Abstract
Hybrid nanofluids (HNFs) are potential fluids that have higher thermophysical properties than conventional nanofluids of heat transfer and viscosity. HNF is a new generation of nanofluid that is produced by dispersing two or more types of dissimilar nanoparticles (NPs) in the base fluid. In this study, the rheological behavior of MWCNT (25%)-MgO (75%)/SAE40 HNF was investigated experimentally, statistically and numerically. Temperature conditions are in the range of T = 50-25 °C, solid volume fractions (SVFs) are in the range of SVF = 0.0625–1% and shear rate (SR) is in the range of SR = 666.5–7998 s−1. This study aims to identify the rheological behavior of HNF based on the effective factors of temperature, SR, and SVF. Various methods show that HNFs exhibit non-Newtonian behavior. The numerical values of the power-law index (n) at T = 50 °C and SVF = 0.75% show the strongest non-Newtonian behavior of HNF and n = 0.9233 is reported. Using laboratory findings, the maximum and minimum viscosities of the base oil increase and decrease by 24% and -8.50%, respectively. Using the response surface methodology (RSM), the relationship between experimental data and modeled data is determined. A quadratic three-variable model with R2 = 0.9994 is used to predict the data.
Keywords: Hybrid nanofluid, Viscosity, Rheological behavior, RSM, MWCNTs, Thermophysical properties, Experimental, Statistical, Numerical, MgO
Hybrid nanofluid; Viscosity; Rheological behavior; RSM.
1. Introduction
The role of nanotechnology in the recent advances in science is very significant and this has led scientists and researchers in different fields to focus on the use of nanotechnology to improve various systems [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. One of the things that is done to improve the properties of different materials is to add additives to the structure of materials in different phases to improve their properties [11, 12, 13, 14, 15, 16]. Nanofluids (NFs) are obtained by suspending nanoparticles (NPs) in base fluids such as water, ethylene glycol (EG), propylene glycol, oil and various fluids. NFs are the evolution of fluid science. NPs have unique mechanical, thermal, magnetic and electrical properties. Many high-tech industries often face cooling technical challenges. Conventional methods such as wide surfaces and microchannels that increase the rate of heat transfer have the disadvantage of increasing the pumping power of the coolant. In recent decades, many studies were conducted to study the thermophysical properties of NFs such as viscosity (μnf) [17, 18], thermal conductivity (TC) [19, 20] and convective heat transfer coefficient [21, 22]. NFs are used in various fields such as air conditioning [23], solar cell [24], automobile [25], nuclear reactors [26], lubrication [27], electrical systems [28], heat exchangers [29]. The TC of solids is higher than that of ordinary fluids, and therefore the dispersion of fine particles is expected to improve the TC of fluids. Many researchers have studied various aspecet of heat transfer and fluid flow properties of NFs in the past 15 years [30, 31, 32] and found that the heat transfer coefficients are increased by adding NPs to the base fluid. However, the enhanced thermal properties of these new fluids should not be offset by additional pumping power. Therefore, it is necessary to investigate the rheological behavior of NFs. Rheological behavior is an important parameter in fluid flow systems. To calculate the required pumping power, the rheological behavior of the fluid is required. Viscosity is one of the important parameters in liquids, which plays an important role in calculating Reynolds number, Prandtl number and heat transfer coefficient. Studies show that various parameters such as solid volume fraction (SVF), particle size, temperature, properties of the base fluid and surfactants affect the TC and μnf. Several studies have been conducted on the μnf of different NFs [33, 34, 35]. Abdullahi et al. [36] reported the changes in rheological behavior and lubrication of CuO–MWCNTs/SAE40 engine oil HNF. Viscosity changes were measured in SVF = 0.0625 and 1 % and T = 25–50 °C and different SRs, the results show that at SVF = 1%, the viscosity of the HNF was 29.47% higher than the viscosity of the base oil. A new relationship was proposed in terms of SVF for each temperature, which estimates the sensitivity of HNF to a 10% increase in SVF. Alidoust et al. [37] studied thermal conductivity of SWCNT (15%)-Fe3O4 (85%)/water HNF. The initial TC increase of the produced HNF compared to water at SVF = 0.03% and T = 30 °C is 0.9%, but the maximum RTC value of 32.20% is reported, which is a significant value. All their studies about viscosity show that the μnf is higher than μbf and increases with the increase of SVF. various base fluids were utilized for all of these studies, but they were all Newtonian fluids. According to the reports in the articles, the dispersion of NPs in Newtonian base fluids has led to the fact that NFs show a Newtonian behavior [38, 39], while many other non-Newtonian NFs show mainly shear-thinning behavior [40, 41]. Some articles reported that the μnf decreases with increasing NP size [42, 43]. The results show that the μnf decreases with increasing temperature [35, 44]. Today, liquids (for coolants) can be used to reduce friction between different parts of moving engine. For example, the use of engine oil increases efficiency and reduces fuel consumption. Oil is one of the most widely used fluids in various components of engines, which has many uses for cooling (See Figure 1).
