Abstract
To clarify the role of cell surface components of Streptococcus mutans in resistance to phagocytosis by human polymorphonuclear leukocytes (PMNs), several isogenic mutants of S. mutans defective in cell surface components were studied with a luminol-enhanced chemiluminescence (CL) assay, a killing assay, and a transmission electron microscope. The CL responses of human PMNs to mutant Xc11 defective in a major cell surface antigen, PAc, and mutant Xc16 defective in two surface glucosyltransferases (GTF-I and GTF-SI) were the same as the response to the wild-type strain, Xc. In contrast, mutant Xc24R, which was defective in serotype c-specific polysaccharide, induced a markedly higher CL response than the other strains. The killing assay showed that human PMNs killed more Xc24R than the parent strain and the other mutants. The transmission electron microscopic observation indicated that Xc24R cells were more internalized by human PMNs than the parental strain Xc. These results may be reflected by the fact that strain Xc24R was more phagocytosed than strain Xc. The CL response of human PMNs to a mutant defective in polysaccharide serotype e or f was similar to the response to Xc24R. Furthermore, mutants defective in serotype-specific polysaccharide were markedly more hydrophobic than the wild-type strains and the other mutants, suggesting that the hydrophilic nature of polysaccharides may protect the bacterium from phagocytosis. We conclude that the serotype-specific polysaccharide, but not the cell surface proteins on the cell surface of S. mutans, may play an important role in the resistance to phagocytosis.
Streptococcus mutans has been strongly implicated as one of the causative organisms of dental caries. Phagocytosis by oral polymorphonuclear leukocytes (PMNs) is one of the most important mechanisms protecting against dental caries (31–34). Furthermore, S. mutans is associated with systemic diseases such as infectious endocarditis-associated glomerulonephritis and rheumatic fever, which can result when whole cells or components of S. mutans are translocated across the epithelial barrier and carried to target organs (36).
Several proteins involved in pathogenicity are located on the cell surface of S. mutans (12). A 190-kDa cell surface protein antigen (PAc) of S. mutans is one of the factors that mediate the binding of the organism to salivary components on tooth surfaces (11). The N-terminal region of this protein possesses an internal repeating amino acid sequence rich in alanine. The C-terminal region of PAc contains a potential transmembrane domain consisting primarily of hydrophobic amino acids, a cytoplasmic tail consisting of five charged amino acids, and a wall-spanning region rich in proline. These were inferred from analogy with streptococcal M protein, which is the major virulence factor of group A streptococci (6, 12). M protein is a fibrillar surface molecule that protects the bacteria from being ingested and killed by the host's phagocytic cells (15, 28). This effect appears to result from the inhibition of phagocytosis and opsonization (41). Therefore, the S. mutans PAc protein antigen is thought to have antiphagocytic activity, like the streptococcal M protein. However, we found no reports on the function of the S. mutans PAc protein in resistance to human phagocytes. In addition, glucosyltransferase I (GTF-I) and glucosyltransferase SI (GTF-SI), which primarily produce water-insoluble glucan, a major cariogenic factor, are also observed on the cell surface of S. mutans (12, 13).
The serotype-specific polysaccharide antigens of S. mutans are cell wall polysaccharides that consist of a backbone structure of 1,2- and 1,3-linked rhamnosyl polymers with glucose side chains (19, 29, 42). S. mutans strains are classified into three serotypes, c, e, and f, whose serotype-specific polysaccharides have different linkages of glucose side chain (19, 29). In vitro stimulation of human monocytes with the serotype f-specific polysaccharide antigen induces the release of inflammatory cytokines, such as tumor necrosis factor alpha and interleukin-1β (35), and provokes nitric oxide production in the rat aorta (20). However, the function of the serotype-specific polysaccharide antigens in phagocytosis of the organism by human phagocytes has not been reported.
We constructed various mutant strains of S. mutans defective in cell-surface components by using insertional inactivated mutagenesis. In this study, we examined the chemiluminescence (CL) responses of human PMNs to these mutants to investigate the role of the cell surface components in the interaction between S. mutans and PMNs. Furthermore the function of the polysaccharide was examined by hydrocarbon partitioning, a phagocytic killing assay, and a transmission electron microscope. In this report, we discuss the function of the serotype-specific polysaccharide of S. mutans in resistance to phagocytosis by human phagocytes.
MATERIALS AND METHODS
Bacterial strains.
The S. mutans strains used in this study are listed in Table 1. S. mutans wild-type strains Xc (serotype c), LM7 (serotype e), KT6219 (serotype f), Xc11 (PAc-defective mutant), and Xc24R (serotype c-specific polysaccharide-defective mutant) were selected from the stock culture collection in the Department of Preventive Dentistry, Kyushu University Faculty of Dentistry, Fukuoka, Japan. Strains of S. mutans were grown at 37°C in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.). For transformants of S. mutans, erythromycin at a final concentration of 10 μg/ml was added.
TABLE 1.
S. mutans strains used in this study
| Strain | Relevant characteristicsa | Source or reference |
|---|---|---|
| Xc | Serotype c wild-type strain | 10 |
| Xc11 | Emr, transformant of Xc; pac; PAc− | 47 |
| Xc16 | Emr, transformant of Xc; gtfB and gtfC; GTF-I− and GTF-SI− | This study |
| Xc24 | Emr, transformant of Xc; rmlB, fusion between gtfB and gtfC; serotype c polysaccharide− | 39 |
| Xc24R | Emr, transformant of Xc; rmlB, intact gtfB and gtfC; serotype c polysaccharide− | 46 |
| LM7 | Serotype e wild-type strain | 21 |
| LM7DR | Emr, transformant of LM7; rmlB; serotype e polysaccharide− | This study |
| KT6219 | Serotype f wild-type strain | This study |
| KT6219DR | Emr, transformant of KT6219; rmlB; serotype f polysaccharide− | This study |
Emr, erythromycin resistance.
