Abstract
Vulvovaginal candidiasis (VVC) is an opportunistic mucosal infection caused by Candida albicans that affects large numbers of otherwise healthy women of childbearing age. Acute episodes of VVC often occur during pregnancy and during the luteal phase of the menstrual cycle, when levels of progesterone and estrogen are elevated. Although estrogen-dependent experimental rodent models of C. albicans vaginal infection are used for many applications, the role of reproductive hormones and/or their limits in the acquisition of vaginal candidiasis remain unclear. This study examined the effects of estrogen and progesterone on several aspects of an experimental infection together with relative cell-mediated immune responses. Results showed that while decreasing estrogen concentrations eventually influenced infection-induced vaginal titers of C. albicans and rates of infection in inoculated animals, the experimental infection could not be achieved in mice treated with various concentrations of progesterone alone. Furthermore, progesterone had no effect on (i) the induction and persistence of the infection in the presence of estrogen, (ii) delayed-type hypersensitivity in primary-infected mice, or (iii) the partial protection from a secondary vaginal infection under pseudoestrus conditions. Other results with estrogen showed that a persistent infection could be established with a wide range of C. albicans inocula under supraphysiologic and near-physiologic (at estrus) concentrations of estrogen and that vaginal fungus titers or rates of infection were similar if pseudoestrus was initiated several days before or after inoculation. However, the pseudoestrus state had to be maintained for the infection to persist. Finally, estrogen was found to reduce the ability of vaginal epithelial cells to inhibit the growth of C. albicans. These results suggest that estrogen, but not progesterone, is an important factor in hormone-associated susceptibility to C. albicans vaginitis.
Vulvovaginal candidiasis (VVC) is a significant problem for women of childbearing age; approximately 75% of all women experience at least one episode of VVC during their lifetime (24, 26). Several exogenous factors, including antibiotic or oral contraceptive usage, pregnancy, hormone replacement therapy (HRT), and uncontrolled diabetes mellitus, predispose women to VVC (24, 26). In the absence of these factors, clinical observations show that VVC most often occurs in women during the luteal phase of the menstrual cycle, when estrogen and progesterone levels are elevated (11). In contrast, premenarchal and postmenopausal women not receiving HRT rarely suffer from VVC (23). There also exists a subset of women (5 to 10%) who experience recurrent VVC (RVVC), defined as 3 to 4 episodes per annum in the absence of any recognized predisposing factors, including menstrual cycle patterns (23, 25). RVVC is presumed to result from some local innate and/or acquired dysfunction in the normal protective immune response most healthy individuals acquire from early exposure to Candida albicans (10, 36, 37). C. albicans, a commensal organism of the gastrointestinal and reproductive tracts, is the causative agent in approximately 85 to 90% of cases of VVC or RVVC (23, 25). Antimycotic therapy is useful for individual attacks of VVC or RVVC but does not prevent recurrence (23), and antifungal drug resistance does not contribute to recurrence (15).
Animal models of experimental vaginal candidiasis have been extremely useful for identifying factors relative to susceptibility to infection (7, 22, 27, 29). In these models, the most important requirement for a persistent infection is a state of pseudoestrus (22, 27, 31). In the absence of estrogen treatments, the infection is short-lived, with a low fungal burden in the vagina (8). In general, it was thought that the estrogen-dependent transition of the epithelial cells from columnar to stratified squamous makes them more permissive for adherence and growth of C. albicans (13; B. L. Powell and D. I. Drutz, Abstr. 23rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 751, p. 222, 1983). Furthermore, yeast cells possess receptors for estrogen that enhance mycelial formation (Powell and Drutz, 23rd ICAAC). Historically, the animal models were used for drug testing under a supraphysiologic state of estrus (17, 22, 27). However, more recently, a near-physiologic state of estrus has been used with similar results (1, 5). No formal study on the role of estrogen has been conducted in these models, however, and the role of progesterone in the infection has not been evaluated.
More recently, the murine model of vaginal candidiasis has been used to study host defense mechanisms against C. albicans. It is generally accepted that cell-mediated immunity (CMI) by T cells and cytokines (specifically a Th1-type response) is the dominant host defense mechanism against C. albicans infection in mucosal tissues (19, 21). The most recent data from the experimental model, however, have questioned whether there is a role for the infection-induced Candida-specific systemic CMI, as well as local CMI, in protection against a vaginal C. albicans infection (5, 6, 8, 9). Although a state of pseudoestrus is considered a requirement to establish and sustain the infection and has no demonstrable effects on in vivo Candida-specific CMI (i.e., delayed-type hypersensitivity [DTH]) (7, 8) it is unclear how reproductive hormones influence host defense in response to the infection. Indeed, estrogen and progesterone have been shown to inhibit aspects of both innate and acquired immunity at the systemic or local level (2, 14, 16, 30, 34, 35), including Candida-specific human peripheral blood lymphocyte (PBL) responses (11, 12) or neutrophil anti-Candida activity (18) in vitro. Furthermore, in vitro Candida-specific PBL responses were reduced in women during the luteal phase of the menstrual cycle concomitant with increased serum-induced germination of C. albicans (11).
