Abstract
Purpose of Review
Inhibition of receptor activator of nuclear factor kappa-B ligand (RANKL) with denosumab is an effective treatment in a number of conditions including osteoporosis where suppression of bone resorption is desired. However, denosumab discontinuation is associated with rebound increase in bone resorption and subsequent loss in bone mass and a rapid return to baseline fracture risk. We review recent data on the rebound increase in bone resorption following denosumab discontinuation and the potential mechanisms behind this phenomenon.
Recent Findings
Osteoclasts have been considered to be highly specialised cells that undergo apoptosis after fulfilling their function of bone resorption. However, recent studies suggest that osteoclasts are longer lived cells which migrate through vasculature and are capable of undergoing fission into a novel cell type (the osteomorph) and re-fusion in a process termed osteoclast recycling.
Summary
The life cycle of the osteoclast is more complex than previously appreciated. Osteoclast recycling provides a novel mechanistic framework to examine changes in osteoclast biology in response to treatment of bone diseases and provides an exciting new avenue towards personalised medicine.
Keywords: Osteoclast, Denosumab discontinuation, Osteoporosis
Introduction
The human skeleton is constantly turning over in a tightly regulated process known as bone remodelling. Bone remodelling occurs at a cellular level within a microscopic unit known as the basic multicellular unit (BMU), which consists of osteoblasts and osteoclasts coupled together on the bone surface, interacting through cell-to-cell contact and local and systemic cytokine networks, to regulate bone resorption and formation and maintain bone homeostasis [1]. Several bone diseases, such as osteoporosis, lead to an imbalance in bone remodelling resulting in net increase in bone resorption and subsequent bone loss. The osteoclast is the primary cell responsible for bone resorption and therefore has been the therapeutic target for several agents directed towards inhibiting bone resorption and increasing bone mass. Historically, osteoclasts have been thought to undergo apoptosis following completion of bone resorption. Alternative osteoclast cell fates such as fission had been postulated but were unable to be confirmed until recently. Advances in lineage tracing, single cell RNA sequencing and imaging technologies have challenged this long-held dogma. This review will briefly outline the historical life cycle of the osteoclast, from formation to fate. We will then focus on how recent discoveries have uncovered an increased life cycle and a novel cell fate, namely osteoclast recycling, and the relevance of this discovery to current and emerging therapies targeting bone resorption.
Osteoclast Formation and Function
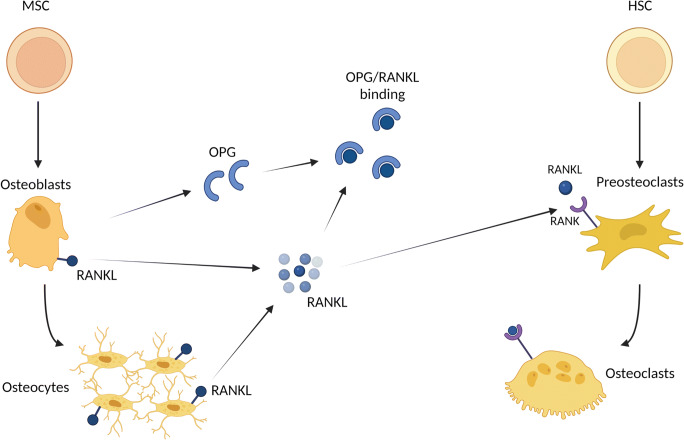
The osteoclast is a highly specialised, multinucleated, tissue-specific macrophage responsible for bone resorption. Bone resorption by the active osteoclast occurs through the development of an acidic environment in which H+ ions are transported via proton pumps [2] and lytic enzymes tartrate resistant acid phosphatase (TRAP) and cathepsin K [3] are released into the resorption compartment. This dissolves hydroxyapatite and allows enzymatic degradation of the bone matrix proteins which are then phagocytosed by the osteoclasts and excreted [4]. Osteoclasts differentiate from monocyte precursors at or near the bone surface. Three molecules: receptor activator of nuclear factor kB ligand (RANKL) and its receptor RANK, and the decoy receptor osteoprotegerin (OPG), form the RANKL/RANK/OPG pathway (Fig. 1) which plays a critical role in the differentiation of osteoclasts from its haematopoietic progenitors.
Fig. 1.
RANKL/RANK/OPG pathway. RANKL is produced by cells of the osteoblast lineage, including matrix-embedded osteocytes. Membrane bound RANKL is cleaved by proteases to form soluble RANKL. OPG is predominantly secreted by osteoblasts to bind to RANKL to suppress its activity and regulate osteoclastic bone resorption. RANKL binding to its receptor RANK promotes the differentiation of mature osteoclasts which are capable of attaching to and resorbing bone. Abbreviations: RANKL, receptor activator of nuclear factor kappa beta ligand, OPG, osteoprotegerin, MSC, mesenchymal stem cells, HSC, haematopoietic stem cells
Osteoclastogenesis and the RANK/RANKL/OPG Pathway
Our current understanding of osteoclast differentiation and activity comes from initial observations made in animals and patients with osteopetrosis, a condition of increased bone mass due to arrested bone resorption. Pioneering parabiosis experiments in the 1970s showed restoration of bone resorption in osteopetrotic mice [5, 6], showing that precursors of these resorptive cells are of haematopoietic origin. Subsequent co-culture experiments [7] provided supporting evidence for a hypothesis that osteoblasts played a key role in mediating osteoclastogenesis [8], revealing that osteoclast formation requires physical contact between haematopoietic precursor cells with specific bone marrow derived stromal cells [9, 10]. This led to the discovery of OPG which plays a key role in osteoclastogenesis.
OPG is a protein of the TNF receptor superfamily and is predominantly secreted by osteoblasts and osteocytes [11]. Transgenic mice overexpressing the gene Tnfrsf11b encoding OPG displayed an osteopetrotic phenotype due to a profound decrease in osteoclasts [12]. Conversely, targeted ablation of OPG in mice led to severe osteoporosis due to marked increase in osteoclastogenesis and bone resorption [13]. Hence, OPG was determined a negative regulator of osteoclastogenesis.
