Graphical abstract
Keywords: CeO2, Formic acid oxidation, Heterostructure, Layered WS2, Ultrasonically intercalated CeO2, Photocatalytic oxidation
Highlights
-
•
Utilisation of ultrasonically exfoliated 2D WS2 as hard template for CeO2 growth.
-
•
Simple hydrothermal approach for synthesising CeO2@WS2 heterojunction.
-
•
Formation of efficient type (II) heterojunction between WS2 and CeO2.
-
•
Proving the Ce-S bonding by XRD and density functional calculations.
-
•
Formic acid photocatalytic oxidation activity, producing 446.7 µmol g −1 CO2.
Abstract
Here, we investigate the band structure, density of states, photocatalytic activity, and heterojunction mechanism of WS2 with CeO2 (CeO2@WS2) as a photoactive heterostructure. In this heterostructure, CeO2′s growth within WS2 layers is achieved through ultrasonicating WS2 and intercalating CeO2′s precursor within the WS2 interlayers, followed by hydrothermal treatment. Through a set of density functional calculations, we demonstrate that CeO2 and WS2 form an interface through a covalent bonding that can be highly stable. The electrochemical impedance spectroscopy (EIS) found that the CeO2@WS2 heterostructure exhibits a remarkably higher conductivity (22.23 mS cm−2) compared to either WS2 and CeO2, assignable to the interface in CeO2@WS2. Furthermore, in a physically mixed CeO2-WS2 where the interaction between particles is noncovalent, the resistance was significantly higher (0.67 mS cm−2), confirming that the heterostructure in the interface is covalently bonded. In addition, Mott-Schottky and the bandgap measurements through Tauc plots demonstrate that the heterojunction in CeO2 and WS2 is type II. Eventually, the CeO2@WS2 heterostructure indicated 446.7 µmol g −1 CO2 generation from photocatalytic oxidation of a volatile organic compound (VOC), formic acid, compared to WS2 and CeO2 alone.
1. Introduction
Solar-driven light in catalysis, i.e. photocatalysis, has a universal appeal for use as a free energy source, which can be incorporated into many applications such as fine chemicals production, energy carriers generation, and pollutants removal [1], [2], [3], [4]. Although many semiconductors have suitable energy band gaps that fall in the UV–vis range for photocatalysis, their photoactivity is often less efficient due to the improper band edge (valence band or conduction band) alignment against the potential redox energy level of the target reaction [5], [6]. Although there are several pathways for improving the photocatalytic performance in such semiconductors [7], [8], among them, a promising solution is a heterojunction within a suitable heterostructure to align the bandgap. Furthermore, the lifetimes of photogenerated electron-hole in most catalysts are short and suffer from fast recombination, which heterojunctions can further improve [9]. However, a heterostructure is only efficient if each of its semiconductor’s band edges is suitably aligned so it can eventually transfer the photogenerated electrons/holes pairs to the reacting species. Another point in a successful heterostructure is the crystalline structure at the interface of the junction [10]. The electron transfer in the boundary will be less efficient if there is no suitable junction due to mismatching, cracked or loose interface. So far, the 2D dichalcogenide WS2 forms promising heterostructures with other photoactive oxides and semiconductors such as TiO2 [11], MoS2 [12], SnS [13], and CuO [14]. However, the heterostructure of CeO2 with WS2 in photocatalysis is rarely investigated [15]. Likewise, cerium oxide has attractive crystal and photocatalytic properties and has been found suitable for the heterostructure through materials nanoarchitecture [16], [17], [18].
Intercalation of (organo)metallic precursors in 2D layered materials’ interlayers is one of the pivotal approaches to synthesising heterostructures with oriented and uniform growth [19], [20]. For such cases, the exfoliation of bulk structures into few/multilayer structures is crucial to intercalate the heterostructure’s precursor. Among the several methods, ultrasound waves as a powerful tool for synthesis of nanomaterials [21], can be also utilised for exfoliating bulk layers are a popular and efficient pathway [22], [23], [24], [25]. Likewise, WS2 is a 2D material that may act as a hard template while it can generate a heterojunction with the grown heterostructure [26]. However, in an efficient heterojunction, along with its well-synthesised architecture, the band edges and the energy bandgaps of the composing compounds must match one another to generate an enhanced photocatalytic activity [27], [28]. Therefore, investigating such parameters in parallel with the synthesis method is vital to reach an optimal photocatalysis result.
Here, we have found that the heterojunction of CeO2 with the WS2 nanosheets leads to a type II heterojunction which generates a remarkable enhancement in the photocatalytic activity of formic acid decomposition with respect to pure WS2 and CeO2. We also demonstrated that the heterostructure in the interface creates a chemical bonding that makes the heterostructure more efficient, stable, and uniform. The Raman spectroscopy and X-ray diffraction results showed the formation of Ce-S bonding in the CeO2@WS2. This heterostructure exhibits better photocatalytic activity, 1.8 times of WS2 and 2.2 times of CeO2. It also reveals sustainability in the UV range toward photooxidation of volatile organic compound (VOC)—formic acid.
2. Materials and methods
2.1. Synthesis of samples
The detailed synthesis procedure of the CeO2@WS2 catalyst is reported in our previous work [15]. In brief, the WS2 nanoflakes were obtained by ultrasound-assisted exfoliation (Sonica ultrasound bath, 300 W, Italy) of bulk WS2 (Sigma-Aldrich, 99 %) in DMF (Merck, >99 %) for 2 h. After centrifuging the sample, the supernatant was removed, washed with EtOH, and dried at room temperature overnight. For synthesising CeO2@WS2, 0.1 g of the synthesised WS2 nanoflakes were added to 60 mL 0.1 mol/L Ce(NO3).6H2O (Merck, >99.99 %) in aqueous solution and sonicated for 3 h. After that, the mixture was added to a Na3PO4·12H2O (Merck, >99.99 %) solution (0.005 mol/L, 20 mL) and was stirred for 30 min. The final mixture was poured into a 100 mL hydrothermal autoclave and kept at 220 °C for 12 h. After cooling the autoclave, the liquid was removed by centrifuge and the remaining was washed with EtOH and deionised water and dried at 60 °C. The synthesis route for pure CeO2, used in this work, was similar to the mentioned approach without adding WS2 nanoflakes.