Figure 1.
Some applications of nanotechnology in different parts of the industry.
One of the factors that lead to energy loss, increased pollution and fuel consumption in cars is the caused friction by the movement of components in the car engine. Using good engine oils can solve these problems. Also, using NPs in engine oil can eliminate these problems. The use of these properties in engine oil leads to improving the lubrication process and repairing worn surfaces and increasing engine power and torque, reducing pollution and gases and saving fuel consumption, which is one of the most important issues in the world. This technology was introduced in various fields of science and different industries; products were created based on this. Meanwhile, engine oil and fuel additives were also influenced by nanotechnology and related products have entered the market. Esfe et al. is one of the prioneer groups in the development of hybrid nanomaterials and nanofluid [45, 46]. The mentioned research group is one of the main research groups of NP sciences, Mono nanofluid and specially hybrid nanofluid. By introducing hybrid nanomaterials, they created a balance between the cost and performance of synthesized nanomaterials. They also began new research to control the μnf after the dispersion of NPs in the base fluid. the rheological behavior of CuO-MWCNT (85%–15%)/10W-40 HNF in the temperature range of T = 5–55 °C and SVF = 0.05–1% were investigated by Hemmat Esfe et al. [47]. The behavior of HNF was determined using the Herschel-Bulkeley (Bingham type) model, so that at T < 45 °C, the HNF shows non-Newtonian pseudo-Bingham behavior. In another study by Hemmat Esfe et al. [48], the dynamic viscosity of MWCNT (40%)-SiO2(60%)/5W50 HNF was experimentally measured in T = 5–55 °C, SVFs between 0 and 1% and SRs from 50 to 800 rpm. Investigation of the rheological behavior of HNF against shear stress shows that HNF has non-Newtonian behavior. Two methods of artificial neural network (ANN) and mathematical correlation were used to present the relationship between dynamic viscosity and independent parameters. The results show that the proposed correlation can estimate the value of dynamic viscosity with acceptable accuracy. The purpose of this research is to add suitable NPs to the engine oil that has a suitable viscosity in different atmospheric conditions. HNFs with these characteristics will increase the efficiency and quality of the produced engine oil and prevent possible damage to the moving parts of the engine. It slows down and increases the life of the engine, which will be of interest to the craftsmen. They found that at all temperatures and SVFs, the μnf was dependent on SR, and the NFs exhibit non-Newtonian shear-thinning behavior. Also, their analytical results show that the μnf decreases and increases with increasing temperature and SVF, respectively.
In this study, laboratory, statistical and numerical analyzes with different objectives were used to investigate the rheological behavior of MWCNT (25%)-MgO (75%)/SAE40 HNF. In the laboratory study, the effect of temperature, SVF and SR on μnf will be investigated. Then, by choosing the appropriate model, two correlations are proposed for the viscosity and prediction of nanofluid so that it can be used for numerical methods. In the final part, it was modeled and predicted by RSM.
2. Laboratory: equipment and measurements
In this research, NPs were prepared in nano dimensions during a material engineering process and in a common two-step method (Figure 2). The first part of preparing NFs is the dispersion of NPs in the base fluid is then stabilizing the suspension. In this work, SAE40 oil was used as the base fluid. Additive NPs consist of MWCNT and MgO NPs with a composition ratio of 25%: 75%. Figure 2a shows MgO NPs and Figure 2b shows MWCNT NPs.
Figure 2.
MgO and MWCNT NPs.
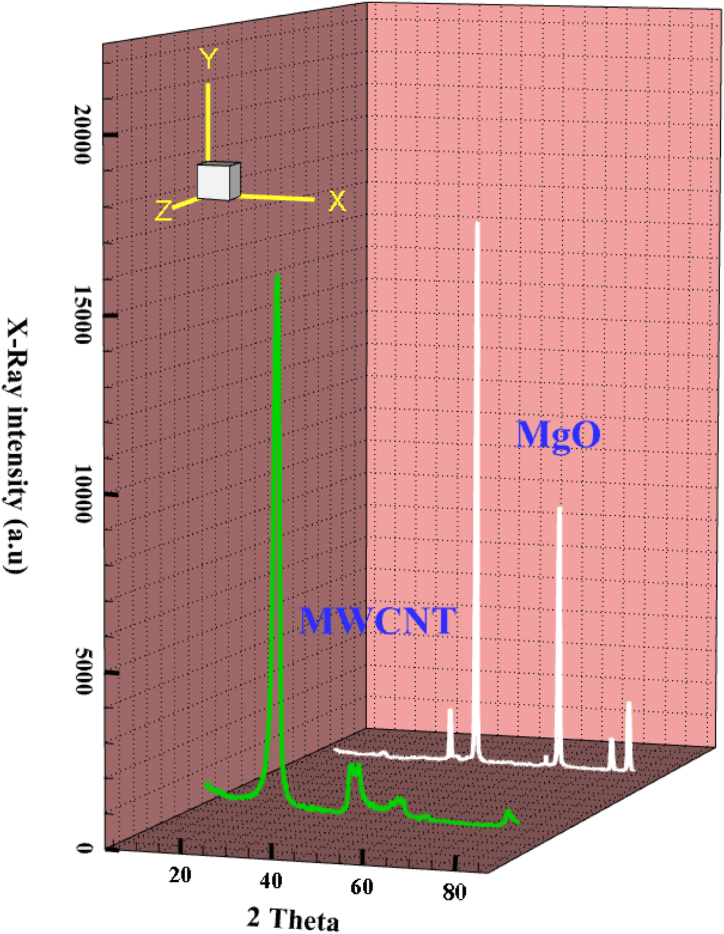
The structural and morphological properties of MgO and MWCNTs were measured using high magnification X-ray diffraction (XRD) as shown in Figure 3
Figure 3.