OPG expression is regulated by Wnt signalling in osteoblasts [14] and local production of OPG by osteoblasts, rather than osteocytes. Interestingly, circulating OPG levels were unchanged in mice with conditional deletion of Tndrsf11b in osteoblasts which exhibited reduced cortical thickness and cancellous bone in the femur, demonstrating that local OPG is essential in regulating bone resorption [15]. OPG therefore was determined to be a decoy receptor for RANKL, blocking RANK and RANKL interaction and subsequent osteoclastogenesis.
RANKL is initially produced as an integral membrane bound protein but can be cleaved by proteases into a functional soluble form [17]. RANKL binds to its receptor RANK on osteoclast progenitors and stimulates osteoclast differentiation and function [16]. RANKL is involved in the fusion of osteoclast precursors into multinucleated cells, differentiation into mature osteoclasts and continued survival [17]. RANKL relies on macrophage-colony stimulating factor (M-CSF, also known as CSF-1) as a co-factor for osteoclast differentiation [18].
Genetic deletion studies show that RANKL is produced at various stages of the osteoblast lineage, including matrix-embedded osteocytes, though the relative contribution of RANKL at each stage remains unclear. Osteopetrosis was demonstrated in mice with RANKL deletion throughout the osteoblast lineage, and less so in mice with deletion restricted to differentiated osteoblasts and osteocytes only [19, 20]. Soluble RANKL, cleaved from its membrane bound form, is measurable in the circulation and increases with stimulated bone resorption [21]. Recent studies have demonstrated that the membrane-bound form of RANKL is responsible for the majority of RANKL functions and more potent than soluble RANKL in stimulating osteoclastogenesis in vitro [22]. This highlights the importance of the local microenvironment and cellular interactions between the different cell types within the BMU.
Elucidation of the RANK/RANKL/OPG pathway has revolutionised our understanding of osteoclastogenesis. Under the influence of RANKL and M-CSF and regulated by OPG, mononuclear haematopoietic cells of the monocyte lineage differentiate and fuse to form multinucleated osteoclasts capable of resorbing bone. However, until recently, little has been known about what happens to osteoclasts following completion of bone resorption.
Osteoclast Cell Fate and Recycling
Following bone resorption, mature osteoclasts are typically thought to undergo apoptosis at the end of their lifespan of approximately 2–3 weeks, and hence, the number of osteoclasts is dependent on the rates of osteoclast differentiation and death [23]. This has been a long-standing dogma, initially described in 1920 where osteoclasts were no longer visible after bone resorption due to degeneration [24] and supported by observations of reduced osteoclast numbers following cessation of bone resorption [25, 26].
The first reported evidence of osteoclast apoptosis in vitro was in 1993 where mature osteoclasts died when the cultures were depleted of M-CSF. The authors concluded that the survival of mature osteoclasts occur through the suppression of apoptosis by factors such as M-CSF [27]. While RANKL and M-CSF are sufficient for osteoclast differentiation and enhance osteoclast survival, several proinflammatory cytokines, including TNFa, IL-1 and IL-6, have been shown to enhance osteoclast survival through activation of the NF-kB pathway [28, 29]. Parathyroid hormone (PTH) is an important hormone in calcium homeostasis as it stimulates osteoclast formation through RANKL expression by osteoblasts and reducing OPG expression by stromal cells [30, 31]. Therefore, proinflammatory cytokines and PTH predominantly act to inhibit osteoclast apoptosis by enhancing RANKL signalling to increase survival.
Conversely, several endogenous inducers of osteoclast apoptosis have also been identified which work through inhibition of RANKL signalling. In cultures, OPG enhances osteoclast apoptosis in a dose-dependent manner and upregulating molecular controllers of programmed cell death, including Fas ligand and caspase [32]. The important role of oestrogen in the regulation of bone homeostasis is evident in the pathophysiology of postmenopausal osteoporosis, where accelerated bone loss occurs with decreased circulating oestrogen in postmenopausal women [33]. Oestrogen appears to have a direct influence on osteoclasts with selective ablation of the oestrogen receptor on osteoclasts leading to trabecular bone loss in female mice and induction of apoptosis and upregulation of Fas ligand [34]. Oestrogen also indirectly promotes osteoclast apoptosis in vivo and in vitro which is mediated in part by transforming growth factor-B (TGF-B) signalling [35].
However, the role of these so called pro-apoptotic factors on osteoclast survival in vivo remains unclear. Indeed OPG has also been shown to suppress osteoclast apoptosis through the inhibition of TNF-related apoptosis inducing ligand (TRAIL) [36]. Oestrogen has been shown to reduce osteoclast numbers attached to bone and changes their morphology and size [37] which was accompanied by increased osteoclasts in the marrow space. This suggests that oestrogen treatment causes dissociation and morphological changes in osteoclasts rather than driving apoptosis, contrary to previous studies showing induction of apoptosis with ablation of the oestrogen receptor [34, 35].
Bisphosphonates are inhibitors of bone resorption and have revolutionised the treatment of a variety of bone diseases where excessive osteoclast activity is a pathological feature [38]. Bisphosphonates are selectively taken up and adsorbed to bone surfaces where they are internalised by osteoclasts, leading to loss of osteoclast function and induction of apoptosis. The mechanism via which bisphosphonates induce apoptosis differs between the two pharmacological classes of bisphosphonates. The nitrogen-containing bisphosphonates (such as alendronate and zoledronate) inhibit protein prenylation in osteoclasts [39], whereas non-nitrogen-containing bisphosphonates (such as clodronate) inhibit adenosine triphosphate (ATP)-dependent enzymes. This leads to osteoclast apoptosis [40], loss of the ruffled border and prevents attachment to bone [41]. Through these mechanisms, bisphosphonates increase bone mass and is now well established as an effective antiresorptive agent in the treatment of osteoporosis.
