Abstract
The purpose of the present study was to determine the immunologic responses, particularly immunopathologic reactions, associated with nasal immunization with the mucosal adjuvant, cholera toxin (CT). BALB/c mice were nasally immunized with tetanus toxoid (TT) combined with CT, and the responses of these mice were determined. After nasal immunization, mice produce a serum antibody response, primarily of the immunoglobulin G (IgG) isotype of predominantly IgG1 subclass, against both TT and CT. Along with the antibody responses, we also found that inflammatory reactions, which could be potentially fatal, developed within the lung. Furthermore, IgE responses were also induced after nasal immunization, and these responses were associated with the detection of interleukin 5 in the serum. Thus, nasal immunization with TT plus CT likely results in the activation of Th2 cells, which may contribute to serious immunopathologic reactions in the lung.
Mucosal immunity constitutes the first line of defense for the host and is a major component of resistance against respiratory infections. The importance of mucosal immunity, specifically secretory immunoglobulin A (S-IgA), in controlling bacterial respiratory infections is exemplified in patients with selective IgA deficiencies. These patients are more prone to respiratory tract infections, including rhinosinusitis, otitis media, tonsillitis, chronic pulmonary infections, and infectious asthma (3–5, 25). Among the effector mechanisms of mucosal immunity in bacterial disease, IgA can inhibit adherence or growth of pathogenic bacteria (14, 15, 17, 34). The importance of mucosal immunity, e.g., IgA, in resistance to respiratory disease is probably best demonstrated for viral infections (7, 8, 26, 27). However, parenteral administration of vaccine does not significantly promote immune responses within the upper respiratory tract, despite development of significant serum antibody responses (6). Circulating antibody, while effective against lower respiratory tract infections, does not play a significant role in protecting the upper respiratory tract (18, 30). However, systemic immunization is the route used for the current Streptococcus pneumoniae and influenza vaccines, and results from our laboratory clearly demonstrate that IgA responses in the upper respiratory tract are not readily produced after systemic immunization (L. Hodge, M. Marinaro, H. Jones, J. R. McGhee, H. Kiyono, and J. W. Simecka, unpublished data). Therefore, generation of mucosal immunity is an obvious area in which notable improvement in vaccination against respiratory pathogens can be made.
Nasal immunization is anticipated to be an optimal route of administration of vaccines against respiratory tract infections. Although oral immunization is an attractive approach to induce mucosal immunity, it has had variable success in protection against upper respiratory tract viral infections. For example, secondary nasal immunization subsequent to primary oral immunization is required for effective protection against viral respiratory disease (19). Several studies in animals and patients demonstrated that vaccination by direct inoculation of the respiratory tract can be effective (22, 28, 37). There also appears to be a significant protective advantage to the nasal route of immunization. Upper respiratory tract infection with the influenza virus was prevented in mice nasally immunized with inactive influenza virus (23). In contrast, there was no noticeable protection after systemic immunization, as viral titers in samples recovered from nasal passages were equivalent for naive (unimmunized) and subcutaneously immunized mice. Another advantage of nasal immunization is the potential generation of cross-protection between related serotypes of respiratory pathogens. Mice previously infected with an aerosol of one strain of influenza virus (e.g., H3N1) were resistant to infection with a different, but cross-reactive, influenza virus (e.g., H3N2) (32, 33). In contrast, systemic immunization with live or inactive virus did not provide protection from the cross-reactive influenza virus. A similar cross-protection between different serotypes or strains of pathogenic bacteria is also likely to be facilitated by the generation of mucosal immune responses. Thus, the nasal route of immunization has clear advantages over systemic routes in protecting the upper respiratory tract from infection, including those caused by cross-reactive pathogens. Importantly, the results obtained by nasal immunization with the cold-adapted influenza virus vaccine (1, 13) establish the feasibility and effectiveness of this route of vaccination in humans.
Immune responses, however, are not readily induced by antigen alone, and to produce an effective immune response against respiratory pathogens at mucosal surfaces, intranasal immunization requires a safe and potent adjuvant. Cholera toxin (CT), an exotoxin of Vibrio cholerae, is the most common adjuvant for intranasal immunization. When intranasally coadministered with an antigen, there is a significant enhancement in both mucosal and systemic immune responses (19, 28, 37). Although CT seems to be an ideal adjuvant for mucosal immunization, oral immunization with CT as a mucosal adjuvant results in IgE responses and hypersensitivity (20, 31). These responses result from the ability of CT to preferentially induce Th2 responses which, through the action of interleukin 4 (IL-4), contributes to development of IgE responses (10). Thus, there is the potential that intranasal immunization with CT can also induce IgE-associated reactions within the respiratory tract.
The purpose of the present study was to determine the extent of immunologic responses, particularly potential immunopathologic reactions, associated with intranasal immunization with the mucosal adjuvant CT. For this study, BALB/c mice were intranasally immunized with tetanus toxoid (TT) combined with CT, and the responses of these mice were determined. For immunization, TT was chosen since past studies by members of our laboratory (16, 20) have described the immunologic reactions after oral immunization. After intranasal immunization, mice produced a serum antibody response against both TT and CT, primarily antibodies of the IgG1 subclass. Although intranasal immunization did induce a good immune response, we found that inflammatory reactions which can be potentially fatal also developed within the lung. Furthermore, IgE responses were also induced after intranasal immunization, and these responses were associated with the detection of IL-5 in the serum. Thus, intranasal immunization with TT plus CT probably results in the activation of Th2 cells, which may contribute to serious immunopathologic reactions.
MATERIALS AND METHODS
Animals.
