analysis
Furthermore, the 80 targets were submitted to Metascape for GO and KEGG enrichment analysis. P < 0.01 was used as the screening criterion. As a result, 1570 GO items were enriched, including 1413 in biological processes (BP), 55 in cellular components (CC), and 102 in molecular functions (MF). At the same time, 191 KEGG pathways were obtained. The visual analysis of the top 10 entries of GO and top 20 pathways of KEGG enrichment analysis is shown in Figure 4.
Figure 4.
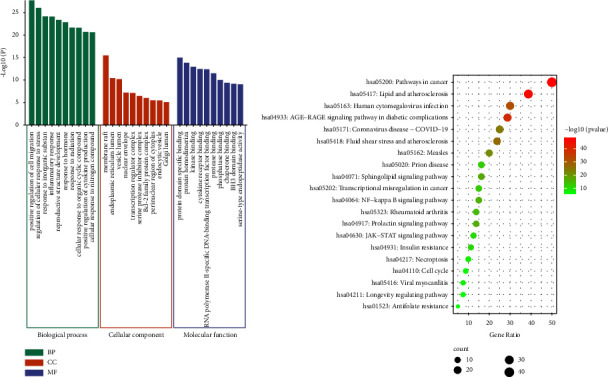
GO (a) and KEGG (b) enrichment analysis of Yinqiao powder in treating for COVID-19.
The GO enrichment analysis results showed that positive regulation of cell migration, regulation of cellular response to stress, response to inorganic substance, inflammatory response, and reproductive structure development were involved in BP. In the aspect of CC, it was mainly included membrane raft, endoplasmic reticulum lumen, vesicle lumen, nuclear envelope, transcription regulator complex, etc. The top 5 items of MF were protein domain specific binding, protein homodimerization activity, kinase binding, cytokine receptor binding, and RNA polymerase II-specific DNA-binding transcription factor binding.
The KEGG enrichment results suggested that the pathways of Yinqiao powder in the treatment for COVID-19 were mainly related to inflammation, such as IL-17 signaling pathway, TNF signaling pathway, PI3K-Akt signaling pathway, NF-kappa B signaling pathway, ErbB signaling pathway, coronavirus disease—COVID-19, and so on (Table S2). COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2). SARS-CoV 2 infects alveolar epithelial cells through the angiotensin-converting enzyme 2 (ACE2) receptor, resulting in increased serum levels of free angiotensin II (Ang II). The increased serum level of free Ang II promotes activation of the NF-kappa B pathway via Ang II type 1 receptor (AT1R), followed by interleukin-6 (IL-6) production. Studies have shown that the serum levels of IL-6, IL-17, and TNF-α in the COVID-19 patients were significantly higher than those in the control group [55, 56]. The damage associated with inflammatory autoimmune diseases can be reduced by inhibiting IL-17 [57]. Robinson et al. retrospectively explored the potential of anti-TNF in modulating COVID-19-related inflammation and concluded that anti-TNF therapies could reduce levels of pro-inflammatory cytokines associated with poor COVID-19 outcomes [58]. In addition, NF-κB is involved in the regulation of immunity, inflammation and cell survival, which could be activated by TNF-α, IL-1, etc. Numerous studies have demonstrated the potential therapeutic effect of inhibiting the NF-κB pathway in relieving severe forms of COVID-19 [59, 60]. The ErbB signaling pathway can regulate cell proliferation, migration, differentiation, apoptosis, and cell movement by mediating the PI3K-Akt pathway, JAK-STAT pathway, and MAPK signaling pathway. It was speculated that Yinqiao powder could inhibit JAK-STAT signaling through IL-6, which could reduce inflammatory responses and alleviate lung injury [61]. EGFR and EGBB2 are members of the epidermal growth factor receptor family. In the ErbB signaling pathway, they can directly or indirectly activate PI3K, thereby improving severe pneumonia by inhibiting the PI3K-Akt signaling pathway. Therefore, Yinqiao powder may inhibit the inflammatory response by inhibiting IL-6, CXCL2, MMP1, TNFα, NF-κB, etc., in the treatment of COVID-19.
3.4. Qualitative Analysis by Fingerprint and Multiple Statistical Strategies
Yinqiao powder and single drug samples were prepared according to the method given in Section 2.5.3, and standard solutions were prepared according to the method given in Section 2.6. The samples were detected under chromatographic conditions of fingerprint analysis in Section 2.3.2; then, the UPLC fingerprint analysis was performed using the “Similarity Evaluation System of Traditional Chinese Medicine Chromatographic Fingerprint” software (2004, edition). The matching results are shown in Figure 5.
Figure 5.
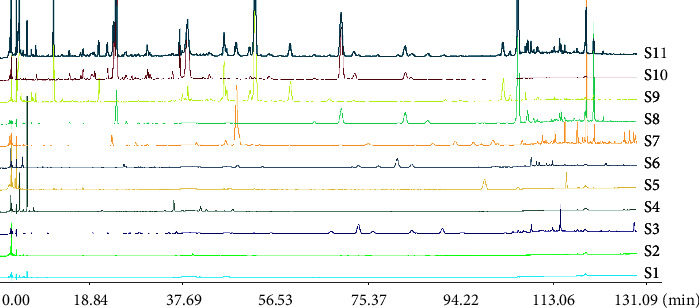
Matching graph of chromatogram between yinqiao powder and single drug. S1. Phragmitis Rhizoma, S2. Platycodonis Radix, S3. Schizonepetae Spica, S4. Lophatheri Herba, S5. Sojae Semen Praeparatum, S6. Menthae Haplocalycis Herba, S7. Glycyrrhizae Radix et Rhizoma, S8. Arctii Fructus, S9. Forsythiae Fructus, S10. Lonicerae Japonicae Flos, S11. Yinqiao powder.
3.4.1. Precision, Stability, and Repeatability
The same batch of Yinqiao powder solution was injected 6 times for the validation of precision and the stability was validated by analyzing the sample of Yinqiao powder at 0, 3, 6, 9, 12, and 24 h at room temperature. It can be seen from the results that RSDs of the retention time of each common peak was 0.01%∼0.08%, and RSDs of relative peak area was 0.32%∼2.19%, indicating that the precision of the instrument was excellent and the sample was stable in 24 hours.
