Abstract
Background
Of patients undergoing transcatheter aortic valve replacement (TAVR), 80–90 % are at extreme, high, or intermediate risk. Patient selection considering futile outcomes in these groups is difficult as significant comorbidity burden is common. Thus, we examined 1-year mortality after TAVR according to age and comorbidities.
Methods
Between 2008 and 2021 all Danish TAVR-patients were included. From a multivariate Cox-regression model, significant characteristics associated with 1-year all-cause mortality were identified. The study population was divided into four groups according to number of significant comorbidities present at baseline: Low (0 comorbidities), mild (1 comorbidity), moderate (2 comorbidities), and high (3 or more comorbidities). The 1-year risk of all-cause mortality with 95 % confidence intervals (CI) was estimated by each group.
Results
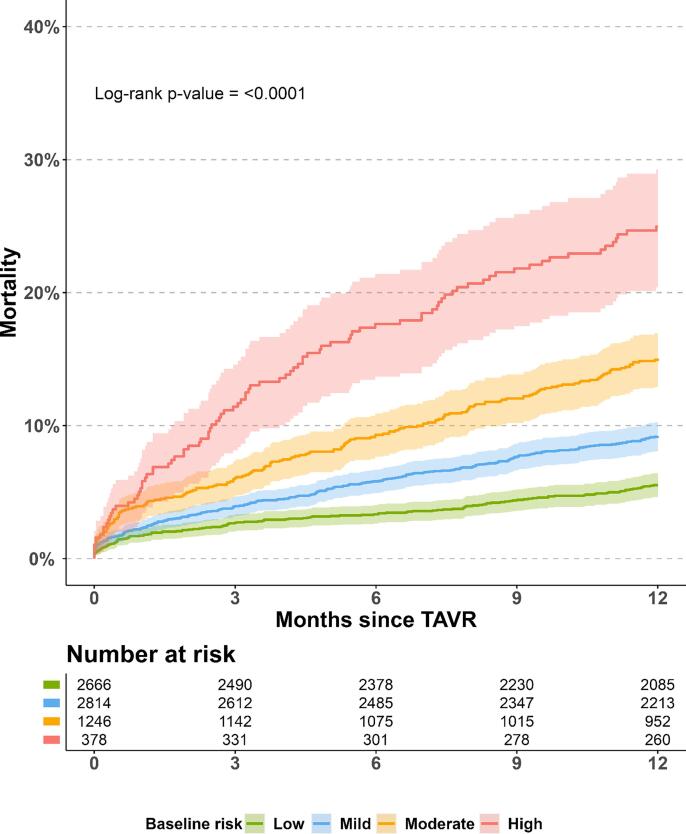
In total, 7,104 patients underwent TAVR. Significant covariates associated with 1-year all-cause mortality were chronic kidney disease, heart failure, chronic obstructive pulmonary disease, peripheral artery disease, and age ≥ 85 years. The four baseline groups comprised low (n = 2,666), mild (n = 2,814), moderate (n = 1,246), and high comorbidity burden (n = 378). The 1-year risk of all-cause mortality was 5.5 % (95 %CI: 4.6–6.4 %) in the low baseline comorbidity burden group. Conversely, the 1-year risk of all-cause mortality was 25.0 % (95 %CI: 20.4–29.3 %) in the high baseline burden group.
Conclusions
In a national sample of TAVR patients, readily available information on age and comorbidities, can be used to identify a high-risk group with 25 % 1-year mortality. This provides physicians and patients with an easy-to-understand view on 1-year prognosis after TAVR and may complement patient selection for improved long-term outcomes.
Keywords: Transcatheter aortic valve replacement, Mortality, Comorbidities, Age, Prognosis
Abbreviations: CI, confidence interval; CKD, Chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ICD-10, International classification of Diseases - 10th edition; IQR, interquartile range; TAVR, Transcatheter aortic valve replacement
1. Introduction
Avoiding futile outcomes and identifying patients with potential long-term benefits of transcatheter aortic valve replacement (TAVR) is a central part of patient selection. Over time, TAVR has been compared with surgical aortic valve replacement across multiple risk groups [1]. As such, the use of TAVR to treat severe, symptomatic aortic stenosis is increasing [2], [3], [4]. TAVR is now also used more than surgical aortic valve replacement in numerous countries and is expanding to lower risk-groups as well as to younger patients; however, 8–9 out of 10 patients remain extreme-, high-, or intermediate-risk patients [2], [3]. In these groups, patient selection is difficult considering the risk of futile outcomes as significant comorbidity burden and frailty is common.
Continuously modified risk scores exist to discriminate between high- and low-surgical risk patients [5], [6]. However, these scores were developed for valvular surgery and pertain only to the surgical risk – not long-term survival [5], [6]. This is reflected in recent guidelines which underline that patient selection for TAVR vs surgical aortic valve replacement is difficult and that an individual patient-centered approach should be performed including the patient’s treatment preferences [7], [8].