The purpose of the present study was to better understand the contribution of estrogen and progesterone in susceptibility to a primary experimental vaginal C. albicans infection and the influence of progesterone on systemic or local immune reactivity in the presence or absence of estrogen.
MATERIALS AND METHODS
Mice.
CBA/J (H-2k) mice, 8 to 10 weeks of age, purchased from the National Cancer Institute, Frederick, Md., were used throughout these studies. All guidelines related to the appropriate care and use of laboratory animals were strictly adhered to.
Hormone treatments, infection, and DTH.
A primary and a secondary C. albicans vaginal infection were used as previously described (6, 7). For primary infection, 72 h prior to inoculation (unless otherwise stated), groups of 5 to 10 animals were treated subcutaneously with 0.1 ml of various concentrations of estradiol valerate (Sigma Chemical Co., St. Louis, Mo.) and/or progesterone (Sigma) dissolved in sesame seed oil. Hormone treatments continued weekly until completion of the study (up to 5 weeks) unless otherwise stated. None of the animals were oophorectomized prior to hormone treatment. However, examination of the vagina, uterus, and fallopian tubes of treated mice showed the predicted presence of swollen tissue under the majority of estrogen concentrations tested and the absence of such swelling under all progesterone concentrations tested. Animals not treated with hormones were given sesame seed oil alone. Animals were inoculated intravaginally with 5 × 104 stationary-phase C. albicans blastoconidia (3153A) (a long-term laboratory-cultivated clinical isolate) in 20 μl of phosphate-buffered saline (PBS) as previously described (7). For secondary infection, animals were inoculated with 5 × 105 stationary-phase C. albicans blastoconidia in the absence of estrogen treatment (6). After 4 weeks, following spontaneous resolution of the primary infection (verified by sterile vaginal lavage fluid), animals were treated with progesterone and/or estrogen as described above and 72 h later were inoculated a second time with 5 × 104 C. albicans blastoconidia. Controls included animals given PBS intravaginally during the first 4 weeks followed by a primary inoculation under estrogen conditions. Estrogen and/or progesterone treatments were continued weekly until completion of the study (10 days). Twenty-four hours prior to sacrifice, animals were footpad challenged with C. albicans culture filtrate antigen (CaCF) (10 μg), and DTH (footpad swelling) was recorded 24 h later as previously described (7). After sacrifice, vaginal lavages were performed by a harsh wash and aspiration of the vaginal cavity using 100 μl of PBS. The lavage fluid was serially diluted and plated on Sabouraud dextrose agar containing 1% gentamicin as described previously (7). CFU were evaluated after 48 h at 35°C. Wet-mount slides of lavage fluid were used to identify hyphae as evidence of infection as previously described (7).
Growth inhibition assay.
Vaginal epithelioid cells were collected from 10 to 12 mice and processed as previously described (28). Briefly, vaginae were excised, the cervix was removed and discarded, and the vaginal tissue was minced and incubated with 0.25% collagenase type IV (Sigma) for 1 h at 37°C in a shaking water bath with intermittent (10 s every 15 min) Stomacher (Tekmar Inc., Cincinnati, Ohio) homogenization. Epithelioid-enriched populations were collected from a 20-μm-pore-size nylon membrane (Small Parts Inc., Miami Lakes, Fla.) with tissue culture medium.
The growth inhibition assay was a [3H]glucose uptake assay (4) modified for use with epithelial cells (28). Briefly, stationary-phase C. albicans blastoconidia were added to individual wells of a microtiter plate at 5 × 104 cells/ml in a volume of 100 μl of tissue culture medium consisting of phytone peptone broth medium (BBL, Cockeysville, Md.) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μ/ml) (all from GIBCO, Grand Island, N.Y.). Vaginal cells suspended in the same medium were added to triplicate wells at various effector-to-target (E:T) ratios in a volume of 100 μl. The culture was incubated for 9 h at 37°C under 7.5% CO2 in the presence of 1 μCi of [3H]glucose (ICN, Costa Mesa, Calif.). Thereafter, bleach was added to the wells for 5 min, and the cell extracts were harvested onto glass fiber filters. The incorporated [3H]glucose was then measured by liquid scintillation. Controls included C. albicans and effector cells cultured in medium alone. The uptake of glucose by C. albicans and effector cells during the 9-h assay was generally 10,000 to 30,000 and 100 to 500 cpm, respectively. Percent growth inhibition was calculated as {1 − [(mean counts per minute in the coculture − mean counts per minute in effector cells)/(mean counts per minute in C. albicans cells)]} × 100.
Statistics.
Where appropriate, comparisons between groups were made by using the Student t test. Significance was defined as a P value of <0.05.
RESULTS
Effects of estrogen concentration on experimental vaginal candidiasis.