XRD analysis of NPs.
The amount of required MWCNT and MgO NPs for different SVFs can be determined using Eq. (1). The NP samples were weighed using a sensitive electronic scale with an accuracy of 1 mg Figure 4.
| (1) |
Figure 4.
Digital scale for weighing NPs.
After weighing the NPs, a magnetic stirrer and ultraonic vibration were used to disperse and suspend the NPs in the base oil. This results in a stable suspension and uniform dispersion. The nanofluid with good dispersion and stability after 25 days were prepared to investigate the rheological behavior.
Figure 5 shows the stabilized HNFs in different SVFs. More information about the properties of MgO and MWCNT NPs is presented in Table 1.
Figure 5.
Stability of HNFs at different SVFs.
Table 1.
Properties of MgO and MWCNT NPs.
| Specifications | NPs |
|
|---|---|---|
| MWCNTs | MgO | |
| Purity | >95 wt% | 99+% |
| Color | Black | white |
| Morphology | Cylindrical | polyhedral |
2.1. Viscosity measurement
To analyze the rheological behavior of HNFs, the viscosity of MgO-MWCNTs/SAE40 HNF with different SVFs (0.0625%, 0.125%, 0.25%, 0.5%, 0.75% and 1%) at T = 25–50 °C was measured by Brookfield CAP2000 viscometer. This device is provided by the Brookfield Engineering Laboratory in America. Before starting to work, the device was calibrated and its error was measured. The viscometer was tested with SAE40 oil at T = 25 °C before measuring the μnf. Table 2 gives the range of conditions for measuring the μnf. To increase the accuracy of the measurements and as a result, provide a more accurate analysis, all the experimental data were repeated at most five times and then their average was recorded. Some measured data are reported in Table 3.
Table 2.
Range of conditions for measuring the μnf
| HNF | Range of laboratory conditions |
||
|---|---|---|---|
| T (°C) | SVF (%) | SR (s−1) | |
| MWCNT-MgO(25:75)/SAE40 | 25–50 | 0.0625–1 | 666.5–7998 |
Table 3.
Some measured data by a CAP2000 + viscometer.
| HNF | SVF (%) | T (°C) | SR (s−1) | μnf (mPa.s) |
|---|---|---|---|---|
| MWCNT-MgO(25:75)/SAE40 | 0.0625 | 25 | 1333 | 321 |
| 0.125 | 30 | 2666 | 233.4 | |
| 0.25 | 35 | 3999 | 188.1 | |
| 0.5 | 40 | 5332 | 154.7 | |
| 0.75 | 45 | 6665 | 119.2 | |
| 1 | 50 | 7998 | 91.6 |
3. Discussion
3.1. Rheological behavior
3.1.1. Effect of SR
In the first step of this study, the rheological behavior of the HNF in Newtonian and non-Newtonian groups was investigated in three-dimensional curves. The τ→SR curve is generally called the flow curve, where the shear stress (τ) is a force that can create continuous and constant deformation in the fluid. Unlike non-Newtonian fluids, Newtonian fluids are fluids in which the ratio of shear stress and SR is linear, and a non-Newtonian fluid is a fluid whose viscosity changes with the applied SR. At the beginning of this study, to determine the flow behavior of HNFs, their rheograms at different temperatures and SVFs are examined and compared. Using rheological curves and matching it with μnf of MWCNT-MgO (25:75)/SAE40 HNF, the flow behavior of HNF was investigated. In the curves of Figure 6 (a-d), the basis of the changes is SR. Hence, Figure 6 (a) is for SVF = 0.0625%, Figure 6 (b) is for SVF = 0.125%, Figure 6 (c) is for SVF = 0.75% and Figure 6 (d) is for SVF = 1%. According to these figures, μnf changes at the studied temperatures (T = 25–50 °C) with changes in SR. The pattern of studied HNF flow behavior corresponds to the non-Newtonian fluids.
Figure 6.
Flow curve at different temperatures.