In summary, patients and physicians face a difficult choice balancing the potential increased life-expectancy and reduced symptom burden with TAVR versus the risk of complications or poor prognosis without TAVR. This is particularly important as up to 30 % of patients experienced limited reduced symptom burden or died within 1 year of TAVR despite high procedural success [9].
Consequently, we wanted to stratify patients in a simple manner according to age and comorbidities and examine 1-year mortality by such simple strata. In doing so, we aimed to provide the clinician and the patient with a readily available and easily understandable view on 1-year prognosis.
2. Methods
2.1. Data collection and definitions of characteristics
This study leveraged data from Danish nationwide registers. A unique personal identification number given to all permanent Danish residents at birth or immigration allowed for crosslinking of information in the following registers: The Danish Civil Registration System [10], The Danish National Patient Register [11], The Danish National Prescription Registry [12], and a database containing blood sample results from 4 out of 5 regions in Denmark, as previously described [13]. The positive predictive value of cardiac diagnoses, procedures, and surgeries is high and appropriate for research [14], [15]. We conducted an observational cohort study in which all patients undergoing first-time TAVR in Denmark between 1 January 2008 and 31 December 2021 were identified.
Baseline characteristics were identified as a hospital contact up to 10 years prior to date of TAVR using International Classification of Diseases − 10th edition (ICD-10) codes (Table S1 for full list of diagnoses codes). Only hospital admissions or outpatient contacts with a primary or secondary diagnosis of an ICD-10 code were included. Further, a claimed prescription of glucose-lowering drugs within 180 days was used as a proxy for diabetes [16]. Likewise, claimed prescriptions for two or more blood pressure lowering drugs within 180 days was used as a proxy for hypertension [17]. For comedication, claimed prescriptions 180 days prior to TAVR was defined as baseline drug use.
2.2. Statistical analyses
Annual procedure volume per TAVR-center is presented. Baseline characteristics are presented with numbers and percentages for categorical values and median and interquartile ranges (IQR) for numerical values. Further, baseline characteristics for each significant covariate associated with 1-year all-cause mortality is presented in Table S2.
2.2.1. Stratification of study population and outcome
Significant characteristics of patients associated with 1-year risk of all-cause death were identified from a multivariate Cox regression model. The following variables were entered into the model based on a clinical assessment of relevant factors for poor long-term survival: Sex, age (categorial: <85 years, ≥85 years), history of stroke, myocardial infarction, heart failure, diabetes, peripheral artery disease, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), and calendar year group of procedure. Based on covariates significantly associated with 1-year mortality (Figure S1 for estimates), the cohort was then divided according to the number of significant comorbidities (including age ≥ 85 years) present at baseline; 0 comorbidities, 1 comorbidity, 2 comorbidities, and 3 or more comorbidities to reflect low, mild, moderate, and high baseline comorbidity burden groups, respectively. However, calendar year of procedure was used only as an adjustment variable – not included in the score. This was done to account for improvements in procedural technique and patient selection not otherwise captured by covariates already included in the model The 1-year risk of all-cause death for each group was non-parametrically estimated and compared using the log-rank test. We aimed to describe mortality rates by these baseline groups. In doing so, patients were followed from date of TAVR procedure until death, emigration, one year of follow-up, or 31 December 2021, whichever came first.
2.2.2. Sensitivity and supplementary analyses
In a sensitivity analysis, we evaluated if the inclusion of patients with an available blood sample of hemoglobin, creatinine, and albumin would refine the identification of patients with poor prognosis. From the baseline creatinine level, the estimated glomerular filtration rate (eGFR) was estimated using the CKD-EPI formula [18]. The same approach was used as in the main analysis, however, the Cox regression model was adjusted for sex, age (categorial: <85 years, ≥85 years), history of stroke, myocardial infarction, heart failure, diabetes, peripheral artery disease, COPD, hemoglobin (categorial: ≤6 mmol/L, >6 mmol/L), eGFR (categorial: ≤29 ml/min/1.73 m2, ≥30 ml/min/1.73 m2), albumin (categorial: ≤29 g/L, ≥30 g/L).
We performed several supplementary analyses:
-
i)
To investigate the association between age and mortality alone, we stratified the population into age quintiles and estimated the 1-year risk of all-cause death within each quintile with the aim of providing insights into the impact of age compared with comorbidities on mortality.
-
ii)
We evaluated the 1-year risk of death individually for each significant comorbidity i.e. heart failure vs. no heart failure. This was done to investigate differences in the importance of each comorbidity.
-
iii)
As CKD was significantly associated with 1-year mortality in both the main analysis using diagnoses codes and the sensitivity analyses including biomarkers, we stratified patients from the sensitivity analyses into four eGFR groups: eGFR ≥ 90, eGFR 60–89, eGFR 30–59, and eGFR < 30 and estimated the 1-year risk of all-cause death within each group. This was done to investigate levels of eGFR on the risk of death.
Data, statistical analyses, and figures were managed, performed, and created in R [19].
2.3. Ethics
The present study was approved by the data responsible institution, Capital Region. We refer to approval number P-2019–191. In Denmark, retrospective registry-based cohort studies do not require further approval from the Research Ethics Committee System.
