Abstract
Microbial carbon use efficiency (CUE)—the balance between microbial growth and respiration—strongly impacts microbial mediated soil carbon storage and is sensitive to many well-studied abiotic environmental factors. However, surprisingly, little work has examined how biotic interactions in soil may impact CUE. Here, we review the theoretical and empirical lines of evidence exploring how biotic interactions affect CUE through the lens of life history strategies. Fundamentally, the CUE of a microbial population is constrained by population density and carrying capacity, which, when reached, causes species to grow more quickly and less efficiently. When microbes engage in interspecific competition, they accelerate growth rates to acquire limited resources and release secondary chemicals toxic to competitors. Such processes are not anabolic and thus constrain CUE. In turn, antagonists may activate one of a number of stress responses that also do not involve biomass production, potentially further reducing CUE. In contrast, facilitation can increase CUE by expanding species realized niches, mitigating environmental stress and reducing production costs of extracellular enzymes. Microbial interactions at higher trophic levels also influence CUE. For instance, predation on microbes can positively or negatively impact CUE by changing microbial density and the outcomes of interspecific competition. Finally, we discuss how plants select for more or less efficient microbes under different contexts. In short, this review demonstrates the potential for biotic interactions to be a strong regulator of microbial CUE and additionally provides a blueprint for future research to address key knowledge gaps of ecological and applied importance for carbon sequestration.
Introduction
Soil microbes are major actors in the terrestrial carbon cycle [1]. Microbial products (e.g. necromass, proteins, DNA) commonly comprise 10–80% of the total soil organic carbon (SOC) stock [2], and the formation and stabilization of these products are a key determinant of ecosystem carbon sequestration. At the same time, microbial activity accounts for approximately 60% of global soil carbon dioxide (CO2) emissions, making microbes an important component of the terrestrial carbon balance [3]. Two fundamental processes influence microbial SOC formation and depletion: growth, which produces biomass that may eventually become SOC; and respiration, which releases SOC as CO2. The balance between microbial respiration and growth is termed microbial carbon use efficiency (CUE; a.k.a. growth efficiency or yield), and is specifically defined as the proportion of assimilated carbon used for building new biomass relative to that lost through respiration and the activity of endogenous metabolism [4, 5]. CUE is one of the few explicit microbial variables in SOC cycling models [6], so accurately predicting it is therefore of considerable interest. Yet, our ability to do so is limited by an incomplete understanding of the factors affecting CUE. Here, we argue that biotic variables, such as competition and facilitation, constrain CUE above and beyond abiotic controls and should be explicitly included in the next phase of CUE research. While the effects of abiotic factors on CUE have been extensively tested, e.g. in [7], much less is known about how biotic factors, such as competition, facilitation, predation, and plant–microbe interactions, affect CUE.
This review aims to guide researchers towards a coherent research direction by linking microbial biotic interactions to microbial physiology with implications for SOC cycling. We draw from multiple lines of theoretical and empirical evidence from the evolution and botanical literature, which collectively suggest that biotic interactions should strongly affect CUE through differences in life history strategies. The most relevant life history frameworks include K- vs. r- selection [8], the competition-stress-ruderal (C-S-R) life history axes [9], and a reimagined microbial C-S-R that considers growth yield (Y), resource acquisition (A), and stress tolerance (S)—referred to as the Y-A-S framework [10]. While all of these life history frameworks directly or indirectly consider biotic interactions in the context of competition for resources, we argue that greater development and additional explicit biotic life history traits could be integrated into these frameworks to better predict CUE. CUE is an emergent property of multiple abiotic and biotic factors that are difficult to disentangle. However, by isolating the contributing effects of specific biotic interactions on CUE, it should be possible to disentangle the mechanisms by which microbes respond to abiotic changes. Doing so could thus allow us to predict microbial physiological performance under changing and/or novel environmental conditions. There is a wide range of biotic interactions occurring among soil microbes, including competition, predation, facilitation, and mutualisms among microbes and with higher trophic level organisms [11]. Hence, CUE may be impacted by many different types of biotic interactions simultaneously. Because microbiomes are typically hyper-diverse in the sense of diversity and functionality, the combined sum of positive (facilitation), neutral (commensal) and negative interactions (competition) will drive CUE at the aggregate community level [12]. Competition relates to negative interactions that deplete a population through the activity of antagonists onto protagonists [13]. It can occur among the same species (intraspecific competition) or among different species (interspecific competition), and can be direct (i.e. interference), indirect (i.e. exploitative), or predator-mediated [14]—though these forms of competition often overlap among microbes [15, 16]. Because competitive interactions commonly require life history strategies that promote fast growth and extensive investments in resource acquisition by antagonists and stress response by protagonists, we hypothesise that competition causes microbes to grow less efficiently. Facilitation relates to myriad positive interactions between organisms that benefit at least one organism and cause no harm to either organism [12, 13]. Facilitation can favour high CUE by (1) ameliorating abiotic stress; (2) creating novel habitats to promote niche partitioning; (3) increasing habitat complexity and heterogeneity; (4) sharing services like producing common goods and (5) increasing the availability of otherwise inaccessible resources [12]. Mutualism is a specialized form of facilitation that benefits both species, such as the exchange of services commonly observed between host plants and rhizobia or mycorrhizal fungi [13]. Because facilitation regularly promotes the exchange of resources and ameliorates stress, we anticipate that positive biotic interactions promote efficient microbial growth and may increase CUE at the community level if the sum of facilitation is greater than the combined sum of commensal plus competitive interactions.
To address these two expectations, we draw on theoretical and empirical lines of evidence to determine how biotic interactions (competition, predation, facilitation, and interactions with plants) drive certain microbial life history strategies, with a particular focus on implications for CUE and SOC cycling. While the focus of this review is on CUE, we also critically evaluate the current state of knowledge on microbial species interactions and life history characteristics relevant to CUE. First, we address intraspecific interactions and density-dependent feedbacks to provide a foundation for understanding relationships between microbial growth and biotic interactions (“CUE Fundamentally Depends On Density Dependence And Carrying Capacity”) section. We then discuss the roles of interspecific direct competition (“Interspecific Direct Competition Induces Metabolic Costs Leading to Low CUE”) section, interspecific indirect competition (“Interspecific Indirect Competition And Its Coincidence With Environmental Heterogeneity”) section, facilitation (“Facilitation Among Microbes Promotes Coexistence and Increases CUE”) section, predation (“Predation Influences Microbial Density and Competitive Outcomes”) section, and plants (“Effects of Plant Community and Plant-Microbe Interactions on CUE”) section on microbial CUE as well the influence of spatial separation among soil organisms (“Effect of Spatial Heterogeneity on CUE”) section—a unique but important characteristic of soil systems.
Effects of Biotic Interactions on Microbial CUE
CUE Fundamentally Depends on Density Dependence and Carrying Capacity
Density-dependent feedbacks on species abundances place important constraints on life history-related differences in CUE. Seminal eco-evolutionary work on microbial growth in culture has demonstrated that if the density of a microbial population is below its carrying capacity, then a slow but efficient metabolism should drive relatively low growth rates and relatively high CUE [17]. Because organisms cannot obtain maximal yield from a substrate at the maximal metabolic rate (Fig. 1); [18, 19], there is a tradeoff between growth efficiency and growth rate under certain environmental conditions [20], which is also reflected by the life history framework regarding r-/K-selection. The selective pressure on species to grow fast versus efficiently depends on whether a species typically reaches carrying capacity and in turn experiences intraspecific competitive exclusion [11, 18]. When cell cultures growing on a single carbon source reach high population densities, competition for shared resources intensifies and favours fast resource use, which is typically inefficient [17]. Evidence from culture studies thus raises numerous important questions regarding soil microbial growth—with an important first question being how often species in soil reach carrying capacity, if at all.
Fig. 1.
