Abstract
Kokumi taste-active compounds enhance salty taste perception. In animal models, sodium (salt) detection is mediated by the amiloride-sensitive epithelial sodium channel, ENaC. This ion channel works as a sodium receptor in the so-called sodium-taste cells. It is not known whether kokumi taste substances are able to affect the activity of functional ENaCs in these cells. Here, we use the patch-clamp technique to study the effect of kokumi-active tripeptides, glutathione (GSH) and γ-glutamyl-valyl-glycine (EVG), on the ENaC-mediated membrane current in rat fungiform sodium-taste cells. GSH and EVG reduced slightly this current and the effect disappeared in the presence of amiloride, a specific ENaC blocker. No effect on membrane current was detected in other taste cells (Type II and Type III cells) that do not express functional ENaC. Our findings suggest that the enhancing effect of kokumi taste-active γ-glutamyl peptides on salt reception is not explained by an increase in the activity of ENaC.
Keywords: GSH, γEVG, Salty taste, Kokumi, ENaC, Patch-clamp recording
Highlights
-
•
Kokumi taste-active γ-glutamyl peptides, GSH and EVG, are tasteless but potentiate salty taste perception.
-
•
To detect salt, taste cells use the amiloride-sensitive epithelial sodium channel (ENaC) as a sodium receptor.
-
•
GSH and EVG exert a small inhibitory effect on ENaC.
-
•
The enhancement of salty taste perception by γ-glutamyl peptides cannot be explained by increased sodium receptor activity.
1. Introduction
Sodium ion (Na+) is a necessary mineral for our bodies because it regulates extracellular fluid osmolarity/volume and is involved in a variety of physiological processes. The taste system is responsible for our ability to detect Na+ in foods: Na + elicits a specific sensation known as salty taste, which guides the ingestion of this vital mineral [1]. However, the widespread use of salt (NaCl) in food products to improve palatability has unavoidably resulted in Na+ consumption above physiological requirements. Increased dietary sodium consumption contributes significantly to the development of hypertension and related diseases [[2], [3], [4]].
It is difficult to reduce salt content in foods without affecting flavor and palatability because low sodium results in a poor savory taste and increased bitterness [5,6]. One strategy for compensating for off-tastes associated with salt reduction is to use so-called salty taste enhancers, which are chemicals capable of increasing the sensation evoked by Na+ and thus making low-salt foods equally savory [7,8]. In recent years, there has been a surge of interest in kokumi taste compounds, which strongly enhance salty taste perception [9]. These substances, γ-glutamyl peptides such as γ-glutamyl-cysteinyl-glycine (GSH, glutathione) and γ-glutamyl-valyl-glycine (EVG), and Maillard-reaction peptides, are tasteless; however, they improve persistency and mouthfulness (mouth-filling sensation) and also enhance some primary taste qualities, including salty taste [[9], [10], [11], [12], [13], [14]]. How kokumi taste-active substances potentiate salt perception, however, is poorly understood.
Food Na+ is detected by specialized sensory cells on the tongue, the taste cells, which use different mechanisms to detect varying salt concentrations [1]. In animal models, it is now well established that the amiloride-sensitive epithelial sodium channel (ENaC) functions as a specific sodium receptor when the salt concentration is ≤ 150 mM [15,16]. This concentration range is preferred by animals and liked by humans [1]. Functional ENaC is found in a subset of taste cells known as sodium-taste cells, which convert changes in salivary sodium concentration into an influx of Na+ (i.e., an ionic current) into the cell. The resulting membrane depolarization leads to action potential firing and neurotransmitter release [16]. ENaC is also found in human taste tissue, though its role as a sodium receptor is not fully understood [17].
There is evidence that small peptides can alter ENaC activity. Studies on human ENaC functionally expressed in Xenopus oocytes have revealed that salt-taste-modulating substances such as the dipeptide argininyl-arginine potentiate the ENaC-mediated sodium current [18]. Xu, Elkaddi, Garcia-Blanco et al. [19] found that some arginyl dipeptides, which are potent salty taste enhancers [20], induce a significant increase in the number of cultured human fungiform taste cells responding to NaCl, most likely via ENaC. Furthermore, they found that blocking calcium-sensing receptor (CaSR) had no effect on ENaC activation by arginyl dipeptides. These findings are significant because CaSR is thought to function as a kokumi taste receptor [9,21,22] in taste tissues [10,[23], [24], [25]].
Here we wanted to test if the well-known kokumi-active γ-glutamyl tripeptides, GSH and EVG [9,26], enhanced the activity of ENaC as arginyl dipeptides did. We used patch-clamp recording to monitor functional ENaC in sodium-taste cells from the rat fungiform papillae. These salt-detecting cells were functionally identified by exploiting the known inhibitory effect of the diuretic drug, amiloride [27,28].