2.2. Characterisation
Scanning electron microscopy (SEM) images were obtained using a TESCAN MIRA3 microscope (TESCAN Ltd, Czech Republic). X-ray diffraction (XRD) patterns were recorded with a SmartLab X-ray diffractometer (Rigaku Co., Japan, via a Cu Kα radiation source). Raman spectroscopy was recorded by the ANDOR Kymera-328i apparatus (Andor Tech. Ltd., United Kingdom, at 532 nm excitation wavelength with 50 × objective, in the range of 100–2000 cm−1). Transmission electron microscopy (TEM) and TEM-based elemental mapping and electron dispersive spectroscopy (TEM-EDS) were all recorded by a JEOL 2100 instrument coupled to an energy-dispersive X-ray analyser (EDX, JEOL Ltd, Japan).
2.3. (Photo)electrochemical measurements
Fluorine-doped tin oxide (FTO) glass with an area of 1.0 × 1.0 cm2 was used as a substrate for the preparation of the working electrode. First, 20 mg of each catalyst sample was dispersed for 45 min in a solution including 1 mL of DI and 3 mL of isopropanol. Then, the FTO was washed and activated by diluted acid (HNO3). 80 µL of stable dispersed catalyst was slowly drop-casted on the FTO and heated to 120 °C on a hotplate for 15 min.
An OrigaFlex-OGF01A Potentiostat/Galvanostat (France) was used to record the electrochemical analysis data. The electrochemical setup included a 0.5 mol/L Na2SO4 electrolyte, an FTO-based working electrode, a platinum plate with an area of 2.0 × 1.0 cm2 as the counter electrode, and a reference electrode of saturated calomel electrode (SCE). Prior to each electrochemical test, the electrolyte solution was degassed by nitrogen bubbling for 20 min.
The electrochemical properties of samples were tested through the photocurrent test by cyclic chronoamperometry (CA) under both dark and light conditions, the EIS, and Mott-Schottky plots to determine the energy of flat band (Efb). Mott-Schottky plots were recorded at a frequency of 1000 Hz and the voltage range of −1 ∼ +1 V vs SCE in dark. The EIS results were obtained using an AC voltage of ± 5 mV at open circuit potential (OCP) and 300 mV vs SCE in a frequency range from 100 kHz to 100 mHz. Photocurrent behaviours were tested under consecutive light on–off cycles (each cycle time: 100 s) for 600 s at 1000 mV vs SCE.
2.4. Computational settings
Spin polarised density functional calculations were performed using VASP code [29], [30] with the projector augmented wave method technique [31]. The electronic correlation-exchange energy was approximated with general gradient approximation within the Perdew-Burke-Ernzerhof formalism [32], [33]. The energy cutoff value and the fast Fourier transform mesh were generated by setting the precision key to Accurate. Ad hoc Hubbard terms, based on the Dudarev implementation [34], were added to Ce and W 5d electrons to account for the strong correlation. For Ce, U and J values were 4.20 eV and 0.00 eV, respectively. For W, U and J values were 2.87 eV and 0.00 eV, respectively. These values were reported to improve the electronic description of CeO2 [35] and WS2 [36], respectively. Van der Waals dispersion energy correction was applied based on the DFT-D3 method [37]. Only Γ point was used for the Brillouin zone sampling. For geometry optimisation, in addition to the atomic coordinates, the lateral lattice parameters, u and v, were also allowed to relax to forces smaller than 0.01 eV/Å. The Bader charge analysis code [38] was used to analyse charge localisations.
2.5. Formic acid photocatalytic oxidation
For formic acid photooxidation, 15 mg of the catalyst was initially dispersed and sonicated for 1 min in 5 mL of a formic acid aqueous solution (5 vol%, Merck, >98 %) in a Pyrex glass test tube (34 mL) and then bubbled with O2 gas for 30 min. The glass tube was sealed with a rubber septum and photo-irradiated by a solar simulator (San-Ei Electric, λ > 300 nm, 1000 W m−2), stirring at 800 rpm. The amount of evolved CO2 in the headspace of the sealed tube was determined by a Shimadzu GC-2010 plus gas chromatograph equipped with a barrier ionisation discharge (BID) detector.
3. Results and discussion
3.1. Materials characterisation
We present the synthesis of ceria by the hydrothermal growth through intercalating Ce4+ in the interlayer space of WS2, exfoliated by sonication in an aqueous solution. According to the XRD pattern, shown in Fig. 1a, the synthesised material indicates a clear set of peaks corresponding to the CeO2, WS2 and CeS structures. Furthermore, given the non-equilibrium growth regime, intense nanostructuring and random growth of CeO2 crystallites, the XRD pattern appears with strong background noise and some unidentified peaks, most likely belonging to the W-S-Ce-O alloys with nonstoichiometric compositions for which we do not have a reference structure for refinement. However, we attempted to refine the identified peaks using the Rietveld refinement Fullprof Suite [39] and the Match! package [40]. With acceptable quality parameters of Bragg factor of 20.3 and a Χ2 of 7.5, the content was found to be 76.86 % (±0.03 %) CeO2, 7.00 % (±0.02 %) WS2, and 16.14 % (±0.02 %) CeS. The lattice parameters of these phases were found to be a = 5.414598 Å (±0.000527 Å) for the cubic CeO2, a = 2.970194 Å (±0.005109 Å) and c = 7.579407 Å (±0.009297 Å) for the hexagonal WS2, and a = 5.799615 Å (±0.004254 Å) for the cubic CeS. Also, the XRD pattern of physically mixed CeO2 and WS2 is shown in Fig. S1. We further investigated the changes in the vibrational modes originating from the heterojunction of CeO2 and WS2 using Raman spectroscopy (Fig. 1b). Accordingly, the peaks at 799, 801, 705, 317, 260, and 122 cm−1 in WS2′s spectra with a slight shift in some to lower wavenumbers and a decrease in the intensity have appeared again in the CeO2@WS2 heterostructure. The 456 cm−1 peak in CeO2′s spectra is characteristic of the CeO2 structure, which was also shifted lower to 442 cm−1 and decreased in intensity in the final CeO2@WS2 heterostructure. Eventually, a new peak appeared at 912 cm−1, which is not originating from either WS2 or CeO2 and is assignable to the newly formed Ce-S bond [41]. Therefore, the Raman spectra prove that there can be a covalent bonding between Ce and S species in the interface of CeO2 and WS2.