Meanwhile, six samples of the same batch of Yinqiao powder were accurately weighed, prepared, and tested in parallel to test the repeatability. The results showed that RSDs of each common peak were less than 0.15% and the relative peak area was less than 2.52%, which indicated that the repeatability was good.
3.4.2. Similarity Evaluation of Fingerprint of Yinqiao Powder
Ten batches of Yinqiao powder sample were prepared and tested, and the chromatograms of each batch were recorded. The HPLC fingerprint analysis was performed using “Similarity Evaluation System of Traditional Chinese Medicine Chromatographic Fingerprint” software (2004, edition). S1 was chosen as the reference peak, the fingerprint of Yinqiao powder was established by median method. The results suggested that the similarity of 10 batches of Yinqiao powder was more than 0.95 (Table S3), indicating that the quality of Yinqiao powder was stable.
3.4.3. Confirmation of Common Peaks in Fingerprint
29 common peaks were determined and 11 common peaks can be identified by comparing the chromatograms of 10 batches of Yinqiao powder. The 11 identified peaks were as follows: peak 3 was neochlorogenic acid, peak 7 was chlorogenic acid, peak 12 was glycyrrhizin, peak 13 was rutin, peak 14 was forsythoside A, peak 15 was cynaroside, peak 17 was isochlorogenic acid A, peak 19 was hesperidin, peak 21 was isochlorogenic acid C, peak 25 was phillyrin, and peak 26 was arctiin. The reference spectrum and fingerprint spectrum of 10 batches of Yinqiao powder are shown in Figures 6(a) and 6(b), the relative retention time is shown in Table S4, and the median retention time and average peak area are shown in Table S5.
Figure 6.
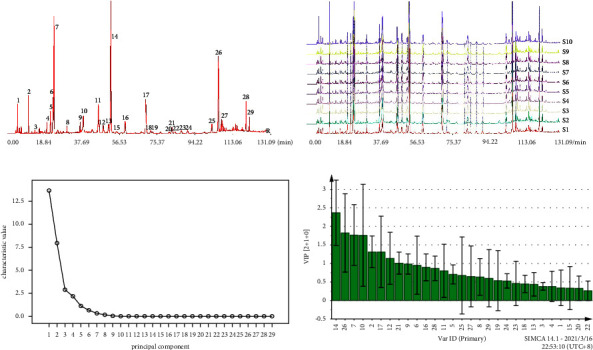
Reference spectrum of Yinqiao powder (a), HPLC fingerprint of 10 batches of yinqiao powder (b), scree plot of principal component of PCA (c), and VIP value of common peaks of PLS-DA (d).
3.4.4. Attribution of Common Peaks in Fingerprint
The reference spectrum of Yinqiao powder was compared with samples of single medicinal materials and negative control medicinal materials. Peak 1 came from Rhizoma Phragmitis and Lophatheri Herba. Peak 7 (chlorogenic acid), peak 17 (isochlorogenic acid A), and peak 21 (isochlorogenic acid C) came from Lonicerae Japonicae Flos and Arctii Fructus. Peak 10 came from Lonicerae Japonicae Flos and Forsythiae Fructus. Peak 19 (hesperidin) was from Menthae Haplocalycis Herba and Schizonepetae Spica, and peak 22 was from Lonicerae Japonicae Flos, Menthae Haplocalycis Herba and Schizonepetae Spica. Peak 28 existed in 10 medicinal materials, and peak 29 came from Forsythiae Fructus, Arctii Fructus and Glycyrrhizae Radix et Rhizoma. Peak 12 (glycyrrhizin) was the exclusive peak of Glycyrrhizae Radix et Rhizoma, peak 24 was the exclusive peak of Schizonepetae Spica, peak 20 was the exclusive peak of Menthae Haplocalycis Herba. Additionally, peaks 3 (neochlorogenic acid), 5, 6, 8, 9, and 18 were the exclusive peaks of Lonicerae Japonicae Flos, and peaks 2, 4, 11, 13, 14 (forsythoside A), 16, 25 (phillyrin), and 27 were the specific peaks of Forsythiae Fructus. Peaks 23 and 26 (arctiin) were the characteristic peaks of Arctii Fructus. The results showed that most of the common peaks came from Lonicerae Japonicae Flos, Forsythiae Fructus and Arctii Fructus, indicating that Lonicerae Japonicae Flos, Forsythiae Fructus, and Arctii Fructus play important roles as monarch and minister drugs in Yinqiao powder.
3.4.5. Principal Component Analysis
SPSS17.0 software was used for principal component analysis. As shown in Table S6 and Table 5, a total of 9 principal components were obtained when the cumulative rate was 100.000% according to the eigenvalue and variance contribution rate. The scree plot (Figure 6(c)) also showed that the slope tends to be gentle after extracting the first 9 components, indicating that these 9 principal components contain most of the chemical information of Yinqiao powder and they were the key components affecting the quality of Yinqiao powder.
Table 5.
Initial factor load matrix analysis of common peaks.