Osteoclast Recycling
While osteoclasts have been thought to undergo apoptosis following bone resorption or under the influence of proapoptotic factors, several historic studies postulated an alternative cell fate. Osteoclast fission had been hypothesised following observations under microscopy, though this was not able to be confirmed [42]. Decreased number of nuclei per osteoclast followed by an increases in the number of osteoclasts was observed in the presence of calcitonin in vivo [25] and while this was thought to be due to fission of pre-existing osteoclasts, again this could not be confirmed. Advances in live cell imaging allowed the novel observation of osteoclast fission in vitro in 2012, where multinucleated osteoclasts were observed to split up into smaller, functional cells [43•].
More recent studies also challenge the long-held dogma that osteoclasts undergo apoptosis following bone resorption after a lifespan of 2–3 weeks and support an alternative cell fate of the osteoclast. Recent parabiosis and cell-fate studies have shown that osteoclasts are longer lived with a lifespan of around 6 months [44•, 45•]. Advances in single cell RNA sequencing and intravital imaging have revealed that osteoclasts behave as long-lived cells that circulate and undergo fusion and fission [46]. Mixed bone marrow chimeras were used to form osteoclasts that are formed through the fusion of bone marrow cells where the cells express a green or red fluorescent protein. The reporter proteins were driven by an osteoclast gene LysozymeM-tdTomato and CSF1R or Blimp-1-GFP leading to formation of multinucleated osteoclasts that express both tdTomato and GFP following cell fusion. These cells were multi-nucleated, secreted cathepsin K and were capable of resorbing fluorescently labelled bisphosphonate from bone, confirming their functional capacity as osteoclasts [47•].
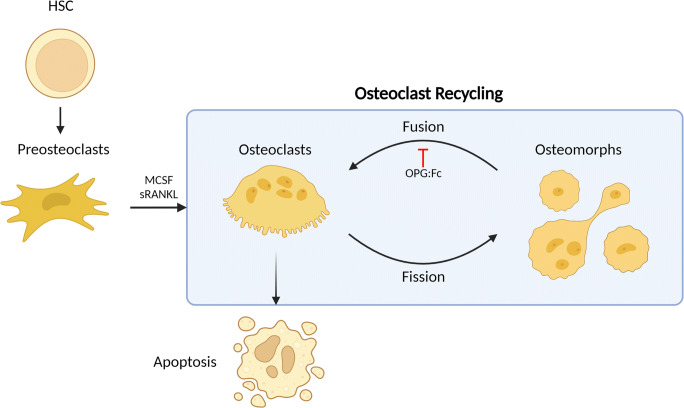
Intravital imaging of these cells under RANKL stimulation revealed that osteoclasts undergo fusion in vivo and provided the first in vivo evidence of osteoclast fission. These fission events were distinct from osteoclast apoptosis. The fate of the fission products was tracked and were demonstrated to fuse with neighbouring osteoclasts and with each other, in a process now termed osteoclast recycling. These recycling cells, termed osteomorphs, are detectable in the blood and bone marrow and are identifiable as a unique cell population expressing 151 unique genes when compared to osteoclasts and osteoclast precursors using scRNA sequencing [47•].
Detailed skeletal phenotyping of mouse lines with single-gene deletions was available through the Origin of Bone and Cartilage Disease program [48], allowing the examination of 40 mouse lines in which one or both copies of an upregulated osteomorphs gene was deleted. A number of these genes were associated with skeletal phenotypes, suggesting that these osteomorphs genes pay a role in the regulation of skeletal structure and function. Analysis of human orthologs showed that osteomorph genes were strongly associated with changes in estimated bone mineral density (BMD) [47•].
Furthermore, inhibition of RANKL with OPG:Fc treatment led to the ablation of osteoclasts and the accumulation of osteoclast precursors and osteomorphs. These results indicate that osteoclast recycling is regulated by RANKL signalling and RANKL inhibition leads to the accumulation of osteomorphs which provides a pool of primed osteoclast precursors capable of re-fusing to form active osteoclasts when RANKL inhibition is discontinued (Fig. 2). This led to rapid resumption of bone resorption and reduced bone mass in these studies [47•]. Elucidating how osteoclast recycling is affected in different disease states could provide new avenues for personalised treatment of bone diseases [49]. In addition to challenging the long-standing dogma that osteoclasts have a linear fate ending in apoptosis, these discoveries also provide a mechanistic framework to examine emerging clinical phenomena observed with anti-RANKL therapies.
Fig. 2.
Osteoclast recycling. Osteoclasts have been thought to undergo apoptosis at the end of its life cycle. Under RANKL stimulation, osteoclasts are capable of undergoing fission into daughter cells termed osteomorphs which can circulate and fuse to re-form osteoclasts in a process termed osteoclast recycling. This is regulated by RANKL signalling and inhibition of RANKL with OPG:Fc leads to the accumulation of osteomorphs and osteoclast precursors (preosteoclasts). Abbreviations: RANKL, receptor activator of nuclear factor kappa beta ligand, OPG, osteoprotegerin, HSC, haematopoietic stem cells, MCSF, macrophage colony stimulating factor
Denosumab Discontinuation and the Rebound Phenomenon
Denosumab
Inhibition of RANKL-RANK signalling leads to increased bone mass through inhibition of osteoclastic bone resorption. RANK- and RANKL-deficient mice display severe osteopetrosis due to arrested osteoclast differentiation [16, 50]. This led to the exploration of OPG as a therapeutic agent in the management of diseases where inhibition of osteoclastic bone resorption is desired. Although initial studies exploring the use of OPG-Fc showed promise in suppressing bone turnover in humans, further development was discontinued due to safety concerns over the development of neutralising immune response to endogenous OPG. Therefore, approaches targeting RANKL activity was preferred over increasing OPG, leading to the development of the anti-RANKL antibody denosumab [51].