DNA manipulation.
Preparation of chromosomal DNA from the S. mutans rmlB mutant strain Xc24R and transformation of S. mutans strains LM7 and KT6219 were carried out as described previously (27). Transformants were isolated on tryptic soy agar plates containing 10 μg of erythromycin per ml.
Immunoblotting.
S. mutans strains were grown to the stationary phase in 20 ml of BHI broth. The bacterial cells were harvested, suspended in 200 μl of 10 mM Tris-HCl buffer (pH 6.8) containing 1% sodium dodecyl sulfate (SDS), 1% mercaptoethanol, and 20% glycerol, and heated at 100°C for 5 min. The cell extracts were then clarified by centrifugation. Seven-microliter samples were subjected to SDS-polyacrylamide gel electrophoresis (PAGE [7.5% polyacrylamide]) and electrophoretically transferred to a nitrocellulose membrane (3, 14). After being blocked with 1% bovine serum albumin in phosphate-buffered saline (PBS [pH 7.4]) plus 0.1% Tween 20, the membranes were treated with rabbit anti-PAc or anti-GTF-I serum. After being washed with PBS containing 0.1% Tween 20, the antibodies bound to proteins immobilized on the membranes were detected with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (IgG) (Zymed Laboratories, South San Francisco, Calif.) and developed by the addition of 5-bromo-4-chloro-3-indolyl phosphate toluidinium salt as the substrate and nitroblue tetrazolium salt as the developer.
Immunological methods.
Serotype-specific antigens were extracted from whole cells of S. mutans strains by the formamide extraction procedure (42). Immunodiffusion was performed in 1% (wt/vol) noble agar in PBS (pH 7.3) (25).
Preparation of complement.
Lyophilized whole cells of S. mutans strain Xc (200 mg) were suspended in 20 ml of cold PBS (pH 7.4) and washed three times with cold PBS. The cells were suspended in 50 ml of fresh human serum from a healthy adult volunteer. The suspension was incubated for 20 min at 4°C. The cells were removed by centrifugation, and then the supernatant was filtered through a 0.45-μm-pore-size membrane filter (Millipore Corp., Bedford, Mass.). This absorption step was repeated five times. The absorbed serum served as the complement source and was stored at −30°C until used.
Preparation of human PMNs.
Human PMNs were prepared from peripheral blood obtained from three healthy volunteers. PMNs were isolated with Mono-Poly resolving medium (Dainippon Pharmaceutical Co., Tokyo, Japan).
CL assay.
Cells of the S. mutans strains were sonicated to disperse streptococcal chains and washed twice with HBSS−− buffer (0.4-mg/ml KCl, 0.06-mg/ml KH2PO4, 8-mg/ml NaCl, 0.35-mg/ml NaHCO3, 0.048-mg/ml Na2HPO4, 1.0-mg/ml glucose [pH 7.3]). They were resuspended at a concentration of 4 × 108 CFU/ml in HBSS−− supplemented with 0.14-mg/ml CaCl2 and 0.10-mg/ml MgSO4 · 7H2O (pH 7.3; HBSS++ buffer). Ten milligrams of luminol (5-amino-2,3-dihydro-1,4-phthalazinedione; Sigma Chemical Co., St. Louis, Mo.) was dissolved in 1 ml of dimethyl sulfoxide and stored at −30°C until just before use. Luminol-enhanced CL was measured as previously described (4, 23). The reaction mixtures contained human PMNs (106 cells/ml), 0.2-mg/ml luminol, and 0 or 10 μl of complement in a total volume of 840 μl of HBSS++ buffer containing 1% gelatin. After the reaction mixtures were allowed to equilibrate at 37°C for 10 min, 160 μl of bacterial suspension (4 × 108 CFU/ml) was added to activate the system. The light emission was recorded continuously for 90 min with a six-channel Biolumat LB9505 luminometer (Berthold, Wildbad, Germany).
Phagocytic killing assay.
The phagocytic killing assay was performed as previously described with minor modifications (44). Human PMNs (106 cells) were incubated with bacteria (105 CFU) in the presence of 1% complement in gelatin-Veronal-buffered saline (pH 7.3) supplemented with 2.5% glucose, 1 mM MgCl2, and 0.15 mM CaCl2 (GGVB++) at 37°C. After incubation with gentle rotation for 90 or 180 min, aliquots were removed, diluted immediately in sterile 1% Triton X-100, and spread on tryptic soy agar plates (Difco) for quantitation of bacterial CFU. As a positive control, 150 μg of rabbit anti-PAc IgG was added to the reaction mixture of Xc with human PMNs. The percent survival was calculated as [(percent CFU to CFU at 0 min in the presence of human PMNs)/(percent CFU to CFU at 0 min in the absence of PMNs)] × 100. Data are expressed as the mean percent survival of three different experiments ± standard deviation.
Microscopy.
