Abstract
Background and Aims
Takotsubo syndrome (TTS), also known as stress cardiomyopathy, is characterized by acute and transient left ventricular dysfunction and has increased during the COVID‐19 pandemic. Herein, we aim to review studies on TTS that were associated with COVID‐19 infection, vaccine, and other COVID‐19‐related etiologies including psychosocial stressors.
Methods
We systematically searched PubMed, EMBASE, and Scopus up to May 12, 2022. We included case reports, case series, and original articles that reported at least one TTS case associated with COVID‐19, or TTS cases after receiving COVID‐19 vaccines, or TTS cases secondary to psychological stress due to the COVID‐19 pandemic. The quality assessment was conducted using the Joanna Briggs Institute checklist.
Results
Sixty‐seven articles including 102 cases were included. Hypertension was the most frequently accompanying comorbidity (N = 67 [65.6%]) and the mean left ventricular ejection fraction was 36.5%. Among COVID‐19 patients, the in‐hospital mortality rate was 33.3%. On the other hand, only one COVID‐19‐negative individual expired (2.3%). The most common presenting clinical symptom was dyspnea in 42 (73.6%) patients. the mean time interval from the first symptom to admission was 7.2 days. The most common chest imaging finding was ground‐glass opacity which was reported in 14 (31.1%) participants. The most common abnormalities were T‐wave inversion in 35 (43.2%) and ST‐segment elevation in 30 (37%). Brain natriuretic peptide and troponin were elevated in 94.7% and 95.9% of participants, respectively.
Conclusion
The TTS in patients with COVID‐19 is almost rare, whereas it could lead to a great mortality and morbidity. An individual with COVID‐19, especially an elderly woman, presented with dyspnea in addition to a rise in brain natriuretic peptide and troponin should be evaluated for TTS.
Keywords: COVID‐19, SARS‐CoV‐2, stress cardiomyopathy, systematic review, takotsubo cardiomyopathy, takotsubo syndrome
1. INTRODUCTION
Takotsubo syndrome (TTS) also known as stress cardiomyopathy is characterized by acute and transient left ventricular dysfunction without coronary obstruction. 1 It is reported to be often triggered by physical or emotional triggers. 1 The clinical presentation of TTS closely imitates that of acute coronary syndrome, which most patients present with chest pain, show ST‐segment elevation on electrocardiogram (ECG), and mild increase in serum troponin levels. 2 Due to a similar clinical picture of TTS and acute coronary syndrome, distinguishing the two conditions still remains crucial; therefore, ischemic cardiomyopathies have to be excluded before TTS diagnosis. 2 Although TTS is often reversible, it can lead to acute heart failure, left ventricle outflow tract obstruction, cardiogenic shock, thrombosis formation, and arrhythmias. 3
Recently, studies have reported an increase in TTS incidence during the COVID‐19 pandemic. 4 A retrospective cohort study indicated that acute coronary syndrome resulting from stress cardiomyopathy was found to be higher during the pandemic period (7.8%) compared to similar periods before the pandemic (1.5%–1.8%). 5 These observations suggested viral mechanism associated with COVID‐19 causing TTS, as well as an increase in TTS in the pandemic due to the associated psychological, social, and economic stress derived from imposed restrictive measures. Therefore, the severe acute respiratory syndrome coronavirus disease 2 (SARS‐CoV‐2) infection potentially induces physical and psychosocial stress in patients, which may lead to an increase in the risk of TTS development. 6 Although the underlying pathophysiology of COVID‐19‐induced TTS still remains unclear, some mechanisms have been proposed in this regard. Direct viral myocardial injury, downregulation of angiotensin‐converting enzyme 2 receptors in myocardium, cytokine storm, surge in catecholamines, and vascular inflammation are proposed to be associated with cardiac injury. 7 , 8 , 9 , 10 Moreover, studies also reported TTS and other cardiomyopathies in non‐COVID‐19 patients after receiving the mRNA‐based and other vaccines despite the low reported rate of cardiovascular complications. 11
A previous systematic review evaluated the effects of COVID‐19 in development of TTS using case reports in 2020. 12 The study did not evaluate the effects of COVID‐19 vaccination in the development of TTS and its results need to be updated. Hence, in this systematic review, we aimed to review studies on TTS that were associated with COVID‐19 infection, vaccine, and other COVID‐19‐related etiologies including psychosocial stressors. The findings could be helpful for clinicians and health authorities for prevention and management of TTS in the COVID‐19 pandemic.
2. METHODS
The present systematic review was prepared based on Preferred Reporting Items for Systematic reviews and Meta‐Analyses guidelines. 13 The study protocol was approved by PROSPERO with the registration code CRD42021282245 (www.crd.york.ac.uk/PROSPERO/). Since ethical approval and the Institutional Review Board (IRB) were reported for each of the included studies, no additional ethical or IRB approvals were required for this systematic review.
2.1. Search strategy
We searched the main medical databases, including PubMed, EMBASE and Scopus, up to May 12, 2022. We used following search terms and combinations: (“takotsubo cardiomyopathy” OR “takotsubo syndrome” OR “stress cardiomyopathy” OR “broken heart syndrome” OR “apical ballooning syndrome”) AND (“COVID‐19” OR “2019‐nCoV disease” OR “coronavirus disease‐19” OR “SARS Coronavirus 2” OR “SARS‐CoV‐2” OR “Wuhan Coronavirus” OR “COVID‐19 Vaccines” OR “SARS‐CoV‐2 Vaccine” OR “Coronavirus Disease 2019 Vaccine” OR “2019‐nCoV Vaccine”). The search strategy for each database is provided in Supporting Information: Table S1. Neither article language nor publication time was restricted. All of the titles and abstracts were screened independently by three reviewers to find potentially eligible studies. Discussions among all of the authors resolved disagreements regarding the inclusion of studies. The full text of those studies found to be eligible for inclusion based on the title and abstract screening were further studied and assessed for inclusion. Backward and forward citation searching was conducted to find any potential additional studies.
2.2. Inclusion and exclusion criteria
We included case reports and case series that reported at least one TTS case associated with COVID‐19, or TTS cases after receiving COVID‐19 vaccines, or TTS cases secondary to psychological stress due to the COVID‐19 pandemic. We also included all observational studies, including, case–control, cohort, and cross‐sectional studies, that investigate the association of TTS to COVID‐19 infection and other COVID‐19‐related stressors. We excluded opinions, book chapters, reviews, letters, and conference abstracts, as well as animal and in‐vitro studies. The diagnosis of TTS is considered in accordance with the criteria outlined by Mayo Clinic. 14
2.3. Data extraction
Three authors separately extracted the following data from studies: study characteristics including author name, publication date, study design, study country, inclusion and exclusion criteria, number of participants and cases, demographic data of participants including age, gender, race and ethnicity, body mass index (BMI), history of menopause, history of neuropsychological disorders, history of cardiovascular risk factors including diabetes, hypertension, and dyslipidemia, history of myocardial infarction, any other medical history, vital signs during presentation including heart rate, blood pressure, respiratory rate, body temperature, and O2 saturation, examination findings, COVID‐19 test results, COVID‐19 symptoms including fever, dyspnea, chest pain, cough, or other presentations, troponin, brain natriuretic peptide (BNP), and creatinine kinase (CK) level, electrocardiogram findings, trans‐thoracic echocardiogram findings, angiography findings, type of TTS, and outcomes. The discrepancies were resolved by discussion or consultation with another author.
2.4. Quality assessment
The quality of all included studies was assessed using the Joanna Briggs Institute (JBI) critical appraisal checklists for case reports, 15 case series, 16 and cohort studies. 17 The checklist for case reports rates the quality of studies by eight major questions which are providing the patient's demographic characteristics, medical history, current clinical condition, description of diagnostic tests, treatment, post‐intervention clinical conditions, adverse events, and mentioning of takeaway lessons. 15 The checklist for case series include items which are providing the inclusion criteria, methods of condition measurement, validity of the diagnostic methods, consecutive inclusion of participants, completeness of participants' inclusion, reporting of the demographic characteristics, clinical information, outcomes, presenting clinic demographic information and the quality of the statistical analysis. 16 The JBI checklist for cohort studies includes 11 items, which are similarity of the two groups, similarity in measurement of exposures, validity and reliability of measuring exposure, identifying confounders, stating strategies for dealing with confounders, lacking of the outcomes at the start of the study, validity and reliability of outcome measurement, completeness and sufficient duration of follow‐up time, using strategies to address incomplete follow‐up, and using appropriate statistical analysis. 17 A higher quality score represents a better quality of that study in the JBI checklists.
3. RESULTS
A total of 1064 studies were identified. After applying the eligibility criteria and title and abstract review followed by detailed evaluations, 67 articles were selected. 4 , 5 , 9 , 11 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 Of these articles, 46 were centered around TTS in patients with COVID‐19, 13 were related to TTS in patients with emotional triggering events, and 8 were linked to TTS in patients with recent COVID‐19 vaccination (Figure 1).
Figure 1.
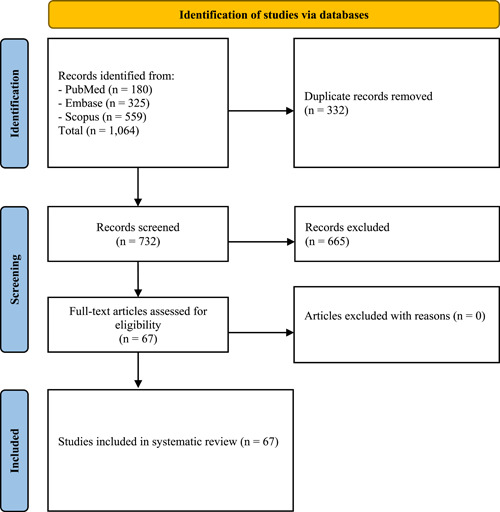
Study selection process
All articles were written in English. Thirty (44.7%) were from the United States. The details on the study characteristic of included articles are represented in Table 1. A total of 102 patients were included in this review which comprised three groups. The first group consists of 60 patients (58.8%) with confirmed COVID‐19 diagnosis, the second group consisted of 34 cases (33.3%) with known emotional triggering events, and the third group consisted of 8 cases (7.8%) who had recently received COVID‐19 vaccination. The mean age of the reported cases was 67.2 (SD = 12.6; range 30–94) years with a female predominance of 68.6%. Among women with TTS, data on patients' age was available in 57 cases, of which 78.9% were above 60 years of age. Hypertension was the most frequently accompanying comorbidity (N = 67 [65.6%]), followed by dyslipidemia (N = 38 [37.2%]) and diabetes (N = 26 [25.4%]). Data on the left ventricular ejection fraction (LVEF) were reported in 60 cases. The overall mean LVEF was 36.5% (males: 37.2% and females: 36.2%) (Table 1). Among COVID‐19 patients, the in‐hospital mortality rate was 33.3%. On the other hand, only one COVID‐19‐negative individual was expired (2.3%) (Table 1).
Table 1.