Fig. 1.
(a) Observed and refined XRD patterns for the synthesised CeO2@WS2. (b) Raman spectra of CeO2, WS2 and CeO2@WS2 heterostructure. SEM images of (c) WS2, (d) CeO2, and (e) CeO2@WS2 heterostructure. (f) Low and high magnification TEM images of the CeO2@WS2. Insets are HRTEM images of the same material.
As shown in Fig. 1c-g, we studied morphology and the structure of the CeO2-intercalated WS2 (CeO2@WS2) through SEM and TEM. In TEM and SEM micrographs (Fig. 1d-h), we can see an agglomeration of uniform nanorods in each particle, hinting to the successful heterostructure formation of CeO2 and WS2 within each nanorod, as all these nanorods are approximately of the same size and shape. Phase separation would have probably caused different nano-shapes for WS2 and CeO2 as these phases have different crystal symmetry. The HRTEM in the inset of Fig. 1g shows two distinct lattice structures belonging to CeO2 and WS2 within the matrix, confirming the presence of the CeO2@WS2 heterostructure. The observed and specified d values in the HRTEM image can be assigned to the planes with Miller indices of CeO2 (1 1 1) and WS2 (1 0 0) [42], [43], [44], indicating the interface in the heterostructures is normal to these planes. TEM-EDS of a selected area under a TEM microscope (Fig. S2) further confirms the co-presence of the related elements in a single particle, showing that the heterostructures have successfully interconnected. TEM-based elemental mapping further proves the presence of the Ce, O, W, and S elements in one particle (Fig. S2).
3.2. (Photo)electrochemical results
Appropriate separation of photogenerated charge carriers at the surface of particles is one of the most critical factors in photocatalytic performance. Photoluminescence (PL) is a valuable method to investigate the charge separation efficiency in materials. As reported before [45], CeO2 possesses a board emission band from 400 to 700 nm with the most intensity in the 470 ∼ 570 nm region. The charge transfer in the CeO2 originated from the electron transfer between the Ce 4f energy level and O 2p orbitals [46]. Therefore, this board peak appears due to the existence of many defect energy levels between the mentioned orbitals [47]. According to our observation in the previous report [15], a remarkably diminished CeO2 emission band appears after heterostructure formation with WS2, converting into CeO2@WS2, attributable to the promoted charge separation, which is absent in CeO2 alone. These good charge separation and adagio recombination rates between the photo-induced electron-hole pairs are the main origins of the boosted photocatalytic efficiency. Moreover, according to our previous study, the CeO2@WS2′s bandgap obtained from Tauc’s plots (2.9 eV) [15], [48] is narrower than CeO2 (∼3.02 eV) and WS2 (3.05 eV) [49]. Lastly, we ought to address the likely lack of contribution from the unintentional CeS phase toward the observed optical properties. CeS has a band gap of ∼ 3.6 eV [50] which is wider than both WS2 and CeO2′s band gaps [49], falling outside the visible range and, thus, is not likely to contribute to photocatalysis.
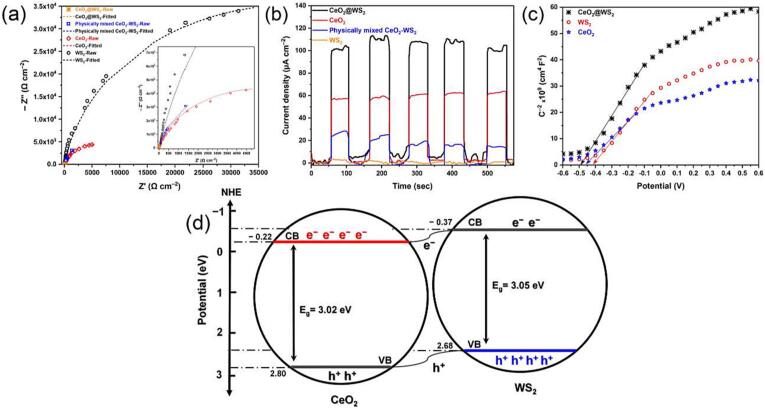
The charge transfer efficiencies of samples were measured by the EIS since it directly influences charge transfer resistance (Rct). As shown in Fig. 2a, the Nyquist plot of WS2 includes a wide semi-circle diameter, demonstrating its higher resistance to charge transfer than other samples. CeO2 also has a greater diameter, showing a high charge transfer resistance. However, the CeO2@WS2 heterostructure revealed a significant reduction in the charge transfer resistance by looking at the diameter of semi-circles which decreases. The Rct of WS2 was found to be ∼ 35000 Ω cm−2, and for CeO2, the Rct value was ∼ 4500 Ω cm−2. Eventually, the CeO2@WS2 heterostructure’s Rct was significantly lower (45 Ω cm−2) than both CeO2 and WS2 (inset of Fig. 2a). Interestingly, the physical mixture of WS2 and CeO2 showed an Rct of 1500 Ω cm−2, confirming that the chemically bonded interface of WS2 and CeO2 plays a substantial role in the electronic structure of the heterostructure. In other words, the charge transport conductivity of the chemically bonded heterostructure (22.23 mS cm−2) is by far higher than WS2, CeO2, and their physical mixture of CeO2-WS2 (0.67 mS cm−2), which confirms the importance of chemical bonding of these two structures at the interface.