3.1.2. Power-law index (n)
Following the path of detecting HNF flow behavior, the power-law contour is shown in Figure 7. Eq. (2) is used to identify the rheological behavior of HNFs. According to the contour of Figure 7, it can be seen that the power-law index is in the range 1 > n > 0. The mentioned values confirm the behavior mentioned in the previous section (effect of parameter SR).
| (2) |
Figure 7.
The contour of the power-law index.
Numerical values of the power-law index are reported in Table 4. HNF [MWCNT-MgO (25:75)/SAE40] at T = 50 °C and SVF = 0.75% has the strongest non-Newtonian behavior and n = 0.9233.
Table 4.
Power-law index values at different SVFs and different temperatures.
| HNF | Power-law index (n) |
||||||
|---|---|---|---|---|---|---|---|
| T = 25 °C | T = 30 °C | T = 35 °C | T = 40 °C | T = 45 °C | T = 50°C | ||
| MWCNT-MgO (25:75)/SAE40 | SVF = 0.0625% | 0.9593 | 0.9599 | 0.9528 | 0.9597 | 0.9431 | 0.9593 |
| SVF = 0.125% | 0.9586 | 0.9559 | 0.9525 | 0.9493 | 0.9472 | 0.9433 | |
| SVF = 0.25% | 0.9611 | 0.9564 | 0.9552 | 0.9528 | 0.9503 | 0.9334 | |
| SVF = 0.5% | 0.9734 | 0.9704 | 0.9736 | 0.9517 | 0.957 | 0.9556 | |
| SVF = 0.75% | 0.9705 | 0.9592 | 0.9623 | 0.9616 | 0.9494 | 0.9233 | |
| SVF = 1% | 0.964 | 0.9652 | 0.9676 | 0.9612 | 0.9604 | 0.9384 | |
3.2. Viscosity comparison
3.2.1. Relative μnf (μr)
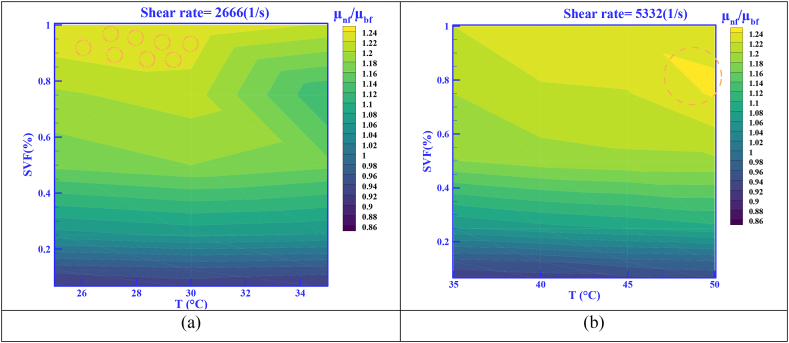
To better understand the viscosity behavior, the μr contour at SR = 2666 (See Figure 8 a) and 5332 s−1 (See Figure 8 b) is shown in Figure 8 (a and b). μr is calculated using Eq. (3).
| (3) |
Figure 8.
μr contour at SR = 2666 and 5332 s−1
In Figure 8 (a) and at SR = 2666 s−1, the largest increase in μr was 1.24, which is equal to +24%. The numerical results of μr are reported in Table 5.
Table 5.
Statistical data on the percentage of μr changes.
|
HNF |
SR (1/s) |
T (°C) |
μr |
|||
|---|---|---|---|---|---|---|
| SVF=0.0625% | SVF=0.125% | SVF=0.75% | SVF=1% | |||
| MWCNT-MgO (25:75)/SAE40 | 2666 (200rpm) | T = 25 | 0.915 (-8.50%) | 0.945 | 1.196 | 1.24 (+24%) |
| T = 30 | 0.927 | 0.953 | 1.21 | 1.237 | ||
| T = 35 | 0.917 | 0.948 | 1.197 | 1.217 | ||
| 5332 (400rpm) | T = 35 | 0.922 | 0.952 | 1.20 | 1.22 | |
| T = 40 | 0.931 | 0.956 | 1.21 | 1.234 | ||
| T = 45 | 0.933 | 0.971 | 1.22 | 1.238 | ||
| T = 50 | 0.944 | 0.976 | 1.231 | 1.235 | ||
3.2.2. μnf→T curve
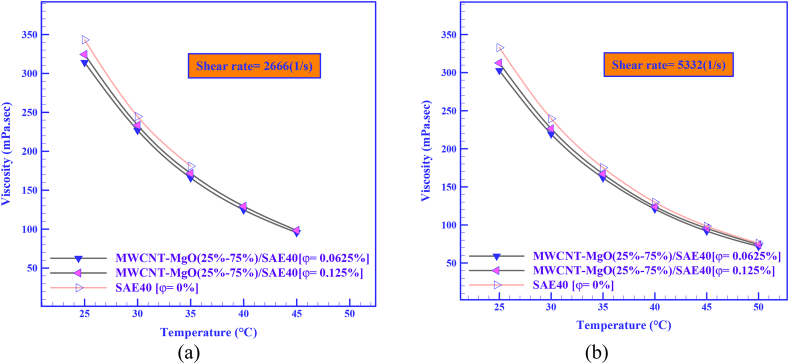
Among other presented comparisons in this scenario, which is very important in determining the optimal HNF, is the examination of viscosity curves in terms of temperature. The most important and effective parameter in this field is temperature. One of the important goals is to compare the difference in the viscosity of HNFs compared to the base fluid at different temperatures. This comparison will ultimately lead to the selection of the most optimal mode in the preparation of HNF. Therefore, for example, in Figure 9 (a and b), the viscosity curves based on the temperature at the highest and lowest SRs (SR = 2666 and 5332 s−1) and SVFs were compared. Figure 9a is for SR = 2666 s−1 and Figure 9b is for SR = 5332 s−1. According to Figure 9, a drop-in viscosity has been seen for both HNFs at all temperatures, but the viscosity is not much different from the base oil.