Specific pathogen-free, female BALB/c mice were obtained from the Frederick Cancer Research Facility (National Cancer Institute, Frederick, Md.). F344 rats were from breeding colonies at University of Alabama at Birmingham. Both groups of animals were from colonies which were specific pathogen free, as determined by serologic and cultural tests for rodent viral and bacterial pathogens. Mice were maintained in horizontal laminar flow cabinets and provided with sterile food and water ad libitum during the experiment. All mice and rats were used between 8 to 12 weeks of age. Prior to experimental manipulation, mice were anesthetized with an intramuscular injection of ketamine-xylazine. For intranasal immunization, mice were allowed to inhale 20 μl of inoculum, which was placed upon the nares. If a volume greater than 20 μl was needed, mice were given multiple inocula (20 μl each), with a 5-min rest between each inoculation. Collection of the serum samples was done by retroorbital bleeding.
Immunogens used.
CT was purchased from List Biological Laboratories, Inc. (Campbell, Calif.). Patricia J. Freda Pietrobon (Connaught Laboratories, Inc., Swiftwater, Pa.) kindly provided TT.
Histopathology.
For the collection of tissues for histologic examination, anesthetized mice were sacrificed by exsanguination by laceration of the femoral artery. The trachea and lungs were removed intact. The lungs were gently inflated with buffered formalin by using a 3-ml syringe with a 20-gauge needle. The lungs were subsequently fixed in buffered formalin, and individual lung lobes were processed for paraffin embedding, sectioning, and hematoxylin and eosin staining. Each lung lobe was examined for histopathologic changes by light microscopy.
TT- and CT-specific antibody ELISA.
TT- and CT-specific antibody titers in sera were determined by enzyme-linked immunosorbent assay (ELISA), as previously described (16). Briefly, Falcon Microtest III assay plates (Becton Dickinson, Oxnard, Calif.) were coated with optimal concentrations of TT (100 μl at 5 μg/ml) or the B subunit of CT (CT-B) (100 μl at 5 μg/ml) in phosphate-buffered saline (PBS). After overnight incubation at 4°C, the plates were washed three times with PBS–0.05% Tween 20 and blocked with PBS–0.05% Tween 20 supplemented with 10% goat serum (Life Technologies, Gibco BRL, Gaithersburg, Md.) for 2 h at room temperature. Serum samples were serially diluted with PBS–0.05% Tween 20–10% goat serum, and 100 μl was placed in duplicate into wells of the antigen-coated plates. After overnight incubation at 4°C, the plates were washed four times with PBS–0.05% Tween 20. The secondary antibodies (biotinylated anti-mouse IgM, IgG, or IgA stock reagents at 0.5 mg/ml; Southern Biotechnology Associates, Birmingham, Ala.) were diluted to 1:4,000 in PBS–0.05% Tween 20–10% goat serum, and 100 μl was added to the appropriate wells. After a 5-h incubation at room temperature, the plates were again washed four times with PBS–0.05% Tween 20, and a 1:2,000 dilution in PBS–0.05% Tween 20–10% goat serum of peroxidase-conjugated streptavidin (Neutravidin; Southern Biotechnology Associates) was added to the wells (100 μl). The plates were incubated at room temperature for 2 h, and the plates were washed twice with PBS–0.05% Tween 20 and twice with PBS. The reactions were developed at room temperature by the addition of 100 μl of 1.1 mM ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) in 0.1 M citrate-phosphate buffer, pH 4.2, containing 0.01% H2O2] in each of the wells. Endpoint titers were expressed as the reciprocal dilution of the last dilution which yielded an optical density at 415 nm of more than 0.1 U above the optical density of the negative controls after a 20-min incubation.
For IgG subclasses, plates were washed three times with PBS and blocked with PBS–10% goat serum–1% bovine serum albumin. The plates were washed three times with PBS–0.05% Tween 20, and serial dilutions of serum starting at 1:64 in blocking buffer were added in duplicate. After 2 h of incubation at 37°C, plates were washed and incubated for 1 h at 37°C with biotinylated monoclonal subclass-specific antibodies (anti-IgG1 [02232D clone A85-1, 1 μg/ml], anti-IgG2a [02012D clone R19-15, 1 μg/ml], anti-IgG2b [02032D clone R-12-3, 0.5 μg/ml], and anti-IgG3 [02062D clone 4082, 1 μg/ml]; PharMingen, San Diego, Calif.). The reaction products were visualized as described above.
Determination of total serum IgE.
Total serum IgE was determined by an ELISA. Plates were coated with capture anti-IgE monoclonal antibody (PharMingen) at 2 μg/ml. After blocking with 10% rat serum in PBS, serial dilutions of murine IgE standard (PharMingen) or serum samples in 3% bovine serum albumin in PBS were added to the wells in duplicate. After overnight incubation at 4°C, biotinylated anti-IgE monoclonal antibody (PharMingen) was added to the wells at a concentration of 4 μg/ml and incubated for 4 h at room temperature. The reaction product was visualized with neutralite avidin-horseradish peroxidase (Southern Biotechnology Associates), followed by addition of substrate 3,3′,5,5′-tetramethylbenzidine (Moss, Inc., Pasadena, Md.). The absorbance readings (450 nm) obtained from the individual serum samples were converted to micrograms of IgE per milliliter by reference to a standard curve produced with dilutions of a standard preparation of murine IgE for each assay. The detection limits for these assays were 125 ng/ml.
PCA assay.
The passive cutaneous anaphylaxis (PCA) assay was used to compare the levels of TT-specific reagenic antibody in serum samples (20). Sera were serially diluted with PBS. A volume of 0.1 ml of each diluted sample was injected intradermally in the back of ether-anesthetized rats. Amounts of 1 ml of 1% Evan's blue and 200 μg of TT were injected intravenously the next day. After 10 min, the highest dilution of sera with a positive (blue) reaction was considered the PCA titer.