Fig. 2.
(a) The EIS plots (inset: the magnified form of EIS plots to see details), (b) Photocurrent response, (c) Mott-Schottky plots, and (d) the proposed type (II) heterojunction diagram.
The transient photocurrent response vs time was measured for each photocatalyst sample as an index of their charge separation and transfer efficiency, as shown in Fig. 2b. In this investigation, the lowest response was observed for WS2 inefficient e−/h+ pairs separation. Albeit, a similar behaviour was obtained for CeO2. As predicted, a higher response for CeO2@WS2 heterostructure was observed, which signifies the heterojunction efficiency in the photoelectrocatalytic performance. However, when WS2 was physically mixed with CeO2, the photocurrent response had a significantly lower response than even the bare CeO2. This observation was further evidence highlighting the role of a stable interface between CeO2 and WS2 through chemical bonding between Ce and S species.
The Mott-Schottky method helps identify the type of semiconductors (p or n) and their flat-band energies (Efb). The Mott-Schottky plots of WS2, CeO2, and CeO2@WS2, depicted in Fig. 2c, show all semiconductors are n-type due to the potential-C−2 plots’ positive slope. Furthermore, the Efb can be determined by interrupting the liner part of potential-C−2 plots with the potential x-axis. The values of Efbs and our previously reported bandgaps (Eg) [15] for these materials are listed in Table S1. In n-type semiconductors, Efb can convert to the conduction band (CB) energy using the ECB = Efb – 0.2 V [51]. Thus, the ECBs of −0.61, −0.46, and −0.69 V were obtained for WS2, CeO2, and CeO2@WS2, respectively. For calculating the valence band (VB) energy, at first, the ECB values convert to the normal hydrogen electrode (NHE) values using ENHE = ESCE + 0.241 V [52]. Then, the EVBs can be obtainable through the EVB = ECB + Eg. All described values are listed in Table S1. Considering the CB and VB levels as depicted in Fig. 2d, a plausible type (II) heterojunction can be proposed for the CeO2@WS2 heterostructure. In such a heterojunction, the photogenerated electrons in WS2′s CB tend to migrate to the CeO2′s CB and make it the main reactive CB site. In contrast, the photogenerated holes (h+) in CeO2′s VB migrate to WS2′s VB and make it a more reactive VB site [53], [54]. Furthermore, the bandgap narrowing in the CeO2@WS2 sample in type (II) heterojunction is also justifiable. It is worth noting that the experimentally-obtained bandgap for CeO2@WS2 through the Tauc equation (2.9 eV) equals a value from CeO2′s ECB to WS2′s EVB, which further confirms the type II heterojunction [15].
3.3. Theoretical insights
Here, we study the interface between CeO2 and WS2. In constructing our model, we considered the HRTEM image of the CeO2@WS2 heterostructure in Fig. 1h. We also restricted our models to high-symmetry and low lattice mismatch configurations. To construct the interface at the heterostructure, we first cleaved the WS2 hexagonal structure (downloaded from Materials Project [55], compound id: mp-224) along the [0 0 1] direction. We then constructed a supercell with (2u ×√2v)R30° dimensions out of the cleaved surface. The resulting surface had an orthogonal cross-section suitable to be interfaced with cubic CeO2 with u′ = 6.381 Å and v′= 5.527 Å. We then cleave the conventional CeO2 structure (a = 5.415 Å) along the [0 0 1] direction. We then interfaced these two surfaces together by expanding WS2 along the [0 0 1] direction to be three sheets deep and expanding CeO2 along the [1 0 0] direction to be 12 atomic layers deep. Since we intended to have only one interface in the supercell, we added an ample vacuum slab of 20 Å to avoid artificial interactions at the non-interfacing facets in the supercell. To reduce the lattice mismatch, we allowed the lateral cell parameters to relax during the geometry optimisation. The lattice mismatch in the relaxed structures had an acceptable average value of ∼ 5.4 %.
Chemically there are two types of interfaces; either an oxygen-cleaved CeO2 facet interfaces with WS2, or a Ce-cleaved facet forms the interface with WS2. The first case has only one high-symmetry configuration, shown in Fig. 3a. The second scenario has two configurations, shown in Fig. 3b and c, respectively. In Fig. 3b, each Ce ion at the interface is coordinated by 3 S ions, while in Fig. 3c, each Ce ion is coordinated by 4 S ions. The Ce-coordinating S ions are marked in the upper row of Fig. 3. Among these three possible interfaces, configuration C was the most stable with total DFT energy of −531.610 eV, while configurations A and B each had higher total energy of −531.209 and −531.178 eV, respectively. In configuration A, the negatively charged facets at the interface create a Coulombic repulsion that reduces stability—The partial charges borne on S and O ions at the interface are given in Fig. 3a-ii. For configuration B, Ce’s undercoordination may be the origin of instability compared to Configuration A, as Ce4+ in a compound is more stable when coordinated by eight anions instead of seven [56].
Fig. 3.
The top (upper rows) and side (lower rows) views of the CeO2@WS2 interface configurations are shown (a), (b), and (c). The interface in (c) was found to be the most stable. (d) The electronic localisation function (η) at the interface region in (c). (e) The η line profile of the Ce–S bond at the CeO2@WS2 interfaces. (f) The partial density of states of the interface shown at (c).
