Abstract
Herein, we have presented a new insight for the synthesis of a hybrid heterogeneous catalyst. For this purpose, phosphonic acid tagged carbon quantum dots of CQDs-N(CH2PO3H2)2 encapsulated and assembled in channels of SBA-15 using a post-modification strategy. The mesoporous catalyst of functionalized carbon quantum dots (CQDs) was characterized by several techniques. CQDs-N(CH2PO3H2)2/SBA-15 as an excellent catalyst was applied for the preparation of novel pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine derivatives by using pyrazole, barbituric acid and indole moieties at 100 °C under the solvent-free condition. The present work shows that a significant increase in the catalytic activity can be achieved by a rational design of mesoporous SBA-15 modified with CQDs for the synthesis of biological active candidates. The synthesized compounds did not convert to their corresponding pyridines via an anomeric-based oxidation mechanism.
Subject terms: Heterogeneous catalysis, Environmental chemistry
Introduction
In recent years, composite materials have become well-known as a catalyst, absorbent and sensor in modern sciences1–3. The research and development in designing a composite with high selectivity, performance and sensitivity is an urgent need to expand new frontiers of knowledge4,5. Quantum dots based on carbon, nitrogen and graphene as nano-materials with small size 10 nm have exhibited great potential uses in photocatalysis, biosensing, heavy metal elements sensing and biomolecule/drug delivery6–9. Despite the success of existing materials and technologies, the stability of CDs especially in harsh chemical and physical conditions and weak thermal stability is still highly desirable. Also, mesoporous silica catalysts are much more functional due to their easy and fast separation from reaction media, which is high surface area, pore volume and tunable pore size10–12. Besides, a composite material is composed of quantum dots and mesoporous silica as a new property which is investigated as a catalyst, sensor, extraction and determination13–16. However, carbon quantum dots (CQDs) encapsulated in the channel of SBA-15 would produce improved or new properties. Therefore, the application of composite materials in the field of catalyst knowledge has a great interest due to their ability to catalyze a wide range of organic synthesis compounds. In this field, we have applied carbon quantum dots (CQDs) and mesoporous silica as a catalyst for the preparation of pyridines, 1,4-dihydropyridines, 4H-pyrans and spiropyran compounds17–19.
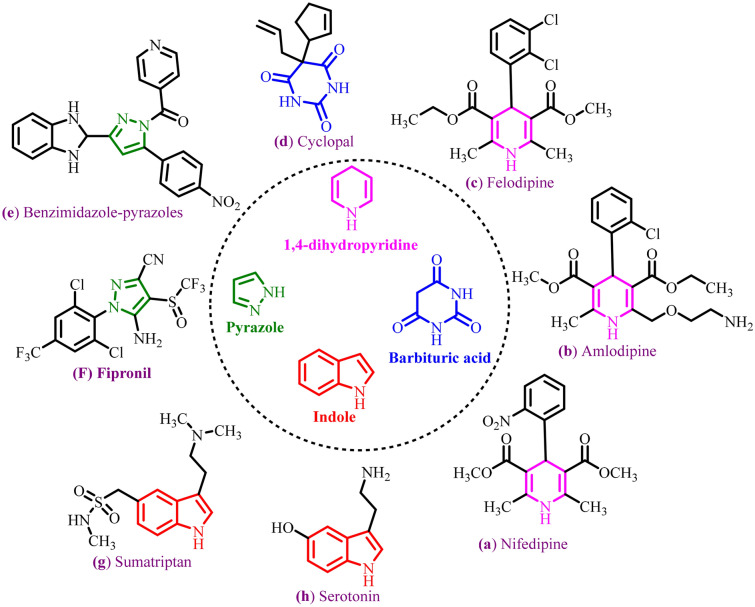
Multicomponent reactions (MCRs) as a versatile method have been also of great interest for the preparation of a wide range of N-heterocycle compounds with biological activity20–29. Therefore, N-heterocycles such as 1,4-dihydropyridines (1,4-DHPs) scaffolds make a variety in the drug structures such as Nifedipine (a), Amlodipine (b) and Felodipine (c) (Fig. 1)30–32. Also, the biological significance of 1,4-DHPs was investigated in antitumor, anti-ischemic, analgesic, anti-Alzheimer, anti-hypertensive, insecticidal, cardiovascular, anti-inflammatory and anti-microbial conditions33–35. Furthermore, diversity of pyrrole, barbituric acid and indole-based scaffolds such as Cyclopal (d), Benzimidazole-pyrazoles (e), Fipronil (f) Sumatriptan (g) and Serotonin (h) are important in the areas of medicine and agriculture36–39. However, some of these methods are accompanied by restrictions including the use of expensive starting materials, harsh reaction conditions, long reaction times and low yields. Therefore, the development of new, efficient and versatile catalysts is still on demand.
Figure 1.
Diversity of pyrrole, barbituric acid and indole based on 1,4-dihydropyridines (1,4-DHPs) structure with biological properties.
In this strategy, we introduced a convenient method to immobilize phosphonic acid tagged carbon quantum dots (CQDs) in the channels of SBA-15. Then, it was used in the synthesis of N-heterocycle compounds with pyrazole, barbituric acid and indole moieties. The novel pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine derivatives were synthesized in the presence of CQDs-N(CH2PO3H2)2/SBA-15 as an excellent mesoporous heterogeneous catalyst (Fig. 2).
Figure 2.
Synthesis of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine derivatives using CQDs-N(CH2PO3H2)2/SBA-15.
Experimental
Synthesis of CQDs-N(CH2PO3H2)2 and SBA-15
Phosphonic acid tagged carbon quantum dots of CQDs-N(CH2PO3H2)2 and SBA-15 were synthesized according to our previously reported works19,40–44.