Denosumab is a fully humanised monoclonal antibody that binds to both soluble and membrane bound RANKL with high affinity and specificity, thereby neutralising the effect of RANKL in a similar mechanism of action to OPG [52]. Denosumab reduces the number of osteoclasts and therefore increases bone mineral density [51]. Denosumab circulates in the bloodstream and is cleared through the reticuloendothelial system with a half-life of approximately 26 days. Unlike bisphosphonates, denosumab is not incorporated into the bone matrix.
Denosumab has revolutionised the management of osteoporosis. The efficacy of denosumab has been demonstrated in the landmark FREEDOM trials and has been associated with reduced fractures [53]. Treatment with denosumab leads to sustained increases in bone mineral density as long as treatment continues which was shown in a 10-year extension study [54]. This appears to be in part attributable to preservation of modelling-based bone formation during treatment [55].
Denosumab is a 6-monthy subcutaneous injection which is administered by a health professional. This is preferred and better tolerated by patients compared to bisphosphonate therapy [56]. This has led to progressive increases in denosumab prescription, and in Australia, denosumab is the most commonly prescribed antiresorptive agent [57]. Current guidelines recommend denosumab as first line therapy for the treatment of osteoporosis [58, 59]. Denosumab’s potent inhibition of osteoclast-mediated bone resorption has been utilised in other clinical settings where suppression of bone resorption is desired, often at higher doses and increased dose frequency. Adjuvant denosumab is used in patients with cancer to reduce the risk of clinical fractures related to cancer therapy [60, 61]. Denosumab is often utilised in paediatric bone diseases where increased BMD, reduced bone turnover and preventing growth of skeletal metastases is desired [62, 63].
Rebound Phenomenon Following Denosumab Discontinuation
Patients receiving denosumab continue to experience BMD gains and fracture prevention. However, there are several clinical scenarios where denosumab discontinuation may be required. Drug “holidays” from antiresorptive therapy have been advocated to reduce the risk of rare, but serious, complications arising from long-term use such as atypical femoral fractures or osteonecrosis of the jaw [64, 65]. Adjuvant denosumab in cancer treatment may be discontinued when cancer treatment has completed. Patients may also develop contraindications to denosumab such as chronic kidney disease or need to consider a change in therapy due to inefficacy and ongoing fractures. Alternatively, patients discontinue denosumab as they are no longer at high risk of fractures or no longer have indications to continue treatment with denosumab. However, discontinuation of denosumab leads to rapid reversal of its therapeutic effect, leading to “rebound” bone loss to baseline bone density and fracture risk [66]. This was also observed in a post hoc analysis of the FREEDOM study cohort, showing an increase in vertebral fracture risk following denosumab discontinuation to the level observed in untreated participants [67].
Upon denosumab cessation and withdrawal of its effect, there is a rebound increase in bone resorption which is highlighted clinically as a rapid rise in the bone turnover markers. Both markers of bone formation, such as procollagen 1 intact N-terminal propeptide (P1NP), and bone resorption including C-terminal telopeptide (CTX) and TRAP-5b are increased following the offset of denosumab’s effect indicating a high bone turnover state [66, 68]. The net effect of this is bone loss, observed as a decrease in BMD which occurs throughout the skeleton and especially in the spine [66]. This has led to increases in the rates of preventable fractures following denosumab discontinuation [69]. Concerningly, prescription data in Australia shows that denosumab treatment is frequently discontinued or interrupted, placing many patients at increased risk of fractures [57].
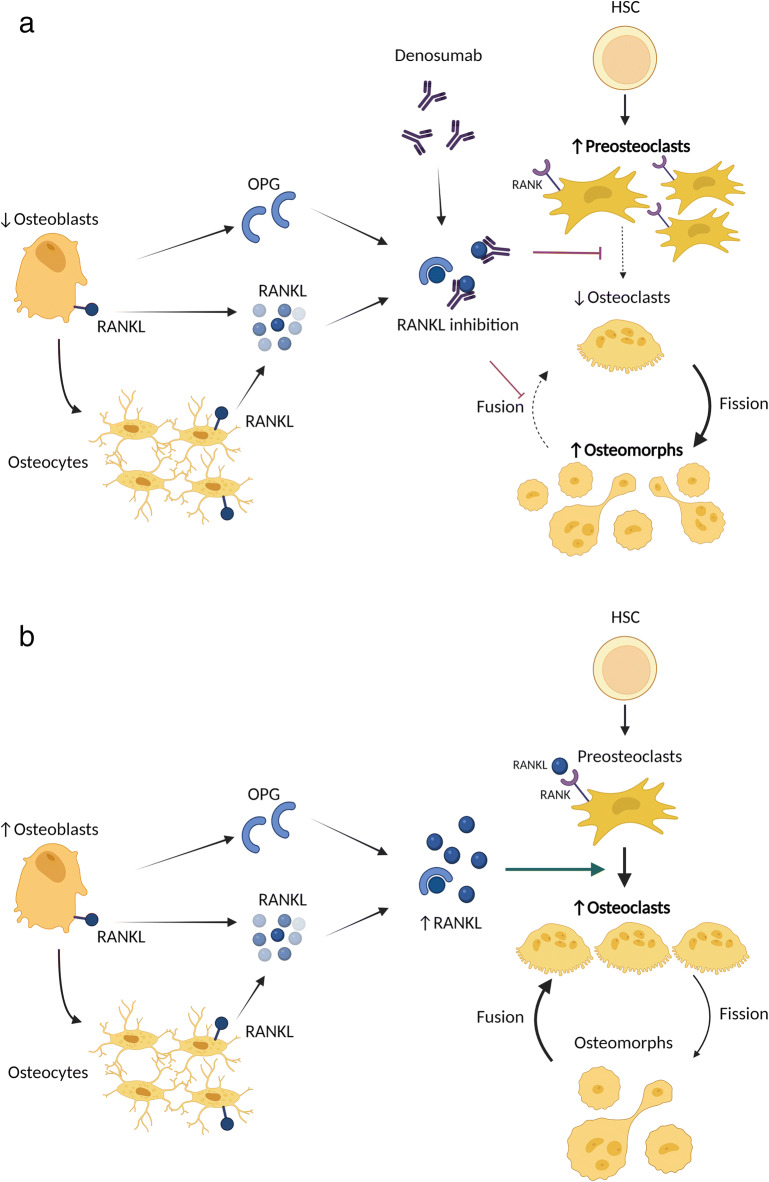
Significant increases in bone remodelling following denosumab discontinuation is accompanied by a rise in serum RANKL, though this only reached statistical significance after 12 months following the loss of effect of denosumab [70]. The mechanism underlying the delay in this RANKL rise is unclear but supports the possible mechanism where osteoclast precursors and osteomorphs form active, resorbing osteoclasts following the offset of denosumab effect (Fig. 3).