Xc and Xc24R cells were washed with HBSS−− buffer and suspended in RPMI 1640 at an optical density at 550 nm (OD550) of 0.2 to 0.25. To assess interaction between human PMNs and bacteria, human complement (200 μl) and human PMNs (107 cells) were added to 10 ml of the bacterial suspension 10-fold diluted in RPMI 1640. The mixtures were incubated at 37°C for 90 min with gentle rotation. After incubation, the uningested bacteria were washed away with HBSS−− buffer, and the washed human PMNs were fixed in 2.5% glutaraldehyde–2% paraformaldehyde at pH 7.4 and postfixed in 1% OsO4. After being washed with 0.1 M sodium phosphate buffer (pH 7.4), cells were concentrated in 1.25% agar and stained with 1% uranyl acetate. Dehydration was accomplished by serial exposure to ethanol (50 to 100%) and then to 100% propylene oxide. After dehydration, these cells were embedded in Epon, and thin or ultrathin sectioned. The thin sections were stained with toluidine blue for light microscopic observation with a VANOX-S light microscope (Olympus, Tokyo, Japan), and the ultrathin sections were used for transmission electron microscopy with a JEM-1200EXII electron microscope (Japan Electron Optics Laboratories Co., Tokyo, Japan).
To examine the bacterial cell surface architecture, Xc and Xc24R cells were washed with 0.1 M sodium phosphate buffer (pH 7.4). The cells were then fixed, stained, and dehydrated under the same conditions as described above. After dehydration, cells were embedded in Epon, ultrathin sectioned, and used for transmission electron microscopy.
Hydrophobicity.
The surface hydrophobicity of S. mutans was examined as described by Koga et al. (11). S. mutans strains were grown at 37°C for 18 h in BHI broth. Bacterial cells were washed and suspended in PUM buffer (11) to an OD550 of 0.6. Three hundred microliters of hexadecane was added to 3-ml samples of the cell suspension, mixed with a vortex mixer for 15 s, and allowed to stand until the phases separated. The OD of the lower aqueous phase was measured. The percentage loss in OD relative to the initial cell suspension, which is due to adsorption of S. mutans strains to hexadecane, was defined to be cell surface hydrophobicity of bacteria.
Statistical analyses.
Differences in the percent survival values and the surface hydrophobicity values were determined by Welch's t test.
RESULTS
Construction of mutant strains of S. mutans.
We previously reported that four rml genes (rmlA, rmlB, rmlC, and rmlD) are involved in dTDP-rhamnose synthesis from glucose 1-phosphate and that dTDP-rhamnose is used as an immediate precursor for the synthesis of the backbone of the serotype-specific polysaccharides of S. mutans (38, 39). Recently, we found that an rmlB-inactivated mutant (Xc24) isolated on mitis salivarius agar exhibited the gene fusion between gtfB and gtfC, which encode GTF-I and GTF-SI, respectively. On the other hand, an rmlB-inactivated mutant which possesses intact gtfB and gtfC (Xc24R) was selected on tryptic soy agar in the absence of sucrose (46). We confirmed that the insertional inactivation of the rmlB gene did not affect the production of PAc, GTF-I, and GTF-SI in Xc24R (Fig. 1). We used Xc24R but not Xc24 as the serotype c-specific polysaccharide-defective mutant, because the surface architecture of Xc24R was considered to be less influenced than that of Xc24 by inactivation of the rmlB gene. In the present study, rmlB-inactivated transformants of LM7 (serotype e) and KT6219 (serotype f) were also isolated on tryptic soy agar by the same procedure used for Xc24R construction. These transformants were designated LM7DR and KT6219DR, as shown in Table 1. Formamide extracts of these two mutants did not react with any serotype-specific antisera in immunodiffusion (Fig. 2). Southern blot analysis revealed that the gtfB and gtfC genes in both two mutants were intact, and normal expression of these two genes was confirmed by Western blot analysis (data not shown). Strain Xc16, in which both gtfB and gtfC are inactivated with a 2.0-kb cat cartridge (47), was constructed by the same principles as described previously (45). The mutation was confirmed following Southern blot analysis of the chromosomal DNA of the mutant Xc16 (data not shown).
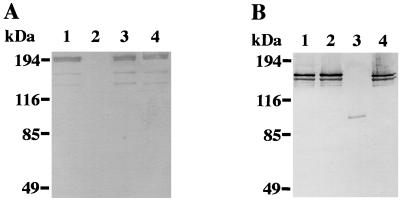
FIG. 1.
Western immunoblot detection of PAc, GTF-I, or GTF-SI expression in S. mutans strains. Surface proteins were extracted from the cell in the stationary phase of growth. The cell extracts were subjected to SDS-PAGE (7.5% polyacrylamide), proteins were blotted onto nitrocellulose membranes, and blots were probed with rabbit serum raised against recombinant PAc protein (A) or GTF-I (B) of S. mutans. Lanes: 1, strain Xc; 2, strain Xc11; 3, strain Xc16; 4, strain Xc24R.
FIG. 2.
Immunodiffusion of formamide extracts of S. mutans strains against rabbit antisera to whole cells of S. mutans strains MT8148 (serotype c) (A), LM7 (serotype e) (B), and OMZ175 (serotype f) (C). The outer wells contain formamide extracts from strains Xc (well 1), Xc11 (well 2), Xc16 (well 3), Xc24R (well 4), LM7 (well 5), LM7DR (well 6), KT6219 (well 7), and KT6219DR (well 8).
Immunoblotting analysis.
