Abstract
Purpose of Review
The purpose of this review is to discuss the molecular mechanisms involved in osteocyte dendrite formation, summarize the similarities between osteocytic and neuronal projections, and highlight the importance of osteocyte dendrite maintenance in human skeletal disease.
Recent Findings
It is suggested that there is a causal relationship between the loss of osteocyte dendrites and the increased osteocyte apoptosis during conditions including aging, microdamage, and skeletal disease. A few mechanisms are proposed to control dendrite formation and outgrowth, such as via the regulation of actin polymerization dynamics.
Summary
This review addresses the impact of osteocyte dendrites in bone health and disease. Recent advances in multi-omics, in vivo and in vitro models, and microscopy-based imaging have provided novel approaches to reveal the underlying mechanisms that regulate dendrite development. Future therapeutic approaches are needed to target the process of osteocyte dendrite formation.
Keywords: Dendrite formation, Osteocyte maturation, Skeletal disease, Osteocyte-neuron similarity
Introduction
Bone-forming osteoblasts can undergo one of at least three fates: death by apoptosis, formation of bone lining cells, and differentiation into osteocytes. Osteocytes are the most abundant and longest-lived cells in bone. Surrounded by mineralized matrix, osteocytes possess an elaborate network of dendrite-like connections that are used for mechanosensing and inter-cellular communication. The mechanisms of how some osteoblasts differentiate into osteocytes remain incompletely understood. Osteoblasts that will become osteocytes are first surrounded by the unmineralized collagenous matrix they have produced (osteoid); next, developing osteocytes initiate dendrite formation prior to matrix mineralization [1, 2]. Following the deposition of calcium and phosphate along collagen fibrils, mature osteocytes are eventually formed by the integration of new dendrites into the existing osteocyte dendrite network. Osteoblasts are cuboidal cells with abundant rough endoplasmic reticulum (ER); in contrast, osteocytes possess cigar-shaped nuclei, scant ER, and large numbers of long, branching dendrites. In addition to the dramatic cellular morphological transition associated with osteocyte maturation, osteocytes acquire distinct and novel functions to control bone strength compared to osteoblasts, including (1) regulating bone remodeling by producing paracrine-acting factors, (2) mechanosensing, and (3) maintaining mineral homeostasis [2–5]. While the characteristics and functions of the lacunar-canalicular network (LCN) have been recently reviewed [6, 7•], the specific role of dendrites and dendrite-dendrite connectivity has received less attention. In this review, we will summarize emerging knowledge on the functions of osteocyte dendrites, the pathways that control their development and maintenance, and the implications of dendritic functions in pathological conditions and human skeletal disease.
Dendrite Morphology and Microstructure
Osteocytes are the only cells whose shape is preserved in fossils through lacunae and canaliculi. Pawlicki first reported the morphology of osteocytes and their dendritic processes in Late Cretaceous dinosaur bones [8]. The osteocyte cell body is enclosed within the lacuna and the osteocyte dendrites pass through the matrix through channels called canaliculi [7•, 9]. Osteocyte dendrites make direct contacts and exhibit periodic, fibrous connections to canalicular walls through so-called tethers or dendritic spines [10]. The heparan sulfate proteoglycan protein perlecan was reported as the major component of the “tethering” structure and is essential for the integrity of the osteocyte lacunar-canalicular network (LCN) [11, 12]. In addition to “tethers,” the “collagen hillock” is another collagen matrix projection structure that directly links the extracellular matrix (ECM) to osteocyte dendrites [13]. Integrin-mediated focal adhesions (FAs) form focal attachments and connect the ECM to cell membranes within “collagen hillocks” and serve the important role of delivering external signals to the cytoskeleton [13, 14].
Osteocyte Dendrites and Their Functions
The overall purpose of this review is to define the functions of osteocyte dendrites and discuss pathways responsible for their development and maintenance. Prior to discussing recent advances in osteocyte dendrite development, first we will briefly review the functions of osteocyte projections (Fig. 1): (1) cell-intrinsic roles in mechano-transduction, (2) homotypic communication with other osteocytes, and (3) heterotypic communication with other cells in the local bone microenvironment. After establishing these functions of osteocyte dendrites, we then will highlight strategies used by osteoblasts to promote the formation of these projections and their subsequent long-term maintenance.
Fig. 1.
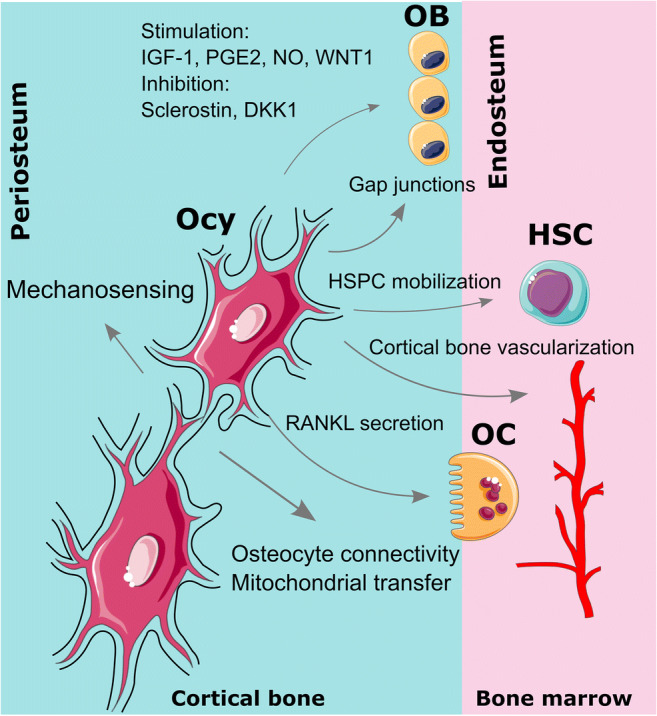
Schematic of osteocyte dendrite function. Osteocytes are the major cell type that responds to mechanical stimulation and transduces the mechanical cues into biochemical signals. Dendrites are more likely to be involved in mechano-transduction compared to osteocyte cell bodies. Osteocyte dendrites are required to maintain the lacunar-canalicular network during bone remodeling, aging, and microdamage. In addition to the osteocyte-osteocyte connectivity, osteocyte processes can regulate osteoblasts via direct contact of gap junctions, stimulate osteoclasts by triggering RANKL expression in dendrites, and control hematopoietic stem/progenitor cell (HSPC) mobilization. Ocy: osteocyte; OB: osteoblast; OC: osteoclast; HSC: hematopoietic stem cell. Part of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
Mechanosensing
Osteocytes have been suggested to be the major cell type that responds to mechanical stimulation and transduces the mechanical cues to the skeleton into biochemical signals [15]. The role of osteocytes in mechano-transduction was reviewed previously [7•]; we will elaborate on how osteocyte dendrites are involved in the process. Dendrites are more likely to be involved in mechano-transduction compared to osteocyte cell bodies [16]. Burra et al. generated a transwell filter system to culture osteocytic MLO-Y4 cells, on which osteocyte cell bodies and dendrites are differentiated. The disruption of the glycocalyx on the dendrite alone is able to decrease the dendrite’s ability to open hemichannels (see below) and transmit signals to the cell body [16]. The Schaffler laboratory utilized an elegant local fluid flow stimulation approach to investigate the mechano-transducing role of dendrites and the relationship between dendrites and cell bodies in response to mechanical cues [17, 18]. Osteocytes utilize dendritic processes to receive mechanical signals, transduce these signals through major cytoskeletal components, and regulate the expression of downstream target genes.
Integrins connect the ECM to the cytoskeleton via focal adhesion (FA)–associated proteins. Integrin β3 associates with αV and is expressed in osteocyte dendrites [13, 18]. One study examined the role of integrin β3 during early corticalization and integrin β3-null mice have significantly reduced femoral length and decreased cortical thickness [19]. Chromatin immunoprecipitation (ChIP) assay performed in the osteoblastic MC3T3-E1 cells showed that the transcription factor Sp7 (see below) binds to the promoter of the Itgb3 gene (encodes integrin β3) [19]. These findings suggest that integrin β3 acts downstream of Sp7 in regulation of corticalization for longitudinal bone growth. Since both Sp7 and integrin β3 are expressed in osteocytes, more studies are needed to explore the specific role of Sp7/Itgb3 axis in osteocyte differentiation and mechanosensing.
