Abstract
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) are being investigated to slow the decline of kidney function in type 2 diabetics with chronic kidney disease (CKD). These agents have proven benefits on cardiac outcomes and all-cause mortality as well as in reducing the incidence of macroalbuminuria. Ours is a case of drug-associated acute interstitial nephritis requiring hemodialysis temporally related to a semaglutide dose increase. This case is unique as the index patient had no underlying CKD. Limited cases of acute kidney injury, superimposed on underlying CKD, in patients taking the GLP-1RA semaglutide have been reported. To our knowledge, there are no existing case reports in the literature of GLP-1RA-associated acute interstitial nephritis in a patient with baseline normal kidney function. Because our prescription of these agents is increasing and is anticipated to increase further with growing scientific evidence for their benefit, we sought to highlight this possible, important serious adverse effect of semaglutide.
Index Words: Acute interstitial nephritis, acute kidney injury, glucagon-like peptide 1 receptor agonists, semaglutide
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), a newer class of antidiabetic agents, have been demonstrated to have pleiotropic benefits including cardioprotection, treatment of obesity, and suggested kidney protective effects.1 A dedicated kidney outcome randomized controlled trial (the FLOW study, NCT03819153) is currently ongoing, studying the effects of weekly subcutaneous semaglutide on the occurrence of persistent estimated glomerular filtration rate decline of ≥50 percent from the trial start, reaching end-stage kidney disease, death from kidney disease, or death from cardiovascular disease in patients with type 2 diabetes and chronic kidney disease (CKD).2 This class has a low side effect profile; however, acute kidney injury (AKI) requiring dialysis is listed as a possible rare adverse reaction (usually occurring in the setting of GLP-1RA induced nausea and vomiting with associated hypovolemia).3,4
There are limited published case reports describing acute interstitial nephritis (AIN) with some frequently used GLP-1RAs including liraglutide and semaglutide.5 A thorough search of the relevant literature yielded only 2 cases of AKI superimposed on CKD (underlying diabetic nephropathy), ascribed to semaglutide-induced AIN.6 AIN is a common cause of AKI, often because of medications in high-income countries such as antibiotics (most common cause), proton pump inhibitors, nonsteroidal anti-inflammatory agents, and diuretics. AIN often has a nonspecific presentation with the classic triad of rash, fever, and eosinophilia seen infrequently. Discontinuation of the offending agent is considered the mainstay of therapy, and the use of corticosteroids to hasten kidney recovery may be beneficial, especially if started early.7 Our case is the first known case description of semaglutide-induced AIN in a patient with no background CKD.
Case Report
A man in his thirties presented via a referral hospital with a 1-week history of malaise and increasing shortness of breath (however, no gastrointestinal disturbance) with a nonoliguric AKI in the context of normal serum creatinine of 1.05 mg/dL (93 μmol/L) with an estimated glomerular filtration rate of 91 mL/min/1.73 m2 and undetectable urinary albumin-creatinine ratio 5 months prior.
His past medical history included morbid obesity, diabetes on 1 oral agent with no known target organ disease, nonischemic cardiomyopathy diagnosed 6 years ago (with the last recorded left ventricular ejection fraction of 40% on transthoracic echocardiogram), and nonsignificant smoking history. His chronic medication for the past 1-2 years had been: carvedilol, spironolactone, furosemide, ascorbic acid, metformin, rosuvastatin, and sacubitril-valsartan at stable doses. He had been prescribed weekly semaglutide injections 6 weeks before presenting, and the dose had increased from 0.25 mg to 0.5 mg 4 weeks prior. He was adherent with all his medication and had no current or remote history of nonsteroidal anti-inflammatory or other medication ingestion.