Immunoblotting analysis showed that although anti-PAc serum detected the band of 190-kDa cell surface protein antigen in the extracts from strains Xc, Xc16, and Xc24R (Fig. 1A, lanes 1, 3, and 4, respectively), the band reacting with anti-PAc serum was not observed in Xc11 (Fig. 1A, lane 2). On the other hand, anti-GTF-I serum detected two prominent bands in the extracts from strains Xc, Xc11, and Xc24R (Fig. 1B, lanes 1, 2, and 4), because of extensive amino acid homology between GTF-I and GTF-SI enzymes (37). Xc16 did not have these two prominent bands but exhibited a smaller band at ∼90 kDa (Fig. 1B, lane 3). The band at ∼90 kDa in Xc16 may be derived from the truncated gene product of the gtfB gene, because half of the 5′-terminus portion of gtfB is still able to be translated in Xc16. Active staining analysis on SDS-PAGE revealed that Xc16 did not exhibit water-insoluble glucan-producing activity (data not shown). These results confirmed that strain Xc11 was a PAc-defective mutant and strain Xc16 was a mutant defective in both GTF-I and GTF-SI.
CL responses of human PMNs to S. mutans strains.
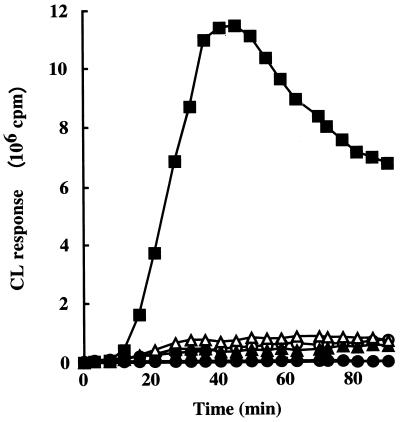
The luminol-enhanced CL responses of human PMNs in the presence or absence of complement were examined when challenged with S. mutans Xc or its transformants. Figure 3 shows typical patterns of the CL response of human PMNs to S. mutans strains in the presence of complement. The CL response of human PMNs to the PAc-defective mutant Xc11 was as weak as it was for wild-type strain Xc. The response to mutant Xc16, which is defective in both GTF-I and GTF-SI, was also very weak. In contrast, the CL response of human PMNs to strain Xc24R, which is defective in serotype c-specific polysaccharide, was significantly stronger than the responses to Xc and the other Xc mutant strains. We also examined the CL responses of human PMNs to these S. mutans strains in the absence of complement. The CL response of human PMNs to strain Xc24R was much stronger than the responses to Xc and the other Xc mutant strains in the absence of complement (data not shown). The CL responses of human PMNs to S. mutans strains LM7 (serotype e) and KT6219 (serotype f) were also much weaker than the responses to their serotype-specific polysaccharide mutants (LM7DR and KT6219DR) in the presence or absence of complement (data not shown). Similar results were obtained from the same experiments by using human PMNs from two other healthy adults (data not shown).
FIG. 3.
Representative CL responses of human PMNs to S. mutans strains in the presence of complement. Human PMNs (106 cells) pretreated with HBSS++ buffer containing 1% gelatin in the presence of 1% complement were unstimulated (●) or stimulated at 37°C for 90 min with whole cells of S. mutans strain (6.4 × 106 CFU) Xc (○), Xc11 (▴), Xc16 (▵), or Xc24R (■). The experiments were performed three times, and similar results were obtained in each experiment.
Killing of serotype c-specific polysaccharide-defective S. mutans mutant.
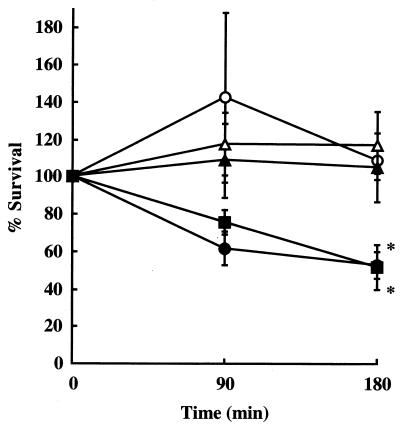
The killing of strains Xc, Xc11, Xc16, and Xc24R by human PMNs in the presence of complement was examined (Fig. 4). Percent survival of Xc11 and Xc16 did not significantly differ from that of parental strain Xc. In contrast, Xc24R was significantly more effectively killed by human PMNs than the parental strain Xc after 180 min (P < 0.01). When rabbit anti-PAc IgG was added to the reaction mixture of Xc, the survival rate of the bacteria was reduced to 50% (Fig. 4). This decrease rate was very near to the rate of Xc24R in the absence of anti-PAc IgG.
FIG. 4.
Phagocytic killing of S. mutans strains by human PMNs in the presence of complement. S. mutans strain Xc (○), Xc11 (▴), Xc16 (▵), or Xc24R (■) (105 CFU) was incubated with or without human PMNs (106 cells) in the presence of 5% complement. As a positive control, 150 μg of rabbit anti-recombinant PAc IgG was added to the reaction mixture of strain Xc with human PMNs as described above (●). Data represent the mean percent survival for three different experiments ± standard deviation. Statistical differences in percent survival values were analyzed by Welch's t test (*, P < 0.01).
Microscopic observation of phagocytic ingestion of S. mutans by human PMNs.