Gap junctions (GJs) are transmembrane channels formed on the surfaces of adjacent cells [20]. Cx43 is the most highly expressed GJs in bone. Work from the Plotkin and Bellido laboratories showed that mice lacking Cx43 in osteoblasts and osteocytes (Oc-Cre; Cx43 fl/−) had increased osteocytic apoptosis in cortical bone [21]. TEM images taken from the femoral midshaft of mutant mice revealed an increased number of empty lacunae and loss of dendrites in apoptotic osteocytes. In contrast, overexpression of Cx43 in osteocytes attenuated cortical bone changes during aging by preserving osteocyte viability and eventually increasing resistance to damage [22]. MLO-Y4 cells exposed to fluid flow shear stress (FFSS) increased the length of the osteocyte dendrites with redistribution of Cx43 from nucleus proximal to punctate spots in osteocyte dendrites [23]. Together, these observations suggest that Cx43 regulates osteocyte apoptosis probably via the interconnected structure of dendrites and plays an important role in bone homeostasis in response to mechanical stimulation.
The osteocyte gene Pdpn, which encodes E11, is increased by FFSS in vivo and in vitro, and contributes to dynamic changes in dendrite elongation seen upon mechanical stimulation [24]. The E11 glycoprotein is highly expressed in the dendrites of osteoid osteocytes and regulates dendrite initiation and elongation [25]. One recent immunocytochemical examination of the early osteoblast-to-osteocyte transition suggested that the interaction between E11 and CD44 on the cell surface, followed by ezrin phosphorylation and actin filament reorganization, may be involved in the osteoblast differentiation to osteocytes during bone remodeling [26].
Maintaining Osteocyte Connectivity During Aging
Optimal osteocyte connectivity is thought to be needed for osteocytes to survive for long periods of time (years in the case of human osteocytes) embedded deep in mineralized bone tissue. Several studies have shown that there is a loss of osteocyte dendrites with aging. Significantly reduced dendrite number was identified in both aged human (females) and mice [27, 28]. Recent studies from the Dallas laboratory also revealed reduced canalicular and dendrite numbers in aging C57BL/6 mice [29]. There are many canaliculi that are not occupied by dendrites in aged mice, so-called empty canaliculi. Moreover, the tethering elements that connect osteocyte dendrites and canalicular walls are reduced in aged animals compared to young mice. While the molecular mechanisms used by osteocyte dendrites to protect cells from metabolic insults remain incompletely understood, it is suggested that autophagy plays an important role. Suppression of autophagy in osteocytes (Dmp1-Cre; Atg7 f/f, Atg7 encodes an E1-like activating enzyme) caused low bone mass [30]. Deletion of Atg7 with Osx1-Cre leads to reduced osteocyte dendrites [31]. Perhaps reduced dendrite number in Osx1-Cre; Atg7 f/f mice further inhibits osteocyte maturation by disrupting the osteocyte-osteocyte connectivity, which suggests the importance of early cell dendrite initiation and embedding.
Mitochondrial Transfer
As the major energy source for eukaryotic cells, mitochondria play a critical role in maintaining tissue homeostasis. At present, the relative role of ATP generation via glycolysis versus oxidative phosphorylation in osteocytes remains an interesting and open question [32], especially since osteocytes reside in a relatively hypoxic environment. Osteocytes may use dendritic projections to exchange mitochondria and thus preserve metabolic capacity. In addition to intra-cellular movement, inter-cellular mitochondrial transfer was first revealed between mesenchymal stem cells (MSCs) to somatic cells with mitochondrial dysfunction [33]. Both mouse primary osteocytes and MLO-Y4 cells transfer mitochondria to adjacent stressed osteocytes. This transfer takes place within osteocyte dendrites and relies on the contact between endoplasmic reticulum (ER) and mitochondria [34••]. With aging, the distribution of mitochondria in dendrites significantly reduces. Therefore, inter-cellular mitochondrial transport is required for the maintenance of the osteocyte dendritic network in aging. Further study of this interesting aspect of osteocyte biology will require identification of factors used by osteocytes to transfer mitochondria across cells.
Regulation of Osteoblasts and Osteo-Progenitor Cells
Several paracrine signals released by osteocytes have regulatory effects on osteoblast activities (reviewed in [5]). The osteocyte-produced paracrine factor sclerostin may also regulate osteoblast progenitors [35] and osteoclasts (reviewed in [4], and see below). In addition to regulating osteoblast activities by secreted factors, osteocytes can directly regulate osteoblasts via gap junctions. In vitro co-culture of osteoblasts and osteocytes showed that under mechanical stimulation, osteocytes communicate with endosteal osteoblasts through dendritic processes and regulate the function of osteoblasts via gap junctions [36]. Osteocyte dendrites are also involved in the regulation of hematopoietic stem/progenitor cell (HSPC) mobilization [37]. Both “osteocyte-less (OL) mice” and klotho hypomorphic (kl/kl) mice models failed to induce HSPC mobilization by granulocyte colony-stimulating factor (G-CSF) when osteocytic dendrites and canaliculi were disrupted.
Regulation of Osteoclasts
One in vitro and two in vivo studies showed that osteocytes are a major source of RANKL during normal bone remodeling [38–40]. Dendrites are likely important for osteocytes to transmit signals to osteoclasts. The subcellular trafficking of RANKL may be mediated by OPG in vitro that interacts with newly synthesized RANKL in lysosomes and then is transmitted to osteoclasts via osteocyte dendrites [41]. In aged female mice, loss of osteocyte dendrites precedes reduced osteocyte numbers and increases in osteoclasts [29]. Another study showed that apoptotic osteocytes in damaged bone regions signal neighboring, healthy osteocytes to secrete RANKL [42, 43]. In summary, under pathological conditions (e.g., aging and microdamage), dendrite defects may cause decreased osteocyte cell viability and increased apoptosis. The release of RANKL triggered by apoptotic osteocytes controls osteoclast localization, increases osteoclast activity, and leads to elevated bone resorption [44, 45]. The Gunzer laboratory recently reported direct physical contacts between osteocyte dendrites, endothelial cells via trans-cortical vessels (TCVs) that traverse cortical bone in a perpendicular orientation, and osteoclasts [46•]. This association between osteocyte dendrites and TCV-associated osteoclasts may induce osteoclast-mediated bone resorption through RANKL signaling and trigger TCV remodeling. This suggests a potential role of osteocyte dendrites in regulating cortical bone vascularization.
Communication Between Osteocytes and Cancer Cells
Several cancers originate from bone, including osteosarcomas (osteoblast lineage) and myeloma (bone marrow), while others that arise from other sites metastasize to bone. Here we will discuss relationships between cancer cells and the osteocyte network. The osteocyte dendrite network is affected in the cancerous microenvironment [47, 48]. A stochastic agent-based model proposed by the Basanta Group predicted the implications of cancer cells to the osteocyte network [49]. The results showed that cancerous microenvironment can either stimulate or inhibit osteocyte dendrite growth. Direct intratibial injection of breast and prostate cancer cell lines led to direct contact between tumor cells and osteocytes [50]. The lacunar-canalicular network is impaired near osteosclerotic lesions, suggesting that bone metastasis can affect bone mechanosensitivity. In a mouse model of multiple myeloma (MM), direct contact between osteocyte dendrites and MM cells and up-regulated Sost expression was observed [51]. MM cells cause osteocyte apoptosis via activation of Notch signaling, and osteocytes enhance MM proliferation also via the Notch pathway [51]. Osteocyte conditioned medium stimulates the proliferation and invasion of several cancer cell lines (breast and prostate) [52]. On the other hand, mechanical loading modulates the effect of breast cancer cells on osteocyte mechanosensing by increasing the number of dendrites per osteocyte and the level of E11 [53]. The gap junction protein Cx43 forms a critical structure to inter-connect dendrites that participates in osteocyte/breast cancer communication [54]. It is likely that these studies represent just the “tip of the iceberg” with respect to how cancers in bone interact with osteocyte projections and the osteocyte network.
Connections Between Osteocytic and Neuronal Projections
The total length of human osteocyte dendrites falls within the same range as the total length of nerve fibers in the human brain [9]. Osteocyte dendrites emanate from osteocyte cell bodies and establish a highly interconnected network. This complex communication network of osteocytes resembles the network of neurons in the brain [9]. We and others have demonstrated the similarities between osteocytic and neuronal transcriptional programs [55, 56••, 57••]. We used lineage-specific Dmp1-Cre transgenic mice crossed to tdTomato reporter mice and developed a digestion protocol to liberate osteo-lineage cells from bone matrix for single-cell RNA-seq. Gene Ontology analysis of the top markers identified from the mature osteocyte cluster revealed enrichment with “neuronal” terms such as cell projection organization and neuron differentiation. To further explore potential similarity between osteocytes and neurons at the transcriptomic level, we performed enrichment analysis of top mature osteocyte markers across cell types in mouse brain [58]. Osteocyte, but not osteoblast, marker genes are significantly enriched in their relative mean expression values in neurons versus other cell types in mouse brain. This finding suggests that osteocytes and neurons share developmental programs and signaling pathways.
