Abstract
Purpose
To assess the planned dose, in vivo dosimetry, acute skin toxicity, pain, and distress using Thermoplastic Elastomer (TPE) bolus for postmastectomy radiotherapy (PMRT).
Material and methods
Thirty-two PMRT patients with TPE bolus (17 patients for 25 fractions, 15 patients for the first 20 fractions) were selected for the study. The acute skin toxicity, pain, and psychological distress were assessed from the first treatment week to the fourth week after the end of treatment. At the first treatment, the MOSFET was used in vivo dosimetry measurement.
Results
In vivo dosimetry with the bolus, the dose deviation ranged from −6.22% to −1.56% for 5 points. The presence of grade 1 and 2 skin toxicity reached its peak (70.0% and 13.3%) in the sixth week. Two patients (6.6%) with 25 fractions bolus experienced moist desquamation in the fifth and seventh week, with pain score 2 and 3, and interruptions of 3 and 5 days, respectively. The incidence of pain score 1, 2, and 3 peaked in the fifth (33.3%), fourth (33.3%), and seventh (10.0%) week. No patients experienced grade 3 skin toxicity and severe pain. One patient had significant anxiety, and two patients had significant depression.
Conclusion
The TPE bolus can accurately fit skin and improve the surface dose to more than 90%. Twenty fractions with TPE bolus had similar skin toxicity and pain to those without bolus and did not increase patients' distress and clinical workload, compared with the literature's data, which is an alternative to the 3D printing bolus for PMRT.
Keywords: TPE bolus, Postmastectomy radiotherapy, In vivo dosimetry, Skin toxicity, Pain
Abbreviations: CI, Conformity Index; PTV, Planning Target Volume; HI, Homogeneity Index; VMAT, Volumetric Modulated Arc Therapy; TPE, Thermoplastic Elastomer; PMRT, Postmastectomy Radiation Therapy; DT, Distress Thermometer; GAD-7, Generalized Anxiety Disorder 7-item Scale; PHQ-9, Patient Health Questionnaire-9; OAR, Organ at Risk; PRV, Planning Risk Volume; CBCT, Cone Beam Computer Tomography; IMRT, Intensity-Modulated Radiation Therapy
Highlights
-
•
First reported the soft and self-viscosity TPE bolus in PMRT.
-
•
Evaluated the planning and in vivo dose in PMRT using TPE bolus.
-
•
TPE bolus had similar skin toxicity to those without bolus compared with the literature's data.
-
•
Using TPE bolus did not increase patients' psychological distress and clinical workload compared with the literature's data.
1. Background
Many studies have demonstrated that postmastectomy radiation therapy (PMRT) reduces the risk of locoregional recurrence and improves survival in selected patients. The radiation therapy target mainly includes the chest wall and regional lymph nodes. Due to the skin-sparing effect of photons, a bolus is usually used to increase the dose to the patient's skin and subcutaneous lymphatic vessels [1].
The conventional bolus does not accurately fit with the chest wall, and there is an air gap, resulting in a dose deviation of the chest wall. 3D printing bolus can accurately fit the surface, which can reduce the dose deviation [2,3], reduce the radiation dose of the distal organ at risk (OAR) (such as heart and lung) [4], and reduce the setup time [2]. However, 3D printing bolus usually requires a second CT scan for simulation [5,6], more than 10 h of segmentation and printing [2,7], and may be more financial costs than commercial bolus [2,[6], [7], [8]]. To avoid the second CT scan and reduce the waiting time for treatment start, Dipasquale G et al. [6] used a high-resolution surface-scanner to produce bolus models to avoid the second CT scan and reduce waiting time for treatment. However, this technique has not been widely used in clinical practice. Therefore, there is a need for a novel bolus that accurately fits the patient's surface, is simple to use, and has no impact on CT simulation.
Thermoplastic Elastomer (TPE) is soft and self viscosity, which can more accurately fit the patient's skin than the traditional bolus. This study aimed to evaluate the planning and in vivo dose in post-mastectomy radiotherapy (PMRT) using TPE bolus. At the same time, we assessed the patients' acute skin toxicity, pain and psychological changes during the whole radiotherapy process.
2. Materials and methods
2.1. Patient data
The Ethics Committee on Biomedical Research, West China Hospital of Sichuan University, approved this study (Number:2020674). Informed consent was obtained from all individual participants included in the study. From October 2020 to July 2021, thirty-two patients receiving postmastectomy chest wall radiation therapy were selected for the study (ranging in age from 36 to 71 years old, with a median age of 51 years). There were 14 patients with left breast cancer and 18 patients with right breast cancer.