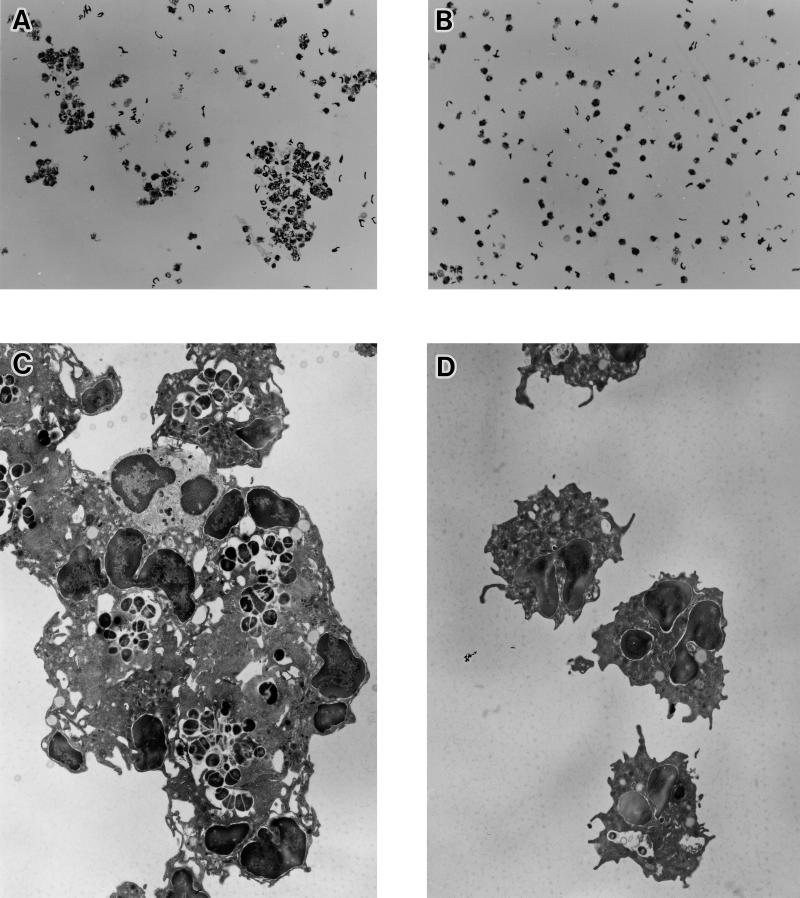
The phagocytic ingestion of Xc or Xc24R by human PMNs was observed with light and electron microscopes. Under a light microscope, many clusters of human PMNs were observed when challenged with Xc24R (Fig. 5A), whereas few clustered human PMNs were observed when challenged with wild-type strain Xc (Fig. 5B). Observations by transmission electron microscopy showed that some human PMNs made clusters, and most of them internalized many Xc24R cells. On the other hand, few human PMNs internalized Xc cells (Fig. 5D).
FIG. 5.
Light (A and B) and transmission electron (C and D) microscopic observations of human PMNs incubated with S. mutans strain Xc24R (A and C) or strain Xc (B and D) in the presence of 2% complement for 90 min. Magnifications, ×80 (A and B) and ×2,500 (C and D).
Cell-surface hydrophobicity.
The cell-surface hydrophobicity of S. mutans strains was examined (Table 2). The surface hydrophobicity of strain Xc11 was significantly lower than that of wild-type strain Xc (P < 0.01), while strain Xc24R was significantly more hydrophobic than strain Xc (P < 0.01). The surface hydrophobicity of strain Xc16 was almost the same as that of strain Xc. Serotype-specific polysaccharide-defective mutants LM7DR and KT6219DR were also significantly more hydrophobic than parental strains LM7 (serotype e) and KT6219 (serotype f), respectively (P < 0.01).
TABLE 2.
Surface hydrophobicity of S. mutans strains
| Strain | Hydrophobicity (%)a |
|---|---|
| Xc | 53.2 ± 2.2 |
| Xc11 | 32.6 ± 2.3b |
| Xc16 | 51.5 ± 3.5 |
| Xc24R | 99.3 ± 0.8b |
| LM7 | 44.2 ± 3.5 |
| LM7DR | 78.6 ± 2.8b |
| KT6219 | 49.9 ± 5.1 |
| KT6219DR | 82.7 ± 1.0b |
The bacterial cell suspension (3 ml, OD550 = 0.6) was mixed with 0.3 ml of hexadecane. The cell surface hydrophobicity was calculated as the percentage loss of OD of the aqueous phase relative to the initial bacterial cell suspension. Each value represents the mean ± standard deviation for assays performed five times.
P < 0.01 compared with the value of the wild-type strain of each serotype.
Differences in cell surface architecture between strains Xc and Xc24R.
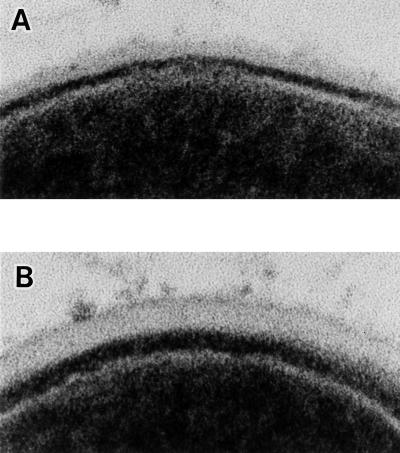
Electron microscopic observation of thin-sectioned cells of Xc and Xc24R indicated that there was a cell wall-like architecture which had two layers on the cell surfaces of both strain Xc and strain Xc24R (Fig. 6). In strain Xc, the outer layer which seemed to contain several cell surface components was wide and rather transparent, and the inner one, which was thought to be a peptidoglycan layer, showed a high density. Both of these layers in strain Xc24R were thinner than those in strain Xc, and Xc24R exhibited a dim contour of the outer layer.