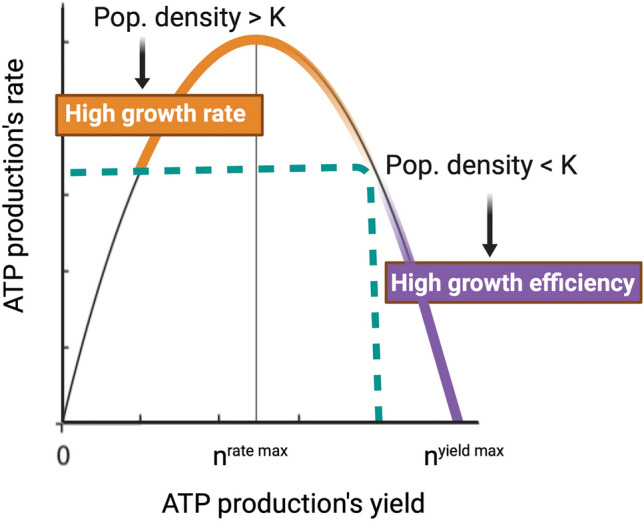
The tradeoff between high growth rate (yellow) and high CUE (purple, a.k.a. growth yield) has its origins in the thermodynamics of ATP production pathway, where the maximal rate of ATP production is obtained at half maximal ATP yield. In the absence of other biotic interactions than intraspecific competition, CUE depends on whether the population density is below or above carrying capacity (K). Figure modified from data in Pfeiffer and Bonhoeffer (2002) (Color figure online)
Carrying capacity is affected by eco-evolutionary forces, as well as by factors influencing how many individuals a habitat can support (i.e. habitat carrying capacity [19]). While individual microbial species carrying capacities are not well quantified in environmental habitats (i.e. soil, plants, animals), there is nonetheless sufficient evidence to indicate that they occur. For example, genetically labelled Curvibacter strains reach a carrying capacity of 2 × 105 cells in their freshwater cnidarian host [21]. Microbial carrying capacities have also been estimated on plastic marine debris [22], absorptive versus transportive fine roots [23], and in the tissues and organs of different animals, such as zebrafish [24] and humans [25]. Furthermore, there is a wealth of studies relating to potting soil development that aim to increase microbial carrying capacities to provide biological control against plant pathogens (e.g. [26]). In order to resist Rhizoctonia pathogens, organic matter in potting mixes must become fully colonized by soil microorganisms, i.e. microbes must reach the habitat carrying capacity [27]. Thus, microorganisms can reach carrying capacity in environmental samples, including soil, but little is known about which environmental and eco-evolutionary forces influence where and which microbes do so.
We hypothesize that only certain soil microbes reach carrying capacity in nature because interspecific competition reduces individual species abundances [28], many species are dispersal limited [29], and, drawing on evidence from plant ecology, stochastic processes limit recruitment [30]. Thus, the probability of reaching carrying capacity may be generally low in natural soils and site-specific in terms of the taxa that are locally dominant. Observations that microbial communities are routinely highly uneven (i.e. extreme dominance by a small number of taxa [31]) suggest that only some of the most dominant species in a community may in fact reach carrying capacity, with the majority of less common taxa being too rare to do so. While many factors can generate uneven community distributions, species evenness and dominance have been used to infer carrying capacity dynamics among plants and animals (e.g. [32]). Further, dominant rather than rare bacteria in the human gut not only reach but exhibit similar carrying capacities across individuals even when overall community composition patterns vary [25]. A hypothesis for future study would thus be that when any dominant microbial species becomes more or less abundant as a consequence of carrying capacity, these taxa have disproportionately strong, yet potentially ephemeral, negative impacts on community-level CUE. While it is likely that species that become dominant in soil harbour certain traits, such as high stress tolerance and carbohydrate metabolism potentials among soil fungi [33] or high growth rates among bacteria (found in culture and soil [23, 34]), any organism that reaches carrying capacity should exhibit reduced CUE. Since microbial communities are routinely dominated by just a few taxa, it may be possible to isolate the growth dynamics of these species if they can be cultured or tracked in natural systems using isotopically labelled water or nutrients.
Another clear axis of importance when considering density dependence is identifying which resources are being metabolized and how they are distributed. Much experimental work on density dependence to date has been conducted using cultures growing on a single, non-limiting, carbon source, whereas in soil, there are many different substrates of varying qualities and quantities that are distributed heterogeneously. In general, microbes grow more efficiently on simple substrates, such as glucose, versus more complex substrates, such as plant residues with high carbon-to-nitrogen ratios (see [35] and [36] where this is discussed in detail). A large body of ecological models suggests that under heterogenous conditions, diffusing populations can reach a higher carrying capacity than under homogenous conditions [37, 38]. How species move in the soil and how heterogenous resources may be distributed could influence whether and to what degree species reach carrying capacity. Further, in the laboratory, yeast cells reach higher population sizes when growing on homogenously versus heterogeneously distributed resources [34]. How resource uniformity versus heterogeneity modulates the carrying capacity of soil microbes therefore remains an important area of future research and could be evaluated using microcosms consisting of patchily versus homogenously distributed resources or under fluctuating versus stable environmental conditions.
Interspecific Direct Competition Induces Metabolic Costs Leading to Low CUE
The impact of direct competition on CUE depends on competitor density and the intensity of antagonistic interactions. Direct competition describes interactions between competitors to obtain space [14]. A clear example of this comes from a study of three different strains of Escherichia coli, which persist in spatially structured habitats that discourage direct competition but cannot co-exist in well-mixed environments as a result of competitive exclusion due to direct competition [11]. The acquisition of space can involve either the production of anti-microbial toxins or interference in the motility and signalling of competitors, which, in turn, alters the behaviour and physiology of both antagonists and protagonists, as seen in culture experiments [11]. For example, streptomycin released by species of the bacterial genus Streptomyces and phenazines produced by species of the bacterial genus Pseudomonas induce metabolic costs to antagonists [11]. In response, protagonists activate essential resistance mechanisms or warn related organisms through the production of volatile organic compounds, both of which impose endogenous metabolic costs to avoid cell damage and death [11]. Thus, high degrees of direct competition may decrease CUE of both protagonists and antagonists, at least in the short term, in comparison to another community in the same environment but without or under less direct competition.
Further experimental work has demonstrated that high competitor density forces microbes to adopt stress-tolerant life history strategies (e.g. by expressing phenotypes, such as sigma-factors or molecular chaperones). The best example of this is among saprotrophic fungi, which lower their CUE under direct competition [39]. When a protagonist is not resistant to an antagonist, the population of the protagonist can be depleted or even entirely replaced by the antagonist, thus altering the microbial community towards a highly competitive, less efficient community composition [40]. It remains unclear whether competitive dominance by antagonists (i.e. exclusion of the protagonist) feeds back to eventually alleviate antagonist investments in competition, allowing the antagonist to grow more efficiently in the context of increased resources due to protagonist exclusion. Nevertheless, protagonists that are particularly sensitive to competition tend to have lower metabolic costs than antagonists resistant to competition because they invest less in toxin production and toxin resistance mechanisms [40], suggesting a higher intrinsic CUE. In summary, antagonists, by negatively impacting sensitive taxa, inducing costly resistance mechanisms in resistant taxa, and by investing in toxin production themselves, decrease the overall CUE of a community (Fig. 1).
Building on general evidence that competition may reduce CUE, there remain a few key areas of uncertainty that future research should examine. One uncertainty is whether an antagonist that excludes a protagonist eventually alleviates investments in competitive strategies and increases its CUE. Tracking CUE over different stages of direct competition, such as prior to engagement, during chemical and physical interactions, and as one species dominates another, would provide new insight into this question. It is additionally unclear whether competition arising from specific contexts, such as low nitrogen availability, could help to select for more efficient species, alleviating competitive costs when compared to the same environment without competition. Beginning to disentangle these complex forms of interspecific competition will allow us to start predicting growth processes in soils inhabited by species known to engage in competition.