Cytokine detection by ELISA.
Cytokine levels in sera were determined by cytokine-specific ELISAs. Anti-murine IL-4, IL-5, and gamma interferon (IFN-γ) capture antibodies, biotinylated anti-cytokine antibodies, and recombinant cytokines, for use as standards, were purchased from PharMingen. The ELISA was performed on serum samples according to manufacturer's recommendations. Diluted (1:3) serum samples were placed in wells coated with anti-IL-4 (11B11), -IL-5 (TRFK5), or -IFN-γ (XMG1.2) capture antibody and incubated overnight at 4°C. The reaction products were visualized with biotinylated anti-IL-4 (BVD6-24G2), -IL-5 (TRFK4), or -IFN-γ (R4-6A2) antibody and neutralite avidin-horseradish peroxidase (Southern Biotechnology Associates), followed by the addition of substrate 3,3′,5,5′-tetramethylbenzidine (Moss, Inc.). The absorbance readings (450 nm) obtained from the individual serum samples were converted to amounts of cytokine by reference to a standard curve produced with dilutions of recombinant murine cytokine for each assay. The detection limits for IL-4, IL-5, and IFN-γ were 15 pg/ml, 1.2 U/ml, and 9.3 U/ml, respectively.
Statistical analysis.
Statistical analysis was performed with the SYSTAT program (Systat, Inc., Evanston, Ill.). Antibody titers were transformed to common logarithms prior to statistical analysis. The data were analyzed by analysis of variance, followed by post-hoc tests for multigroup comparisons, as needed. The data were also analyzed by Student's t test or an unpaired Mann-Whitney U test. A probability (P) of 0.05 or less was accepted as significant.
RESULTS
Intranasal immunization results in pathologic changes.
To examine the development of host responses after intranasal immunization with TT and CT as the adjuvant, anesthetized mice were intranasally given 250 μg of TT combined with 10 μg of CT. These doses were previously shown to be effective for oral immunization (16). Seven days later, mice were intranasally immunized a second time with the same doses of TT and CT.
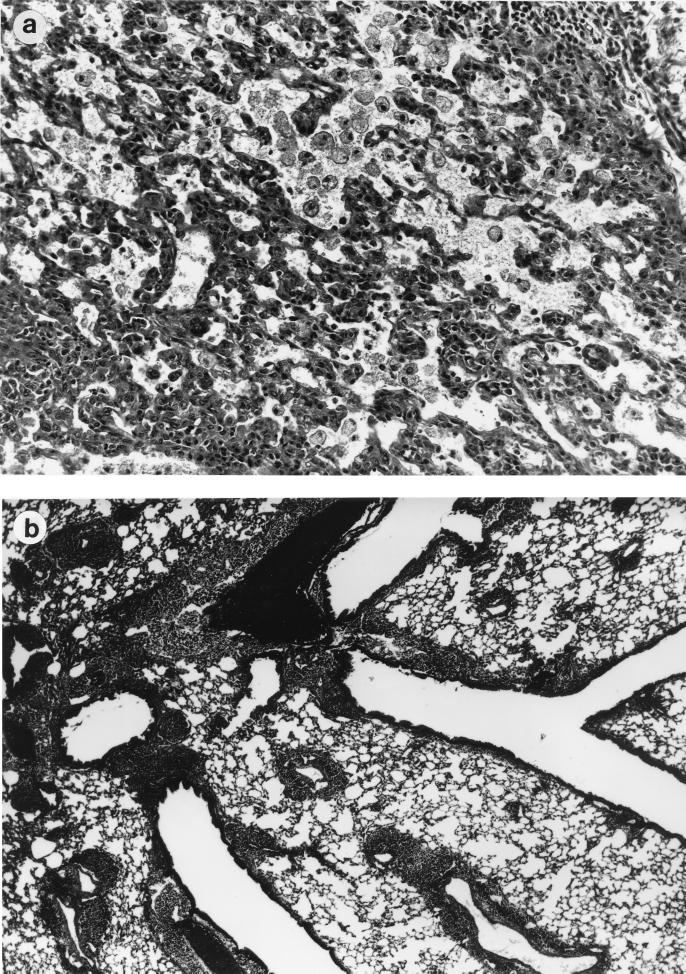
Intranasal immunization of mice with these high doses of TT and CT resulted in potentially fatal, pathologic changes in the lungs. In the initial experiment, three out of five animals died within 10 min of the second intranasal immunization with a full dose of antigen (250 μg of TT plus 10 μg of CT), even though the mice seemed to tolerate the first immunization well. By gross examination, lungs of the dead animals did not deflate even after removal from the chest cavity. Histologic examination of the lungs of the animals that died revealed the presence of edema and an increase in the number of macrophages in the alveoli (Fig. 1). Furthermore, there was evidence of an infiltration of mononuclear cells into the submucosa of the pulmonary airways.
FIG. 1.
Histopathologic changes in lungs of mice intranasally immunized with TT plus CT. BALB/c mice were intranasally immunized with TT in combination with CT on days 0 and 7. (a) The alveoli of mice, who died after receiving a high dosage of TT (250 μg) and CT (10 μg) for the second immunization, contained large numbers of macrophages and edema (magnification, 360×). Also, thickening of the alveolar walls was evident. (b) In mice receiving one-third of the primary dose for their second inoculation, there was a massive infiltration of cells around the airways and blood vessels (magnification, 36×). The infiltration also involved alveoli surrounding the vessels and airways.