2. Materials and methods
2.1. Ethical approval
Experiments were performed in compliance with the Italian law on animal care No. 116/1992, and in accordance with the European Community Council Directive (EEC/609/86). This study was approved by the institutional review board, ‘Organismo Preposto al Benessere degli Animali (OPBA)'.
2.2. Animals
Sprague-Dawley rat represents an animal model for salt taste, e.g. Ref. [29]. Thus, we used Sprague-Dawley rats, male and female, 50–70 days of age, 200–350 g of weight. Animals were housed 2 per cage on a 12-h light/dark cycle in climate-controlled conditions with ad libitum access to water and food. To isolate taste tissue, animals were deeply anesthetized by inhalation of isoflurane (Merial Italia, Milan, Italy), and then euthanized by cervical dislocation.
2.3. Fungiform taste cells
Electrophysiological experiments were performed on single taste cells in isolated taste buds. The procedure to isolate taste buds from rat fungiform papillae closely followed published protocols, e.g. Refs. [[30], [31], [32]]. In brief, the tongue was injected with an enzyme cocktail containing: 1 mg collagenase A (Merck, Milan, Italy), 2.5 mg dispase II (Merck), and 1 mg trypsin inhibitor type I–S (Merck) in 1 mL of Tyrode solution (see below). The tongue was incubated in divalent-free Tyrode solution for 30–40 min at 25 °C with air bubbling. The lingual epithelium was then gently peeled away from the underlying tissue. The isolated epithelium was placed in a Sylgard-lined Petri dish and examined under a dissecting microscope for the presence of fungiform taste buds. During taste bud isolation, amiloride (10 μM) was added to all solutions to avoid enzymatic degradation of ENaCs, e.g. Ref. [30]. Taste buds were plated on the bottom of a chamber consisting of a standard glass slide onto which a silicon ring 1–2 mm thick and 15 mm ID was pressed. We used glass slides pre-coated with poly-lysine (Fisher Scientific, Milan, Italy) to improve adherence of isolated taste buds to the bottom of the chamber. The chamber was placed on the stage of an upright Olympus microscope (model BHWI), and taste buds were viewed with Nomarski optics using a water immersion objective. During the experiments, isolated taste buds were continuously bathed with Tyrode's solution (containing in mM: 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 sodium pyruvate, and 10 HEPES; pH 7.4) by means of a gravity-driven system.
2.4. Patch-clamp recording
Whole cell patch-clamp recordings were made from cells in isolated taste buds as described previously, e.g. Refs. [[30], [31], [32]]. Signals were filtered at 5 kHz with the patch-clamp amplifier (Axopatch 1-D; Molecular Devices, Sunnyvale, CA). The sampling rate was adjusted to the type of membrane current: 100 kHz for fast currents (such as voltage-gated sodium currents) and 100 Hz for slow currents (such as amiloride responses). Data were acquired and analyzed with the pCLAMP9 software (Molecular Devices). Recording pipettes were made from soda lime glass capillaries (Globe Scientific, Paramus, NJ) on a 2-stage vertical puller (PP-830, Narishige, Tokyo, Japan). Typical pipette resistances were 2–3 MΩ when filled with a standard pipette solution containing (in mM) 120 CsCl, 1 CaCl2, 2 MgCl2, 10 HEPES, 11 EGTA, and 2 ATPNa2, pH 7.2 adjusted with CsOH. The liquid junction potential of ∼4 mV measured between pipette solution and Tyrode (bath) solution was neglected. Patch pipette was positioned onto taste cells by means of a water hydraulic micromanipulator (MHW-3, Narishige).
2.5. Amiloride-sensitive epithelial sodium channel (ENaC)
The presence of functional ENaCs in taste cells was monitored by studying the effect of bath-applied amiloride, a selective blocker of ENaC at submicromolar concentrations, on the whole-cell current recorded at the holding potential of −80 mV [27]. To assure specificity, we used as a probe an amiloride concentration of 1 μM, which is above the inhibition constant for ENaCs in these cells and below the dose range affecting other membrane transporters [33,34].
2.6. Chemicals
All drugs were purchased from Merck. EVG (purity >97%) was synthesized by Lugen Sci (Seoul, Korea). Amiloride was dissolved in dimethyl sulfoxide at a concentration of 0.5 M, and then diluted to its final concentration of 1 μM. GSH and EVG were dissolved in 50-μL Tyrode's at a concentration of 20 mM, and stored at −20 °C. For experimental tests, one aliquot was diluted to a final concentration of 20 μM. We chose this concentration based on experiments performed in vitro using HEK293 cells expressing human CaSR. Specifically, both GSH and EVG at 20 μM produce a maximal response (see Supplementary Fig. 1). The solutions were delivered via gravity-driven flow perfusion, which ensured a solution exchange in less than 1 min.