Interspecific Indirect Competition and its Coincidence with Environmental Heterogeneity
Indirect competition impacts CUE by reducing resource availability. Indirect competition consists of competitors blocking or limiting access to resources [11]. This is critical because resource limitation in any form can reduce CUE (see review by [5]). When several species compete for the same resource, similarly to intraspecific competition, resource use must be faster and relatively less efficient than in the absence of competition [17]. However, in many natural environments, resources are patchily distributed in space and time. The degree of resource heterogeneity can also be enhanced by resource competition [41]. Patchy soil resource availability can lead to co-existence because the chances of taxa existing on the same spectrum of multiple fluctuating resources are rather low, as observed in computational experiments [41]. Ecosystem heterogeneity can therefore increase species co-existence by alleviating indirect competition, and this should lead to higher emergent CUE (Fig. 2).
Fig. 2.
A summary of biotic interactions occurring in soil with implications for CUE. High CUE communities are characterized by facilitation, and, in specific contexts, interspecific competition and predation. Low CUE communities are characterized by high metabolic costs that do not generate growth (e.g. S- and A-strategy phenotypes), induce increases in growth rates (e.g. in response to direct competition), interspecific competition (in many instances), and during specific predator–prey interactions. Interspecific competition does not negatively impact CUE when population density is lower than carrying capacity (K). Green arrows represent an expansion of a species’ realized niche via facilitative effects. The black dashed arrows represent how a certain biotic interaction can change due to cascading effect of biotic interactions
Environmental heterogeneity can promote microbial niche partitioning and thereby reduce competition for common substrates [42, 43]. This mechanism was a pioneering conclusion of the “Paradox of the Plankton” proposed by [44], who observed high species diversity of plankton in lakes despite species possessing similar nutrient requirements. While lakes appear to be well-mixed environments, they are in fact composed of heterogenous micro-patches of nutrients, making them somewhat analogous to soil. Moreover, as species evolve, they develop different competitive abilities and specialize to decompose distinct substrates from their neighbours [45, 46]. It is important to acknowledge that there is still a considerable degree of functional redundancy among microbial communities [47, 48], but trait overlap does not necessarily translate to redundancies in the functioning of communities, as observed in freshwater ecosystems [49]. High levels of resource heterogeneity can also favour niche partitioning, which can promote diversity in soil by encouraging microbes to exit dormancy [45, 50]. This could increase CUE since microbial dormancy is generally believed to be inefficient [51]. The logic behind such a mechanism is well demonstrated, in that dormant microbes exhibit minimal anabolism but must invest in maintenance and repair costs [52]. Thus, for a number of reasons high environmental heterogeneity should promote high CUE.
One putative mechanism for a connection between environmental heterogeneity and CUE is high microbial diversity via niche partitioning. Recent evidence that microbial diversity corresponds to increases in CUE was documented by Domeignoz-Horta et al. [53], who assessed the direct effects of microbial diversity on CUE by establishing a microbial diversity gradient at two moisture levels and temperatures in an artificial soil environment. Adding cellobiose as a carbon source, they tracked CUE and correlated it with bacterial and fungal diversity. Bacterial phylogenetic diversity was positively correlated with CUE, but only under high soil moisture contents. These results suggest that, under high soil moisture, organisms may interact more cooperatively by sharing resources and that this is not possible when moisture limitations physically restrict microbial interactions. A key component of niche partitioning includes facilitative effects, and it would be interesting for future studies to consider how facilitation might vary with environmental conditions, including resource heterogeneity. While experimental evidence for diversity–CUE relationships is still rudimentary, recent experimental work not only suggests that microbial diversity is related to CUE, but also that positive species interactions (e.g. facilitation) may be an important underlying mechanism. Future work must now investigate the relationship between CUE and diversity using natural communities and under different abiotic conditions to identify specific facilitative mechanisms influencing CUE.
Facilitation Among Microbes Promotes Co-existence and Increases CUE
Facilitation describes an interaction whereby the presence of one organism (the facilitator) benefits another (the facilitated) by improving its local environment [13]. Facilitation can increase CUE at population and community levels under certain circumstances in three ways: (1) increasing the size and the number of realized niches of facilitated species, thereby increasing local species richness; (2) mitigating stressful environmental conditions which constrain anabolism and (3) decreasing the production costs of extracellular enzymes. Perhaps most obviously, facilitators can enlarge facilitated species niches by directly improving the surrounding energy and nutrient contents required by the facilitated species [12, 13]. For instance, the release of microbial products, such as dead microbial cells or the remains of extracellular enzymes, can enlarge the realized niches of facilitated microbes by providing them with nutrient-rich microbial products, as observed in a computational model representing litter decomposition in soil [54]. This mechanism has been empirically shown in cultures using different populations of interacting E. coli, which can exhibit complementary metabolism by sharing hydrogen, acetate, amino acids, nitrogen and glucose, promoting the growth of each population [55]. While pervasive nutrient limitations are known to decrease CUE [5], a recent modelling study also demonstrated that co-existence increases with facilitative chemical interactions because microbial products, including metabolites and waste-products, provide limiting nutrients to other microbes [56]. Further, Kästner et al. [57] suggested in a recent review that microbes feeding on microbial necromass have a high CUE because necromass provides nutrients with similar stoichiometric ratios to living microbial biomass. Thus, resources can be recycled within a community, enlarging realized niches, and—if sufficiently widespread—increasing CUE at population and community levels.
Facilitative interactions can also promote survival during stressful abiotic conditions, such as drought [12]. Indeed, facilitation appears to be especially important in harsh and stressful conditions because it alleviates essential metabolic constraints on the facilitated species [13]. For instance, in a culture experiment, the presence of microbial species resistant to competition increased the survivorship of other microbial species less resistant to competition [58]. While the exact mechanism of such induced resistance remains unclear, horizontal transfer of genes conferring resistance to antibiotics has been repeatedly observed among bacteria in soil [59, 60] and is very likely to be one of a number of mechanisms by which resistance against anti-microbial compounds is obtained by otherwise sensitive species. Ultimately, when microbes are not forced to respond to stress, they can invest relatively more energy into growth (or other processes), and we therefore expect this to increase the CUE of facilitated species (Fig. 2).
Finally, facilitation can reduce the energetic costs required to break down complex substrates. For instance, a recent simulation study demonstrated that aquatic bacterial communities grow more efficiently by aggregating enzyme production at the community level [61]. In extreme cases, microbial products, such as enzymes or molecules that scavenge iron (siderophores), can lead to “cheaters”, which are organisms that realize the benefits of such facilitative interactions without producing extracellular enzymes or siderophores [62, 63]. Although the presence of cheaters may negatively affect some ecosystem processes, such as mycorrhizal nutrient transfer, they nevertheless increase total microbial community biomass relative to extracellular enzyme production, resulting in higher CUE at the community level [63]. However, when the density of non-cheating microbes exceeds a certain threshold, each individual receives less per capita resource, which leads to enhanced competition and subsequently promotes rapid growth rates, lowering community-level CUE [61, 62]. In short, when facilitative processes lower the costs of enzyme production for specific community members or the whole community without dramatically reducing the amount of resources per capita, the CUE of the community may increase.
Predation Influences Microbial Density and Competitive Outcomes
Microbial predators indirectly affect carbon cycling by altering microbial densities and community composition and can increase CUE by alleviating competition among microbes. Microbes are consumed by carnivorous organisms, including other microbes and soil animals, such as nematodes, arthropods, and soil vertebrates [64]. Microbes are also consumed by omnivorous organisms, such as earthworms and springtails, which feed on living microbes and microbial detritus [64]. A seminal study on protozoan predation found that predators at high grazing intensity increased the CUE of the soil community [65]. Despite such findings, surprisingly little work has followed up on these results, so it remains unclear how effects of predation on microbial CUE vary across environmental conditions and involving different types of grazers and prey.
Predator density has well-known effects on microbial processes related to CUE, such as respiration, decomposition and growth [16, 66]. For example, a study on earthworm invasions in two deciduous forests showed that earthworms reduced microbial biomass by 42% and soil respiration by 32%, potentially leading to decreased CUE via increased water stress induced by earthworms [66]. The negative effect of earthworms on respiration and microbial biomass was greatest at the edge of the invasion but had less pronounced effects in areas where earthworm biomass was higher. Thus, one possibility is that high earthworm densities have a smaller effect on CUE than intermediate earthworm densities and, at least in this study, this occurs due to reduced soil moisture at the invasion edge [66]. Interestingly, in another study by [67], high predator density decreased both decomposition and respiration rates [67]. Corroborating these results, hyphal extension of soil fungi is restricted at high predator densities, whereas at low predator densities, fungal growth rates increase, thereby potentially also reducing CUE [16]. These findings collectively suggest that while any form of predation can impact microbial growth processes, CUE may be particularly reduced at low compared to high predator densities. Future work should test this hypothesis by directly measuring CUE in the context of different grazer densities.