Figure 9.
Difference between μnf and μbf at SR = 2666 and 5332 s−1 and different SVFs.
A comparison of the difference between μnf and μbf in SR = 2666 and 5332 s−1 and SVF = 0.0625 % and 0.125% is reported in Table 6. According to Table 6, at SR = 2666 s−1 and SVF = 0.0625 % at T = 25 °C, the HNF has the lowest viscosity percentage of -8.45%.
Table 6.
A comparison of the difference between the μnf and the μbf at SR = 2666 and 5332 s−1 and SVF = 0.0625% and 0.125%.
| SR (s−1) | T (°C) |
|
|
|---|---|---|---|
| MWCNT-MgO (25:75)/SAE40 | MWCNT-MgO (25:75)/SAE40 | ||
| 2666 | 25 °C | -29.00 (-8.45%) | -18.70 |
| 30 °C | -17.80 | -11.30 | |
| 35 °C | -15.00 | -9.30 | |
| 5332 | 40 °C | -8.90 | -5.60 |
| 45 °C | -6.50 | -2.80 | |
| 50 °C | -4.20 | -1.80 |
4. Impractical results
4.1. Correlation model of the RSM
RSM is one of the important procedures in examining the target response to achieve the relationship between the independent variable and the dependent variable. The RSM was used for dependent variable analysis where one or more dependent variables (as a response) were affected by many variables and the objective is to optimize the response. In RSM, the governing equation for the rheological behavior of HNFs and the effective parameters and interactions are determined, and finally, the target response can be optimized based on the effective parameters. In this research, the optimization process was carried out using the three-variable model with the coefficient of determination of R2 = 0.9994, and then a non-linear mathematical relationship with the parameters of temperature (T), SVF and SR were presented to predict the experimental data (Eq. 4). In Tables 7 and 8, the related values to the parameters affecting the performance of the modeler and the accuracy of the equation are reported.
| μnf = +1439.18463–74.55874T + 680.60734SVF-0.023008SR-18.93092T∗SVF +9.20862E-004T∗SR+1.41458T2-335.84851SVF2+1.64364E-007SR2+0.14964T2SVF-1.03952E-005 T2∗SR+3.83013 T∗SVF2-9.38513E-003 T3 + 68.56743SVF3 | (4) |
Table 7.
ANOVA for Response Surface Reduced Cubic model.
| ANOVA table [Partial sum of squares - Type III] | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value |
P-value |
|
| Prob > F | ||||||
| Model | 1.684E+006 | 13 | 1.295E+005 | 22252.48 | <0.0001 | significant |
| A-T | 1.297E+005 | 1 | 1.297E+005 | 22279.76 | <0.0001 | |
| B-SVF | 2035.45 | 1 | 2035.45 | 349.69 | <0.0001 | |
| C-SR | 669.38 | 1 | 669.38 | 115.00 | <0.0001 | |
| AB | 17589.04 | 1 | 17589.04 | 3021.76 | <0.0001 | |
| AC | 319.22 | 1 | 319.22 | 54.84 | <0.0001 | |
| A2 | 62812.91 | 1 | 62812.91 | 10791.11 | <0.0001 | |
| B2 | 8369.61 | 1 | 8369.61 | 1437.88 | <0.0001 | |
| C2 | 49.06 | 1 | 49.06 | 8.43 | 0.0042 | |
| A2B | 1603.49 | 1 | 1603.49 | 275.48 | <0.0001 | |
| A2C | 211.61 | 1 | 211.61 | 36.35 | <0.0001 | |
| AB2 | 1247.53 | 1 | 1247.53 | 214.32 | <0.0001 | |
| A3 | 1912.26 | 1 | 1912.26 | 328.52 | <0.0001 | |
| B3 | 322.94 | 1 | 322.94 | 55.48 | <0.0001 | |
| Residual | 931.33 | 160 | 5.82 | |||
| Cor Total | 1.685E+006 | 173 | ||||
In this test, the R2 is equal to 0.9999 and the P-value is less than 0.0001, which indicates the appropriateness of the proposed correlation.