In subsequent experiments, mice were given one-third of the primary dose of TT plus CT for their secondary immunization (83.3 μg of TT plus 3.33 μg of CT). Although mice given the lower secondary dose of TT plus CT survived, there were obvious clinical signs (e.g., lack of activity, ruffled fur, and wasting) in mice after immunization. Three days after the secondary immunization, the lungs of the mice were removed and inspected. As described above, the lungs did not deflate as did those from naive mice or mice given TT alone. By histologic examination, there was a dramatic infiltration of mononuclear cells around every airway and blood vessel in lungs of mice immunized with TT plus CT (Fig. 1). Similar histopathologic reactions were found in lungs of mice given CT alone.
Serum antibody responses after intranasal immunization with TT plus CT.
Antigen-specific serum antibody responses were evaluated in mice intranasally immunized with TT plus CT. Mice were intranasally immunized with either TT (250 μg) alone or TT plus CT (250 μg of TT plus 10 μg of CT). A group of naive mice were included as controls. Seven days later, the mice were reimmunized with a one-third dose (83.3 μg of TT plus 3.33 μg of CT [TT plus CT] or 83.3 μg of TT [TT alone]) of the initial inoculum. Three days after the secondary immunization, serum was collected from each of the mice, and titers of antibody against TT and CT in the serum samples were measured by an ELISA.
Antibody responses to TT and CT were detected in the serum of mice 3 days after the secondary immunization (10 days after primary immunization). The anti-TT-specific IgG and IgM antibody responses were present in serum samples from immunized mice (Table 1). However, only low levels of IgG against CT were present, and anti-CT-specific IgM responses were undetectable. The immune responses of the IgA isotype to either antigen were undetectable at this time point. Antibody to either antigen was not detected in serum from unimmunized (naive) mice.
TABLE 1.
Serum antibody responses in mice 3 days after secondary intranasal immunization with TT plus CT
| Pooled seruma | Antigenb | Antigen-specific serum antibody titersc
|
||
|---|---|---|---|---|
| IgM | IgG | IgA | ||
| Expt 1 | TT | 512 | 8,192 | <128 |
| CT-B | <128 | 256 | <128 | |
| Expt 2 | TT | 256 | 8,192 | <128 |
| CT-B | <128 | 128 | <128 | |
Serum from mice (n = 5) was pooled.
Antigen used in coating ELISA plates.
Antigen-specific antibody titers were determined by ELISA using endpoint titration.
Kinetics and subclass of serum antibody responses after intranasal immunization with TT and CT.
To further examine antibody responses in mice after intranasal immunization, we compared the development of serum antibody responses in mice immunized with TT alone and TT in combination with CT. Mice were immunized on days 0 (a full dose of 250 μg of TT with or without 10 μg of CT), 7 (one-third dose), and 14 (one-third dose), and serum samples were collected on days 7, 14, and 21. Serum antibody titers were determined by endpoint ELISA assays for each of the antibody isotypes.
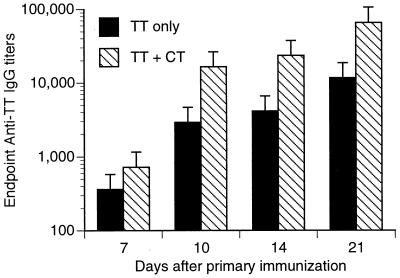
Mice immunized with TT plus CT developed greater anti-TT IgG responses than mice immunized with TT only (Fig. 2). At 10 days after immunization, serum IgG responses against TT were close to 10-fold higher than when CT was included as an adjuvant. TT-specific IgM responses were greater in mice given TT plus CT (titer of 4,096 at 10-day time point) than in mice immunized with TT alone (titer of 512 at 10-day time point). Anti-TT IgA responses were low (titer of 256) or undetectable in the sera from either group of immunized mice.
FIG. 2.
Anti-TT IgG responses after intranasal immunization. Mice were intranasally immunized with TT alone or TT in combination with CT (TT + CT) on days 0, 7, and 14. Serum samples were taken at 7, 10, 14, and 21 days after the primary immunization. Serum antibody titers were determined by a TT-specific IgG ELISA. Sera from animals (n = 9 for each time point except for day 10 [n = 3]) were pooled.
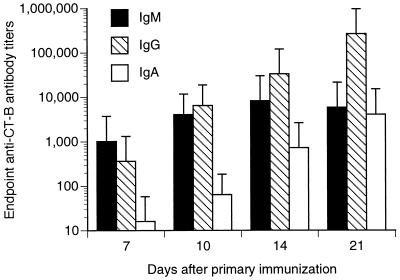
Mice immunized with TT alone had no antibody response to CT. However, mice immunized with TT plus CT developed significant anti-CT IgM antibody responses by 7 days after immunization, which plateaued by day 14 (Fig. 3). Anti-CT IgG responses were present at 7 days after infection, but continued to increase throughout the course of these experiments. Serum IgA responses against CT began to appear 14 days after primary immunization.
FIG. 3.
Anti-CT antibody responses after intranasal immunization with TT plus CT. Mice were intranasally immunized with TT in combination with CT (TT + CT) on days 0, 7, and 14. Serum samples were at 7, 10, 14, and 21 days after the primary immunization. Serum antibody titers were determined by a CT-B-specific ELISA. Sera were pooled from animals (n = 9 for each time point except for day 10 [n = 3]).