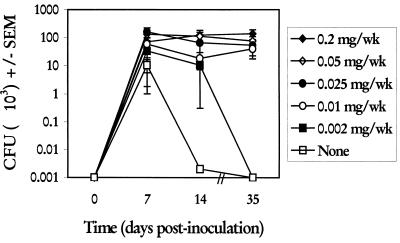
To determine the limits of estrogen required to induce and sustain a vaginal infection, estrogen was given to mice in concentrations ranging from 0.2 to 0.002 mg/mouse (ms)/week beginning 72 h prior to vaginal inoculation. The vaginal fungal burden was quantified at days 7, 14, and 35 postinoculation. Results in Fig. 1 show that estrogen given to mice at concentrations between 0.2 and 0.01 mg/ms/week resulted in a persistent infection with high fungal titers in the vagina through the 35-day observation period that were not statistically different. In contrast, estrogen given to mice at 0.002 mg/ms/week resulted in high fungal titers in the vagina early (day 7), followed by variable titers at day 14 and undetectable levels at day 35. Mice not given estrogen had comparable vaginal fungal titers at day 7 that sharply declined at day 14 (P < 0.0007), with similarly undetectable levels at day 35. Rates of infection were high on day 7 postinoculation in groups of mice given 0.01 to 0.2 mg of estrogen/ms/week (94 to 100%), whereas lower rates were observed in untreated mice or those given 0.002 mg/ms/week (50 and 69%, respectively). At later times, the rates of infection in those mice given 0.05 to 0.2 mg/ms/week remained high (87 to 100%), whereas rates of infection in mice that were left untreated or given <0.025 mg/ms/week were much lower (0 to 70%).
FIG. 1.
Effect of estrogen concentration on the induction and maintenance of experimental vaginal C. albicans infection. Animals were treated subcutaneously with various concentrations of estradiol valerate or sesame seed oil alone (none) 72 h prior to vaginal inoculation and weekly thereafter until completion of the study. At days 7, 14, and 35 postinoculation, groups of five to six randomly selected animals per group were sacrificed, and vaginal fungal burden was determined by quantitative vaginal lavage culture. CFU ± standard errors of the means (SEM) from five separate experiments are shown.
Effects of progesterone on experimental vaginal candidiasis.
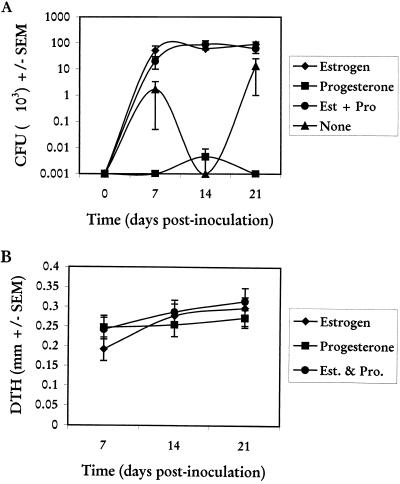
To determine the effects of progesterone on the induction of a primary vaginal infection and the resulting DTH as a measure of Candida-specific systemic CMI, progesterone (1.0 mg/ms/week) was given in the presence or absence of estrogen (0.2 mg/ms/week) beginning 72 h prior to vaginal inoculation. Control animals received vehicle alone. Results in Fig. 2A show that progesterone treatment had no effect on the high fungal titers in the vagina through 21 days of infection in the presence of estrogen. In the absence of exogenous estrogen, vaginal fungal titers in progesterone-treated mice were not different from those in mice treated with vehicle only (throughout the 21-day period, 7% of progesterone-treated animals versus 13% of vehicle-treated controls had detectable vaginal fungal burdens). DTH results (Fig. 2B) showed that mice treated with progesterone alone had DTH responses similar to those of mice treated with estrogen alone or with both progesterone and estrogen. Similar results for vaginal fungal titers and DTH were observed when progesterone was given at threefold-higher concentrations (1.0 mg/ms/2 days) (data not shown).
FIG. 2.
Effects of progesterone on experimental C. albicans vaginal infections. Animals were treated with estrogen (Est; 0.2 mg/mouse) and/or progesterone (Pro; 1.0 mg/mouse) or sesame seed oil alone (none) subcutaneously 72 h prior to vaginal inoculation and weekly thereafter until completion of the study. At days 6, 13, and 20 postinoculation, five to six randomly selected animals per group were footpad challenged with CaCF, and DTH was measured 24 h later. Thereafter, mice were sacrificed and vaginal fungal burden was determined by quantitative vaginal lavage culture. (A) CFU ± standard errors of the means (SEM) from two experiments. (B) DTH (difference in thickness of footpad given Candida antigen versus footpad given PBS) ± SEM from estrogen-treated, progesterone-treated, or estrogen-plus-progesterone-treated mice.
To determine if the lack of effect of progesterone on vaginal fungal burden in the presence of estrogen was due to the high concentrations of estrogen used, a similar experiment was performed with a 10-fold-lower concentration of estrogen (0.02 mg/ms/week). Results, expressed as vaginal fungal burdens, showed that compared with treatment with estrogen alone and inoculation (1.5 × 104 ± 1.4 × 104 CFU on day 7; 1.4 × 104 ± 1.3 × 104 CFU on day 21), the addition of progesterone had no effect when given once (3.9 × 104 ± 2.9 × 104 CFU on day 7; 2.4 × 104 ± 9.8 × 103 CFU on day 21) or three times (4.4 × 104 ± 3.1 × 104 CFU on day 7; 3.3 × 104 ± 3.0 × 104 CFU on day 21) per week over a 21-day period.