2.7. Assessment of the drug effect on the ENaC-mediated stationary current
When the membrane of taste cells is held at −80 mV, functional ENaCs produce a stationary inward current (IS) [27]. It is customary to observe the behavior of IS during drug application to determine whether a given chemical had any effect on ENaCs. An upward deflection in the baseline current is observed if the tested chemical reduces IS. This is the case with amiloride, which blocks ENaC [27]. On the other hand, if the tested chemical increases IS, the baseline current deflects downward. This is true for substances such as choline chloride, which increases the current passing through ENaC [18]. Our goal was to see if GSH or EVG caused any changes in IS when applied to taste cells. To monitor the drug effect, the baseline current must be stable and without drift prior to drug application. All of the recordings reported in this study met this requirement. The amplitude of current deflection was measured in comparison to the IS level when the effect was at its peak (Supplementary Fig. 2).
2.8. Statistics
Analysis and plotting were performed using Prism 9.0 software (Graph Pad Software, La Jolla, USA). Data comparisons were made with Mann–Whitney test or Wilcoxon matched-pairs test. Spearman test was used to establish correlation between variables.
3. Results
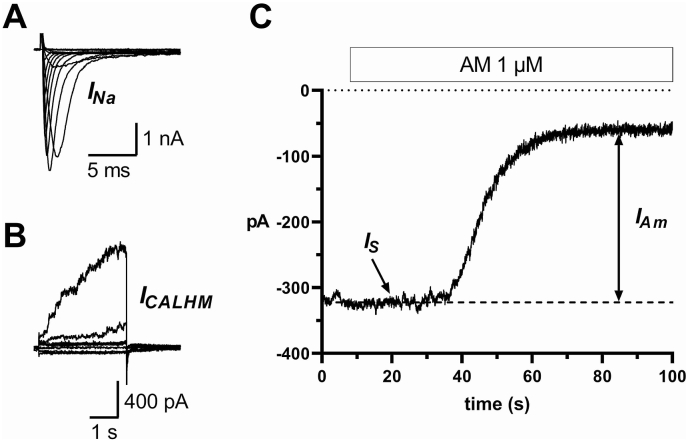
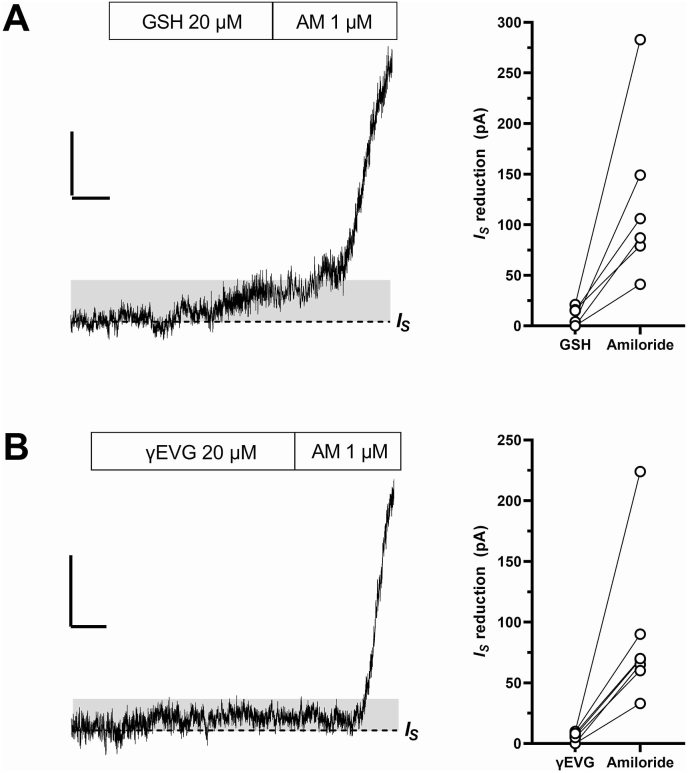
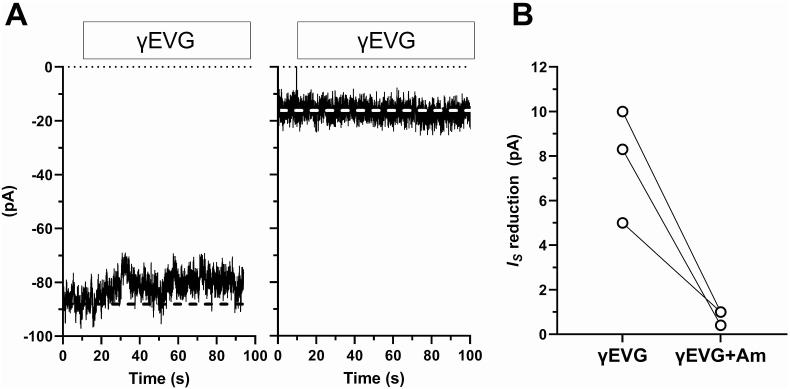
Fungiform sodium-taste cells were identified electrophysiologically by the presence of voltage-gated sodium current (Fig. 1A), CALHM current (Fig. 1 B), and the “response to amiloride” (Fig. 1C) [27,28]. Amiloride-sensitive sodium channels (ENaCs) are constitutively open: in Tyrode's solution, it is possible to record a stationary inward current (IS, Fig. 1C) due to the influx of Na+ into taste cells when the cell membrane is held at −80 mV. Bath application of 1 μM amiloride reduces this stationary current by blocking ENaCs, and this reduction is the response to amiloride (IAm, Fig. 1C). In sodium-taste cells held at −80 mV, bath-application of 20 μM GSH (Fig. 2A) or 20 μM EVG (Fig. 2B) produced a small decrease of IS (upward deflection in the current records). On the contrary, application of 1 μM amiloride resulted in a sharp IS reduction (Fig. 2). Supplementary Fig. 2 shows the complete recording for the traces shown in Fig. 2. Even though it was slight, the upward deflection of IS in the presence of either GSH or EVG differed significantly from the current level recorded without the drug (Supplementary Fig. 3). The effect of kokumi taste-active γ-glutamyl peptides was related to the magnitude of the response to amiloride: the larger IAm, the greater the effect (Spearman correlation coefficient r = 0.6640, P = 0.0161). Supplementary