One fundamental cell biology mechanism shared by both osteocytes and neurons involves development of elaborate cell projections. Our recent work demonstrated that the transcription factor Sp7 plays a crucial role in osteocyte dendrite formation. Sp7 is required for osteoblast lineage commitment [59]; however, its role in osteocyte development was poorly studied other than the observation that postnatal Sp7 ablation led to severe osteocyte morphology defects [60]. Therefore, we studied the role of Sp7 in mature osteoblasts and their descendants by deleting this gene using Dmp1-Cre. Surprisingly, late-stage Sp7 deletion led to osteocytes with nearly absent dendrite, increased apoptosis, and cortical porosity. These results prompted us to determine the cell-specific role of Sp7 in osteocyte dendrite development. We performed RNA-seq and ChIP-seq to determine osteocyte-specific Sp7 target genes, and identified a small group of neuronal-related genes including osteocrin. Osteocrin (encodes by the Ostn gene) promotes osteocyte dendrite formation downstream of Sp7 [57••]. Ostn overexpression rescues dendrite defects caused by Sp7 deficiency both in vitro and in vivo. Ostn was initially identified based on its expression in osteoblasts and early embedding osteocytes [61, 62]. OSTN mRNA is induced in primate (but not rodent) neocortical excitatory neurons upon depolarization. In primate neurons, OSTN regulates dendritic branch number and complexity, which suggests that OSTN inhibits neuron dendritic growth in response to excessive membrane depolarization [63].
Osteocytes and neurons may also use other common mechanisms to regulate cell survival and/or apoptosis. For example, Semaphorin 3A (Sema3A) is a secreted factor that suppresses axon growth and promotes neuronal dendrite formation via cGMP signaling (interestingly, osteocrin also potentiates cGMP signaling) [64]. Sema3A functions in bone by inhibiting bone resorption and increasing bone formation [65•]. Both postnatal global deletion of Sema3A and conditional deletion in osteoblasts and osteocytes result in osteoporotic phenotypes including reduced osteocyte number [66]. Sema3A activates the soluble guanylate cyclase (sGC)-cGMP signaling to promote osteocyte survival. At present, whether Sema3A signaling in osteocytes controls cellular morphogenesis or regulates dendrite maintenance remains unknown.
Shared signaling pathways could also contribute to the transcriptomic, morphologic, and functional similarities between osteocytes and neurons. Many studies reported that the ERK signaling pathway regulates neuron neurite outgrowth, number, and dendrite branching [67]. Using MLO-Y4 cells, Kyono et al. demonstrated that Fgf2 regulates osteocyte differentiation via an ERK/MAPK-dependent manner. Osteocytes in Prx1-Cre; ERK1 −/−; ERK2 fl/fl mice have very low Dmp1 expression and lack dendrites, indicating that the inactivation of ERK signaling pathways disrupts osteocyte maturation and dendrite formation [68]. Further in vitro studies showed that ERK activation regulates E11 expression downstream of Fgf2 [69]. Consistent with this notion, our work also demonstrated that osteocrin potentiates C-type natriuretic peptide (CNP) signaling in vitro by enhancing downstream ERK1/2 phosphorylation [57••].
Subcellular RNA localization is relatively well-studied in neurons where many RNAs are actively transported to neuronal projections for local protein translation [70, 71]. Active transport of RNA takes place along cytoskeletal scaffolds [72]. Subcellular RNA trafficking is often regulated by RNA regulatory elements (often located in the 3′ untranslated region of mRNAs) and RNA-binding proteins (RBPs) [73]. In neurons, RNA localization and local translation enhance signal transmission; defects in mRNA trafficking are linked to intellectual disability in patients with fragile X syndrome [74]. Testing whether mRNA localization also occurs in osteocyte dendrites and whether this mechanism is regulated by similar regulatory elements and RBPs identified in neurons may be an interesting future direction.
Current Progress on Osteocyte Dendrite Formation
While many studies reported the diverse functions of osteocyte dendrites, little is known about the mechanisms that regulate dendrite formation and osteocyte maturation. In this section, we will summarize genes and signaling pathways identified and involved in osteocyte dendrite formation (Fig. 2).
Fig. 2.
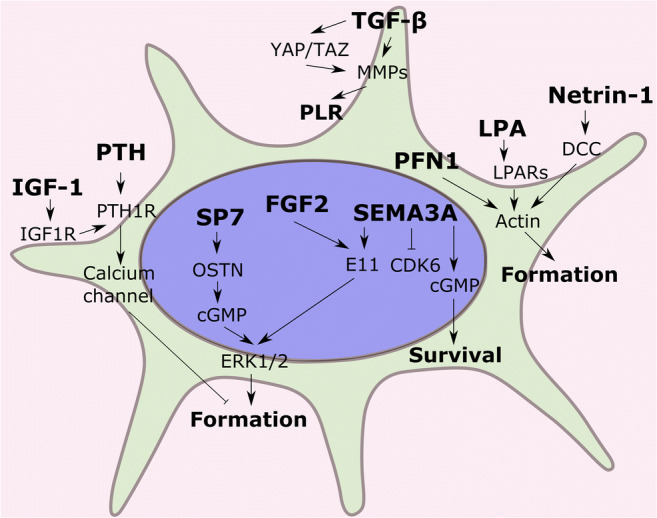
Genes and signaling pathways that are involved in osteocyte dendrite development. Several mechanisms are proposed: (1) genes (SP7, FGF2, SEMA3A) regulate osteocyte dendrite formation via cGMP levels and ERK signaling; (2) factors including lysophosphatidic acid (LPA), netrin-1, profilin1 (PFN1), and PTH/IGF-1 mediate osteocyte projection outgrowth via the actin polymerization dynamics; (3) TGF-β and YAP/TAZ pathways are essential for the perilacunar/canalicular remodeling (PLR)
Lysophosphatidic Acid (LPA)
The lipid growth factor lysophosphatidic acid (LPA) can induce osteocyte dendrite outgrowth [75]. Transcriptomic and proteomic analyses performed in LPA-treated MLO-Y4 cells both showed that genes and/or proteins up-regulated by LPA treatment are linked to actin microfilament dynamics, protein distribution, and membrane outgrowth [76]. This suggests that osteocyte dendrite formation is a membrane- and cytoskeleton-driven process. This is consistent with LPA-related findings in the neurons. LPA can rearrange actin cytoskeleton and microtubule in neurons [77, 78]. More studies have demonstrated the ability of LPA to induce neurite retraction and neurite branching [79, 80]. Interestingly, local LPA generation in bone has been linked to FGF-23 production in the setting of acute kidney injury [81], highlighting potential roles for LPA signaling in several aspects of osteocyte biology.
Netrin-1
Netrins were first studied as guidance cues in neuron axon migrating [82]. Several studies indicate that netrin-1 regulates neuron axon growth through the DCC receptor [83, 84]. The Nakano laboratory recently developed a novel inkjet printing platform that contains a cross pattern of fibronectin and netrin-1 [85••]. By culturing primary osteocytes with the designed micropatterned substrates, elongation of osteocyte dendrites is selectively induced by netrin-1. This is an example of the molecular and functional similarity between osteocytic and neuronal dendrites. More in vivo studies are needed to fully understand how netrin-1/DCC signaling contributes to osteocyte dendrite elongation.
Profilin1
Profilin1 (encoded by the Pfn1 gene) is an actin-binding protein and is required in actin fiber polymerization [86]. Deletion of Pfn1 increases alkaline phosphatase activity and represses dendrite formation in MLO-Y4 cells [87]. Pfn1-deficient mice (Dmp1-Cre; Pfn1 fl/fl) show decreased bone mineral density (BMD) and reduced trabecular bone. It is intriguing to examine whether profilin1 regulates bone mass via modulating normal osteocyte dendrite formation in the future.
Sema3A/CDK6
Sema3A is another interesting factor that functions in osteocyte dendrite formation. As discussed above, Sema3A regulates neuron axon and dendrite growth [64]. The Yoda Group demonstrated that Sema3A promotes osteocyte dendrite elongation in vitro (MLO-Y4 cells) by down-regulation of CDK6 [88]. CDK6 is a G1 cell cycle kinase and plays an important role in tissue homeostasis and differentiation [89]. Though CDK6 down-regulation is essential for osteoblast differentiation [90], further studies are needed to elucidate the specific function of CDK6 in osteocyte maturation. Coordinating cell cycle exit may represent an important aspect of terminal osteoblast and osteocyte maturation.