Synthesis of CQDs-N(CH2PO3H2)2/SBA-15 composite
For this purpose, in a 100 mL round-bottomed flask, a mixture of CQDs-N(CH2PO3H2)2 (1 g) and SBA-15 (1 g) and toluene (50 mL) was stirred under reflux conditions for 12 h. After this time, the reaction mixture slowly cooled down to room temperature and participate was separated by a centrifuge (2 × 4000 rpm). Then, the residual small amount of toluene was evaporated and the brown participate was triturated with EtOH (2 × 5 mL) and dried under a powerful vacuum at 70 °C. We have synthesized CQDs-N(CH2PO3H2)2/SBA-15 composite under the ambient and air atmosphere. Therefore, the environment of the reaction does not affect the synthesis of catalyst.
General procedure for the preparation of N-heterocycle compounds with pyrazole barbituric acid and indole tags using CQDs-N(CH2PO3H2)2/SBA-15
In the first step, the 5-(1H-Indol-3-yl)-2H-pyrazol-3-ylamine was synthesized according to the procedure reported in the literature (Fig. 2)45. In the second step, in a 10 mL round-bottomed flask, a mixture of aldehydes (1 mmol), pyrimidine-2,4,6(1H,3H,5H)-trione derivatives (1 mmol) and as-synthesized 5-(1H-indol-3-yl)-1H-pyrazol-3-amine (1 mmol) were mixed in the presence of 10 mg of CQDs-N(CH2PO3H2)2/SBA-15 as a novel heterogeneous catalyst. Then, the mixture temperature was slowly raised up to 100 °C and it was stirred at 100 °C under the solvent-free condition for appropriate time (Tables 2 and 3). After the completion of the reactions which were monitored by the TLC technique, 10 mL of hot EtOH was added to the reaction mixture and the solid catalyst was separated by centrifugation (4000 rpm for 10 min). Finally, after the evaporation of the solvent at room temperature, the pure product was obtained and washed with cool ethanol for several times.
Table 2.
Synthesis of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines using CQDs–N(CH2PO3H2)2/SBA15 under solvent free condition.
Table 3.
Synthesis of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines using CQDs-N(CH2PO3H2)2/SBA15 under solvent free condition.
Results and discussion
In continuation of our investigation on the mesoporous SBA-15 and CQDs as catalysts in organic synthesis, herein we wish to develop the knowledge of catalysts, consisting of mesoporous SBA-15 and CQDs as highly active composite catalysts19,46–48. With this aim, CQDs-N(CH2PO3H2)2/SBA-15 as a novel heterogeneous catalyst was synthesized, characterized and applied in the synthesis of target organic molecules (Fig. 3). To determine the structural nature and morphology of the CQDs-N(CH2PO3H2)2/SBA-15 various techniques such as XRD, SEM, TEM, N2 adsorption–desorption isotherms, FT-IR, energy dispersive X-ray (EDS) and SEM-elemental mapping were applied.
Figure 3.
Schematic preparation of CQDs-N(CH2PO3H2)2/SBA-15 as a desired catalyst.
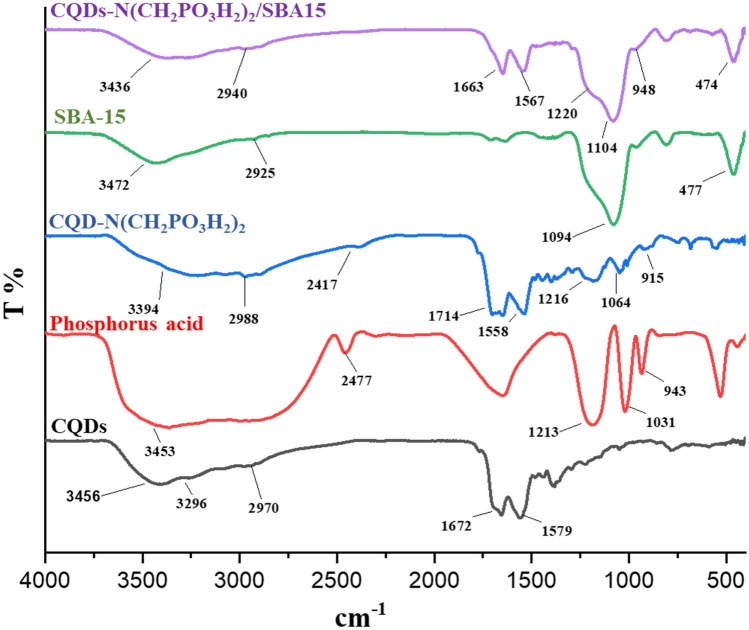
The FT-IR spectrum of CQDs, phosphorus acid, CQD-N(CH2PO3H2)2, SBA-15 and CQDs-N(CH2PO3H2)2/SBA-15 as a desired catalyst were compared in Fig. 4. The broad peak at 2600–3400 cm−1 indicates the OH of PO3H2 functional group. The aromatic C-H and C = C stretches bands are, respectively, at 2940 and 1663 cm−1. The absorption bands at 948 and 1058 cm−1 are related to P–O bond stretching and the band at 1104 cm−1 is related to P=O. The peak in the 1714 cm−1 indicates the CO of the carbonyl group in the CQDs-N(CH2PO3H2). Also, the absorption band at 1094 cm−1 was linked to the stretching vibrational of SiO2 group in the SBA-15. The changes in different stages of synthesis indicated the preparation of the catalyst.
Figure 4.
FT-IR spectra of CQDs, phosphorus acid, CQDs-N(CH2PO3H2)2, SBA-15 and CQDs-N(CH2PO3H2)2/SBA-15.
