Abstract
Given the high rate of depression associated with narcolepsy or obstructive sleep apnea (OSA), this analysis compared effects of solriamfetol treatment of excessive daytime sleepiness (EDS) in participants with/without a history of depression (DHx+/DHx−). This secondary analysis included data from two randomized, controlled trials in which participants were randomized to 12 weeks placebo or solriamfetol 37.5 (OSA only), 75, 150, or 300 mg/day. Efficacy/safety (combined solriamfetol doses) was summarized for DHx+/DHx−subgroups. 27.5% (65/236) with narcolepsy and 23.4% (111/474) with OSA were DHx+. In narcolepsy (DHx+ and DHx−), 40-min Maintenance of Wakefulness Test (MWT40) mean sleep latency increased (5.4 and 7.0 min), Epworth Sleepiness Scale (ESS) score decreased (3.8 and 3.5 points), and percentage of participants improved on Patient Global Impression of Change (PGI-C) was higher (31.7% and 39.4%) relative to placebo. In OSA (DHx+ and DHx−), MWT40 mean sleep latency increased (7.7 and 10.7 min), ESS decreased (3.5 and 3.7 points), and percentage of participants improved on PGI-C was higher (41.1% and 29.4%) relative to placebo. Common treatment-emergent adverse events (headache, decreased appetite, nausea, anxiety) were similar in DHx+/DHx−. This study suggests that safety and efficacy of solriamfetol for treating EDS in narcolepsy and OSA are not affected by depression history. Moreover, the findings emphasize the high prevalence of depression in people with sleep disorders and suggest that increased awareness of this association may have clinical significance.
Keywords: Sunosi, JZP-110, Wakefulness, Antidepressant agents
1. Introduction
Excessive daytime sleepiness (EDS) can have many etiologies, including lack of sleep, medications, substance abuse, psychiatric disorders, medical disorders, or sleep disorders. EDS is a fundamental symptom of narcolepsy and is required for its diagnosis. Long-term management generally includes symptomatic treatment, including pharmacotherapy for EDS (Thorpy, 2020). In patients with obstructive sleep apnea (OSA), EDS can persist despite primary therapy for the underlying airway obstruction, thus adjunctive pharmacotherapy may be useful. Population-based studies have estimated that 9%–22% of patients with OSA continue to experience EDS despite normalization of breathing, oxygenation, and sleep quality with continuous positive airway pressure (CPAP) (the gold standard treatment for OSA) (Gasa et al., 2013; Patil et al., 2019; Pepin et al., 2009).
Depression commonly co-occurs with both narcolepsy and OSA. The prevalence of depression is estimated to be 32% in narcolepsy and 22% in untreated OSA (Acker et al., 2017; Jackson et al., 2019; Li et al., 2021). In patients with narcolepsy, risk factors for comorbid depression have not been well established, although studies have shown that prevalence is higher in adults than children and adolescents (Li et al., 2021). It has been postulated that worsening of narcolepsy symptoms (eg, deterioration in functioning and poor quality of life) may lead to depressive symptoms. In patients with OSA, population-based, longitudinal studies have shown that the severity of OSA significantly predicts the odds of developing depression (Peppard et al., 2006). Specifically, patients who have even minimal (apnea-hypopnea index [AHI] >0 to <5/h) or mild (AHI 5–14/h) OSA are twice as likely to have depression than those who do not have OSA; those who have moderate to severe (AHI ≥15/h) OSA are nearly three times as likely to have depression (Peppard et al., 2006).
Given these rates of depression in the narcolepsy and OSA populations (Acker et al., 2017; Jackson et al., 2019; Li et al., 2021), some patients with either current depression, a history of depression, or taking antidepressants will be administered treatments for EDS associated with narcolepsy or OSA. Solriamfetol is a dopamine and norepinephrine re-uptake inhibitor (DNRI) indicated for the treatment of adults with EDS associated with narcolepsy (75–150 mg/day) or OSA (37.5–150 mg/day) (Sunosi, 2021; Schweitzer et al., 2019; Thorpy et al., 2019). Notably, there is overlap in the mechanisms of action between solriamfetol and some antidepressant medications (eg, serotonin and norepinephrine reuptake inhibitors [SNRIs] and DNRIs), and individuals prone to depression may have a heightened risk for experiencing suicidal ideation, anxiety, or triggering of depressive or manic episodes in response to medication for EDS associated with narcolepsy or OSA (Nuvigil 2018; Provigil 2018; Sunosi 2021; Wakix 2021).
As such, this analysis was undertaken to explore whether the therapeutic and adverse effects of solriamfetol treatment are affected by the presence of depression, having a history of depression (depression is an episodic phenomenon and a patient may not be symptomatic when assessed at a particular point in time), and/or the concomitant use of antidepressant medications (American Psychiatric Association, 2013). Specifically, these post hoc analyses examined the efficacy and safety of solriamfetol compared with placebo for the treatment of EDS associated with narcolepsy or OSA in subgroups of participants with or without a history of depression, as well as in subgroups of participants who were or were not on concomitant antidepressants.
2. Methods
2.1. Study Design
This post hoc analysis included data from two 12-week, randomized, placebo-controlled, parallel-group studies (TONES 2, TONES 3) that evaluated the safety and efficacy of solriamfetol in treating EDS in adults with narcolepsy (Thorpy et al., 2019) or OSA (Schweitzer et al., 2019). Both were approved by institutional review boards or ethics committees at each institution and were conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. Detailed methods were published with the primary results (Schweitzer et al., 2019; Thorpy et al., 2019) and are summarized here.
2.2. Participants
Eligible participants in TONES 2 were adults (aged 18–75 years) with narcolepsy (diagnosed with International Classification of Sleep Disorders – Third Edition [ICSD-3] or Diagnostic and Statistical Manual of Mental Disorders, 5th ed [DSM-5] criteria)(American Academy of Sleep Medicine, 2014; American Psychiatric Association, 2013) and EDS (baseline Epworth Sleepiness Scale [ESS] scores ≥10) (Johns, 1991). Eligible participants in TONES 3 were adults (aged 18–75 years) with OSA (diagnosed with ICSD-3) and EDS (baseline ESS scores ≥10) (Johns, 1991). Additional key inclusion criteria were baseline mean sleep latency <25 min (narcolepsy) or <30 min (OSA) on the Maintenance of Wakefulness Test (MWT) (Littner et al., 2005), usual nightly total sleep duration ≥6 h, and, for participants with OSA, current or prior use of a primary OSA therapy (CPAP, oral appliance, or surgical intervention).