Sp7/Ostn
Our recent work reported the key role of Sp7/Ostn axis in controlling osteocyte dendrite formation [57••]. Sp7 may cooperate with distinct binding factors to regulate cell type–specific gene expression in osteocytes. Future studies are needed to understand how Sp7 orchestrates distinct gene expression programs in different cell types in bone (hypertrophic chondrocytes, early osteoprogenitors, mature osteoblasts, and osteocytes). Careful analysis of human disease–associated SP7 mutations [91–97] may provide key insights into this important question.
PTH and IGF
It has long been understood that PTH (parathyroid hormone) can change the osteocyte cytoskeletal structure in vivo [98]. More recent work on PTH treatment of mature IDG-SW3 cell cultures alters the osteocyte projection morphology and increases osteocyte mobility [99]. Downstream analysis revealed that the change of dendrite phenotype responding to PTH is dependent on calcium signaling (increasing L-type calcium expression and decreasing T-type calcium expression). The L-type calcium channel has relatively higher expression level in osteoblasts compared to osteocytes, while the T-type calcium channel expression level is higher in osteocytes [100]. Blockage of L-type calcium channels can prevent the morphology and mobility changes caused by PTH. IGF-1 (insulin-like growth factor type 1) is expressed in osteoprogenitors, osteoblasts, and osteocytes [101]. One recent study revealed a novel PTH-IGF-1 interaction that regulates osteocyte dendrite outgrowth [102••]. IGF1R directly phosphorylates the PTH receptor (PTH1R) at tyrosine 494 in the receptor’s C-terminal tail. This specific phosphorylation targets PTH1R to the ends of actin filaments, simulates actin polymerization, and further increases osteocyte dendrite outgrowth. It remains unknown how the phosphorylated PTH1R migrates from cell membrane to the dendrite cytoskeleton.
TGF-ß and YAP/TAZ
Perilacunar/canalicular remodeling (PLR) is a homeostatic mechanism that maintains the lacunar-canalicular network (LCN) [103]. Many factors play the crucial role in PLR, including matrix metalloproteinases (e.g., Mmp13) and cathepsin K (Ctsk) [104, 105]. Mmp13-deficient mice (Mmp13−/−) exhibit collagen organization and mineralization defects, and a disrupted lacunar-canalicular network, indicating that Mmp13 is essential for osteocyte perilacunar remodeling [104]. The Alliston Group described the regulatory role of the TGF-β (transforming growth factor beta) signaling pathway in osteocyte PLR [106]. Pharmacologic inhibition of TGF-β in mice results in significant reduction of canalicular length and decreased expression of PLR enzymes (Mmps, Ctsk, and Acp5). Ablation of TGF-β receptor II in osteocytes (Dmp1-Cre; TβRII f/f) causes severe deterioration of the osteocyte canalicular network. TGF-β signaling interacts with YAP/TAZ signaling in many cell types [107, 108]. To examine whether YAP/TAZ regulates osteocyte-mediated bone remodeling and PLR, YAP/TAZ were conditionally deleted in osteocytes (Dmp1-Cre; YAP f/f; TAZ f/f). YAP/TAZ double knockout mice have increased number of empty lacunae, increased apoptotic osteocytes, and increased canalicular length compared to wild-type littermates [109•]. Further in vitro (IDG-SW3 cells) inhibition of YAP/TAZ transcriptional activity abrogates the expression of TGF-β-induced genes (Ctgf, Cyr61) and PLR-related enzymes, which suggests that YAP/TAZ may act downstream of TGF-β signaling to control perilacunar/canalicular remodeling.
Dendrites and Human Skeletal Disease
Though osteocytes are buried deeply in mineralized bone matrix, the LCN provides a structural foundation for these cells to communicate and connect. Patients with skeletal disease have disrupted bone remodeling and mineral homeostasis. The molecular mechanism of how osteocyte dendrites regulate bone development and remodeling under different bone diseases remains under-studied. We will summarize the effect and importance of osteocyte dendrites in skeletal disease in the following section (Table 1).
Table 1.
Major human skeletal disease with osteocyte dendrite defects
| Disease | Dendrite defects | References |
|---|---|---|
| Osteoporosis |
Decreased osteocyte connectivity, disrupted dendrite orientation, and higher dendrite tortuosity in osteoporosis patients Larger lacunar-canalicular porosity and increased effective canalicular size in ovariectomized (OVX, mimic postmenopausal osteoporosis) rats |
[112, 116] |
| Osteoarthritis (OA) |
Deformed osteocytes with fewer and disorganized dendrites in the subchondral bone of OA patients Decreased osteocyte viability and reduced dendrite connectivity in the femoral neck of OA patients |
[112, 119, 121] |
| Osteogenesis imperfecta (OI) |
Defective dendrites including reduced dendrite number and length in OI patients homologous of the SP7 R316C variant Defective dendritic processes in Bmp1 and Tll1 double knockout mice |
[57••, 128] |
| Glucocorticoids | Degeneration of the lacunar-canalicular network, including loss of dendrites and rearrangement of cytoskeleton during in vitro and ex vivo culture | [136] |
Osteoporosis
Osteoporosis is a common skeletal disease characterized by decreased bone mineral density (BMD) and increased fracture risk and affects more than 200 million people worldwide. Critical osteocyte-derived factors have been reported by GWAS to show strong genetic association to BMD and fracture risk, including RANKL and SOST [110, 111]. Osteoporosis patients have decreased osteocyte connectivity, disrupted dendrite orientation (not oriented in the direction of blood supply), and higher dendrite tortuosity when compared to healthy controls [112]. At present, the genetic basis underlying osteocyte dendrite defects in osteoporosis remains unknown. Since the “osteocyte transcriptome” is enriched in genes linked to BMD variation [56••, 57••, 113], it is possible that specific genetic variants predispose certain individuals to accelerated deterioration of the osteocyte network over time. Future study is needed to better define how osteocyte-expressed BMD-associated genes control cellular morphology and dendrite homeostasis in the setting of skeletal disease. As an aging-related disease, osteoporosis results in fragility fractures in both male and female populations [114]. The ovariectomized (OVX) rat is commonly used as the model for postmenopausal osteoporosis [115]. OVX rats (estrogen deficiency) have altered lacunar-canalicular microenvironment including the larger lacunar-canalicular porosity and increased effective canalicular size [116].
Osteoarthritis
Osteoarthritis (OA) is one of the most prevalent skeletal diseases and is characterized by articular cartilage degeneration, remodeling of the underlying bone, and inflammation of the synovium [117]. Subchondral bone (SCB) is considered the first region where the earliest change in osteoarthritic joints occurs and further triggers the degeneration of articular cartilage [118]. OA patients have deformed osteocytes in SCB with fewer and disorganized dendrites, disrupted sclerostin expression, and increased dentin matrix protein 1 expression [119, 120]. Another study also showed that the femoral neck of OA patients exhibits decreased osteocyte viability and reduced osteocytic dendrite connectivity [112]. As the factor regulating dendrite initiation and elongation [25], E11 is enriched in the sclerotic lesions of OA patients [121, 122]. Elevated E11 level in early osteocyte dendrite forming phase may inhibit bone resorption in OA femoral head bone. Future studies are needed to precisely define “cause and effect” regarding changes in osteocyte biology in subchondral bone in the setting of OA progression.
Osteogenesis Imperfecta
Osteogenesis imperfecta (OI) is a relatively common skeletal dysplasia, affecting between 15,000 and 20,000 patients in the USA [123]. Though the majority of OI cases are caused by variants in COL1A1 and COL1A2 genes, a large number of genes, including genes crucial for osteoblast and osteocyte function, have been identified to cause skeletal fragility and a phenotype similar to “classic” (collagen-mutated) OI [124, 125]. For example, rare SP7 mutations cause recessive forms of OI [94, 95]. Homozygous SP7 R316C patients are characterized by short stature, recurrent fractures, and high cortical porosity. Recent studies from our laboratory demonstrated reduced osteocyte dendrite length and number in homozygous SP7 R316C patients compared to age-matched controls [57••]. BMP1 is another candidate gene identified in recessive OI by exome sequencing [126, 127]. Postnatal deletion of Bmp1 and Tll1 (encodes Tolloid Like 1) in mice showed defective dendritic processes [128]. In vivo transcriptomic analysis performed in OI mouse models (CrtapKO and oim/oim) identified differentially expressed genes that are significantly enriched in “Cell Projection” and “Neuron Projection,” which suggests that the connectivity of osteocyte dendritic network may be affected in OI [129]. Taken together, these observations indicate that osteocyte dendrite defects may be a common feature across multiple forms of OI, and suggest that targeted interventions to restore dendrite viability may represent a novel treatment strategy for this serious and debilitating skeletal disease.