2.2. CT simulation and treatment planning
The vacuum bags were placed on a wedge plate for immobilization patients [9]. The physician marked the surgical scar of the chest wall using lead wire and then covered the thermoplastic elastomer bolus (TPE) (thickness of 5 mm, density 0.83, CT value −180, size 26 × 26 cm) (Shenzhen To-create Medical Technology co., LTD, Shenzhen, China)on the involved chest wall. Composition of TPE bolus includes cosmetic grade white oil, Styrene-Ethylene/Butylene-Styrene (SEBS) Block copolymer, polyethylene, polyethylene glycol terephthalate, and antibacterial agent. If there is an air gap of more than 5 mm between the patient's skin and the TPE, adjust it manually or trim it with scissors (Fig. 1). Patients were simulated using GE Revolution™ CT (128 slices) with a slice thickness of 5 mm from the chin to the lower edge of the liver with intravascular contrast. The target area of radiotherapy included the chest wall and axillary level 3 and 4 lymph nodes, as recommended by ESTRO. All post-mastectomy radiotherapy (PMRT) patients received 50 Gy in 25 fractions [1] with Intensity-Modulated Radiation Therapy (IMRT) or Volumetric Modulated Arc Therapy (VMAT) treatment plan (17 patients with bolus for all 25 fractions, and the other 15 patients with bolus for the first 20 fractions at the discretion of the treating radiation oncologist) [10]. The OAR constraints protocol as follows: ipsilateral lung V5<55%, V20<30%,V30<18% s; contralateral lung V5<30%, V20<10%; spinal cord Dmax<1200 cGy; Dmax of spinal cord PRV<1300 cGy; heart V30<20 cc,V40<10 cc; contralateral breast Dmax<800 cGy; brachial plexus Dmax<5500 cGy. The homogeneity index (HI) of PTV was calculated as HI = D2% −D98% ∕D50%. The conformity index (CI) of the PTV was evaluated as CI = (VPTV50)2∕VPTV × V50, where VPTV is the target volume, V50 is the volume of the prescribed isodose value, and VPTV50 is the volume of the target covered by the prescribed isodose value [4].
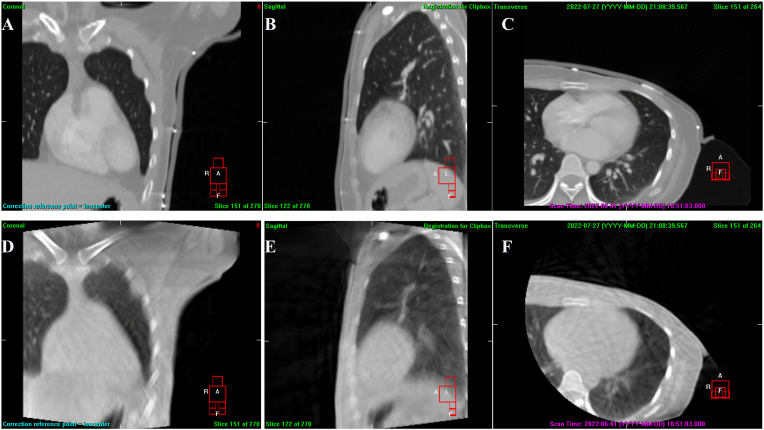
Fig. 1.
CT and cone beam CT (CBCT) images of one patient; in A-C, which were the coronal, sagittal, and transverse view of CT images, due to the presence of marking lead wire, there was a relatively small air gap between bolus and patient surface; in D-F which were the coronal, sagittal and transverse view of CBCT images, without lead markers, the bolus fitted well with the skin.
2.3. In vivo dosimetry
At the first treatment, the physicist chose 5 points on the patient's chest wall for placement of a metal-oxide-semiconductor-field-effect-transistor (MOSFET) (Best Medical Canada, Ottawa, ON, Canada) (Fig. 2. A and B). After placing MOSFET with adhesive tape, cover the TPE bolus on the involved chest wall. Once the bolus was judged to fit the skin accurately, the daily cone beam computer tomography (CBCT) was performed for image guidance (Synergy, Elekta, Crawley, UK). The CBCT scanning parameters were as follows: tube voltage of 100 kV, tube current of 36.1 mA, S20 F1 filter, acquisition speed of 5.5 frames/s and acquisition gantry angle of 50°–210° or 310°–130°.