FIG. 6.
Transmission electron microscopic observation of surface architecture of strain Xc24R (A) or strain Xc (B). Magnification, ×360,000.
DISCUSSION
Phagocytosis of bacterial pathogens by phagocytes, such as neutrophil leukocytes and macrophages, is one of the most important defense mechanisms in the oral cavity (2, 31, 32, 34). This function may regulate S. mutans in crevicular fluid and prevent dental caries, particularly root caries. There is evidence that patients with neutropenia are more susceptible to root caries (26), suggesting an important role of phagocytic cells in the host resistance to dental caries. Therefore, it is very important to study phagocytosis of S. mutans by human PMNs.
The luminol-enhanced CL method, which reflects phagocytic oxidative metabolism in PMNs, is often used for assessing phagocytosis (9). In this study, we examined the roles of cell surface components of S. mutans in resistance to phagocytosis by human PMNs by using a CL assay. The CL responses of human PMNs to parent strain Xc, PAc-defective mutant Xc11, and mutant Xc16, which is defective in both GTF-I and GTF-SI, were very weak (Fig. 3). The protein PAc is structurally similar to the M protein (6, 12). Therefore, it would be reasonable to assume that this protein may have antiphagocytic activity, like the M protein. However, the results of the CL assay and killing assay were not consistent with this hypothesis. In contrast, strain Xc24R defective in serotype c-specific polysaccharide induced a markedly strong CL response (Fig. 3). In addition, strain Xc24R was more efficiently killed by human PMNs than strains Xc, Xc11, and Xc16 (Fig. 4). Under light microscopy, many human PMNs were observed to form clusters when human PMNs were incubated with Xc24R (Fig. 5A). On the other hand, most of the human PMNs could not aggregate when incubated with strain Xc (Fig. 5B). Henricks et al. (8) reported that human PMNs aggregate during phagocytosis of bacteria. Electron microscopic observation demonstrated that the clustered human PMNs ingested a lot of strain Xc24R cells (Fig. 5C). In contrast, the nonaggregating human PMNs internalized few cells of strain Xc (Fig. 5D). Furthermore, mutants defective in serotype e- and f-specific polysaccharides also induced markedly higher CL responses than their wild-type strains (data not shown). These results suggest that the major antiphagocytic cell surface component of S. mutans is the serotype-specific polysaccharide antigen, but not the PAc protein. Xc16 still produces truncated GTF-I protein. However Xc16 did not change its antiphagocytic properties at all, even though a C-terminal half of the GTF-I protein and the whole GTF-SI protein were lost. Although we could not rule out a role of the GTF-I N terminus in antiphagocytic effect against PMNs, our findings suggest that GTF proteins may not play an important role in antiphagocytic effect.
Bacterial hydrophobicity is known to play an important role in the interaction between bacteria and phagocytic cells and subsequent phagocytosis (1, 7, 24, 40, 43). In this study, the cell surface of the serotype c-specific polysaccharide-defective mutant Xc24R is markedly more hydrophobic than that of the wild-type strain Xc (Table 2). This was consistent with the results of the CL assay (Fig. 3). The cell surface hydrophobicity of Xc16 (defective in both GTF-I and GTF-SI) was the same as that of strain Xc, while that of the PAc-defective mutant Xc11 was much lower. These results do not completely match those of the CL assay. The CL response of human PMNs to strain Xc was the same as the background response in the absence of bacteria (Fig. 3). Moreover, the degrees of killing of Xc and Xc11 by human PMNs were very low, and the difference between both data was not significant (Fig. 4). These findings suggest that even wild-type strain Xc is rarely ingested by human PMNs. In the assays used in this study, it is difficult to show that Xc11 is more resistant to human phagocytic cells than Xc. Therefore, the results of the CL assay and the killing assay may not contradict those related to the cell surface hydrophobicity of S. mutans strains.
The electron microscopic observation of cell surfaces of S. mutans strains Xc and Xc24R indicated that the cell wall of Xc24R was thinner than that of parental strain Xc (Fig. 6). This difference is considered to be derived from the defectiveness of serotype-specific polysaccharide. Gram-positive bacteria such as S. mutans have a thick cell wall which is mainly composed of peptidoglycan and lipoteichoic acid (LTA). It was reported that LTA is the major factor responsible for cell surface hydrophobicity of group A streptococci and that LTA specifically binds to human PMNs (5, 22). The markedly high cell surface hydrophobicity of Xc24R may be due to the exposure of LTA on the cell surface. Furthermore the exposure of LTA on the cell surface may elicit binding of Xc24R to human PMNs and subsequent phagocytosis.
The serum opsonic activity for S. mutans was reported to be closely related to protection against dental caries (34). Scully et al. (33) suggested that the opsonized S. mutans may fail to proliferate and may be phagocytosed and killed by local neutrophils and complement. Lehner et al. (16–18) also suggested that local passive immunization by monoclonal antibody may be a strong weapon for preventing dental caries. Although serotype-specific polysaccharide antigens of mutans streptococci were reported to be poorly immunogenic, oral administration of serotype c polysaccharide encapsulated in liposomes to human volunteers was reported to induce serum IgG and salivary IgA antibodies (30). This finding suggests that immunization with serotype-specific polysaccharide may prevent dental caries.