Key exclusion criteria included usual bedtime later than 1:00 A.M.; occupations requiring nighttime or variable shift work; any other clinically relevant medical, behavioral, or psychiatric disorder associated with EDS; history or presence of bipolar disorder, bipolar-related disorders, schizophrenia, or schizophrenia spectrum disorders; and moderate or severe substance use disorders (current or within past 2 years). Participants with current depression were excluded if the depression was acutely unstable, associated with EDS, and/or if active suicidal ideation or behavior was present (history of suicidal ideation/behavior was not specifically exclusionary); suicidal ideation (lifetime and over the past 12 months) and behavior (lifetime and over the past 5 years) were assessed with the Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2011) at the screening visit. Excessive caffeine use (>600 mg/day) 1 week prior to and during the study was also exclusionary, as was use of medication that could affect EDS evaluation, such as sleep aids or stimulants (eg, amphetamines), wake-promoting agents (eg, modafinil, armodafinil), pemoline, trazodone, hypnotics, benzodiazepines, barbiturates, and opioids. The TONES 2 study excluded use of medication that could affect the evaluation of cataplexy (eg, selective serotonin reuptake inhibitors [SSRIs], SNRIs, tricyclic antidepressants, monoamine oxidase inhibitors, anticonvulsant agents, sodium oxybate). As such, SSRIs and SNRIs being used for the management of cataplexy were excluded or washed out if deemed appropriate by the investigator. SSRIs and SNRIs were not exclusionary in TONES 3, and other antidepressants (eg, bupropion, mirtazapine) were not exclusionary in either study. Participants with urine drug screen positive for illicit drugs of abuse, with the exception of prescribed drugs, at screening or at any point throughout the duration of the study were excluded.
2.3. Treatment
Participants were randomly assigned to receive placebo or solriamfetol 37.5 mg (OSA only), 75 mg, 150 mg, or 300 mg once daily for 12 weeks.
2.4. Assessments
EDS was assessed objectively with the 40-min MWT (Littner et al., 2005) and by self-report with the ESS (Johns, 1991). Change in overall condition was assessed with the Patient Global Impression of Change (PGI-C) (Guy, 1976). Safety and tolerability assessments included treatment-emergent adverse events (TEAEs) and the C-SSRS (Posner et al., 2011) (using the Since Last Visit version at post-screening visits, including the baseline visit). Concomitant medication use was summarized.
2.5. Statistical analysis
This analysis evaluated efficacy and safety outcomes in groups of participants with or without a history of depression. History of depression was identified by the presence of the terms affective disorder, depression, depressed mood, major depression, postpartum depression, and seasonal affective disorder in the complete medical history taken at screening. Sensitivity analyses were performed in which participants who were taking antidepressants but had no noted history of depression were excluded from the data set. To determine the impact of concomitant antidepressant use, efficacy and safety outcomes were also analyzed in groups of participants who were or were not taking concomitant antidepressant medications, regardless of history of depression.
Efficacy endpoints included change from baseline to week 12 in mean sleep latency on the MWT, change from baseline to week 12 in ESS score, and percentage of participants reported as improved (defined as minimally, much, or very much improved) on the PGI-C at week 12. Safety and tolerability were assessed throughout each study.
MWT, ESS, and PGI-C analyses were based on the modified intent-to-treat (mITT) populations, defined as participants who received ≥1 dose of study drug and had ≥1 post-baseline MWT or ESS assessment. Differences from placebo were analyzed with mixed-model repeated measures for the MWT and ESS or with χ2 tests for the PGI-C. Comparisons were not prespecified and did not control for multiplicity; therefore, P values presented are nominal. Safety endpoints were summarized descriptively for the safety populations, defined as participants who received ≥1 dose of study drug. Data from the two trials were analyzed separately.
3. Results
3.1. Participant population
A total of 236 participants with narcolepsy were included in the safety population, of whom 120 (50.8%) had cataplexy. A total of 474 participants with OSA were included in the safety population, of whom 344 (72.6%) used a primary OSA therapy (318 [92.4%] used PAP, 5 [1.5%] used another type of primary OSA therapy device, and 21 [6.1%] did not specify the type of device they used). A history of surgical intervention for OSA was reported in 14.6% of participants.
In the safety populations (narcolepsy, n = 236; OSA, n = 474), 27.5% (65/236) of participants with narcolepsy and 23.4% (111/474) of those with OSA had a history of depression (Supplemental Table 1). In the mITT populations (narcolepsy, n = 231; OSA, n = 459), 28.1% (65/231) of participants with narcolepsy and 23.5% (108/459) of those with OSA had a history of depression.
In the safety populations, 2 participants with narcolepsy (placebo, n = 1; solriamfetol 150 mg, n = 1) and 2 participants with OSA (placebo, n = 1; solriamfetol 300 mg, n = 1) had a history of suicide attempt.
At baseline, mean MWT sleep latency and mean ESS score were similar for participants with or without a history of depression within each study population (Table 1). Baseline mean MWT sleep latency was numerically shorter (more impaired) and baseline mean ESS score was numerically higher (more impaired) in participants with narcolepsy compared with participants with OSA, regardless of depression history (Table 1).
Table 1.
Baseline demographics and clinical characteristics.a
| Narcolepsy |
OSA |
|||||||
|---|---|---|---|---|---|---|---|---|
| History of Depression |
No History of Depression |
History of Depression |
No History of Depression |
|||||
| Placebo (N=17) | Combined Solriamfetol (N=48) | Placebo (N=42) | Combined Solriamfetol (N=129) | Placebo (N=26) | Combined Solriamfetol (N=85) | Placebo (N=93) | Combined Solriamfetol (N=270) | |
| Age, years, mean (SD) | 37.6 (12.45) | 37.1 (10.95) | 35.3 (16.23) | 36.0 (13.02) | 58.1 (11.96) | 55.7 (10.98) | 53.0 (11.06) | 53.3 (10.63) |
| Sex, male, n (%) | 7 (41.2) | 15 (31.3) | 17 (40.5) | 43 (33.3) | 15 (57.7) | 38 (44.7) | 62 (66.7) | 182 (67.4) |
| Race, n (%) | ||||||||
| White | 13 (76.5) | 43 (89.6) | 34 (81.0) | 99 (76.7) | 23 (88.5) | 78 (91.8) | 64 (68.8) | 196 (72.6) |
| African | 3 (17.6) | 3 (6.3) | 7 (16.7) | 20 (15.5) | 2 (7.7) | 4 (4.7) | 24 (25.8) | 59 (21.9) |
| American | ||||||||
| Asian | 0 | 2 (4.2) | 0 | 4 (3.1) | 0 | 1 (1.2) | 4 (4.3) | 12 (4.4) |
| Other | 1 (5.9) | 0 | 1 (2.4) | 6 (4.7) | 1 (3.8) | 2 (2.4) | 1 (1.1) | 3 (1.1) |
| BMI, kg/m2, mean (SD) | 29.49 (6.03) | 27.06 (5.64) | 28.97 (5.99) | 28.35 (5.85) | 32.89 (5.81) | 32.89 (5.38) | 33.15 (5.08) | 33.44 (5.272) |
| MWT sleep latency, min, mean (SD)a | (n = 17) 7.60 (6.69) | (n = 48) 8.86 (5.72) | (n = 40) 5.54 (5.16) | (n = 122) 7.71 (5.77) | (n = 24) 11.99 (5.66) | (n = 81)13.01 (7.01) | (n = 87) 12.75 (7.52) | (n = 258)12.40 (7.47) |
| ESS score, mean (SD)a | (n = 17) 16.59 (2.27) | (n = 48) 16.56 (3.27) | (n = 41) 17.54 (3.05) | (n = 125) 17.38 (3.29) | (n = 24) 14.92 (3.28) | (n = 84)14.52 (3.12) | (n = 90) 15.72 (3.32) | (n = 261)15.28 (3.36) |
Safety population, except MWT and ESS (modified intent-to-treat population). Note: Higher mean sleep latency on the MWT indicates greater ability to stay awake and less severe EDS, whereas higher scores on the ESS represent more severe EDS (Johns, 1991; Littner et al., 2005). BMI, body mass index; ESS, Epworth Sleepiness Scale; MWT, Maintenance of Wakefulness Test; OSA, obstructive sleep apnea; SD, standard deviation.