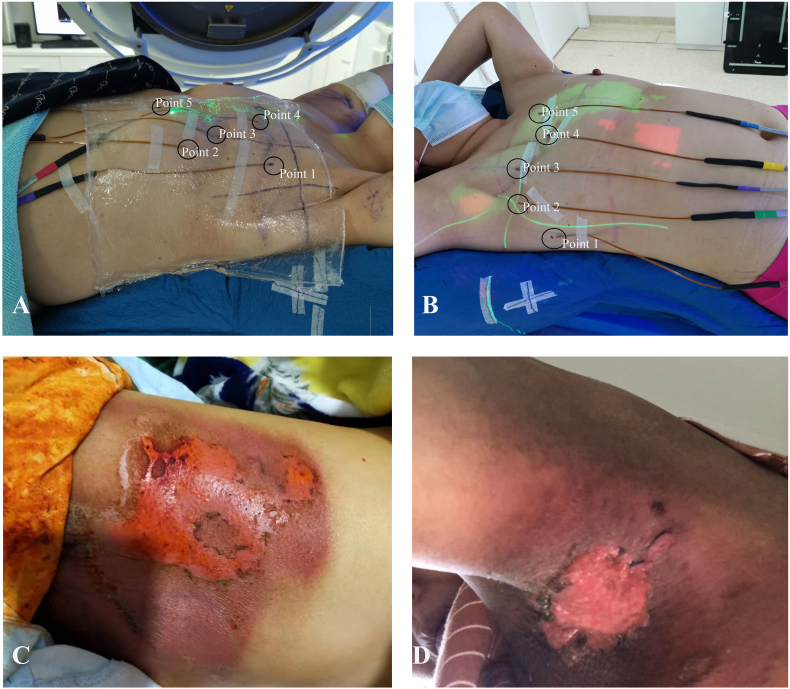
Fig. 2.
A–B Using MOSFETs to measure 5 points of skin dose for patients, in B the MOSFET in point 1 was located outside the area directly covered by the treatment field; C grade 2 skin toxicity, erythema; D grade 2 skin toxicity, moist desquamation in the skin folder of the axillary.
2.4. The skin acute toxicity and pain assessment
Research radiation therapists evaluated the skin's acute toxicity (The RTOG acute radiation morbidity scoring criteria) [11] and pain (The visual analogue scale (VAS) from a score of 0 (no pain) to 10 (worst pain)). Severe pain was defined as pain impacting the activity of daily life (score≥4) [12]. Patients filled out the questionnaire during the first to the fifth week of treatment, and the radiotherapist gave skin care recommendations. Skin care includes keeping dry (no touching water even if showering), no scratching the chest wall, and using povidone iodine solution in cases of Grade 2 toxicity. The treatment will be interrupted if the patient experiences grade 3 skin toxicity and severe pain. The radiation oncologist will evaluate whether add the treatment fraction if the interruption is more than 14 days [13]. When the treatment finished, telephone follow-up was conducted weekly (from the sixth to the ninth week).
2.5. Psychological assessment
The Distress Thermometer of the National Comprehensive Cancer Network (DT) and adapted problem list were used weekly to assess patients’ distress on a scale of 0 (no distress) to 10 (worst distress). Meanwhile, anxiety and depression in the past two weeks (the first, third, and fifth weeks) were assessed with the GAD-7 (Generalized Anxiety Disorder 7-item Scale) and the PHQ-9 (Patient Health Questionnaire-9). With higher scores indicating more severe anxiety and depression and scores of 10 or greater indicating moderate anxiety or depression [14].
3. Results
The patients’ target and adjacent OAR dosimetric characteristics are shown in Table 1. Regarding the treatment plan, the mean values of D98%, D2%, CI, and HI of PTV, were 4879.13 cGy, 5381.75 cGy, 0.75, and 0.10, respectively. The mean dose of the heart and the V5, V10, V20, V30 and mean dose of the ipsilateral lung were 348.69 cGy, 51.35%, 36.72%, 25.66%, 19.00%, and 1375.94 cGy. In vivo dosimetric measurement, the skin surface dose at the first treatment of 16 of the 32 patients was measured [9,12] (Fig. 2.). In point 1 for one patient, the deviation of the in vivo dosimetry results was −92.25%. If we remove the result, the mean and the percentage deviation from the planned dose are 188.67 cGy (−5.67%), 187.56 cGy (−6.22%), 196.88 cGy (−1.56%), 193.50 cGy (−3.25%), 192.75 cGy (- 3.63%) for point 1 to 5 respectively (Table 2).
Table 1.