Fig. 3.
The effect of denosumab on osteoclast recycling. A Inhibition of RANKL with denosumab prevents the differentiation of preosteoclasts and leads to fission of osteoclasts into osteomorphs. Osteomorph fusion into osteoclasts is also inhibited by denosumab, leading to the accumulation of osteomorphs during denosumab treatment. B Denosumab discontinuation and its subsequent withdrawal of RANKL inhibition leads to increased RANKL and the resumption of osteoclast differentiation and osteomorphs fusion into osteoclasts. The accumulation of preosteoclasts and osteomorphs provide a pool of primed cells from which bone-resorbing osteoclasts can form. Abbreviations: RANKL, receptor activator of nuclear factor kappa beta ligand, OPG, osteoprotegerin, HSC, haematopoietic stem cells, MCSF, macrophage colony-stimulating factor
This is supported by an in vivo study examining the effect of mouse anti-RANKL monoclonal antibody which showed significantly increased TRAP-positive mononuclear cells in the bone marrow compared to controls in femoral sections following treatment discontinuation [71]. These mononuclear cells were predominantly found near, but not on, trabecular bone surfaces which may represent the fission of osteoclasts and the accumulation of osteoclast precursors and osteomorphs with anti-RANKL treatment. There was also increased expression of pro-osteoclastic genes including C-fms, RANK and RANKL in the bone marrow [71] suggesting a pro-osteoclastogenic bone marrow environment during RANKL inhibition. Thereby following treatment discontinuation, this leads to resumption of bone resorption and bone loss. This is consistent with the process of osteoclast recycling, where inhibition of RANKL with OPG:Fc led to the fission of osteoclasts into osteomorphs which then recycled to form resorbing osteoclasts once the RANKL inhibition was withdrawn.
Further evidence of this in humans is recent reports of accumulating of osteoclast precursors in the circulation of postmenopausal women receiving denosumab [72]. This supports the concept of a block in differentiation of osteoclast precursors and the fission of osteoclasts into osteomorphs during denosumab therapy. This may therefore lead to a pool of osteoclast precursors and osteomorphs primed to differentiate into osteoclasts and resorb bone once their inhibition by denosumab is withdrawn (Fig. 3).
Rebound increase in bone resorption following treatment discontinuation is not typically observed in patients treated with bisphosphonates. In ovariectomised (OVX) mice, risedronate demonstrated increased bone mass and suppression of bone turnover, whereas OVX mice that discontinued anti-RANKL antibody treatment experienced bone loss associated with an increase in bone turnover [73]. This may be due to the differences in the offset of antiresorptive activity between bisphosphonates, which are embedded into the bone matrix and therefore have a long duration of effect, compared to anti-RANKL antibodies which display a more rapid offset of effect as the drug is cleared from circulation. How bisphosphonates affect osteoclast recycling is yet unclear. Examination of osteoclast precursors in postmenopausal women treated with bisphosphonates showed a reduction of osteoclast precursors with treatment compared to healthy untreated controls, and there was no significant effect on RANKL and OPG levels [74]. As bisphosphonates inhibit bone resorption by predominantly acting on mature osteoclasts, it may not have a direct effect on osteoclast recycling. However, an apparent rebound increase in bone resorption following treatment discontinuation in OVX mice treated with NE-58025, a bisphosphonate with a low hydroxyapatite binding affinity, has been observed but the mechanism behind this remains unclear [75]. Studies to directly examine the effect of bisphosphonates on osteoclast recycling are therefore warranted.
Bone biopsies allow visualisation of histomorphometric changes that occur following denosumab discontinuation. Iliac crest bone biopsies from patients who experienced rebound fractures following denosumab discontinuation were compared to biopsies from patients receiving denosumab and treatment-naïve patients. This study demonstrated elevated bone turnover in patients discontinuing denosumab, with increased number of osteoclasts and eroded bone surface, as well as higher osteoblast numbers and osteoblast-covered bone surface [76]. Furthermore, there was a reduction in cortical and trabecular bone structure, with reduced cortical thickness and significantly lower trabecular bone volume, demonstrating compromised bone structure in patients following denosumab discontinuation [76]. In addition, there were alterations in osteocyte histomorphometry with a significant reduction in viable osteocytes with denosumab treatment which persisted at 12 months post-denosumab discontinuation, highlighting the accumulation of apoptotic osteocytes and retention of old bone during treatment due to suppressed bone turnover. These changes in osteocyte morphology following denosumab discontinuation highlight the complex cellular interaction between osteoclasts and the cells of the osteoblast lineage, particularly given the latter’s role in local RANKL and OPG production. This presents potential therapeutic opportunities to prevent the rebound phenomenon following withdrawal of RANKL inhibition.