Glucocorticoids
Glucocorticoids (GCs) are widely used as anti-inflammatory drugs to treat inflammatory diseases including rheumatoid arthritis, multiple sclerosis, asthma, lupus, and inflammatory bowel disease [130]. However, long-term GC treatment is associated with skeletal side effects including bone loss, fracture, osteoporosis, and osteonecrosis [131]. Both in vivo and in vitro studies showed that excess GCs induce osteocytes apoptosis [132–135]. In glucocorticoid-treated mice, perilacunar remodeling is suppressed due to inhibition of matrix metalloprotease expression, which eventually causes degeneration of the osteocyte lacunar-canalicular network [135]. It is suggested that GCs regulate osteocytes in a dose-dependent manner: osteocytes undergo the autophagy pathway with lower GC dose, while high GC dose induces osteocyte apoptosis [134]. Go et al. reported that dexamethasone (Dex) inhibits Cx43 expression at sites of inter-cellular connections via dendrite tips [136]. Dex-administration during in vitro and ex vivo culture causes loss of osteocyte dendrites and cytoskeletal rearrangement due to autophagy-medicated Cx43 degradation [136]. Therefore, preserving or regenerating osteocytic dendrite network during GC excess may provide a novel direction in preventing GC-induced skeletal complications. Doing so will require a more detailed understanding of how glucocorticoids lead to osteocyte dendrite loss.
Gaps and Limitations in Studying Osteocyte Dendrites
Current methods to visualize osteocytes and their dendrites are somewhat challenging. Silver nitrate can be used to stain osteocyte cell bodies and processes dark brown [106, 137]. Conjugated forms of phalloidin, which stains filamentous actin, can be used to visualize the osteocyte cytoskeleton coursing throughout acellular bone matrix [138]. We and others have applied phalloidin staining in cultured osteocytes to visualize dendrite development and osteoblast-to-osteocyte differentiation [57••, 139, 140]. The Dallas Group has developed multiplexed confocal imaging methods for imaging different aspects of osteocytes (DAPI: nucleus; phalloidin: cytoskeleton; mineralized matrix: alizarin red; collagen: 2nd harmonic generation) combined with fluorescence-conjugated dextran dye that permeates the lacunar-canalicular system [141]. In addition, the Dallas laboratory has also applied live cell confocal imaging to visualize the dynamics of osteocyte embedding and dendrite formation in mice co-expressing GFPtopaz-tagged collagen and Dmp1-Cre/tdTomato [142]. The combination of confocal imaging, live imaging, and streamlined methods for image quantification and analysis will provide novel insights into the formation of osteocyte dendrites and the lacunar-canalicular system.
Conclusions
Osteocyte dendrites serve as functional structures that regulate osteocyte function and bone health. Evidence has suggested a causal relationship between the loss of osteocyte dendrites and the increased osteocyte apoptosis. Apoptotic osteocytes eventually trigger bone loss and bone fracture. An enhanced understanding of osteocyte dendrite development and maintenance will highlight new ways to regenerate these structures in skeletal disease. Novel therapeutic approaches are needed to target the processes of dendrite formation and maintenance. Osteocytes and neurons share similarities at the morphological, transcriptional, and functional levels. Osteocytes may repurpose neuronal molecular control pathways to regulate dendrite formation, cell survival, and mRNA transport to dendrites. Leveraging knowledge from neuroscience research is likely to accelerate understanding of how the osteocyte network forms and functions.
Acknowledgements
M.N.W. acknowledges funding support from the National Institute of Health (P01DK011794, R01DK116716). J.S.W. acknowledged funding support from the National Institute of Health (T32DK007028).
Compliance with Ethical Standards
Conflict of Interest
MNW receives research funding from Radius Health and holds equity in and is a scientific advisory board member of Relation Therapeutics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Osteocytes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dynam. 2005;235:176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- 2.Plotkin LI, Bellido T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat Rev Endocrinol. 2016;12:593–605. doi: 10.1038/nrendo.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... and more. Endocr Rev. 2013;34:658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. 2020;82:485–506. doi: 10.1146/annurev-physiol-021119-034332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaffler MB, Cheung W-Y, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2013;94:5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiede-Lewis LM, Dallas SL. Changes in the osteocyte lacunocanalicular network with aging. Bone. 2019;122:101–113. doi: 10.1016/j.bone.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.•.Qin L, Liu W, Cao H, Xiao G. Molecular mechanosensors in osteocytes. Bone Res. 2020;8:23. An up-to-date review on osteocytes as key mechanosensory cells in bone. [DOI] [PMC free article] [PubMed]
- 8.Pawlicki R. Morphological differentiation of the fossil dinosaur bone cells. Cells Tissues Organs. 1978;100:411–418. doi: 10.1159/000144925. [DOI] [PubMed] [Google Scholar]
- 9.Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone. 2015;75:144–150. doi: 10.1016/j.bone.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 10.You L, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol. 2004;278A:505–513. doi: 10.1002/ar.a.20050. [DOI] [PubMed] [Google Scholar]
- 11.Thompson WR, Modla S, Grindel BJ, Czymmek KJ, Kirn-Safran CB, Wang L, et al. Perlecan/Hspg2 deficiency alters the pericellular space of the lacunocanalicular system surrounding osteocytic processes in cortical bone. J Bone Miner Res. 2011;26:618–629. doi: 10.1002/jbmr.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Lai X, Price C, Thompson WR, Li W, Quabili TR, et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2014;29:878–891. doi: 10.1002/jbmr.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat Rec. 2009;292:355–363. doi: 10.1002/ar.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geoghegan IP, Hoey DA, McNamara LM. Integrins in osteocyte biology and mechanotransduction. Curr Osteoporos Rep. 2019;17:195–206. doi: 10.1007/s11914-019-00520-2. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs CR, Temiyasathit S, Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Annu Rev Biomed Eng. 2010;12:369–400. doi: 10.1146/annurev-bioeng-070909-105302. [DOI] [PubMed] [Google Scholar]
- 16.Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, et al. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci USA. 2010;107:13648–13653. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu D, Schaffler MB, Weinbaum S, Spray DC. Matrix-dependent adhesion mediates network responses to physiological stimulation of the osteocyte cell process. Proc Natl Acad Sci USA. 2013;110:12096–12101. doi: 10.1073/pnas.1310003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thi MM, Suadicani SO, Schaffler MB, Weinbaum S, Spray DC. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require αVβ3 integrin. Proc NatL Acad Sci USA. 2013;110:21012–21017. doi: 10.1073/pnas.1321210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon YJ, Yun C-Y, Choi H, Kim JR, Park B-H, Cho E-S. Osterix regulates corticalization for longitudinal bone growth via integrin β3 expression. Exp Mol Med. 2018;50:1–11. doi: 10.1038/s12276-018-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buo AM, Stains JP. Gap junctional regulation of signal transduction in bone cells. FEBS Lett. 2014;588:1315–1321. doi: 10.1016/j.febslet.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis HM, Aref MW, Aguilar-Perez A, Pacheco-Costa R, Allen K, Valdez S, et al. Cx43 overexpression in osteocytes prevents osteocyte apoptosis and preserves cortical bone quality in aging mice. JBMR Plus. 2018;2:206–216. doi: 10.1002/jbm4.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 24.Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–4552. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prideaux M, Loveridge N, Pitsillides AA, Farquharson C. Extracellular matrix mineralization promotes E11/gp38 glycoprotein expression and drives osteocytic differentiation. PLoS ONE. 2012;7:e36786. doi: 10.1371/journal.pone.0036786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai T, Hasegawa T, Yimin, Yamamoto T, Hongo H, Abe M, et al. Immunocytochemical assessment of cell differentiation of podoplanin-positive osteoblasts into osteocytes in murine bone. Histochem Cell Biol. 2021;155:369–380. [DOI] [PubMed]