Baseline and clinical characteristics of the reported patients
| Study, year | Study design | Study country | Age (years) | Gender | Past medical history | LVEF (%) | Outcome | Follow‐up echocardiography |
|---|---|---|---|---|---|---|---|---|
| Takotsubo after COVID‐19 articles | ||||||||
| Nguyen et al. (2020) 19 | Case report | Belgium | 71 | Female | HTN, DLP | NR | Discharged in good condition | NR |
| Panchal et al. (2020) 20 | Case report | USA | 65 | Male | DM, HTN, AF | NR | Deceased | N/A |
| Kariyanna et al. (2020) 21 | Case report | USA | 72 | Female | DM, HTN, DLP, obesity | NR | Deceased | N/A |
| Alizadehasl et al. (2022) 22 | Case report | Iran | 57 | Male | HTN, DLP | NR | Discharged in good condition | On 6 weeks follow‐up: mild biventricular residual systolic dysfunction |
| Fujisaki et al. (2021) 23 | Case report | USA | 60 | Male | DM, HTN, DLP | 15% | Discharged in good condition | On 37 days follow‐up: resolution of the wall motion abnormalities and the LVEF was 55% |
| Demertzis et al. (2020) 24 | Case series | USA | 76 | Female | HTN, obstructive sleep apnea | 40% | Deceased | N/A |
| 67 | Female | Nonischemic dilated cardiomyopathy with preservedEF = 50%, HTN | N/A | Discharged in good condition | On 3 weeks follow‐up: resolution of the wall motion abnormalities with an EF of 63% | |||
| Torabi et al. (2021) 25 | Case report | USA | 42 | Female | Crohn's disease | 20% | Deceased | N/A |
| Ortuno et al. (2021) 26 | Case report | France | 79 | Male | DM, HTN, CKD | 40% | Deceased | Restoration of LVEF with decrease of apical ballooning aspect |
| Hegde et al. (2020) 27 | Case series | USA | 71 | Female | DM, HTN, DLP | 15% | Deceased | N/A |
| 78 | Male | DM, HTN, DLP, CVA, AF | 53% | Discharged in good condition | NR | |||
| 70 | Female | DM, HTN, DLP | 45% | Discharged in good condition | NR | |||
| 78 | Female | DM, HTN, DLP, CVA, AF | 20% | Deceased | N/A | |||
| 88 | Male | DM, HTN, DLP, CKD, CVA, AF | 30% | Deceased | N/A | |||
| 58 | Male | DLP | 40% | Discharged in good condition | NR | |||
| 56 | Male | HTN, DLP, CVA, AF, schizophrenia | 45% | Deceased | N/A | |||
| Hoepler et al. (2021) 28 | Case series | Austria | 67 | Female | HTN, DLP, CKD | 65% | Discharged in good condition | On 4 weeks follow‐up: normal left ventricular function without any regional differences and normal heart valves |
| 60 | Female | COPD, depression | 68% | Still under medical care | NR | |||
| 73 | Female | Osteoporosis, chronic pain syndrome | 20% | Still under medical care | On 2 weeks follow‐up: normal global systolic left ventricular function | |||
| Alshamam et al. (2021) 4 | Case report | USA | 86 | Female | HTN, osteoporosis, anemia | 35%‐40% | Deceased | N/A |
| Bernardi et al. (2020) 29 | Case report | Italy | 74 | Male | Impaired fasting blood sugar, HTN, DLP | 30% | Discharged in good condition | On 14 days follow‐up: resolution of the 2 thrombi and a complete restoration of LVEF (57%) |
| Sattar et al. (2020) 30 | Case report | USA | 67 | Female | DM, HTN | 30% | Discharged in good condition | NR |
| Tsao et al. (2020) 31 | Case report | USA | 59 | Female | None | 36% | Discharged in good condition | On 10 days follow‐up: resolution of the stress cardiomyopathy, with normal biventricular systolic function |
| Gomez et al. (2020) 32 | Case report | USA | 57 | Female | Crohn's disease, morbid obesity | 25%–30% | Discharged in good condition | On 18 days follow‐up: resolution of left ventricular dysfunction with no appreciable regional wall abnormalities (LVEF 70%–75%) |
| Belli et al. (2021) 18 | Case report | Italy | 53 | Female | CKD | 30% | Still under medical care | On following week follow‐up: improvement of left ventricular systolic function and motion abnormalities |
| Titi et al. (2021) 33 | Case report | Italy | 83 | Male | DM, HTN, DLP, COPD | NR | Deceased | N/A |
| Faqihi et al. (2020) 34 | Case report | Kingdom of Saudi Arabia | 40 | Male | None | 30% | Discharged in good condition | NR |
| Solano‐López et al. (2020) 35 | Case report | Spain | 50 | Male | Asymptomatic benign mediastinal tumor since childhood | NR | Discharged in good condition | On discharge: significant improvement of left ventricular contractility |
| Pasqualetto et al. (2020) 36 | Case series | Italy | 84 | Male | DM, HTN | 53% | Discharged in good condition | NR |
| 85 | Female | HTN | 30% | Deceased | N/A | |||
| 81 | Male | DM, HTN | 42% | Discharged in good condition | NR | |||
| Koh et al. (2021) 37 | Case report | Singapore | 34 | Male | None | 30% | Discharged in good condition | NR |
| Dave et al. (2020) 38 | Case report | USA | 59 | Female | HTN, COPD | 26% | Deceased | N/A |
| Van Osch et al. (2020) 39 | Case report | UK | 72 | Female | AF | 30% | Discharged in good condition | On 3 months follow‐up: normal contractility of the apical myocardial segments, with normalization of the left ventricular systolic function (LVEF: 55%) |
| Bhattacharyya et al. (2020) 40 | Case report | India | 32 | Female | None | 38% | Discharged in good condition | On 13 days follow‐up: normalization of the LV regional wall motion abnormalities (LVEF: 51%) |
| Taza et al. (2020) 41 | Case report | USA | 52 | Male | DM, HTN, schizophrenia | 45% | Discharged in good condition | NR |
| Bottiroli et al. (2020) 42 | Case report | Italy | 76 | Female | None | 25% | Discharged in good condition | On 62 days follow‐up: improvement of the wall motion of apical segments and complete recovery of LVEF up to normal values |
| Roca et al. (2020) 43 | Case report | Italy | 87 | Female | Breast cancer | 48% | Discharged in good condition | NR |
| Oyarzabal et al. (2020) 44 | Case report | Spain | 82 | Male | DM, HTN, DLP, CKD | NR | Discharged in good condition | NR |
| Minhas et al. (2020) 45 | Case report | USA | 58 | Female | DM, HTN, DLP | 20% | Discharged in good condition | On 6 days follow‐up: improvement noted in overall wall motion and LVEF 55% |
| Kong et al. (2021) 46 | Case series | USA | 88 | Male | Prostate cancer under chemotherapy, dementia | Severe LV systolic dysfunction | Deceased | N/A |
| 79 | Female | MS, non‐obstructive CAD | 28.9% | Discharged in good condition | NR | |||
| Park et al. (2020) 47 | Case series | Korea | 78 | Female | None | Severe LV systolic dysfunction | Deceased | N/A |
| 73 | Female | None | Severe LV systolic dysfunction | Deceased | N/A | |||
| Meyer et al. (2020) 48 | Case report | Switzerland | 83 | Female | HTN | NR | Discharged in good condition | Only mild residual apical hypokinesis on the day of discharge |
| Eftekharzadeh et al. (2022) 49 | Case report | USA | 94 | Female | Anxiety disorder | NR | Deceased | N/A |
| Frynas‐Jończyk et al. (2022) 50 | Case report | Poland | 76 | Female | HTN, Asthma, DVT | 30% | Discharged in good condition | On 2 months follow‐up: resolution of apical ballooning and improvement of EF to 58% |
| Fujiyoshi et al. (2022) 51 | Case report | Japan | 71 | Female | HTN, anxiety disorder | 58% | Discharged in good condition | On 2 weeks follow‐up: normal LV wall motion with trivial apical hypertrophy and EF: 63% |
| Kimura et al. (2021) 52 | Case report | Japan | 68 | Female | HTN | 50% | Discharged in good condition | On 66 days follow‐up: marked decrease in apical ballooning of the left ventricle, indicating good recovery |
| Mishra et al. (2021) 53 | Case report | USA | 70 | Male | HTN, DLP, DM, COPD, AF, status post cardioversion, ablation | NR | Deceased | N/A |
| Namburu et al. (2021) 54 | Case report | USA | 69 | Male | HTN | 45% | Discharged in good condition | On week 1 follow‐up: improvement in LVEF (60%) with resolution of right atrial thrombus |
| Rivera et al. (2021) 55 | Case report | Spain | 94 | Female | HTN, paroxysmal AF, CVA | NR | Discharged in good condition | At 2 months follow‐up TTE demonstrated recovery of ventricular contractility |
| Wildermann et al. (2022) 56 | Case report | Germany | 39 | Female | MS | NR | Discharged in good condition | NR |
| Bapat et al. (2020) 57 | Case report | USA | 67 | Female | HTN, DM, asthma | 61% | Discharged in good condition | NR |
| Chao et al. (2020) 58 | Case report | USA | 49 | Male | None | 40% | Discharged in good condition | Normalization of LVEF to 55% and marked improvement in regional wall motion abnormalities |
| Dabbagh et al. (2020) 59 | Case report | USA | 67 | Female | Nonischemic cardiomyopathy | 40% | Discharged in good condition | Stable ejection fraction and resolution of pericardial effusion |
| Manzur‐Sandoval et al. (2021) 60 | Case report | USA | 54 | Female | HTN, DM | NR | Discharged in good condition | Reversal of regional wall‐motion abnormalities in the apical two‐chamber view and in the left ventricular longitudinal strain |
| Sang et al. (2020) 61 | Case report | USA | 58 | Female | HTN, COPD, RA | Poor | Deceased | N/A |
| Tutor et al.(2021) 62 | Case series | USA | 78 | Male | HTN, CAD, CKD, AF | 25% | Deceased | N/A |
| 76 | Male | HTN, DM, DLP | 30% | Discharged in good condition | 4 months follow‐up: LVEF 55%–60% with no wall motion abnormality | |||
| Takotsubo after social stress articles | ||||||||
| Habedank et al. (2020) 63 | Case report | Germany | 63 | Female | HTN, anxiety, depression | 35% | Discharged in good condition | On 4 days follow‐up: still moderate hypokinesia in the mid‐anterior section and LVEF recovered too normal |
| Giannitsi et al. (2020) 64 | Case report | Greece | 79 | Female | HTN | 35% | Discharged in good condition | NR |
| Parker et al. (2020) 65 | Case report | Australia | 69 | Female | Lung cancer | 34% | Discharged in good condition | NR |
| Uhe et al. (2020) 66 | Case report | Germany | 81 | Female | HTN, CKD | 45% | Discharged in good condition | On 2 months follow‐up: full recovery of LV function |
| Chadha. (2020) 67 | Case report | USA | 85 | Female | None | 35% | Discharged in good condition | On 5 days follow‐up: complete recovery of the LV systolic function |
| Rivers et al. (2020) 68 | Case report | Australia | 71 | Female | None | NR | Discharged in good condition | NR |
| Koutroumpakis et al. (2020) 69 | Case report | USA | 65 | Female | None | 30% | Discharged in good condition | On 6 weeks follow‐up: normalization of the left ventricular function |
| Jabri et al. (2020) 5 | Cohort (N = 20 participants) | USA | Mean: 63 | Female (N = 13), Male (N = 7) | DM (N = 3), HTN (N = 19), DLP (N = 14), CAD (N = 5), AF (N = 3), CKD (N = 2), COPD (N = 2) | 30 (IQR: 25‐35) | Deceased (N = 1), discharged in good condition (N = 19) | NR |
| Dolci et al. (2021) 70 | Case report | Italy | 65 | Female | HTN, DLP | NR | Discharged in good condition | On 6 weeks follow‐up: patient was asymptomatic with full recovery of LV function |
| Moady et al. (2021) 71 | Case series | Israel | 81 | Female | DLP, hypothyroidism | 38% | Discharged in good condition | On discharge: mild apical hypokinesia without left ventricular outflow tract obstruction (LVEF: 44%) |
| 70 | Female | Hypothyroidism, AML | 42% | Discharged in good condition | On discharge: normal cardiac anatomy and function | |||
| Kir et al. (2021) 9 | Case series | USA | 85 | Female | DM, HTN, DLP, CKD | 45%–50% | Discharged in good condition | On 4 weeks follow‐up: complete resolution of her cardiomyopathy |
| 70 | Female | HTN | 30% | Discharged in good condition | On 1 month follow‐up: normal left ventricular wall motion, confirming her prior cardiomyopathy to be stress‐mediated (LVEF: 60%–65%) | |||
| Mohammed et al. (2020) 72 | Case report | USA | 60 | Female | DLP, anemia | 22% | Discharged in good condition | NR |
| Ben Ammar et al. (2021) 73 | Case report | Tunis | 59 | Male | DM, HTN, DLP, CVA | 40% | Discharged in good condition | NR |
| Takotsubo after COVID‐19 vaccination articles | ||||||||
| Vidula et al. (2021) 11 | Case series | USA | 60 | Female | CAD | 44% | Discharged in good condition | NR |
| Boscolo Berto et al. (2021) 74 | Case report | Switzerland | 63 | Female | None | 40% | Discharged in good condition | NR |
| Fearon et al. (2021) 75 | Case report | USA | 73 | Female | HTN, CKD, COPD, RA, asthma, hepatocellular carcinoma | 20% | Discharged in good condition | On 3 days follow‐up: mild Improvement in biventricular function (LVEF: 35%–40%) |
| Crane et al. (2021) 76 | Case report | Australia | 72 | Male | DM, HTN, DLP, CABG, UC | 38% | Discharged in good condition | On 5 days follow‐up: complete resolution of systolic dysfunction and wall motion abnormalities (EF: 52%) |
| Stewart et al. (2022) 77 | Case report | UK | Early 50s (52.5) | Female | COPD | NR | Discharged in good condition | Normal left ventricular (LV) systolic function and resolution of previously noted wall motion abnormalities |
| Tedeschi et al. (2022) 78 | Case report | Italy | 71 | Female | Congenital LQTS (mutation in KCHNQ 1 gene), catheter ablation for paroxysmal atrial fibrillation, mitral prolapse with mild mitral regurgitation | 38% | Discharged in good condition | On Day 21 after the first dose: improvement of the LVEF up to 50% |
| Toida et al. (2022) 79 | Case report | Japan | 80 | Female | ESRD (renal sclerosis), HTN, secondary hyperparathyroidism with hyperphosphatemia | 48% | Discharged in good condition | Normalized contractility of the apical myocardial segment, with the normalization of LV ejection fraction systolic function of 63% |
| Yamaura et al. (2022) 80 | Case report | Japan | 30 | Female | NR | NR | Discharged in good condition | On 15 day, The LV contraction had returned to normal range during follow‐up transthoracic Doppler echocardiography examination |
Abbreviations: AF, atrial fibrillation; AML, acute myeloid leukemia; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease, 2019; CVA, cerebrovascular accident; DLP, dyslipidemia; DM, diabetes mellitus; DVT, deep vein thrombosis; ECG, electrocardiogram; ESRD, end‐stage renal disease; HTN, hypertension; LV, left ventricular; LVEF, left ventricular ejection fraction; MS, multiple sclerosis; NR, not reported; RA, rheumatoid arthritis; TTE, transthoracic echocardiogram; UC, ulcerative colitis.
Among patients with COVID‐19, the most common presenting clinical symptoms were dyspnea in 42/57 (73.6%), fever in 37/57 (64.9%), and cough in 29/57 (50.8%). However, among patients with emotional triggers, and patients with COVID‐19 vaccination, chest pain in 19/22 cases (86.3%) was the most common symptom (Table 2). In COVID‐19‐positive patients, the mean time interval from the first symptom to admission was 7.2 days (range 1–14 days), while it was 3.1 (range 0.08–10 days) in COVID‐19‐negative cases (Table 2). Out of 60 cases with COVID‐19, chest imaging, including chest X‐ray (CXR) or chest computed tomography scan, was performed in 45 cases. The most common chest imaging finding was ground‐glass opacity (GGO) which was reported in 14 (31.1%) participants (Table 2). During hospitalization, mechanical ventilation was required in 37/53 patients with COVID‐19 (69.8%). Of these patients, one was kept at continuous positive airway pressure and another used bilevel positive airway pressure. COVID‐19‐negative patients did not require oxygen support (Table 2). Among patients with COVID‐19, the most commonly reported in‐hospital complications were cardiogenic shock in 9/42 (21.4%), acute respiratory distress syndrome in 7/42 (16.6%), and acute kidney injury in 7/42 (16.6%). Among COVID‐19‐negative individuals, only two reported complications. One was cardiac arrest and the other was a gradual decline in the patient's visual acuity (Table 2).
Table 2.