Sequential Therapy Following Denosumab Discontinuation
Current approaches following denosumab discontinuation aim to prevent the rebound changes in bone remodelling to avoid bone loss and reduce risk of fractures [68]. Randomised controlled trials of sequential bisphosphonate, which prevent mature osteoclasts from resorbing bone, have not succeeded in consistently prevent rebound bone loss following denosumab discontinuation [77]. Sequential treatment with the PTH-analogue teriparatide following denosumab discontinuation leads to accelerated BMD loss [78]. PTH mediates bone homeostasis in a coupled manner, affecting both bone formation and bone resorption. PTH signalling in osteoblasts and osteocytes increases the RANKL/OPG ratio which recruits osteoclast precursors and stimulates osteoclastogenesis [79]. Therefore, the observed changes in BMD with transition from denosumab to teriparatide may reflect an accumulation of osteoclast precursors and osteomorphs during denosumab treatment and the formation of active resorbing osteoclasts, accelerated in the presence of PTH.
Targeting osteoblasts with newer therapies such as the sclerostin inhibitor romosozumab provides an alternate therapeutic target in the setting of denosumab discontinuation. Local production of OPG by mature osteoblasts play a critical role in suppressing RANKL activity and osteoclastogenesis [15]. Transgenic mice treated with denosumab showed almost complete absent osteoclasts and osteoblasts on the bone surface and gene expression analysis showed a striking reduction in OPG mRNA expression [80]. These findings highlight that the lack of osteoblasts and the OPG produced by these cells may contribute to the rebound bone resorption following denosumab discontinuation. Therefore, increasing osteoblastogenesis could be an alternative strategy for sequential therapy.
The effect of prior treatment on treatment response to romosozumab was examined in a real-world observational study in Japan which showed significant attenuation of bone mineral density response in patients with prior denosumab use [81]. The cellular changes that occur with transition from denosumab to ROMO remains unclear but most likely involves changes in RANKL and OPG signalling given that sclerostin promotes osteoclastogenesis via a RANKL-dependent pathway [82]. There is also documented decrease in RANKL:OPG following anti-sclerostin treatment [83]. Given the complex cellular interaction at play, a multi-pronged approach targeting not only osteoclasts, but also cells of the osteoblastic lineage may prove to be the most effective in preventing the rebound phenomenon following denosumab discontinuation. Furthermore, investigation into how osteoclast recycling could be directly targeted and also how it is affected by anabolic therapies would contribute significant insight into the optimal sequential treatment approach in these patients.
Conclusion
The discovery of osteoclast recycling provides a novel mechanistic framework to examine osteoclast biology and their role in skeletal diseases. Osteoclasts are long-lived cells with a complex life cycle and are capable of fission into osteomorphs, circulating and recycling to maintain bone homeostasis. Changes in osteoclast recycling, including the accumulation of the novel osteomorphs and their fusion to form mature osteoclasts, provides a mechanism underlying the rebound phenomenon following denosumab discontinuation and paves the way to a better understanding of the cellular responses to therapies targeting bone. As we improve our understanding of how other bone-targeted therapeutics impact osteoclast recycling, we will optimise sequential therapy approaches to prevent denosumab withdrawal induced bone loss. Finally, this paradigm shift in osteoclast biology will lead to more targeted and optimal treatment strategies in patients with skeletal diseases in which osteoclast recycling may be implicated.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work is supported by the University Postgraduate Award (ASK), the Westmead Charitable Trust Early Career Grant (CMG), Cancer Institute NSW and Cancer Council NSW grants (MMM). Figures in this manuscript was generated using BioRender.
Compliance with Ethical Standards
Conflict of Interest
The authors have no competing interests to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors for this manuscript.
Footnotes
This article is part of the Topical Collection on Osteoimmunology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3:481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245(4920):855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 3.Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, Hastings G, Gowen M. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271(21):12511–12516. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H. Membrane trafficking in osteoblasts and osteoclasts: new avenues for understanding and treating skeletal diseases. Traffic. 2012;13(10):1307–1314. doi: 10.1111/j.1600-0854.2012.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker DG. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975;190(4216):784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- 6.Walker DG. Control of bone resorption by hematopoietic tissue. The induction and reversal of congenital osteopetrosis in mice through use of bone marrow and splenic transplants. J Exp Med. 1975;142(3):651–663. doi: 10.1084/jem.142.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123(5):2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 8.Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- 9.Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- 10.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87(18):7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12(1):17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 13.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12(9):1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce BF, Xing L, Chen D. Osteoprotegerin, the bone protector, is a surprising target for beta-catenin signaling. Cell Metab. 2005;2(6):344–345. doi: 10.1016/j.cmet.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawley KM, Bustamante-Gomez NC, Guha AG, MacLeod RS, Xiong J, Gubrij I, et al. Local production of osteoprotegerin by osteoblasts suppresses bone resorption. Cell Rep. 2020;32(10):108052. doi: 10.1016/j.celrep.2020.108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, Smedt TD, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi N, Udagawa N, Akatsu T, Tanaka H, Shionome M, Suda T. Role of colony-stimulating factors in osteoclast development. J Bone Miner Res Off J Am Soc Bone Miner Res. 1991;6(9):977–985. doi: 10.1002/jbmr.5650060912. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 20.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitaura H, Marahleh A, Ohori F, Noguchi T, Shen W-R, Qi J, Nara Y, Pramusita A, Kinjo R, Mizoguchi I. Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int J Mol Sci. 2020;21(14):E5169. doi: 10.3390/ijms21145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong J, Cawley K, Piemontese M, Fujiwara Y, Zhao H, Goellner JJ, O’Brien CA. Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss. Nat Commun. 2018;9(1):2909. doi: 10.1038/s41467-018-05244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis*. Endocr Rev. 2000;21(2):115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 24.Hall BK. The origin and fate of osteoclasts. Anat Rec. 1975;183(1):1–11. doi: 10.1002/ar.1091830102. [DOI] [PubMed] [Google Scholar]