Predators also impact the spatial organization of microbial communities by enhancing dispersal of propagules (spores, hyphae) via predator gut passages and faecal deposition, as well as through passive transport on predator exoskeletons [16, 68]. In some contexts, dispersal may enhance CUE since dispersed microbes can accidentally be deposited into less stressful or less competitive environments [69]. Indeed, many microbes produce volatile organic compounds in order to attract animals that disperse their propagules [70]. It is possible that dispersal through predation is highly stochastic, but the common observation of preferential grazing, i.e. the preference of predators for specific prey, indicates that it may be possible to predict which microbes are most likely to be consumed by predators and thus have their propagules dispersed. For instance, selective grazing for weak and strong microbial competitors has been repeatedly observed, such as collembola preferring to consume less competitive fungi [16, 68, 71]. In contrast to enhancing CUE, there is also evidence that by increasing the spatial range of prey species, predators may force microbes to forage on low quality, nutrient-limited substrates [68], thus reducing CUE. In short, the consequences of higher trophic level-mediated dispersal on CUE likely depend on context, predator preference, the life history strategy of the prey and stochastic processes. However, this has not been empirically tested, and future studies should explicitly measure microbial CUE in different prey–predator systems and in animal-based studies of dispersal.
Effects of Plant Community and Plant–Microbe Interactions on CUE
Plant–microbe interactions have the potential to impact CUE by regulating microbial metabolism, shaping microbial community composition, as well as by physically altering the soil environment. Plants (autotrophic organisms) and soil microbes (usually heterotroph organisms) often enter into reciprocal interactions, whereby microbes consume plant root exudates, mineralize organic matter (including plant litter) and liberate nutrients for plants [72]. Different plants produce distinct profiles of exudates that select for specific microbial species in the rhizosphere [72, 73]. Microbial mutualists of plants include—but are not limited to—nitrogen-fixing bacteria, arbuscular mycorrhizal fungi and ectomycorrhizal fungi [73]. All are abundant in certain rhizospheres and can profoundly impact ecosystem functioning. Presumably, these mutualists also impact the CUE of the free-living soil microbial community via changes to nutrient availabilities and competitive interactions, and may function to lower heterotrophic microbial CUE in the rhizosphere. Nevertheless, plants also affect the CUE of their microbial symbionts and may even enhance CUE by providing soil conditions beneficial to microbial growth.
Plants, by shaping soil conditions, such as moisture content, nutrient inputs and habitat space via litter and root inputs, fuel microbial metabolism. In some contexts, plants may create conditions that enhance CUE. This is evidenced by the fact that the presence of plants in comparison to bare fallow increases soil water content [74], which tends to increase microbial CUE by reducing stress, increasing substrate diffusion and promoting cooperation among microbes [53, 75]. Further, by exuding carbon compounds, plants provide microbes with labile energy sources [73] that have the capacity to increase the CUE of carbon-limited free-living microbes. However, such a mechanism could alternatively represent a metabolic cost to nitrogen-limited microbes, reducing their CUE. Meier et al. [76] demonstrated that root exudates increase nitrogen mineralization and investment in nitrogen acquisition enzymes, increasing nitrogen availability but not microbial biomass. The amount and types of plant exudates released are also plant species-specific [75] and vary with local plant diversity, which may explain why diverse plant systems have higher microbial biomass and lower respiration rates than plant monocultures [77]. Plants may increase microbial CUE if they modify abiotic conditions that reduce stress and balance microbial stoichiometric demands, particularly if plant diversity is high; thus, plant effects are likely highly localized and context dependent.
There is also evidence for plants to reduce microbial CUE, with recent evidence showing that plants select for less efficient microbial communities in the rhizosphere compared to microbes living in bulk soil [78]. This can occur through three mechanisms. First, plants alter soil nutrient availability by competing with microbes for resources [73, 79]. For instance, Moreau et al. [79] demonstrated that the abundance of nitrate-reducing bacteria decreased as a function of plant nitrogen-use efficiency. If plants deplete bioavailable nitrogen pools, microbes must invest in more intensively into nutrient acquisition strategies in order to mine nitrogen from SOM or mineral complexes, which can reduce CUE [5]. Second, by directly altering soil nutrient availability [80], root exudates can induce microbial nitrogen limitation and lead to reduced microbial CUE of N-limited microbes [as discussed above; 5]. Finally, plants select for specific microbial species in the rhizosphere that may be either r-strategists [73] or K-strategists [81], which can impact CUE at the community level if r-strategists grow less efficiently [82]. Ultimately, by reducing nutrient availability and selecting for unique microbial communities in the rhizosphere, plants may favour the proliferation of fast-growing microbes via indirect competition and select for microbes with high investments in resource acquisition (A-strategy). This can decrease CUE at the community level, but such an effect may additionally depend on the plant species involved and environmental context of the rhizosphere.
Some plants additionally produce secondary metabolites (e.g. allelochemicals) that induce stress in microbial communities and suppress microbial growth [83]. For example, the release of arabinogalactan-proteins, jasmonic acid, salicylic acid, and flavonoids serves as important plant defence compounds that inhibit fungal growth [84], which includes pathogens that are commonly facultative saprotrophs [85]. Some plants also deploy toxic compounds to suppress symbiotic microbes that associate with plant competitors [86]. For example, Alliaria petiolata produces flavonoids and aliphatic glucosinolates that suppress the growth of arbuscular and ectomycorrhizal fungi [86, 87]. Arabidopsis thaliana produces indolic glucosinolates that strongly reduce mycorrhizal colonization [88]. Some plant species, such as Cucumis sativus, also exude compounds, such as the amino acid tryptophan, to enhance colonization of plant growth-promoting rhizobacterium—the presence of which suppresses other soil community members [83]. Plant growth-promoting rhizobacteria suppresses soil-borne pathogens by releasing antibiotic compounds and is also competitive for soil micronutrients, both of which could lower community CUE [83]. Plant chemicals that induce stress and reduce the growth of key components of the microbial community could have potentially substantial impacts on CUE. Even if microbes do adapt to such stressful conditions, then they have adopted an S-based life history strategy, which is associated with metabolic costs. While empirical studies of the costs induced by resistant species are needed to validate such a hypothesis, it could explain why rhizosphere microbes grow less efficiently than bulk soil microbes among certain plant species [78].
Plant–microbe symbioses may affect microbial CUE by altering interactions between symbiotic microbes and free-living microbes. Whether microbial symbionts increase or decrease community CUE depends on the ecology and physical habitat of symbionts. For example, it has been suggested that microbial endophytes, by colonizing the inside of the root, experience reduced competition against other microbes living in the rhizosphere [73]. Soil systems dominated by arbuscular mycorrhizal fungi, which are relatively weak decomposers, are associated with higher CUE than those dominated by ectomycorrhizal fungi, which have retained a high decomposing ability and are strong competitors against saprotrophic fungi [69]. Indeed, it has been shown that the CUE of ectomycorrhizal fungal mycelium across eight boreal forests ranges from less than 5% to 20% [89], which is twice as low as the average observed for free-living microbes (50%; [35]). Thus, symbionts that compete with free-living saprotrophs—such as ectomycorrhizal fungi—may reduce overall community CUE, whereas those that physically avoid competition and are not strong competitors—like arbuscular mycorrhizal fungi or rhizobacterium—may increase community CUE.