Fig. 4A shows the distribution of IS reduction induced by 20 μM GSH or EVG and 1 μM amiloride. The median value for the amiloride effect was almost ten times larger than the one for GSH or EVG (horizontal bars in Supplementary Fig. 4A). To establish whether the small IS reduction induced by kokumi taste peptides was due to an effect on ENaC, we repeated the experiments with EVG in the presence of amiloride. The left current record in Fig. 3A depicts the effect of EVG alone. After washing out EVG, we applied amiloride and waited for its maximum effect on IS (not shown). At this point, we applied EVG once more in the presence of an ongoing amiloride application (right current record). When ENaC was blocked by amiloride, EVG was unable to reduce IS. The results from three different cells are shown in Fig. 3B. It should be noted that the current noise was significantly reduced in the presence of amiloride (Fig. 3A, right current record). This is due to amiloride's strong blocking effect on ENaC [27] (see also Supplementary Fig. 2).
Fig. 1.
Electrophysiological identification of sodium-taste cells in rat fungiform taste buds. All the recordings are from the same cell and were obtained with the whole-cell configuration of the patch-clamp technique. (A) Voltage-gated sodium currents (INa) were elicited by the application of depolarizing voltage steps (10-mV increments from −70 mV to 50 mV) from a holding potential −80 mV. INa appears as downward deflections in the current records. (B) Currents mediated by calcium homeostasis modulator (CALHM) channels (ICALHM) were elicited by applying 20-mV voltage steps (from −80 mV to + 80 mV) from a holding potential of −40 mV. ICALHM were activated by membrane potentials larger than 0 mV (upward deflections in the current records). (C) Response to amiloride (IAm) obtained by bath-applying 1 μM amiloride (box over the record) to the cell membrane held at −80 mV. IS: stationary inward current.
Fig. 2.
Effect of kokumi-active γ-glutamyl tripeptides on the stationary inward current (IS) in sodium-taste cells. Cells were held at −80 mV in the whole-cell configuration of the patch-clamp technique. (A) left: Bath-application of 20 μM glutathione (GSH) reduces IS (grey shadow). Application of 1 μM amiloride (AM) soon after induces a sharp IS reduction, which is shown only in part. right: Distribution of the IS reduction value during application of GSH and then of amiloride in 6 sodium-taste cells. (B) left: Bath-application of 20 μM γ-glutamyl-valyl-glycine (γEVG) reduces IS (grey shadow). Application of 1 μM amiloride (AM) soon after induces a sharp IS reduction, which is shown only in part. right: Distribution of the IS reduction value during application of γEVG and then of amiloride in 7 sodium-taste cells. Vertical bars: 50 pA; horizontal bars: 20 s.
Fig. 3.