- 25.Baron R, Vignery A. Behavior of osteoclasts during a rapid change in their number induced by high doses of parathyroid hormone or calcitonin in intact rats. Metab Bone Dis Relat Res. 1981;2(5):339–346. doi: 10.1016/0221-8747(81)90020-5. [DOI] [Google Scholar]
- 26.Tonna EA. Periosteal osteoclasts, skeletal development and ageing. Nature. 1960;185(4710):405–407. doi: 10.1038/185405a0. [DOI] [PubMed] [Google Scholar]
- 27.Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med. 1993;178(5):1733–1744. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77(4):477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 29.Soysa NS, Alles N. Positive and negative regulators of osteoclast apoptosis. Bone Rep. 2019;11:100225. doi: 10.1016/j.bonr.2019.100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142(9):4047–4054. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 31.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, Nissenson RA. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res Off J Am Soc Bone Miner Res. 2004;19(2):235–244. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Xu C, Zhao H, Xia P, Song R, Gu J, Liu X, Bian J, Yuan Y, Liu Z. Osteoprotegerin induces apoptosis of osteoclasts and osteoclast precursor cells via the Fas/Fas ligand pathway. PLoS ONE. 2015;10(11):e0142519. doi: 10.1371/journal.pone.0142519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators — mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2(10):1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 36.Chamoux E, Houde N, L’Eriger K, Roux S. Osteoprotegerin decreases human osteoclast apoptosis by inhibiting the TRAIL pathway. J Cell Physiol. 2008;216(2):536–542. doi: 10.1002/jcp.21430. [DOI] [PubMed] [Google Scholar]
- 37.Liu CC, Howard GA. Bone-cell changes in estrogen-induced bone-mass increase in mice: dissociation of osteoclasts from bone surfaces. Anat Rec. 1991;229(2):240–250. doi: 10.1002/ar.1092290211. [DOI] [PubMed] [Google Scholar]
- 38.Russell RGG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25(1):97–106. doi: 10.1016/S8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 39.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res Off J Am Soc Bone Miner Res. 1998;13(4):581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 40.Frith JC, Rogers MJ. Antagonistic effects of different classes of bisphosphonates in osteoclasts and macrophages in vitro. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18(2):204–212. doi: 10.1359/jbmr.2003.18.2.204. [DOI] [PubMed] [Google Scholar]
- 41.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(S12):2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 42.Hancox NM. Motion picture observations on osteoclasts in vitro. J Physiol. 1949;110(1–2):205–206.1. doi: 10.1113/jphysiol.1949.sp004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.•.Jansen IDC, Vermeer JAF, Bloemen V, Stap J, Everts V. Osteoclast fusion and fission. Calcif Tissue Int. 2012;90(6):515–22. Advances in live cell imaging techniques have allowed the authors to provide the first in vitro evidence of osteoclast fission. [DOI] [PMC free article] [PubMed]
- 44.•.Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, Eitler J, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568(7753):541–5. This parabiosis study showed that osteoclasts are long lived cells and their function is maintained through fusion and long-term gene transfer following transfusion. [DOI] [PMC free article] [PubMed]
- 45.•.Yahara Y, Barrientos T, Tang YJ, Puviindran V, Nadesan P, Zhang H, et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat Cell Biol. 2020;22(1):49–59. This cell-fate study showed that erythromyeloid progenitor derived osteoclasts were long lived cells which remained alive at 6 months following birth and were capable of contributing to bone remodelling in adult mice. [DOI] [PMC free article] [PubMed]
- 46.McDonald MM, Kim AS, Mulholland BS, Rauner M. New Insights Into Osteoclast Biology. JBMR Plus. 2021 Sep;5(9):e10539. [DOI] [PMC free article] [PubMed]
- 47.••.McDonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021;184(5):1330-1347.e13. This pivotal study first described the novel process of osteoclast recycling where osteoclasts undergo fission into smaller daughter cells termed osteomorphs, which are transcriptionally distinct from osteoclasts. Osteoclast recycling provides a novel framework in which changes in osteoclast biology in skeletal disorders and responses to bone directed therapies can be visualised.
- 48.Freudenthal B, Logan J, Croucher PI, Williams GR, Bassett JHD. Rapid phenotyping of knockout mice to identify genetic determinants of bone strength. J Endocrinol. 2016;231(1):R31–R46. doi: 10.1530/JOE-16-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mabilleau G, Libouban H, Geoffroy V. Osteomorphs as a tool for personalized medicine. Trends Endocrinol Metab. 2021;32(9):655–656. doi: 10.1016/j.tem.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 51.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, et al. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. 2012;11(5):401–419. [DOI] [PubMed]
- 52.Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, Chen C, Li L, Cattley RC, van G, Scully S, Elliott R, Grisanti M, Morony S, Tan HL, Asuncion F, Li X, Ominsky MS, Stolina M, Dwyer D, Dougall WC, Hawkins N, Boyle WJ, Simonet WS, Sullivan JK. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res Off J Am Soc Bone Miner Res. 2009;24(2):182–195. doi: 10.1359/jbmr.081112. [DOI] [PubMed] [Google Scholar]
- 53.Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 54.Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. The lancet Diabetes & endocrinology. 2017;5(7):513–23. [DOI] [PubMed]