| Common peak no. | Component | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 1 | 0.480 | 0.621 | 0.115 | −0.489 | −0.122 |
| 2 | 0.941 | −0.240 | 0.220 | −0.021 | 0.070 |
| 3 | 0.991 | 0.034 | 0.108 | −0.029 | −0.019 |
| 4 | 0.799 | −0.155 | −0.244 | 0.378 | 0.330 |
| 5 | −0.341 | 0.921 | −0.133 | −0.008 | 0.095 |
| 6 | −0.221 | 0.668 | −0.438 | 0.316 | 0.331 |
| 7 | 0.764 | −0.462 | 0.319 | −0.267 | −0.125 |
| 8 | 0.867 | 0.474 | 0.022 | 0.063 | 0.008 |
| 9 | 0.969 | −0.178 | 0.123 | −0.060 | 0.041 |
| 10 | 0.742 | −0.624 | 0.189 | −0.093 | 0.030 |
| 11 | −0.438 | 0.567 | 0.594 | −0.222 | −0.219 |
| 12 | 0.919 | 0.284 | 0.138 | −0.202 | −0.013 |
| 13 | −0.448 | 0.750 | 0.433 | 0.183 | 0.081 |
| 14 | −0.172 | −0.133 | 0.730 | 0.598 | −0.083 |
| 15 | 0.201 | 0.644 | −0.105 | 0.408 | −0.454 |
| 16 | −0.511 | −0.232 | 0.688 | 0.418 | 0.107 |
| 17 | 0.898 | 0.356 | 0.067 | −0.143 | 0.147 |
| 18 | 0.483 | −0.846 | −0.043 | 0.111 | −0.177 |
| 19 | −0.623 | −0.697 | −0.148 | 0.242 | 0.106 |
| 20 | −0.730 | −0.380 | 0.173 | −0.456 | 0.091 |
| 21 | 0.959 | −0.076 | 0.001 | 0.096 | −0.185 |
| 22 | 0.015 | −0.522 | 0.287 | −0.283 | 0.619 |
| 23 | 0.548 | 0.794 | 0.037 | 0.162 | 0.174 |
| 24 | −0.931 | 0.104 | 0.313 | −0.016 | 0.039 |
| 25 | 0.495 | −0.680 | 0.408 | 0.346 | −0.021 |
| 26 | 0.935 | 0.287 | 0.061 | −0.173 | 0.041 |
| 27 | 0.520 | 0.750 | 0.171 | 0.283 | 0.219 |
| 28 | 0.949 | 0.018 | 0.014 | 0.236 | 0.036 |
| 29 | 0.178 | −0.605 | −0.623 | 0.275 | −0.093 |
The identified common peaks were assigned according to the matrix analysis of the first five components. Among them, the first principal components were peak 3 (neochlorogenic acid), peak 7 (chlorogenic acid), peak 12 (liquiritin), peak 17 (isochlorogenic acid A), peak 21 (isochlorogenic acid C), peak 25 (phillyrin), and peak 26 (arctiin). The second principal components were peak 13 (rutin) and peak 15 (cynaroside). The third principal components were peak 14 (forsythoside A) and peak 19 (hesperidin). The abovementioned components were distributed in the first three principal components, indicating that these components were the key components to ensure the quality of Yinqiao powder, especially the first principal components.
3.4.6. Partial Least Squares Discriminant Analysis
Partial least squares discriminant analysis (PLS-DA) was performed by SIMCA 14.1 software with common peak area (Figure 6(d)). In this model, the VIP value diagram can directly reflect the contribution of each chromatographic peak. The VIP value greater than 1 was usually used as the criterion for screening indicators. The VIP values of peak 14 (forsythoside A), peak 26, peak 7 (chlorogenic acid), peak 10, peak 2, peak 17 (isochlorogenic acid A), peak 12 (liquiritin), and peak 21 (isochlorogenic acid C) were greater than 1. It was speculated that these components were the key components affecting the quality of Yinqiao powder and played important roles in the quality control.
3.5. Quantitative Analysis by QAMS
In quantitative analysis of Yinqiao powder, the 7 reference standards were selected according to the results of UPLC-Q-Exactive-Orbitrap-MS and network pharmacology. Although the results of the network pharmacology showed that kaempferol, luteolin, quercetin, etc., may also be the possible components of Yinqiao powder for the treatment of COVID-19, the peak area was relatively small according to the UPLC-Q-Exactive-Orbitrap-MS results. So, it was indicated that the content of these components in Yinqiao powder was low, especially kaempferol, it was not detected in the extract solution of Yinqiao powder.
3.5.1. Investigation of System Applicability and Specificity
The chromatograms of Yinqiao powder samples and negative samples at 237 nm and 327 nm are shown in Figure 7.
Figure 7.
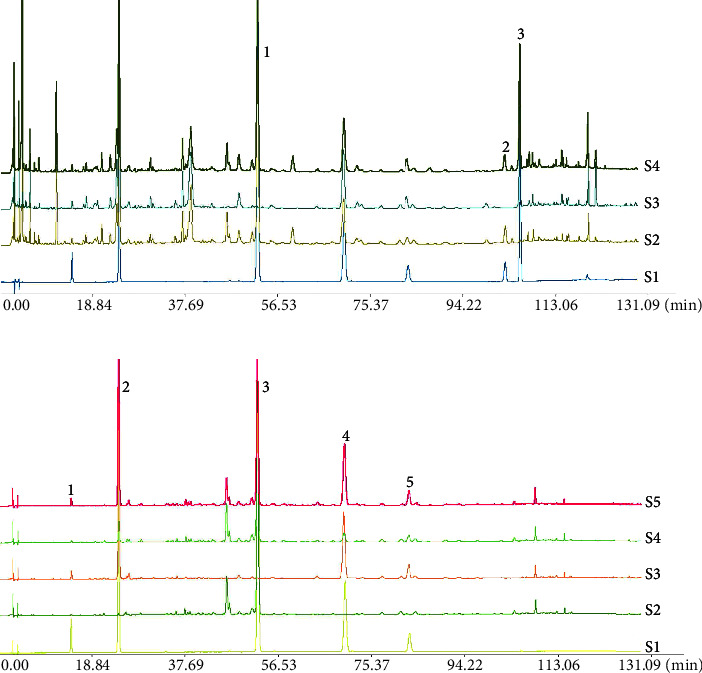
Chromatograms of yinqiao powder and negative control samples at 237 nm (a) and 327 nm (b). In panel a, 1. forsythoside A 2. phillyrin, 3. arctiin, S1. mixed reference, S2. negative control sample of Arctii Fructus, S3. negative control sample of Forsythiae Fructus, S4. Yinqiao powder. In panel b, 1. neochlorogenic acid, 2. chlorogenic acid, 3. forsythoside A, 4. isochlorogenic acid A, 5. isochlorogenic acid C, S1. mixed reference, S2. negative control sample of Lonicerae Japonicaeflos and Arctii Fructus, S3. negative control sample of Forsythiae Fructus, S4. negative control sample of Lonicerae japonicaeflos, S5. Yinqiao powder.
3.5.2. Linearity
Two kinds of mixed reference solutions were analyzed, respectively, and the calibration curves were plotted with different density solution (X, μg) and corresponding peak area (Y, A). The results (Table 6) showed that there was a good correlation between the injection volume and peak area for each compound.
Table 6.