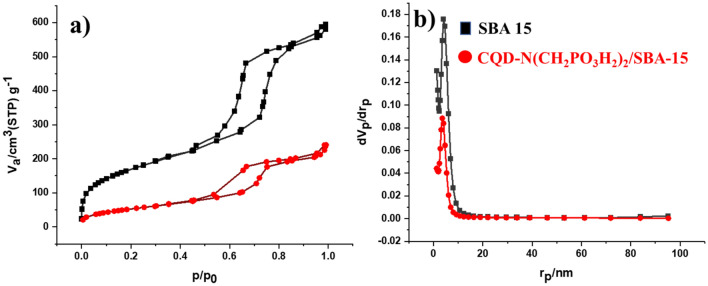
The synthesis of CQDs-N(CH2PO3H2)2 encapsulated in SBA-15 as a composite material was also checked by XRD patterns of the corresponding starting material components (Figs. 5 and 6). For this purpose, XRD patterns exhibited distinguished three bands inclusive of a sharp band (100) and small bands indexed (110) and (200), which are in a close agreement with the previously reported data18. As shown in Fig. 5, the broad peak of CQDs-N(CH2PO3H2)2/SBA-15 corresponds to diffraction lines 002 in carbon quantum dots (CQDs). Therefore, the broad band in 2Ɵ = 15–35° indicated a successful immobilization of CQDs-N(CH2PO3H2)2 into SBA-15. The textural properties of SBA-15 and CQDs-N(CH2PO3H2)2/SBA-15 were also studied by N2 adsorption–desorption isotherms (Fig. 7a,b). A hysteresis loop is observed indicating the presence of mesoporous in the sample. The calculated surface areas for SBA-15 and catalyst based on BET equation and total pore volumes are 596.55 m2 g−1, 192.07 m2 g−1 and 0.8564 cm3 g−1, 0.3709 cm3 g−1, respectively. The pore size distribution of CQDs-N(CH2PO3H2)2/SBA15 based on BJH method is shown in Fig. 7. This plot clearly shows pores diameter of SBA-15 was 4.03 nm and reached 3.53 in CQDs-N(CH2PO3H2)2/SBA-15.
Figure 5.
Comparison Low angle and PXRD of CQDs-N(CH2PO3H2)2/SBA-15 with SBA-15.
Figure 6.
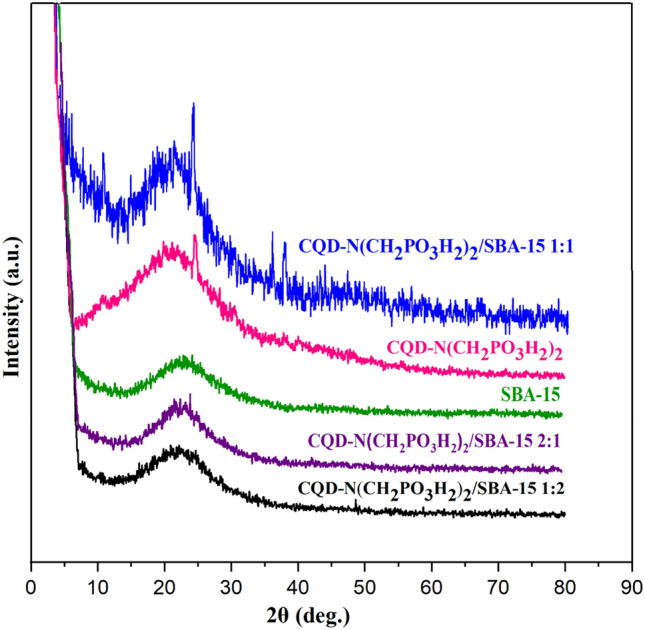
Comparison PXRD of CQDs-N(CH2PO3H2)2/SBA-15 (1:1), CQD-N(CH2PO3H2)2, SBA-15, CQDs-N(CH2PO3H2)2/SBA-15 (2:1) and CQDs-N(CH2PO3H2)2/SBA-15 (1:2).
Figure 7.
(a) Nitrogen adsorption–desorption isotherm and (b) BJH of SBA-15 and CQDs-N(CH2PO3H2)2/SBA-15.
The surface of CQDs-N(CH2PO3H2)2/SBA-15 was determined by SEM images (Fig. 8). SEM images of the catalyst revealed that the particles have not completely agglomerated and particles of observed in tubular nano rod shape of silica template (Fig. 8a,b). In another investigation, the schaffold CQDs-N(CH2PO3H2)2/SBA-15 is composed of C, N, O, Si and P according to the energy dispersive X-ray spectroscopy (EDX) technique (Fig. 8c). Also, distribution of elements including P (red), Si (blue), O (green), N (yellow) and C (orange) on the surface of the catalyst was investigated and verified by SEM-elemental technique (Fig. 8d). Therefore, energy dispersive X-ray spectroscopy (EDX) and SEM-elemental mapping spectroscopy of all the expected elements confirm the uniform distribution of the elements on the surface.
Figure 8.
(a,b) Scanning electron microscope (SEM) images of CQDs-N(CH2PO3H2)2/SBA-15. (c) Energy dispersive X-ray analysis (EDX) and (d) SEM-elemental technique of CQDs-N(CH2PO3H2)2/SBA-15.
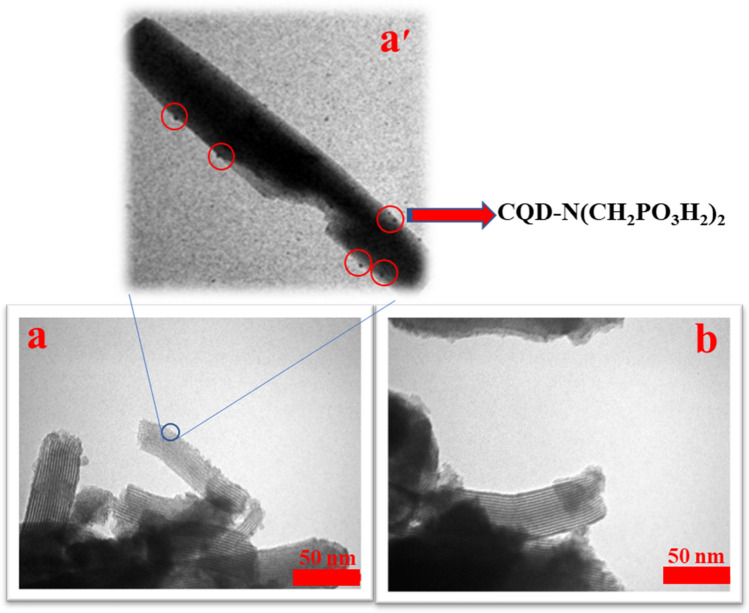
The morphology and surface of CQDs-N(CH2PO3H2)2/SBA-15 was determined by TEM analysis (Fig. 9). The TEM images of the CQDs-N(CH2PO3H2)2/SBA-15 were shown to be well fit with the two-dimensional hexagonal meso-structures. Based on these images, the scaffold of mesoporous template has been assembled with carbon quantum dots (CQDs) (Fig. 9), which agreed well with the results of low-angle XRD and N2 adsorption–desorption isotherms characterizations.