3.2. Concomitant use of medications
Concomitant medication use varied with indication and depression history (Table 2). As expected, use of antidepressants was considerably higher in participants with a history of depression than in those without a history of depression. Use of antihistamines, nonsteroidal anti-inflammatory drugs, drugs for peptic ulcer and gastroesophageal reflux, and thyroid preparations was somewhat higher in some groups of participants with a history of depression (Table 2).
Table 2.
| n (%) | Narcolepsy |
OSA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| History of Depression |
No History of Depression |
History of Depression |
No History of Depression |
||||||
| Placebo (N=17) | Combined Solriamfetol (N=48) | Placebo (N=42) | Combined Solriamfetol (N=129) | Placebo (N=26) | Combined Solriamfetol (N=85) | Placebo (N=93) | Combined Solriamfetol (N=270) | ||
| Any concomitant medication | 17 (100) | 42 (88) | 33 (79) | 103 (80) | 25 (96) | 84 (99) | 84 (90) | 252 (93) | |
| ACE inhibitor | 0 | 5 (10) | 4 (10) | 7 (5) | 5 (19) | 10 (12) | 12 (13) | 55 (20) | |
| Antidepressantc | 2 (12) | 18 (38) | 3 (7) | 4 (3) | 14 (54) | 50 (59) | 3 (3) | 20 (7) | |
| Antihistamine, systemic | 1 (6) | 8 (17) | 4 (10) | 19 (15) | 7 (27) | 24 (28) | 16 (17) | 45 (17) | |
| Anti-inflammatory, nonsteroidal | 6 (35) | 19 (40) | 11 (26) | 41 (32) | 10 (39) | 35 (41) | 36 (39) | 84 (31) | |
| Anxiolytic | 0 | 4 (8) | 2 (5) | 5 (4) | 1 (4) | 1 (1) | 0 | 6 (2) | |
| Drug for peptic ulcer or gastroesophageal reflux | 3 (18) | 7 (15) | 5 (12) | 11 (9) | 6 (23) | 28 (33) | 21 (23) | 52 (19) | |
| Lipid-modifying agent, | 0 | 3 (6) | 4 (10) | 9 (7) | 14 (54) | 33 (39) | 28 (30) | 97 (36) | |
| plain | |||||||||
| Opioid | 0 | 0 | 1 (2) | 0 | 3 (12) | 6 (7) | 0 | 10 (4) | |
| Other analgesic or antipyretic | 2 (12) | 12 (25) | 5 (12) | 22 (17) | 8 (31) | 19 (22) | 14 (15) | 44 (16) | |
| Psychostimulantd | 1 (6) | 0 | 3 (7) | 12 (9) | 1 (4) | 2 (2) | 0 | 2 (1) | |
| Thyroid preparation | 1 (6) | 5 (10) | 4 (10) | 4 (3) | 3 (12) | 15 (18) | 7 (8) | 30 (11) | |
ACE, angiotensin-converting enzyme; OSA, obstructive sleep apnea.
Safety population.
Concomitant medication defined as medication with stop date on or after first dose of study drug or any medication that is ongoing.
Reported antidepressants included selective serotonin reuptake inhibitors (citalopram, escitalopram, fluoxetine, paroxetine, and sertraline), serotonin and norepinephrine reuptake inhibitors (desvenlafaxine, duloxetine, and venlafaxine), tricyclic antidepressants (amitriptyline and nortriptyline), bupropion, nefazodone, and vortioxetine.
Generally, cases of psychostimulant use were taken after last dose of study drug and on or before the early termination visit or week 14. These patients were
included in the modified intent-to-treat population.
3.3. Efficacy
3.3.1. Efficacy in participants with or without a medical history of depression
Mean MWT sleep latency increased (improved) from baseline to week 12 with solriamfetol in participants with narcolepsy or OSA, regardless of depression history (Fig. 1A). In solriamfetol-treated participants with narcolepsy, least squares (LS) mean changes from baseline to week 12 in mean MWT sleep latency ranged from 4.38 to 12.13 min in participants with a history of depression and from 5.14 to 12.44 min in participants without a history of depression. For the combined solriamfetol group in participants with narcolepsy, LS mean difference from placebo (95% confidence interval [CI]) in mean MWT sleep latency was 5.4 min (−0.2, 11.1) in those with a history of depression and 7.0 min (3.3, 10.7) in those without a history of depression. Results generally were dose-dependent, with greater improvements observed with higher doses of solriamfetol, relative to placebo, regardless of whether participants had a history of depression (LS mean difference from placebo [95% CI]: 75 mg, 2.25 min [−4.53, 9.04]; 150 mg, 5.78 min [−0.61, 12.17]; 300 mg, 10.00 min [2.24, 17.75]) or no history of depression (LS mean difference from placebo [95% CI]: 75 mg, 2.73 [−1.71, 7.17]; 150 mg, 8.43 [3.83, 13.03]; 300 mg, 10.02 [5.59, 14.46]). In solriamfetol-treated participants with OSA, LS mean changes in mean MWT sleep latency from baseline to week 12 ranged from 3.24 to 14.04 min in participants with a history of depression and from 5.05 to 12.88 min in participants without a history of depression. For the combined solriamfetol group in participants with OSA, LS mean difference from placebo (95% CI) in mean MWT sleep latency was 7.7 min (3.2, 12.3) in those with a history of depression and 10.7 min (8.0, 13.3) in those without a history of depression. Results generally were dose-dependent, with greater improvements observed with higher doses of solriamfetol, relative to placebo, regardless of whether participants had a history of depression (LS mean difference from placebo [95% CI]: 37.5 mg, 1.39 min [−6.22, 9.00]; 75 mg, 5.26 min [−0.54, 11.06]; 150 mg, 9.15 min [3.99, 14.32]; 300 mg, 12.19 min [5.75, 18.63]) or no history of depression (LS mean difference from placebo [95% CI]: 37.5 mg, 5.29 min [1.48, 9.10]; 75 mg, 10.33 min [6.30, 14.36]; 150 mg, 11.03 min [7.83, 14.23]; 300 mg, 13.12 min [9.99, 16.26]) (Fig. 1A). Results were similar when participants without a history of depression who were taking antidepressants were excluded (Supplemental Fig. 1A).
Fig. 1.