Linear regression equation of seven reference substances.
| Wavelength (nm) | Reference substance | Regression equations | R | Linear range (μg) |
|---|---|---|---|---|
| 237 | Forsythoside A | y = 26.30x + 0.073 | 0.9995 | 0.1776–2.96 |
| Phillyrin | y = 21.89x + 0.024 | 0.9995 | 0.0165–0.275 | |
| Arctiin | y = 22.68x + 0.073 | 0.9995 | 0.0678–1.130 | |
|
| ||||
| 327 | Forsythoside A | y = 43.42x + 0.065 | 0.9995 | 0.1776–2.960 |
| Neochlorogenic acid | y = 94.10x + 0.048 | 0.9995 | 0.00462–0.077 | |
| Chlorogenic acid | y = 70.02x + 0.027 | 0.9995 | 0.0576–0.960 | |
| Isochlorogenic acid A | y = 68.79x − 0.420 | 0.9995 | 0.039–0.650 | |
| Isochlorogenic acid C | y = 78.88x − 0.090 | 0.9995 | 0.00912–0.152 | |
3.5.3. Precision, Stability, Repeatability, and Accuracy
The precision was measured by continuous injection of two kinds of mixed reference solutions for six times at different wavelengths. The stability was validated by analyzing the sample solution of Yinqiao powder at 0, 3, 6, 9, 12, and 24 h at room temperature. Six identical samples of Yinqiao powder were prepared accurately and peak area at different wavelengths were determined in these parallel samples. The accuracy was determined through adding a certain amount of reference substances to six identical samples of Yinqiao powder with a known content. The recovery rate was calculated using the equation (1):
| (1) |
As shown in Table S7, the RSD of each component was 0.11%∼2.72% and the recovery rate of seven compounds was 96.32%∼101.79%, indicating that this method could accurately determine the content of seven components in the sample.
3.5.4. Calculation of Relative Correction Factors (RCFs, ƒx)
RCFs were calculated using the equation (2):
| (2) |
In this equation, Wm indicates the concentration of a specific reference substance, and Am indicates the peak area of reference substance. Forsythoside A was selected as the internal standard reference to calculate the RCFs of phillyrin and arctiin at 237 nm, and the RCFs of neochlorogenic acid, chlorogenic acid, isochlorogenic acid A and isochlorogenic acid C were calculated at 327 nm (Table 7).
Table 7.
The RCFs of components at 237 nm and 327 nm.
| 237 nm | 327 nm | |||||
|---|---|---|---|---|---|---|
| Phillyrin | Arctiin | Neochlorogenic acid | Chlorogenic acid | Isochlorogenic acid A | Isochlorogenic acid C | |
| 1 | 0.832 | 0.867 | 2.179 | 1.613 | 1.567 | 1.793 |
| 2 | 0.836 | 0.869 | 2.185 | 1.614 | 1.567 | 1.800 |
| 3 | 0.838 | 0.867 | 2.178 | 1.613 | 1.566 | 1.796 |
| 4 | 0.841 | 0.867 | 2.181 | 1.611 | 1.564 | 1.803 |
| 5 | 0.840 | 0.869 | 2.181 | 1.614 | 1.566 | 1.802 |
| 6 | 0.838 | 0.867 | 2.187 | 1.614 | 1.566 | 1.795 |
| Mean | 0.838 | 0.868 | 2.182 | 1.613 | 1.566 | 1.798 |
| RSD | 0.41% | 0.13% | 0.17% | 0.06% | 0.06% | 0.23% |
3.5.5. Reproducibility Investigation of RCFs
The reproducibility of RCFs was investigated under the condition of different instruments and different chromatographic columns. The result showed that the RCFs had a good reproducibility in different instruments and columns (Table S8).
3.5.6. Comparison between QAMS and ESM
The relative errors (REs) built by calculating deviations between the QAMS and ESM ranged from −0.83% to 1.08%. The result showed that there was no significant difference on RE of the two methods, indicating that QAMS was feasible to control the quality of Yinqiao powder (Tables 8 and 9).
Table 8.
The calculation results of the ESM and QAMS method at 237 nm.
| Batch | Forsythoside A | Phillyrin | Arctiin | ||||
|---|---|---|---|---|---|---|---|
| ESM | ESM | QAMS | RE % | ESM | QAMS | RE % | |
| 1 | 8.689 | 0.868 | 0.862 | −0.69 | 5.077 | 5.062 | −0.30 |
| 2 | 9.311 | 1.292 | 1.283 | −0.70 | 4.763 | 4.748 | −0.31 |
| 3 | 8.260 | 1.146 | 1.138 | −0.70 | 6.034 | 6.016 | −0.30 |
| 4 | 8.193 | 1.107 | 1.099 | −0.72 | 6.024 | 6.005 | −0.32 |
| 5 | 8.224 | 1.043 | 1.036 | −0.67 | 6.195 | 6.176 | −0.31 |
| 6 | 9.462 | 1.125 | 1.117 | −0.71 | 5.878 | 5.860 | −0.31 |
| 7 | 9.346 | 1.110 | 1.103 | −0.63 | 5.653 | 5.635 | −0.32 |
| 8 | 9.316 | 1.229 | 1.220 | −0.73 | 5.445 | 5.428 | −0.31 |
| 9 | 8.376 | 0.993 | 0.987 | −0.60 | 4.713 | 4.699 | −0.30 |
| 10 | 8.653 | 1.114 | 1.107 | −0.63 | 6.257 | 6.238 | −0.30 |
Table 9.