In conclusion, this study suggests that the serotype-specific polysaccharide antigens of S. mutans play an important role in resistance to phagocytosis and consequent killing by human PMNs. The serotype-specific polysaccharide antigens of S. mutans may make the cell surface more hydrophilic, which interferes with the interaction between this organism and the host phagocytic cells. It is speculated that inhibition of the rmlB gene product may be an effective method of controlling S. mutans. Antibodies against serotype-specific polysaccharide antigens of S. mutans may also be useful for controlling the organism and preventing dental caries.
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aid for Developmental Scientific Research (04557095 and 07557134) from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Absolom D R. The role of bacterial hydrophobicity in infection: bacterial adhesion and phagocytic ingestion. Can J Microbiol. 1988;34:287–298. doi: 10.1139/m88-054. [DOI] [PubMed] [Google Scholar]
- 2.Attström R, Egelberg J. Emigration of blood neutrophils and monocytes into the gingival crevices. J Periodontal Res. 1970;5:48–55. doi: 10.1111/j.1600-0765.1970.tb01837.x. [DOI] [PubMed] [Google Scholar]
- 3.Burnette W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 4.China B, N'Guyen B T, de Bruyere M, Cornelis G R. Role of YadA in resistance of Yersinia enterocolitica to phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1994;62:1275–1281. doi: 10.1128/iai.62.4.1275-1281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtney H, Ofek I, Simpson W A, Beachey E H. Characterization of lipoteichoic acid binding to polymorphonuclear leukocytes of human blood. Infect Immun. 1981;32:625–631. doi: 10.1128/iai.32.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 7.Genco C A, Schifferle R E, Njoroge T, Forng R-Y, Cutler C W. Resistance of a Tn4351-generated polysaccharide mutant of Porphyromonas gingivalis to polymorphonuclear leukocyte killing. Infect Immun. 1995;63:393–401. doi: 10.1128/iai.63.2.393-401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henricks P A J, van der Tol M E, Verhoef J. Aggregation of human polymorphonuclear leucocytes during phagocytosis of bacteria. Immunology. 1984;52:671–678. [PMC free article] [PubMed] [Google Scholar]
- 9.Hosker H S R, Kelly C, Corris P A. Assessment of phagocytic function using chemiluminescence. Blood Rev. 1989;3:88–93. doi: 10.1016/0268-960x(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 10.Koga T, Asakawa H, Okahashi N, Takahashi I. Effect of subculturing on expression of a cell-surface protein antigen by Streptococcus mutans. J Gen Microbiol. 1989;135:3199–3207. doi: 10.1099/00221287-135-12-3199. [DOI] [PubMed] [Google Scholar]
- 11.Koga T, Okahashi N, Takahashi I, Kanamoto T, Asakawa H, Iwaki M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990;58:289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koga T, Yamashita Y, Nakano Y, Kawasaki M, Oho T, Yu H, Nakai M, Okahashi N. Surface proteins of Streptococcus mutans. Dev Biol Stand. 1995;85:363–369. [PubMed] [Google Scholar]
- 13.Kuramitsu H K, Smorawinska M, Nakano Y J, Shimamura A, Lis M. Analysis of glucan synthesis by Streptococcus mutans. Dev Biol Stand. 1995;85:303–307. [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lancefield R C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 16.Lehner T, Caldwell J, Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect Immun. 1985;50:796–799. doi: 10.1128/iai.50.3.796-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehner T, Challacombe S J, Caldwell J. Immunological and bacteriological basis for vaccination against dental caries in rhesus monkeys. Nature. 1975;254:517–520. doi: 10.1038/254517a0. [DOI] [PubMed] [Google Scholar]
- 18.Lehner T, Challacombe S J, Wilton J M A, Caldwell J. Cellular and humoral immune responses in vaccination against dental caries in monkeys. Nature. 1976;264:69–72. doi: 10.1038/264069a0. [DOI] [PubMed] [Google Scholar]
- 19.Linzer R, Reddy M S, Levine M J. Immunochemical aspects of serotype carbohydrate antigens of Streptococcus mutans. In: Hamada S, Michalek S M, Kiyono H, Menaker L, McGhee J R, editors. Molecular microbiology and immunology of Streptococcus mutans. Amsterdam, The Netherlands: Elsevier Science Publishers; 1986. pp. 29–38. [Google Scholar]
- 20.Martin V, Kleschyov A L, Klein J-P, Beretz A. Induction of nitric oxide production by polyosides from the cell walls of Streptococcus mutans OMZ 175, a gram-positive bacterium, in the rat aorta. Infect Immun. 1997;65:2074–2079. doi: 10.1128/iai.65.6.2074-2079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maryanski J H, Wittenberger C L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975;124:1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miörner H, Johansson G, Kronvall G. Lipoteichoic acid is the major cell wall component responsible for surface hydrophobicity of group A streptococci. Infect Immun. 1983;39:336–343. doi: 10.1128/iai.39.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mundi H, Björkstén B, Svanborg C, Öhman L, Dahlgren C. Extracellular release of reactive oxygen species from human neutrophils upon interaction with Escherichia coli strains causing renal scarring. Infect Immun. 1991;59:4168–4172. doi: 10.1128/iai.59.11.4168-4172.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ofek I, Goldhar J, Keisari Y, Sharon N. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 25.Ouchterlony Ö. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- 26.Pernu H E, Pajari U H, Lanning M. The importance of regular dental treatment in patients with cyclic neutropenia. Follow-up of 2 cases. J Periodontol. 1996;67:454–459. doi: 10.1902/jop.1996.67.4.454. [DOI] [PubMed] [Google Scholar]