3. Results
3.1. Population characteristics
During the study period (2008–2021), 7,104 patients underwent first-time TAVR in Denmark. Significant baseline characteristics associated with 1-year mortality were the five covariates: CKD, COPD, heart failure, peripheral artery disease, and age ≥ 85 years (Figure S1). Patients were divided into 4 groups according to baseline comorbidity burden: low baseline comorbidity burden (none of the five risk factors, n = 2,666), mild baseline comorbidity burden (one of the five risk factors, n = 2,814), moderate comorbidity burden (two of the five risk factors, n = 1,246), and high baseline comorbidity burden (at least three of the five risk factors, n = 378). Procedure volume increased over time with three out of four centers performing>50 first-time TAVR procedures annually from 2011 (Figure S2). All centers performed>50 first-time TAVR procedures from 2016.
The baseline characteristics of patients within each group are shown in Table 1. Patients in the low baseline comorbidity burden group were the youngest (median age 80 IQR [75–82] years). Contrary, patients in the high baseline comorbidity burden group were the oldest (median age 85 IQR [79–87] years). A temporal trend towards treating lower comorbidity burden patients was observed; in 2008–2010 the high baseline comorbidity burden group comprised 35/393 (8.9 %) patients compared with 65/1,970 (3.2 %) in 2020–2021. However, the absolute number of high baseline comorbidity burden patients increased over time.
Table 1.
Characteristics according to baseline groups.
| Baseline comorbidity burden | Low | Mild | Moderate | High | Total |
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 or more | ||
| No. | 2,666 | 2,814 | 1,246 | 378 | 7,104 |
| Male (%) | 1,442 (54.1) | 1,496 (53.2) | 743 (59.6) | 258 (68.3) | 3,939 (55.4) |
| Age (years), median [IQR] | 80 [75–82] | 83 [78–87] | 85 [78–87] | 85 [79–87] | 81 [77–85] |
| Year group* | |||||
| 2008–2010 | 108 (27.5) | 151 (38.4) | 99 (25.2) | 35 (8.9) | 393 (1 0 0) |
| 2011–2013 | 238 (27.7) | 374 (43.5) | 179 (20.8) | 69 (8.0) | 860 (1 0 0) |
| 2014–2016 | 500 (32.9) | 606 (39.9) | 310 (20.4) | 103 (6.8) | 1,519 (1 0 0) |
| 2017–2019 | 923 (39.1) | 939 (39.8) | 394 (16.7) | 106 (4.5) | 2,362 (1 0 0) |
| 2020–2021 | 897 (45.5) | 744 (37.8) | 264 (13.4) | 65 (3.3) | 1,970 (1 0 0) |
| Comorbidities, No. (%) | |||||
| Stroke/systemic embolism | 325 (12.2) | 378 (13.4) | 186 (14.9) | 68 (18.0) | 957 (13.5) |
| Myocardial infarction | 222 (8.3) | 308 (10.9) | 202 (16.2) | 95 (25.1) | 827 (11.6) |
| Ischemic heart disease | 1,014 (38.0) | 1,254 (44.6) | 674 (54.1) | 263 (69.6) | 3,205 (45.1) |
| Heart failure | 0 (0) | 779 (27.7) | 856 (68.7) | 337 (89.2) | 1,972 (27.8) |
| Peripheral artery disease | 0 (0) | 271 (9.6) | 347 (27.8) | 211 (55.8) | 829 (11.7) |
| Previous PCI | 508 (19.1) | 604 (21.5) | 368 (29.5) | 136 (36.0) | 1,616 (22.7) |
| Previous CABG | 106 (4.0) | 107 (3.8) | 66 (5.3) | 34 (9.0) | 313 (4.4) |
| Atrial fibrillation | 728 (27.3) | 984 (35.0) | 532 (42.7) | 201 (53.2) | 2,445 (34.4) |
| Diabetes | 490 (18.4) | 506 (18.0) | 252 (20.2) | 103 (27.2) | 1,351 (19.0) |
| COPD | 0 (0) | 359 (12.8) | 367 (29.5) | 235 (62.2) | 961 (13.5) |
| Chronic kidney disease | 0 (0) | 217 (7.7) | 280 (22.5) | 202 (53.4) | 699 (9.8) |
| Hemoglobin ≤ 6 mmol/L | 1,727 (91.0) | 1,682 (86.7) | 708 (82.8) | 206 (84.8) | 4,323 (87.6) |
| Missing | 769 (28.8) | 875 (31.1) | 391 (31.4) | 135 (35.7) | 2,170 (30.5) |
| eGFR < 30 ml/min/1,73 m2 | 1,883 (98.5) | 1,857 (95.1) | 732 (85.3) | 180 (74.1) | 4,652 (93.7) |
| Missing | 755 (28.3) | 862 (30.6) | 388 (31.1) | 135 (35.7) | 2,140 (30.1) |
| Albumin < 30 g/L | 1,423 (90.5) | 1,436 (85.9) | 629 (83.9) | 177 (80.1) | 3,665 (87.0) |
| Missing | 1,094 (41.0) | 1,142 (40.6) | 496 (39.8) | 157 (41.5) | 2,889 (40.7) |
Abbreviations: CABG: Coronary artery bypass graft, COPD: Chronic obstructive pulmonary disease, eGFR: Estimated glomerular filtration rate, IQR: Interquartile range, PCI: Percutaneous coronary intervention.