Baseline characteristics and clinical presentations of COVID‐19
| Study, year | Clinical presentation | Time from symptom onset to admission | Heart rate (beats/min) | Blood pressure (mmHg) | Respiratory rate (breaths/min) | Saturation without O2 (%) | Saturation with O2 (%) | Temperature (°C) | Physical examination | Chest imaging | Oxygen support | In‐hospital complication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takotsubo after COVID‐19 | ||||||||||||
| Nguyen et al. (2020) 19 | Dyspnea | NR | 75 | 119/76 | NR | 89% | 100% | NR | NR | CT scan: ground glass opacity involving 10–20% of the lungs | Mechanical ventilation | NR |
| Panchal et al. (2020) 20 | Fever, dyspnea, cough, malaise | 14 days | 64 | 159/70 | 23 | NR | 87% | 39.3 | NR | CXR: multifocal pneumonia | Mechanical ventilation | Pulseless electrical arrest |
| Kariyanna et al. (2020) 21 | Cough, stroke presentation, right‐sided gaze, loss of appetite | 4 days | 98 | 146/97 | 32 | NR | 89% | 37 | NR | CXR: diffuse bilateral infiltrates | Mechanical ventilation | AKI, pulseless electrical arrest |
| Alizadehasl et al. (2022) 22 | Fever, dyspnea, chest pain, cough, diaphoresis | 5 days | 115 | 90/65 | 28 | 77% | NR | 37.9 | NR | CT scan: ground glass opacities compatible and congestion | Nasal mask | NR |
| Fujisaki et al. (2021) 23 | Fever, dyspnea | 14 days | 148 | 145/88 | 30 | NR | 75% | 38.4 | Bilateral crackles and tachycardia | CXR: diffuse opacities throughout the lung fields | Mechanical ventilation | ARDS, AKI, septic shock, cardiogenic shock |
| Demertzis et al. (2020) 24 | Fever, dyspnea, diarrhea, myalgia | 2 days | NR | NR | NR | 55% | NR | 38.5 | Bilateral crackles with end‐expiratory rhonchi, and systolic murmur along the left sternal border unchanged with respiration | CXR: bilateral multifocal patchy opacities | Mechanical ventilation | Cardiogenic shock |
| Dyspnea, Orthopnea, cough | 7 days | NR | NR | NR | NR | NR | NR | Bilateral crackles on auscultation, and muffled heart sounds | CXR: significant enlargement of the cardiac silhouette | NR | NR | |
| Torabi et al. (2021) 25 | Fever, altered mental status | 7 days | 139 | 93/62 | NR | NR | 89% | 38.2 | Diffuse crackles and no cardiac murmurs | CXR: patchy consolidative opacities in the lung fields | Mechanical ventilation | Septic shock, cardiogenic shock |
| Ortuno et al. (2021) 26 | Fever, dyspnea, cough | 5 days | NR | NR | NR | NR | 93% | 37.2 | Bilateral diffuse crackling | CT scan: typical bilateral opacity | Mechanical ventilation | ARDS, AKI, cardiogenic shock |
| Hegde et al. (2020) 27 | Cough, myalgia | NR | NR | NR | NR | NR | NR | NR | NR | NR | Mechanical ventilation | AKI, shock, AF RVR |
| Fever, altered mental status | NR | NR | NR | NR | NR | NR | NR | NR | NR | Mechanical ventilation | AKI | |
| Dyspnea | NR | NR | NR | NR | NR | NR | NR | NR | NR | Mechanical ventilation | ARDS, chronic respiratory failure | |
| Fever, dyspnea, cough | NR | NR | NR | NR | NR | NR | NR | NR | NR | Nasal mask | ARDS, shock | |
| Dyspnea, malaise | NR | NR | NR | NR | NR | NR | NR | NR | NR | Mechanical ventilation | Bilateral pleural effusion status post thoracentesis | |
| Dyspnea | NR | NR | NR | NR | NR | NR | NR | NR | NR | Mechanical ventilation | Bilateral pneumothorax status post chest tube placement, transient transaminitis | |
| Fever, dyspnea | NR | NR | NR | NR | NR | NR | NR | NR | NR | Mechanical ventilation | AKI, metabolic encephalopathy | |
| Hoepler et al. (2021) 28 | Chest pain | NR | NR | NR | NR | NR | NR | NR | NR | CXR: unremarkable | Nasal mask | None |
| Dyspnea | NR | NR | NR | NR | NR | NR | NR | Severely compromised regarding respiration | NR | Nasal mask | None | |
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | Mechanical ventilation | Respiratory failure, cardiogenic shock, cardiac arrest | |
| Alshamam et al. (2021) 4 | Dyspnea | 1 day | Normal range | Normal range | 30 | 50% | 80% | Normal range | Respiratory distress, diffuse bilateral pulmonary crackles, mild jugular venous distention, and minimal bilateral pitting edema | CXR: diffuse bilateral opacification attributing to pneumonia and/or pulmonary edema | BiPAP | ARDS, AKI, Severe microcytic anemia |
| Bernardi et al. (2020) 29 | Fever, dyspnea | NR | 95 | 135/85 | NR | NR | NR | 38 | NR | CXR: diffuse hazy densities | CPAP | Cardiogenic shock |
| Sattar et al. (2020) 30 | Fever, cough, malaise, myalgia | 14 days | 114 | 133/64 | 24 | 92% | NR | 36.9 | Bilateral coarse crackles most prominent in the lower lung fields | CXR: bibasilar mixed ground glass opacities | Nasal mask | Atrial fibrillation |
| Tsao et al. (2020) 31 | Fever, cough, fatigue, myalgia | NR | NR | NR | NR | NR | NR | NR | NR | Mechanical ventilation | ARDS, vasodilatory shock | |
| Gomez et al. (2020) 32 | Fever, dyspnea, cough, sore throat, rhinorrhea | 5 days | 89 | 118/70 | 18 | 93% | NR | 39.7 | Wheezes on pulmonary examination | CXR: diffuse bilateral alveolar infiltrates without cephalization or prominent pulmonary vascular markings | Mechanical ventilation | ARDS, cardiogenic shock, multiorgan failure |
| Belli et al. (2021) 18 | NR | NR | NR | NR | NR | NR | NR | N/A | NR | CT scan: right‐sided ground glass opacities and left‐sided dense ground glass with consolidation | Mechanical ventilation | NR |
| Titi et al. (2021) 33 | Fever, dyspnea, diarrhea | 3 days | 95 | 120/80 | NR | 95% | NR | 37 | Bilateral, basal crackles and decreased breath sounds | CT scan: lung ground glass opacities and subpleural patchy areas of consolidation | Mechanical ventilation | Cardiogenic shock, pericardial effusion |
| Faqihi et al. (2020) 34 | Chest pain, cough, myalgia | 4 days | Normal range | Normal range | Normal range | Normal range | Normal range | Normal range | Mild tachypnea and decreased breath sound at the lung bases | CXR: interstitial infiltrates and consolidations | Mechanical ventilation | Cardiogenic shock |
| Solano, López et al. (2020) 35 | Fever, dyspnea, chest pain, cough | 8 days | NR | SBP < 90 | NR | NR | NR | N/A | NR | CXR: bilateral infiltrates, CT scan: perihilar ground‐glass opacities | NR | NR |
| Pasqualetto et al. (2020) 36 | Fever, dyspnea, chest pain, cough | 10 days | NR | 220/100 | NR | NR | NR | N/A | NR | CT scan: ground‐glass opacities and bilateral consolidation in the lungs | Nasal mask | NR |
| Fever, dyspnea, chest pain, cough | 10 days | NR | NR | NR | NR | NR | N/A | NR | CT scan: ground‐glass opacities and bilateral consolidation in the lungs | Mechanical ventilation | None | |
| Fever, dyspnea, chest pain, cough | 10 days | NR | NR | NR | NR | NR | N/A | NR | CT scan: ground‐glass opacities and bilateral consolidation in the lungs | Nasal mask | NR | |
| Koh et al. (2021) 37 | Fever, dyspnea, chest pain, cough, diarrhea, nausea, and vomiting | 2 days | 125 | 82/45 | 30 | 95% | NR | 39.4 | NR | NR | Mechanical ventilation | NR |
| Dave et al. (2020) 38 | Fever, dyspnea, myalgia, diarrhea | 5 days | Normal range | Normal range | Tachypnea | Normal range | NR | Normal range | NR | CXR: right middle and lower lung infiltrates | Mechanical ventilation | None |
| Van Osch et al. (2020) 39 | Fever, dyspnea | NR | 70 | 150/70 | 25 | 92% | NR | N/A | Bilateral inspiratory crackles and expiratory rhonchi | CXR: bilateral consolidations | Mechanical ventilation | NR |
| Bhattacharyya et al. (2020) 40 | Dyspnea | 3 days | Normal range | 150/100 | Normal range | Normal range | NR | Normal range | NR | NR | NR | NR |
| Taza et al. (2020) 41 | Fever, dyspnea | NR | Normal range | NR | Tachypnea | hypoxic | NR | Febrile | NR | NR | Mechanical ventilation | NR |
| Bottiroli et al. (2020) 42 | Fever, cough | 9 days | 81 | 135/65 | 22 | NR | 90% | 38.2 | NR | CXR: diffuse opacities mainly in the right lung, CT scan: diffuse bilateral ground glass opacities | Mechanical ventilation | NR |
| Roca et al. (2020) 43 | Fever, dyspnea, cough, fatigue | 14 days | Tachycardia | NR | NR | NR | 91% | N/A | NR | CXR: multiple patchy shadows in both lungs and parenchymal thickening with bilateral basal alveolar interstitial infiltrates | Nasal mask | NR |
| Oyarzabal et al. (2020) 44 | Chest pain | NR | NR | NR | NR | NR | NR | N/A | NR | CXR: unremarkable | NR | NR |
| Minhas et al. (2020) 45 | Fever, cough, fatigue, diarrhea | 5 days | 130 | 156/95 | 24 | NR | 82% | 38.7 | Diffuse rhonchi | CXR: lower lobe predominant bilateral infiltrates | NR | NR |
| Kong et al. (2021) 46 | Fever, fatigue, loss of appetite | Several days | Tachycardia | NR | NR | NR | NR | N/A | NR | NR | Mechanical ventilation | None |
| NR | NR | Tachycardia | NR | NR | hypoxic | NR | 39.1 | NR | NR | Mechanical ventilation | NR | |
| Park et al. (2020) 47 | Fever, dyspnea, sore throat | 7 days | 112 | 114/76 | 24 | 60 | NR | 38.4 | NR | CXR: diffuse infiltration of whole lung fields | Mechanical ventilation | None |
| Fever, cough | 13 days | 70 | 148/78 | 28 | NR | NR | 37.6 | NR | CXR: typical diffuse ground‐glass appearance, CT scan: diffuse infiltration of bilateral lung fields | Mechanical ventilation | None | |
| Meyer et al. (2020) 48 | Fever, dyspnea, chest pain, cough | 3 days | Normal range | Normal range | Normal range | Normal range | NR | Normal range | Normal | CXR: clear bilateral lung opacities | Nasal mask | NR |
| Eftekharzadeh et al. (2022) 49 | Dyspnea | 7 days | 118 | 196/93 | 46 | 70% | 96% | 37.2 | Accessory muscle use with rales and rhonchi | CXR: clear lungs with cardiomegaly | None | None |
| Frynas‐Jończyk et al. (2022) 50 | Fever, cough, dyspnea | 14 days | 60 | 134/76 | NR | 95% | NR | NR | NR | CT scan: multiple peripheral, small areas of ground‐glass opacities | None | None |
| Fujiyoshi et al. (2022) 51 | Fever, dyspnea | NR | NR | NR | NR | NR | NR | NR | NR | CT scan: trivial peripheral consolidations | None | None |
| Kimura et al. (2021) 52 | Dysarthria, gait disturbance, fever, cough | 14 days | 120 | 152/114 | NR | NR | NR | 33.2 | NR | CT scan: bilateral pneumonia | Mechanical ventilation | None |
| Mishra et al. (2021) 53 | Dyspnea, fever | 7 days | 88 | 146/64 | 28 | NR | 85% | NR | Reduced breath sounds bilaterally, along with mild infrascapular crackles | CT scan: bilateral ground glass opacities and infiltrates | NR | None |
| Namburu et al. (2021) 54 | Chest pain, dyspnea | 7 days | 124 | 132/88 | 28 | 83% | 95% | 37.1 | Moderate to severe respiratory distress with bilateral basilar crackles and sinus tachycardia | CXR: bilateral patchy opacities most prominent at left lung base with minimal left pleural effusion | None | Bilateral pulmonary embolism with right heart strain |
| Rivera et al. (2020) 55 | Dyspnea, cough | 2 days | NR | NR | NR | NR | NR | NR | NR | CXR: bilateral basal pneumonia | None | None |
| Wildermann et al. (2022) 56 | Malaise, fatigue, nystagmus, dizziness, headache, cough, dyspnea | 10 days | NR | NR | NR | NR | NR | NR | NR | CT scan: multifocal central and peripheral ground glass opacities involving both pulmonary lobes | NR | None |
| Bapat et al. (2020) 57 | Dyspnea, nausea | 4 days | 118 | NR | NR | <90% | NR | NR | NR | CXR: bilateral predominantly peripherally distributed patchy opacities | Mechanical ventilation | None |
| Chao et al. (2020) 58 | Fever, cough | NR | NR | NR | NR | NR | NR | NR | NR | CXR: severe diffuse bilateral pulmonary infiltrates consistent with ARDS | Mechanical ventilation | None |
| Dabbagh et al. (2020) 59 | Cough, dyspnea, and left shoulder pain | NR | 122 | 118/82 | 24 | Normal | Normal | 36.8 | Normal | Unremarkable | None | None |
| Manzur‐Sandoval et al. (2021) 60 | Cough, fever, dyspnea | 3 days | 75 | 100/60 | NR | 82% | NR | NR | Diffuse pulmonary rales | CXR: bilateral diffuse interstitial infiltrates | Mechanical ventilation | None |
| Sang et al. (2020) 61 | Fever, dyspnea | NR | >200 | NR | NR | NR | NR | NR | NR | CXR: bronchiectasis with interstitial thickening | Mechanical ventilation | None |
| Tutor et al. (2021) 62 | Confusion, dyspnea | NR | Tachycardia | 90/50 | 20 | 85% | NR | 39 | NR | CXR: bilateral pulmonary infiltrates | Mechanical ventilation | Multiorgan failure |
| Cough, dyspnea, fever | NR | Tachycardia | Normal | 14 | 90% | NR | Normal | NR | CXR: bilateral multifocal infiltrates | Nasal mask | NR | |
| Takotsubo after social stress | ||||||||||||
| Habedank et al. (2020) 63 | Chest pain | NR | NR | NR | NR | NR | NR | NR | NR | NR | None | Cardiac arrest |
| Giannitsi et al. (2020) 64 | Chest pain | NR | 75 | 130/70 | NR | 99 | NR | NR | Normal | NR | None | None |
| Parker et al. (2020) 65 | Chest pain | NR | NR | NR | NR | NR | NR | NR | NR | NR | None | None |
| Uhe et al. (2020) 66 | Dyspnea, chest pain | NR | 78 | 130/73 | NR | NR | NR | 37.2 | NR | NR | None | None |
| Chadha et al. (2020) 67 | Chest pain | NR | NR | NR | NR | NR | NR | NR | Normal | CXR: unremarkable | None | None |
| Rivers et al. (2020) 68 | Chest pain | NR | NR | NR | NR | NR | NR | NR | NR | NR | None | None |
| Koutroumpakis et al. (2020) 69 | Chest pain, diaphoresis | NR | 63 | 142/77 | NR | NR | NR | NR | Normal | NR | None | None |
| Jabri et al. (2020) 5 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Dolci et al. (2020) 70 | Chest pain | NR | NR | NR | NR | NR | NR | NR | NR | NR | None | None |
| Moady et al. (2021) 71 | Chest pain | 2 days | 100 | 100/60 | NR | NR | NR | NR | Apical systolic heart murmur with no signs of heart failure | NR | None | None |
| Chest pain | Few hours | 95 | 170/80 | NR | NR | NR | NR | Normal | NR | None | None | |
| Kir et al. (2021) 9 | Dyspnea, chest pain | 2 h | 120 | 108/45 | 15 | 95 | NR | 36.9 | Tachycardic, with normal pulses, normal cardiac exam with no significant murmur or rub on auscultation, lungs were clear to auscultation | NR | None | None |
| Dyspnea, chest pain, diaphoresis | 1 day | NR | NR | NR | NR | NR | NR | NR | NR | None | Gradual decline in her visual acuity | |
| Mohammed et al. (2020) 72 | Dyspnea, chest pain, diaphoresis, disorientation | 7 days | 115 | 188/82 | NR | NR | NR | NR | NR | CXR: unremarkable | None | None |