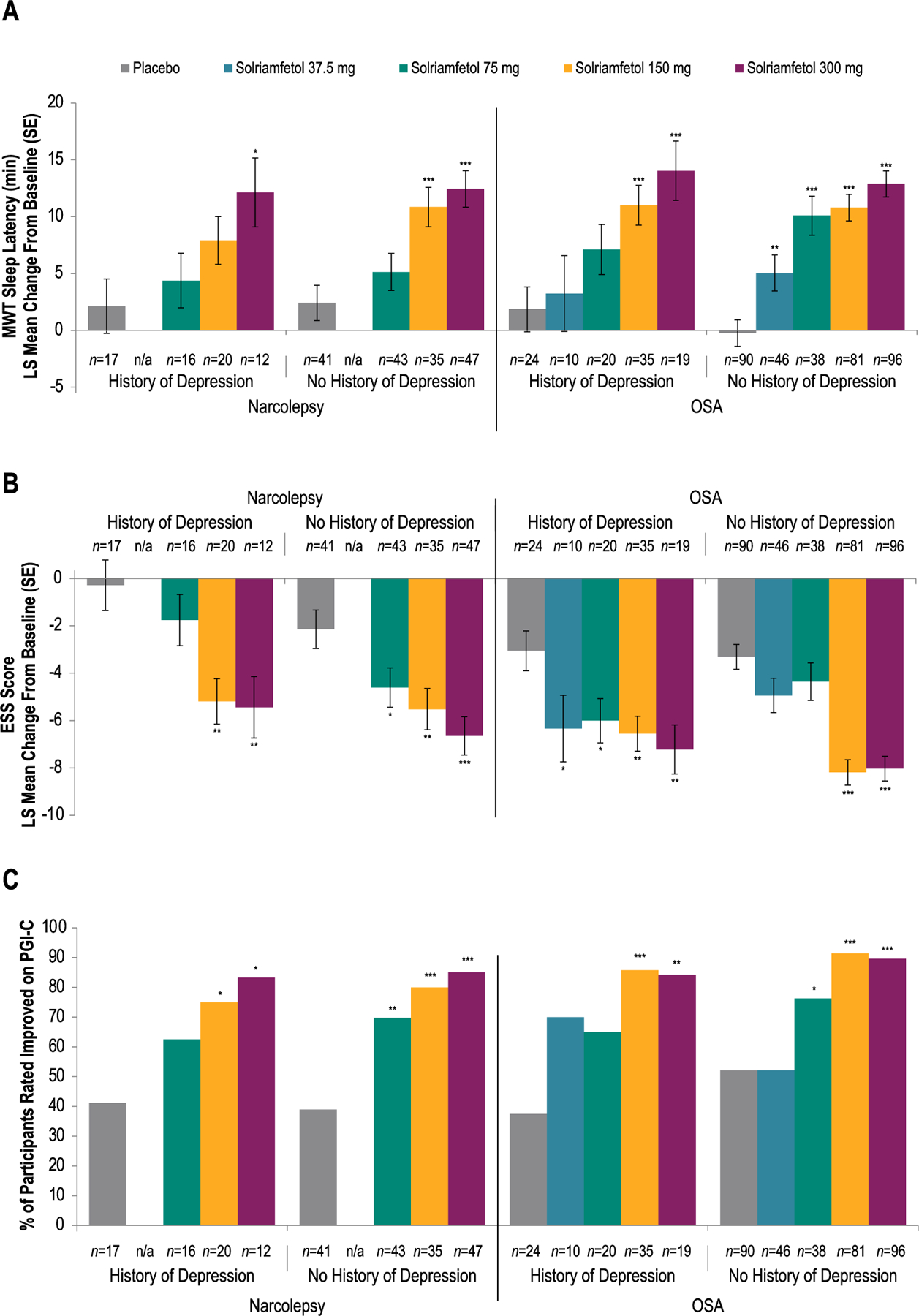
Efficacy of Solriamfetol in Participants With or Without a History of Depression
(A) Mean Sleep Latency on Maintenance of Wakefulness Test
(B) Epworth Sleepiness Scale Scores
(C) Improvement on Patient Global Impression of Changea
aImprovement defined as minimally, much, or very much improved. *P < 0.05 vs placebo. **P < 0.01 vs placebo. ***P < 0.0001 vs placebo. P values are nominal. Note: Higher mean sleep latency on the MWT indicates greater ability to stay awake and less severe EDS, whereas higher scores on the ESS represent more severe EDS (Johns, 1991; Littner et al., 2005). ESS, Epworth Sleepiness Scale; LS, least squares; MWT, Maintenance of Wakefulness Test; n/a, not applicable; OSA, obstructive sleep apnea; PGI-C, Patient Global Impression of Change; SE, standard error.
ESS scores decreased (improved) from baseline to week 12 with solriamfetol in participants with narcolepsy or OSA, regardless of depression history (Fig. 1B). In solriamfetol-treated participants with narcolepsy, LS mean changes from baseline to week 12 in ESS score ranged from −1.76 to −5.44 in participants with a history of depression and from −4.61 to −6.65 in participants without a history of depression. For the combined solriamfetol group in participants with narcolepsy, LS mean difference from placebo (95% CI) in ESS score was −3.8 (−6.3, −1.2) in those with a history of depression and −3.5 ( −5.3, −1.6) in those without a history of depression. Results generally were dose-dependent, with greater improvements observed with higher doses of solriamfetol, relative to placebo, regardless of whether participants had a history of depression (LS mean difference from placebo [95% CI]: 75 mg, −1.47 [−4.50, 1.57]; 150 mg, −4.90 [−7.76, −2.03]; 300 mg, −5.15 [−8.50, −1.79]) or no history of depression (LS mean difference from placebo [95% CI]: 75 mg, −2.45 [−4.75, −0.16]; 150 mg, −3.37 [−5.72, −1.01]; 300 mg, 4.50 [ 6.76, 2.23]). In solriamfetol-treated participants with OSA, LS mean changes from baseline to week 12 in ESS score ranged from 6.01 to 7.22 in participants with a history of depression and from 4.36 to 8.19 in participants without a history of depression. For the combined solriamfetol group in participants with OSA, LS mean difference from placebo (95% CI) in ESS score was 3.5 ( 5.4, 1.6) in those with a history of depression and −3.7 (−4.9, −2.5) in those without a history of depression. Solriamfetol-treated participants with OSA showed improvements relative to placebo whether they had a history of depression (LS mean difference from placebo [95% CI]: 37.5 mg, −3.28 [−6.51, −0.05]; 75 mg, −2.95 [−5.41, −0.48]; 150 mg, −3.50 [−5.68, −1.32]; 300 mg, −4.16 [−6.79, −1.53]) or had no history of depression (LS mean difference from placebo [95% CI]: 37.5 mg, −1.62 [−3.37, 0.12]; 75 mg, −1.05 [−2.90, 0.79]; 150 mg, −4.88 [−6.34, −3.43]; 300 mg, −4.72 [−6.14, −3.30]) (Fig. 1B). Results were similar when participants without a history of depression who were taking antidepressants were excluded from the analysis (Supplemental Fig. 1B).