There was a qualitative difference in IgG subclass responses between mice immunized with TT alone and mice given TT plus CT (Table 2). Serum IgG1 antibody responses developed in mice immunized with TT alone, but no other IgG subclasses were detected. In contrast, mice immunized with TT plus CT produced TT-specific responses by all IgG subclasses, although IgG1 predominated. Similarly, IgG1, IgG2a, IgG2b, and IgG3 responses against CT were present in mice intranasally immunized with TT plus CT. However, it should be noted that the highest titers of TT- and CT-specific IgG1 subclass responses were noted when mice were intranasally immunized with TT in the presence of CT. Anti-CT antibody was not detected in mice immunized with TT alone.
TABLE 2.
Titers of anti-TT IgG subclass antibodies in sera from intranasally immunized micea
| Immunization | Antibody subclass | Antibody titersb
|
|
|---|---|---|---|
| Anti-TT | Anti-CT-B | ||
| TT only | IgG1 | 4,096 | <64 |
| IgG2a | <64 | <64 | |
| IgG2b | <64 | <64 | |
| IgG3 | <64 | <64 | |
| TT + CT | IgG1 | 16,384 | 8,192 |
| IgG2a | 256 | 4,096 | |
| IgG2b | 512 | 4,096 | |
| IgG3 | 256 | 512 | |
Titers of TT-specific antibodies in sera from mice intranasally immunized with TT only or TT in combination with CT (time point, day 21) were determined by ELISA using endpoint titration.
Sera from animals (n = 9) were pooled. The results are the mean titers of the groups.
Intranasal immunization with TT combined with CT results in an IgE response.
We examined the sera from control and immunized mice for the levels of total IgE and the presence of TT-specific reagenic antibody. In mice intranasally immunized with TT plus CT, the levels of total IgE in sera were higher than those found in sera from naive mice (Table 3). The average level of total IgE in two experiments was 2,200 ng of IgE/ml of serum from immunized mice. In contrast, IgE was not detected in control (unimmunized) mice. As the detection limits of these assays, the control mice had levels of IgE in serum that were less than 125 ng of IgE/ml of serum. Thus, there was a minimum of a 17-fold increase in total IgE of the immunized mice above that of the control mice after intranasal immunization. Furthermore, we were able to detect the presence of specific reagenic antibody, presumably of the IgE class, by the PCA assay (Table 3).
TABLE 3.
IgE production after intranasal immunization with TT plus CT
| Expt no. | Serum sample | Total IgE (μg/ml) | PCA titera |
|---|---|---|---|
| 1 | Control (naive) | NDb | 0 |
| Immune | 2.20 | 90 | |
| 2 | Control | ND | 0 |
| Immune | 2.20 | 30 |
The PCA assay was used to compare the levels of TT-specific reagenic antibodies in these sera. Sera were serially diluted with PBS. A volume of 0.1 ml of each diluted sample was injected subcutaneously in the backs of F344 rats. Amounts of 1 ml of 1% Evan's blue and 200 μg of TT were injected intravenously the next day. The last dilution of sera with a positive reaction was considered the PCA titer. PCA reactivity was depleted by adsorption of sera with anti-IgE coated microbeads (data not shown).
ND, not detected. For total IgE assay, the detection limit was 250 ng/ml.
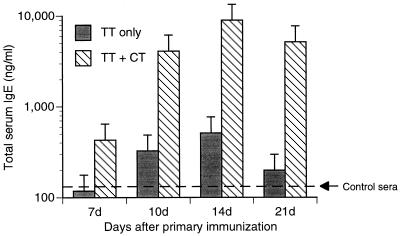
We also measured the levels of total IgE and PCA antibody titers in mouse serum at various times after primary immunization. By ELISA, the amount of total serum IgE increased 7 days after primary immunization with TT plus CT, whereas serum IgE levels in mice given TT alone were not above those in serum from naive mice (Fig. 4). Levels of total IgE increased at subsequent time points in the sera of both immunized groups of mice (TT only and TT plus CT). However, the TT-plus-CT immunized mice had dramatically higher amounts of IgE in their sera than the TT-only immunized mice. There was a transient increase in the TT-specific reagenic antibody response, presumably of the IgE class, in the serum of these mice, which peaked at day 10 after immunization with TT plus CT (Table 4). In contrast, there was no TT-specific reagenic antibody detected by PCA in the sera of mice immunized with TT alone.
FIG. 4.
Total IgE in serum after intranasal immunization. Mice were intranasally immunized with TT alone or TT in combination with CT (TT + CT) on days 0, 7, and 14. Serum samples were obtained 7, 10, 14, and 21 days after the primary immunization. Total IgE in pooled serum samples was determined by ELISA. Sera from unimmunized mice (control sera) contained 143 ± 25.2 (mean ± standard deviation) ng of IgE/ml. Sera were pooled from animals (n = 9 for each time point except for day 10 [n = 3]).
TABLE 4.
PCA titers in sera from intranasally immunized micea
| Day | Mean titer of immunization group
|
|
|---|---|---|
| TT only | TT + CT | |
| 7 | <30 | <30 |
| 10 | <30 | 90–270 |
| 14 | <30 | 30 |
| 21 | <30 | ≤30 |
Sera from mice intranasally immunized with TT only or TT in combination with CT (days 0, 7, and 14) were assayed for reagenic antibody by PCA reaction. Sera from animals (n = 9 for each time point except for day 10 [n = 3]) were pooled.
IL-5 is detected in sera from mice immunized with TT plus CT.
Serum IL-4, IL-5, or IFN-γ levels were measured in mice intranasally immunized with TT plus CT. Mice were intranasally immunized with TT plus CT (250 μg of TT plus 10 μg of CT). A group of naive mice were included as controls. Seven days later, the mice were reimmunized with a one-third dose (83.3 μg of TT plus 3.33 μg of CT) of the initial inoculum, as done in earlier experiments. Three days after the secondary immunization, serum was collected from each of the mice, and cytokine levels in the serum samples were measured by ELISA.