Further, we argue that there must be flexibility in the effects of symbionts on CUE via their direct growth responses to plant compounds. A suite of plant genes encode for particular compounds that establish and regulate rhizobia and mycorrhizal symbiosis [90]. For instance, strigolactone is exudated by roots of arbuscular mycorrhizal associated host plants to facilitate mycorrhizal colonization [91]. Strigolactone has been found to stimulate arbuscular mycorrhizal fungal hyphal branching [92], and it would thus be interesting to investigate its effects on CUE. It was also recently shown that the fatty acid myristate permits a free-living life cycle in a model arbuscular mycorrhizal fungus widely considered to be an obligate biotroph [93]. Via myristate uptake, arbuscular mycorrhizal fungal growth may become unlinked to host plant carbon and lipid allocation as arbuscular mycorrhizal fungi act more independently, potentially compete with free-living microbes and alter their growth modality [94]. How plants communicate with microbial symbionts can drastically affect microbial symbiont growth, but, to our knowledge, no study has yet examined how symbiosis communication impacts either population or community-based microbial CUE.
As a final point, autotroph–heterotroph interactions are also not limited to plants and include interactions among algae and some types of bacteria. For instance, lichen is a symbiosis between fungi and photoautotrophic partners (i.e. green algae, cyanobacteria) that can occur in soil and that typically shows the pattern of K-selected organisms with a slow growth and decomposition rate [95]. The ground cover of some ecosystems is covered with mats of lichen that may play important roles in affecting CUE, particularly in extreme environments where lichen are most common. Fungi comprise most of the lichen body (i.e. thallus), which provides a controlled level of sunlight and facilitates gas exchange, while the photoautotroph redistributes energy-rich nutrients [96]. The lichen symbiosis confers an outstanding tolerance to desiccation, radiation and extreme temperature—an adaptation that may lower the cost of coping with stress in hostile habitats, including compacted soil or desert [96]. In addition to being K-selected, lichens are therefore highly stress tolerant. Further, the mass of lichen is up to 30% secondary metabolites, mostly of fungal origin [97]. High stress tolerance and substantial investment in secondary metabolites versus biomass production together suggest that lichen may be an example of facilitation that reduces the overall CUE of a system. However, this is a highly understudied area of research, and its importance in typical mineral soils that usually support low lichen populations remains unknown.
Effect of Spatial Heterogeneity on CUE
We argue that detailed consideration should now be given to the impact of abiotic factors on biotic interactions and how this, in turn, affects CUE. For instance, soil spatial distribution affects how water, gases, nutrients, organic matter and microorganisms move through soil and in turn interact to affect CUE. Soil pore networks are especially important regulators of effective distances between species with implications for species interactions. For example, in well-connected soils with fine-particle size fractions, the rod-forming bacteria Bacillus out-competed the filament-forming bacteria Streptomyces due its faster growth rate, but in poorly-connected soils, Streptomyces out-competed Bacillus because of its ability to produce hyphae and exploit far-off resources [98]. The development of 30–150 μm pores in soil promotes connectivity and microbial enzyme activities which enhance decomposition [99]. Connectivity may reduce CUE by promoting faster microbial growth and increasing resource acquisition, both of which can constrain CUE, as discussed in this review. However, under generally anoxic conditions, the development of 30–150 μm pores promotes aerobic conditions and may increase CUE [100]. Further, pores of this size can transport molecules into active bindings sites that stabilize soil carbon [99]. Thus, even if greater connectivity reduces CUE, the positive effects of pore formation on carbon stabilization may outweigh reductions in CUE, but this remains an open question.
Soil structure also affects physical access by different sized soil organisms and protection via the exclusion of larger organisms. As an example, the survival of rhizobia was higher in soil with pores smaller versus larger than 6 µm because these small pores protected rhizobia from protozoan grazing [101]. Ritz and Young (2004) [102] proposed that fungi inhabiting soil pores smaller than the body sizes of grazers experience protection. Depending on fungal biomass and carrying capacity, this could enhance the prey’s CUE if biomass is below carrying capacity by protecting fungi from grazing or reduce CUE at carrying capacity by promoting intraspecific competition and rapid growth (see chapter 1.5). Further, Six et al. [103] suggest that pore space could reduce carbon and nitrogen decomposition by offering protection of fungi and protozoa from nematodes predation, thereby stabilizing carbon and increasing the system’s CUE.
Lastly, soil spatial distribution also includes soil biofilms serving as microhabitats for interacting species. Often microbes produce biofilms cooperatively, generating favourable conditions for efficient growth [104]. For example, diverse bacterial species in biofilms are often auxotrophic and rely on the cross-exchange of different amino acids and vitamins in order to grow [105]. Mixed species biofilms also reduce stress more than single-species biofilms by promoting tolerance to anti-microbial compounds [106]. Collectively, this suggests that biofilms create favourable spatial habitats for high CUE. However, there are also contexts in which biofilms promote competition and may reduce CUE. Notably, competition is promoted within biofilms if species are more closely related or have generalist metabolic strategies [107] and when labile resource availability is high [61]. Soil spatial distribution is strongly impacted by microbial biofilms that provide key habitats for species interactions affecting CUE. Much of this work is based on simple mesocosm studies and models and has not been explicitly linked to CUE. Tracking the role of soil spatial distributions on CUE in actual soils is an important area of future research.
Conclusion and Further Considerations
Biotic interactions influence CUE by affecting the allocation of carbon to different metabolic processes, changing environmental conditions, and inducing shifts in microbial community composition. However, a huge variety of biotic interactions take place in soil, making it challenging to predict CUE without having a complete picture of their cumulative impacts. While we broadly show that competition is a “negative” interaction that reduces CUE, we also present evidence that indirect competition can, in some cases, positively impact CUE. At the same time, facilitation is a “positive” interaction that is generally expected to increase CUE, especially when microbial population densities are low. Although plant–microbe interactions are often facilitative, such interactions can reduce CUE in the rhizosphere and potentially more widely in ectomycorrhizal fungal-dominated systems. Furthermore, we know that biotic interactions can have complex cascading effects, with some interactions generating new interactions (see Fig. 2). For example, indirect competition can increase the probability of direct competition and facilitation can decrease the probability of direct competition or predation. Importantly, cascading effects of biotic interactions are widely known to introduce apparent stochasticity to microbial communities, which may be difficult to predict [108]. Future research must ultimately focus on teasing such apparent complexity apart.
In conclusion, by reviewing literature spanning the fields of microbiology, evolution, and botany, we analysed how soil microbial CUE is affected by a key set of biotic interactions. This is important because while there is a concerted effort to understand the abiotic factors regulating microbial CUE, surprisingly few studies have addressed the additional role of biotic interactions in soil ecosystems. Of course, studying soil microbial interactions comes with substantial technical challenges. Several methods are used to measure CUE and they differ in meaningful ways depending on whether the CUE is measured at the population, community or ecosystem scale, or in cultures, mesocosms, or actual field soil (see [109]). Creating generalizable CUE frameworks is therefore challenging when working across different scales and media. While much of the evidence presented here considering biotic interactions and CUE yields more questions than answers, this review was written to organize a path forwards. By summarizing the key theoretical biotic interactions that should affect CUE and pairing this with available supporting evidence, we were able to provide concrete research suggestions for the future. Despite addressing a new area of CUE research, we argue that understanding how biotic interactions shape microbial CUE is important not only for conceptually, but also for managing natural systems, such as to identify strategies in agricultural soils that favour biotic interactions known to increase CUE (see [43]). In our opinion, considering biotic interactions alongside abiotic drivers of CUE will ultimately improve both mechanistic insights and predictive power in ecosystem ecology and management.
Acknowledgements
We thank Helena Mühlhaus and William Verbiest for helpful feedback on an earlier version of the manuscript.