The calculation results of the ESM and QAMS method at 327 nm.
| Batch | Forsythoside A | Neochlorogenic acid | Chlorogenic acid | Isochlorogenic acid A | Isochlorogenic acid C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESM | ESM | QAMS | RE % | ESM | QAMS | RE % | ESM | QAMS | RE % | ESM | QAMS | RE % | |
| 1 | 8.603 | 0.063 | 0.063 | 0.00 | 3.633 | 3.629 | −0.11 | 2.502 | 2.502 | 0.00 | 0.436 | 0.433 | −0.69 |
| 2 | 9.202 | 0.070 | 0.070 | 0.00 | 4.283 | 4.279 | −0.09 | 2.349 | 2.349 | 0.00 | 0.540 | 0.536 | −0.74 |
| 3 | 8.165 | 0.095 | 0.095 | 0.00 | 4.438 | 4.433 | −0.11 | 2.871 | 2.872 | 0.03 | 0.669 | 0.665 | −0.60 |
| 4 | 8.082 | 0.094 | 0.094 | 0.00 | 4.590 | 4.585 | −0.11 | 2.760 | 2.761 | 0.04 | 0.685 | 0.680 | −0.73 |
| 5 | 8.133 | 0.093 | 0.094 | 1.08 | 4.360 | 4.355 | −0.11 | 2.828 | 2.828 | 0.00 | 0.691 | 0.687 | −0.58 |
| 6 | 9.424 | 0.087 | 0.087 | 0.00 | 4.305 | 4.301 | −0.09 | 2.792 | 2.792 | 0.00 | 0.616 | 0.612 | −0.65 |
| 7 | 9.300 | 0.086 | 0.086 | 0.00 | 4.198 | 4.194 | −0.10 | 2.753 | 2.753 | 0.00 | 0.604 | 0.599 | −0.83 |
| 8 | 9.234 | 0.086 | 0.086 | 0.00 | 4.389 | 4.384 | −0.11 | 2.589 | 2.590 | 0.04 | 0.642 | 0.638 | −0.62 |
| 9 | 8.317 | 0.064 | 0.064 | 0.00 | 3.816 | 3.812 | −0.10 | 2.342 | 2.343 | 0.04 | 0.485 | 0.482 | −0.62 |
| 10 | 8.549 | 0.096 | 0.096 | 0.00 | 4.394 | 4.389 | −0.11 | 2.826 | 2.826 | 0.00 | 0.707 | 0.703 | −0.57 |
4. Conclusions
In this study, network pharmacology and UPLC-Q-Exactive-Orbitrap-MS were combined with HPLC fingerprints and QAMS for the quality evaluation of Yinqiao powder for the first time. This quality evaluation method covered four aspects: chemical compositions, pharmacological effects, qualitative analysis, and quantitative analysis.
Network pharmacology and UPLC-Q-Exactive-Orbitrap-MS were used to screen the potential active components of Yinqiao powder. The network pharmacology results showed that Yinqiao powder may inhibit the inflammatory response by suppressing IL-6, CXCL2, TNFα, NF-κB, etc., in the treatment of COVID-19. A total of 53 compounds were identified in Yinqiao powder extract and 33 blood components were identified in rat serum using UPLC-Q-Exactive-Orbitrap-MS. The combination of network pharmacology and serum pharmacochemistry could lay the foundation of the medicinal material for Yinqiao powder. In the HPLC fingerprint analysis of Yinqiao powder, 11 compounds were identified. It was clear that the main components were from Lonicerae Japonicae Flos, Forsythiae Fructus, and Arctii Fructus by comparing the chromatograms of single herbs. The multivariate statistical analysis results showed that chlorogenic acid, neochlorogenic acid, isochlorogenic acid A, isochlorogenic acid C, arctiin, phillyrin, and forsythoside A were key components affecting the quality of Yinqiao powder. For the quantitative analysis of Yinqiao powder, the QAMS method was carried out at double-wavelength (237 nm, 327 nm). The content of forsythoside A, phillyrin, arctiin, neochlorogenic acid, chlorogenic acid, isochlorogenic acid A, and isochlorogenic acid C was simultaneously determined using forsythoside A as the internal standard reference. The result of reproducibility investigation of RCFs showed that the reproducibility was good and the RSDs were all less than 3%. In order to verify the reliability and accuracy of the QAMS method established in this experiment, the experimental results measured by QAMS method were compared with those measured by ESM. The comparison results of QAMS and ESM showed that there was no significant difference between the two methods, which indicated that the QAMS method was economical and efficient.
This study did not conduct a more in-depth exploration due to the limited time. In conclusion, the quality evaluation method established in our study could comprehensively and scientifically reflect the stability of the internal quality of Yinqiao powder, which is conducive to the improvement of the quality standard of Yinqiao powder and provides a beneficial guarantee for the clinical treatment of COVID-19.
Acknowledgments
This research was funded by the Key Research and Development Program of Shandong Province (no. 2018GSF119018), National Key R & D Program of China (no. 2019YFE0117800), and Collaborative Innovation Center for quality control and whole industry chain construction of Traditional Chinese Medicine In Colleges and Universities of Shandong Province (no. CYLXTCX2021-01).
Abbreviations
- HPLC:
High-performance liquid chromatography
- UPLC:
Ultrahigh-performance liquid chromatography
- QAMS:
Quantitative analysis of multi-components by single marker
- ESM:
External standard method
- COVID-19:
Coronavirus disease 2019
- TCM:
Traditional Chinese medicine
- SARS:
Severe acute respiratory syndrome
- OB:
Oral bioavailability
- DL:
Drug-likeness
- Ch. P:
Pharmacopoeia of the People's Republic of China
- PPI:
Protein-protein interaction
- GO:
Gene Ontology
- KEGG:
Kyoto Encyclopedia of Genes and Genomes
- BP:
Biological processes
- CC:
Cellular components
- MF:
Molecular functions
- NCE:
Normalized collision energy
- UAE:
Ultrasonic assisted extraction
- SNR:
Signal-to-noise ratio
- TIC:
Total ion chromatogram
- RSD:
Relative standard deviation
- PCA:
Principal component analysis
- PLS-DA:
Partial least squares discriminant analysis
- RCF:
Relative correction factor
- RE:
Relative error.
Contributor Information
Qingjun Li, Email: liqjxxhh@126.com.
Xinyan Qu, Email: qxy0117@126.com.
Ping Wang, Email: wangpingjinan@163.com.
Data Availability
The data used to support the findings of this study are included within the article and supplementary information file.
Conflicts of Interest
Author Aijun Zhang is employed by Shandong Hongjitang Pharmaceutical Group Co., Ltd. The remaining authors declare that they have no conflicts of interest.
Authors' Contributions
Huimin Zhang, Lin Xu, and Jian Song contributed equally to this work. Huimin Zhang, Jian Song, Aijun Zhang, Qingjun Li, Xinyan Qu, and Ping Wang conceived and designed the experiment. Huimin Zhang, Lin Xu, and Jian Song performed the experiments. Huimin Zhang, Lin Xu, and Xiao Zhang analyzed data and wrote and revised the manuscript. Huimin Zhang, Jian Song, Qingjun Li, Xinyan Qu, and Ping Wang supervised all work. All authors revised, read, and approved the submitted version.