We were unable to detect IL-4 or IFN-γ in the sera of immunized mice at 10 days after the primary immunization. We found an average of 4.16 U of IL-5/ml of these serum samples (Table 5). However, IL-5 levels in serum from naive mice were below the detection limit of the assay (<1.2 U/ml).
TABLE 5.
Detection of IL-5 in sera from intranasally immunized micea
| Expt no. | Serum sample | IL-5 (U/ml) |
|---|---|---|
| 1 | Control (naive) | NDb |
| Immune | 3.88 | |
| 2 | Control | ND |
| Immune | 4.44 |
Sera from mice intranasally immunized with 250 μg of TT plus 10 μg of CT (days 0 and 7) were assayed for IL-4, IL-5, and IFN-γ by ELISA. Significant levels of IL-5, but not IL-4 or IFN-γ, were detected. Sera from animals (n = 5) were pooled.
ND, not detected. The detection limit was 0.39 U/ml.
DISCUSSION
Although nasal immunizations with live vaccines are currently done in clinical trials (1, 9), there are significant advantages with using inactive vaccine antigens, in contrast to live vaccines, for nasal immunization. Inactive vaccines should not be a safety issue for individuals immunocompromised due to disease, chemotherapy, or age. Furthermore, in the case of viral vectors, immune responses against the vector can limit the effectiveness of immunization (35), preventing the widespread utilization of these vectors for use in multiple vaccines. Notably, the ability to immunize with an inactive vaccine also allows the use of a variety of vaccine antigens, including polysaccharide-protein conjugates, which may prove impossible to produce with a viral vector. This should allow for the adaptation of this vaccination approach against numerous bacterial and viral respiratory pathogens. Thus, intranasal immunization with inactive vaccines has many attractive features that should result in an extremely powerful approach to prevent respiratory infection and disease.
The purpose of the present study was to determine the extent of immunologic responses, particularly immunopathologic reactions, associated with intranasal immunization with the mucosal adjuvant CT. For this study, BALB/c mice were intranasally immunized with TT combined with CT, and the responses of these mice were determined. For immunization, TT was chosen since studies by members of our laboratory (16, 20) described the immunologic reactions against this immunogen after oral immunization. However, the doses of TT and CT, although ideal for oral immunization, are probably too high for intranasal immunization. In ongoing studies (data to be published), similar results were obtained with a different antigen (influenza vaccine) and lower doses of CT.
It is clear that CT is an effective adjuvant for the intranasal immunization of mice. In our studies, CT promoted the development of specific serum antibody responses after intranasal immunization with TT. The levels of anti-TT IgG were about four to eight times higher in sera from mice immunized with TT combined with CT than in mice immunized with TT alone. Others have demonstrated similar results with other immunogens, such as Sendai virus and respiratory syncytial virus (19, 28, 37). We found that IgG1 responses were particularly enhanced after intranasal immunization, which is consistent with results obtained after oral immunization of healthy mice with TT and CT (24).
Although intranasal immunization is an effective route of immunization, we found that potentially fatal, pathologic changes occurred in the lungs after antigen and CT were deposited in the lower respiratory tract. At higher doses of CT and TT, some mice died a short time after the secondary immunization, and this was associated with edema and an increase in the number of macrophages in the alveoli. Although they rarely died, mice given lower secondary doses of TT plus CT also had clinical and pathologic signs. The lungs of these mice remained inflated even after their removal. This suggests that the airways were constricted and/or blocked with exudate, which thereby inhibited airflow. Histologically, we confirmed that there was a dramatic infiltration of mononuclear cells almost exclusively around pulmonary airways and vessels. To illustrate the magnitude of the cellular response, we were able to recover more than six times as many cells from the lungs of these mice as from naive mice (data not shown). These effects were seen only in mice inoculated with TT plus CT and not in those given TT alone. In addition to the pathologic effects, there was a large, transient increase in the TT-specific IgE level present in sera from mice immunized with TT plus CT, but not in mice immunized with TT alone. These effects are not unique to TT, as a similar phenomenon of IgE responses associated with peribronchial and perivascular cell infiltration occurs with a different antigen (e.g., influenza vaccine) in combination with lower doses (0.1 μg) of CT (Hodge et al., unpublished data). Overall, the changes in the lung, increases in cell numbers from respiratory tissues, and the production of antigen-specific reagenic antibody (IgE) suggest that an IgE-associated hypersensitivity response developed in the lung after the intranasal immunization of mice with a mixture of TT and CT, but not after the intranasal immunization with TT alone.
The immunopathologic effects of intranasal immunization with TT plus CT are probably associated with the activation of Th2 cells. Th2 cells are characterized by their support of humoral immunity through the secretion of selected cytokines, such as IL-4, IL-5, and IL-6 (21). Murine Th2 cells preferentially promote the production of IgG1 over other IgG subclasses, and IL-4 produced by Th2 cells aids in the development of IgE responses (10, 21). In the present studies, the early and predominant production of antigen-specific IgG of the IgG1 subclass and IgE responses are consistent with the activation of Th2 cells after intranasal immunization with TT plus CT. Furthermore, significant levels of IL-5, a cytokine produced by Th2 cells (21), were detected in sera from immunized mice. These observations are consistent with our previous studies describing the preferential activation of Th2 cells after oral immunization with TT plus CT (20). Although Th2 cells are mediators of humoral immunity, Th2-cell responses also appear to contribute to the lung pathology associated with viral diseases (12), asthma (29), cryptogenic fibrosing alveolitis (36), and adverse reactions associated with vaccination against respiratory viruses (11). Some of these effects may be due to Th2-cell-induced IgE production, which is supported by earlier studies demonstrating that pulmonary challenge of rats sensitized with antigen-specific IgE produces a peribronchial and alveolar infiltration of mononuclear cells (2). Thus, our results are consistent with the idea that the activation of Th2 cells is responsible for the intense immunopathologic reactions observed in mice intranasally immunized with TT combined with CT.