Percentages represent row percentages.
3.2. Mortality according to age and comorbidity burden
The unadjusted 1-year risk of all-cause mortality for all patients was 9.7 % (95 % confidence interval (CI): 9.0 % to 10.5 %) (Figure S3). Fig. 1 illustrates the 1-year absolute risk of death for each burden group. The risk of death increased with increasing burden group. Specifically, patients in the low baseline risk group had the lowest risk of death with a 1-year risk of all-cause death of 5.5 % (95 %CI: 4.6 % to 6.4 %). Conversely, patients in the high baseline risk group had the highest risk of death with a 1-year risk of all-cause death of 25.0 % (95 %CI: 20.4 % to 29.3 %).
Fig. 1.
Title: Absolute risk of 1-year all-cause mortality. Legend: The absolute risk of all-cause mortality for each burden group after TAVR. Numbers beneath plot represents patients at risk 0, 3, 6, 9, and 12 months after TAVR, respectively. Colored areas represent 95 % confidence intervals.
When analyzing mortality according to each comorbidity factor (Figure S4), only history of CKD conferred a point estimate of>20 % 1-year risk of death (20.2 % [95 % CI: 17.1 % to 23.2 %]). Stratifying patients according to age into two groups did not confer significant differences in risk of death and the CI overlapped. Further, when stratifying patients into quintiles based on age groups, the 1-year risk of death increased gradually up to 12.1 % (95 % CI: 10.2 % to 13.9 %) in the oldest age quintile: 87–100 years (Figure S5).
3.3. Sensitivity analysis with biomarkers
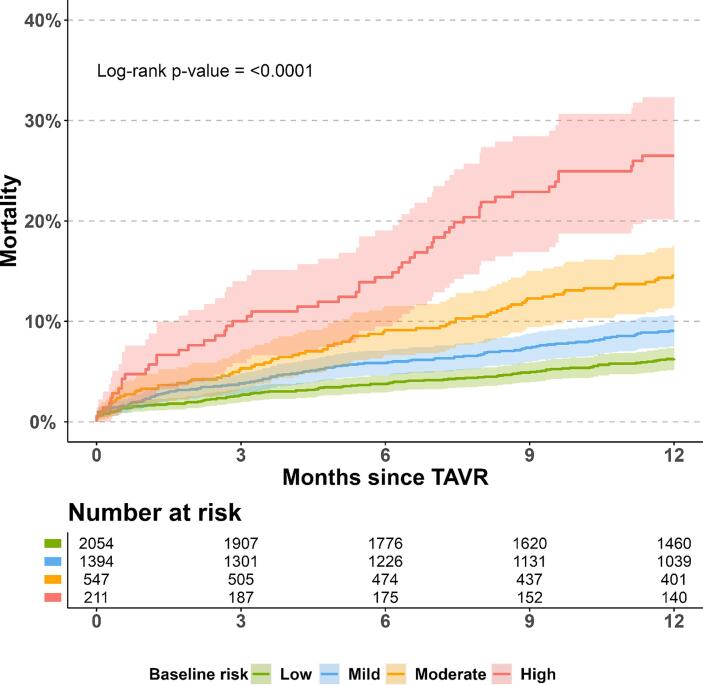
The sensitivity analysis comprised 4,206 TAVR patients with an available sample of hemoglobin, eGFR, and albumin. In the Cox model, heart failure, COPD, and the three biomarkers (hemoglobin ≤ 6 mmol/L, eGFR < 30 ml/min/1.73 m2, albumin < 30 g/L) were all significantly associated with an increased hazard ratio of death (Figure S6). As with the main analysis, the 1-year risk of all-cause mortality increased with increasing baseline comorbidity burden group (Fig. 2). When stratifying patients solely according to eGFR, the 1-year absolute risk of all-cause death was below 15 % for all groups except for eGFR < 30, which was associated with marked increased risk of death of 21.3 % (95 %CI: 16.3 % to 25.9 %) (Figure S7).
Fig. 2.
Title: Absolute risk of 1-year all-cause mortality with biomarkers. Legend: The absolute risk of all-cause mortality for each burden group after TAVR in a population with biomarkers. Numbers beneath plot represents patients at risk 0, 3, 6, 9, and 12 months after TAVR, respectively. Colored areas represent 95 % confidence intervals. The included biomarkers were hemoglobin, estimated glomerular filtration rate, and albumin.
4. Discussion
In this nationwide, observational cohort study, investigating the 1-year risk of all-cause death as a function of baseline age and comorbidity burden, the main findings can be summarized as: i) The proportion of high baseline comorbidity burden patients decreased over time, however, the absolute number of patients in this group increased. ii) When dividing patients according to age and comorbidity burden, a 4-fold increased risk of death was found between high baseline comorbidity burden patients and low baseline comorbidity burden patients. iii) Patients with a one in four risk of death within one year can be identified from few clinical risk factors.