Effects of progesterone on protection against a secondary vaginal infection.
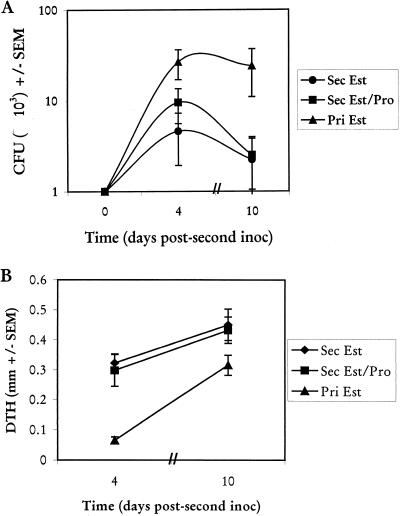
To determine whether progesterone had any effect on the partial protection against a secondary vaginal infection, groups of animals were given the standard vaginal inoculum in the absence of estrogen. At week 4, when the low-grade vaginal infection had spontaneously resolved, animals were randomized to receive a second vaginal inoculation in the presence of estrogen (0.02 mg/ms/week) alone or with progesterone (1 mg/ms/week). Positive-control mice received PBS intravaginally in the absence of estrogen and then were given a primary infection in the presence of estrogen when the other mice were given the secondary challenge (week 4). Vaginal fungal burdens and DTH responses on days 4 and 10 in the three groups of mice are shown in Fig. 3. Progesterone had no effect on the partial protection against the second infection (Fig. 3A) (P < 0.04 for primary versus secondary infection in the presence of estrogen), nor did it affect the anamnestic DTH response normally observed as a result of the second vaginal exposure to C. albicans (Fig. 3B) (P < 0.00005 for primary versus secondary infections in the presence of estrogen) (6).
FIG. 3.
Effect of progesterone (Pro) on secondary (Sec) vaginal C. albicans infections. Animals were vaginally inoculated in the absence of estrogen. Four weeks later the mice received the first of weekly injections of estrogen (Est; 0.02 mg/mouse) or estrogen plus progesterone (1.0 mg/mouse), and they were inoculated intravaginally a second time 72 h later. Controls included animals given a primary C. albicans inoculation in the presence of estrogen (0.02 mg/mouse) (Pri Est) at the time of secondary challenge. At days 3 and 9 postinoculation, 10 mice per group were footpad challenged with CaCF, and DTH was measured 24 h later. Thereafter the mice were sacrificed, and vaginal fungal burdens were determined by quantitative vaginal lavage culture. (A) CFU ± standard errors of the means (SEM) from three experiments. (B) DTH ± SEM from the same mice.
Studies to further examine the estrogen dependence of experimental vaginal candidiasis.
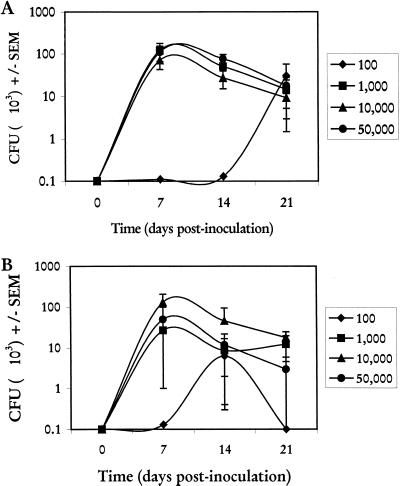
Taking into account the dominant role of estrogen in vaginal candidiasis, a series of experiments to determine the properties of the hormone dependence was conducted. The first study evaluated the limits of organism inoculum in the presence of estrogen. For this, animals were given the first of weekly injections of 0.2 or 0.02 mg of estradiol/ms/week 72 h prior to inoculation. The mice were inoculated with 5 × 104, 1 × 104, 1 × 103, and 1 × 102 blastoconidia, and vaginal fungal burdens were quantified over 21 days. Figure 4A shows that in the presence of 0.2 mg of estradiol/ms/week, there were no significant differences in vaginal fungal burden down to an inocula of 1 × 103 blastoconidia. There were also no differences in the rates of infection (data not shown). In contrast, only 7% of mice had detectable vaginal fungal burdens over the 21-day period from an inocula of 1 × 102 blastoconidia. Similar results were observed in the presence of 0.02 mg of estradiol/mouse (Fig. 4B).
FIG. 4.
Effect of C. albicans inocula on induction and maintenance of experimental C. albicans vaginal infections. Animals received the first of weekly subcutaneous injections of estradiol valerate at 0.2 (A) or 0.02 (B) mg/mouse and were inoculated intravaginally 72 h later with various concentrations of C. albicans blastoconidia in a volume of 20 μl of PBS. At days 7, 14, and 21 postinoculation, five to six randomly selected animals per group were sacrificed, and vaginal fungal burdens were determined by quantitative vaginal lavage culture. Shown are CFU ± standard errors of the means (SEM) for two experiments.
A second study evaluated the time limits of initiating pseudoestrus on the experimental infection. For this, the first estrogen treatment (0.02 mg/ms/week) was initiated 72 h prior to, or 24, 48, 72, or 120 h after, vaginal inoculation. Treatments were continued weekly thereafter, and vaginal fungal burdens were assessed in groups of mice at 7 and 14 days postinoculation. Results showed that vaginal fungal titers were not statistically different irrespective of when the estrogen treatments were initiated (data not shown). Rates of infection were similar in each group.