Clinical examination was unremarkable. He was normotensive and afebrile with no rash and was assessed as euvolemic with no evidence of decompensated heart failure or fluid overload. His laboratory examinations at presentation were as follows: serum blood urea nitrogen level of 165.53 mg/dL (58.7 mmol/L), serum creatinine 12.86 mg/dL (1138 μmol/L), serum potassium 6.2 mmol/L, C-reactive protein 7.34 mg/dL (73.4 mg/L), brain natriuretic peptide level 160 pg/mL, urinary albumin-creatinine ratio 91.1 mg/g (10.3 mg/mmol), white cell count 11.1 × 103/μL (slight leukocytosis, normal eosinophil count), pH 7.22, bicarbonate (venous) 14 mmol/L, anion gap 21 mEq/L (lactate 5.4 mg/dL, 0.3 mmol/L, beta-hydroxybutyrate <0.10 mmol/L). His hemoglobin A1c was 7.7%. Urinalysis revealed occasional hyaline and granular casts, hemoglobin, and trace proteinuria. Complement C3 and C4, anti-double-stranded DNA, antineutrophil cytoplasm antibodies, and serum protein electrophoresis were all negative. Acute intermittent hemodialysis was initiated and subsequently continued for a week because of the metabolic parameters.
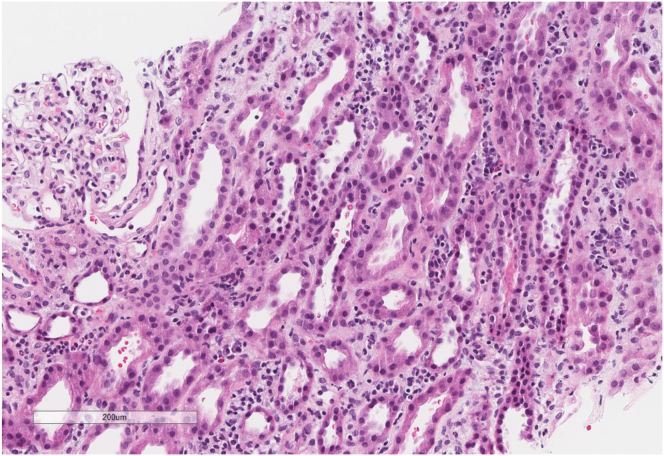
An ultrasound-guided kidney biopsy showed patchy interstitial inflammatory infiltrates (lymphocytes, plasma cells, mild eosinophils), mild tubulitis, diffuse acute tubular injury, and no evidence of immune complex-mediated glomerulonephritis by immunofluorescence or electron microscopy (Fig 1). He was started on prednisone 1 mg/kg after preliminary information from his biopsy and discharged on a tapering oral prednisone regimen. The following medications were held on admission and discharge: sacubitril-valsartan, spironolactone, furosemide, rosuvastatin, semaglutide, and metformin.
Figure 1.
Interstitial inflammatory infiltrates composed of lymphocytes, plasma cells, and occasional eosinophils, and acute tubular injury. Hematoxylin and eosin, 10×.
The patient was documented to have recovered kidney function 10 days after his initial presentation, serum creatinine 1.32 mg/dL (117 μmol/L) and urinary albumin-creatinine ratio <8.84 mg/g (<1.0 mg/mmol). At his most recent heart failure clinic appointment 1 month after discharge, his creatinine was at baseline, and he had been re-established on sacubitril-valsartan and spironolactone. He continued on tapering prednisone with cholecalciferol, famotidine and sulfamethoxazole-trimethoprim.