Solriamfetol-treated participants with narcolepsy or OSA reported improvement in their overall condition on the PGI-C at week 12, regardless of depression history (Fig. 1C). Of solriamfetol-treated participants with narcolepsy, 62.5%–83.3% with a history of depression and 69.8%–85.1% without a history of depression reported improvement on the PGI-C compared with 41.2% and 39.0%, respectively, of those receiving placebo. For the combined solriamfetol group in participants with narcolepsy, the mean percentage difference from placebo (95% CI) in percentage of participants improved on PGI-C was 31.7% (5.2, 58.3) in those with a history of depression and 39.4% (22.8, 56.0) in those without a history of depression. Of solriamfetol-treated participants with OSA, 65.0%–85.7% with a history of depression and 52.2%– 91.4% without a history of depression reported improvement on the PGI-C compared with 37.5% and 52.2%, respectively, of those receiving placebo (Fig. 1C). For the combined solriamfetol group in participants with narcolepsy, the mean percentage difference from placebo (95% CI) in percentage of participants improved on PGI-C was 41.1% (19.8, 62.3) in those with a history of depression and 29.4% (18.1, 40.7) in those without a history of depression. Results were similar when participants without a history of depression who were taking antidepressants were excluded (Supplemental Fig. 1C).
3.3.2. Efficacy in participants with or without concomitant antidepressant use
Results were similar when efficacy was analyzed in subgroups of participants who were or were not using concomitant antidepressants. Mean MWT sleep latency increased (improved) and ESS scores decreased (improved) from baseline to week 12 with solriamfetol in participants with narcolepsy or OSA, regardless of concomitant antidepressant use (Fig. 2A; Fig. 2B). Additionally, solriamfetol-treated participants with narcolepsy or OSA reported improvement in their overall condition on the PGI-C at week 12, regardless of concomitant antidepressant use (Fig. 2C). An interaction analysis indicated that the interaction between antidepressant use and the effect of solriamfetol was not significant for the MWT (narcolepsy, F1,211 = 1.54; P = 0.2167; OSA, F1,425 = 2.36; P = 0.1254) or ESS (narcolepsy, F1,225 = 0.64; P = 0.4238; OSA, F1,453 = 0.27; P = 0.6048).
Fig. 2.
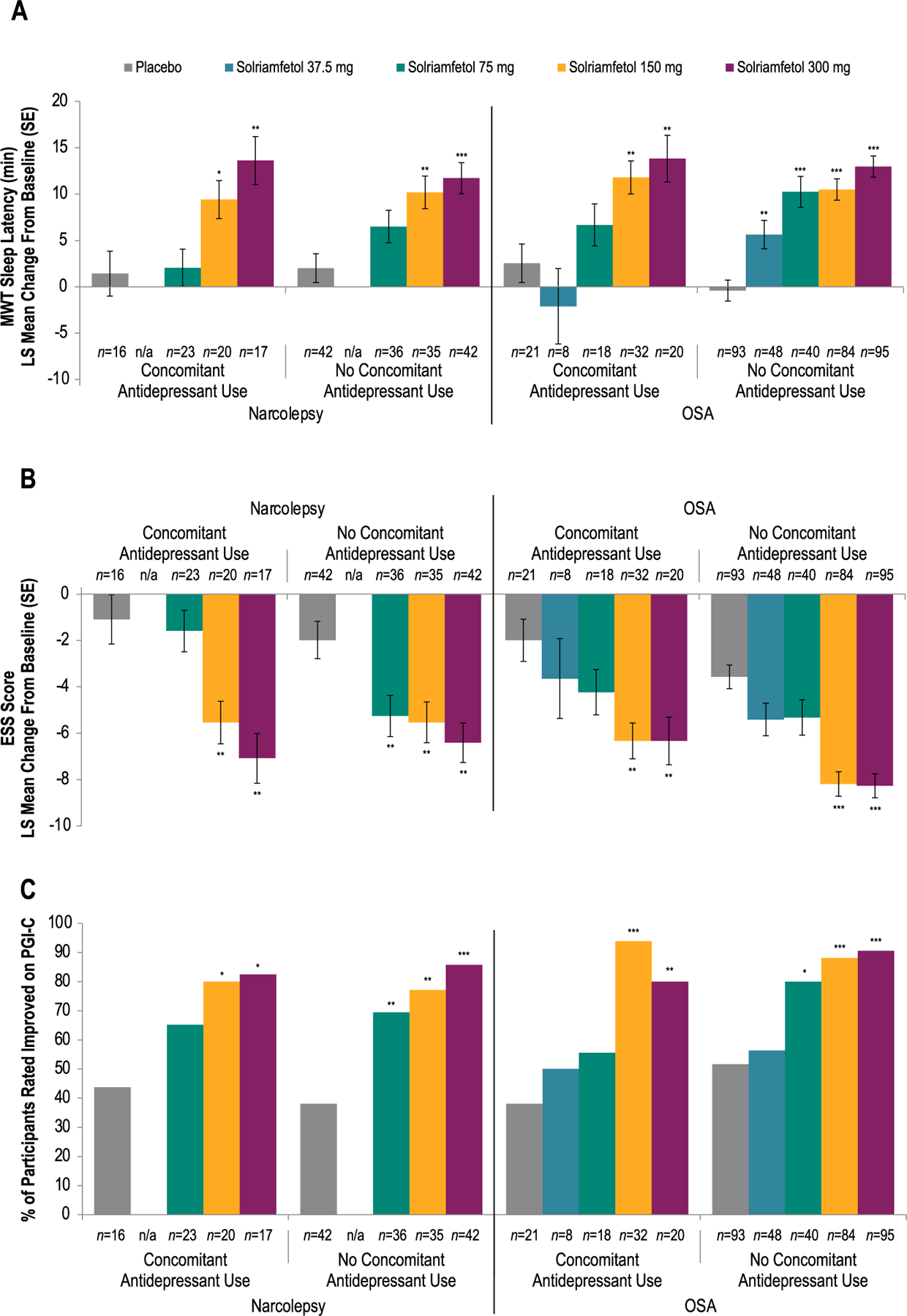
Efficacy of Solriamfetol in Participants With or Without Concomitant Antidepressant Use
(A) Mean Sleep Latency on Maintenance of Wakefulness Test
(B)Epworth Sleepiness Scale Scores
(C) Improvement on Patient Global Impression of Changea
aImprovement defined as minimally, much, or very much improved. *P < 0.05 vs placebo. **P < 0.01 vs placebo. ***P < 0.0001 vs placebo. P values are nominal. Note: Higher mean sleep latency on the MWT indicates greater ability to stay awake and less severe EDS, whereas higher scores on the ESS represent more severe EDS (Johns, 1991; Littner et al., 2005). ESS, Epworth Sleepiness Scale; LS, least squares; MWT, Maintenance of Wakefulness Test; n/a, not applicable; OSA, obstructive sleep apnea; PGI-C, Patient Global Impression of Change; SE, standard error.