Figure 9.
Transmission electron microcopy (TEM) images of CQDs-N(CH2PO3H2)2/SBA-15.
After successful synthesis and characterization of the CQDs-N(CH2PO3H2)2/SBA-15, it was used to prepare novel pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine derivatives. The above-mentioned catalyst was achieved one-pot reaction of between 4-chloro benzaldehyde (1 mmol, 0.14 g), pyrimidine-2,4,6(1H,3H,5H)-trione (1 mmol, 0.128 g) and 5-(1H-indol-3-yl)-1H-pyrazol-3-amine (1 mmol, 0.198 g) as a model reaction. The model reaction was tested using different amounts of catalysts, solvents and temperatures. The results of the obtained products are summarized in Table 1. The best of choice for the synthesis of novel pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines was achieved in the presence of catalytic amount of CQDs-N(CH2PO3H2)2/SBA-15 (10 mg) at 100 °C under the solvent-free condition (Entry 2, Table 1). The model reaction was also tested using different organic solvents such as such EtOH, DMF, H2O, CH3CN, n-Hexane, CHCl3, Toluene, MeOH, CH2Cl2, EtOAc (5 mL) and water which is results of the reaction did not improve) Entry 8–17, Table 1). The results show that CQDs-N(CH2PO3H2)2/SBA-15 is suitable for the preparation of novel pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine derivatives.
Table 1.
Effect of various amounts of catalysts, temperature and solvents in synthesis of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines.

| |||||
|---|---|---|---|---|---|
| Entry | Solvent | Catalyst (mg) | Temp. (˚C) | Time (min.) | Yield (%) |
| 1 | – | 5 | 100 | 40 | 70 |
| 2 | – | 10 | 100 | 15 | 91 |
| 3 | – | 20 | 100 | 35 | 72 |
| 4 | – | – | 100 | 120 | - |
| 5 | – | 10 | 110 | 20 | 85 |
| 6 | – | 10 | 50 | 60 | 46 |
| 7 | – | 10 | 25 | 70 | 40 |
| 8 | EtOH | 10 | Reflux | 45 | 70 |
| 9 | DMF | 10 | 100 | 100 | 30 |
| 10 | H2O | 10 | Reflux | 120 | – |
| 11 | CH3CN | 10 | Reflux | 120 | Trace |
| 12 | n-Hexane | 10 | Reflux | 120 | – |
| 13 | CHCl3 | 10 | Reflux | 100 | 35 |
| 14 | Toluene | 10 | Reflux | 120 | – |
| 15 | MeOH | 10 | Reflux | 60 | 60 |
| 16 | CH2Cl2 | 10 | Reflux | 65 | 35 |
| 17 | EtOAc | 10 | Reflux | 120 | – |
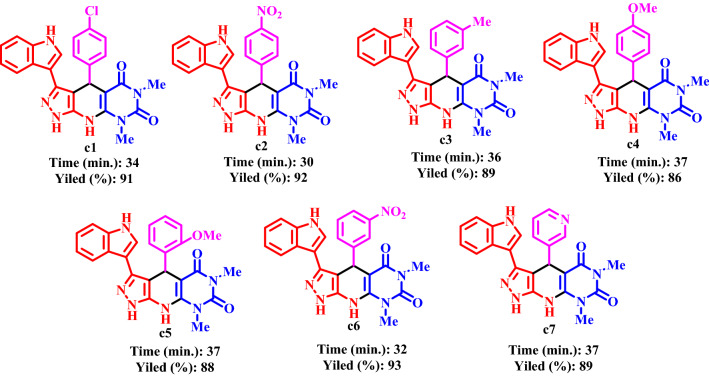
After the optimization of the reaction conditions, the efficiency and applicability of CQDs-N(CH2PO3H2)2/SBA-15 were studied for the preparation of novel pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines. The results are summarized in Tables 2 and 3. As Tables 2 and 3 indicate, pyrimidine-2,4,6(1H,3H,5H)-trione derivatives and 5-(1H-indol-3-yl)-1H-pyrazol-3-amine as starting materials and various aldehydes including bearing electron-donating, electron-withdrawing and halogens groups afforded desired products (b1-b15), (c1-c7) excellent yields (75–94%) and short reaction times (10–40 min.), respectively.
In a suggested mechanism, the aldehyde is activated by the catalyst. The enol form of the pyrimidine-2,4,6(1H,3H,5H)-trione and activated aldehyde is produced intermediate I. Then, by reaction of intermediate I as a Michael acceptor and 5-(1H-indol-3-yl)-1H-pyrazol-3-amine leads to the intermediate II. In the following, intermediate II creates intermediate III during the intramolecular reaction and cyclization reaction. Finally, intermediate III loss of molecule H2O to prepared product (Fig. 10).
Figure 10.
Proposed mechanism for the synthesis of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines.
In another section, to determine efficiency of CQDs-N(CH2PO3H2)2/SBA-15 as a catalyst, we have tested the model reaction using previously reported catalysts such as organic, magnetic, solid acid and basic catalysts. The results of the reactions showed that CQDs-N(CH2PO3H2)2/SBA-15 is the best catalyst for the synthesis of novel pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine derivatives (short reaction time and high yield) (Table 4). Also, the recyclability and reusability of CQDs-N(CH2PO3H2)2/SBA-15 as catalyst were also studied on a model reaction under the above-mentioned reaction conditions. The results shown in Fig. 11, CQDs-N(CH2PO3H2)2/SBA-15 can be reused up to five runs in the reaction without a significant reduction in product yield.