- 27.Milovanovic P, Zimmermann EA, Hahn M, Djonic D, Püschel K, Djuric M, et al. Osteocytic canalicular networks: morphological implications for altered mechanosensitivity. ACS Nano. 2013;7:7542–7551. doi: 10.1021/nn401360u. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi K, Nojiri H, Saita Y, Morikawa D, Ozawa Y, Watanabe K, et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci Rep. 2015;5:9148. doi: 10.1038/srep09148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiede-Lewis LM, Xie Y, Hulbert MA, Campos R, Dallas MR, Dusevich V, et al. Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging Albany NY. 2017;9:2190–2208. doi: 10.18632/aging.101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onal M, Piemontese M, Xiong J, Wang Y, Han L, Ye S, et al. Suppression of autophagy in osteocytes mimics skeletal aging*. J Biol Chem. 2013;288:17432–17440. doi: 10.1074/jbc.M112.444190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piemontese M, Onal M, Xiong J, Han L, Thostenson JD, Almeida M, et al. Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Sci Rep. 2016;6:24262. doi: 10.1038/srep24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karthik V, Guntur AR. Energy metabolism of osteocytes. Curr Osteoporos Rep. 2021;19:444–451. doi: 10.1007/s11914-021-00688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.••.Gao J, Qin A, Liu D, Ruan R, Wang Q, Yuan J, et al. Endoplasmic reticulum mediates mitochondrial transfer within the osteocyte dendritic network. Sci Adv. 2019;5:eaaw7215. This study demonstrated the function of inter-cellular mitochondrial transport in the maintenance of the osteocyte dendritic network. [DOI] [PMC free article] [PubMed]
- 35.Balani DH, Trinh S, Xu M, Kronenberg HM. Sclerostin antibody administration increases the numbers of Sox9creER+ skeletal precursors and their progeny. J Bone Miner Res. 2021;36:757–767. doi: 10.1002/jbmr.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2007;292:C545–C552. doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- 37.Asada N, Katayama Y, Sato M, Minagawa K, Wakahashi K, Kawano H, et al. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell. 2013;12:737–747. doi: 10.1016/j.stem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 38.You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 40.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honma M, Ikebuchi Y, Kariya Y, Hayashi M, Hayashi N, Aoki S, et al. RANKL subcellular trafficking and regulatory mechanisms in osteocytes: RANKL SUBCELLULAR TRAFFICKING IN OSTEOCYTES. J Bone Miner Res. 2013;28:1936–1949. doi: 10.1002/jbmr.1941. [DOI] [PubMed] [Google Scholar]
- 42.Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res Off J Am Soc Bone Miner Res. 2009;24:597–605. doi: 10.1359/jbmr.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung WY, Fritton JC, Morgan SA, Seref-Ferlengez Z, Basta-Pljakic J, Thi MM, et al. Pannexin-1 and P2X7-receptor are required for apoptotic osteocytes in fatigued bone to trigger RANKL production in neighboring bystander osteocytes. J Bone Miner Res. 2016;31:890–899. doi: 10.1002/jbmr.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, et al. Osteocyte apoptosis caused by hindlimb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J Bone Miner Res. 2016;31:1356–1365. doi: 10.1002/jbmr.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plotkin LI, Gortazar AR, Davis HM, Condon KW, Gabilondo H, Maycas M, et al. Inhibition of osteocyte apoptosis prevents the increase in osteocytic receptor activator of nuclear factor κB ligand (RANKL) but does not stop bone resorption or the loss of bone induced by unloading*. J Biol Chem. 2015;290:18934–18942. doi: 10.1074/jbc.M115.642090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.•.Grüneboom A, Hawwari I, Weidner D, Culemann S, Müller S, Henneberg S, et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metab. 2019;1:236–50. This study revealed the association between osteocyte dendrites and trans-cortical vessels (TCV)–associated osteoclasts. [DOI] [PMC free article] [PubMed]
- 47.Stinson JC. The ailing mythical osteocyte. Med Hypotheses. 1975;1:186–190. doi: 10.1016/0306-9877(75)90049-3. [DOI] [PubMed] [Google Scholar]
- 48.Eisenberger S, Ackermann K, Voggenreiter G, Sültmann H, Kasperk C, Pyerin W. Metastases and multiple myeloma generate distinct transcriptional footprints in osteocytes in vivo. J Pathol. 2008;214:617–626. doi: 10.1002/path.2322. [DOI] [PubMed] [Google Scholar]
- 49.Taylor-King JP, Buenzli PR, Chapman SJ, Lynch CC, Basanta D. Modeling osteocyte network formation: healthy and cancerous environments. Front Bioeng Biotechnol. 2020;8:757. doi: 10.3389/fbioe.2020.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemmatian H, Conrad S, Furesi G, Mletzko K, Krug J, Faila AV, et al. Reorganization of the osteocyte lacuno-canalicular network characteristics in tumor sites of an immunocompetent murine model of osteotropic cancers. Bone. 2021;152:116074. doi: 10.1016/j.bone.2021.116074. [DOI] [PubMed] [Google Scholar]
- 51.Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, et al. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016;76:1089–1100. doi: 10.1158/0008-5472.CAN-15-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui Y-X, Evans BAJ, Jiang WG. New roles of osteocytes in proliferation, migration and invasion of breast and prostate cancer cells. Anticancer Res. 2016;36:1193–1201. [PubMed] [Google Scholar]
- 53.Wang W, Sarazin BA, Kornilowicz G, Lynch ME. Mechanically-loaded breast cancer cells modify osteocyte mechanosensitivity by secreting factors that increase osteocyte dendrite formation and downstream resorption. Front Endocrinol. 2018;9:352. doi: 10.3389/fendo.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou JZ, Riquelme MA, Gu S, Kar R, Gao X, Sun L, et al. Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis. Oncogene. 2016;35:5597–5607. doi: 10.1038/onc.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–692. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.••.Youlten SE, Kemp JP, Logan JG, Ghirardello EJ, Sergio CM, Dack MRG, et al. Osteocyte transcriptome mapping identifies a molecular landscape controlling skeletal homeostasis and susceptibility to skeletal disease. Nat Commun. 2021;12:2444. This study reported the transcriptomic similarity between osteocytes and neurons and its relevance for skeletal diseases. [DOI] [PMC free article] [PubMed]
- 57.••.Wang JS, Kamath T, Mazur CM, Mirzamohammadi F, Rotter D, Hojo H, et al. Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin. Nat Commun. 2021;12:6271. This paper described a Sp7/Ostn axis that regulates osteocyte dendrite formation in vivo and in vitro. [DOI] [PMC free article] [PubMed]
- 58.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, Rivera H de, et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015-1030.e16. [DOI] [PMC free article] [PubMed]
- 59.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 60.Zhou X, Zhang Z, Feng JQ, Dusevich VM, Sinha K, Zhang H, et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci USA. 2010;107:12919–12924. doi: 10.1073/pnas.0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas G, Moffatt P, Salois P, Gaumond M-H, Gingras R, Godin É, et al. Osteocrin, a novel bone-specific secreted protein that modulates the osteoblast phenotype. J Biol Chem. 2003;278:50563–50571. doi: 10.1074/jbc.M307310200. [DOI] [PubMed] [Google Scholar]
- 62.Bord S, Ireland DC, Moffatt P, Thomas GP, Compston JE. Characterization of osteocrin expression in human bone. J Histochem Cytochem. 2005;53:1181–1187. doi: 10.1369/jhc.4C6561.2005. [DOI] [PubMed] [Google Scholar]
- 63.Ataman B, Boulting GL, Harmin DA, Yang MG, Baker-Salisbury M, Yap E-L, et al. Evolution of osteocrin as an activity-regulated factor in the primate brain. Nature. 2016;539:242–247. doi: 10.1038/nature20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shelly M, Cancedda L, Lim BK, Popescu AT, Cheng P, Gao H, et al. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron. 2011;71:433–446. doi: 10.1016/j.neuron.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.•.Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by Semaphorin 3A. Nature. 2012;485:69–74. This paper demonstrated that the neuronal gene Sema3A regulates osteocyte survival via soluble guanylate cyclase (sGC)-cGMP signaling. [DOI] [PubMed]