Effect of γEVG on the stationary inward current (IS) in sodium-taste cells. Cell membrane was held at −80 mV in the whole-cell configuration of the patch-clamp technique. (A) Effect of 20 μM γ-glutamyl-valyl-glycine either alone (left) or in the presence of 1 μm amiloride (right) on IS (dashed line). Note the marked upward shift of IS in the presence of amiloride: from about 86 pA (left) to about 16 pA (right). No effect by γEVG was detected after reduction of IS by amiloride (right). Note that IS shifts toward 0 mV when amiloride is present because of channel blockage by this drug. (B) Distribution of the IS reduction values during application of γEVG alone (γEVG) or in the presence of amiloride (γEVG + Am) in 3 sodium-taste cells.
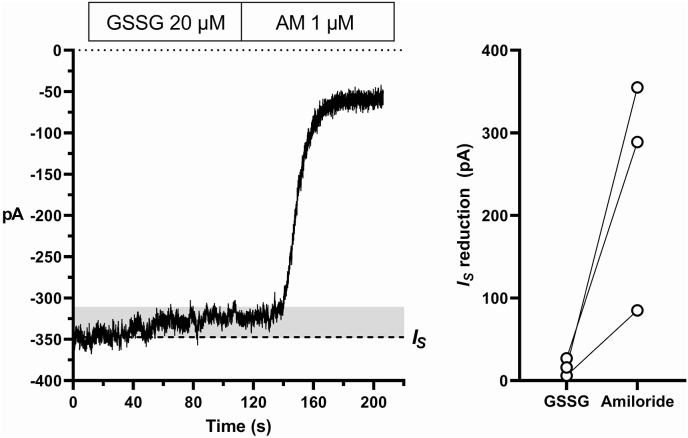
In alveolar epithelial cells of the rat lung, bath-applied oxidized glutathione (GSSG) inhibits ENaC activity [35]. We therefore tested whether GSSG also affected IS in rat fungiform sodium-taste cells. Indeed, 20 μM GSSG mimicked the effect observed with the kokumi taste peptides (Fig. 4). Although the sample size was limited (n = 3), GSSG seemed to produce a larger current reduction (median value of 16 pA) compared to GSH and EVG (median value: 9.5 pA and 6.5 pA, respectively; horizontal bars in Supplementary Fig. 4A). However, after normalization to the amiloride-induced current reduction, the effect of GSSG (median value of 7.1%) was comparable to that of GSH or EVG (median values of 5.1% and 8.3%, respectively; horizontal bars in Supplementary Fig. 4B).
Fig. 4.
Effect of oxidized glutathione (GSSG) on the stationary inward current (IS) in sodium-taste cells. Whole-cell configuration of the patch-clamp technique, holding potential: 80 mV. (A) Bath-application of 20 μM GSSG reduces IS (grey shadow). Application of 1 μM amiloride (AM) soon after induces a larger IS reduction. (B) Distribution of the IS reduction value during application of GSSG and then of amiloride in 3 sodium-taste cells.
To explore the specificity of the effect on ENaC expressed in sodium-taste cells, we tested GSH or EVG on cell types lacking the response to amiloride: Type II and Type III cells. These cells can be identified electrophysiologically based on the occurrence of specific voltage-gated ion currents (Supplementary Figs. 5 and 6). Type II cells sense sweet, bitter, and umami substances, while Type III cells detect acids and hypertonic salt solutions [[36], [37], [38], [39]]. Supplementary Fig. 7A shows that in Type II cells, GSH had no effect on the membrane current when the cell was held at −80 mV, that is, in the experimental conditions used with sodium-taste cells (Fig. 2). The same finding was obtained with Type III cells (Supplementary Fig. 7B). Note that these cell types do not exhibit functional ENaCs, as indicated by the lack of the amiloride response (Supplementary Fig. 7). GSH was unable to affect the holding current at −80 mV in all tested cells (4 Type II cells and 5 Type III cells). Similar results were obtained also with EVG (5 Type II and 4 Type III cells) (data not shown). The statistical analysis of pooled data for the effect of γ-glutamyl tripeptides on IS in sodium cells and Type II/III cells is shown in Supplementary Fig. 8.
4. Discussion
As a whole, our findings suggest that GSH and EVG slightly inhibit ENaC in sodium-taste cells. Thus, the enhancing effect of these substances on salt reception does not appear to be mediated by an increase in the activity of the sodium receptor, as it has been suggested for arginyl dipeptides [18,19].
CaSR senses kokumi substances in taste tissues and seems to be expressed by Type II and Type III cells [9,10,[21], [22], [23], [24]]. In a subset of Type III cells, CaSR is functional and is involved in the regulation of serotonin release [25]. It is not known whether CaSR is expressed in sodium-taste cells. Further experiments are required to elucidate its role, if any, in the inhibitory effect of GSH or EVG on ENaC.