Table 4.
Synthesis of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines in the presence of various catalysts.

| ||||
|---|---|---|---|---|
| Entry | Catalyst | (mol%) | Time (min.) | Yield (%) |
| 1 | FeCl3 | 10 | 90 | 20 |
| 2 | H2SO4 | 10 | 120 | 25 |
| 3 | Fe3O4 | 10 mg | 120 | Trace |
| 4 | NH4NO3 | 10 | 100 | 20 |
| 5 | CF3SO3H | 10 | 80 | 25 |
| 6 | GTBSA29 | 10 | 120 | 20 |
| 7 | MIL-100(Cr)/NHEtN(CH2PO3H2)249 | 10 | 90 | 45 |
| 8 | H3[p(W3O10)4].XH2O | 10 | 120 | Trace |
| 9 | SBA-15/(CH2)3 N(CH2PO3H2)(CH2)2-N(CH2PO3H2)218 | 10 | 60 | 52 |
| 10 | [PVI-SO3H]FeCl450 | 10 | 120 | 30 |
| 11 | p-TSA | 10 | 120 | 20 |
| 12 | SSA51 | 10 mg | 100 | 30 |
| 13 | Et3N | 10 | 120 | – |
| 14 | MHMHPA27,28 | 10 | 100 | 30 |
| 15 | [Py-SO3H]Cl52 | 10 | 120 | 20 |
| 16 | APVPB53 | 10 mg | 70 | 40 |
| 17 | Fe3O4@Co(BDC)-NH254 | 10 mg | 85 | 45 |
| 18 | H3PO3 | 10 | 70 | 55 |
| 19 | CQDs55 | 10 mg | 40 | 60 |
| 20 | CQDs-N(CH2PO3H2)219 | 10 mg | 30 | 75 |
| 21 | CQDs-N(CH2PO3H2)2/SBA-15 | 10 mg | 15 | 91 |
Figure 11.
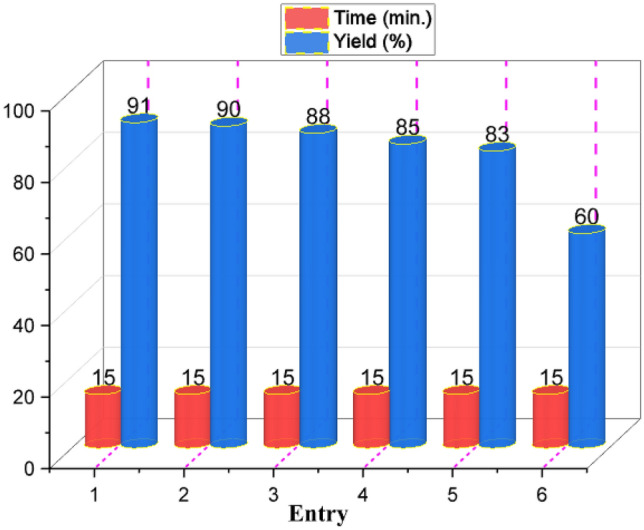
Recyclability of CQDs-N(CH2PO3H2)2/SBA-15 at the synthesis of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines.
A hot filtration test was conducted to synthesize 1,4-dihydropyridines (1,4-DHPs) as a model reaction under the optimized reaction condition. While running the fresh batch, the catalyst was filtered off at 25 min. At this stage, the reaction yield was 45%. Another reaction was run for 25 min and reaction mixture was filtered off. The filtrate was stirred for another 25 min under the same conditions. Incidentally, the reaction afforded no augmentation in its yield. Therefore, it can be concluded that the structure of the catalyst is not decomposed and the catalyst can be considered a heterogeneous catalyst.
Conclusion
In summary, a novel porous composite catalyst consists of carbon quantum dots (CQDs) and the mesoporous silica namely CQDs-N(CH2PO3H2)2/SBA-15 was introduced and fully characterized by several techniques. This novel porous catalyst was investigated via a single step reaction for the synthesis of a wide range of novel pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine derivatives as biological active candidates. The major advantages of described methodology are the high yield of the isolated products, short reaction time, eco-friendly way, mild and green reaction conditions.
Supplementary Information
Acknowledgements
We thank the Bu-Ali Sina University and Iran National Science Foundation (INSF) (Grant Number: 98001912) for financial support.
Author contributions
M.M.R. and H.S.; methodology, validation, investigation. M.Z. investigation and writing the original draft. M.A.Z.; supervision, resources, project administration, funding acquisition, conceptualization, writing-review. S.R. writing-review and editing.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahmoud Zarei, Email: mahmoud8103@yahoo.com.
Mohammad Ali Zolfigol, Email: zolfi@basu.ac.ir, Email: mzolfigol@yahoo.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-24553-3.