| Ben Ammar et al. (2021) 73 | Weird behavior, psychomotor agitation | NR | 100 | 140/80 | NR | 99% | NR | 37 | NR | NR | None | None |
| Takotsubo after COVID‐19 vaccination articles | ||||||||||||
| Vidula et al. (2021) 11 | Chest pain | 4 days | NR | NR | NR | NR | NR | NR | NR | NR | None | None |
| Boscolo Berto et al. (2021) 74 | Fever, dyspnea | 1 day | NR | NR | NR | NR | NR | NR | NR | CT scan: revealed no pulmonary embolism but did show signs of heart failure | None | None |
| Fearon et al. (2021) 75 | Dyspnea, chest pain | 17 h | 118 | 108/57 | 24 | Normal range | NR | Normal range | Jugular venous distention | NR | None | None |
| Crane et al. (2021) 76 | Dyspnea, chest pain | 3 days | 99 | 130/65 | Tachypnoea | Normal | NR | 36.1 | Normal | CT angiography: excluded any pulmonary embolism | None | None |
| Stewart et al., 2021 77 | Chest pain, diaphoresis, dyspnea, vomiting | NR | NR but normal | NR but normal | NR but normal | NR but normal | NR but normal | NR but normal | NR but normal | Chest radiograph: absence of consolidation or pleural effusion | None | None |
| Tedeschi et al. (2022) 78 | Chest pain, dyspnea | 10 days | NR | NR | NR | 93% | NR | 37.2 | Bilateral diffuse crackles | NR | None | None |
| Toida et al. (2022) 79 | Hypotension during dialysis | 3 days | 114 | 82/47 | NR | NR | NR | NR | Systolic murmur of Levine 2/6 at the second left sternal border, clear lung sounds, and no leg edema | NR | None | None |
| Yamaura et al. (2023) 80 | Chest pain, diaphoresis | Few hours | NR | NR | NR | NR | NR | NR | NR | NR | None | None |
Abbreviations: AF, atrial fibrillation; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; BiPAP, bilevel positive airway pressure; COVID‐19: coronavirus disease, 2019, CPAP, continuous positive airway pressure; CT, computed tomography; CXR, chest X‐ray; ECG: electrocardiogram, N/A, not/applicable; NR, not reported; RVR, rapid ventricular rate.
Electrocardiogram (ECG) findings were reported in 81 cases. The most common abnormalities were T‐wave inversion in 35 (43.2%), ST‐segment elevation in 30 (37%) and QT interval prolongation in 13 (16%). In addition, atrial fibrillation was reported in 6 (7.4%). Out of 82 cases, apical ballooning was reported in 31 (37.8%) (Table 3). Regarding cardiac biomarkers, BNP was measured in 38 cases and was found to be elevated in 36 (94.7%). Troponin was measured in 74 cases and was found to have been raised in 71 (95.9%). Also, CK was measured in 28 cases and found to be raised in 14 (50%) (Table 3).
Table 3.
Cardiac laboratory and paraclinical findings
| Study, year | ECG findings | TTE findings | CA findings | Troponin elevation | BNP elevation | Creatine kinase elevation |
|---|---|---|---|---|---|---|
| Takotsubo after COVID‐19 | ||||||
| Nguyen et al. (2020) 19 | Sinus rhythm with prolonged QT interval (QTc 521 ms) | NR | Significant lesions on the proximal LAD and the first diagonal arteries. The ventriculogram showed regional wall motion abnormality unrelated to the coronary lesions, compatible with a median takotsubo. | Positive | NR | NR |
| Panchal et al. (2020) 20 | Nonspecific ST‐T wave abnormality (QTc 420 ms, QTc 439 ms on admission) | New left ventricular (LV) regional wall motion abnormality with hypokinesis of the basal to midsegments and hyperkinetic apical segments | NR | NR | NR | NR |
| Kariyanna et al. (2020) 21 | Normal range sinus rhythm, Q waves in V1–V2 leads and Q waves with ST segment elevation V3, V4, V5 and deep T wave inversion in V6 | Diffuse hypokinesis with distinct regional wall motion abnormality, apical dyskinesis or apical systolic ballooning suggestive of stress induce cardiomyopathy | NR | Positive | Positive | NR |
| Alizadehasl et al. (2022) 22 | Sinus tachycardia with mild dynamic ST segment depression in precordial leads | Akinesia in mid‐to‐apical segments of both ventricles compatible with biventricular apical ballooning syndrome | NR | Positive | NR | NR |
| Fujisaki et al. (2021) 23 | Atrial fibrillation, poor R progression, and negative T waves in lead I, aVL, and V2–V6 | Severe hypokinetic biventricular apical and mid segments | NR | Positive | Positive | NR |
| Demertzis et al. (2020) 24 | Normal range sinus rhythm, QTc 387 ms | New reduced EF (40%) and severe hypokinesis of the basal–mid inferoseptal, inferior, anteroseptal, and anterior walls | NR | Positive | NR | Positive |
| Normal sinus rhythm, QTc 427 ms | Large pericardial effusion with signs of right ventricular dysfunction and apical hypokinesis | NR | Positive | Positive | NR | |
| Torabi et al. (2021) 25 | Low voltage in the limb leads | Hyperdynamic left ventricle and a hemodynamically significant moderate‐sized pericardial effusion with right atrial systolic collapse. The LV apex was dilated with systolic hypokinesis and basal segments had preserved contraction | Normal range coronaries and mildly elevated left ventricular end diastolic pressure | Positive | Positive | NR |
| Ortuno et al. (2021) 26 | Non‐elevated ST segment, prolonged QT interval, T wave inversion | Left ventricular failure with reduced ejection fraction (LVEF 40%) and typical apical ballooning suggesting | Positive | NR | NR | NR |
| Hegde et al. (2020) 27 | Atrial flutter RVR with diffuse ST elevations | Left ventricular EF: 15% | NR | Positive | Positive | NR |
| Atrial fibrillation, with RVR, diffuse deep T‐wave inversions | Left ventricular EF: 53% | NR | Positive | Positive | Positive | |
| Sinus rhythm with diffuse ST‐T changes | Left ventricular EF: 45% | NR | Negative | Negative | Negative | |
| Sinus rhythm with deep T‐wave inversions | Left ventricular EF: 20% | NR | Positive | Positive | Negative | |
| Atrial fibrillation, with diffuse ST‐T changes | Left ventricular EF: 30% | NR | Positive | Positive | Negative | |
| Sinus tachycardia with PACs and T‐wave inversions | Left ventricular EF: 40% | NR | Positive | Negative | Negative | |
| Sinus tachycardia with diffuse ST‐T changes | Left ventricular EF: 45% | NR | Positive | Positive | Positive | |
| Hoepler et al. (2021) 28 | Complete right bundle branch block (QRS 160 ms) with T wave inversions in leads I, aVL, and V3–V6. Three days later, the QRS complex was normal range again but T wave inversions were more pronounced | Severe hypo‐ to akinesia in parts of the apical and the inferoapical wall with hypercontractility of the basal segments of the heart | Normal‐range coronary arteries | Positive | NR | Negative |
| ST‐elevations and negative T waves in leads V4 and V5 and isolated negative T waves in leads II, III, aVF, and V6 (the patient later developed persistent T wave inversions in II, III, aVF, and V2 to V6) | Moderately reduced systolic function and apical, anterior, and posterolateral akinesia | Severe three‐vessel coronary artery disease (CAD), but also TT cardiomyopathy with classic apical ballooning and hyperkinesia of the basal segments | Positive | Positive | Positive | |
| Hyperacute T waves, which were later replaced by deep T wave inversions in V3 to V6 | Severe apical akinesia with hyperkinesia of the basal segments and a minimum ejection fraction (EF) of 20% after it had been only moderately reduced 2 days earlier | Positive | Positive | Positive | Negative | |
| Alshamam et al. (2021) 4 | ST‐segment elevation in leads V1–V5 and T‐wave inversions in leads I and aVL | Mid to apical left ventricular (LV) akinesia with preserved function in the proximal and segment, aortic valve sclerosis, reduced excursion of Trileaflet valve (without stenosis), and mild‐to‐moderate tricuspid regurgitation with moderate pulmonary artery systolic pressure (PASP) | NR | Positive | Positive | Positive |
| Bernardi et al. (2020) 29 | ST‐segment elevation in anterolateral leads, suggesting an acute myocardial infarction | Dilated left ventricle with akinesis of the mid and apical ventricle segments with hyperkinesis of the basal segments and severe systolic dysfunction (left ventricle ejection fraction calculated by Simpson's biplane method [LVEF]: 30%); first‐grade diastolic dysfunction; partial left ventricle outflow tract obstruction determining a late maximal gradient of 56 mmHg with systolic anterior motion of the mitral valve and associated moderate to severe mitral regurgitation; and, finally, 2 large apical thrombotic formations: the positive terior one was elongated (maximum: 31 mm) and mobile, and the anterior one was wide and oval | Positive | Positive | Positive | Negative |
| Sattar et al. (2020) 30 | Atrial fibrillation with a rapid ventricular response, right bundle branch block (RBBB), and T‐wave inversions in the inferolateral leads | Left ventricle ejection fraction (LVEF) of 30% with diffuse anterior wall and apical akinesia and apical ballooning | Positive | NR | NR | NR |
| Tsao et al. (2020) 31 | Slight ST‐segment elevations diffusely with nonspecific T‐wave inversions | Severe hypokinesis of the mid‐left ventricular cavity, with normal range‐to‐hyperdynamic contractility of basal and apical left ventricular segments and a moderately reduced biplane ejection fraction of 36% | Positive | Negative | Positive | Negative |
| Gomez et al. (2020) 32 | Sinus tachycardia without ST‐wave or T‐wave changes, prolonged QTc interval of 516 ms and low‐voltage QRS in the precordial leads | Depressed left ventricular ejection fraction of 25%–30%, with severe hypokinesis of the mid‐to‐apical segments and preserved basal myocardial function | Positive | Positive | Positive | Negative |
| Belli et al. (2021) 18 | ST elevation with biphasic T waves and Q waves | Complete apical ballooning and extensive akinesia spanning multiple coronary territories with a global LV systolic function impairment | Nonsignificant 30% stenosis of the left anterior descending coronary artery with otherwise smooth coronary arteries | Positive | Positive | NR |
| Titi et al. (2020) 33 | Diffuse ST segment elevation, more evident in the precordial leads (V3–V5), and Q waves in precordial and peripheral inferior leads | Severe global reduction of the left ventricular contractility with mild pericardial effusion | 70% stenosis in the posterolateral branch which originated from the circumflex (left dominance) | NR | NR | NR |
| Faqihi et al. (2020) 34 | Sinus tachycardia (115 beats/min) and nonspecific ST‐segment and T‐wave abnormalities in the precordial leads | LV basal and midventricular akinesia with apical sparing | NR | Positive | NR | Positive |
| Solano, López et al. (2020) 35 | 2 mm ST elevation Inf and Lat lids | Akinesia of all basal segments | Normal range coronary arteries, left ventricular angiography presented basal segment akinesia and hypercontractility of the mid‐apical segments with elevated diastolic pressure | Positive | Positive | NR |
| Pasqualetto et al. (2020) 36 | Diffuse negative T waves on precordial leads with QT interval prolongation | Dyskinesia of the left ventricle apex (apical ballooning) and basal wall hypercontractility with systolic dysfunction, global preserved left ventricular ejection fraction (EF) of 53% | The autopsy confirmed a normal‐range coronary anatomy. | Positive | Positive | NR |
| Diffuse negative T waves on precordial leads with QT interval prolongation | Dyskinesia of the left ventricle apex (apical ballooning) and basal wall hypercontractility with systolic dysfunction, LVEF (30%) | Negative for significant coronary stenosis | Positive | Positive | NR | |
| Diffuse negative T waves on precordial leads with QT interval prolongation | Dyskinesia of the left ventricle apex (apical ballooning) and basal wall hypercontractility with systolic dysfunction, moderately impaired LVEF (42%) | Negative for significant coronary stenosis | Positive | Positive | NR | |
| Koh et al. (2021) 37 | There were diffuse ST‐segment elevations and PR‐segment depressions in the inferolateral leads. There were also ST‐segment depressions and PR‐ segment elevations in leads V1, aVR | Biventricular systolic dysfunction with a left ventricular ejection fraction (LVEF) of around 30%. This demonstrated global left ventricular hypokinesia (biplane LVEF 32.7% with average left ventricular [LV] global longitudinal strain of −8.7%). There was moderate right ventricular systolic dysfunction with a tricuspid annular plane systolic excursion of 11.7 mm, estimated pulmonary artery systolic pressure of 42 mmHg, borderline pulmonary artery acceleration time (120 ms) and echocardiographic estimated pulmonary vascular resistance of 3.41 wood units. A small pericardial effusion was also found. CMR showed an improved LVEF of 66%. There was maximal LV wall thickness of 8 mm at the basal anteroseptal segment, normal range right ventricular systolic function and indexed volumes and there was no late gadolinium enhancement (LGE) in the myocardium of both ventricles or myocardial edema | Normal range epicardial vessels with slow coronary flow. Left ventriculography revealed global left ventricular hypokinesia with severe left ventricular systolic dysfunction | Positive | NR | NR |
| Dave et al. (2020) 38 | Sinus tachycardia and nonspecific T‐wave abnormality in the lateral leads | Normal range right ventricular function, left ventricular ejection fraction (LVEF) 26% with preserved basal function, and apical ballooning consistent with takotsubo cardiomyopathy | NR | Positive | Positive | NR |
| Van Osch et al. (2020) 39 | Negative T‐waves were observed at the monitor and a 12‐lead ECG was obtained which showed sinus rhythm with diffuse, new, deeply negative T‐waves and a prolonged QTc interval of 505 ms | A poor left ventricular systolic function [left ventricular ejection fraction (LVEF) approximately 30%] with circumferential akinesia of the apex in the mid‐ventricular and apical segments and circumferential hyperdynamic contractions of the basal segments consistent with the diagnosis takotsubo cardiomyopathy | Low calcium score and a nonsignificant stenosis (<50%) in the proximal left anterior descending | Positive | NR | NR |