Author Contributions
Conceptualizations: H.I., M.A., Writing—Original Draft, Review & Editing: H.I., M.A., T.W., Supervision: M.A.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich. No funding was received to assist with the preparation of this manuscript.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lehmann J, Kleber M. The contentious nature of soil organic matter. Nature. 2015;528:60–68. doi: 10.1038/nature16069. [DOI] [PubMed] [Google Scholar]
- 2.Liang C, Amelung W, Lehmann J, Kästner M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Change Biol. 2019;25:3578–3590. doi: 10.1111/gcb.14781. [DOI] [PubMed] [Google Scholar]
- 3.Lal R. Soil carbon sequestration impacts on global climate change and food security. Science. 2004;304:1623–1627. doi: 10.1126/science.1097396. [DOI] [PubMed] [Google Scholar]
- 4.Manzoni S, Taylor P, Richter A, et al. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils: research review. New Phytol. 2012;196:79–91. doi: 10.1111/j.1469-8137.2012.04225.x. [DOI] [PubMed] [Google Scholar]
- 5.Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A. Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett. 2013;16:930–939. doi: 10.1111/ele.12113. [DOI] [PubMed] [Google Scholar]
- 6.Kallenbach CM, Grandy AS, Frey SD, Diefendorf AF. Microbial physiology and necromass regulate agricultural soil carbon accumulation. Soil Biol Biochem. 2015;91:279–290. doi: 10.1016/j.soilbio.2015.09.005. [DOI] [Google Scholar]
- 7.Allison SD, Wallenstein MD, Bradford MA. Soil-carbon response to warming dependent on microbial physiology. Nat Geosci. 2010;3:336–340. doi: 10.1038/ngeo846. [DOI] [Google Scholar]
- 8.Lipson DA. The complex relationship between microbial growth rate and yield and its implications for ecosystem processes. Front Microbiol. 2015 doi: 10.3389/fmicb.2015.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grime JP. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat. 1977;111:1169–1194. doi: 10.1086/283244. [DOI] [Google Scholar]
- 10.Malik AA, Martiny JBH, Brodie EL, et al. Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J. 2020;14:1–9. doi: 10.1038/s41396-019-0510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntire EJB, Fajardo A. Facilitation as a ubiquitous driver of biodiversity. New Phytol. 2014;201:403–416. doi: 10.1111/nph.12478. [DOI] [PubMed] [Google Scholar]
- 13.Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends Ecol Evol. 2003;18:119–125. doi: 10.1016/S0169-5347(02)00045-9. [DOI] [Google Scholar]
- 14.Buchkowski RW, Bradford MA, Grandy AS, et al. Applying population and community ecology theory to advance understanding of belowground biogeochemistry. Ecol Lett. 2017;20:231–245. doi: 10.1111/ele.12712. [DOI] [PubMed] [Google Scholar]
- 15.Boddy L. Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiol Ecol. 2000;31:185–194. doi: 10.1111/j.1574-6941.2000.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 16.Crowther TW, Boddy L, Hefin Jones T. Functional and ecological consequences of saprotrophic fungus–grazer interactions. ISME J. 2012;6:1992–2001. doi: 10.1038/ismej.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeiffer T, Bonhoeffer S. Evolutionary consequences of tradeoffs between yield and rate of ATP production. Z Phys Chem. 2002 doi: 10.1524/zpch.2002.216.1.051. [DOI] [Google Scholar]
- 18.Bachmann H, Bruggeman FJ, Molenaar D, et al. Public goods and metabolic strategies. Curr Opin Microbiol. 2016;31:109–115. doi: 10.1016/j.mib.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Lanfear R, Kokko H, Eyre-Walker A. Population size and the rate of evolution. Trends Ecol Evol. 2014;29:33–41. doi: 10.1016/j.tree.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Lipson DA, Monson RK, Schmidt SK, Weintraub MN. The trade-off between growth rate and yield in microbial communities and the consequences for under-snow soil respiration in a high elevation coniferous forest. Biogeochemistry. 2009;95:23–35. doi: 10.1007/s10533-008-9252-1. [DOI] [Google Scholar]
- 21.Wein T, Dagan T, Fraune S, et al. Carrying capacity and colonization dynamics of curvibacter in the hydra host habitat. Front Microbiol. 2018;9:443. doi: 10.3389/fmicb.2018.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao S, Zettler ER, Amaral-Zettler LA, Mincer TJ. Microbial carrying capacity and carbon biomass of plastic marine debris. ISME J. 2021;15:67–77. doi: 10.1038/s41396-020-00756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King WL, Yates CF, Guo J, et al. The hierarchy of root branching order determines bacterial composition, microbial carrying capacity and microbial filtering. Commun Biol. 2021;4:483. doi: 10.1038/s42003-021-01988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jemielita M, Taormina MJ, Burns AR, et al. Spatial and temporal features of the growth of a bacterial species colonizing the zebrafish gut. MBio. 2014 doi: 10.1128/mBio.01751-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons SM, Kearney SM, Smillie CS, Alm EJ. Two dynamic regimes in the human gut microbiome. PLoS Comput Biol. 2017;13:e1005364. doi: 10.1371/journal.pcbi.1005364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause MS, Madden LV, Hoitink HAJ. Effect of potting mix microbial carrying capacity on biological control of rhizoctonia damping-off of radish and rhizoctonia crown and root rot of poinsettia. Phytopathology®. 2001;91:1116–1123. doi: 10.1094/PHYTO.2001.91.11.1116. [DOI] [PubMed] [Google Scholar]
- 27.Nelson EB, Hoitkin HAJ. Factors affecting suppression of Rhizoctonia solani in container media. Phytopathology. 1982;72(3):275–279. [Google Scholar]
- 28.Mallon CA, Roux XL, van Doorn GS, et al. The impact of failure: unsuccessful bacterial invasions steer the soil microbial community away from the invader’s niche. ISME J. 2018;12:728–741. doi: 10.1038/s41396-017-0003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peay KG, Garbelotto M, Bruns TD. Evidence of dispersal limitation in soil microorganisms: isolation reduces species richness on mycorrhizal tree islands. Ecology. 2010;91:3631–3640. doi: 10.1890/09-2237.1. [DOI] [PubMed] [Google Scholar]
- 30.Hurtt GC, Pacala SW. The Consequences of recruitment limitation: reconciling chance, history and competitive differences between plants. J Theor Biol. 1995;176(1):1–12. doi: 10.1006/jtbi.1995.0170. [DOI] [Google Scholar]
- 31.Dumbrell AJ, Nelson M, Helgason T, et al. Idiosyncrasy and overdominance in the structure of natural communities of arbuscular mycorrhizal fungi: is there a role for stochastic processes? J Ecol. 2010;98:419–428. doi: 10.1111/j.1365-2745.2009.01622.x. [DOI] [Google Scholar]
- 32.Del Monte-Luna P, Brook BW, Zetina-Rejón MJ, Cruz-Escalona VH. The carrying capacity of ecosystems: Carrying capacity of ecosystems. Glob Ecol Biogeogr. 2004;13:485–495. doi: 10.1111/j.1466-822X.2004.00131.x. [DOI] [Google Scholar]
- 33.Egidi E, Delgado-Baquerizo M, Plett JM, et al. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat Commun. 2019;10:2369. doi: 10.1038/s41467-019-10373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang B, Kula A, Mack KML, et al. Carrying capacity in a heterogeneous environment with habitat connectivity. Ecol Lett. 2017;20:1118–1128. doi: 10.1111/ele.12807. [DOI] [PubMed] [Google Scholar]
- 35.Qiao Y, Wang J, Liang G, et al. Global variation of soil microbial carbon-use efficiency in relation to growth temperature and substrate supply. Sci Rep. 2019;9:5621. doi: 10.1038/s41598-019-42145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manzoni S, Schimel JP, Porporato A. Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology. 2012;93:930–938. doi: 10.1890/11-0026.1. [DOI] [PubMed] [Google Scholar]
- 37.Holt RD. Population dynamics in two-patch environments: Some anomalous consequences of an optimal habitat distribution. Theor Popul Biol. 1985;28:181–208. doi: 10.1016/0040-5809(85)90027-9. [DOI] [Google Scholar]