- 66.Hayashi M, Nakashima T, Yoshimura N, Okamoto K, Tanaka S, Takayanagi H. Autoregulation of osteocyte Sema3A orchestrates estrogen action and counteracts bone aging. Cell Metab. 2019;29:627-637.e5. [DOI] [PubMed]
- 67.Romano R, Bucci C. Role of EGFR in the nervous system. Cells. 2020;9:1887. doi: 10.3390/cells9081887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyono A, Avishai N, Ouyang Z, Landreth GE, Murakami S. FGF and ERK signaling coordinately regulate mineralization-related genes and play essential roles in osteocyte differentiation. J Bone Miner Metab. 2012;30:19–30. doi: 10.1007/s00774-011-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikpegbu E, Basta L, Clements DN, Fleming R, Vincent TL, Buttle DJ, et al. FGF-2 promotes osteocyte differentiation through increased E11/podoplanin expression. J Cell Physiol. 2018;233:5334–5347. doi: 10.1002/jcp.26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taliaferro JM, Vidaki M, Oliveira R, Olson S, Zhan L, Saxena T, et al. Distal alternative last exons localize mRNAs to neural projections. Mol Cell. 2016;61:821–833. doi: 10.1016/j.molcel.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zappulo A, Bruck D van den, Mattioli CC, Franke V, Imami K, McShane E, et al. RNA localization is a key determinant of neurite-enriched proteome. Nat Commun. 2017;8:583. [DOI] [PMC free article] [PubMed]
- 72.Engel KL, Arora A, Goering R, Lo HG, Taliaferro JM. Mechanisms and consequences of subcellular RNA localization across diverse cell types. Traffic. 2020;21:404–418. doi: 10.1111/tra.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nostrand ELV, Freese P, Pratt GA, Wang X, Wei X, Xiao R, et al. A large-scale binding and functional map of human RNA-binding proteins. Nature. 2020;583:711–719. doi: 10.1038/s41586-020-2077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karagiosis SA, Karin NJ. Lysophosphatidic acid induces osteocyte dendrite outgrowth. Biochem Bioph Res Co. 2007;357:194–199. doi: 10.1016/j.bbrc.2007.03.121. [DOI] [PubMed] [Google Scholar]
- 76.Waters KM, Jacobs JM, Gritsenko MA, Karin NJ. Regulation of gene expression and subcellular protein distribution in MLO-Y4 osteocytic cells by lysophosphatidic acid: relevance to dendrite outgrowth. Bone. 2011;48:1328–1335. doi: 10.1016/j.bone.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fukushima N, Ishii I, Habara Y, Allen CB, Chun J. Dual regulation of actin rearrangement through lysophosphatidic acid receptor in neuroblast cell lines: actin depolymerization by Ca2+-α-actinin and polymerization by Rho. Mol Biol Cell. 2002;13:2692–2705. doi: 10.1091/mbc.01-09-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fukushima N, Morita Y. Actomyosin-dependent microtubule rearrangement in lysophosphatidic acid-induced neurite remodeling of young cortical neurons. Brain Res. 2006;1094:65–75. doi: 10.1016/j.brainres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Jang Y, Lee MH, Lee J, Jung J, Lee SH, Yang D-J, et al. TRPM2 mediates the lysophosphatidic acid-induced neurite retraction in the developing brain. Pflügers Archiv Eur J Physiol. 2014;466:1987–1998. doi: 10.1007/s00424-013-1436-4. [DOI] [PubMed] [Google Scholar]
- 80.Furuta D, Yamane M, Tsujiuchi T, Moriyama R, Fukushima N. Lysophosphatidic acid induces neurite branch formation through LPA3. Mol Cell Neurosci. 2012;50:21–34. doi: 10.1016/j.mcn.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Simic P, Kim W, Zhou W, Pierce KA, Chang W, Sykes DB, et al. Glycerol-3-phosphate is an FGF23 regulator derived from the injured kidney. J Clin Invest. 2020;130:1513–1526. doi: 10.1172/JCI131190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun KLW, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138:2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 83.Laumonnerie C, Silva RVD, Kania A, Wilson SI. Netrin 1 and Dcc signalling are required for confinement of central axons within the central nervous system. Development. 2014;141:594–603. doi: 10.1242/dev.099606. [DOI] [PubMed] [Google Scholar]
- 84.Liu G, Beggs H, Jürgensen C, Park H-T, Tang H, Gorski J, et al. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.••.Matsugaki A, Yamazaki D, Nakano T. Selective patterning of netrin-1 as a novel guiding cue for anisotropic dendrogenesis in osteocytes. Mater Sci Eng C. 2020;108:110391. A key study demonstrated the repurposing of neurite outgrowth signaling pathway in osteocyte dendrite elongation. [DOI] [PubMed]
- 86.Böttcher RT, Wiesner S, Braun A, Wimmer R, Berna A, Elad N, et al. Profilin 1 is required for abscission during late cytokinesis of chondrocytes. EMBO J. 2009;28:1157–1169. doi: 10.1038/emboj.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin W, Izu Y, Smriti A, Kawasaki M, Pawaputanon C, Böttcher RT, et al. Profilin1 is expressed in osteocytes and regulates cell shape and migration. J Cell Physiol. 2018;233:259–268. doi: 10.1002/jcp.25872. [DOI] [PubMed] [Google Scholar]
- 88.Niimura M, Sato T, Enoki Y, Okubo M, Kokabu S, Takeda S, et al. Semaphorin 3A promotes dendrite elongation of osteocytes in association with down-regulation of CDK6. Vivo Athens Greece. 2016;30:231–236. [PubMed] [Google Scholar]
- 89.Tigan A-S, Bellutti F, Kollmann K, Tebb G, Sexl V. CDK6—a review of the past and a glimpse into the future: from cell-cycle control to transcriptional regulation. Oncogene. 2016;35:3083–3091. doi: 10.1038/onc.2015.407. [DOI] [PubMed] [Google Scholar]
- 90.Ogasawara T, Kawaguchi H, Jinno S, Hoshi K, Itaka K, Takato T, et al. Bone morphogenetic protein 2-induced osteoblast differentiation requires Smad-mediated down-regulation of Cdk6. Mol Cell Biol. 2004;24:6560–6568. doi: 10.1128/MCB.24.15.6560-6568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, et al. New sequence variants associated with bone mineral density. Nat Genet. 2009;41:15–17. doi: 10.1038/ng.284. [DOI] [PubMed] [Google Scholar]
- 92.Timpson NJ, Tobias JH, Richards JB, Soranzo N, Duncan EL, Sims A-M, et al. Common variants in the region around osterix are associated with bone mineral density and growth in childhood. Hum Mol Genet. 2009;18:1510–1517. doi: 10.1093/hmg/ddp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rivadeneira F, Styrkársdottir U, Estrada K, Halldórsson BV, Hsu Y-H, Richards JB, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41:1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lapunzina P, Aglan M, Temtamy S, Caparrós-Martín JA, Valencia M, Letón R, et al. Identification of a frameshift mutation in osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet. 2010;87:110–114. doi: 10.1016/j.ajhg.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fiscaletti M, Biggin A, Bennetts B, Wong K, Briody J, Pacey V, et al. Novel variant in Sp7/Osx associated with recessive osteogenesis imperfecta with bone fragility and hearing impairment. Bone. 2018;110:66–75. doi: 10.1016/j.bone.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 96.Lui JC, Raimann A, Hojo H, Dong L, Roschger P, Kikani B, et al. A neomorphic variant in SP7 alters sequence specificity and causes a high-turnover bone disorder. Nat Commun. 2022;13:700. doi: 10.1038/s41467-022-28318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ludwig K, Ward LM, Khan N, Robinson M-E, Miranda V, Bardai G, et al. Dominant osteogenesis imperfecta with low bone turnover caused by a heterozygous SP7 variant. Bone. 2022;160:116400. doi: 10.1016/j.bone.2022.116400. [DOI] [PubMed] [Google Scholar]
- 98.Matthews JL, Talmage RV. Influence of parathyroid hormone on bone cell ultrastructure. Clin Orthop Relat R. 1981:27–38. [PubMed]
- 99.Prideaux M, Dallas SL, Zhao N, Johnsrud ED, Veno PA, Guo D, et al. Parathyroid hormone induces bone cell motility and loss of mature osteocyte phenotype through L-calcium channel dependent and independent mechanisms. PLoS ONE. 2015;10:e0125731. doi: 10.1371/journal.pone.0125731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shao Y, Alicknavitch M, Farach-Carson MC. Expression of voltage sensitive calcium channel (VSCC) L-type Cav1.2 (α1C) and T-type Cav3.2 (α1H) subunits during mouse bone development. Dev Dyn. 2005;234:54–62. doi: 10.1002/dvdy.20517. [DOI] [PubMed] [Google Scholar]
- 101.Yakar S, Isaksson O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: lessons from mouse models. Growth Horm Igf Res. 2016;28:26–42. doi: 10.1016/j.ghir.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.••.Qiu T, Crane JL, Xie L, Xian L, Xie H, Cao X. IGF-I induced phosphorylation of PTH receptor enhances osteoblast to osteocyte transition. Bone Res. 2018;6:5. This study demonstrated that IGF1R directly phosphorylates PTH receptor and simulates actin polymerization to increase osteocyte dendrite outgrowth. [DOI] [PMC free article] [PubMed]