3.4. Safety/tolerability
The most common TEAEs across both studies were headache, decreased appetite, nausea, and anxiety (Table 3). In participants with OSA, the frequency and type of common TEAEs generally were similar in those with or without a history of depression. In participants with solriamfetol-treated participants with narcolepsy, TEAEs occurred more frequently among those with a history of depression (75 mg, n = 11 [69%]; 150 mg, n = 18 [90%]; 300 mg, n = 11 [92%]; combined solriamfetol, n = 40 [83%]) than among those without a history of depression (75 mg, n = 23 [54%]; 150 mg, n = 29 [74%]; 300 mg, n = 29 [62%]; combined solriamfetol, n = 81 [63%]). Results were similar when participants without a history of depression who were taking antidepressants were excluded (Supplemental Table 2). In participants with narcolepsy or OSA, frequency and type of TEAEs were generally similar between those using or not using concomitant antidepressants (Table 4).
Table 3.
Rates of common TEAEs in participants with or without a history of depression.a
| Preferred Term, n (%) | Narcolepsy |
OSA |
||||||
|---|---|---|---|---|---|---|---|---|
| History of Depression |
No History of Depression |
History of Depression |
No History of Depression |
|||||
| Placebo (N=17) | Combined Solriamfetol (N=48) | Placebo (N=42) | Combined Solriamfetol (N=129) | Placebo (N=26) | Combined Solriamfetol (N=85) | Placebo (N=93) | Combined Solriamfetol (N=270) | |
| Any TEAE | 10 (59) | 40 (83) | 17 (40) | 81 (63) | 11 (42) | 57 (67) | 46 (49) | 184 (68) |
| Headache | 2 (12) | 11 (23) | 1 (2) | 27 (21) | 2 (8) | 4 (5) | 8 (9) | 32 (12) |
| Decreased appetite | 1 (6) | 8 (17) | 0 | 11 (9) | 0 | 5 (6) | 1 (1) | 22 (8) |
| Nausea | 1 (6) | 7 (15) | 0 | 12 (9) | 0 | 7 (8) | 7 (8) | 21 (8) |
| Anxiety | 0 | 5 (10) | 1 (2) | 4 (3) | 0 | 8 (9) | 0 | 17 (6) |
| Insomnia | 0 | 4 (8) | 0 | 1 (1) | 0 | 2 (2) | 2 (2) | 13 (5) |
| Upper respiratory tract infection | 1 (6) | 4 (8) | 0 | 1 (1) | 0 | 3 (4) | 3 (3) | 0 |
| Dry mouth | 0 | 3 (6) | 2 (5) | 10 (8) | 0 | 7 (8) | 2 (2) | 9 (3) |
| Fatigue | 0 | 3 (6) | 0 | 2 (2) | 1 (4) | 0 | 1 (1) | 4 (2) |
| Nasopharyngitis | 2 (12) | 5 (10) | 1 (2) | 11 (9) | 2 (8) | 3 (4) | 6 (6) | 15 (6) |
Common TEAEs are those with incidence ≥5% in ≥1 solriamfetol subgroup. OSA, obstructive sleep apnea; TEAE, treatment-emergent adverse event.
Table 4.
TEAEs in participants with or without concomitant antidepressant use.
| Preferred Term n (%) | Narcolepsy |
OSA |
||||||
|---|---|---|---|---|---|---|---|---|
| Concomitant Antidepressant Use |
No Concomitant Antidepressant Use |
Concomitant Antidepressant Use |
No Concomitant Antidepressant Use |
|||||
| Placebo (n = 16) | Combined Solriamfetol (n = 61) | Placebo (n = 43) | Combined Solriamfetol (n = 116) | Placebo (n = 23) | Combined Solriamfetol (n = 79) | Placebo(n = 96) | Combined Solriamfetol (n =276) | |
| Any TEAE, n (%) | 7 (43.8) | 45 (73.8) | 20 (46.5) | 76 (65.5) | 9 (39.1) | 50 (63.3) | 48 (50.0) | 191 (69.2) |
| Any serious TEAE, n (%) | 0 | 1 (1.6) | 0 | 0 | 1 (4.3) | 0 | 1 (1.0) | 3 (1.1) |
| TEAE leading to discontinuation, n (%) | 1 (6.3) | 2 (3.3) | 0 | 7 (6.0) | 0 | 9 (11.4) | 4 (4.2) | 16 (5.8) |
| Most common TEAEs (≥5%),a n (%) | ||||||||
| Headache | 0 | 13 (21.3) | 3 (7.0) | 25 (21.6) | 2 (8.7) | 4 (5.1) | 8 (8.3) | 32 (11.6) |
| Nasopharyngitis | 1 (6.3) | 11 (18.0) | 2 (4.7) | 5 (4.3) | 1 (4.3) | 3 (3.8) | 7 (7.3) | 15 (5.4) |
| Decreased appetite | 0 | 10 (16.4) | 1 (2.3) | 9 (7.8) | 0 | 5 (6.3) | 1 (1.0) | 22 (8.0) |
| Nausea | 0 | 8 (13.1) | 1 (2.3) | 11 (9.5) | 0 | 7 (8.9) | 7 (7.3) | 21 (7.6) |
| Dry mouth | 0 | 7 (11.5) | 2 (4.7) | 6 (5.2) | 0 | 5 (6.3) | 2 (2.1) | 11 (4.0) |
| Anxiety | 0 | 6 (9.8) | 1 (2.3) | 3 (2.6) | 0 | 7 (8.9) | 0 | 18 (6.5) |
| Diarrhea | 0 | v | 1 (2.3) | 4 (3.4) | 0 | 3 (3.8) | 1 (1.0) | 14 (5.1) |
| Feeling jittery | 0 | 1 (1.6) | 0 | 2 (1.7) | 0 | 5 (6.3) | 0 | 9 (3.3) |
| Dyspepsia | 0 | 0 | 0 | 6 (5.2) | 0 | 1 (1.3) | 0 | 2 (0.7) |
| Insomnia | 0 | 3 (4.9) | 0 | 2 (1.7) | 1 (4.3) | 1 (1.3) | 1 (1.0) | 14 (5.1) |
Common TEAEs are those with incidence ≥5% in ≥1 solriamfetol-treated subgroup. OSA, obstructive sleep apnea; TEAE, treatment-emergent adverse event.