- 38.Lou Y. On the effects of migration and spatial heterogeneity on single and multiple species. J Differ Equ. 2006;223:400–426. doi: 10.1016/j.jde.2005.05.010. [DOI] [Google Scholar]
- 39.Maynard DS, Crowther TW, Bradford MA. Fungal interactions reduce carbon use efficiency. Ecol Lett. 2017;20:1034–1042. doi: 10.1111/ele.12801. [DOI] [PubMed] [Google Scholar]
- 40.Czaran TL, Hoekstra RF, Pagie L. Chemical warfare between microbes promotes biodiversity. Proc Natl Acad Sci. 2002;99:786–790. doi: 10.1073/pnas.012399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakavara A, Tsirtsis G, Roelke DL, et al. Lumpy species coexistence arises robustly in fluctuating resource environments. Proc Natl Acad Sci USA. 2018;115:738–743. doi: 10.1073/pnas.1705944115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell JR. Deterministic processes vary during community assembly for ecologically dissimilar taxa. Nat Commun. 2015;6(1):1–10. doi: 10.1038/ncomms9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kallenbach CM, Wallenstein MD, Schipanksi ME, Grandy AS. Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and a Path Forward. Front Microbiol. 2019;10:1146. doi: 10.3389/fmicb.2019.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutchinson GE. The paradox of the plankton. Am Nat. 1961;95:137–145. doi: 10.1086/282171. [DOI] [Google Scholar]
- 45.Sturm A, Dworkin J. Phenotypic diversity as a mechanism to exit cellular dormancy. Curr Biol. 2015;25:2272–2277. doi: 10.1016/j.cub.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dodds WK, Whiles MR. Freshwater ecology. Amsterdam: Elsevier; 2020. Nutrient use and remineralization; pp. 503–535. [Google Scholar]
- 47.Rineau F, Courty P-E. Secreted enzymatic activities of ectomycorrhizal fungi as a case study of functional diversity and functional redundancy. Ann For Sci. 2011;68:69–80. doi: 10.1007/s13595-010-0008-4. [DOI] [Google Scholar]
- 48.Louca S, Polz MF, Mazel F, et al. Function and functional redundancy in microbial systems. Nat Ecol Evol. 2018;2:936–943. doi: 10.1038/s41559-018-0519-1. [DOI] [PubMed] [Google Scholar]
- 49.Delgado-Baquerizo M, Giaramida L, Reich PB, et al. Lack of functional redundancy in the relationship between microbial diversity and ecosystem functioning. J Ecol. 2016;104:936–946. doi: 10.1111/1365-2745.12585. [DOI] [Google Scholar]
- 50.Allesina S, Levine JM. A competitive network theory of species diversity. Proc Natl Acad Sci. 2011;108:5638–5642. doi: 10.1073/pnas.1014428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang G, Jagadamma S, Mayes MA, et al. Microbial dormancy improves development and experimental validation of ecosystem model. ISME J. 2015;9:226–237. doi: 10.1038/ismej.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price PB, Sowers T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc Natl Acad Sci. 2004;101:4631–4636. doi: 10.1073/pnas.0400522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Domeignoz-Horta LA, Pold G, Liu X-JA, et al. Microbial diversity drives carbon use efficiency in a model soil. Nat Commun. 2020;11:3684. doi: 10.1038/s41467-020-17502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaiser C, Franklin O, Dieckmann U, Richter A. Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol Lett. 2014;17:680–690. doi: 10.1111/ele.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wintermute EH, Silver PA. Emergent cooperation in microbial metabolism. Mol Syst Biol. 2010;6:7. doi: 10.1038/msb.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niehaus L, Boland I, Liu M, et al. Microbial coexistence through chemical-mediated interactions. Nat Commun. 2019;10:2052. doi: 10.1038/s41467-019-10062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kästner M, Miltner A, Thiele-Bruhn S, Liang C. Microbial necromass in soils—linking microbes to soil processes and carbon turnover. Front Environ Sci. 2021;9:756378. doi: 10.3389/fenvs.2021.756378. [DOI] [Google Scholar]
- 58.Gallardo-Navarro ÓA, Santillán M. Three-way interactions in an artificial community of bacterial strains directly isolated from the environment and their effect on the system population dynamics. Front Microbiol. 2019 doi: 10.3389/fmicb.2019.02555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsu J-T, Chen C-Y, Young C-W, et al. Prevalence of sulfonamide-resistant bacteria, resistance genes and integron-associated horizontal gene transfer in natural water bodies and soils adjacent to a swine feedlot in northern Taiwan. J Hazard Mater. 2014;277:34–43. doi: 10.1016/j.jhazmat.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 60.Baquero F, Coque TM, Martínez J-L, et al. Gene transmission in the one health microbiosphere and the channels of antimicrobial resistance. Front Microbiol. 2019;10:1–14. doi: 10.3389/fmicb.2019.02892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ebrahimi A, Schwartzman J, Cordero OX. Cooperation and spatial self-organization determine rate and efficiency of particulate organic matter degradation in marine bacteria. Proc Natl Acad Sci USA. 2019;116:23309–23316. doi: 10.1073/pnas.1908512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.West SA, Buckling A. Cooperation, virulence and siderophore production in bacterial parasites. Proc R Soc Lond B. 2003;270:37–44. doi: 10.1098/rspb.2002.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaiser C, Franklin O, Richter A, Dieckmann U. Social dynamics within decomposer communities lead to nitrogen retention and organic matter build-up in soils. Nat Commun. 2015;6:8960. doi: 10.1038/ncomms9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steffan SA, Dharampal PS. Undead food-webs: Integrating microbes into the food-chain. Food Webs. 2019;18:e00111. doi: 10.1016/j.fooweb.2018.e00111. [DOI] [Google Scholar]
- 65.Frey SD. Protozoan grazing affects estimates of carbon utilization efficiency of the soil microbial community. Soil Biol. 2001 doi: 10.1016/S0038-0717(01)00101-8. [DOI] [Google Scholar]
- 66.Eisenhauer N, Schlaghamerský J, Reich PB, Frelich LE. The wave towards a new steady state: effects of earthworm invasion on soil microbial functions. Biol Invasions. 2011;13:2191–2196. doi: 10.1007/s10530-011-0053-4. [DOI] [Google Scholar]
- 67.Crowther TW, Boddy L, Jones TH. Outcomes of fungal interactions are determined by soil invertebrate grazers: grazers alter fungal community. Ecol Lett. 2011;14:1134–1142. doi: 10.1111/j.1461-0248.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- 68.Livingston G, Fukumori K, Provete DB, et al. Predators regulate prey species sorting and spatial distribution in microbial landscapes. J Anim Ecol. 2017;86:501–510. doi: 10.1111/1365-2656.12639. [DOI] [PubMed] [Google Scholar]
- 69.Anthony MA, Crowther TW, Maynard DS, et al. Distinct assembly processes and microbial communities constrain soil organic carbon formation. One Earth. 2020;2:349–360. doi: 10.1016/j.oneear.2020.03.006. [DOI] [Google Scholar]
- 70.Bitas V, Kim H-S, Bennett JW, Kang S. Sniffing on microbes: diverse roles of microbial volatile organic compounds in plant health. MPMI. 2013;26:835–843. doi: 10.1094/MPMI-10-12-0249-CR. [DOI] [PubMed] [Google Scholar]
- 71.Newell K. Interaction between two decomposer basidiomycetes and a collembolan under Sitka spruce: distribution, abundance and selective grazing. Soil Biol Biochem. 1984;16:227–233. doi: 10.1016/0038-0717(84)90006-3. [DOI] [Google Scholar]
- 72.de Vries FT, Wallenstein MD. Below-ground connections underlying above-ground food production: a framework for optimising ecological connections in the rhizosphere. J Ecol. 2017;105:913–920. doi: 10.1111/1365-2745.12783. [DOI] [Google Scholar]
- 73.Hartmann A, Schmid M, van Tuinen D, Berg G. Plant-driven selection of microbes. Plant Soil. 2009;321:235–257. doi: 10.1007/s11104-008-9814-y. [DOI] [Google Scholar]