References
- 1.Zhang Y, Zhu X, Liu Y, Liu L, Xu Q, Liu H, Wang W, Chen L. Ultra-stretchable monofilament flexible sensor with low hysteresis and linearity based on MWCNTs/Ecoflex composite materials. Macromol. Mater. Eng. 2021;306:2100113. doi: 10.1002/mame.202100113. [DOI] [Google Scholar]
- 2.Tiuc AE, Nemeş O, Vermeşan H, Toma A. New sound absorbent composite materials based on sawdust and polyurethane foam. C. Compos. B. Eng. 2019;165:120–130. doi: 10.1016/j.compositesb.2018.11.103. [DOI] [Google Scholar]
- 3.Li X, Xiong J, Gao X, Huang J, Feng Z, Chen Z, Zhu Y. Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J. Alloys Compd. 2019;802:196–209. doi: 10.1016/j.jallcom.2019.06.185. [DOI] [Google Scholar]
- 4.Dinu MV, Dinu IA, Lazar MM, Dragan ES. Chitosan-based ion-imprinted cryo-composites with excellent selectivity for copper ions. Carbohydr. Polym. 2018;186:140–149. doi: 10.1016/j.carbpol.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Gu K, Wang S, Li Y, Zhao X, Zhou Y, Gao C. A facile preparation of positively charged composite nanofiltration membrane with high selectivity and permeability. J. Membr. Sci. 2019;581:214–223. doi: 10.1016/j.memsci.2019.03.057. [DOI] [Google Scholar]
- 6.Su W, Guo R, Yuan F, Li Y, Li X, Zhang Y, Zhou S, Fan L. Red-emissive carbon quantum dots for nuclear drug delivery in cancer stem cells. J. Phys. Chem. 2020;11:1357–1363. doi: 10.1021/acs.jpclett.9b03891. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Li J, Tu X, Liu H, Shu M, Si R, Ferguson CTJ, Zhang K, Li R. Single atomically anchored cobalt on carbon quantum dots as efficient photocatalysts for visible light-promoted oxidation reactions. Chem. Mater. 2019;32:734–743. doi: 10.1021/acs.chemmater.9b03708. [DOI] [Google Scholar]
- 8.Algar WR, Wegner D, Huston AL, Blanco-Canosa JB, Stewart MH, Armstrong A, Medintz IL. Quantum dots as simultaneous acceptors and donors in time-gated Förster resonance energy transfer relays: Characterization and biosensing. J. Am. Chem. Soc. 2012;134:1876–1891. doi: 10.1021/ja210162f. [DOI] [PubMed] [Google Scholar]
- 9.Zhao DL, Chung TS. Applications of carbon quantum dots (CQDs) in membrane technologies: A review. Water Res. 2018;147:43–49. doi: 10.1016/j.watres.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Linares N, Silvestre-Albero AM, Serrano E, Silvestre-Albero J, García-Martínez J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014;43:7681–7717. doi: 10.1039/C3CS60435G. [DOI] [PubMed] [Google Scholar]
- 11.Perego C, Millini R. Porous materials in catalysis: Challenges for mesoporous materials. Chem. Soc. Rev. 2013;42:3956–3976. doi: 10.1039/C2CS35244C. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Hartmann M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem. Soc. Rev. 2013;42:3894–3912. doi: 10.1039/c3cs60059a. [DOI] [PubMed] [Google Scholar]
- 13.Ziarani GM, Moradi R, Mohajer FA. Badiei, 2-Chloroquinoline-3-carbaldehyde modified nanoporous SBA-15-propylamine (SBA-Pr-NCQ) as a selective and sensitive Ag+ ion sensor in aqueous media. J. Phys. Chem. Solids. 2022;161:110399. doi: 10.1016/j.jpcs.2021.110399. [DOI] [Google Scholar]
- 14.Li J, Tang Y, Li Z, Ding X, Yu B, Lin L. Largely enhancing luminous efficacy, color-conversion efficiency, and stability for quantum-dot white LEDs using the two-dimensional hexagonal pore structure of SBA-15 mesoporous particles. ACS Appl. Mater. Interfaces. 2019;11:18808–18816. doi: 10.1021/acsami.8b22298. [DOI] [PubMed] [Google Scholar]
- 15.Chang Q, Yang S, Xue C, Li N, Wang Y, Li Y, Wang H, Yang J, Hu S. Nitrogen-doped carbon dots encapsulated in the mesoporous channels of SBA-15 with solid-state fluorescence and excellent stability. Nanoscale. 2019;11:7247–7255. doi: 10.1039/C9NR01224A. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Li X, Li X, Wang Y, Han Q, Li J. Determination of 2,4,6-trinitrophenol by in-situ assembly of SBA-15 with multi-hydroxyl carbon dots. Anal. Chim. Acta. 2020;1098:170–180. doi: 10.1016/j.aca.2019.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Zarei M, Zolfigol MA, Moosavi-Zare AR, Noroozizadeh E, Rostamnia S. Three-component synthesis of spiropyrans using SBA-15/En bonded phosphorous acid [SBA-15/Pr-NH1-y(CH2PO3H2)y-Et-NH2-x(CH2PO3H2)x] as a new nanoporous heterogeneous catalyst. ChemistrySelect. 2018;3:12144–12149. doi: 10.1002/slct.201802525. [DOI] [Google Scholar]
- 18.Jalili F, Zarei M, Zolfigol MA, Rostamnia S, Moosavi-Zare AR. SBA-15/PrN(CH2PO3H2)2 as a novel and efficient mesoporous solid acid catalyst with phosphorous acid tags and its application on the synthesis of new pyrimido[4,5-b]quinolones and pyrido[2,3-d]pyrimidines via anomeric based oxidation. Microporous Mesoporous Mater. 2020;294:109865. doi: 10.1016/j.micromeso.2019.109865. [DOI] [Google Scholar]