- 103.Qing H, Bonewald LF. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci. 2009;1:59–65. doi: 10.4248/ijos.09019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang SY, Herber R, Ho SP, Alliston T. Matrix metalloproteinase–13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012;27:1936–1950. doi: 10.1002/jbmr.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lotinun S, Ishihara Y, Nagano K, Kiviranta R, Carpentier V, Neff L, et al. Cathepsin K-deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression. J Clin Invest. 2019;129:3058–3071. doi: 10.1172/JCI122936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, et al. Osteocyte-intrinsic TGF-β signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017;21:2585–2596. doi: 10.1016/j.celrep.2017.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 108.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The crumbs complex couples cell density sensing to hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 109.•.Kegelman CD, Coulombe JC, Jordan KM, Horan DJ, Qin L, Robling AG, et al. YAP and TAZ mediate osteocyte perilacunar/canalicular remodeling. J Bone Miner Res. 2020;35:196–210. This study reported that YAP/TAZ regulates osteocyte-mediated bone remodeling and perilacunar/canalicular remodeling. [DOI] [PMC free article] [PubMed]
- 110.Sabik OL, Farber CR. Using GWAS to identify novel therapeutic targets for osteoporosis. Transl Res. 2017;181:15–26. doi: 10.1016/j.trsl.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kemp JP, Morris JA, Medina-Gomez C, Forgetta V, Warrington NM, Youlten SE, et al. Identification of 153 new loci associated with heel bone mineral density and functional involvement of GPC6 in osteoporosis. Nat Genet. 2017;49:1468–1475. doi: 10.1038/ng.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tate MLK, Adamson JR, Tami AE, Bauer TW. The osteocyte. Int J Biochem Cell Biology. 2004;36:1–8. doi: 10.1016/S1357-2725(03)00241-3. [DOI] [PubMed] [Google Scholar]
- 113.Kaya S, Schurman CA, Dole NS, Evans DS, Alliston T. Prioritization of genes relevant to bone fragility through the unbiased integration of aging mouse bone transcriptomics and human GWAS analyses. J Bone Miner Res. 2022;37:804–817. doi: 10.1002/jbmr.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 115.Frost HM, Jee WSS. On the rat model of human osteopenias and osteoporoses. Bone Miner. 1992;18:227–236. doi: 10.1016/0169-6009(92)90809-R. [DOI] [PubMed] [Google Scholar]
- 116.Sharma D, Ciani C, Marin PAR, Levy JD, Doty SB, Fritton SP. Alterations in the osteocyte lacunar–canalicular microenvironment due to estrogen deficiency. Bone. 2012;51:488–497. doi: 10.1016/j.bone.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Felson DT. Osteoarthritis: new insights. Part 1: The disease and its risk factors. Ann Intern Med. 2000;133:635. [DOI] [PubMed]
- 118.Mansell JP, Collins C, Bailey AJ. Bone, not cartilage, should be the major focus in osteoarthritis. Nat Clin Pract Rheum. 2007;3:306–307. doi: 10.1038/ncprheum0505. [DOI] [PubMed] [Google Scholar]
- 119.Jaiprakash A, Prasadam I, Feng JQ, Liu Y, Crawford R, Xiao Y. Phenotypic characterization of osteoarthritic osteocytes from the sclerotic zones: a possible pathological role in subchondral bone sclerosis. Int J Biol Sci. 2012;8:406–417. doi: 10.7150/ijbs.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu L, Guo H, Sun K, Zhao X, Ma T, Jin Q. Sclerostin expression in the subchondral bone of patients with knee osteoarthritis. Int J Mol Med. 2016;38:1395–1402. doi: 10.3892/ijmm.2016.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ilas DC, Churchman SM, Baboolal T, Giannoudis PV, Aderinto J, McGonagle D, et al. The simultaneous analysis of mesenchymal stem cells and early osteocytes accumulation in osteoarthritic femoral head sclerotic bone. Rheumatology. 2019;58:1777–1783. doi: 10.1093/rheumatology/kez130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Staines KA, Ikpegbu E, Törnqvist AE, Dillon S, Javaheri B, Amin AK, et al. Conditional deletion of E11/podoplanin in bone protects against load-induced osteoarthritis. BMC Musculoskelet Disord. 2019;20:344. doi: 10.1186/s12891-019-2731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCarthy EF. Genetic diseases of bones and joints. Semin Diagn Pathol. 2011;28:26–36. doi: 10.1053/j.semdp.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 124.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016;387:1657–1671. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marini JC, Forlino A, Bächinger HP, Bishop NJ, Byers PH, Paepe AD, et al. Osteogenesis imperfecta. Nat Rev Dis Primers. 2017;3:17052. doi: 10.1038/nrdp.2017.52. [DOI] [PubMed] [Google Scholar]
- 126.Asharani PV, Keupp K, Semler O, Wang W, Li Y, Thiele H, et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet. 2012;90:661–674. doi: 10.1016/j.ajhg.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martínez-Glez V, Valencia M, Caparrós-Martín JA, Aglan M, Temtamy S, Tenorio J, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat. 2012;33:343–350. doi: 10.1002/humu.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Muir AM, Ren Y, Butz DH, Davis NA, Blank RD, Birk DE, et al. Induced ablation of Bmp1 and Tll1 produces osteogenesis imperfecta in mice. Hum Mol Genet. 2014;23:3085–3101. doi: 10.1093/hmg/ddu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zimmerman SM, Dimori M, Heard-Lipsmeyer ME, Morello R. The osteocyte transcriptome is extensively dysregulated in mouse models of osteogenesis imperfecta. JBMR Plus. 2019;3:e10171. doi: 10.1002/jbm4.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang T, Yu X, He C. Pro-inflammatory cytokines: cellular and molecular drug targets for glucocorticoid-induced-osteoporosis via osteocyte. Curr Drug Targets. 2019;20:1–15. doi: 10.2174/1389450119666180405094046. [DOI] [PubMed] [Google Scholar]
- 131.Weinstein RS. Glucocorticoids, osteocytes, and skeletal fragility: the role of bone vascularity. Bone. 2010;46:564–570. doi: 10.1016/j.bone.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O’Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 134.Jia J, Yao W, Guan M, Dai W, Shahnazari M, Kar R, et al. Glucocorticoid dose determines osteocyte cell fate. FASEB J. 2011;25:3366–3376. doi: 10.1096/fj.11-182519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fowler TW, Acevedo C, Mazur CM, Hall-Glenn F, Fields AJ, Bale HA, et al. Glucocorticoid suppression of osteocyte perilacunar remodeling is associated with subchondral bone degeneration in osteonecrosis. Sci Rep. 2017;7:44618. doi: 10.1038/srep44618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao J, Cheng TS, Qin A, Pavlos NJ, Wang T, Song K, et al. Glucocorticoid impairs cell-cell communication by autophagy-mediated degradation of connexin 43 in osteocytes. Oncotarget. 2016;7:26966–26978. doi: 10.18632/oncotarget.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, et al. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2004;118:147–156. doi: 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- 138.Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28:145–149. doi: 10.1016/S8756-3282(00)00421-X. [DOI] [PubMed] [Google Scholar]
- 139.Nasello G, Alamán-Díez P, Schiavi J, Pérez MÁ, McNamara L, García-Aznar JM. Primary human osteoblasts cultured in a 3D microenvironment create a unique representative model of their differentiation into osteocytes. Front Bioeng Biotechnol. 2020;8:336. doi: 10.3389/fbioe.2020.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Naqvi SM, Pérez JAP, Kumar V, Verbruggen ASK, McNamara LM. A Novel 3D osteoblast and osteocyte model revealing changes in mineralization and pro-osteoclastogenic paracrine signaling during estrogen deficiency. Front Bioeng Biotechnol. 2020;8:601. doi: 10.3389/fbioe.2020.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kamel-ElSayed SA, Tiede-Lewis LM, Lu Y, Veno PA, Dallas SL. Novel approaches for two and three dimensional multiplexed imaging of osteocytes. Bone. 2015;76:129–140. doi: 10.1016/j.bone.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shiflett LA, Tiede-Lewis LM, Xie Y, Lu Y, Ray EC, Dallas SL. Collagen dynamics during the process of osteocyte embedding and mineralization. Front Cell Dev Biol. 2019;7:178. doi: 10.3389/fcell.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]