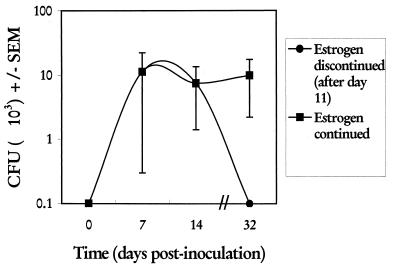
We next determined the consequences of discontinuing estrogen on vaginal fungal burden. For this, estrogen treatment (0.02 mg/week) was initiated 72 h prior to vaginal inoculation and vaginal fungal burden was monitored over a 14-day period. Thereafter, estrogen treatments were discontinued for one group and continued weekly for the other. Vaginal fungal burdens were assessed on day 32 (3 weeks following the last estrogen treatment). Figure 5 shows that discontinuing estrogen for 3 weeks resulted in the resolution of infection.
FIG. 5.
Effect of estrogen withdrawal on maintenance of experimental vaginal C. albicans infections. Two groups of animals were treated with the first of weekly subcutaneous injections of estradiol valerate (0.02 mg/mouse) and were inoculated intravaginally 72 h later. On days 7 and 14 postinoculation, randomly selected animals (5 mice/group/day) were sacrificed, and vaginal fungal burdens were determined by quantitative vaginal lavage culture. For the remaining mice, estrogen treatments were discontinued after day 11 (three injections) in one group, while the other group had two additional treatments (days 18 and 25). Animals were sacrificed on day 32, and vaginal fungal burdens were determined. Shown are CFU ± standard errors of the means (SEM) for two experiments.
Effects of estrogen on epithelial-cell-mediated anti-Candida activity.
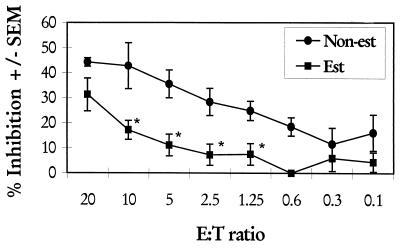
We recently showed that murine, human, and primate vaginal epithelial cells inhibit the growth of C. albicans in vitro (28, 29). To determine if estrogen affected the vaginal epithelial-cell-mediated growth inhibition of C. albicans, mice were treated either with estrogen (0.2 mg/mouse) or with sesame seed oil weekly for 2 weeks and then sacrificed, and their vaginal epithelial cells were collected and examined by an established [3H]glucose uptake assay for inhibition of the growth of C. albicans. Sesame seed oil-treated mice visibly in estrus (3) were added to the estrogen-treated group. Figure 6 shows that epithelial cells from estrogen-treated mice had significantly less ability to inhibit the growth of C. albicans at a range of E:T ratios (P < 0.016 for the E:T ratio of 10; P < 0.003 for the E:T ratio of 5, P < 0.006 for the E:T ratio of 2.5, and P < 0.005 for the E:T ratio of 1.2).
FIG. 6.
Effects of pseudoestrus on vaginal epithelial-cell-mediated anti-Candida activity. Groups of 10 to 12 mice were treated with 2 weekly subcutaneous injections of estradiol valerate (0.2 mg/mouse) (Est) or sesame seed oil alone (Non-est). Animals were sacrificed, and vaginal epithelial cells were isolated from excised vaginas. Epithelial cells were cultured in triplicate together with C. albicans blastoconidia for 9 h at various E:T ratios in the presence of [3H]glucose. At the conclusion of the culture period, the cultures were harvested and [3H]glucose uptake by C. albicans was determined by liquid scintillation. Each point is the mean percent inhibition ± standard error of the mean (SEM) for three experiments. Asterisks, significant differences (P < 0.05).
DISCUSSION
While it has been clear for some time that C. albicans vaginal infections are often dependent on the presence of reproductive hormones (23, 25), the specific roles of estrogen and progesterone are not known. In animals too, although experimental C. albicans vaginal infections are dependent on a state of pseudoestrus, no formal studies have been conducted on the limits of estrogen and what role progesterone may have on the infection. We found considerable flexibility with the use of estrogen. Near-physiologic concentrations of estrogen (1 to 5 μg in serum at estrus) were as capable as supraphysiologic concentrations of sustaining experimental infections (limit, 0.010 to 0.025 mg/ms/week) induced by a wide range of C. albicans inocula (limit, 103 blastoconidia/mouse). Additionally, we found that a persistent infection (with high rates of infection) could equally occur if estrogen treatments were initiated several days before or after inoculation. These results suggest that infections can be established equally from initial contact with columnar or squamous epithelial cells. Therefore, the estrogen receptor on C. albicans cells (13; Powell and Drutz, 23rd ICAAC) may not be required for initial adherence to vaginal tissue. In support of this, we recently found that a C. albicans strain lacking the estrogen receptor (homozygous knockout) was equally capable of causing a persistent experimental vaginal infection in the presence of estrogen (R. K. Swoboda, U. Riese, J. Brinkman, T. Munder, and P. L. Fidel, Jr., submitted for publication). However, estrogen is clearly required within 7 to 10 days postinoculation, as is evident by the rapid decline and resolution of the infection between 7 and 14 days in the absence of estrogen treatments and the lack of ability of estrogen to induce an infection once the organism is undetectable (6). Furthermore, the requirement for a maintained state of pseudoestrus was confirmed by the rapid clearance of the infection when the estrogen treatments were removed.