4.1. Long-term survival after TAVR
Existing models for patient selection have limited implications for identifying TAVR patients at high risk of 1-year mortality [20]. In this study, we found that a simple approach based on age and simple comorbidity burden was able to identify a subgroup of patients associated with a 25 % risk of death within one year of TAVR. This provides physicians and patients with a readily available tool to discuss prognosis after TAVR.
Other studies have evaluated 1-year mortality in patients undergoing TAVR [21], [22]. Hermiller et al. found that home oxygen use, low albumin, falls in past 6 months, STS PROM score > 7 %, and severe Charlson score predicted 1-year mortality [22]. This study found an overall mortality that was higher (22.8 %) compared to the present study most likely due to inclusion of only high-risk and extreme-risk patients. They found a subset of patients with 36.6 % 1-year risk of death, however, as stated by the authors, the applicability of the results to everyday clinical practice is limited as the predictors included in the study are time consuming to gather and not routinely collected [22]. Kiani et al. found 1-year mortality rates ranging from around 7 % to 27 % depending on the number of frailty components (low albumin, anemia, and slow walking speed) present at time of TAVR. In our sensitivity analysis including blood samples, we also found that low albumin and low hemoglobin was associated with an increased risk of 1-year all-cause death. Laboratory work is routinely collected in clinical practice before patients undergo TAVR and might improve patient selection. Further, albumin and hemoglobin levels are increasingly recognized as markers of frailty and has been associated with an increased risk of death in previous studies [21], [22], [23], [24], [25].
4.2. Comorbidities and age in relation to mortality
We found that CKD whether based on a diagnosis code or baseline eGFR ≤ 30 was associated with the greatest increase in the 1-year risk of death. Patients with CKD represents an important subgroup, as patients with severe CKD or dialysis were excluded or underrepresented in all landmark TAVR trials [1]. By contrast, CKD is prevalent in TAVR patients in real world cohorts [26], [27], [28], [29]. In our analysis using only diagnosis codes, CKD was associated with a > 20 % 1-year risk of death. However, this estimate may be higher than what is attributable to CKD alone as residual comorbidity burden was highest for patients with CKD compared to patients with the other significant covariates (Table S2). Nevertheless, when restricting the analysis according to eGFR groups, the 1-year risk of death increased with reduced baseline kidney function as expected, however, there was a marked increase in risk of death particularly for patients with eGFR ≤ 30 consistent with previous findings [26], [27], [28], [30]. The most prevalent comorbidity in the score was heart failure. Heart failure is common in TAVR patients [31]. Further, reduced ejection fraction has been associated with an increased risk of short- and long-term mortality despite TAVR improving ejection fraction in low ejection fraction patients [32].
Interestingly, stratifying patients according to age > 85 and 85 years and younger, the survival curves overlapped with only a limited difference in the 1-year mortality point estimates. Further, in our supplementary analysis stratifying patients into age quintiles, the survival curves once again overlapped with only the greatest quintile, age 87–100 years, having a relatively higher point estimate compared to other age groups. Importantly, the mortality in this age group was still lower compared to CKD, heart failure, peripheral artery disease, and COPD. The results indicate a limited ability to discriminate patients based on chronological age alone most likely explained by selection and healthy survivor bias consistent with other findings [33], [34], [35], [36].
5. Strengths and limitations
This study leveraged data from Danish nationwide administrative registers. In doing so, all patients undergoing TAVR in Denmark were included thereby minimizing selection bias. Further the data sources in combination with the unique personal identification number allowed for almost complete follow-up for all patients enrolled except for administrative censoring (end of study period). In Denmark, only four high-volume centers perform TAVR through a tax-funded health care system ensuring universal, free access to TAVR minimizing selection bias across socioeconomic groups. Further, high-volume centers with on-site cardiac surgeons facilitates optimal outcomes of TAVR procedures [37].
Some limitations apply: This was not a prospective study. Information on procedural characteristics such as type of bioprosthetic valve and paravalvular leakage are lacking. Important unmeasured confounders were not available in the registers (e.g. smoking habits and frailty indices). Using ICD-10 codes limits the model discrimination i.e. degree of COPD and types of heart failure could not be incorporated in the model. For the sensitivity analysis including laboratory works, not all patients had available information. Changes in kidney function over time were not included. We were not able to investigate mortality among patients who did not undergo TAVR which could have provided valuable information for comparison.
5.1. Conclusions
In a retrospective nationwide cohort study national sample of patients undergoing TAVR, we showed that readily available information on age and medical history could easily identify a high-risk patient group with 25 % mortality by 1-year. This may provide physicians and patients with a readily available view on 1-year prognosis after TAVR. Our results suggest that TAVR in patients with chronic kidney disease should be thoroughly reconsidered, but more studies are needed to guide patient selection to secure best patient-level but also society-economic outcomes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
None.