Supplementary Materials
Table S1: Information of the standard materials; Table S2: The KEGG enrichment results of Yinqiao powder in treating for COVID-19; Table S3: Similarity evaluation of 10 batches of Yinqiao powder; Table S4: Relative retention time and RSD of common peaks; Table S5: Median retention time and average peak area of common peaks; Table S6: Variance contribution rates of principal components; Table S7: Results of method validation; Table S8: RCFs in different instruments and columns.
References
- 1.Li D. X. A clinical analysis of treating children’s HFMD with the Zhuye Shigao decoction. Clinical Journal of Chinese Medicine . 2017;9:63–65. [Google Scholar]
- 2.Guo R., Zhao M., Liu H., et al. Uncovering the pharmacological mechanisms of Xijiao Dihuang decoction combined with Yinqiao powder in treating influenza viral pneumonia by an integrative pharmacology strategy. Biomedicine & Pharmacotherapy . 2021;141 doi: 10.1016/j.biopha.2021.111676.111676 [DOI] [PubMed] [Google Scholar]
- 3.Fan Y., Liu W., Wan R., et al. Efficacy and safety of yinqiao powder combined with western medicine in the treatment of pneumonia: a systematic review and meta-analysis. Complementary Therapies in Clinical Practice . 2021;42 doi: 10.1016/j.ctcp.2020.101297.101297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y. L., Lin L. F., Luo Y. T., Wu Y. Q., Li H. Research progress of heat-clearing and detoxifying traditional Chinese medicine in treatment of corona virus disease 2019. Chinese archives of traditional Chinese medicine . 2021;39:181–186. [Google Scholar]
- 5.Cao L. H., Jia X. Y., He H. J., et al. Using a system pharmacology method to search for the potential targets and pathways of Yinqiaosan against COVID-19. Journal of Healthcare Engineering . 2022;2022 doi: 10.1155/2022/9248674.9248674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China . Beijing, China: China Medical Science Press; 2020. [Google Scholar]
- 7.Ma F. X., Xue P. F., Wang Y. Y., Wang Y. N., Xue S. Y. Research progress of serum pharmacochemistry of traditional Chinese medicine. China Journal of Chinese Materia Medica . 2017;42:1265–1270. doi: 10.19540/j.cnki.cjcmm.20170224.010. [DOI] [PubMed] [Google Scholar]
- 8.Sang Q., Jia Q., Zhang H., et al. Chemical profiling and quality evaluation of Zhishi-Xiebai-Guizhi Decoction by UPLC-Q-TOF-MS and UPLC fingerprint. Journal of Pharmaceutical and Biomedical Analysis . 2021;194 doi: 10.1016/j.jpba.2020.113771.113771 [DOI] [PubMed] [Google Scholar]
- 9.Li C., Cui Y., Lu J., et al. Spectrum-effect relationship of immunologic activity of Ganoderma lucidum by UPLC-MS/MS and component knock-out method. Food Science and Human Wellness . 2021;10:278–288. doi: 10.1016/j.fshw.2021.02.019. [DOI] [Google Scholar]
- 10.Jiang M., Cao J., Zhang C., et al. A comprehensive strategy for quality evaluation of Wushe Zhiyang Pills by integrating UPLC-DAD fingerprint and multi-ingredients rapid quantitation with UPLC-MS/MS technology. Journal of Pharmaceutical and Biomedical Analysis . 2022;210 doi: 10.1016/j.jpba.2021.114556.114556 [DOI] [PubMed] [Google Scholar]
- 11.Peng Y., Dong M., Zou J., Liu Z. Analysis of the HPLC fingerprint and QAMS for sanhuang gypsum soup. journal of analytical methods in chemistry . 2018;2018 doi: 10.1155/2018/5890973.5890973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren K. J., Xu J., He S. L. Quality control of seven components in bushen kangle capsules based on quantitative analysis of multi-components by single marker. China pharmacist . 2020;23:1539–1544. [Google Scholar]
- 13.Liu Y. X., Feng C. P., Liu L. L. Simultaneous determination of 8 components in Erzhi pills by QAMS method. Chinese Journal of Pharmaceutical Analysis . 2021;41:210–218. [Google Scholar]
- 14.Yao Z., Yu J., Tang Z., et al. Multi-evaluating strategy for siji-kangbingdu mixture: chemical profiling, fingerprint characterization, and quantitative analysis. Molecules . 2019;24 doi: 10.3390/molecules24193545.3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Liu X., Wang J., et al. Study on multiple fingerprint profiles control and quantitative analysis of multi-components by single marker method combined with chemometrics based on Yankening tablets. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy . 2021;253 doi: 10.1016/j.saa.2021.119554.119554 [DOI] [PubMed] [Google Scholar]
- 16.Yin C., Wang H. F., Xiang H. X., Wang S. Simultaneous determination of 4 index components in yinqiao powder by UPLC. Chinese journal of hospital pharmacy . 2016;36:2170–2172. [Google Scholar]
- 17.Yin C., Long H. N., Liu L. Study on UPLC fingerprint of yinqiao powder. journal of Chinese medicinal materials . 2017;40:379–383. [Google Scholar]
- 18.Zhang L., Wang Y., Zheng F., Zhu D., Liang Y., Shi Q. Influence exerted by the solvent effect on the mobility peak of 1, 8-naphthalic anhydride in ion mobility spectrometry. Journal of the American Society for Mass Spectrometry . 2022;33:457–462. doi: 10.1021/jasms.1c00299. [DOI] [PubMed] [Google Scholar]
- 19.Zhao C., Cheng J., Li C., et al. Quality evaluation of Acanthopanax senticosus via quantitative analysis of multiple components by single marker and multivariate data analysis. Journal of Pharmaceutical and Biomedical Analysis . 2021;201 doi: 10.1016/j.jpba.2021.114090.114090 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z. H., Zhong P. P., Xu Y. Comparison of chemical compositions between tanreqing capsule and tanreqing injection by HPLC-ESI-MS/MS. Chinese journal of experimental traditional medical formulae . 2017;23:44–51. [Google Scholar]