| Bhattacharyya et al. (2020) 40 | Inferolateral ST‐segment elevation | Hypokinetic mid and akinetic apical left ventricular (LV) segments and hypercontractile basal segments with prominent apical ballooning typical of takotsubo cardiomyopathy (TTC). Two‐dimensional speckle tracking echocardiography revealed LV global longitudinal strain (GLS) of −13.9 and ejection fraction (EF%) of 38% | Non‐obstructive coronary artery disease (CAD) involving the left anterior descending artery | Positive | Positive | NR |
| Taza et al. (2020) 41 | ST segment elevations in the inferior leads (II, III, aVF) | NR | Non‐obstructive coronary arteries and apical ballooning on ventriculography, consistent with takotsubo syndrome | Negative | NR | NR |
| Bottiroli et al. (2020) 42 | ST‐segment elevation with loss of R‐waves in leads V2 to V4 | Normal range size of ventricular chambers, severe left ventricular (LV) systolic dysfunction with an LV ejection fraction (EF) of 25%, and akinesia of middle and apical segments (apex ballooning) with hyperkinetic motion of basal segments | NR | Positive | Positive | NR |
| Roca et al. (2020) 43 | The electrocardiogram showed negative T waves and repolarization phase alterations | Alterations in the left ventricle: apical akinetic expansion (apical ballooning) and hypokinesia of the mid‐ventricular segments with slightly reduced systolic function (ejection fraction slightly reduced to 48%) | NR | Positive | NR | Positive |
| Oyarzabal et al. (2020) 44 | The electrocardiogram showed a 1 mm ST segment elevation in leads V2–V3 and DI‐AVL | The findings of ventriculography were confirmed by echocardiography | Coronary angiography showed coronary arteries free of lesions and cardiac ventriculography was performed. This showed a very reduced left ventricular ejection fraction with extensive apical akinesia | NR | NR | NR |
| Minhas et al. (2020) 45 | Sinus tachycardia and 1‐mm upsloping ST‐segment elevations in leads I and aVL, mild diffuse PR interval depressions, and diffuse ST‐T wave changes | Akinetic middle to distal anterior, anteroseptal, antero‐lateral, and apical segments, moderately hypokinetic middle and distal inferolateral segments, and hyper‐dynamic basal segments. Apical ballooning was also noted. Left ventricular (LV) ejection fraction was 20%. The distal third or apical right ventricular (RV) free wall was akinetic, with hyperdynamic RV basal wall motion. RV function was mildly reduced | NR | Positive | NR | NR |
| Kong et al. (2021) 46 | Anteroseptal ST‐segment elevations | NR | Mild non‐obstructive coronary artery disease, and a left ventriculogram was performed which demonstrated preserved basal function with apical akinesis, consistent with TTS | Positive | NR | NR |
| New ST‐segment elevations in the anterolateral leads | Apical hypokinesis, consistent with ventriculogram findings | Non‐obstructive coronary artery disease. A left ventriculogram demonstrated significantly reduced ejection fraction with preserved basal function and apical ballooning and akinesis, consistent with TTS. Right heart catheterization showed elevated biventricular filling pressures with reduction in CO and CI | Positive | NR | NR | |
| Park et al. (2020) 47 | T wave inversion appeared | Apical ballooning with severe LV systolic function | NR | Positive | Positive | NR |
| NR | Apical ballooning with dyskinetic movement and severe LV systolic dysfunction | NR | Positive | NR | Positive | |
| Meyer et al. (2020) 48 | <1 mm ST‐segment elevation in all precordialleads with deep T‐wave inversions | Typical left ventricular apical ballooning with hyperkinetic basal segments | Nonsignificant lesions with a typical takotsubo syndrome (TTS) image on ventriculography | Positive | NR | NR |
| Eftekharzadeh et al. (2022) 49 | Tachycardia and ST‐segment elevation in inferior lateral leads II, III, aVF, and V5 | NR | A 40% proximal to mid‐left anterior descending (LAD) lesion without any severe obstruction, moderate left ventricular (LV) dysfunction with apical ballooning was noted during the left heart chamber assessment | Positive | Positive | NR |
| Frynas‐Jończyk et al. (2022) 50 | Sinus rhythm of 80/min, left anterior fascicular block, ST‐segment depression in V1‐V5 leads, and negative T waves in II, III, aVF, V1–V6 leads | Apical dyskinesis resulting in apical ballooning and hypo‐akinesia of the mid‐ventricular segments with severely reduced left ventricular ejection fraction (LVEF) of 30% | Mild, non‐obstructive atherosclerotic plaques in the coronary arteries | Positive | Positive | Negative |
| Fujiyoshi et al. (2022) 51 | Deep T‐wave inversions in all precordial leads | Hypokinesis with hypertrophy in the apical region and hyperkinesis in the basal region with estimated LV ejection fraction of 58% | NR | Positive | NR | NR |
| Kimura et al. (2021) 52 | Inverted T‐waves in leads I, II, aVF, and V1–V6 | Apical akinesis with preserved basal function and a depressed ejection fraction of around 50% | NR | Positive | Positive | Positive |
| Mishra et al. (2021) 53 | New onset T wave inversion across V1–V6 | Hypokinesis of the basal region of the left ventricle with hyperkinesis of the apical region of the left ventricle consistent with a reverse takotsubo cardiomyopathy | NR | NR | NR | NR |
| Namburu et al. (2021) 54 | ST elevations in V1–V3 leads consistent with the diagnosis of ST‐elevation myocardial infarction (STEMI) | Left ventricular ejection fraction (LVEF) of 45%, enlarged right ventricle (RV), and a right atrial thrombus | Non‐obstructive coronary artery disease with apical ballooning of the left ventricle, a ventriculogram characteristic of TTC | Positive | NR | NR |
| Rivera et al. (2020) 55 | Atrial fibrillation with rapid ventricular response and ST‐segment elevation in the anterolateral leads | NR | Absence of obstructive coronary lesions and ventriculography showed severe ventricular dysfunction with anterolateral, apical, and inferior dyskinesia and hypercontractility of the basal segments, compatible with takotsubo syndrome (TTS) | NR | NR | NR |
| Wildermann et al. (2022) 56 | Intermittent ventricular bigeminy | NR | Cardiac catheterization showed no coronary artery disease but regional wall motion abnormalities compatible with atypical TTC | Positive | NR | NR |
| Bapat et al. (2020) 57 | Persistence of T wave inversions and progressive prolongation in the QT interval | Preserved left ventricular ejection fraction of 61% but with new apical hypokinesis | Not performed | Positive | NR | NR |
| Chao et al. (2020) 58 |
Narrow QRS, precordial T‐wave inversion, QTc of 467 ms 3 days later: right bundle branch block, prolonged QTc of 539 ms, and mild diffuse ST elevation |
Mild to moderately reduced LVEF of 40% with marked hypokinesis of basal and mid segments and pre‐served wall motion of apical segments | Not performed | Positive | Positive | Positive |
| Dabbagh et al. (2020) 59 |
Low voltage in the limb leads with nonspecific ST‐segment changes serial ECG revealed deep T‐wave inversions in precordial leads (V2–V6) |
Large pericardial effusion circumferentially around the entire heart with signs of early right ventricular diastolic collapse, dilated but collapsing inferior vena cava, and mitral valve inflow variation of 31% on pulsed wave Doppler. LVEF was mildly reduced at 40%, with no regional wall motion abnormalities, similar to TTE 1 year prior. Serial TTE demonstrated resolution of the pericardial effusion; however, the patient was found to have new hypokinesis of the apical and periapical walls concerning for takotsubo cardiomyopathy (TTC) |
Not performed | Positive | NR | NR |
| Manzur‐Sandoval et al. (2021) 60 | Pulse rate 75 beats/min; PR interval 160 ms; QRS interval 100 ms; prolonged QTc interval 551 ms; QRS axis –30°; poor R‐wave progression; giant inverted T waves at V2– V6, DI, and AVL; and Q waves at DII and AVF. | In the apical 2‐chamber view, apical ballooning with normal contraction of the basal segments was observed; left ventricular longitudinal strain was decreased in the mid and apical segments | Not performed | Positive | Positive | Positive |
| Sang et al. (2020) 61 | Septal infarction pattern | Severely reduced left ventricular systolic function with global hypokinesis of the left ventricle. The apical segments had disproportionately poor function compared with the basal segments, a finding consistent with stress‐induced (takotsubo) cardiomyopathy | Not performed | Positive | Positive | NR |
| Tutor et al. (2021) 62 | Nonspecific ST‐T wave changes | Depressed LVEF of 25% with basal‐sparing and severe apical akinesis | NR | Positive | NR | Negative |
| Diffuse t‐wave inversions | Severely depressed LV systolic function, and global hypokinesis with akinesis of the apex and basal sparing | NR | Positive | NR | Positive | |
| Takotsubo after social stress | ||||||
| Habedank et al. (2020) 63 | ST elevations 0.4 mV from J‐point in leads V2 to V4, 0.1 mV in lead aVL, and a QTc = 522 ms by Bazett's resp. 477 ms by Fridericia's formula | Moderate hypokinesia in the mid‐anterior section and cardiac MRI proving significant edema in the entire anterior and septal wall. severe hypokinesia in the mid‐apical segments and hyperdynamic basal segments | Moderate coronary sclerosis | Positive | NR | Negative |
| Giannitsi et al. (2020) 64 | Diffuse ST segment elevation | NR | Excluded stenotic lesions | Positive | NR | NR |
| Parker et al. (2020) 65 | Q waves and ST elevation in the inferior leads | NR | Chronic occlusion of right coronary artery, left ventricular ejection fraction of 34%, and basal hyperkinesis with mid‐ventricular and apical dyskinesis | Positive | NR | NR |
| Uhe et al. (2020) 66 | Negative T‐waves in II, III, aVF, and V3‐6 | Wall motion abnormalities with apical septal dyskinesis, mid ubiquitous akinesia and basal septal and anterior hypokinesis. Left ventricular ejection fraction was reduced to 45%. Global longitudinal strain with a typical strain pattern of apical ballooning was 8% | No signs of artery disease (CAD) | Positive | NR | Positive |
| Chadha et al. (2020) 67 | A septal q‐ST pattern in leads V1–V3 | Basal hyperkinesis and apical ballooning | Nonsignificant coronary artery disease | Positive | NR | NR |
| Rivers et al. (2020) 68 | Diffuse ST elevation | A dilated left ventricle with an akinetic apex and preserved contraction of the basal segments | No obstructive lesions | Positive | NR | NR |
| Koutroumpakis et al. (2020) 69 | A sinus rhythm at 63 beats/min, with marked T wave inversion in the inferior and anterolateral leads | Left ventricular systolic dysfunction with apical ballooning. basal hyperkinesis with dyskinesis of the apex | TIMI 2 flow down the left anterior descending artery | Positive | NR | NR |
| Jabri et al. (2020) 5 | NR | NR | NR | NR | NR | NR |
| Dolci et al. (2020) 70 | Regular sinus rhythm with nonspecific ST‐segment alterations in the inferior leads |
Hypokinesia of the left ventricle (LV) mid segments with normal range apical and basal contraction resulting in mild reduction of LV ejection fraction. Normal range epicardial coronary arteries and confirmed mid‐ventricular ballooning with Normal range contraction of basal and apical segments |
Normal range epicardial coronary arteries and confirmed mid‐ventricular ballooning with Normal range contraction of basal and apical segments | Positive | NR | NR |
| Moady et al. (2021) 71 | Normal sinus rhythm with diffuse ST segment elevation, most prominent in the anterior leads with no reciprocal changes | Moderately reduced global systolic left ventricular function with a typical pattern of apical ballooning and left ventricular outflow obstruction | Basal hypercontractility and apical ballooning were obvious during left ventriculography | Positive | Positive | NR |
| Normal sinus rhythm with anterior ST segment elevation | Reduced apical contraction with estimated ejection fraction of 42% and hyperkinetic basal segments of the left ventricle | Normal arteries | Positive | Positive | NR | |
| Kir et al. (2021) 9 | Sinus tachycardia with inferior Q waves, poor R wave progression, and nonspecific ST‐segment changes |
Basal hyperkinesis with severe apical hypokinesis. An ejection fraction of 45%–50%, with severe hypokinesis of the apical segments with apical ballooning and basal hyperkinesis |
Negative for any significant obstructive coronary artery disease | Positive | Positive | NR |
| New deep T wave inversions in the precordial leads and subtle ST elevation in the inferior leads |
Severe hypokinesis of the antero‐apical wall, concerning for anterior myocardial infarction. severely depressed left ventricular ejection fraction of 30% with antero‐apical and infero‐apical wall akinesis |
Mild calcification and nonobstructive disease in the coronary arteries with a right dominant circulation. Myocardial bridge was noted in the proximal mid left anterior descending artery. Left ventricular end‐diastolic pressure was elevated at 22 mmHg. | NR | NR | NR | |
| Mohammed et al. (2020) 72 | Sinus tachycardia and left bundle branch block | A newly depressed ejection fraction (EF) of 22%, a moderately increased left ventricular (LV) cavity size, and moderate hypokinesis of the mid‐distal left ventricular wall with preservation of basal LV contractility | Normal coronary arteries | Positive | Positive | NR |
| Ben Ammar et al. (2021) 73 | Elevated ST‐segment in leads V3 to V6 | Reduced left ventricular ejection fraction which was 40%, There was also a decrease in the global longitudinal strain with a marked decrease in the apical segments | Severe multivessel disease, tight stenosis in the posterior‐right coronary artery. On the left, there were insignificant lesions in the mid and distal left anterior descending arteries, as well as insignificant lesions in the mid‐circumflex and second obtuse marginal arteries. | Positive | NR | NR |