In contrast to estrogen, progesterone treatment alone could not support an experimental vaginal infection for any significant period of time. In fact, vaginal fungal burdens in progesterone-treated animals were lower than those in untreated animals. This may have been due to a lack of or reduced influence by endogenous estrogen. Alternatively, infiltrating leukocytes may have enhanced the clearance of Candida by clumping of germ tubes, a mechanism similar to that observed by Kinsman and Collard (13) in rats, although differences in cellular contents of lavage fluids between progesterone-treated and untreated mice were not observed in this study (data not shown). Interestingly, progesterone had no effect on the titers of C. albicans in the vagina, rates of infection, or chronicity of the vaginal infection in the presence of estrogen. This was true as well if progesterone was administered three times as often (3 doses/week). It is unlikely that the concentrations of progesterone used were not high enough to compete with estrogen, since the ratio of progesterone to estrogen in the extreme case was 150:1, exceeding that expected during diestrus. Taken together, estrogen is clearly dominant in supporting the experimental vaginal infection and is unaffected by the added presence of progesterone.
Based on these data in animals, estrogen is predicted to be the primary factor in the susceptibility to vaginitis during the luteal phase of the menstrual cycle, despite higher concentrations of progesterone than estrogen during that time. This is also consistent with the lack of prevalence of Candida vaginitis in women taking progesterone contraceptives (e.g., Depo Provera) (K. Ginsburg, personal communication). On the other hand, one may speculate that it is the peak levels of estrogen during the short ovulatory phase of the menstrual cycle that precipitate the vaginal infection and that the symptomatic infection does not fully present itself until the luteal phase. Similarly, based on our data, one would predict that despite high levels of progesterone during pregnancy, the high incidence of vaginitis in pregnant women is more likely due to estrogen. This dominant role of estrogen over progesterone in inducing and supporting a vaginal infection, however, is not consistent with the fact that sera from women in the luteal phase had a stronger effect in promoting C. albicans hyphal germination than sera from women in the ovulatory phase (11). However, this hyphal-germination-promoting effect may be unrelated to the presence of progesterone or estrogen alone in sera.
Another important component of this study was the effects of reproductive hormones on immunity associated with vaginal C. albicans infections. We had previously shown that estrogen treatment of mice had no effect on Candida-specific systemic CMI (i.e., in vivo DTH and in vitro proliferation of lymph node cells in response to Candida antigens) (8). Here we show as well that progesterone has no effect on Candida-specific DTH during a primary infection or on the anamnestic response during a secondary infection. Moreover, the similar levels of positive DTH in animals treated with progesterone alone compared to those in animals treated with estrogen suggests that the animals are similarly exposed to Candida antigen(s) irrespective of hormone treatment and resulting vaginal fungal titers. This effectively challenges previous in vitro studies showing several inhibitory effects of both estrogen and progesterone on systemic immune responsiveness (2, 14, 16, 30, 34, 35), including Candida-specific responses (11, 12, 18).
With respect to mucosal immunity, protection against a secondary vaginal infection in the murine model is partial at best but is considered to be locally acquired (6). Since estrogen is required to sustain an infection, the measurement of protection must be evaluated in the presence of estrogen. However, it does not appear that the level of protection is significantly affected by the concentration of estrogen, since protection was equally observed when near-physiologic (present study) and supraphysiologic (6) concentrations of estrogen were used. Thus, if estrogen is inhibiting a more profound local immune response leading to inefficient protection, it is doing so even at near-physiologic concentrations. Reported effects of estrogen on vaginal immunity, to date, include inhibition of antigen presentation by vaginal epithelial cells in vitro (33) and decreased immunoglobulins in vaginal secretions (20, 32, 35). On the other hand, progesterone has been reported to reverse many of the effects of estrogen (35). However, the results of this study suggest that at least for vaginal anti-Candida immunity, progesterone has no effect on the lack of protection during a primary infection or on the efficiency of protection during a secondary infection in the presence of estrogen. Perhaps these differences are due to the in vitro versus in vivo conditions under which the experiments were conducted.
We recently reported that the lack of clearance of the experimental infection in the presence of estrogen was not accompanied by T-cell infiltration into the vaginal mucosa or by changes in local T cells (5). Interestingly, the same lack of cellular activity is observed when the infection spontaneously resolves in the absence of estrogen (5). Thus it would not appear that estrogen is responsible for this lack of demonstrable T-cell activity. A recent observation that transforming growth factor β, a potent down-regulatory cytokine, is present in high concentrations in the vaginal tissues of naïve, estrogen-treated, or infected mice (B. N. Taylor, M. Saavedra, and P. L. Fidel, Jr., submitted for publication) suggests a potential mechanism for the lack of expected CMI against a primary infection, and possibly the inefficient protection against a secondary infection, irrespective of reproductive hormones.