Funding
This work was supported by “Torben og Alice Frimodts Fond, Denmark”; “Dagmar Marshalls Fond, Denmark”; “Eva og Henry Frænkels Mindefond, Denmark”; and “Snedkermester Sophus Jacobsen og Hustru Astrid Jacobsens Fond, Denmark”. None of the funders had any influence on the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Disclosures
JES, CSP, EHB, GG: The authors report no relationships that could be construed as a conflict of interest; LK: Speakers honorarium from Novo, Novartis, AstraZenca, Boehringer and Bayer all unrelated to this manuscript; ELF: Independent research grant from Novo Nordisk Foundation; JBO: Speaker honoraria or consultancy fees from Bayer, Bristol-Myers Squibb, and Pfizer all unrelated to this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2022.101157.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.G.C.M. Siontis, P. Overtchouk, T.J. Cahill, T. Modine, B. Prendergast, F. Praz, T. Pilgrim, T. Petrinic, A. Nikolakopoulou, G. Salanti, L. Sondergaard, S. Verma, P. Juni, S. Windecker, Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis, Eur Hear. J. 40 (2019) 3143–3153. https://doi.org/10.1093/eurheartj/ehz275. [DOI] [PubMed]
- 2.Strange J.E., Sindet-Pedersen C., Gislason G.H., Torp-Pedersen C., Kragholm K.H., Lundahl C., Fosbøl E.L., Butt J.H., Køber L., Søndergaard L., Olesen J.B. Temporal trends in utilization of transcatheter aortic valve replacement and patient characteristics: a nationwide study. Am. Heart J. 2021;243:140–146. doi: 10.1016/j.ahj.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Carroll J.D., Mack M.J., Vemulapalli S., Herrmann H.C., Gleason T.G., Hanzel G., Deeb G.M., Thourani V.H., Cohen D.J., Desai N., Kirtane A.J., Fitzgerald S., Michaels J., Krohn C., Masoudi F.A., Brindis R.G., Bavaria J.E. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 4.Durko A.P., Osnabrugge R.L., Van Mieghem N.M., Milojevic M., Mylotte D., Nkomo V.T., Pieter Kappetein A. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur. Hear. J. 2018;39:2635–2642. doi: 10.1093/eurheartj/ehy107. [DOI] [PubMed] [Google Scholar]
- 5.Nashef S.A.M., Roques F., Sharples L.D., Nilsson J., Smith C., Goldstone A.R., Lockowandt U. Euroscore II. Eur. J. Cardio-Thoracic Surg. 2012;41:734–745. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A., Sato K., Narayanswami J., Banerjee K., Andress K., Lokhande C., Mohananey D., Anumandla A.K., Khan A.R., Sawant A.C., Menon V., Krishnaswamy A., Tuzcu E.M., Jaber W.A., Mick S., Svensson L.G., Kapadia S.R. Current Society of Thoracic Surgeons Model Reclassifies Mortality Risk in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2018;11:e006664. doi: 10.1161/CIRCINTERVENTIONS.118.006664. [DOI] [PubMed] [Google Scholar]
- 7.A. Vahanian, F. Beyersdorf, F. Praz, M. Milojevic, S. Baldus, J. Bauersachs, D. Capodanno, L. Conradi, M. De Bonis, R. De Paulis, V. Delgado, N. Freemantle, M. Gilard, K.H. Haugaa, A. Jeppsson, P. Jüni, L. Pierard, B.D. Prendergast, J.R. Sádaba, C. Tribouilloy, W. Wojakowski, F.-J. Neumann, P. Myers, M. Abdelhamid, S. Achenbach, R. Asteggiano, F. Barili, M.A. Borger, T. Carrel, J.-P. Collet, D. Foldager, G. Habib, C. Hassager, A. Irs, B. Iung, M. Jahangiri, H.A. Katus, K.C. Koskinas, S. Massberg, C.E. Mueller, J.C. Nielsen, P. Pibarot, A. Rakisheva, M. Roffi, A. Rubboli, E. Shlyakhto, M. Siepe, M. Sitges, L. Sondergaard, M. Sousa-Uva, G. Tarantini, J.L. Zamorano, F. Praz, M. Milojevic, S. Baldus, J. Bauersachs, D. Capodanno, L. Conradi, M. De Bonis, R. De Paulis, V. Delgado, N. Freemantle, M. Gilard, K.H. Haugaa, A. Jeppsson, P. Jüni, L. Pierard, B.D. Prendergast, J.R. Sádaba, C. Tribouilloy, W. Wojakowski, 2021 ESC/EACTS Guidelines for the management of valvular heart disease, Eur. Heart J. (2021) 1–72. https://doi.org/10.1093/eurheartj/ehab395.