- 19.Rasooll MM, Zarei M, Zolfigol MA, Sepehrmansourie H, Omidi A, Hasani M, Gu Y. Novel nano-architectured carbon quantum dots (CQDs) with phosphorous acid tags as an efficient catalyst for the synthesis of multisubstituted 4H-pyran with indole moieties under mild conditions. RSC Adv. 2021;11:25995–26007. doi: 10.1039/D1RA02515E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalhor S, Zarei M, Zolfigol MA, Sepehrmansourie H, Nematollahi D, Alizadeh S, Shi H, Arjomandi J. Anodic electrosynthesis of MIL-53(Al)-N(CH2PO3H2)2 as a mesoporous catalyst for synthesis of novel (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines via a cooperative vinylogous anomeric based oxidation. Sci. Rep. 2021;11:19370. doi: 10.1038/s41598-021-97801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babaee S, Zarei M, Zolfigol MA, Khazalpour S, Hasani M, Rinner U, Schirhagl R, Norouzi N, Rostamnia S. Synthesis of biological based hennotannic acid-based salts over porous bismuth coordination polymer with phosphorous acid tags. RSC Adv. 2021;11:2141–2157. doi: 10.1039/D0RA06674E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavakoli E, Sepehrmansourie H, Zarei M, Zolfigol MA, Khazaei A, Hosseinifard M. Applications of novel composite UiO-66-NH 2/Melamine with phosphorous acid tags as a porous and efficient catalyst for the preparation of novel spiro-oxindoles. New J. Chem. 2022;46:19054–19061. doi: 10.1039/D2NJ03340B. [DOI] [Google Scholar]
- 23.Kalhor S, Zarei M, Sepehrmansourie H, Zolfigol MA, Shi H, Wang J, Arjomandi J, Hasani M, Schirhagl R. Novel uric acid-based nano organocatalyst with phosphorous acid tags: Application for synthesis of new biologically-interest pyridines with indole moieties via a cooperative vinylogous anomeric based oxidation. Mol. Catal. 2021;507:111549. doi: 10.1016/j.mcat.2021.111549. [DOI] [Google Scholar]
- 24.Sepehrmansourie H, Zarei M, Zolfigol MA, Gu Y. A new approach for the synthesis of bis (3-Indolyl) pyridines via a cooperative vinylogous anomeric based oxidation using ammonium acetate as a dual reagent-catalyst role under mild and green condition. Polycycl. Aromat. Compd. 2022 doi: 10.1080/10406638.2022.2128830. [DOI] [Google Scholar]
- 25.Jalili F, Zarei M, Zolfigol MA, Khazaei A. Application of novel metal–organic framework [Zr-UiO-66-PDC-SO3H]FeCl4 in the synthesis of dihydrobenzo[g]pyrimido[4,5-b]quinoline derivatives. RSC Adv. 2022;12:9058–9068. doi: 10.1039/D1RA08710J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naseri AM, Zarei M, Alizadeh S, Babaee S, Zolfigol MA, Nematollahi D, Arjomandi J, Shi H. Synthesis and application of [Zr-UiO-66-PDC-SO3H]Cl MOFs to the preparation of dicyanomethylene pyridines via chemical and electrochemical methods. Sci. Rep. 2021;11:16817. doi: 10.1038/s41598-021-96001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afsar J, Zolfigol MA, Khazaei A, Zarei M, Gu Y, Alonso DA, Khoshnood A. Synthesis and application of melamine-based nano catalyst with phosphonic acid tags in the synthesis of (3′-indolyl)pyrazolo[3,4-b]pyridines via vinylogous anomeric based oxidation. Mol. Catal. 2020;482:110666. doi: 10.1016/j.mcat.2019.110666. [DOI] [Google Scholar]
- 28.Danishyar B, Sepehrmansourie H, Zarei M, Zolfigol MA, As’ Habi MA, Gu Y. Synthesis and application of novel magnetic glycoluril tetrakis (methylene phosphorous acid) as a nano biological catalyst for the preparation of nicotinonitriles via a cooperative vinylogous anomeric-based oxidation. Polycycl. Aromat. Compd. 2022 doi: 10.1080/10406638.2022.2126506. [DOI] [Google Scholar]
- 29.Zarei M, Sepehrmansourie H, Zolfigol MA, Karamian R, Farida SHM. Novel nano-size and crab-like biological-based glycoluril with sulfonic acid tags as a reusable catalyst: Its application to the synthesis of new mono- and bis-spiropyrans and their in vitro biological studies. New J. Chem. 2018;42:14308–14317. doi: 10.1039/C8NJ02470G. [DOI] [Google Scholar]
- 30.Solaimanzadeh I. Acetazolamide, nifedipine and phosphodiesterase inhibitors: Rationale for their utilization as adjunctive countermeasures in the treatment of coronavirus disease 2019 (COVID-19) Cureus. 2019;12:3. doi: 10.7759/cureus.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiqi FH, Menzies FM, Lopez A, Stamatakou E, Karabiyik C, Ureshino R, Ricketts T, Jimenez-Sanchez M, Esteban MA, Lai L, Tortorella M, Luo Z, Liu H, Metzakopian E, Fernandes H, Bassett A, Karran E, Miller B, Fleming A, Rubinsztein D. Felodipine induces autophagy in mouse brains with pharmacokinetics amenable to repurposing. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-09494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solaimanzadeh I. Nifedipine and amlodipine are associated with improved mortality and decreased risk for intubation and mechanical ventilation in elderly patients hospitalized for COVID-19. Cureus. 2020;12:5. doi: 10.7759/cureus.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra AP, Bajpai A, Rai AK. 1,4-Dihydropyridine: A dependable heterocyclic ring with the promising and the most anticipable therapeutic effects. Mini. Rev. Med. Chem. 2019;19:1219–1254. doi: 10.2174/1389557519666190425184749. [DOI] [PubMed] [Google Scholar]
- 34.Gündüz MG, Armaković SJ, Dengiz C, Tahir MN, Armaković S. Crystal structure determination and computational studies of 1,4-dihydropyridine derivatives as selective T-type calcium channel blockers. J. Mol. Struct. 2021;1230:129898. doi: 10.1016/j.molstruc.2021.129898. [DOI] [Google Scholar]
- 35.Taheri-Ledari R, Rahimi J, Maleki A. Synergistic catalytic effect between ultrasound waves and pyrimidine-2,4-diamine-functionalized magnetic nanoparticles: Applied for synthesis of 1,4-dihydropyridine pharmaceutical derivatives. Ultrason. Sonochem. 2019;59:104737. doi: 10.1016/j.ultsonch.2019.104737. [DOI] [PubMed] [Google Scholar]
- 36.Singh TP, Singh OM. Recent progress in biological activities of indole and indole alkaloids. Mini. Rev. Med. Chem. 2018;18:9–25. doi: 10.2174/1389557517666170807123201. [DOI] [PubMed] [Google Scholar]