With respect to innate defenses, polymorphonuclear leukocytes (PMNs) were recently shown to have minimal effects on vaginal fungal burden in vivo (1, 5) despite their ability to kill C. albicans in vitro (19) and their frequent presence in vaginal lavage fluid during infection. Interestingly, while PMN anti-Candida activity in vitro has been shown to be reduced in the presence of progesterone, but not estrogen (18), our data showed that PMNs have little effect against C. albicans in vivo irrespective of whether estrogen or progesterone is present (mice in pseudoestrus or in diestrus as part of a normal cycle, respectively) (5).
Innate vaginal host defense against Candida was also recently suggested by the discovery that vaginal epithelial cells inhibit the growth of C. albicans in vitro (28, 29). This study extended this finding to include the fact that estrogen treatment of mice significantly reduced epithelial-cell-mediated anti-Candida activity. This is consistent with the observations we made with primates (29) and suggests that stratified squamous epithelial cells may not be as effective at controlling C. albicans growth if indeed epithelial cells represent a form of innate resistance against C. albicans at the vaginal mucosa. Thus, a reduced or absent epithelial-cell-mediated anti-Candida response in the presence of estrogen may be an additional factor associated with estrogen-dependent susceptibility to infection. Moreover, in the absence of changes in local T cells during a primary infection in animals, it is interesting to speculate that the resolution of infection in the absence of estrogen is due to epithelial-cell-mediated anti-Candida activity.
In summary, the results of this study show that estrogen is the dominant reproductive hormone that supports and sustains an experimental vaginal C. albicans infection and reduces the inhibitory activity of epithelial cells against Candida. Progesterone, on the other hand, has no demonstrable effect on the vaginal infection or on systemic and/or local immune responsiveness associated with the infection. Taken together, these results suggest that estrogen, but not progesterone, is important in the reproductive-hormone-associated susceptibility to vaginal C. albicans infection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-32556 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Black C A, Eyers F M, Russell A, Dunkley M L, Clancy R L, Beagley K W. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect Immun. 1998;66:1273–1275. doi: 10.1128/iai.66.3.1273-1275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlsten H, Holmdahl R, Tarkowski A. Analysis of the genetic encoding of oestradiol suppression of delayed-type hypersensitivity in (NZB × NZW) F1 mice. Immunology. 1991;73:186–190. [PMC free article] [PubMed] [Google Scholar]
- 3.Champlin A K, Dorr D L, Gates A H. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1974;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- 4.Djeu J Y, Parapanissios A, Halkias D, Friedman H. A rapid 3H-glucose incorporation assay for determination of lymphoid cell-mediated inhibition of Candida albicans growth. J Immunol Methods. 1986;92:73–77. doi: 10.1016/0022-1759(86)90505-3. [DOI] [PubMed] [Google Scholar]
- 5.Fidel P L, Jr, Luo W, Steele C, Chabain J, Baker M, Wormley F L. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect Immun. 1999;67:3135–3140. doi: 10.1128/iai.67.6.3135-3140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidel P L, Jr, Lynch M E, Conaway D H, Tait L, Sobel J D. Mice immunized by primary vaginal Candida albicans infection develop acquired vaginal mucosal immunity. Infect Immun. 1995;63:547–553. doi: 10.1128/iai.63.2.547-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidel P L, Jr, Lynch M E, Sobel J D. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:1990–1995. doi: 10.1128/iai.61.5.1990-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidel P L, Jr, Lynch M E, Sobel J D. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidel P L, Jr, Lynch M E, Sobel J D. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect Immun. 1995;63:2403–2408. doi: 10.1128/iai.63.7.2403-2408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidel P L, Jr, Sobel J D. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335–348. doi: 10.1128/cmr.9.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalo-Klein A, Witkin S S. Candida albicans: cellular immune system interactions during different stages of the menstrual cycle. Am J Obstet Gynecol. 1989;161:1132–1136. doi: 10.1016/0002-9378(89)90649-2. [DOI] [PubMed] [Google Scholar]
- 12.Kalo-Klein A, Witkin S S. Regulation of the immune response to Candida albicans by monocytes and progesterone. Am J Obstet Gynecol. 1991;164:1351–1354. doi: 10.1016/0002-9378(91)90712-z. [DOI] [PubMed] [Google Scholar]
- 13.Kinsman O S, Collard A E. Hormonal factors in vaginal candidiasis in rats. Infect Immun. 1986;53:498–504. doi: 10.1128/iai.53.3.498-504.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klink M, Rozalaska B, Rudnicka W. Weakness of cellular response to Listeria antigens in pregnant mice. Med Dosw Mikrobiol. 1993;45:51–54. . (In Polish.) [PubMed] [Google Scholar]