- 21.Dong T., Zhou J. L., Zhao W. L., Zhang W. T., Huang Q. W. Quality evaluation of yinqiao jiedu granules by HPLC fingerprint. Chinese journal of modern applied pharmacy . 2018;35:1337–1341. [Google Scholar]
- 22.Zhao L. J., Gao W. Y., Gu X. R., Wang H. J., Zhao H. Y., Bian B. L. Identification and attribution of chemical compounds of pudilan antiphlogistic oral liquid. China journal of Chinese materia medica . 2019;44:1573–1587. doi: 10.19540/j.cnki.cjcmm.20181221.009. [DOI] [PubMed] [Google Scholar]
- 23.Li C., Lv J., Yang L. F., Zhao B. N., Gao Y. Integrate network pharmacology to explore the anti-RSV pneumonia mechanism of Lonicera Japonica Thunb. based upon UPLC-Q-Exactive-Orbitrap-MS. Chinese Journal of Hospital Pharmacy . 2021;41:769–776. [Google Scholar]
- 24.Zhang W. X., Feng M., Miao Y. L., Zhang W. Z. Analysis of chemical components of huanbei zhike prescription based on UPLC-Q-TOF-MS/MS technology. China journal of Chinese materia medica . 2019;44:3022–3034. doi: 10.19540/j.cnki.cjcmm.20190410.304. [DOI] [PubMed] [Google Scholar]
- 25.Li L. L., Li Y., Lu H., Wang X. Metabolomics analysis of Lonicera japonica in different flowering stages based on liquid chromatography-mass spectrometry. Journal of Instrumental Analysis . 2020;39:1501–1507. [Google Scholar]
- 26.Yan J., Zhao C. F., Li B. P. Chemical constituents and antioxidant activity of radix isatidis. journal of Chinese mass spectrometry society . 2017;38:248–255. [Google Scholar]
- 27.Lv X., Sun J. Z., Xu S. Z., Cai Q., Liu Y. Q. Rapid characterization and identification of chemical constituents in Gentiana radix before and after wine-processed by UHPLC-LTQ-Orbitrap MS(n), Molecules. Molecules . 2018;23(12) doi: 10.3390/molecules23123222.3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C. X., Liu S. X., Zhao Y. M., Zhang T. J., Liu D. L. Analysis on chemical constituents from Forsythiae Fructus by HPLC-Q-TOF-MS. Chinese traditional and herbal drugs . 2016;47:2053–2060. [Google Scholar]
- 29.Xiang Q., Wang X. H., Lin H., Lin J., Xu W. Qualitative and quantitative analysis of major constituents in yinhuang granules by HPLC-DAD-Q-TOF-MS/MS. Chinese traditional patent medicine . 2015;37:105–112. [Google Scholar]
- 30.An X. X., Li Y. Y., Zhan Y. F. Analysis of the contents of total flavonoids in Dandelion and determination of main components based on UPLC-Q-Exactive and HPLC. Traditional Chinese Drug Research and Clinical Pharmacology . 2019;30:99–105. [Google Scholar]
- 31.Zhang N., Gao X., Zhou Y., Geng T. Rapid identification of chemical components in xingbei zhike keli by UPLC-Q-TOF-MS/MS. China journal of Chinese materia medica . 2018;43:4439–4449. doi: 10.19540/j.cnki.cjcmm.20180807.004. [DOI] [PubMed] [Google Scholar]
- 32.Fang G., Zhang P., Ye X. L., Zhu X., Zhao X., Fan Gr. Electron spray ion trap mass spectrometry of isoflavones and isoflavone aglycones of Semen Sojae Praeparatum. Academic Journal of Second Military Medical University . 2013;33:1108–1115. doi: 10.3724/sp.j.1008.2013.01108. [DOI] [Google Scholar]
- 33.Zhou X. J., Li Y. F., Chen Y., Chen X. H., Zeng R. Rapid establishment of Q-Marker database for Qingreling Granules with UPLC coupled with hybrid quadrupole-orbitrap mass spectrometry. Chinese Traditional and Herbal Drugs . 2017;48:67–74. [Google Scholar]
- 34.Yang D., Li F. T., Liu M. Analysis of chemical constituents in physalis Calyx seu Fructus using UPLC-Q-orbitrap MS/MS. journal of Chinese mass spectrometry society . 2021;42:253–260. [Google Scholar]
- 35.Zhou M. Y., Huo J. H., Sun G. D., Wang W. M. Identification of 45 kinds of chemical components of Forsythia suspensa by UPLC-Q-TOF-MS. China Pharmacy . 2019;30:3067–3073. [Google Scholar]
- 36.Yang L., Yuan F. R., Cao L. Identification of chemical constituents in chaishi tuire granules by UPLC-ESI-Q-TOF-MS/MS. Chinese journal of experimental traditional medical formulae . 2021;27:152–159. [Google Scholar]
- 37.Liu J. T., Zhang Y., Bu R. Z. Identification of chemical components and blood components of biqi capsules by UPLC-Q/TOF-MS. Chinese traditional and herbal drugs . 2021;52:5496–5513. [Google Scholar]
- 38.Xu W., Wu X., Huang M. Q., et al. Analysis on pharmacodynamic constituents in Gualou Guizhi Decoction by serum pharmacochemistry. Chinese Traditional and Herbal Drugs . 2017;48(10):2033–2043. [Google Scholar]
- 39.Jin H., Mou J. J., Xia N. Identification and analysis of absorbed components in rat plasma after oral administration of Jinqi Jiangtang Tablets by UPLC-ESI-MS. Drug Evaluation Research . 2018;41:2227–2231. [Google Scholar]
- 40.Zhou W., Shan J. J., Meng M. X. A two-step ultra-high-performance liquid chromatography-quadrupole/time of flight mass spectrometry with mass defect filtering method for rapid identification of analogues from known components of different chemical structure types in Fructus Gardeniae-Fructus Forsythiae herb pair extract and in rat’s blood. Journal of Chromatography A . 2018;1563:99–123. doi: 10.1016/j.chroma.2018.05.067. [DOI] [PubMed] [Google Scholar]
- 41.Kumar G., Paliwal P., Mukherjee S., Patnaik N., Krishnamurthy S., Patnaik R. Pharmacokinetics and brain penetration study of chlorogenic acid in rats. Xenobiotica . 2019;49:339–345. doi: 10.1080/00498254.2018.1445882. [DOI] [PubMed] [Google Scholar]