Table 8.
Values of measurements accuracy of regression analysis.
| Std. Dev. | 2.41 | R-Squared | 0.9994 |
|---|---|---|---|
| Mean | 188.50 | Adj R-Squared | 0.9994 |
| C.V. % | 1.28 | Pred R-Squared | 0.9993 |
| PRESS | 1139.49 | Adeq Precision | 541.007 |
| -2 Log Likelihood | 785.69 | BIC | 857.91 |
To check the appropriateness and significance of the model, Fisher's test parameters (F-test), probability (P-value) and coefficient of variation (R-square) were used in the analysis of variance (ANOVA).
As can be seen in Figure 10, the modeled data provide good agreement and correlation with the corresponding empirical data, which indicates the acceptable validity of the proposed relationship.
Figure 10.
Correlation of modeled data with experimental data.
5. Conclusion
In this study, the rheological behavior of MWCNT-MgO(25%:75%)/SAE40 HNF as laboratory and statistical analysis with different objectives at T = 25–50 °C and SVF = 0.0625, 0.125, 0.25, 0.5, 0.75 and 1% were measured and reported. The results show that traditional models are not suitable for predicting the viscosity of MWCNT-MgO/SAE40 HNFs. The main results of this study are classified as follows:
-
•
Temperature and SVF are two important and effective parameters of viscosity. The effect of temperature on viscosity is superior to the effect of SVF on viscosity.
-
•
It was found that the relationship between shear stress and SR is nonlinear and viscosity has non-Newtonian behavior.
-
•
An increase in temperature weakens van der Waals forces. Therefore, an increase in temperature led to a decrease in viscosity. At T = 25 °C and SR = 2666 s−1, the viscosity has the lowest percentage and is equal to -8.45%.
-
•
The statistical results of the laboratory study show that the maximum μnf and minimum μnf increase and decrease by -8.50% and 24%, respectively, compared to the base oil.
-
•
Using RSM, a non-linear relationship with acceptable accuracy and quality with R2 = 0.9994 was presented to predict the experimental data and establish the relationship between the response function and the independent variables.
-
•
The studied HNFs with these characteristics will increase the efficiency and quality of the produced engine oil and prevent possible damage to the moving parts of the engine. It reduces the speed and increases the life of the engine, which will be of interest to the craftsmen.
Declarations
Author contribution statement
Mohammad Hemmat Esfe, Davood Toghraie, Soheyl Alidoust, Fatemeh Amoozadkhalili, Erfan Mohammadnejad Ardeshiri: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Sun L., Wang G., Zhang C., Jin Q., Song Y. On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid. Nanotechnol. Rev. 2021;10(1):1339–1348. [Google Scholar]
- 2.Asleshirin S., Mazaheri H., Omidkhah M., Hassani Joshaghani A. Investigation of thermophysical properties of io nanofluids containing multi-walled carbon nanotubes and graphene. Iran. J. Chem. Chem. Eng. (Int. Engl. Ed.) 2022;41(2):380–391. [Google Scholar]
- 3.Zahmatkesh R., Mohammadiun H., Mohammadiun M., Dibaei Bonab M., Sadi M. Theoretical investigation of entropy generation in axisymmetric stagnation point flow of nanofluid impinging on the cylinder axes with constant wall heat flux and uniform transpiration. Iran. J. Chem. Chem. Eng. (Int. Engl. Ed.) 2021;40(6):1893–1908. [Google Scholar]
- 4.Bakhshkandi R., Ghoranneviss M. Investigating the synthesis and growth of titanium dioxide nanoparticles on a cobalt catalyst. J. Res. Sci. Eng. Tech. 2019;7(4):1–3. [Google Scholar]
- 5.Asif Muhammad Adnan. A theoretical study of the size effect of carbon nanotubes on the removal of water chemical contaminants. J. Res. Sci. Eng. Tech. 2018;6(4):21–27. [Google Scholar]
- 6.Shojaie M. One-pot Multicomponent synthesis of pyrano[2,3-c]pyrazoles catalyzed by Copper oxide nanoparticles (CuO NPs) J. Synth. Chem. 2022;1:125–131. [Google Scholar]
- 7.Shafik M.S. CuI nanoparticles immobilized on magnetic nanoparticles catalyzed synthesis of diaryl ethers through C-O cross-coupling of phenols with aryl iodides. J. Synth. Chem. 2022;1:132–136. [Google Scholar]
- 8.Cui W., Li X., Li X., Si T., Lu L., Ma T.…Wang Q. Thermal performance of modified melamine foam/graphene/paraffin wax composite phase change materials for solar-thermal energy conversion and storage. J. Clean. Prod. 2022;367 [Google Scholar]