The inclusion of CT most likely has a qualitative effect on immune responses after intranasal immunization, in addition to its adjuvant effects. In contrast to results for mice given TT alone, IgG2a responses, particularly against CT, were also found in serum from mice intranasally immunized with TT plus CT. As the production of IgG2a is mediated by IFN-γ production, a product of Th1 cells (19), this suggests that intranasal immunization with CT results in the activation of Th1 cells. In support, ongoing studies suggest that both Th1 and Th2 cells are activated in lungs after intranasal immunization with the adjuvant, CT, whereas intranasal immunization with antigen alone results in a predominantly Th2 response. Thus, the selection of adjuvants used for intranasal immunization could have a significant effect on the nature of immunity generated and influence the potential for inflammatory reactions associated with immunization. However, additional studies are needed to determine if and how T-helper subset activation contributes to these adverse immunologic reactions.
In summary, it is clear that CT is an effective adjuvant for intranasal immunization with TT in mice. However, our results also demonstrate that intranasal immunization with CT as an adjuvant can result in the development of IgE responses which may contribute to potentially fatal, pathologic changes in the lungs of mice. Most likely, this precludes the use of CT as a mucosal adjuvant for man, especially in atopic individuals. The present studies used relatively high doses of antigen and CT, and lower doses do reduce, but do not eliminate, these adverse reactions without compromising immunogenicity (data to be published). In addition, other adjuvants need to be examined for their adjuvant activity. However, it is possible, given the preferential production of IL-4 by lung cells (data to be published), that IgE production will be a major component of any response to a soluble antigen given by this route. As locally produced cytokines are likely to be quickly absorbed or inactivated, the lack of IL-4 and IFN-γ in serum does not necessarily reflect their lack of production in the respiratory tract after intranasal immunization. By employing this immunization model, we will be able to more fully characterize the activation of CD4+ T cells and cytokine production, which promote the development of IgE responses and recruitment of cells to the lungs. Future studies can then determine if these responses can be beneficially altered by treatment with recombinant cytokines or other modulators that downregulate IgE production or cellular recruitment, leading to vaccine-adjuvant combinations which induce appropriate protective immune responses.
ACKNOWLEDGMENTS
We would like to thank the other members of Mucosal Immunization Research Group, including Gail Cassell, Mariarosario Marinara, and Michell Coste for their support and useful discussion. We also appreciate the review of the manuscript and useful comments by Lisa Hodge and Harlan Jones. We also thank Patricia J. Freda Pietrobon and Connaught Laboratories, Inc. for the generous supply of tetanus toxoid. We also appreciate the excellent technical support by Padma Patel and Haifa Al-Khatib.
This work was supported by the American Lung Association of Texas (J.W.S.) and Public Health Service grant AI15128 from the National Institutes of Health (J.R.M.).
REFERENCES
- 1.Belshe R B, Mendelman P M, Treanor J, King J, Gruber W C, Piedra P, Bernstein D I, Hayden F G, Kotloff K, Zangwill K, Iacuzio D, Wolff M. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 2.Blythe S, England D, Esser B, Junk P, Lemanske R F., Jr IgE antibody mediated inflammation of rat lung: histologic and bronchoalveolar lavage assessment. Am Rev Respir Dis. 1986;134:1246–1251. doi: 10.1164/arrd.1986.134.5.1246. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P, Karlsson G, Hansson G, Petruson B, Bjorkander J, Hanson L A. The clinical condition of IgA-deficient patients is related to the proportion of IgD- and IgM-producing cells in their nasal mucosa. Clin Exp Immunol. 1987;67:626–636. [PMC free article] [PubMed] [Google Scholar]
- 4.Calvo M, Grob K, Marin F, Bertoglio J, Neira J, Arellano P, Anido M. Evaluation of secretory IgA childhood respiratory diseases. Allergol Immunopathol. 1988;16:157–161. [PubMed] [Google Scholar]
- 5.Casterline C L, Evans III R, Battista V C, Talamo R C. Selective IgA deficiency and Pi ZZ-antitrypsin deficiency. Association with recurrent sinopulmonary infections, emphysema, and bronchiectasis. Chest. 1978;73:885–886. doi: 10.1378/chest.73.6.885. [DOI] [PubMed] [Google Scholar]
- 6.Clements M L. Influenza vaccines. Bio/Technology. 1992;20:129–150. doi: 10.1016/b978-0-7506-9265-6.50012-9. [DOI] [PubMed] [Google Scholar]
- 7.Clements M L, Betts R F, Tierney E L, Murphy B R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements M L, O'Donnell S, Levine M M, Chanock R M, Murphy B R. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortment vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983;40:1044–1051. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doepel, L. 29 January 1998. Novel concepts put to the test in three new AIDS vaccine trials. NIAID News. [PubMed]
- 10.Finkelman F D, Urban J F, Jr, Beckmann M P, Schooley K A, Holmes J M, Katona I M. Regulation of murine in vivo IgG and IgE responses by a monoclonal anti-IL-4 receptor antibody. Int Immunol. 1991;3:599–607. doi: 10.1093/intimm/3.6.599. [DOI] [PubMed] [Google Scholar]