Further examination of the most stable interface of configuration C in Fig. 3c-ii shows that the interface separation between CeO2 and WS2 is 2.74 Å, and the Ce–S bond is 2.93 Å long. This bond length is very similar to the Ce–S bond length in CeS, which is 2.89 Å [57]. Furthermore, the electronic localisation function (η) along the Ce–S bond (Fig. 3d, e) indicates two peaks around both Ce and S centres. This η profile deviates from a perfect ionic bond where η approaches 1 near the anion while approaching 0 near the cation [58]. This profile, however, is not perfectly covalent either. In covalent bonding, η is maximum at the bond centre and tapers off at both ends [58]. Consequently, we conclude that the bonding nature at the CeO2@WS2 interface is only partially ionic (or partially covalent).
The partial density of states of the interfacing layers in Fig. 3f shows that the Ce 4f states are all empty and located above the Fermi level (marked with a green circle). However, some of the Ce 5d states are still occupied and located below the Fermi level (marked with a cyan arrow), explaining the less than + 4 oxidation state indicated by Bader charge analysis (Fig. 3c-ii). Similarly, some of W’s 5d states are also below the Fermi level, as marked by a blue arrow, indicating a deviation from the pure ionic + 4 oxidation state. This deviation is probably caused by the covalency between S and W. Partial oxidation of W and S, in turn, raises the Fermi level to the bottom of the conduction band, intercepting W’s 5d states and creating n-type carriers, facilitating the observed photocatalytic activity.
3.4. Photocatalytic experiments
We studied the impact of the heterostructure in the WS2 intercalated CeO2 layered materials for the photocatalytic formic acid decomposition. When the heterostructure of two or more different structures is constructive, the photocatalytic activity boosts due to their longer electron/hole lifetime and efficient charge separation. Consequently, the photocatalytic activity improves. The oxidation of formic acid into CO2 under photocatalytic conditions is a benchmark reaction that can be useful to judge the photocatalytic capability of the semiconductors that are supposed to be photocatalysts. This photocatalytic test is also suitable for comparing the photocatalysts under identical conditions.
The photocatalytic activity of CeO2@WS2 was investigated under UV–vis range irradiation. The formic acid oxidation power of the CeO2@WS2 was first investigated in the absence and then the presence of light (Fig. 4a). We observed that, within 90 min, the dispersion of CeO2@WS2 results in 6.7 µmol (or 446.7 µmol g-1) CO2 under UV–vis irradiation and 2.2 µmol (146.7 µmol g-1) CO2 in the dark, three fold higher CO2 higher evolution rate originating from light irradiation. After 30 min, when there was no light irradiation, the reaction has minor progress and almost stops while in the presence of light; CO2 continued to evolve, further proving that CeO2@WS2 is photo-catalytically active under UV–vis range irradiation. In the next step, we tried to understand the photoactivity of CeO2@WS2 under visible light irradiation. Formic acid photooxidation under the visible range is almost similar to dark conditions (Fig. 4a). This observation indicates that the photocatalytic activity is minimal under the visible range. Therefore, we can claim that the photocatalytic activity of CeO2@WS2 majorly originates from the UV range irradiation.
Fig. 4.
(a) Time-course formic acid decomposition in the presence and absence of light through the catalysis of CeO2@WS2 (15 wt%). (b) Comparing the photocatalytic activity of the CeO2@WS2 (15 wt%) with CeO2 and WS2 nanosheets. (c) Photocatalytic activity of the CeO2@WS2 with three different amounts of WS2 loading, including 5, 10, and 15 wt%. All photocatalytic tests have been performed under the UV–vis range in the aqueous solution of formic acid (5 vol%) in the presence of a catalyst (15 mg) at room temperature.
Lastly, we compared the photocatalytic activity of CeO2@WS2 with CeO2 and WS2 on their own (Fig. 4b). We also studied the effect of different amounts of CeO2 loading on WS2 (Fig. 4c). We realised that CeO2 loaded on WS2 (15 wt%) causes more photocatalytic activity than the 5 wt% and 10 wt% loading amounts. Accordingly, the CO2 production rates for CeO2 and WS2 were 2.9 µmol and 3.5 µmol in 90 min, respectively, which are significantly lower than the ceria-loaded WS2. At last, the produced CO2 in this work is comparable to the previous reports [59], [60], [61], e.g., anatase TiO2, layered titanate, protonated layered titanate, and 2D layered titanate-based photocatalysts [59], [61]. In another example [60], a newly developed titania-based catalyst (namely, Ti@PMO-Bipy) reports the production of 5.5 µmol CO2 in 90 min under optimal conditions while our developed catalyst produces a greater value (6.7 µmol) of CO2 within 90 min.
4. Conclusions
WS2, once exfoliated through ultrasonic waves, could efficiently form chemical bonds with CeO2 during the hydrothermal synthesis, creating a promising heterostructure. The chemical bonding between Ce and S at the heterojunction was determined by Raman spectroscopy and XRD. Our density functional calculations could identify the most stable CeO2/WS2 interface configuration, further confirming the covalence bonding at the interface. Our simulation predicted a 2.74 Å interface separation between phases with a 2.93 Å Ce–S bond length. The density of states at the interface showed the formation of n-type carriers, matching with that of Mott-Schottky plot. This ultrasonically-synthesised CeO2@WS2 heterostructure emerged as a superior photocatalyst compared to WS2, CeO2, and their physical mixture. Using the Mott-Schottky plots and comparing the Egs of the heterostructure with the pure phases through the Tauc plots, we showed that the CeO2@WS2 heterojunction was type (II). We speculate that several other transition metal oxides can be synthesised in the interlayers of WS2, further building on the work presented here.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the University of Tabriz and the National Institute for Materials Science (NIMS) for their support. Especially, the authors gratefully appreciated the central laboratory of University of Tabriz for all its support. Also, E.D. acknowledges the TÜBITAK and Horizon-2020 Marie Skłodowska Curie for providing financial support in Co-Funded Brain Circulation Program (Project No. 120C057) framework.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106245.