Few participants reported depression or a similar TEAE, regardless of depression history. In participants with narcolepsy, among those with a history of depression, AEs of depression (placebo, n = 1 [5.9%]; solriamfetol, n = 1 [2.1%]) and depressed mood (placebo, n = 0; solriamfetol, n = 1 [2.1%]) were reported. Among those without a history of depression, depressive symptoms (placebo, n = 0; solriamfetol, n = 1 [0.8%]) and dysthymic disorder (placebo, n = 0; solriamfetol n = 1 [0.8%]) were reported. In participants with OSA, among those with a history of depression, depressed mood (placebo, n = 0; solriamfetol, n = 1 [1.2%]) was reported. In those without a history of depression, depressed mood (placebo, n = 1 [1.1%]; solriamfetol, n = 1 [0.4%]), depression (placebo, n = 2 [2.2%]; solriamfetol, n = 0), and suicidal ideation (placebo, n = 2 [2.2%]; solriamfetol, n = 0) were reported. A total of 5 participants with narcolepsy and 6 participants with OSA experienced other psychiatric events (3 of these participants [narcolepsy, n = 2; OSA, n = 1] had a history of depression, as noted below): altered mood (narcolepsy: solriamfetol 75 mg, n = 1), mood swings (narcolepsy: solriamfetol 75 mg, n = 1), affect lability (narcolepsy: placebo, n = 1 [history of depression]; solriamfetol 150 mg, n = 1; OSA: solriamfetol 150 mg, n = 1), pressure of speech (narcolepsy: solriamfetol 150 mg, n = 1 [history of depression]), disinhibition (OSA: solriamfetol 75 mg, n = 1), euphoric mood (OSA: solriamfetol 300 mg, n = 1), tachyphrenia (OSA: solriamfetol 300 mg, n = 1), emotional disorder (OSA: solriamfetol 150 mg, n = 1 [history of depression]), and hypervigilance (OSA: solriamfetol 300 mg, n = 1). None of the events were considered acute manic or psychotic behavior or suggested abuse potential.
Reports of suicidal ideation or behavior, as assessed with the C-SSRS, were uncommon, regardless of depression history. Transient shifts from baseline in suicidal ideation on the C-SSRS were recorded for three participants with narcolepsy who received solriamfetol (1.7%; a history of depression was noted for one of these participants), and three participants with OSA (n = 2 [1.7%] receiving placebo, n = 1 [0.3%] receiving solriamfetol; none had a recorded history of depression). All three of the solriamfetol-treated participants with narcolepsy had shifts from 0 (“no suicidal ideation/behavior”) at baseline to 1 (“wish to be dead”) post-baseline (weeks 4, 8, or 12). The solriamfetol-treated participant with OSA had a shift from 0 at baseline to 1 at week 8. Of the two placebo-treated participants with OSA, one had a shift from 0 at baseline to 1 at week 4 and a shift to 3 (“active suicidal ideation without intent”) at weeks 8 and 12; the other participant had a shift from 0 at baseline to 2 (“non-specific active suicidal thoughts”) at week 8. No shifts from baseline in suicidal behavior were observed in either study. Among all participants who experienced a shift from baseline in suicidal ideation on the C-SSRS, the following TEAEs related to depression/suicidal ideation were reported: worsening of depression (n = 1; narcolepsy, solriamfetol 75 mg), depressive symptoms (n = 1; narcolepsy, solriamfetol 300 mg), situational depression (n = 1; OSA, placebo), suicidal ideation (n = 2; OSA, placebo), depressed mood (n = 1; OSA, solriamfetol 300 mg). Suicidal behavior was not reported for any participants during either study.
4. Discussion
In this secondary analysis, the prevalence of a history of depression in the narcolepsy (28.1%) and OSA (23.5%) study populations was consistent with prevalence rates reported in prior clinic-based studies (Acker et al., 2017; Jackson et al., 2019; Li et al., 2021). Results demonstrated the therapeutic effects of solriamfetol compared with placebo in subgroups of participants with or without a history of depression, leading to a conclusion that solriamfetol was effective in treating EDS in participants with narcolepsy or OSA regardless of medical history of depression. Results were similar when outcomes were examined in subgroups of participants who were or were not using antidepressants, regardless of depression history, indicating that antidepressant use did not affect the efficacy of solriamfetol. Findings were consistent across efficacy measures examined, including the MWT, the ESS, and the PGI-C. Rates of common TEAEs were generally similar in participants with or without a history of depression and in participants who were or were not using concomitant antidepressants in both the narcolepsy and OSA populations.
Given the likelihood of differential use of concomitant medications in participants with a history of depression compared with participants without a history of depression and the potential impact of concomitant medication use on sleep-related symptoms (Doghramji and Jangro, 2016; Wichniak et al., 2017) and, thereby, the therapeutic and adverse effects of solriamfetol treatment, concomitant medication use was analyzed. As expected, use of antidepressants was more common in those with a history of depression than those without a history of depression. However, the same was true for use of antihistamines, nonsteroidal anti-inflammatory drugs, drugs for peptic ulcer and gastroesophageal reflux, and thyroid preparations. Importantly, TEAEs were similar in those taking concomitant antidepressants and those who were not, suggesting concomitant use of antidepressants did not impact the tolerability of solriamfetol treatment.
4.1. Limitations
There were several limitations to the current analyses. First, these were secondary analyses of primary data from studies that evaluated the safety and efficacy of solriamfetol in participants with EDS associated with narcolepsy or OSA (Schweitzer et al., 2019; Thorpy et al., 2019). As participants were not assessed for depression during the studies, conclusions cannot be drawn regarding the effect of solriamfetol on current depressive symptoms. Second, the presence or absence of a history of depression was determined with an unstructured history taken at screening rather than with a structured diagnostic tool or structured interview. Importantly, the relationship between sleep disorders and psychiatric disorders, particularly depression, is complex. Sleep disorders, especially when untreated, can negatively impact mental and physical health (eg, mood, quality of life, and daily functioning), thereby contributing to or masquerading as depression. Further, narcolepsy and OSA share many symptoms with depression, such as EDS, disturbed sleep, impaired cognition, irritability, weight changes, mood changes, and decreased motivation, complicating recognition of depression in these patients (American Academy of Sleep Medicine, 2014; American Psychiatric Association, 2013). Thus, some misclassification in the current study was possible if not likely. Another possible downstream effect of reliance on medical history is the potential for racial bias. For instance, few African American participants with OSA were identified as having a history of depression, potentially limiting generalizability of findings to this population. Third, use of antidepressants was not used to differentiate subgroups of participants with or without a history of depression, as many of these agents are used for treatment of other conditions. As a result, the subgroup without a history of depression included individuals who were taking antidepressants, which could have included some with current or past depression. However, results from the sensitivity analysis that excluded participants without a history of depression who were taking antidepressants were similar to the main analysis for safety and efficacy outcomes. Finally, participants were not stratified by concomitant medication use, and concomitant medication use was not standardized in a way that would allow analyses of the impact of specific medications on the safety/efficacy of solriamfetol treatment. Instead, analyses were performed at the medication-group level (eg, antidepressants, antihistamines), which may not have detected effects of specific medications. Additionally, these analyses may have been underpowered to detect an effect of antidepressant use on response to solriamfetol treatment, as the power analyses for the original studies did not account for an interaction term.
5. Conclusions
These analyses confirmed earlier findings of a high prevalence (20%– 35%) of a clinical history of depression in participants with narcolepsy or OSA and suggested that its appearance in the clinical record does not affect the response to solriamfetol treatment, relative to placebo, in these populations. These findings also suggest that solriamfetol has a similar safety profile in those with and without a history of depression.
Supplementary Material
Acknowledgments
Diane Menno, PhD, and Nalina Dronamraju, PhD, of Jazz Pharmaceuticals, PLC, provided assistance with statistical analysis. Under the direction of the authors, Diane Sloan, PharmD, and Christopher Jaworski of Peloton Advantage, LLC, an OPEN Health company, provided medical writing and editorial support for this research paper, which was funded by Jazz Pharmaceuticals.