- 9.Ruhani B., et al. Statistical investigation for developing a new model for rheological behavior of ZnO–Ag (50%–50%)/Water hybrid Newtonian nanofluid using experimental data. Phys. Stat. Mech. Appl. 2019;525:741–751. [Google Scholar]
- 10.Ruhani B., Barnoon P., Toghraie D. Statistical investigation for developing a new model for rheological behavior of Silica–ethylene glycol/Water hybrid Newtonian nanofluid using experimental data. Phys. Stat. Mech. Appl. 2019;525:616–627. [Google Scholar]
- 11.Zhang Z., Yang F., Zhang H., Zhang T., Wang H., Xu Y., Ma Q. Influence of CeO2 addition on forming quality and microstructure of TiCx-reinforced CrTi4-based laser cladding composite coating. Mater. Char. 2021;171 [Google Scholar]
- 12.Abdul-Hameed M.H. A coated of Ca/Fe layered hydroxide onto a synthesized adsorbent from (banana peels) for removal of cadmium from simulated wastewater. Caspian J. Environ. Sci. 2021;19(5):825–827. [Google Scholar]
- 13.Gupta Sonal, Jain Renuka, Goyal Deepti. Recent trends in conversion of c6-sugars into value-added chemicals over solid catalysts. European Chem. Bulletin. 2021;10(2):80–84. [Google Scholar]
- 14.Müssig J., Graupner N. Test methods for fibre/matrix adhesion in cellulose fibre-reinforced thermoplastic composite materials: a critical review. Rev. Adhesion Adhesives. 2021;8(No. 2):68–129. [Google Scholar]
- 15.Rakshe S., Nimje S.V., Panigrahi S.K. Optimization of adhesively bonded spar-wingskin joints of laminated FRP composites subjected to pull-off load: a critical review. Rev. Adhesion Adhesi. 2021;8(1):29–46. [Google Scholar]
- 16.Zhang L., Li J., Xue J., Zhang C., Fang X. Vol. 291. Guildford); Fuel: 2021. (Experimental Studies on the Changing Characteristics of the Gas Flow Capacity on Bituminous Coal in CO2-ECBM and N-2-ECBM). [Google Scholar]
- 17.Dehghani Y., et al. Experimental investigation toward obtaining a new correlation for viscosity of WO3 and Al2O3 nanoparticles-loaded nanofluid within aqueous and non-aqueous basefluids. J. Thermal Analy. Calorimetry. 2019;135(1):713–728. [Google Scholar]
- 18.Eshaghi A., Mojab M. Hydrophilicity of silica nano-porous thin films. calc fects of mult walled carbon nanotubes on rheological behavior of engine ination temperature effects. J Nanostruct. behavior of engine ination temperature effects. J Nanostruct. 2017;7(2):127–133. [Google Scholar]
- 19.Esfe M.H., Sarlak M.R. Experimental investigation of switchable behavior of CuO-MWCNT (85%–15%)/10W-40 hybrid nano-lubricants for applications in internal combustion engines. J. Mol. Liq. 2017;242:326–335. [Google Scholar]
- 20.Sundar L.S., et al. Thermal conductivity and viscosity of stabilized ethylene glycol and water mixture Al2O3 nanofluids for heat transfer applications. An Experiment. Study. 2014;56:86–95. [Google Scholar]
- 21.Salari E., et al. Thermal behavior of aqueous iron oxide nano-fluid as a coolant on a flat disc heater under the pool boiling condition. Heat Mass Transf. 2017;53(1):265–275. [Google Scholar]
- 22.Arya A., et al. Thermal performance analysis of a flat heat pipe working with carbon nanotube-water nanofluid for cooling of a high heat flux heater. Heat Mass Transf. 2018;54(4):985–997. [Google Scholar]
- 23.Hatami M., Domairry G., Mirzababaei S. Experimental investigation of preparing and using the H2O based nanofluids in the heating process of HVAC system model. Int. J. Hydrogen Energy. 2017;42(12):7820–7825. [Google Scholar]
- 24.Javadi M., Abdi Y., Arzi E.J.S.e. Local collection efficiency in the nano-crystalline solar cells. Solar Energy. 2016;133:549–555. [Google Scholar]
- 25.Hemmat Esfe M. Designing an artificial neural network using radial basis function (RBF-ANN) to model thermal conductivity of ethylene glycol–water-based TiO2 nanofluids. J. Therm. Anal. Calorim. 2017;127(3):2125–2131. [Google Scholar]
- 26.Ansarifar G., Ebrahimian M.J. Vol. 87. 2016. Design and Neutronic Investigation of the Nano Fluids Application to VVER-1000 Nuclear Reactor with Dual Cooled Annular Fuel; pp. 39–47. .A.o.N.E. [Google Scholar]