| Takotsubo after COVID‐19 vaccination | ||||||
| Vidula et al. (2021) 11 | Inferolateral T wave inversions | Mildly reduced LV function with apical akinesis | A patent LAD stent and no obstructive disease | Positive | NR | NR |
| Boscolo‐Berto et al. (2021) 74 | Negative T waves over the inferior/anterior leads |
Apical ballooning. mid‐ventricular to apical ballooning (asterisk) with preserved basal contraction (blues arrows) and a moderately impaired left ventricular ejection fraction of 40% |
No coronary artery disease | Positive | Positive | Negative |
| Fearon et al. (2021) 75 | ST wave changes concerning for infero lateral ischemia and new poor anterior R wave progression | Mid‐ventricular ballooning of the LV, EF 20% with a Grade I diastolic dysfunction, mild mitral regurgitation, and severe right ventricular dysfunction associated with functional severe tricuspid regurgitation | No significant coronary artery disease | Positive | Positive | NR |
| Crane et al. (2021) 76 | Sinus tachycardia with first degree and right bundle branch block without acute or dynamic ischemic changes | New moderately severe segmental systolic dysfunction with an estimated ejection fraction of 37%–39% with hyperdynamic base, akinesis of the mid‐distal left ventricular segments and severe hypokinesis of the apical cap with apical ballooning | Patent grafts, no new flow limiting coronary disease and left ventriculography consistent with transthoracic echocardiogram finding with apical ballooning and reduced cardiac function in the antero‐apical regions | Positive | NR | NR |
| Stewart et al. (2021) 77 | Anterior T wave inversion with a corrected QT interval of 480ms, which evolved over 48 h | Hypokinesia of the mid‐cavity anteroseptum and the apical septum with overall mildly impaired left ventricular systolic contraction. No significant valvular heart disease, normal dimensions of the atria and ventricles and good right ventricular systolic contraction were noted | Left ventricular hypokinesia of the mid‐cavity anterior wall with no significant coronary artery disease present and left dominant coronary arteries | Positive | NR | NR |
| Tedeschi et al. (2022) 78 | Sinus rhythm with normal atrioventricular conduction, deep and symmetric T‐wave inversion in all leads except for aVL and aVF, and prolongation of corrected QT (QTc) >600 ms | Moderate depression of left ventricular contraction (LVEF 38%) in the presence of hypokinesia of apical and mid‐distal walls consistent with the apical ballooning syndrome | Non‐obstructive coronary artery disease | Positive | NR | NR |
| Toida et al. (2022) 79 | Atrial fibrillation with a normal axis, negative T‐waves in I, aVL, and V3‐6, and a prolonged QTc interval of 495 ms | Akinesia of the apical segments of the LV with apical ballooning and sparing of the base of each wall as well as a reduced ejection fraction of 48%. Systolic anterior motion of the mitral valve (MV) and LV outfow tract obstruction with basal hyperkinesia were detected in addition to mild‐moderate mitral regurgitation. Peak fow velocity and the mean pressure gradient of the LV outfow tract were 4.2 m/s and 71 mmHg, respectively | Not performed (coronary computed tomography showed no significant stenosis of the coronary arteries and extensive akinesis in the apical portion and hyperkinesia in the basal portion of LV with apical ballooning) | Positive | NR | Negative |
| Yamaura et al. (2022) 80 | ST‐segment depression on the V4–V6 leads | Akinesis at the basal portion of the left ventricle (LV) and hypercontraction at the apex | Not performed (coronary computed tomography angiography showed no significant stenosis in epicardial coronary arteries or aortic dissection. Coronary computed tomography angiography depicted akinesis at the basal portion of the LV, as visualized using transthoracic Doppler echo‐cardiography) | Positive | NR | Positive |
Abbreviations: BNP, brain natriuretic peptide; NR, not reported; RVR, rapid ventricular rate; TTE, transthoracic echocardiogram.
Neurologic or psychiatric disorders (i.e., multiple sclerosis, cerebrovascular accident, dementia, schizophrenia, anxiety, depression, chronic pain syndrome, obstructive sleep apnea, and Bickerstaff brainstem encephalitis) were reported in 16/82 cases (19.5%) (Table 4). Among the case reports, the quality scores ranged from 5 to 8, in which 41 articles had the complete scores and providing the demographic characteristics and diagnostic tests were the items with the highest quality (Supporting Information: Table S2). Among the case series, the scores ranged from 6 to 10 with an average of 7.4. Validity of measuring the condition, consecutive, and complete inclusion of participants were those with the lowest quality in the included articles (Supporting Information: Table S3). The cohort study received the highest score for all of the items (Supporting Information: Table S4).
Table 4.
Different physical and psychological etiologies associated with takotsubo in patients with COVID‐19
| Study, Year | Study design | Transient left ventricular dysfunction in echocardiography | Emotional, physical, or combined trigger | Psychiatric/Neurologic disorders | New ECG abnormalities | Elevated cardiac biomarkers | Menopause |
|---|---|---|---|---|---|---|---|
| Takotsubo after COVID‐19 | |||||||
| Nguyen et al. (2020) 19 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Panchal et al. (2020) 20 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Negative | N/A |
| Kariyanna et al. (2020) 21 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Alizadehasl et al. (2022) 22 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Fujisaki et al. (2021) 23 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Demertzis et al. (2020) 24 | Case series | Positive | Positive COVID‐19 | Positive | Negative | Positive | Positive |
| Positive | Positive COVID‐19 | Negative | Negative | Positive | Positive | ||
| Torabi et al. (2021) 25 | Case report | Positive | Positive COVID‐19 | Negative | Negative | Positive | NR |
| Ortuno et al. (2021) 26 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Hegde et al. (2020) 27 | Case series | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Positive | Positive COVID‐19 | Positive | Positive | Positive | N/A | ||
| Positive | Positive COVID‐19 | Negative | Positive | Negative | Positive | ||
| Positive | Positive COVID‐19 | Positive | Positive | Negative | Positive | ||
| Positive | Positive COVID‐19 | Positive | Positive | Positive | N/A | ||
| Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A | ||
| Positive | Positive COVID‐19 | Positive | Positive | Positive | N/A | ||
| Hoepler et al. (2021) 28 | Case series | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Positive | Positive COVID‐19 | Positive | Positive | Positive | Positive | ||
| Positive | Positive COVID‐19 | Positive | Positive | Positive | Positive | ||
| Alshamam et al. (2021) 4 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Bernardi et al. (2020) 29 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Sattar et al. (2020) 30 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | NR |
| Tsao et al. (2020) 31 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | NR |
| Gomez et al. (2020) 32 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | NR |
| Belli et al. (2021) 18 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | NR |
| Titi et al. (2020) 33 | Case report | Positive | Positive COVID‐19 | Negative | Positive | NR | N/A |
| Faqihi et al. (2020) 34 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Solano‐López et al. (2020) 35 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Pasqualetto et al. (2020) 36 | Case series | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive | ||
| Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A | ||
| Koh et al. (2021) 37 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Dave et al. (2020) 38 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Van Osch et al. (2020) 39 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Bhattacharyya et al. (2020) 40 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Negative |
| Taza et al. (2020) 41 | Case report | Positive | Positive COVID‐19 | Positive | Positive | Negative | N/A |
| Bottiroli et al. (2020) 42 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Roca et al. (2020) 43 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Oyarzabal et al. (2020) 44 | Case report | Positive | Positive COVID‐19 | Negative | Positive | NR | N/A |
| Minhas et al. (2020) 45 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Kong et al. (2021) 46 | Case series | Positive | Positive COVID‐19 | Positive | Positive | Positive | N/A |
| Positive | Positive COVID‐19 | Positive | Positive | Positive | Positive | ||
| Park et al. (2020) 47 | Case series | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Positive | Positive COVID‐19 | Negative | NR | Positive | Positive | ||
| Meyer et al. (2020) 48 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Eftekharzadeh et al. (2022) 49 | Case report | Positive | Positive COVID‐19 | Positive | Positive | Positive | Positive |
| Frynas‐Jończyk et al. (2022) 50 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Fujiyoshi et al. (2022) 51 | Case report | Positive | Positive COVID‐19 | Positive | Positive | Positive | Positive |
| Kimura et al. (2021) 52 | Case report | Positive | Positive COVID‐19 | Positive | Positive | Positive | Positive |
| Mishra et al. (2021) 53 | Case report | Positive | Positive COVID‐19 | Negative | Positive | NR | N/A |
| Namburu et al. (2021) 54 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Rivera et al. (2020) 55 | Case report | NR (but positive for angiography) | Positive COVID‐19 | Negative | Positive | NR | Positive |
| Wildermann et al. (2022) 56 | Case report | NR (but positive for angiography) | Positive COVID‐19 | Positive | Positive | Positive | Negative |
| Bapat et al. (2020) 57 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Chao et al. (2020) 58 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Dabbagh et al. (2020) 59 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Manzur‐Sandoval et al. (2021) 60 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | NR |
| Sang et al. (2020) 61 | Case report | Positive | Positive COVID‐19 | Negative | Positive | Positive | Positive |
| Tutor et al. (2021) 62 | Case series | Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A |
| Positive | Positive COVID‐19 | Negative | Positive | Positive | N/A | ||
| Takotsubo after social stress | |||||||
| Habedank et al. (2020) 63 | Case report | Positive | Social stress/social isolation | Positive | Positive | Positive | Positive |
| Giannitsi et al. (2020) 64 | Case report | Positive | Death anxiety | Negative | Positive | Positive | Positive |
| Parker et al. (2020) 65 | Case report | Positive | phone consult which the patient was informed that the lung biopsy demonstrated recurrence of lung adenocarcinoma | Negative | Positive | Positive | Positive |
| Uhe et al. (2020) 66 | Case report | Positive | Death anxiety | Negative | Positive | Positive | Positive |
| Chadha et al. (2020) 67 | Case report | Positive | Social stress & death anxiety | Negative | Positive | Positive | Positive |
| Rivers et al. (2020) 68 | Case report | Positive | Social stress/social isolation | Negative | Positive | Positive | Positive |
| Koutroumpakis et al. (2020) 69 | Case report | Positive | Death anxiety | Negative | Positive | Positive | Positive |
| Jabri et al. (2020) 5 | Cohort | NR | Social stress/social isolation | NR | NR | Positive | NR |
| Dolci et al. (2020) 70 | Case report | Positive | Social stress/social isolation | Negative | Positive | Positive | Positive |
| Moady et al. (2021) 71 | Case series | Positive | Social stress/social isolation | Negative | Positive | Positive | Positive |
| Positive | Social stress & death anxiety | Negative | Positive | Positive | Positive | ||
| Kir et al. (2021) 9 | Case series | Positive | Social stress/social isolation | Negative | Positive | Positive | Positive |
| Positive | Death anxiety | Negative | Positive | NR | Positive | ||
| Mohammed et al. (2020) 72 | Case report | Positive | Increased workload | Negative | Positive | Positive | Positive |
| Ben Ammar et al. (2021) 73 | Case report | Positive | Social stress/social isolation | Positive | Positive | Positive | N/A |
| Takotsubo after COVID‐19 vaccination | |||||||
| Vidula et al. (2021) 11 | Case series | Positive | Receiving second dose of the BNT162b2 (Pfizer‐BioNTech) vaccine | Negative | Positive | Positive | Positive |
| Tedeschi et al. (2022) 78 | Case report | Positive | Receiving first dose of the BNT162b2 (Pfizer–BioNTech) vaccine | Negative | Positive | Positive | Positive |
| Toida et al. (2022) 79 | Case report | Positive | Receiving first dose of the BNT162b2 (Pfizer‐BioNTech) vaccine | Negative | Positive | Positive | Positive |
| Yamaura et al. (2022) 80 | Case report | Positive | Receiving second dose of the BNT162b2 (Pfizer‐BioNTech) vaccine | Negative | Positive | Positive | Negative |
| Boscolo Berto et al. (2021) 74 | Case report | Positive | Receiving the first of two mRNA‐1273 (Moderna) COVID‐19 vaccinations | Negative | Positive | Positive | Positive |
| Fearon et al. (2021) 75 | Case report | Positive | Receiving first dose of the mRNA‐1273 (Moderna) vaccine | Negative | Positive | Positive | Positive |
| Crane et al. (2021) 76 | Case report | Positive | Receiving first dose of the ChadOX1 nCOV‐19 (AstraZeneca) vaccine | Negative | Negative | Positive | N/A |
| Stewart et al. (2021) 77 | Case report | Positive | Receiving second dose of the ChadOX1 nCOV‐19 (AstraZeneca) vaccine | Negative | Positive | Positive | Positive |
Abbreviations: COVID‐19, coronavirus disease, 2019; ECG, electrocardiogram; N/A, not/applicable; NR, not reported.