- 11.Graham B S, Henderson G S, Tang Y W, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 12.Graham M B, Braciale V L, Braciale T J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruber W C, Darden P M, Still J G, Lohr J, Reed G, Wright P F. Evaluation of bivalent live attenuated influenza A vaccines in children 2 months to 3 years of age: safety, immunogenicity and dose-response. Vaccine. 1997;15:1379–1384. doi: 10.1016/s0264-410x(97)00032-7. [DOI] [PubMed] [Google Scholar]
- 14.Haas L, Petit-Phar M, Terzidis H, Kapel N, Meillet D, Gobert J G, Rostoker G. IgA subclass distribution of IgA anti-gliadin antibodies in feces of patients with coeliac disease. Adv Exp Med Biol. 1995;371B:1349–1353. [PubMed] [Google Scholar]
- 15.Hbabi-Haddioui L, Roques C. Inhibition of Streptococcus pneumoniae adhesion by specific salivary IgA after oral immunisation with a ribosomal immunostimulant. Drugs. 1997;54:29–32. doi: 10.2165/00003495-199700541-00008. [DOI] [PubMed] [Google Scholar]
- 16.Jackson R J, Fujihashi K, Xu-Amano J, Kiyono H, Elson C O, McGhee J R. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect Immun. 1993;61:4272–4279. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurono Y, Shimamura K, Shigemi H, Mogi G. Inhibition of bacterial adherence by nasopharyngeal secretions. Ann Otol Rhinol Laryngol. 1991;100:455–458. doi: 10.1177/000348949110000605. [DOI] [PubMed] [Google Scholar]
- 18.Liang S C, Simecka J W, Lindsey J R, Cassell G H, Davis J K. Antibody responses after Sendai virus infection and their role in upper and lower respiratory tract disease in rats. Lab Anim Sci. 1999;49:385–394. [PubMed] [Google Scholar]
- 19.Liang X P, Lamm M E, Nedrud J G. Cholera toxin as a mucosal adjuvant for respiratory antibody responses in mice. Reg Immunol. 1989;2:244–248. [PubMed] [Google Scholar]
- 20.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P N, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 21.Mosmann T, Coffman R. TH1 and TH2: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 22.Murphy B R, Clements M L, Johnson P R, Wright P F. The mucosal and systemic immune responses of children and adults to live and inactivated influenza A virus vaccines. In: Strober W, Lamm M E, McGhee J R, James S P, editors. Mucosal immunity and infections at mucosal surfaces. New York, N.Y: Oxford University Press; 1988. p. 303. [Google Scholar]
- 23.Novak M, Moldoveanu Z, Schafer D P, Mestecky J, Compans R W. Murine model for evaluation of protective immunity to influenza virus. Vaccine. 1993;11:55–60. doi: 10.1016/0264-410x(93)90339-y. [DOI] [PubMed] [Google Scholar]
- 24.Okahashi N, Yamamoto M, Vancott J L, Chatfield S N, Roberts M, Bluethmann H, Hiroi T, Kiyono H, McGhee J R. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostergaard P A. IgA levels, bacterial carrier rate, and the development of bronchial asthma in children. Acta Pathol Microbiol Scand Sect C. 1977;85:187–195. [PubMed] [Google Scholar]
- 26.Renegar K B, Small P A., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renegar K B, Small P A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 28.Reuman P D, Keely S P, Schiff G M. Similar subclass antibody responses after intranasal immunization with UV-inactivated RSV mixed with cholera toxin or live RSV. J Med Virol. 1991;35:192–197. doi: 10.1002/jmv.1890350309. [DOI] [PubMed] [Google Scholar]
- 29.Robinson D S, Durham S R, Kay A B. Cytokines. 3. Cytokines in asthma. Thorax. 1993;48:845–853. doi: 10.1136/thx.48.8.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small P A., Jr Influenza: pathogenesis and host defense. Hosp Pract. 1990;25:51–54. doi: 10.1080/21548331.1990.11704033. , 57–62. [DOI] [PubMed] [Google Scholar]
- 31.Snider D P, Marshall J S, Perdue M H, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein antigen and cholera toxin. J Immunol. 1994;153:647–657. [PubMed] [Google Scholar]
- 32.Tamura S, Ito Y, Asanuma H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992;149:981–988. [PubMed] [Google Scholar]
- 33.Tamura S I, Asanuma H, Ito Y, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T, Oya A. Superior cross-protective effect of nasal vaccination to subcutaneous inoculation with influenza hemagglutinin vaccine. Eur J Immunol. 1992;22:477–481. doi: 10.1002/eji.1830220228. [DOI] [PubMed] [Google Scholar]
- 34.Taylor G, Howard C J. Protection of mice against Mycoplasma pulmonis infection using purified mouse immunoglobulins: comparison between protective effect and biological properties of immunoglobulin classes. Immunology. 1981;43:519–525. [PMC free article] [PubMed] [Google Scholar]
- 35.van Ginkel F W, Liu C, Simecka J W, Dong J Y, Greenway T, Frizzell R A, Kiyono H, McGhee J R, Pascual D W. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and beta-galactosidase. Hum Gene Ther. 1995;6:895–903. doi: 10.1089/hum.1995.6.7-895. [DOI] [PubMed] [Google Scholar]
- 36.Wallace W A, Ramage E A, Lamb D, Howie S E. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA) Clin Exp Immunol. 1995;101:436–441. doi: 10.1111/j.1365-2249.1995.tb03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh E E. Mucosal immunization with a subunit respiratory syncytial virus vaccine in mice. Vaccine. 1993;11:1135–1138. doi: 10.1016/0264-410x(93)90075-9. [DOI] [PubMed] [Google Scholar]