- 15.Lynch M E, Sobel J D, Fidel P L., Jr Role of antifungal drug resistance in the pathogenesis of recurrent vulvovaginal candidasis. J Med Vet Mycol. 1996;34:337–339. doi: 10.1080/02681219680000571. [DOI] [PubMed] [Google Scholar]
- 16.Mazumder D N, Ghose N, Mirta J, Dutta G, Santra A. Immunological status of women with prolonged oral contraceptives and occurrence of giardiasis. J Indian Med Assoc. 1990;88(5):129–131. [PubMed] [Google Scholar]
- 17.McRipley R J, Erhard P J, Scwind R A, Whitney R R. Evaluation of vaginal antifungal formulations in vivo. Postgrad Med J. 1979;55:648–652. doi: 10.1136/pgmj.55.647.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nohmi T, Abe S, Dobashi K, Tansho S, Yamaguchi H. Suppression of anti-Candida activity of murine neutrophils by progesterone in vitro: a possible mechanism in pregnant women's vulnerability to vaginal candidiasis. Microbiol Immunol. 1995;39:405–409. doi: 10.1111/j.1348-0421.1995.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 19.Odds F C. Candida and candidosis. Baltimore, Md: University Park Press; 1988. Chronic mucocutaneous candidiosis; pp. 104–110. [Google Scholar]
- 20.Parr M B, Parr E L. Mucosal immunity in the female and male reproductive tracts. In: Ogra P L, Mestecky J J, Lamm M E, editors. Handbook of mucosal immunity. San Diego, Calif: Academic Press; 1994. pp. 677–689. [Google Scholar]
- 21.Romani L, Puccetti P, Bistoni F. Biological role of Th cell subsets in candidiasis. In: Romagnani S, editor. Th1 and Th2 cells in health and disease. Farmington, Conn: Karger; 1996. pp. 114–137. [PubMed] [Google Scholar]
- 22.Ryley J F, McGregor S. Quantitation of vaginal Candida albicans infections in rodents. J Med Vet Mycol. 1986;24:455–460. [PubMed] [Google Scholar]
- 23.Sobel J D. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci. 1988;544:547–557. doi: 10.1111/j.1749-6632.1988.tb40450.x. [DOI] [PubMed] [Google Scholar]
- 24.Sobel J D. Vaginal infections in adult women. Sex Transm Dis. 1990;74:1573–1601. doi: 10.1016/s0025-7125(16)30496-5. [DOI] [PubMed] [Google Scholar]
- 25.Sobel J D. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992;14:S148–S153. doi: 10.1093/clinids/14.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 26.Sobel J D, Faro S, Force R, Foxman B, Ledger W J, Nyirjesy P R, Reed B D, Summers P R. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178:203–211. doi: 10.1016/s0002-9378(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 27.Sobel J D, Muller G, McCormick J F. Experimental chronic vaginal candidosis in rats. Sabouraudia. 1985;23:199–206. doi: 10.1080/00362178585380301. [DOI] [PubMed] [Google Scholar]
- 28.Steele C, Ozenci H, Luo W, Scott M, Fidel P L., Jr Growth inhibition of Candida albicans by vaginal cells from naive mice. J Med Mycol. 1999;37:251–260. [PubMed] [Google Scholar]
- 29.Steele C, Ratterree M, Fidel P L., Jr Differential susceptibility to experimental vaginal candidiasis in macaques. J Infect Dis. 1999;180:802–810. doi: 10.1086/314964. [DOI] [PubMed] [Google Scholar]
- 30.Szekeres-Bartho J. Endrocine regulation of the immune system during pregnancy. Arch Immunol Ther Exp. 1990;38:125–140. [PubMed] [Google Scholar]
- 31.Taschdjian C L, Reiss F, Kozinn P J. Experimental vaginal candidiasis in mice; its implications for superficial candidiasis in humans. J Investig Dermatol. 1960;34:89–94. [PubMed] [Google Scholar]
- 32.Wira C R, Prabhala R H. The female reproductive tract is an inductive site for immune responses: effect of oestradiol and antigen on antibody and secretory component levels in uterine and cervico-vaginal secretions following various routes of immunization. In: Griffin P D, Johnson P M, editors. Scientific basis of fertility regulation: local immunity in reproductive tract tissues. New York, N.Y: Oxford University Press; 1993. pp. 271–293. [Google Scholar]
- 33.Wira C R, Rossoll R M. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–508. [PMC free article] [PubMed] [Google Scholar]
- 34.Wira C R, Sandoe C P. Sex steroid hormone regulation of IgA and IgG in rat uterine secretions. Nature. 1977;268:534–536. doi: 10.1038/268534a0. [DOI] [PubMed] [Google Scholar]
- 35.Wira C R, Stern J E. Endocrine regulation of the mucosal immune system in the female reproductive tract. Control of IgA, IgG, and secretory component during the reproductive cycle, at implantation, and throughout pregnancy. In: Pasqualini J R, Scholler R, editors. Hormones and fetal pathophysiology. M. New York, N.Y: Dekker; 1992. pp. 343–367. [Google Scholar]
- 36.Witkin S S. Immunology of recurrent vaginitis. Am J Reprod Immunol Microbiol. 1987;15:34–37. doi: 10.1111/j.1600-0897.1987.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 37.Witkin S S. Immunologic factors influencing susceptibility to recurrent candidal vaginitis. Clin Obstet Gynecol. 1991;34:662–668. [PubMed] [Google Scholar]