GSSG inhibits alveolar ENaC activity by reducing the channel open probability, PO [35]. The GSSG effect likely results from reversible S-glutathionylation of the channel protein [35]. These findings would explain why the inhibitory effect in sodium-taste cells is quite small compared to the effect of amiloride (Fig. 4). The positive charge-bearing guanidinium group of amiloride interacts with part of the ENaC channel pore, causing channel blockage [40] and a strong inhibition of the stationary current. On the contrary, GSSG likely reduces the channel activity (PO) without any pore blockage. It is tempting to speculate that also GSH and EVG may interact with the ENaC protein to reduce its activity without blocking the channel. It is worth noting that while arginyl dipeptides seem to enhance ENaC activity in heterologous expression system [18,19], γ-glutamyl peptides do not in sodium-taste cells, that is, in situ (present data). This disparity could be explained by the different experimental and methodological approaches used. In rat fungiform taste cells, all three classical α-, β-, and γ-ENaC subunits have been identified [41,42]. It is unknown, however, how they come together to form a functional channel in sodium-taste cells. Differences in the subunit stoichiometry in the channel protein could explain the discrepancy among results. In any case, it is clear that a comprehensive picture of how kokumi taste-active peptides work will require further investigations. Species differences may also be involved and should be taken into account. For example, GSH works as kokumi substance for humans but not for cats [43].
Enzymatic cleavage by serine proteases, such as trypsin, can activate ENaC [44]. Because our procedure for isolating taste buds from the rat tongue required enzymatic treatment (see Materials and Methods), ENaCs could have been cleaved during tissue preparation and thus fully activated. This may prevent further activation by kokumi taste-active peptides. However, it is worth noting that Baquero and Gilbertson [45] demonstrated an increase in ENaC-mediated stationary current by insulin in mouse fungiform taste cells using the same enzymatic cocktail we used in this study. The presence of a trypsin inhibitor in the enzymatic mixture may have protected ENaC from enzymatic cleavage by external proteases. Nonetheless, future experiments will be required to address these concerns.
5. Conclusions
Our findings suggest that the enhancing effect of kokumi taste-active γ-glutamyl peptides, GSH and EVG, on salt reception is most likely not due to an increase in the activity of the amiloride-sensitive sodium receptor, ENaC. It is conceivable that other mechanisms, involving perhaps cross-talk between taste cells or taste cells and trigeminal nerve endings [11,46], could be responsible for the kokumi sensation of salty food.
Funding
This work was supported in part by Università di Modena e Reggio Emilia (FAR Dipartimentale 2018, 2019, 2020) and by the National Research Foundation of Korea (NRF) [grant number NRF2020R1A2C2004661].
Declaration of competing interest
None.
Acknowledgements
We are grateful to Mr. Claudio Frigeri (Università di Modena e Reggio Emilia) and Ms. Yiseul Kim (Korea Food Research Institute) for their excellent technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2022.101400.
Contributor Information
Albertino Bigiani, Email: albertino.bigiani@unimore.it.
MeeRa Rhyu, Email: mrrhyu@kfri.re.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Bigiani A. In: The Senses: A Comprehensive Reference. Fritzsch B., Meyerhof W., editors. Elsevier; 2020. Salt taste; pp. 247–263. [Google Scholar]
- 2.Batuman V. Salt and hypertension: why is there still a debate? Kidney Int. Suppl. 2011;3:316–320. doi: 10.1038/kisup.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delahaye F. Should we eat less salt? Arch. Cardiovasc. Dis. 2013;106:324–332. doi: 10.1016/j.acvd.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Ha S.K. vol. 12. Electrolyte Blood Press; 2014. pp. 7–18. (Dietary Salt Intake and Hypertension). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breslin P.A., Beauchamp G.K. Salt enhances flavour by suppressing bitterness. Nature. 1997;387:563. doi: 10.1038/42388. [DOI] [PubMed] [Google Scholar]
- 6.Wise P.M., Damani S., Breslin P.A.S. Sodium, but not potassium, blocks bitterness in simple model chicken broths. J. Food Sci. Technol. 2019;56:3151–3156. doi: 10.1007/s13197-019-03770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dötsch M., Busch J., Batenburg M., Liem G., Tareilus E., Mueller R., Meijer G. Strategies to reduce sodium consumption: a food industry perspective. Crit. Rev. Food Sci. Nutr. 2009;49:841–851. doi: 10.1080/10408390903044297. [DOI] [PubMed] [Google Scholar]