4. DISCUSSION
The findings of the present study showed that the most common comorbidities and clinical presentation among those with TTS and COVID‐19 were hypertension and dyspnea, respectively. Moreover, the most common ECG findings of patients with COVID‐19 who developed TTS were ST elevation and T inversion. Elevated troponin, followed by BNP were the cardiac biomarkers which had the highest frequency among the patients. TTS leads to complications in which cardiogenic shock is the most common one. Comparing the patients with TTS after COVID‐19 infection and those with conventional TTS associated with emotional triggers shows that the most common presentation in the former group is dyspnea, some of them need mechanical ventilation, and the most common complication is cardiogenic shock, while in the latter group they mostly presented with chest pain, there is no need for mechanical ventilation, and the frequency of complications is lower.
In accordance with our study which found that most cases of TTS were developed in patients with COVID‐19 above 60 years of age, a systematic review conducted by Singh et al. 12 on 12 case reports of TTS in patients with COVID‐19 showed a mean age of 70.8 years and 66.6% had age of above 60 years old. Moreover, the TTS was more common among women with COVID‐19 than males (66.6% vs. 33.4%), 16 as it was also revealed in our study (69.5% vs. 30.5%). The same study also showed that hypertension (66.7%), followed by diabetes (41.6%), and dyslipidemia (16.6%) were the most common comorbidities. 12 Similarly, we found that hypertension with a frequency of 65% was the most common comorbidity in these patients. The study also showed a mean time interval of 8.3 days from the first clinical presentation to admission which was almost in accordance with our study that was 7.2 days. 12 In addition, Haussner et al. 81 showed that the mean age of patients with COVID‐19 who developed TTS were higher than those with other COVID‐19‐assocaited cardiomyopathies (58.9 vs. 53.8 years). 81
A systematic review on 99 studies, including 108 patients, showed that dyspnea (70.5%), chest pain (24.8%), and syncope (2.9%) had the highest frequency in patients with coexisting respiratory disease and TTS. 82 In patients receiving mRNA COVID‐19 vaccines which lead to cardiac consequences, the most common signs/symptoms were chest pain (96.1%) and fever (38.2%). 83 TTS can also clinically be presented with chest pain and dyspnea. 84 Although most of the studies reported almost similar frequency in clinical presentations, minor differences could be as a result of differences in mean age, sex, and underlying diseases of the patients included in the different studies.
The ST‐T abnormalities, in particular ST‐segment elevation, were the most common ECG finding in patients with COVID‐19. 85 Moreover, TTS could lead to occurrence of ST‐segment abnormalities. 86 Diffuse ST elevation (43.8%) followed by PR depression (9.5%) were the most common ECG findings of those patients with cardiac complications after mRNA COVID‐19 vaccine administration. 83 Among patients with COVID‐19 and TTS, only three‐fourth of patients had abnormal ECG in which ST elevation (50%), T inversion (50%), and prolonged QT‐interval (50%) were the most common findings. 12 Similarly, the included studies in the current systematic review reported ST‐segment elevation, T‐wave inversion, and QT interval prolongation in 36.9%, 44.0%, and 16.6% of patients, respectively. The differences between the studies could be as a result of variations in the frequency of comorbidities among the included participants in the study which affect the ECG abnormalities.
In echocardiography, the mean LVEF was 36.4% and the GGO feature was reported in CXR of 31.9% of participants with COVID‐19 who developed TTS. In this regard, Singh et al. 12 reported a mean 40.6% of LVEF of included 12 patients, and bilateral opacities (72.7%) and GGO (54.5%) were the most frequent findings in CXR. 12 The minor differences between the studies could be as a result of higher number of included cases in our study than the previously conducted one in 2020. 12 A preprint systematic review on eight case reports which aimed to evaluate the features of TTS following COVID‐19 vaccine administration showed that all cases had abnormal ECG and an elevated troponin test, while all of them had LVEFs of above 50%. 87 In a study on 1216 patients with COVID‐19 who underwent echocardiography, evidence in favor of TTS was the least frequent cardiac complications that only found in solely 2% of them. 88 The typical features of TTS in echocardiography are basal hypercontractility and apical ballooning. 89 In this regard, we found the atypical ballooning in 38.8% of participants. Moreover, another systematic review on patients with COVID‐19 and TTS revealed atypical ballooning and basal segment hypo‐ or akinesia in 58.3% and 33.3% of participants, respectively. 12
Elevated troponin, BNP, and CK were found to be increased in 96.1%, 95.0%, and 48.2% of included participants, respectively. Moreover, cardiac biomarkers, including NT‐proBNP, CK, troponin, and CK‐MB were increased in 28%, 18%, 17%, and 12% of patients with COVID‐19, respectively. 90 Among those presented with cardiac complications following receiving COVID‐19 vaccines, CK‐MB, troponin, and NT‐proBNP were increased in 100%, 99.5%, and 78.3% of participants, respectively. 83 The previously mentioned systematic review on patients with TTS and COVID‐19 also reported elevated troponin in 91.6% of participants. 12 Therefore, troponin might be useful to be measured in patients with clinical presentations compatible with TTS in patients with positive COVID‐19. However, it needs to be confirmed with further diagnostic measures like echocardiography.
The TTS in patients with COVID‐19 could be life‐threatening as we found a mortality rate of 36.3%. Regarding the outcomes of patients with TTS and COVID‐19, the study by Singh et al. 12 also found that 11 out of 12 patients developed at least one of the following complications: cardiac tamponade, heart failure, myocarditis, hypertensive crisis, septic shock, and cardiogenic shock. Nevertheless, the rate of recovery was almost high (90.9%). 12 These differences could be due to including a higher number of cases in our systematic review and also differences in the number of patients with different past medical history and the medications used by them. Additionally, a systematic review of 123 patients with TTS during the COVID‐19 pandemic showed an in‐hospital morality rate of 23.3% which was significantly different by sex (38.7% in males and 13.9% in females; p = 0.03). 91
There are some limitations in the present study which should be considered in the interpretation of the results. Firstly, because of the limited original papers on the association between TTS and COVID‐19, we only included case reports and case series. It could lead to bias since these types of papers are not indicative. Additionally, we did not exclude those studies with a past medical or family history of SARS‐CoV‐2 infection or cardiac diseases, so the previous infection with SARS‐CoV‐2 or a family history of cardiac diseases could susceptible the individuals to development of TTS and lead to bias in interpretation of the findings. Secondly, there is a probability of missing some relevant articles, although we used a comprehensive search strategy for electronic databases and gray literature search, as well as backward and forward citation searching. Thirdly, the relevant data for some items like CXR, ECG findings, or cardiac biomarkers, and complications were not reported by some studies. Fourthly, most of the included studies evaluated the TTS in patients with COVID‐19, while the TTS among those receiving COVID‐19 vaccines has not been comprehensively evaluated in the present study. Fifthly, most of the included studies were conducted in the United States, so due to the racial effects on the outcomes of TTS, 92 the findings cannot be generalized to other races/ethnicities. Sixthly, pathological feature of TTS in patients with COVID‐19 or their cardiac computed tomography scans or magnetic resonance imaging findings were not reported in this systematic review. In addition, the treatments or drugs which were used for treatment of the individuals were not reported in the present study.
5. CONCLUSIONS
The TTS in patients with COVID‐19 is almost rare, whereas it could lead to a great mortality and morbidity. If an individual with COVID‐19, especially an elderly woman presented with dyspnea, ECG abnormality (e.g., ST elevation and T inversion) in addition to a rise in BNP and troponin and a decrease in LVEF, TTS should be considered as a differential diagnosis. Further observational studies and meta‐analyses on these studies are required to determine the association between COVID‐19 and TTS. Furthermore, the effects of COVID‐19 or history of previous SARS‐CoV‐2 infection on TTS development should be evaluated in future research. Future research should also focus on mechanisms related to TTS caused by COVID‐19 and if DNA samples can be extracted from these cases, it could lead to a new breakthrough in mechanistic research on COVID‐19‐induced TTS.
AUTHOR CONTRIBUTIONS
Hoomaan Ghasemi: Conceptualization; methodology; project administration; visualization; writing – original draft; writing – review & editing. Sina Kazemian: Conceptualization; methodology; project administration; supervision; visualization; writing – original draft; writing – review & editing. Seyed Aria Nejadghaderi: Methodology; visualization; writing – original draft; writing – review & editing. Mahan Shafie: Conceptualization; investigation; methodology; project administration; supervision; validation; visualization; writing – original draft; writing – review & editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Since the ethical approval and the Institutional Review Board (IRB) were reported for each of the included studies, no additional ethical or IRB approvals were required for this systematic review.
TRANSPARENCY STATEMENT
The lead author Mahan Shafie affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Ghasemi H, Kazemian S, Nejadghaderi SA, Shafie M. Takotsubo syndrome and COVID‐19: a systematic review. Health Sci Rep. 2022;6:e972. 10.1002/hsr2.972
Hoomaan Ghasemi and Sina Kazemian contributed as co‐first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Brenner ZR, Powers J. Takotsubo cardiomyopathy. Heart Lung. 2008;37(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 2. Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6(7):602‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523‐1529. [DOI] [PubMed] [Google Scholar]
- 4. Alshamam MS, Nso N, Idrees Z, Nassar M, Munira MS. Coronavirus disease 2019 (COVID‐19)‐induced takotsubo cardiomyopathy prognosis in geriatric setting. Cureus. 2021;13(7):e16211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jabri A, Kalra A, Kumar A, et al. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Network Open. 2020;3(7):e2014780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desai HD, Sharma K, Jadeja DM, Desai HM, Moliya P. COVID‐19 pandemic induced stress cardiomyopathy: a literature review. IJC Heart Vasc. 2020;31:100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sattar Y, Ullah W, Rauf H, et al. COVID‐19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. IJC Heart Vasc. 2020;29:100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turshudzhyan A. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐induced cardiovascular syndrome: etiology, outcomes, and management. Cureus. 2020;12(6):e8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kir D, Beer N, De Marchena EJ. Takotsubo cardiomyopathy caused by emotional stressors in the coronavirus disease 2019 (COVID‐19) pandemic era. J Card Surg. 2021;36(2):764‐769. [DOI] [PubMed] [Google Scholar]
- 10. Mahan S, Mahsa M, Hamed H, Mahnaz A. Medical sciences' students responses during the late phase of the COVID‐19 pandemic in Iran: a comprehensive investigation of the risk perception and information exposure. Acta Med Iranica. 2021;59(12):704‐712. [Google Scholar]
- 11. Vidula MK, Ambrose M, Glassberg H, et al. Myocarditis and other cardiovascular complications of the mRNA‐based COVID‐19 vaccines. Cureus. 2021;13(6):e15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh S, Desai R, Gandhi Z, et al. Takotsubo syndrome in patients with COVID‐19: a systematic review of published cases. SN Compr Clin Med. 2020;2(11):2102‐2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Madhavan M, Prasad A. Proposed Mayo Clinic criteria for the diagnosis of Tako‐Tsubo cardiomyopathy and long‐term prognosis. Herz. 2010;35(4):240‐244. [DOI] [PubMed] [Google Scholar]
- 15. Institute JB . Checklist for case reports. 2020. https://jbi.global/critical-appraisal-tools
- 16. Institute JB . Checklist for case series. 2020. https://jbi.global/critical-appraisal-tools
- 17. Institute JB . Checklist for cohort studies. 2020. https://jbi.global/critical-appraisal-tools
- 18. Belli O, Ardissino M, Bottiroli M, et al. Emergency cardiac imaging for coronavirus disease 2019 (COVID‐19) in practice: a case of takotsubo stress cardiomyopathy. Cardiovasc Ultrasound. 2021;19(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen D, Nguyen T, De Bels D, Castro Rodriguez J. A case of takotsubo cardiomyopathy with COVID 19. Eur Heart J Cardiovasc Imaging. 2020;21(9):1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panchal A, Kyvernitakis A, Biederman R. An interesting case of COVID‐19 induced reversed takotsubo cardiomyopathy and insight on cardiac biomarkers. Cureus. 2020;12(11):e11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kariyanna PT, Chandrakumar HP, Jayarangaiah A, et al. Apical takotsubo cardiomyopathy in a COVID‐19 patient presenting with stroke: a case report and pathophysiologic insights. Am J Med Case Rep. 2020;8(10):350‐357.32704530 [Google Scholar]