- 74.Alvarez R, Steinbach HS, De Paepe JL. Cover crop effects on soils and subsequent crops in the pampas: a meta-analysis. Soil Tillage Res. 2017;170:53–65. doi: 10.1016/j.still.2017.03.005. [DOI] [Google Scholar]
- 75.Manzoni S, Taylor P, Richter A, et al. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils: research review. New Phytol. 2018;196:79–91. doi: 10.1111/j.1469-8137.2012.04225.x. [DOI] [PubMed] [Google Scholar]
- 76.Meier IC, Finzi AC, Phillips RP. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol Biochem. 2017;106:119–128. doi: 10.1016/j.soilbio.2016.12.004. [DOI] [Google Scholar]
- 77.Anderson T-H, Domsch KH. Soil microbial biomass: The eco-physiological approach. Soil Biol Biochem. 2010;42:2039–2043. doi: 10.1016/j.soilbio.2010.06.026. [DOI] [Google Scholar]
- 78.Chen X, Xia Y, Rui Y, et al. Microbial carbon use efficiency, biomass turnover, and necromass accumulation in paddy soil depending on fertilization. Agr Ecosyst Environ. 2020;292:0167–8809. doi: 10.1016/j.agee.2020.106816. [DOI] [Google Scholar]
- 79.Moreau D, Pivato B, Bru D, et al. Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology. 2015;96:2300–2310. doi: 10.1890/14-1761.1. [DOI] [PubMed] [Google Scholar]
- 80.Chen B, Liu E, Tian Q, et al. Soil nitrogen dynamics and crop residues. Rev Agron Sustain Dev. 2014;34:429–442. doi: 10.1007/s13593-014-0207-8. [DOI] [Google Scholar]
- 81.Zhalnina K, Louie KB, Hao Z, et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol. 2018;3:470–480. doi: 10.1038/s41564-018-0129-3. [DOI] [PubMed] [Google Scholar]
- 82.Shi S, Nuccio E, Herman DJ, et al. Successional trajectories of rhizosphere bacterial communities over consecutive seasons. MBio. 2015;6:e00746–e815. doi: 10.1128/mBio.00746-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rolfe SA, Griffiths J, Ton J. Crying out for help with root exudates: adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr Opin Microbiol. 2019;49:73–82. doi: 10.1016/j.mib.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Watson BS, Bedair MF, Urbanczyk-Wochniak E, et al. Integrated metabolomics and transcriptomics reveal enhanced specialized metabolism in Medicago truncatula root border cells. Plant Physiol. 2015;167:1699–1716. doi: 10.1104/pp.114.253054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zanne AE, Abarenkov K, Afkhami ME, et al. Fungal functional ecology: bringing a trait-based approach to plant-associated fungi. Biol Rev. 2020;95:409–433. doi: 10.1111/brv.12570. [DOI] [PubMed] [Google Scholar]
- 86.Cantor A, Hale A, Aaron J, et al. Low allelochemical concentrations detected in garlic mustard-invaded forest soils inhibit fungal growth and AMF spore germination. Biol Invasions. 2011;13:3015–3025. doi: 10.1007/s10530-011-9986-x. [DOI] [Google Scholar]
- 87.Wolfe BE, Rodgers VL, Stinson KA, Pringle A. The invasive plant Alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range. J Ecol. 2008;96:777–783. doi: 10.1111/j.1365-2745.2008.01389.x. [DOI] [Google Scholar]
- 88.Anthony MA, Celenza JL, Armstrong A, Frey SD. Indolic glucosinolate pathway provides resistance to mycorrhizal fungal colonization in a non-host Brassicaceae. Ecosphere. 2020;11:e03100. doi: 10.1002/ecs2.3100. [DOI] [Google Scholar]
- 89.Hagenbo A, Hadden D, Clemmensen KE, et al. Carbon use efficiency of mycorrhizal fungal mycelium increases during the growing season but decreases with forest age across a Pinus sylvestris chronosequence. J Ecol. 2019;107:2808–2822. doi: 10.1111/1365-2745.13209. [DOI] [Google Scholar]
- 90.Delaux P-M, Varala K, Edger PP, et al. Comparative phylogenomics uncovers the impact of symbiotic associations on host genome evolution. PLoS Genet. 2014;10:e1004487. doi: 10.1371/journal.pgen.1004487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kobae Y, Kameoka H, Sugimura Y, et al. Strigolactone biosynthesis genes of rice are required for the punctual entry of arbuscular mycorrhizal fungi into the roots. Plant Cell Physiol. 2018;59:544–553. doi: 10.1093/pcp/pcy001. [DOI] [PubMed] [Google Scholar]
- 92.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 93.Sugiura Y, Akiyama R, Tanaka S, et al. Myristate as a carbon and energy source for the asymbiotic growth of the arbuscular mycorrhizal fungus Rhizophagus irregularis. Microbiology. 2019 doi: 10.1073/pnas.2006948117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rillig MC, Aguilar-Trigueros CA, Anderson IC, et al. Myristate and the ecology of AM fungi: significance, opportunities, applications and challenges. New Phytol. 2020 doi: 10.1111/nph.16527. [DOI] [PubMed] [Google Scholar]
- 95.Grube M, Berg G. Microbial consortia of bacteria and fungi with focus on the lichen symbiosis. Fungal Biol Rev. 2009;23:72–85. doi: 10.1016/j.fbr.2009.10.001. [DOI] [Google Scholar]
- 96.Grimm M, Grube M, Schiefelbein U, et al. The Lichens’ microbiota, still a mystery? Front Microbiol. 2021;12:623839. doi: 10.3389/fmicb.2021.623839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goga M, Elečko J, Marcinčinová M, et al. Lichen metabolites: an overview of some secondary metabolites and their biological potential. In: Mérillon J-M, Ramawat KG, et al., editors. Co-evolution of secondary metabolites. Cham: Springer International Publishing; 2020. pp. 175–209. [Google Scholar]
- 98.Wolf AB, Vos M, de Boer W, Kowalchuk GA. Impact of matric potential and pore size distribution on growth dynamics of filamentous and non-filamentous soil bacteria. PLoS ONE. 2013;8:e83661. doi: 10.1371/journal.pone.0083661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kravchenko AN, Guber AK, Razavi BS, et al. Microbial spatial footprint as a driver of soil carbon stabilization. Nat Commun. 2019;10:3121. doi: 10.1038/s41467-019-11057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keiluweit M, Wanzek T, Kleber M, et al. Anaerobic microsites have an unaccounted role in soil carbon stabilization. Nat Commun. 2017;8:1771. doi: 10.1038/s41467-017-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heijnen CE, Veen JA. A determination of protective microhabitats for bacteria introduced into soil. FEMS Microbiol Lett. 1991;85:73–80. doi: 10.1111/j.1574-6968.1991.tb04699.x. [DOI] [Google Scholar]
- 102.Ritz K, Young IM. Interactions between soil structure and fungi. Mycologist. 2004;18:52–59. doi: 10.1017/S0269915X04002010. [DOI] [Google Scholar]
- 103.Six J, Frey SD, Thiet RK, Batten KM. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J. 2006;70:555–569. doi: 10.2136/sssaj2004.0347. [DOI] [Google Scholar]
- 104.Cai P, Sun X, Wu Y, et al. Soil biofilms: microbial interactions, challenges, and advanced techniques for ex-situ characterization. Soil Ecol Lett. 2019;1:85–93. doi: 10.1007/s42832-019-0017-7. [DOI] [Google Scholar]
- 105.Zengler K, Zaramela LS. The social network of microorganisms —how auxotrophies shape complex communities. Nat Rev Microbiol. 2018;16:383–390. doi: 10.1038/s41579-018-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee KWK, Periasamy S, Mukherjee M, et al. Biofilm development and enhanced stress resistance of a model, mixed-species community biofilm. ISME J. 2014;8:894–907. doi: 10.1038/ismej.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Russel J, Røder HL, Madsen JS, et al. Antagonism correlates with metabolic similarity in diverse bacteria. Proc Natl Acad Sci USA. 2017;114:10684–10688. doi: 10.1073/pnas.1706016114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Powell JR, Bennett AE. Unpredictable assembly of arbuscular mycorrhizal fungal communities. Pedobiologia. 2016;59:11–15. doi: 10.1016/j.pedobi.2015.12.001. [DOI] [Google Scholar]
- 109.Geyer KM, Kyker-Snowman E, Grandy AS, Frey SD. Microbial carbon use efficiency: accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry. 2016;127:173–188. doi: 10.1007/s10533-016-0191-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.