- 8.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., Gentile F., Jneid H., Krieger E.V., Mack M., McLeod C., O’Gara P.T., Rigolin V.H., Sundt T.M., Thompson A., Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5) doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 9.Lindman B.R., Alexander K.P., O’Gara P.T., Afilalo J. Futility, benefit, and transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2014;7:707–716. doi: 10.1016/j.jcin.2014.01.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen C.B. The Danish Civil Registration System. Scand. J. Public Heal. 2011;39:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 11.Lynge E., Sandegaard J.L., Rebolj M. The Danish National Patient Register. Scand. J. Public Heal. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 12.A. Pottegard, S.A.J. Schmidt, H. Wallach-Kildemoes, H.T. Sorensen, J. Hallas, M. Schmidt, Data Resource Profile: The Danish National Prescription Registry, Int. J. Epidemiol. 46 (2017) 798-798f. https://doi.org/10.1093/ije/dyw213. [DOI] [PMC free article] [PubMed]
- 13.Strange J.E., Sindet-Pedersen C., Staerk L., Grove E.L., Gerds T.A., Torp-Pedersen C., Gislason G.H., Olesen J.B. All-cause mortality, stroke, and bleeding in patients with atrial fibrillation and valvular heart disease. Eur. Hear. J. Cardiovasc. Pharmacother. 2021;7(FI1):f93–f100. doi: 10.1093/ehjcvp/pvaa011. [DOI] [PubMed] [Google Scholar]
- 14.Sundbøll J., Adelborg K., Munch T., Frøslev T., Sørensen H.T., Bøtker H.E., Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adelborg K., Sundbøll J., Munch T., Frøslev T., Sørensen H.T., Bøtker H.E., Schmidt M. Positive predictive value of cardiac examination, procedure and surgery codes in the Danish National Patient Registry: a population-based validation study. BMJ Open. 2016;6(12):e012817. doi: 10.1136/bmjopen-2016-012817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carstensen B., Kristensen J.K., Marcussen M.M., Borch-Johnsen K. The National Diabetes Register. Scand. J. Public Health. 2011;39:58–61. doi: 10.1177/1403494811404278. [DOI] [PubMed] [Google Scholar]
- 17.Olesen J.B., Lip G.Y.H., Hansen M.L., Hansen P.R., Tolstrup J.S., Lindhardsen J., Selmer C., Ahlehoff O., Olsen A.-M.- S., Gislason G.H., Torp-Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342(jan31 1) doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., Coresh J. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/., (n.d.).
- 20.Wang T.K.M., Wang M.T.M., Gamble G.D., Webster M., Ruygrok P.N. Performance of contemporary surgical risk scores for transcatheter aortic valve implantation: A meta-analysis. Int. J. Cardiol. 2017;236:350–355. doi: 10.1016/j.ijcard.2016.12.188. [DOI] [PubMed] [Google Scholar]
- 21.Kiani S., Stebbins A., Thourani V.H., Forcillo J., Vemulapalli S., Kosinski A.S., Babaliaros V., Cohen D., Kodali S.K., Kirtane A.J., Hermiller J.B., Stewart J., Lowenstern A., Mack M.J., Guyton R.A., Devireddy C. The Effect and Relationship of Frailty Indices on Survival After Transcatheter Aortic Valve Replacement. JACC. Cardiovasc. Interv. 2020;13(2):219–231. doi: 10.1016/j.jcin.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Hermiller J.B., Yakubov S.J., Reardon M.J., Deeb G.M., Adams D.H., Afilalo J., Huang J., Popma J.J. Predicting Early and Late Mortality After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016;68(4):343–352. doi: 10.1016/j.jacc.2016.04.057. [DOI] [PubMed] [Google Scholar]
- 23.Afilalo J., Lauck S., Kim D.H., Lefèvre T., Piazza N., Lachapelle K., Martucci G., Lamy A., Labinaz M., Peterson M.D., Arora R.C., Noiseux N., Rassi A., Palacios I.F., Généreux P., Lindman B.R., Asgar A.W., Kim C.A., Trnkus A., Morais J.A., Langlois Y., Rudski L.G., Morin J.-F., Popma J.J., Webb J.G., Perrault L.P. Frailty in Older Adults Undergoing Aortic Valve Replacement. J. Am. Coll. Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Kundi H., Valsdottir L.R., Popma J.J., Cohen D.J., Strom J.B., Pinto D.S., Shen C., Yeh R.W. Impact of a Claims-Based Frailty Indicator on the Prediction of Long-Term Mortality After Transcatheter Aortic Valve Replacement in Medicare Beneficiaries. Circ. Cardiovasc. Qual. Outcomes. 2018;11:e005048. doi: 10.1161/CIRCOUTCOMES.118.005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debonnaire P., Fusini L., Wolterbeek R., Kamperidis V., Van Rosendael P., Van Der Kley F., Katsanos S., Joyce E., Tamborini G., Muratori M., Gripari P., Bax J.J., Marsan N.A., Pepi M., Delgado V. Value of the “tAVI2-SCORe” versus surgical risk scores for prediction of one year mortality in 511 patients who underwent transcatheter aortic valve implantation. Am. J. Cardiol. 2015;115:234–242. doi: 10.1016/j.amjcard.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Ferro C.J., Chue C.D., De Belder M.A., Moat N., Wendler O., Trivedi U., Ludman P., Townend J.N. Impact of renal function on survival after transcatheter aortic valve implantation (TAVI): An analysis of the UK TAVI registry. Heart. 2015;101:546–552. doi: 10.1136/heartjnl-2014-307041. [DOI] [PubMed] [Google Scholar]
