Abstract
Sotorasib is a KRASG12C inhibitor that recently received approval for use in locally advanced or metastatic KRASG12C-mutated NSCLC. CodeBreaK100, the phase 2 clinical trial leading to the approval of sotorasib, excluded patients with untreated brain metastases; there have been no reports describing efficacy of sotorasib on untreated brain metastases. We present a case of a patient with active untreated brain metastases with resulting disorientation and weakness who has radiographic response and complete resolution of neurologic symptoms with sotorasib. Our case illustrates the intracranial activity of sotorasib, but additional studies are needed to characterize the intracranial response rate and duration of response in these patients.
Keywords: Sotorasib, KRAS G12C, Non–small cell lung cancer, Brain metastases, Case report
Introduction
Activating KRAS mutations are found in approximately 20% to 35% of lung adenocarcinoma, making it one of the most prevalent genomic drivers in NSCLC.1 Mutations in KRAS lead to cellular dysregulation and oncogenic transformation of the signaling pathways, resulting in uncontrolled cell proliferation. Although targeted therapy for other activating drivers in NSCLC has become standard, efforts to target KRAS mutations in NSCLC have been disappointing until recently. Sotorasib (AMG 510, Amgen, Thousand Oaks, CA) is a small molecule that specifically binds inactive GDP-bound KRASG12C, trapping it in an inactive state and preventing oncogenic signaling.2 Sotorasib was granted accelerated approval by the U.S. Food and Drug Administration in May 2021 for KRASG12C-mutated locally advanced or metastatic NSCLC after at least one line of systemic therapy on the basis of the results of the phase 2 CodeBreaK100 study. The objective response rate was 37.1%, with a disease control rate of 80.6% and a median duration of response (DoR) of 11.1 months.3 Although the trial included patients with evidence of metastatic brain disease, patients with active, untreated brain metastases were excluded. Here, we report a case of KRASG12C-mutated NSCLC with active, untreated brain metastases having profound clinical and radiographic response to sotorasib.
Case Presentation
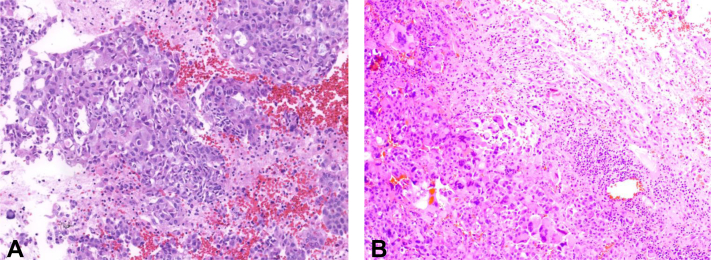
A 61-year-old man presenting with worsening headaches was found to have a presumed left occipital hematoma. A chest computed tomography scan revealed a 4.0 cm right upper lobe (RUL) lung mass and bulky right hilar and mediastinal lymphadenopathy. He underwent endobronchial ultrasound bronchoscopy with a transbronchial fine-needle aspiration of the right paratracheal and hilar lymph nodes, which revealed poorly differentiated lung adenocarcinoma (Fig. 1A). In the setting of subsequent progressive headaches and confusion, a repeat magnetic resonance imaging (MRI) of the brain revealed a mildly rim-enhancing, left occipital lesion with mass effect that was increased in size compared with imaging 2 weeks earlier. He was treated with dexamethasone and subsequently underwent a surgical left occipital craniotomy with tumor resection. Pathologic result revealed metastatic poorly differentiated adenocarcinoma of the lung primary origin (Fig. 1B). Tumoral programmed death-ligand 1 expression (Dako 22C3) was high in both the brain and the lymph node at 80% and 90%, respectively. Blood-based next-generation sequencing (Guardant 360, Guardant Health, Redwood, CA) revealed a KRASG12C mutation without other genomic alterations. Tissue-based next-generation sequencing (Caris Life Sciences, Phoenix, AZ) of the right paratracheal node revealed KRAS G12C, TP53 V157F, and ARID2 E101∗ mutations.
Figure 1.
H&E stain of cell block preparation of the right paratracheal lymph node (A), revealing solid sheet/nest of malignant cells, some with intracytoplasmic mucin. H&E stain of sections from the right parietal brain tumor (B) revealing malignant cells with large pleomorphic nuclei, frequent multinucleation, and increased mitoses. Tumor cells are positive for cytokeratin 7, TTF-1, and Napsin A (not pictured), supporting metastasis from pulmonary primary origin. The adjacent brain parenchyma has abundant reactive changes including reactive gliosis, chronic inflammation, and patchy hemorrhage. H&E, hematoxylin and eosin.