- 8.Hoppu U., Hopia A., Pohjanheimo T., Rotola-Pukkila M., Mäkinen S., Pihlanto A., Sandell M. Effect of salt reduction on consumer acceptance and sensory quality of food. Foods. 2017;6:103. doi: 10.3390/foods6120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohsu T., Amino Y., Nagasaki H., Yamanaka T., Takeshita S., Hatanaka T., Maruyama Y., Miyamura N., Eto Y. Involvement of the calcium-sensing receptor in human taste perception. J. Biol. Chem. 2010;285:1016–1022. doi: 10.1074/jbc.M109.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maruyama Y., Yasuda R., Kuroda M., Eto Y. Kokumi substances, enhancers of basic tastes, induce responses in calcium-sensing receptor expressing taste cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhyu M.R., Song A.Y., Kim E.Y., Son H.J., Kim Y., Mummalaneni S., Qian J., Grider J.R., Lyall V. Kokumi taste active peptides modulate salt and umami taste. Nutrients. 2020;12:1198. doi: 10.3390/nu12041198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y., Amin M.S., Li Q., Bak K.H., Lametsch R. In: Biologically Active Peptides. Toldrá F., Wu J., editors. Academic Press; 2021. Chapter 23 - applications in nutrition: peptides as taste enhancers; pp. 569–580. [Google Scholar]
- 13.Yan F., Cui H., Zhang Q., Hayat K., Yu J., Hussain S., Tahir M.U., Zhang X., Ho C.-T. Small peptides hydrolyzed from pea protein and their maillard reaction products as taste modifiers: saltiness, umami, and kokumi enhancement, food bioproc. Tech. 2021;14:1132–1141. [Google Scholar]
- 14.Yu B., Wu W., Wang B., Zhang N., Bak K.H., Soladoye O.P., Aluko R.E., Zhang Y., Fu Y. Maillard-reacted peptides from glucosamine-induced glycation exhibit a pronounced salt taste-enhancing effect. Food Chem. 2022;374 doi: 10.1016/j.foodchem.2021.131776. [DOI] [PubMed] [Google Scholar]
- 15.Chandrashekar J., Kuhn C., Oka Y., Yarmolinsky D.A., Hummler E., Ryba N.J., Zuker C.S. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomura K., Nakanishi M., Ishidate F., Iwata K., Taruno A. All-electrical Ca2+-independent signal transduction mediates attractive sodium taste in taste buds. Neuron. 2020;106:816–829. doi: 10.1016/j.neuron.2020.03.006. e6. [DOI] [PubMed] [Google Scholar]
- 17.Bigiani A. Does ENaC work as sodium taste receptor in humans? Nutrients. 2020;12:1195. doi: 10.3390/nu12041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stähler F., Riede K., Demgensky S., Neumann K., Dunkel A., Täubert A., Raab B., Behrens M., Raguse J.-D., Hofmann T., Meyerhof T.W. A role of the epithelial Sodium Channel in human salt taste transduction?, chemosens. Perception. 2008;1:78–90. [Google Scholar]
- 19.Xu J.J., Elkaddi N., Garcia-Blanco A., Spielman A.I., Bachmanov A.A., Chung H.Y., Ozdener M.H. Arginyl dipeptides increase the frequency of NaCl-elicited responses via epithelial sodium channel alpha and delta subunits in cultured human fungiform taste papillae cells. Sci. Rep. 2017;7:7483. doi: 10.1038/s41598-017-07756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindler A., Dunkel A., Stähler F., Backes M., Ley J., Meyerhof W., Hofmann T. Discovery of salt taste enhancing arginyl dipeptides in protein digests and fermented fish sauces by means of a sensomics approach. J. Agric. Food Chem. 2011;59:12578–12588. doi: 10.1021/jf2041593. [DOI] [PubMed] [Google Scholar]
- 21.Amino Y., Nakazawa M., Kaneko M., Miyaki T., Miyamura N., Maruyama Y., Eto Y. Structure-CaSR-activity relation of kokumi γ-glutamyl peptides. Chem. Pharm. Bull. 2016;64:1181–1189. doi: 10.1248/cpb.c16-00293. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M., Terada Y., Motoyama T., Shibata M., Saito T., Ito K. N-terminal [Glu]3 moiety of γ-glutamyl peptides contributes largely to the activation of human calcium-sensing receptor, a kokumi receptor. Biosci. Biotechnol. Biochem. 2020;84:1497–1500. doi: 10.1080/09168451.2020.1743169. [DOI] [PubMed] [Google Scholar]
- 23.San Gabriel A., Uneyama H., Maekawa T., Torii K. The calcium-sensing receptor in taste tissue. Biochem. Biophys. Res. Commun. 2009;378:414–418. doi: 10.1016/j.bbrc.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 24.Bystrova M.F., Romanov R.A., Rogachevskaja O.A., Churbanov G.D., Kolesnikov S.S. Functional expression of the extracellular-Ca2+-sensing receptor in mouse taste cells. J. Cell Sci. 2010;123:972–982. doi: 10.1242/jcs.061879. [DOI] [PubMed] [Google Scholar]