Role of the funding source
This study was supported by Jazz Pharmaceuticals. At the time that the study was conducted, Jazz Pharmaceuticals had worldwide development, manufacturing, and commercialization rights to solriamfetol, excluding certain jurisdictions in Asia. Jazz Pharmaceuticals completed the divestiture of Sunosi® (solriamfetol) in the US to Axsome Therapeutics, Inc. on May 9, 2022. SK Biopharmaceuticals, the discoverer of the compound (also known as SKL-N05), maintains rights in 12 Asian markets, including Korea, China, and Japan.
Biography
A Krystal has received research grant support from NIH, Janssen, Jazz Pharmaceuticals, Axsome Therapeutics, and Reveal Biosensors; and has served as a consultant for Adare Pharmaceuticals, Angelini, Axsome Therapeutics, Big Health, Evecxia, Eisai Pharmaceuticals, Ferring Pharmaceuticals, Galderma, Harmony, Idorsia Pharmaceuticals, Jazz Pharmaceuticals, Janssen, Takeda, Merck, Millennium, Neurawell, Neurocrine, Pernix, and Physician’s Seal, Sage Pharmaceuticals.
R Benca has received grant support from Eisai, American Academy of Sleep Medicine Foundation, and NIH and has served as a consultant for Eisai, Genomind, Idorsia Pharmaceuticals, Janssen, Jazz Pharmaceuticals, Merck, Sage, and Sunovion.
R Rosenberg has received consultancy fees from Eisai; honoraria from Merck; research funding from Jazz Pharmaceuticals, Merck, Actelion, Eisai, and Philips Respironics; and has served on the speakers’ bureau for Merck and as an advisory board member for Jazz Pharmaceuticals.
PK Schweitzer has received consultancy fees from Jazz Pharmaceuticals and Apnimed, Inc; her institution has received research funding from Jazz Pharmaceuticals, Apnimed, Inc, Avadel-Flamel, Harmony Biosciences, Inspire Medical Systems, and Suven Life Sciences.
A Malhotra is funded by NIH. He reports income related to medical education from LIvanova, Equillium, and Corvus. He has been PI of Jazz studies but has not received personal income from the company. ResMed provided a philanthropic donation to UC San Diego in support of a sleep center.
K Babson is an employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc.
L Lee is a former employee of Jazz Pharmaceuticals who, in the course of this employment, received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc.
S Bujanover is a former employee of Jazz Pharmaceuticals who, in the course of this employment, received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc.
KP Strohl has served as an advisory board member and is a principal investigator for Jazz; is a site principal investigator for Inspire Medical Systems; and has received consultancy fees from Sommetrics, GSK (Galvani Bioelectronics), and 7 Dreamers.
Footnotes
Credit author statement
Andrew D. Krystal, Ruth M. Benca, Russell Rosenberg, Paula K. Schweitzer, Atul Malhotra, Kimberly Babson, Lawrence Lee, Shay Bujanover, Kingman P. Strohl: Data interpretation, Review and revisions, Final approval. Atul Malhotra: Study Design, Study investigator, Enrolled patients. Paula K. Schweitzer: Principal investigator, Enrolled patients, Collection and assembly of data.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2022.08.018.
References
- Acker J, Richter K, Piehl A, Herold J, Ficker JH, Niklewski G, 2017. Obstructive sleep apnea (OSA) and clinical depression-prevalence in a sleep center. Sleep Breath 21, 311–318. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine, 2014. International Classification of Sleep Disorders, third ed. American Academy of Sleep Medicine, Darien, IL. [Google Scholar]
- American Psychiatric Association, 2013, 5th ed.. Diagnostic and Statistical Manual of Mental Disorders, DSM American Psychiatric Publishing, Washington, DC. [Google Scholar]
- Doghramji K, Jangro WC, 2016. Adverse effects of psychotropic medications on sleep. Psychiatr. Clin. North. Am 39, 487–502. [DOI] [PubMed] [Google Scholar]
- Gasa M, Tamisier R, Launois SH, Sapene M, Martin F, Stach B, Grillet Y, Levy P, Pepin JL, Pneumology-FFP SCo.t.S.R.o.t.F.F.o, 2013. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J. Sleep Res 22, 389–397. [DOI] [PubMed] [Google Scholar]
- Guy W, 1976. ECDEU Assessment Manual for Psychopharmacology. Revised. U.S. Department of Health, Education and Welfare, Rockville, MD. [Google Scholar]
- Jackson ML, Tolson J, Bartlett D, Berlowitz DJ, Varma P, Barnes M, 2019. Clinical depression in untreated obstructive sleep apnea: examining predictors and a meta-analysis of prevalence rates. Sleep Med 62, 22–28. [DOI] [PubMed] [Google Scholar]
- Johns MW, 1991. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 14, 540–545. [DOI] [PubMed] [Google Scholar]
- Li X, Sanford LD, Zong Q, Zhang Y, Tan L, Li T, Ren R, Zhou J, Han F, Tang X, 2021. Prevalence of depression or depressive symptoms in patients with narcolepsy: a systematic review and meta-analysis. Neuropsychol. Rev 31, 89–102. [DOI] [PubMed] [Google Scholar]
- Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, Hirshkowitz M, Daniel LL, Bailey D, Berry RB, Kapen S, Kramer M, 2005. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 28, 113–121. [DOI] [PubMed] [Google Scholar]
- Nuvigil, 2018. Package Insert. Teva Pharmaceuticals [Google Scholar]
- Patil SP, Ayappa IA, Caples SM, R.J. Kimoff, S.R. Patel, Harrod, CG, 2019. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J. Clin. Sleep Med 15, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin JL, Viot-Blanc V, Escourrou P, Racineux JL, Sapene M, Levy P, Dervaux B, Lenne X, Mallart A, 2009. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur. Respir. J 33, 1062–1067. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Szklo-Coxe M, Hla KM, Young T, 2006. Longitudinal association of sleep-related breathing disorder and depression. Arch. Intern. Med 166, 1709–1715. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ, 2011. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatr 168, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provigil, 2018. Package Insert. Teva Pharmaceuticals
- Schweitzer PK, Rosenberg R, Zammit GK, Gotfried M, Chen D, Carter LP, Wang H, Lu Y, Black J, Malhotra A, Strohl KP, 2019. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): a randomized controlled trial. Am. J. Respir. Crit. Care Med 199, 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunosi™ (solriamfetol) tablets, 2021. Prescribing Information. Jazz Pharmaceuticals
- Thorpy MJ, 2020. Recently approved and upcoming treatments for narcolepsy. CNS Drugs 34, 9–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpy MJ, Shapiro C, Mayer G, Corser BC, Emsellem H, Plazzi G, Chen D, Carter LP, Wang H, Lu Y, Black J, Dauvilliers Y, 2019. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann. Neurol 85, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakix, 2021. Package Insert. Harmony Biosciences
- Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W, 2017. Effects of antidepressants on sleep. Curr. Psychiatr. Rep 19, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