He received postoperative stereotactic radiosurgery to the cranial resection cavity and started treatment with pembrolizumab 200 mg every 3 weeks. An MRI of the brain obtained 1 month after initiation of pembrolizumab revealed postsurgical changes without evidence of enhancing lesions. His clinical course was complicated by thyroiditis with progression to grade 2 hypothyroidism, for which he received thyroid replacement therapy. On restaging imaging, the RUL mass was stable by Response Evaluation Criteria in Solid Tumors version 1.1, after four cycles of pembrolizumab. Cycle five was delayed owing to hospitalization for weakness and fatigue thought to be secondary to grade 3 immunotherapy-related hypothyroidism. His dose of thyroid replacement therapy was increased, and he was discharged after improvement with supportive care.
Within a week of hospital discharge, he presented with severe disorientation and profound weakness, prompting readmission. Workup for reversible causes of encephalopathy was unrevealing. A repeat MRI of the brain revealed considerable progression with innumerable brain metastases (Fig. 2A and D) and leptomeningeal enhancement consistent with leptomeningeal disease, and the patient was subsequently initiated on corticosteroid therapy. Given these findings and the poor prognosis, his family elected for open-access hospice care but agreed to a trial of sotorasib 960 mg daily, starting 2 months after his last dose of pembrolizumab. No further radiation to the brain was delivered.
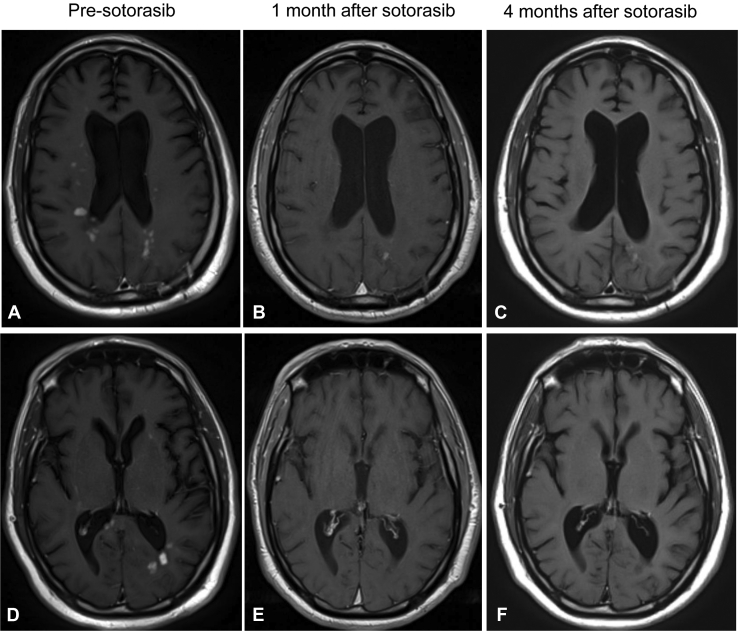
Figure 2.
Magnetic resonance imaging, T1 post-contrast phase, axial brain. Pre-sotorasib (A, D), 1 month after sotorasib treatment (B, E), and 4 months after sotorasib initiation (C, F). Numerous metastases were found in multiple levels in A and D, with improvement in B, C, E, and F.
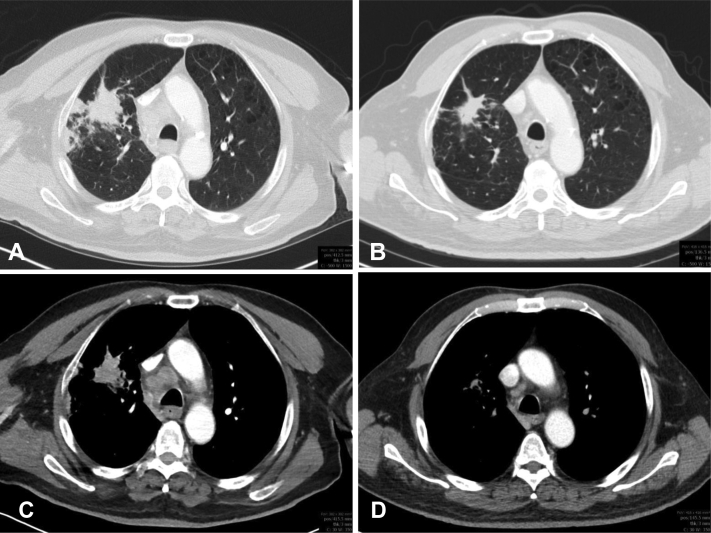
After 2 weeks of sotorasib, the patient had remarkable clinical improvement in his mental status and strength. An MRI of the brain after 1 month of sotorasib revealed clear radiographic improvement in the numerous enhancing lesions, with most lesions resolved or significantly smaller (Fig. 2B and E). A computed tomography scan of the chest at that time revealed a reduction in size of the RUL mass (Fig. 3A-D) and right hilar and subcarinal lymph nodes. The patient’s clinical course was complicated by the development of grade 3 transaminitis, which occurred 2 months after initiation of sotorasib. Sotorasib was held, and he was treated with a course of corticosteroids with improvement in transaminitis. As he had a remarkable response to sotorasib, the patient was rechallenged at lower doses of sotorasib (720 mg daily, then later 480 mg daily). On each rechallenge, his transaminitis worsened, leading to permanent discontinuation of sotorasib. He received sotorasib for 3.5 months before discontinuation. A repeat brain MRI 1 month after discontinuation of sotorasib revealed ongoing intracranial response (Fig. 2C and F).