- 25.Cherkashin A.P., Rogachevskaja O.A., Kabanova N.V., Kotova P.D., Bystrova M.F., Kolesnikov S.S. Taste cells of the type III employ CASR to maintain steady serotonin exocytosis at variable Ca2+ in the extracellular medium. Cells. 2022;11:1369. doi: 10.3390/cells11081369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda M., Miyamura N. Mechanism of the perception of “kokumi” substances and the sensory characteristics of the “kokumi” peptide, γ-Glu-Val-Gly. Flavour. 2015;4:11. [Google Scholar]
- 27.Bigiani A. Electrophysiology of sodium receptors in taste cells. J. Biomed. Sci. Eng. 2016;9:367–383. [Google Scholar]
- 28.Bigiani A. Calcium homeostasis modulator 1-like currents in rat fungiform taste cells expressing amiloride-sensitive sodium currents. Chem. Senses. 2017;42:343–359. doi: 10.1093/chemse/bjx013. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto T., Fujii A., Saito N., Kondo H., Ohuchi A. Alteration of amiloride-sensitive salt taste nerve responses in aldosterone/NaCl-induced hypertensive rats. Neurosci. Res. 2016;108:60–66. doi: 10.1016/j.neures.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Doolin R.E., Gilbertson T.A. Distribution and characterization of functional amiloride-sensitive sodium channels in rat tongue. J. Gen. Physiol. 1996;107:545–554. doi: 10.1085/jgp.107.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kossel A.H., McPheeters M., Lin W., Kinnamon S.C. Development of membrane properties in taste cells of fungiform papillae: functional evidence for early presence of amiloride-sensitive sodium channels. J. Neurosci. 1997;17:9634–9641. doi: 10.1523/JNEUROSCI.17-24-09634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L., Hansen D.R., Kim I., Gilbertson T.A. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am. J. Physiol. Cell Physiol. 2005;289:C868–C880. doi: 10.1152/ajpcell.00115.2005. [DOI] [PubMed] [Google Scholar]
- 33.Lindemann B. Taste reception. Physiol. Rev. 1996;76:719–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- 34.Halpern B.P. Amiloride and vertebrate gustatory responses to NaCl. Neurosci. Biobehav. Rev. 1998;23:5–47. doi: 10.1016/s0149-7634(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 35.Downs C.A., Kreiner L., Zhao X.M., Trac P., Johnson N.M., Hansen J.M., Brown L.A., Helms M.N. Oxidized glutathione (GSSG) inhibits epithelial sodium channel activity in primary alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308:L943–L952. doi: 10.1152/ajplung.00213.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhari N., Roper S.D. The cell biology of taste. J. Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bigiani A., Prandi S. Functional diversity of taste cells. A review. Flavour Fragrance J. 2011;26:214–217. [Google Scholar]
- 38.Roper S.D., Chaudhari N. Taste buds: cells, signals and synapses. Nat. Rev. Neurosci. 2017;18:485–497. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinnamon S.C., Finger T.E. Recent advances in taste transduction and signaling. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.21099.1. Faculty Rev-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kellenberger S., Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 41.Kretz O., Barbry P., Bock R., Lindemann B. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J. Histochem. Cytochem. 1999;47:51–64. doi: 10.1177/002215549904700106. [DOI] [PubMed] [Google Scholar]
- 42.Lin W., Finger T.E., Rossier B.C., Kinnamon S.C. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J. Comp. Neurol. 1999;405:406–420. doi: 10.1002/(sici)1096-9861(19990315)405:3<406::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 43.Laffitte A., Gibbs M., Hernangomez de Alvaro C., Addison J., Lonsdale Z.N., Giribaldi M.G., Rossignoli A., Vennegeerts T., Winnig M., Klebansky B., Skiles J., Logan D.W., McGrane S.J. Kokumi taste perception is functional in a model carnivore, the domestic cat (Felis catus) Sci. Rep. 2021;11 doi: 10.1038/s41598-021-89558-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughey R.P., Carattino M.D., Kleyman T.R. Role of proteolysis in the activation of epithelial sodium channels. Curr. Opin. Nephrol. Hypertens. 2007;16:444–450. doi: 10.1097/MNH.0b013e32821f6072. [DOI] [PubMed] [Google Scholar]
- 45.Baquero AF A.F., Gilbertson T.A. Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells. Am. J. Physiol. Cell Physiol. 2011;300:C860–C871. doi: 10.1152/ajpcell.00318.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhyu M.R., Kim Y., Lyall V. Interactions between chemesthesis and taste: role of TRPA1 and TRPV1. Int. J. Mol. Sci. 2021;22:3360. doi: 10.3390/ijms22073360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.