- 37.Faria JV, Vegi PF, Miguita AGC, Dos Santos MS, Boechat N, Bernardino AMR. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017;25:5891–5903. doi: 10.1016/j.bmc.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 38.Marinescu M. Synthesis of antimicrobial benzimidazole-pyrazole compounds and their biological activities. Antibiotics. 2021;10:1002. doi: 10.3390/antibiotics10081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moussier N, Bruche L, Viani F, Zanda M. Fluorinated barbituric acid derivatives: synthesis and bio-activity. Curr. Org. Chem. 2003;7:1071–1080. doi: 10.2174/1385272033486567. [DOI] [Google Scholar]
- 40.Rostamnia S, Doustkhah E, Golchin Hossieni H, Luque R. Covalently bonded PIDA on SBA-15 as robust Pd support: Water-tolerant designed catalysts for aqueous suzuki couplings. ChemistrySelect. 2017;2:329–334. doi: 10.1002/slct.201600624. [DOI] [Google Scholar]
- 41.Rostamnia S, Doustkhah E. Covalently bonded zwitterionic sulfamic acid onto the SBA-15 (SBA-15/PrEn-NHSO3H) reveals good Bronsted acidity behavior and catalytic activity in N-formylation of amines. J. Mol. Catal. A: Chem. 2016;411:317–324. doi: 10.1016/j.molcata.2015.11.002. [DOI] [Google Scholar]
- 42.Doustkhah E, Rostamnia S. Single site supported N-sulfonic acid and N-sulfamate onto SBA-15 for green and sustainable oxidation of sulfides. Mater. Chem. Phys. 2016;177:229–265. doi: 10.1016/j.matchemphys.2016.04.023. [DOI] [Google Scholar]
- 43.Rostamnia S, Hassankhani A. Covalently bonded ionic liquid-type sulfamic acid onto SBA-15: SBA-15/NHSO3H as a highly active, reusable, and selective green catalyst for solvent-free synthesis of polyhydroquinolines and dihydropyridines. Synlett. 2014;25:2753–2756. doi: 10.1055/s-0034-1379477. [DOI] [Google Scholar]
- 44.Rostamnia S, Xin H. Pd(OAc)2@SBA-15/PrEn nanoreactor: A highly active, reusable and selective phosphine-free catalyst for Suzuki-Miyaura cross-coupling reaction in aqueous media. Appl. Organometal. Chem. 2013;27:348–352. doi: 10.1002/aoc.2986. [DOI] [Google Scholar]
- 45.El-Mekabaty A, Etman HA, Mosbah A. Synthesis of some new fused pyrazole derivatives bearing indole moiety as antioxidant agents. J. Heterocycl. Chem. 2016;53:894–900. doi: 10.1002/jhet.2218. [DOI] [Google Scholar]
- 46.Doustkhah E, Lin J, Rostamnia S, Len C, Luque R, Luo X, Bando Y, Wu K, Kim J, Yamauchi Y, Ide Y. Development of sulfonic-acid-functionalized mesoporous materials: Synthesis and catalytic applications. Eur. J. Chem. 2019;25:1614–1635. doi: 10.1002/chem.201802183. [DOI] [PubMed] [Google Scholar]
- 47.Dindar MH, Yaftian MR, Rostamnia S. Potential of functionalized SBA-15 mesoporous materials for decontamination of water solutions from Cr(VI), As(V) and Hg(II) ions. J. Environ. Chem. Eng. 2015;3:986–995. doi: 10.1016/j.jece.2015.03.006. [DOI] [Google Scholar]
- 48.Doustkhah E, Mohtasham H, Farajzadeh M, Rostamnia S, Wang Y, Arandiyan H, Assadi MHN. Organosiloxane tunability in mesoporous organosilica and punctuated Pd nanoparticles growth; theory and experiment. Microporous Mesoporous Mater. 2020;293:109832. doi: 10.1016/j.micromeso.2019.109832. [DOI] [Google Scholar]
- 49.Sepehrmansouri H, Zarei M, Zolfigol MA, Moosavi-Zare AR, Rostamnia S, Moradi S. Multilinker phosphorous acid anchored En/MIL-100(Cr) as a novel nanoporous catalyst for the synthesis of new N-heterocyclic pyrimido[4,5-b]quinolines. Mol. Catal. 2020;481:110303. doi: 10.1016/j.mcat.2019.01.023. [DOI] [Google Scholar]
- 50.Sepehrmansourie H, Zarei M, Taghavi R, Zolfigol MA. Mesoporous ionically tagged cross-linked poly(vinyl imidazole)s as novel and reusable catalysts for the preparation of N-heterocycle spiropyrans. ACS Omega. 2019;4:17379–17392. doi: 10.1021/acsomega.9b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zolfigol MA. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron. 2001;57:9509–9511. doi: 10.1016/S0040-4020(01)00960-7. [DOI] [Google Scholar]
- 52.Moosavi-Zare AR, Zolfigol MA, Zarei M, Khakyzadeh V, Hasaninejad A. Design, characterization and application of new ionic liquid 1-sulfopyridinium chloride as an efficient catalyst for tandem Knoevenagel-Michael reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one with aldehydes. Appl. Catal. A Gen. 2013;467:61–68. doi: 10.1016/j.apcata.2013.07.004. [DOI] [Google Scholar]
- 53.Noroozizadeh E, Moosavi-Zare AR, Zolfigol MA, Zarei M, Karamian R, Asadbegy M, Yari S, Farida SHM. Synthesis of bis-coumarins over acetic acid functionalized poly(4-vinylpyridinum) bromide (APVPB) as a green and efficient catalyst under solvent-free conditions and their biological activity. J. Iran. Chem. Soc. 2018;15:471–481. doi: 10.1007/s13738-017-1247-1. [DOI] [Google Scholar]
- 54.Sepehrmansourie H, Zarei M, Zolfigol MA, Babaee S, Rostamnia S. Application of novel nanomagnetic metal-organic frameworks as a catalyst for the synthesis of new pyridines and 1,4-dihydropyridines via a cooperative vinylogous anomeric based oxidation. Sci. Rep. 2021;11:5279. doi: 10.1038/s41598-021-84005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider J, Reckmeier CJ, Xiong Y, von Seckendorff M, Susha AS, Kasák P, Rogach AL. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C. 2017;121:2014–2022. doi: 10.1021/acs.jpcc.6b12519. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.