Contributor Information
Esmail Doustkhah, Email: edoustkhahheragh@ku.edu.tr.
Alireza Khataee, Email: a_khataee@tabrizu.ac.ir.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Arcudi F., Ðorđević L., Schweitzer N., Stupp S.I., Weiss E.A. Selective visible-light photocatalysis of acetylene to ethylene using a cobalt molecular catalyst and water as a proton source. Nat. Chem. 2022;14(9):1007–1012. doi: 10.1038/s41557-022-00966-5. [DOI] [PubMed] [Google Scholar]
- 2.Yoon T.P., Ischay M.A., Du J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010;2(7):527–532. doi: 10.1038/nchem.687. [DOI] [PubMed] [Google Scholar]
- 3.Hassandoost R., Pouran S.R., Khataee A., Orooji Y., Joo S.W. Hierarchically structured ternary heterojunctions based on Ce3+/ Ce4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019;376:200–211. doi: 10.1016/j.jhazmat.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Giannakoudakis D.A., Chatel G., Colmenares J.C. Mechanochemical Forces as a Synthetic Tool for Zero- and One-Dimensional Titanium Oxide-Based Nano-photocatalysts. Top. Curr. Chem. 2019;378:2. doi: 10.1007/s41061-019-0262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J., Wang D., Han H., Li C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013;46(8):1900–1909. doi: 10.1021/ar300227e. [DOI] [PubMed] [Google Scholar]
- 6.Chen L., Tang J., Song L.-N., Chen P., He J., Au C.-T., Yin S.-F. Heterogeneous photocatalysis for selective oxidation of alcohols and hydrocarbons. Appl. Catal. B. 2019;242:379–388. [Google Scholar]
- 7.Yuan L., Weng B., Colmenares J.C., Sun Y., Xu Y.J. Multichannel charge transfer and mechanistic insight in metal decorated 2D–2D Bi2WO6–TiO2 cascade with enhanced photocatalytic performance. Small. 2017;13:1702253. doi: 10.1002/smll.201702253. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z., Wang B., Chu D., Cazorla C. First-principles high-throughput screening of bulk piezo-photocatalytic materials for sunlight-driven hydrogen production. J. Mater. Chem. A. 2022;10:18132–18146. [Google Scholar]
- 9.Shao B., Wang J., Liu Z., Zeng G., Tang L., Liang Q., He Q., Wu T., Liu Y., Yuan X. Ti3C2Tx MXene decorated black phosphorus nanosheets with improved visible-light photocatalytic activity: experimental and theoretical studies. J. Mater. Chem. A. 2020;8(10):5171–5185. [Google Scholar]
- 10.He J., Gao P., Ling Z., Ding L., Yang Z., Ye J., Cui Y. High-efficiency silicon/organic heterojunction solar cells with improved junction quality and interface passivation. ACS Nano. 2016;10:11525–11531. doi: 10.1021/acsnano.6b07511. [DOI] [PubMed] [Google Scholar]
- 11.Shi L.i., Li Z., Ju L., Carrasco-Pena A., Orlovskaya N., Zhou H., Yang Y. Promoting nitrogen photofixation over a periodic WS2@TiO2 nanoporous film. J. Mater. Chem. A. 2020;8(3):1059–1065. [Google Scholar]
- 12.Kośmider K., Fernández-Rossier J. Electronic properties of the MoS2-WS2 heterojunction. Phys. Rev. B. 2013;87 [Google Scholar]
- 13.Browning R., Plachinda P., Padigi P., Solanki R., Rouvimov S. Growth of multiple WS2/SnS layered semiconductor heterojunctions. Nanoscale. 2016;8(4):2143–2148. doi: 10.1039/c5nr08006a. [DOI] [PubMed] [Google Scholar]
- 14.Luo H., Shi J., Liu C., Chen X., Lv W., Zhou Y., Zeng M., Yang J., Wei H., Zhou Z., Su Y., Hu N., Yang Z. Design of p–p heterojunctions based on CuO decorated WS2 nanosheets for sensitive NH3 gas sensing at room temperature. Nanotechnology. 2021;32(44):445502. doi: 10.1088/1361-6528/ac1800. [DOI] [PubMed] [Google Scholar]
- 15.Yousef Tizhoosh N., Khataee A., Hassandoost R., Darvishi Cheshmeh Soltani R., Doustkhah E. Ultrasound-engineered synthesis of WS2@CeO2 heterostructure for sonocatalytic degradation of tylosin. Ultrason. Sonochem. 2020;67:105114. doi: 10.1016/j.ultsonch.2020.105114. [DOI] [PubMed] [Google Scholar]
- 16.Zeng J., Xu L., Luo X., Peng B., Ma Z., Wang L.-L., Yang Y., Shuai C. A novel design of SiH/CeO2(111) van der Waals type-II heterojunction for water splitting. PCCP. 2021;23:2812–2818. doi: 10.1039/d0cp05238h. [DOI] [PubMed] [Google Scholar]
- 17.Yuan K., Wang C.-Y., Zhu L.-Y., Cao Q., Yang J.-H., Li X.-X., Huang W., Wang Y.-Y., Lu H.-L., Zhang D.W. Fabrication of a micro-electromechanical system-based acetone gas sensor using CeO2 nanodot-decorated WO3 nanowires. ACS Appl. Mater. Interfaces. 2020;12:14095–14104. doi: 10.1021/acsami.9b18863. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X., Mofarah S.S., Cazorla C., Daiyan R., Esmailpour A.A., Scott J., Yao Y., Lim S., Wong V., Chen E.Y. Decoupling the Impacts of Engineering Defects and Band Gap Alignment Mechanism on the Catalytic Performance of Holey 2D CeO2−x-Based Heterojunctions. Adv. Funct. Mater. 2021;31:2103171. [Google Scholar]