- 42.Law A. H. Y., Yang C. L. H., Lau A. S. Y., Chan G. C. F. Antiviral effect of forsythoside A from Forsythia suspensa (Thunb.) Vahl fruit against influenza A virus through reduction of viral M1 protein. Journal of Ethnopharmacology . 2017;209:236–247. doi: 10.1016/j.jep.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Ma Q., Li R., Pan W., et al. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway. Phytomedicine . 2020;78 doi: 10.1016/j.phymed.2020.153296.153296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin H., Li C. L., Yen L. J., et al. Forsythoside A alleviates imiquimod-induced psoriasis-like dermatitis in mice by regulating Th17 cells and IL-17a expression. Journal of Personalized Medicine . 2022;12:p. 62. doi: 10.3390/jpm12010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu L. L., Xiao Y., Zhi X. Screening active ingredients of Lonicerae Japonicae Flos against influenza based on Box-Behnken response surface method. Modern Chinese Medicine . 2016;18:1454–1457. [Google Scholar]
- 46.Barragán-Zarate G. S., Alexander-Aguilera A., Lagunez-Rivera L., Solano R., Soto-Rodríguez I. Bioactive compounds from Prosthechea karwinskii decrease obesity, insulin resistance, pro-inflammatory status, and cardiovascular risk in Wistar rats with metabolic syndrome. Journal of Ethnopharmacology . 2021;279 doi: 10.1016/j.jep.2021.114376.114376 [DOI] [PubMed] [Google Scholar]
- 47.Liu X. T., Gu L. G., Deng D. Y. Effects of baicalin and luteolin-7-O-glucoside on NF-κB signaling pathway in A549 cells infected with influenza virus H1N1 in vitro. China Journal of Traditional Chinese Medicine . 2016;31:1937–1941. [Google Scholar]
- 48.Kalita B., Das M. K. Rutin–phospholipid complex in polymer matrix for long-term delivery of rutin via skin for the treatment of inflammatory diseases. Artificial Cells, Nanomedicine, and Biotechnology . 2018;46:41–56. doi: 10.1080/21691401.2017.1411931. [DOI] [PubMed] [Google Scholar]
- 49.Tejada S., Pinya S., Martorell M. Potential anti-inflammatory effects of hesperidin from the genus citrus. current medicinal chemistry . 2018;25:4929–4945. doi: 10.2174/0929867324666170718104412. [DOI] [PubMed] [Google Scholar]
- 50.Zhai K. F., Duan H., Cui C. Y., et al. Liquiritin from Glycyrrhiza uralensis attenuating rheumatoid arthritis via reducing inflammation, suppressing angiogenesis, and inhibiting MAPK signaling pathway. Journal of Agricultural and Food Chemistry . 2019;67:2856–2864. doi: 10.1021/acs.jafc.9b00185. [DOI] [PubMed] [Google Scholar]
- 51.Tan S., Gao J., Li Q., et al. Synergistic effect of chlorogenic acid and levofloxacin against Klebsiella pneumonia infection in vitro and in vivo. Scientific Reports . 2020;10 doi: 10.1038/s41598-020-76895-5.20013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao X. H., Zhang S. D., Wang L. T., et al. Anti-inflammatory effects of neochlorogenic acid extract from Mulberry Leaf (Morus alba L.) against LPS-stimulated inflammatory response through mediating the AMPK/Nrf2 signaling pathway in A549 cells. Molecules . 2020;25 doi: 10.3390/molecules25061385.1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma R., Ma R. Q., Chen B., Wang L. Y., Fan X. Y. Compound cocktail inhibits influenza viral pneumonia via phospholipase Cγ1 phosphorylation-related necroptosis and partial autophagy in natural killer cells. Planta Medica . 2021;87(07):538–549. doi: 10.1055/a-1353-6672. [DOI] [PubMed] [Google Scholar]
- 54.Lu Z. B., Liu S. H., Ou J. Y., et al. Forsythoside A inhibits adhesion and migration of monocytes to type II alveolar epithelial cells in lipopolysaccharide-induced acute lung injury through upregulating miR-124. Toxicology and Applied Pharmacology . 2020;407 doi: 10.1016/j.taap.2020.115252.115252 [DOI] [PubMed] [Google Scholar]
- 55.Ghazavi A., Ganji A., Keshavarzian N., Rabiemajd S., Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine . 2021;137 doi: 10.1016/j.cyto.2020.155323.155323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Q., Zhu Z., Tan C., et al. Changes of serum IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 in COVID‐19 patients. International Journal of Clinical Practice . 2021;75(9) doi: 10.1111/ijcp.14462.e14462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pacha O., Sallman M. A., Evans S. E. COVID-19: a case for inhibiting IL-17? Nature reviews. Immunology . 2020;20:345–346. doi: 10.1038/s41577-020-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson P. C., Liew D. F. L., Liew J. W., et al. The potential for repurposing anti-TNF as a therapy for the treatment of COVID-19. Medical Times . 2021;1 doi: 10.1016/j.medj.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MontalvanToledo V., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clinical Neurology and Neurosurgery . 2020;194 doi: 10.1016/j.clineuro.2020.105921.105921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hariharan A., Hakeem A. R., Radhakrishnan S., Reddy M. S., Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology . 2021;29:91–100. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radigan K. A., Nicholson T. T., Welch L. C., et al. Influenza A virus infection induces muscle wasting via IL-6 regulation of the E3 ubiquitin ligase atrogin-1. The Journal of Immunology . 2019;202:484–493. doi: 10.4049/jimmunol.1701433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Information of the standard materials; Table S2: The KEGG enrichment results of Yinqiao powder in treating for COVID-19; Table S3: Similarity evaluation of 10 batches of Yinqiao powder; Table S4: Relative retention time and RSD of common peaks; Table S5: Median retention time and average peak area of common peaks; Table S6: Variance contribution rates of principal components; Table S7: Results of method validation; Table S8: RCFs in different instruments and columns.
Data Availability Statement
The data used to support the findings of this study are included within the article and supplementary information file.