Figure 3.
Computed tomography contrast-enhanced axial scan of the right upper lobe lung mass in the lung window (A and B) and soft tissue window (C and D). Pre-sotorasib (A, C; 4.0 × 2.1 cm) and 1 month after sotorasib (B, D; 3.2 × 1.5 cm) treatment.
After 6 weeks of sotorasib discontinuation, the patient began third-line carboplatin, pemetrexed, and bevacizumab and remains on third-line therapy at the time of submission. A timeline of the patient’s treatment course is summarized in Figure 4.
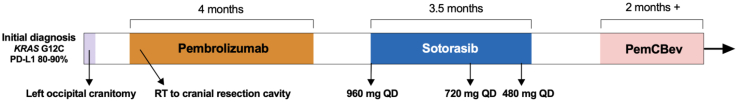
Figure 4.
Timeline of the patient’s treatment course spanning from the initial diagnosis to the most recent follow-up at time of the report. PD-L1, programmed death-ligand 1; PemCBev, carboplatin, pemetrexed, bevacizumab; QD, twice daily; RT, radiotherapy.
Discussion
Here, we present a patient with KRASG12C-mutated lung adenocarcinoma with metastasis to the brain who experienced a remarkable intracranial response to sotorasib. To the best of our knowledge, this is the first report to reveal activity of sotorasib for symptomatic, untreated brain metastases. The patient exhibited clinical improvement shortly after initiation of sotorasib, with imaging revealing an objective response to sotorasib.
Brain metastases are a relatively common occurrence in KRASG12C-mutated NSCLC. A retrospective analysis from the Massachusetts General Hospital identified 149 patients with KRASG12C-mutant NSCLC; 60 of those patients (40%) developed brain metastases.4 The 12-month cumulative incidence of brain metastases from the time of diagnosis of metastatic KRASG12C-mutant NSCLC was 48.2%. Most brain metastases were identified at the time of initial diagnosis.
Limited data on central nervous system (CNS) activity of sotorasib in metastatic NSCLC exist. In the CodeBreaK100 trial, patients with active, untreated brain metastases were excluded.3 In a post hoc analysis of CodeBreaK100, patients with stable brain metastases previously treated with radiation or surgery had a median overall survival of 8.3 months and a median progression-free survival of 5.3 months with a disease control rate of 77.5% and a median DoR of 11.1 months.5 Of note, 14 of 16 patients (87.5%) with assessable brain metastases achieved intracranial disease control. These results suggest that patients with previously treated brain metastases benefit from sotorasib with continued intracranial stabilization, but the effect of sotorasib on treatment-naive metastatic disease has not been determined. In CodeBreaK101 (NCT04185883), there is a cohort for patients with active, untreated brain metastases, which will help to elucidate the intracranial activity of sotorasib.
Adagrasib (MRTX849, Mirati Therapeutics, San Diego, CA) is another small molecule KRASG12C inhibitor which has promise for use in KRASG12C-mutated NSCLC with brain metastases. Adagrasib was found to have cerebrospinal fluid penetration and extend survival in preclinical models and brain metastasis regression in two patients with NSCLC and untreated brain metastases.4 In the KRYSTAL-1 trial, 25 patients with active, untreated CNS metastases received adagrasib. The intracranial objective response rate per modified RANO criteria by blinded independent central review was 32%, suggesting CNS-specific activity of adagrasib.6 Prospective data are needed to confirm these early signals of efficacy and to determine the depth and durability of CNS response to small molecule inhibitors in KRASG12C-mutated NSCLC.
The continued use of sotorasib in our patient was limited owing to grade 3 immune-mediated hepatitis. Begum et al.7 first reported a case of severe immune checkpoint-mediated hepatotoxicity triggered by sotorasib in a patient who had received pembrolizumab 14 weeks before sotorasib initiation. Grade 3 or higher transaminitis was reported at a rate of approximately 6% in CodeBreaK100 and 8% in CodeBreaK200, while occurring at a rate as high as 31% in a retrospective study of 32 patients who had received sotorasib within 90 days of immune checkpoint therapy.3,8,9 Clear guidance for the management of hepatotoxicity with sotorasib is an unmet area of need, particularly in patients who have received previous immune checkpoint inhibitor therapy.