- 27.Esfe M.H., Rostamian H., Rejvani M., Emami M.R.S. Rheological behavior characteristics of ZrO2-MWCNT/10w40 hybrid nano-lubricant affected by temperature, concentration, and shear rate: an experimental study and a neural network simulating. Phys. E Low-dimens. Syst. Nanostruct. 2018;102:160–170. [Google Scholar]
- 28.Li Y., et al. Structure models and nano energy system design for proton exchange membrane fuel cells in electric energy vehicles. Renew. Sust. Energy Rev. 2017;67:160–172. [Google Scholar]
- 29.Jafari S.M., et al. Vol. 42. 2017. Nano-fluid thermal Processing of Watermelon Juice in a Shell and Tube Heat Exchanger and Evaluating its Qualitative Properties; pp. 173–179. [Google Scholar]
- 30.Yu W., et al. Review and comparison of nanofluid thermal conductivity and heat transfer enhancements. Heat Transfer Eng. 2008;29(5):432–460. [Google Scholar]
- 31.Kakaç S., Pramuanjaroenkij A. m. transfer, Review of convective heat transfer enhancement with nanofluids. Int. J. Heat Mass Transfer. 2009;52(13-14):3187–3196. [Google Scholar]
- 32.Godson L., et al. Enhancement of heat transfer using nanofluids—an overview. 2010;14(2):629–641. [Google Scholar]
- 33.Chandrasekar M., et al. Experimental investigations and theoretical determination of thermal conductivity and viscosity of Al2O3/water nanofluid. 2010;34(2):210–216. [Google Scholar]
- 34.Timofeeva E.V., Routbort J.L., Singh D. Particle shape effects on thermophysical properties of alumina nanofluids. 2009;106(1) [Google Scholar]
- 35.Garg P., et al. An experimental study on the effect of ultrasonication on viscosity and heat transfer performance of multi-wall carbon nanotube-based aqueous nanofluids. Int. J. Heat Mass Transfer. 2009;52(21–22):5090–5101. [Google Scholar]
- 36.Abdollahi Moghaddam M., Motahari K. Experimental investigation, sensitivity analysis and modeling of rheological behavior of MWCNT-CuO (30-70)/SAE40 hybrid nano-lubricant. Appl. Therm. Eng. 2017;8:3815–8349. [Google Scholar]
- 37.Alidoust S., AmoozadKhalili F., Hamedi S. Investigation of effective parameters on relative thermal conductivity of SWCNT (15%)-Fe3O4 (85%)/water hybrid ferro-nanofluid and presenting a new correlation with response surface methodology. Colloids Surf. A Physicochem. Eng. Asp. 2022;645 [Google Scholar]
- 38.Namburu P.K., et al. Viscosity of copper oxide nanoparticles dispersed in ethylene glycol and water mixture. Exp. Therm. Fluid Sci. 2007;32(2):397–402. [Google Scholar]
- 39.Chevalier J., Tillement O., Ayela F. Structure and rheology of SiO2 nanoparticle suspensions under very high shear rates. 2009;80(5) doi: 10.1103/PhysRevE.80.051403. [DOI] [PubMed] [Google Scholar]
- 40.Chen H., Ding Y., Lapkin A. Rheological behaviour of nanofluids containing tube/rod-like nanoparticles. Powder Technol. 2009;194(1–2):132–141. [Google Scholar]
- 41.Phuoc T.X., Massoudi M.J. Experimental observations of the effects of shear rates and particle concentration on the viscosity of Fe2O3–deionized water nanofluids. Int. J. Therm. Sci. 2009;48(7):1294–1301. .I.J.o.T.S. [Google Scholar]
- 42.Jia-Fei Z., et al. Dependence of nanofluid viscosity on particle size and pH value. Chinese Phys. Lett. 2009;26(6) [Google Scholar]
- 43.Kang H.U., Kim S.H., Oh J. Estimation of thermal conductivity of nanofluid using experimental effective particle. Exp. Heat Transfer. 2006;19(3):181–191. [Google Scholar]
- 44.Duangthongsuk W., Wongwises S., science f. Measurement of temperature-dependent thermal conductivity and viscosity of TiO2-water nanofluids. Exp. therm. fluid sci. 2009;33(4):706–714. [Google Scholar]
- 45.Esfe M.H. Designing a neural network for predicting the heat transfer and pressure drop characteristics of Ag/water nanofluids in a heat exchanger. Appl. Therm. Eng. 2017;126:559–565. [Google Scholar]
- 46.Esfe M.H., Sarlak M.R. A novel study on rheological behavior of ZnO-MWCNT/10w40 nanofluid for automotive engines. J. Mol. Liq. 2018;254:406–413. [Google Scholar]
- 47.Esfe M.H., Sarlak M.R. Experimental investigation of switchable behavior of CuO-MWCNT (85%–15%)/10W-40 hybrid nano-lubricants for applications in internal combustion engines. J. Mol. Liq. 2017;242:326–335. [Google Scholar]
- 48.Esfe M.H., Arani A.A.A. An experimental determination and accurate prediction of dynamic viscosity of MWCNT (% 40)-SiO2 (% 60)/5W50 nano-lubricant. J. Mol. Liq. 2018;259:227–237. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.