- 27.T.E. Oguri A, Yamamoto M, Mouillet G, Gilard M, Laskar M, Eltchaninoff H, Fajadet J, Iung B, Donzeau-Gouge P, Leprince P, Leguerrier A, Prat A, Lievre M, Chevreul K, Dubois-Rande J, Impact of chronic kidney disease on the outcomes of transcatheter aortic valve implantation: results from the FRANCE 2 registry, Catheter. Cardiovasc. Interv. 85 (2015) 448–449. https://doi.org/10.1002/ccd.25809. [DOI] [PubMed]
- 28.Dumonteil N., Van Der Boon R.M.A., Tchetche D., Chieffo A., Van Mieghem N.M., Marcheix B., Buchanan G.L., Vahdat O., Serruys P.W., Fajadet J., Colombo A., De Jaegere P.P.T., Carrié D. Impact of preoperative chronic kidney disease on short- and long-term outcomes after transcatheter aortic valve implantation: A pooled-rotterdam- milano-toulouse in collaboration plus (Pragmatic-Plus) initiative substudy. Am. Heart J. 2013;165:752–760. doi: 10.1016/j.ahj.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Beohar N., Doshi D., Thourani V., Jensen H., Kodali S., Zhang F., Zhang Y., Davidson C., McCarthy P., Mack M., Kapadia S., Leon M., Kirtane A. Association of transcatheter aortic valve replacement with 30-day renal function and 1-year outcomes among patients presenting with compromised baseline renal function experience from the PARTNER 1 trial and registry. JAMA Cardiol. 2017;2:742–749. doi: 10.1001/jamacardio.2017.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto M., Hayashida K., Mouillet G., Hovasse T., Chevalier B., Oguri A., Watanabe Y., Dubois-Randé J.L., Morice M.C., Lefèvre T., Teiger E. Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2013;62:869–877. doi: 10.1016/j.jacc.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 31.Himbert D., Vahanian A. Transcatheter aortic valve replacement for patients with heart failure. Heart Fail. Clin. 2015;11:231–242. doi: 10.1016/j.hfc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Sannino A., Gargiulo G., Schiattarella G.G., Brevetti L., Perrino C., Stabile E., Losi M.A., Toscano E., Giugliano G., Scudiero F., Chiacchio E., Trimarco B., Esposito G. Increased mortality after transcatheter aortic valve implantation (TAVI) in patients with severe aortic stenosis and low ejection fraction: A meta-analysis of 6898 patients. Int. J. Cardiol. 2014;176:32–39. doi: 10.1016/j.ijcard.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Arsalan M., Szerlip M., Vemulapalli S., Holper E.M., Arnold S.V., Li Z., Dimaio M.J., Rumsfeld J.S., Brown D.L., Mack M.J. Should Transcatheter Aortic Valve Replacement Be Performed in Nonagenarians? Insights from the STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2016;67:1387–1395. doi: 10.1016/j.jacc.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blumenstein J., Möllmann H., Bleiziffer S., Bauer T., Ensminger S., Bekeredjian R., Walther T., Frerker C., Beyersdorf F., Hamm C., Beckmann A. Transcatheter aortic valve implantation in nonagenarians: insights from the German Aortic Valve Registry (GARY) Clin. Res. Cardiol. 2020;109:1099–1106. doi: 10.1007/s00392-020-01601-4. [DOI] [PubMed] [Google Scholar]
- 35.McNeely C., Zajarias A., Robbs R., Markwell S., Vassileva C.M. Transcatheter Aortic Valve Replacement Outcomes in Nonagenarians Stratified by Transfemoral and Transapical Approach. Ann. Thorac. Surg. 2017;103:1808–1814. doi: 10.1016/j.athoracsur.2017.02.056. [DOI] [PubMed] [Google Scholar]
- 36.Attinger-Toller A., Ferrari E., Tueller D., Templin C., Muller O., Nietlispach F., Toggweiler S., Noble S., Roffi M., Jeger R., Huber C., Carrel T., Pilgrim T., Wenaweser P., Togni M., Cook S., Heg D., Windecker S., Goy J.J., Stortecky S. Age-Related Outcomes After Transcatheter Aortic Valve Replacement: Insights From the SwissTAVI Registry. JACC Cardiovasc. Interv. 2021;14:952–960. doi: 10.1016/j.jcin.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 37.Bavaria J.E., Tommaso C.L., Brindis R.G., Carroll J.D., Michael Deeb G., Feldman T.E., Gleason T.G., Horlick E.M., Kavinsky C.J., Kumbhani D.J., Craig Miller D., Allen Seals A., Shahian D.M., Shemin R.J., Sundt T.M., Thourani V.H. AATS/ACC/SCAI/STS expert consensus systems of care document: Operator and institutional recommendations and requirements for transcatheter aortic valve replacement: A Joint Report of the American Association for Thoracic Surgery, American College of. Catheter. Cardiovasc. Interv. 2018;93(2019):E153–E184. doi: 10.1002/ccd.27811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.