Conclusion
In summary, our case reveals the intracranial activity of sotorasib in a patient with KRASG12C-mutated NSCLC and active, untreated brain metastases complicated by leptomeningeal disease. Further studies are needed to understand the intracranial response rate and DoR associated with sotorasib in patients with KRASG12C-mutated NSCLC.
CRediT Authorship Contribution Statement
Justin Yeh: Writing—original draft, Investigation.
Jennifer A. Marks, Emily A. Sloan, Peter J. Bergquist: Writing—review and editing, Investigation.
Reshma Varghese, Nitika Paudel, Joshua E. Reuss, Stephen V. Liu: Writing—review and editing.
Ali H. Alzeer: Investigation.
Chul Kim: Supervision, Writing—review and editing, Conceptualization.
Acknowledgments
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Footnotes
Disclosure: Dr. Reuss served as a consultant or advisory board member for Oncocyte, Genentech/Roche, Sanofi/Genzyme, and Personalis; received research funding (to institution) from Genentech/Roche and Verastem; and received speaking fees from AstraZeneca. Dr. Liu served as a consultant or advisory board member for Amgen, AstraZeneca, Bayer, BeiGene, Blueprint, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Elevation Oncology, Genentech/Roche, Gilead, Guardant Health, Janssen, Jazz Pharmaceuticals, Eli Lilly, Merck/Merck Sharp & Dohme, Novartis, Regeneron, Sanofi, Takeda, and Turning Point Therapeutics and received research funding (to institution) from Alkermes, Bayer, Blueprint, Bristol-Myers Squibb, Elevation Oncology, Genentech, Gilead, Lilly, Merck, Merus, Nuvalent, Pfizer, Rain Therapeutics, RAPT, and Turning Point Therapeutics. Dr. Kim served as a consultant or advisory board member for Novartis, Janssen, AstraZeneca, Sanofi, PierianDx, Diffuse Pharmaceuticals, Mirati, Jazz Pharmaceuticals, and Arcus Biosciences and received research funding (to institution) from AstraZeneca, Bristol-Myers Squibb, Novartis, Genentech, Janssen, Regeneron, Debiopharm, and Karyopharm. The remaining authors declare no conflict of interest.
Cite this article as: Yeh J, Marks JA, Alzeer AH, et al. Remarkable intracranial response to sotorasib in a patient with KRASG12C-mutated lung adenocarcinoma and untreated brain metastases: a case report. JTO Clin Res Rep. 2022;3:100428.
References
- 1.Judd J., Karim N.A., Khan H., et al. Characterization of KRAS mutation subtypes in non–small cell lung cancer. Mol Cancer Ther. 2021;20:2577–2584. doi: 10.1158/1535-7163.MCT-21-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrem J.M., Peters U., Sos M.L., Wells J.A., Shokat K.M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoulidis F., Li B.T., Dy G.K., et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabari J.K., Velcheti V., Shimizu K., et al. Activity of adagrasib (MRTX849) in brain metastases: preclinical models and clinical data from patients with KRASG12C-mutant non-small cell lung cancer. Clin Cancer Res. 2022;28 doi: 10.1158/1078-0432.CCR-22-0383. 3381–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramalingam S., Skoulidis F., Govindan R., et al. P52. 03 Efficacy of sotorasib in KRAS p.G12C-mutated NSCLC with stable brain metastases: a post-hoc analysis of CodeBreaK 100. J Thorac Oncol. 2021;16(suppl):S1123. [Google Scholar]
- 6.Sabari J.K., Spira A.I., Heist R.S., et al. Activity of adagrasib (MRTX849) in patients with KRASG12C-mutated NSCLC and active, untreated CNS metastases in the KRYSTAL-1 trial. J Clin Oncol. 2022;40(suppl) LBA9009–LBA9009. [Google Scholar]
- 7.Begum P., Goldin R.D., Possamai L.A., Popat S. Severe immune checkpoint inhibitor hepatitis in KRAS G12C-mutant NSCLC potentially triggered by sotorasib: case report. JTO Clin Res Rep. 2021;2 doi: 10.1016/j.jtocrr.2021.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rakshit S., Bansal R., Potter A., et al. MA13.09 Time from immune checkpoint inhibitor to sotorasib use correlates with risk of hepatotoxicity in nonsmall cell lung cancer. J Thorac Oncol. 2022;17(suppl):S92. doi: 10.1016/j.ctarc.2023.100743. [DOI] [PubMed] [Google Scholar]
- 9.Johnson M.L., De Langen J., Waterhouse D.M., et al. Sotorasib versus docetaxel for previously treated non-small cell lung cancer with KRAS G12C mutation: CodeBreaK 200 phase III study. Ann Oncol. 2022;33(suppl 7):S808–S869. [Google Scholar]