- 27.Perry D, Wondrack L M, Kuramitsu H K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983;41:722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips G N, Jr, Flicker P F, Cohen C, Manjula B N, Fischetti V A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci USA. 1981;78:4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchard D G, Gregory R L, Michlek S M, McGhee J R. Biochemical aspects of serotype carbohydrate antigens of Streptococcus mutans. In: Hamada S, Michalek S M, Kiyono H, Menaker L, McGhee J R, editors. Molecular microbiology and immunology of Streptococcus mutans. Amsterdam, The Netherlands: Elsevier Science Publishers; 1986. pp. 39–49. [Google Scholar]
- 30.Russell M W. Immunization against dental caries. Curr Opin Dent. 1992;2:72–80. [PubMed] [Google Scholar]
- 31.Scully C, Challacombe S J. The migration of 111Indium-labelled polymorphonuclear leucocytes into the oral cavity in the rhesus monkey. J Periodontal Res. 1979;14:475–481. doi: 10.1111/j.1600-0765.1979.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 32.Scully C M. Comparative opsonic activity for Streptococcus mutans in oral fluids, and phagocytic activity of blood, crevicular, and salivary polymorphonuclear leucocytes in rhesus monkeys. Immunology. 1980;39:101–107. [PMC free article] [PubMed] [Google Scholar]
- 33.Scully C M, Lehner T. Opsonization, phagocytosis and killing of Streptococcus mutans by polymorphonuclear leukocytes, in relation to dental caries in the rhesus monkey (Macaca mulatta) Arch Oral Biol. 1979;24:307–312. doi: 10.1016/0003-9969(79)90093-1. [DOI] [PubMed] [Google Scholar]
- 34.Scully C M, Russell M W, Lehner T. Specificity of opsonizing antibodies to antigens of Streptococcus mutans. Immunology. 1980;41:467–473. [PMC free article] [PubMed] [Google Scholar]
- 35.Soell M, Lett E, Holveck F, Schöller M, Wachsmann D, Klein J P. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-alpha release. J Immunol. 1995;154:851–860. [PubMed] [Google Scholar]
- 36.Stinson M W, Albini B, Nisengart R J. Adverse effects of Streptococcus mutans antigens on host tissues. In: Hamada S, Michalek S M, Kiyono H, Menaker L, McGhee J R, editors. Molecular microbiology and immunology of Streptococcus mutans. Amsterdam, The Netherlands: Elsevier Science Publishers; 1986. pp. 307–318. [Google Scholar]
- 37.Tanzer J M, Freedman M L, Fitzgerald R J, Larson R H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974;10:197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsukioka Y, Yamashita Y, Nakano Y, Oho T, Koga T. Identification of a fourth gene involved in dTDP-rhamnose synthesis in Streptococcus mutans. J Bacteriol. 1997;179:4411–4414. doi: 10.1128/jb.179.13.4411-4414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukioka Y, Yamashita Y, Oho T, Nakano Y, Koga T. Biological function of the dTDP-rhamnose synthesis pathway in Streptococcus mutans. J Bacteriol. 1997;179:1126–1134. doi: 10.1128/jb.179.4.1126-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Oss C J, Absolom D R, Neumann A W. Surface forces in phagocytosis. In: Reichard S M, Filkins W, editors. The reticuloendothelial system. Vol. 7. New York, N.Y: Plenum Press; 1984. pp. 3–35. [Google Scholar]
- 41.Weis J J, Law S K, Levine R P, Cleary P P. Resistance to phagocytosis by group A streptococci: failure of deposited complement opsonins to interact with cellular receptors. J Immunol. 1985;134:500–505. [PubMed] [Google Scholar]
- 42.Wetherell J R, Jr, Bleiweis A S. Antigens of Streptococcus mutans: characterization of a polysaccharide antigen from walls of strain GS-5. Infect Immun. 1975;12:1341–1348. doi: 10.1128/iai.12.6.1341-1348.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams P, Lambert P A, Haigh C G, Brown M R W. The influence of the O and K antigens of Klebsiella aerogenes on surface hydrophobicity and susceptibility to phagocytosis and antimicrobial agents. J Med Microbiol. 1986;21:125–132. doi: 10.1099/00222615-21-2-125. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi N, Kawasaki M, Yamashita Y, Nakashima K, Koga T. Role of the capsular polysaccharide-like serotype-specific antigen in resistance of Actinobacillus actinomycetemcomitans to phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1995;63:4589–4594. doi: 10.1128/iai.63.12.4589-4594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita Y, Bowen W H, Burne R A, Kuramitsu H K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita Y, Tomihisa K, Nakano Y, Shimazaki Y, Oho T, Koga T. Recombination between gtfB and gtfC is required for survival of a dTDP-rhamnose synthesis-deficient mutant of Streptococcus mutans in the presence of sucrose. Infect Immun. 1999;67:3693–3697. doi: 10.1128/iai.67.7.3693-3697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita Y, Tsukioka Y, Nakano Y, Shibata Y, Koga T. Molecular and genetic analysis of multiple changes in the levels of production of virulence factors in a subcultured variant of Streptococcus mutans. FEMS Microbiol Lett. 1996;144:81–87. doi: 10.1111/j.1574-6968.1996.tb08512.x. [DOI] [PubMed] [Google Scholar]