- 19.Han J.H., Lee S., Cheon J. Synthesis and structural transformations of colloidal 2D layered metal chalcogenide nanocrystals. Chem. Soc. Rev. 2013;42:2581–2591. doi: 10.1039/c2cs35386e. [DOI] [PubMed] [Google Scholar]
- 20.Doustkhah E., Hassandoost R., Khataee A., Luque R., Assadi M.H.N. Hard-templated metal–organic frameworks for advanced applications. Chem. Soc. Rev. 2021;50:2927–2953. doi: 10.1039/c9cs00813f. [DOI] [PubMed] [Google Scholar]
- 21.Chatel G., Colmenares J.C. Sonochemistry: from Basic Principles to Innovative Applications. Top. Curr. Chem. 2017;375:8. doi: 10.1007/s41061-016-0096-1. [DOI] [PubMed] [Google Scholar]
- 22.Liu X., Jia Q., Fu Y., Zheng T. Exfoliation of metal-organic framework nanosheets using surface acoustic waves. Ultrason. Sonochem. 2022;83 doi: 10.1016/j.ultsonch.2022.105943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arefi-Oskoui S., Khataee A., Safarpour M., Orooji Y., Vatanpour V. A review on the applications of ultrasonic technology in membrane bioreactors. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104633. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W., Zhang X. The effect of ultrasound on synthesis and energy storage mechanism of Ti3C2Tx MXene. Ultrason. Sonochem. 2022;89 doi: 10.1016/j.ultsonch.2022.106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassandoost R., Kotb A., Movafagh Z., Esmat M., Guegan R., Endo S., Jevasuwan W., Fukata N., Sugahara Y., Khataee A., Yamauchi Y., Ide Y., Doustkhah E. Nanoarchitecturing bimetallic manganese cobaltite spinels for sonocatalytic degradation of oxytetracycline. Chem. Eng. J. 2022;431 [Google Scholar]
- 26.Kreizman R., Hong S., Sloan J., Popovitz-Biro R., Albu-Yaron A., Tobias G., Ballesteros B., Davis B., Green M.H., Tenne R. Core-Shell PbI2@WS2 Inorganic Nanotubes from Capillary Wetting. Angew. Chem. Int. Ed. 2009;48(7):1230–1233. doi: 10.1002/anie.200803447. [DOI] [PubMed] [Google Scholar]
- 27.Li P., Zhao X., Jia C.-J., Sun H., Sun L., Cheng X., Liu L., Fan W. ZnWO4/BiOI heterostructures with highly efficient visible light photocatalytic activity: the case of interface lattice and energy level match. J. Mater. Chem. A. 2013;1:3421–3429. [Google Scholar]
- 28.He Z., Kim C., Lin L., Jeon T.H., Lin S., Wang X., Choi W. Formation of heterostructures via direct growth CN on h-BN porous nanosheets for metal-free photocatalysis. Nano Energy. 2017;42:58–68. [Google Scholar]
- 29.Kresse G., Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54(16):11169–11186. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 30.Kresse G., Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996;6(1):15–50. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 31.Blöchl P.E. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953–17979. doi: 10.1103/physrevb.50.17953. [DOI] [PubMed] [Google Scholar]
- 32.Perdew J.P., Burke K., Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 33.Perdew J.P., Burke K., Ernzerhof M. Generalized Gradient Approximation Made Simple [Phys. Rev. Lett. 77, 3865 (1996)] Phys. Rev. Lett. 1997;78 doi: 10.1103/PhysRevLett.77.3865. 1396-1396. [DOI] [PubMed] [Google Scholar]
- 34.Dudarev S.L., Botton G.A., Savrasov S.Y., Humphreys C.J., Sutton A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B. 1998;57:1505–1509. [Google Scholar]
- 35.Fronzi M., Assadi M.H.N., Hanaor D.A.H. Theoretical insights into the hydrophobicity of low index CeO2 surfaces. Appl. Surf. Sci. 2019;478:68–74. [Google Scholar]
- 36.Bui V.Q., Pham T.-T., Le D.A., Thi C.M., Le H.M. A first-principles investigation of various gas (CO, H2O, NO, and O2) absorptions on a WS2 monolayer: stability and electronic properties. J. Phys. Condens. Matter. 2015;27 doi: 10.1088/0953-8984/27/30/305005. [DOI] [PubMed] [Google Scholar]
- 37.Grimme S., Antony J., Ehrlich S., Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010;132 doi: 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- 38.Tang W., Sanville E., Henkelman G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter. 2009;21(8):084204. doi: 10.1088/0953-8984/21/8/084204. [DOI] [PubMed] [Google Scholar]
- 39.J. Rodriguez-Carvajal, FULLPROF: a program for Rietveld refinement and pattern matching analysis, Satellite meeting on powder diffraction of the XV congress of the IUCr, (IUCr) International Union of Crystallography, Toulouse, France, 1990.