Discussion
To our knowledge, this is the first reported case of biopsy-proven AIN related to semaglutide in a patient with no pre-existing CKD. This case is relevant as, amidst emerging evidence of improved cardiovascular and obesity outcomes, it is anticipated that GLP1-RAs will be used more frequently in patients with and without kidney disease and may soon be added to the growing armamentarium of agents to slow the progression of diabetic kidney disease. Although multiple cases of AKI with GLP-1RAs have been described in a pooled analysis of cardiovascular outcome trials, GLP-1RAs do not increase the risk of AKI.8 In a meta-analysis of the novel glucose-lowering agent, GLP-1RAs had a neutral effect on the risk of AKI; this is in contrast to sodium/glucose cotransporter 2 inhibitors, which were associated with a lower risk of AKI.9 However, in a systematic review of case reports of adverse drug reactions related to GLP-1RAs, 120 cases were found of which 23 were kidney-related, mostly in patients with pre-existing kidney disease.4
Our patient had no known baseline CKD and presented with a nonoliguric AKI requiring acute hemodialysis because of biopsy-proven drug-induced AIN. Because of the time correlation between semaglutide initiation and dose increase and AKI presentation, it was highly suggestive that this was due to semaglutide therapy. We could not determine another cause for his AIN; he had been on all his other medications at the same doses for at least 1 year. Although being exposed to multiple medications that could have been contributory, drug-induced AIN typically occurs 7-10 days after exposure to the drug and with some agents after a few weeks or months, making semaglutide the only possible causative agent.7 Our patient luckily recovered kidney function and came off dialysis. Although rare semaglutide-related AIN is a described serious adverse effect, it should be considered in the differential diagnosis in applicable patients.
Currently, with semaglutide prescription, monitoring of kidney function is limited to patients with severe adverse gastrointestinal reactions.10 The authors suggest that regular clinical follow-up and careful dose escalation with monitoring of kidney function be considered with future prescribing guidelines, especially in the CKD population.
Article Information
Authors’ Full Names and Academic Degrees
Megan Borkum, MD, Wynnie Lau, BPharm, Paula Blanco, MD, MSc, and Myriam Farah, MD, FRCPC.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that they have obtained consent from the patient reported in this article for publication of the information about him that appears within this Case Report.
Peer Review
Received July 6, 2022. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form August 28, 2022.
Footnotes
Complete author and article information provided before references.
References
- 1.Giugliano D., Scappaticcio L., Longo M., et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20(1):189. doi: 10.1186/s12933-021-01366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams D.M., Evans M. Semaglutide: charting new horizons in GLP-1 analogue outcome studies. Diabetes Ther. 2020;11(10):2221–2235. doi: 10.1007/s13300-020-00917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smits M.M., Van Raalte D.H. Safety of semaglutide. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.645563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shetty R., Basheer F.T., Poojari P.G., Thunga G., Chandran V.P., Acharya L.D. Adverse drug reactions of GLP-1 agonists: a systematic review of case reports. Diabetes Metab Syndr. 2022;16(3) doi: 10.1016/j.dsx.2022.102427. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhury N.T., Liarakos A.L., Gopalakrishnan K., Ayub W., Murthy N., Rao R. Antidiabetic medication-induced acute interstitial nephritis: case report and literature search. Br J Diabetes. 2021;21:228–232. [Google Scholar]
- 6.Leehey D.J., Rahman M.A., Borys E., Picken M.M., Clise C.E. Acute kidney injury associated with semaglutide. Kidney Med. 2021;3(2):282–285. doi: 10.1016/j.xkme.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perazella M.A., Markowitz G.S. Drug-induced acute interstitial nephritis. Nat Rev Nephrol. 2010;6(8):461–470. doi: 10.1038/nrneph.2010.71. [DOI] [PubMed] [Google Scholar]
- 8.Patoulias D., Boulmpou A., Papadopoulos C.E., Doumas M. Glucagon-like peptide-1 receptor agonists and the risk of acute kidney injury: alarming, or not? Kidney Med. 2021;3(4):674–675. doi: 10.1016/j.xkme.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao M., Sun S., Huang Z., Wang T., Tang H. Network meta-analysis of novel glucose-lowering drugs on risk of acute kidney injury. Clin J Am Soc Nephrol. 2020;16(1):70–78. doi: 10.2215/CJN.11220720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novo Nordisk Canada Ozempic: product monograph including patient information. Published January 4, 2018. Revised January 4, 2022. https://www.novonordisk.ca/content/dam/nncorp/ca/en/products/ozempic-product-monograph.pdf