- 55.Dempster DW, Chines A, Bostrom MP, Nieves JW, Zhou H, Chen L, Pannacciulli N, Wagman RB, Cosman F. Modeling-based bone formation in the human femoral neck in subjects treated with denosumab. J Bone Miner Res Off J Am Soc Bone Miner Res. 2020;35(7):1282–1288. doi: 10.1002/jbmr.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freemantle N, Satram-Hoang S, Tang E-T, Kaur P, Macarios D, Siddhanti S, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23(1):317–326. doi: 10.1007/s00198-011-1780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naik-Panvelkar P, Norman S, Elgebaly Z, Elliott J, Pollack A, Thistlethwaite J, Weston C, Seibel MJ. Osteoporosis management in Australian general practice: an analysis of current osteoporosis treatment patterns and gaps in practice. BMC Fam Pract. 2020;21(1):32. doi: 10.1186/s12875-020-01103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis— 2020 Update Executive Summary. Endocr Pract. 2020;26(5):564–570. doi: 10.4158/GL-2020-0524. [DOI] [PubMed] [Google Scholar]
- 59.Kanis JA, Cooper C, Rizzoli R, Reginster J-Y, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, Wette V, Balic M, Haslbauer F, Melbinger E, Bjelic-Radisic V, Artner-Matuschek S, Fitzal F, Marth C, Sevelda P, Mlineritsch B, Steger GG, Manfreda D, Exner R, Egle D, Bergh J, Kainberger F, Talbot S, Warner D, Fesl C, Singer CF, Austrian Breast and Colorectal Cancer Study Group Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. The Lancet. 2015;386(9992):433–443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 61.Smith MR, Egerdie B, Toriz NH, Feldman R, Tammela TLJ, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyce AM. Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep. 2017;15(4):283–292. doi: 10.1007/s11914-017-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhou YJJ, Wang C-J, Nguyen M, Vanderniet JA, Munns CF, Coleman H, Kim J, Holmes-Walker DJ, Lim L, Girgis CM. Clinical and radiologic response of central giant cell granuloma to denosumab: a 6-year prospective observational study. Calcif Tissue Int. 2022 Apr;110(4):464–74. [DOI] [PubMed]
- 64.Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O'Keefe R, Papapoulos S, Howe TS, van der Meulen MCH, Weinstein RS, Whyte MP. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 65.Diz P, López-Cedrún JL, Arenaz J, Scully C. Denosumab-related osteonecrosis of the jaw. J Am Dent Assoc 1939. 2012;143(9):981–4. [DOI] [PubMed]
- 66.McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM. Observations following discontinuation of long-term denosumab therapy. Osteoporos Int. 2017;28(5):1723–1732. doi: 10.1007/s00198-017-3919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen J-EB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- 68.Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. 2017;32(6):1291–1296. doi: 10.1002/jbmr.3110. [DOI] [PubMed] [Google Scholar]
- 70.Fassio A, Adami G, Benini C, Vantaggiato E, Saag KG, Giollo A, Lippolis I, Viapiana O, Idolazzi L, Orsolini G, Rossini M, Gatti D. Changes in Dkk-1, sclerostin, and RANKL serum levels following discontinuation of long-term denosumab treatment in postmenopausal women. Bone. 2019;123:191–195. doi: 10.1016/j.bone.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 71.Kuroshima S, Al-Salihi Z, Yamashita J. Mouse anti-RANKL antibody delays oral wound healing and increases TRAP-positive mononuclear cells in bone marrow. Clin Oral Investig. 2016;20:727–736. doi: 10.1007/s00784-015-1550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fontalis A, Gossiel F, Schini M, Walsh J, Eastell R. The effect of denosumab treatment on osteoclast precursor cells in postmenopausal osteoporosis. Bone Reports. 2020 Oct 1;13:100457. [DOI] [PubMed]
- 73.Omiya T, Hirose J, Omata Y, Tominari T, Inada M, Watanabe H, Miyamoto T, Tanaka S. Sustained anti-osteoporotic action of risedronate compared to anti-RANKL antibody following discontinuation in ovariectomized mice. Bone Rep. 2020;13:100289. doi: 10.1016/j.bonr.2020.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gossiel F, Hoyle C, McCloskey EV, Naylor KE, Walsh J, Peel N, et al. The effect of bisphosphonate treatment on osteoclast precursor cells in postmenopausal osteoporosis: The TRIO study. Bone. 2016;92:94–99. doi: 10.1016/j.bone.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 75.Coffman AA, Basta-Pljakic J, Guerra RM, Ebetino FH, Lundy MW, Majeska RJ, Schaffler MB. A bisphosphonate with a low hydroxyapatite binding affinity prevents bone loss in mice after ovariectomy and reverses rapidly with treatment cessation. JBMR Plus. 2021;5(4):e10476. doi: 10.1002/jbm4.10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jähn-Rickert K, Wölfel EM, Jobke B, Riedel C, Hellmich M, Werner M, McDonald MM, Busse B. Elevated bone hardness under denosumab treatment, with persisting lower osteocyte viability during discontinuation. Front Endocrinol. 2020;11:250. doi: 10.3389/fendo.2020.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sølling AS, Harsløf T, Langdahl B. Treatment with zoledronate subsequent to denosumab in osteoporosis: a 2-year randomized study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2021;36(7):1245–1254. doi: 10.1002/jbmr.4305. [DOI] [PubMed] [Google Scholar]
- 78.Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, Burnett-Bowie SAM. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet Lond Engl. 2015;386(9999):1147–1155. doi: 10.1016/S0140-6736(15)61120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu Q, Bustamante-Gomez C, Guha AG, Gubrij I, Kim H, O’Brien CA. Low osteoblast number and OPG levels may contribute to rebound resorption after discontinuation of Denosumab administration. J Bone Min Res 36 Suppl 1. Available at https://www.asbmr.org/meetings/annualmeeting/AbstractDetail?aid=10776d27-3ea4-420a-9495-ef47c9687c49. Accessed February 2, 2022.
- 81.Ebina K, Hirao M, Tsuboi H, Nagayama Y, Kashii M, Kaneshiro S, Miyama A, Nakaya H, Kunugiza Y, Okamura G, Etani Y, Takami K, Goshima A, Nakata K. Effects of prior osteoporosis treatment on early treatment response of romosozumab in patients with postmenopausal osteoporosis. Bone. 2020;140:115574. doi: 10.1016/j.bone.2020.115574. [DOI] [PubMed] [Google Scholar]
- 82.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE. 2011;6(10):e25900. doi: 10.1371/journal.pone.0025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allison H, Holdsworth G, McNamara LM. Scl-Ab reverts pro-osteoclastogenic signalling and resorption in estrogen deficient osteocytes. BMC Mol Cell Biol. 2020;21(1):1–15. doi: 10.1186/s12860-020-00322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]