- 40.Putz H., Brandenburg K. Brandenburg GbR; Bonn, Germany: 2022. Match! - Phase Analysis using Powder Diffraction. [Google Scholar]
- 41.Zahid M., Yasmin N., Ashiq M.N., Safdar M., Mirza M. Fabrication and performance evaluation of cerium sesquisulfide/graphene oxide composite for electrochemical application with high power density and energy density. Phys. B Condens. Matter. 2022;624:413359. [Google Scholar]
- 42.Rout C.S., Joshi P.D., Kashid R.V., Joag D.S., More M.A., Simbeck A.J., Washington M., Nayak S.K., Late D.J. Superior Field Emission Properties of Layered WS2-RGO Nanocomposites. Sci. Rep. 2013;3:3282. doi: 10.1038/srep03282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang N., Qian W., Chu W., Wei F. Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming. Catal. Sci. Technol. 2016;6(10):3594–3605. [Google Scholar]
- 44.Gunawan C., Lord M.S., Lovell E., Wong R.J., Jung M.S., Oscar D., Mann R., Amal R. Oxygen-Vacancy Engineering of Cerium-Oxide Nanoparticles for Antioxidant Activity. ACS Omega. 2019;4(5):9473–9479. doi: 10.1021/acsomega.9b00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang C., Li Q., Xia Y., Lv K., Li M. Enhanced visible-light photocatalytic CO2 reduction performance of Znln2S4 microspheres by using CeO2 as cocatalyst. Appl. Surf. Sci. 2019;464:388–395. [Google Scholar]
- 46.Wang L., Meng F., Li K., Lu F. Characterization and optical properties of pole-like nano-CeO2 synthesized by a facile hydrothermal method. Appl. Surf. Sci. 2013;286:269–274. [Google Scholar]
- 47.Aslam M., Qamar M.T., Soomro M.T., Ismail I.M.I., Salah N., Almeelbi T., Gondal M.A., Hameed A. The effect of sunlight induced surface defects on the photocatalytic activity of nanosized CeO2 for the degradation of phenol and its derivatives. Appl. Catal. B. 2016;180:391–402. [Google Scholar]
- 48.Vangelista S., Piagge R., Ek S., Sarnet T., Ghidini G., Martella C., Lamperti A. Structural, chemical and optical properties of cerium dioxide film prepared by atomic layer deposition on TiN and Si substrates. Thin Solid Films. 2017;636:78–84. [Google Scholar]
- 49.Guo S., Arwin H., Jacobsen S.N., Järrendahl K., Helmersson U. A spectroscopic ellipsometry study of cerium dioxide thin films grown on sapphire by rf magnetron sputtering. J. Appl. Phys. 1995;77:5369–5376. [Google Scholar]
- 50.Kariper İ.A. Synthesis and characterization of cerium sulfide thin film. Prog. Nat. Sci.: Mater. Int. 2014;24:663–670. [Google Scholar]
- 51.Li Y., Jin Z., Zhao T. Performance of ZIF-67 – derived fold polyhedrons for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2020;382 [Google Scholar]
- 52.Yang X., Guo W., Shen Q. Formation of disinfection byproducts from chlor(am)ination of algal organic matter. J. Hazard. Mater. 2011;197:378–388. doi: 10.1016/j.jhazmat.2011.09.098. [DOI] [PubMed] [Google Scholar]
- 53.Kang Y., Mao Z., Wang Y., Pan C., Ou M., Zhang H., Zeng W., Ji X. Design of a two-dimensional interplanar heterojunction for catalytic cancer therapy. Nat. Commun. 2022;13:2425. doi: 10.1038/s41467-022-30166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong K.T., Kim S.C., Yun K., Choong C.E., Nah I.W., Jeon B.-H., Yoon Y., Jang M. Understanding the potential band position and e–/h+ separation lifetime for Z-scheme and type-II heterojunction mechanisms for effective micropollutant mineralization: Comparative experimental and DFT studies. Appl. Catal. B. 2020;273:119034. [Google Scholar]
- 55.Jain A., Ong S.P., Hautier G., Chen W., Richards W.D., Dacek S., Cholia S., Gunter D., Skinner D., Ceder G., Persson K.A. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013;1 [Google Scholar]
- 56.Mofarah S.S., Adabifiroozjaei E., Pardehkhorram R., Assadi M.H.N., Hinterstein M., Yao Y., Liu X., Ghasemian M.B., Kalantar‐Zadeh K., Mehmood R., Cazorla C., Shahmiri R., Bahmanrokh G., Bhattacharyya S., Spadaro M.C., Arbiol J., Lim S., Xu Y., Arandiyan H., Scott J., Koshy P., Sorrell C.C. Coordination Polymer to Atomically Thin, Holey, Metal-Oxide Nanosheets for Tuning Band Alignment. Adv. Mater. 2019;31(52):1905288. doi: 10.1002/adma.201905288. [DOI] [PubMed] [Google Scholar]
- 57.Wyckoff R.W. Interscience Publishers; New York: 1963. Crystal structures. [Google Scholar]
- 58.Savin A., Silvi B., Colonna F. Topological analysis of the electron localization function applied to delocalized bonds. Can. J. Chem. 1996;74(6):1088–1096. [Google Scholar]
- 59.Mani D., Tahawy R., Doustkhah E., Shanmugam M., Arivanandhan M., Jayavel R., Ide Y. A rutile TiO2 nanobundle as a precursor of an efficient visible-light photocatalyst embedded with Fe2O3. Inorg. Chem. Front. 2021;8(19):4423–4430. [Google Scholar]
- 60.Ahadi A., Alamgholiloo H., Rostamnia S., Liu X., Shokouhimehr M., Alonso D.A., Luque R. Layer-Wise Titania Growth Within Dimeric Organic Functional Group Viologen Periodic Mesoporous Organosilica as Efficient Photocatalyst for Oxidative Formic Acid Decomposition. ChemCatChem. 2019;11(19):4803–4809. [Google Scholar]
- 61.Esmat M., Farghali A.A., El-Dek S.I., Khedr M.H., Yamauchi Y., Bando Y., Fukata N., Ide Y. Conversion of a 2D Lepidocrocite-Type Layered Titanate into Its 1D Nanowire Form with Enhancement of Cation Exchange and Photocatalytic Performance. Inorg. Chem. 2019;58:7989–7996. doi: 10.1021/acs.inorgchem.9b00722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.