- 22. Alizadehasl A, Soleimani A, Peighambari MM, Mostafavi A. Biventricular apical ballooning in patient with COVID‐19. J Echocardiogr. 2022;20:243‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujisaki T, Kassim F, Kassim G, Bandyopadhyay D, Singh V, Kim B. Biventricular takotsubo syndrome with COVID‐19 in an Asian male. J Cardiol Cases. 2021;24(1):6‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demertzis ZD, Dagher C, Malette KM, et al. Cardiac sequelae of novel coronavirus disease 2019 (COVID‐19): a clinical case series. Eur Heart J. 2020;4(FI1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torabi AJ, Villegas‐Galaviz J, Guglin M, Frick K, Rao R. Cardiogenic shock following cardiac tamponade and takotsubo in COVID‐19. Future Cardiol. 2021;17(4):631‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ortuno S, Jozwiak M, Mira J‐P, Nguyen LS. Case report: takotsubo syndrome associated with novel coronavirus disease 2019. Front Cardiovasc Med. 2021;8:614562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hegde S, Khan R, Zordok M, Maysky M. Characteristics and outcome of patients with COVID‐19 complicated by takotsubo cardiomyopathy: case series with literature review. Open Heart. 2020;7(2):e001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoepler W, Traugott MT, Christ G, et al. Clinical and angiographic features in three COVID‐19 patients with takotsubo cardiomyopathy. case report. SN Compr Clin Med. 2021;3(1):263‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bernardi N, Calvi E, Cimino G, et al. COVID‐19 pneumonia, takotsubo syndrome, and left ventricle thrombi. JACC Case Rep. 2020;2(9):1359‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sattar Y, Connerney M, Ullah W, et al. COVID‐19 presenting as takotsubo cardiomyopathy complicated with atrial fibrillation. IJC Heart Vasc. 2020;29:100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsao CW, Strom JB, Chang JD, Manning WJ. COVID‐19‐associated stress (takotsubo) cardiomyopathy. Circ Cardiovasc Imaging. 2020;13(7):e011222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomez JMD, Nair G, Nanavaty P, Rao A, Marinescu K, Suboc T. COVID‐19‐associated takotsubo cardiomyopathy. BMJ Case Rep. 2020;13(12):e236811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Titi L, Magnanimi E, Mancone M, et al. Fatal takotsubo syndrome in critical COVID‐19 related pneumonia. Cardiovasc Pathol. 2021;51:107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faqihi F, Alharthy A, Alshaya R, et al. Reverse takotsubo cardiomyopathy in fulminant COVID‐19 associated with cytokine release syndrome and resolution following therapeutic plasma exchange: a case‐report. BMC Cardiovasc Disord. 2020;20(1):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solano‐López J, Sánchez‐Recalde A, Zamorano JL. SARS‐CoV‐2, a novel virus with an unusual cardiac feature: inverted takotsubo syndrome. Eur Heart J. 2020;41(32):3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pasqualetto MC, Secco E, Nizzetto M, et al. Stress cardiomyopathy in COVID‐19 disease. Eur J Case Rep Intern Med. 2020;7(6):001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koh MCY, Li TYW, Ong JSY, Somani J, Ambhore AA. Stress cardiomyopathy with transient biventricular dysfunction following recent COVID‐19 infection. Acta Cardiol Sin. 2021;37(2):204‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dave S, Thibodeau JT, Styrvoky K, Bhatt SH. Takotsubo cardiomyopathy in a coronavirus disease‐2019‐positive patient: a case report. A&A Pract. 2020;14(11):e01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Osch D, Asselbergs FW, Teske AJ. Takotsubo cardiomyopathy in COVID‐19: a case report. haemodynamic and therapeutic considerations. Eur Heart J Case Rep. 2020;4(Fi1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhattacharyya PJ, Attri PK, Farooqui W. Takotsubo cardiomyopathy in early term pregnancy: a rare cardiac complication of SARS‐CoV‐2 infection. BMJ Case Rep. 2020;13(9):e239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taza F, Zulty M, Kanwal A, Grove D. Takotsubo cardiomyopathy triggered by SARS‐CoV‐2 infection in a critically ill patient. BMJ Case Rep. 2020;13(6):e236561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bottiroli M, De Caria D, Belli O, et al. Takotsubo syndrome as a complication in a critically ill COVID‐19 patient. ESC Heart Fail. 2020;7(6):4297‐4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roca E, Lombardi C, Campana M, et al. Takotsubo syndrome associated with COVID‐19. Eur J Case Rep Intern Med. 2020;7(5):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oyarzabal L, Gómez‐Hospital JA, Comin‐Colet J. Síndrome de tako‐tsubo asociado con COVID‐19. Rev Esp Cardiol. 2020;73(10):846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Minhas AS, Scheel P, Garibaldi B, et al. Takotsubo syndrome in the setting of COVID‐19. JACC Case Rep. 2020;2(9):1321‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kong N, Singh N, Mazzone S, Burkhart R, Anchan R, Blair J. Takotsubo syndrome presenting as cardiogenic shock in patients with COVID‐19: a case series and review of current literature. Cardiovasc Revasc Med. 2021;28s:50‐53. [DOI] [PubMed] [Google Scholar]
- 47. Park JH, Moon JY, Sohn KM, Kim YS. Two fatal cases of stress‐induced cardiomyopathy in COVID‐19 patients. J Cardiovas Imaging. 2020;28(4):300‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meyer P, Degrauwe S, Van Delden C, Ghadri JR, Templin C. Typical takotsubo syndrome triggered by SARS‐CoV‐2 infection. Eur Heart J. 2020;41(19):1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eftekharzadeh P, Patel A, Sokolova E, Rodas A, Ahmed S. Takotsubo cardiomyopathy: a COVID‐19 complication. Cureus. 2022;14(3):e22803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Frynas‐Jończyk K, Wiek‐Rębowska E, Filipiak‐Strzecka D, Szymczyk E, Kasprzak JD. COVID‐tsubo: Takotsubo syndrome in patient hospitalized due to the SARS‐CoV‐2 infection. Pol Arch Intern Med. 2022;132:16255. [DOI] [PubMed] [Google Scholar]
- 51. Fujiyoshi K, Ako J, Ishida K, Ishida M, Minami Y, Inomata T. Tako‐tsubo‐like left ventricular dysfunction in a patient with COVID‐19 demonstrated by non‐invasive multi‐modality imaging. J Nucl Cardiol. 2022;29(2):863‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kimura M, Hashiguchi S, Tanaka K, et al. Case report: takotsubo cardiomyopathy in Bickerstaff brainstem encephalitis triggered by COVID‐19. Front Neurol. 2021;12:822247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mishra AK, Dai Q, Sahu KK, ElMeligy A. Atypical takotsubo cardiomyopathy in COVID‐19. Am J Med Sci. 2021;362(5):e41‐e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Namburu L, Bhogal S, Ramu VK. COVID‐19‐Induced takotsubo cardiomyopathy with concomitant pulmonary embolism. Cureus. 2021;13(10):e18693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rivera K, Fernández‐Rodríguez D, Zielonka M, Casanova‐Sandoval J. Diagnosis of takotsubo syndrome in the COVID‐19 era. Rev Port Cardiol. 2021;40(11):899‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wildemann B, Jarius S, Lehmann LH, et al. COVID‐19‐related severe MS exacerbation with life‐threatening takotsubo cardiomyopathy in a previously stable patient and interference of MS therapy with long‐term immunity against SARS‐CoV‐2. J Neurol. 2022;269(3):1138‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bapat A, Maan A, Heist EK. Stress‐induced cardiomyopathy secondary to COVID‐19. Case Rep Cardiol. 2020;2020:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chao CJ, DeValeria PA, Sen A, et al. Reversible cardiac dysfunction in severe COVID‐19 infection, mechanisms and case report. Echocardiography. 2020;37(9):1465‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dabbagh MF, Aurora L, D'Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID‐19. JACC Case Rep. 2020;2(9):1326‐1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Manzur‐Sandoval D, Carmona‐Levario P, García‐Cruz E. Giant inverted T waves in a patient with COVID‐19 infection. Ann Emerg Med. 2021;77(2):264‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sang CJ 3rd, Heindl B, Von Mering G, et al. Stress‐Induced cardiomyopathy precipitated by COVID‐19 and influenza A coinfection. JACC Case Rep. 2020;2(9):1356‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tutor A, Unis G, Ruiz B, Bolaji OA, Bob‐Manuel T. Spectrum of suspected cardiomyopathy due to COVID‐19: A case series. Curr Probl Cardiol. 2021;46(10):100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Habedank D, Thieme R, Bublak A, Heinemann F, Spencker S, Atmowihardjo I. Ventricular fibrillation and takotsubo cardiomyopathy triggered by media panic on COVID‐19: a case report. Clin Case Rep. 2021;9(1):72‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Giannitsi S, Tsinivizov P, Poulimenos LE, et al. [Case report] Stress induced (takotsubo) cardiomyopathy triggered by the COVID‐19 pandemic. Exp Ther Med. 2020;20(3):2812‐2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Parker J, Niranjan S, Sriram KB. A case of broken heart syndrome via the telephone: socially distant outpatient clinics in the COVID‐19 pandemic. Intern Med J. 2020;50(11):1429‐1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Uhe T, Hagendorff A, Wachter R, Laufs U. Collateral damage: fear from SARS‐CoV2‐infection causing takotsubo cardiomyopathy. Clin Res Cardiol. 2020;109(12):1588‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chadha S. COVID‐19 pandemic' anxiety‐induced takotsubo cardiomyopathy. QJM. 2020;113(7):488‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rivers J, Ihle JF. COVID‐19 social isolation‐induced takotsubo cardiomyopathy. Med J Aust. 2020;213(7):336‐e1. [DOI] [PubMed] [Google Scholar]
- 69. Koutroumpakis E, Taylor T, Damaraju S, Badruddin Mawji S. “Covidsubo”: stress‐induced cardiomyopathy by novel coronavirus disease 2019. Cardiology. 2020;145(12):779‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dolci G, Prevedello F, Lobascio I, Mugnai G, Dalla Valle C, Bilato C. Mid‐ventricular takotsubo syndrome ‘lockdown'‐related during coronavirus disease 2019 outbreak: a case report. J Cardiovasc Med. 2021;22(5):414‐416. [DOI] [PubMed] [Google Scholar]
- 71. Moady G, Atar S. Quarantine‐induced stress cardiomyopathy (takotsubo syndrome) during the COVID‐19 pandemic. Isr Med Assoc J. 2021;23(3):149‐152. [PubMed] [Google Scholar]
- 72. Mohammed M, Zakhour S, Devgun J, Lee J, Keimig T, Wang DD. Takotsubo cardiomyopathy in a healthcare worker during the COVID‐19 pandemic: caused by the virus or the demands of the many being placed on the few? Eur J Case Rep Intern Med. 2020;7(12):002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ben Ammar H, Bouguira E, Brahmi L, et al. Simultaneous occurrence of a takotsubo syndrome and paranoia delirium, related to Covid‐19 pandemic: a case report. Clin Case Rep. 2021;9(11):e05026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boscolo Berto M, Spano G, Wagner B, et al. Takotsubo cardiomyopathy after mRNA COVID‐19 vaccination. Heart Lung Circ. 2021;30(12):e119‐e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fearon C, Parwani P, Gow‐Lee B, Abramov D. Takotsubo syndrome after receiving the COVID‐19 vaccine. J Cardiol Cases. 2021;24(5):223‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Crane P, Wong C, Mehta N, Barlis P. Takotsubo (stress) cardiomyopathy after ChAdOx1 nCoV‐19 vaccination. BMJ Case Rep. 2021;14(10):e246580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stewart C, Gamble DT, Dawson D. Novel case of takotsubo cardiomyopathy following COVID‐19 vaccination. BMJ Case Rep. 2022;15(1):e247291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tedeschi A, Camilli M, Ianni U, et al. Takotsubo syndrome after BNT162b2 mRNA Covid‐19 vaccine: emotional or causative relationship with vaccination? IJC Heart Vasc. 2022;40:101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Toida R, Uezono S, Komatsu H, et al. Takotsubo cardiomyopathy after vaccination for coronavirus disease 2019 in a patient on maintenance hemodialysis. CEN Case Rep. 2022;11(2):220‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yamaura H, Ishikawa H, Otsuka K, Kasayuki N. Reverse takotsubo cardiomyopathy as a cause of acute chest pain in a young woman following COVID‐19 vaccination. Circ Cardiovasc Imaging. 2022;15(1):e013661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Haussner W, DeRosa AP, Haussner D, et al. COVID‐19 associated myocarditis: a systematic review. Am J Emerg Med. 2022;51:150‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li P, Wang Y, Liang J, et al. Takotsubo syndrome and respiratory diseases: a systematic review. Eur Heart J Open. 2022;2(2):oeac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fazlollahi A, Zahmatyar M, Noori M, et al. Cardiac complications following mRNA COVID‐19 vaccines: a systematic review of case reports and case series. Rev Med Virol. 2022;32:e2318. [DOI] [PubMed] [Google Scholar]
- 84. Amin HZ, Amin LZ, Pradipta A. Takotsubo cardiomyopathy: a brief review. J Med Life. 2020;13(1):3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mehraeen E, Seyed Alinaghi SA, Nowroozi A, et al. A systematic review of ECG findings in patients with COVID‐19. Indian Heart J. 2020;72(6):500‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pelliccia F, Pasceri V, Patti G, et al. Long‐term prognosis and outcome predictors in takotsubo syndrome. JACC: Heart Fail. 2019;7(2):143‐154. [DOI] [PubMed] [Google Scholar]
- 87. Ahmed SK, Mohamed MG, Essa RA, et al. Global reports of takotsubo (stress) cardiomyopathy following COVID‐19 vaccination: a systematic review and meta‐analysis. J Cardiol Heart Vasc. 2022;43:101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID‐19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moady G, Atar S. Takotsubo syndrome during the COVID‐19 pandemic: state‐of‐the‐art review. CJC Open. 2021;3(10):1249‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shafi AMA, Shaikh SA, Shirke MM, Iddawela S, Harky A. Cardiac manifestations in COVID‐19 patients—a systematic review. J Card Surg. 2020;35(8):1988‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chang A, Wang YG, Jayanna MB, Wu X, Cadaret LM, Liu K. Mortality correlates in patients with takotsubo syndrome during the COVID‐19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1050‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zaghlol R, Dey AK, Desale S, Barac A. Racial differences in takotsubo cardiomyopathy outcomes in a large nationwide sample. ESC Heart Fail. 2020;7(3):1056‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