The planning dose parameter.
| Dose parameter | Mean | Max | Min | Median | |
|---|---|---|---|---|---|
| PTV | Volume(cm3) | 723.07 | 1472.95 | 431.58 | 699.24 |
| D99% (cGy) | 4755.31 | 4912.00 | 4424.00 | 4804.50 | |
| D98% (cGy) | 4879.13 | 4964.00 | 4650.00 | 4901.00 | |
| D95% (cGy) | 4997.06 | 5045.00 | 4865.00 | 5000.50 | |
| Mean (cGy) | 5190.06 | 5226.00 | 5147.00 | 5182.00 | |
| D50% (cGy) | 5203.50 | 5245.00 | 5155.00 | 5193.00 | |
| D2% (cGy) | 5381.75 | 5466.00 | 5314.00 | 5377.00 | |
| D1% (cGy) | 5401.88 | 5491.00 | 5338.00 | 5393.50 | |
| CI | 0.75 | 0.84 | 0.58 | 0.76 | |
| HI | 0.10 | 0.14 | 0.07 | 0.09 | |
| Spinal cord | Max (cGy) | 1644.00 | 2259.00 | 1036.00 | 1536.00 |
| Spinal cord PRV | Max (cGy) | 2467.19 | 3183.00 | 1705.00 | 2434.00 |
| Lung (ipsilateral) | V5 (%) | 51.35% | 60.00% | 42.00% | 52.25% |
| V10 (%) | 36.72% | 40.00% | 31.00% | 37.50% | |
| V20 (%) | 25.66% | 29.39% | 21.34% | 26.33% | |
| V30 (%) | 19.00% | 22.12% | 15.05% | 19.46% | |
| Mean (cGy) | 1375.94 | 1496.00 | 1224.00 | 1387.00 | |
| Lung (contralateral) | V5 (%) | 7.06% | 16.13% | 0.04% | 6.98% |
| V10 (%) | 0.83% | 2.37% | 0.00% | 0.42% | |
| V20 (%) | 0.03% | 0.21% | 0.00% | 0.00% | |
| V30 (%) | 0.00% | 0.02% | 0.00% | 0.00% | |
| Mean (cGy) | 212.25 | 319 | 110 | 203.5 | |
| Heart | Mean (cGy) | 348.69 | 796.00 | 178.00 | 329.00 |
| Breast (contralateral) | Mean (cGy) | 410.23 | 609.00 | 122.00 | 466.00 |
Notes: D1%, D2%, D50%, D95%, D98%, and D99% is the dose of 1%, 2%, 50%, 95%, 98%, and 99% PTV volume respectively. PTV, planning target volume. PRV, planning risk volume. V5, V10, V20, and V30 is the percentage volume receiving 5, 10, 20, and 30 Gy, respectively. HI, homogeneity index. CI, conformity index.
Table 2.
The surface dose measured in vivo.
| Patients | Point 1 |
Point 2 |
Point 3 |
Point 4 |
Point 5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose (cGy) | Differ (%) | Dose (cGy) | Differ (%) | Dose (cGy) | Differ (%) | Dose (cGy) | Differ (%) | Dose (cGy) | Differ (%) | |
| 1 | 193.00 | −3.50 | 193.00 | −3.50 | 201.00 | 0.50 | 196.00 | −2.00 | 206.00 | 3.00 |
| 2 | 186.00 | −7.00 | 180.00 | −10.00 | 191.00 | −4.50 | 182.00 | −9.00 | 181.00 | −9.50 |
| 3 | 178.00 | −11.00 | 172.00 | −14.00 | 196.00 | −2.00 | 178.00 | −11.00 | 181.00 | −9.50 |
| 4 | 183.00 | −8.50 | 183.00 | −8.50 | 188.00 | −6.00 | 184.00 | −8.00 | 180.00 | −10.00 |
| 5 | 210.00 | 5.00 | 196.00 | −2.00 | 207.00 | 3.50 | 204.00 | 2.00 | 207.00 | 3.50 |
| 6 | 194.00 | −3.00 | 196.00 | −2.00 | 188.00 | −6.00 | 193.00 | −3.50 | 187.00 | −6.50 |
| 7 | 194.00 | −3.00 | 194.00 | −3.00 | 194.00 | −3.00 | 199.00 | −0.50 | 201.00 | 0.50 |
| 8 | 213.00 | 6.50 | 201.00 | 0.50 | 210.00 | 5.00 | 215.00 | 7.50 | 211.00 | 5.50 |
| 9 | 194.00 | −3.00 | 205.00 | 2.50 | 192.00 | −4.00 | 191.00 | −4.50 | 199.00 | −0.50 |
| 10 | 205.00 | 2.50 | 196.00 | −2.00 | 209.00 | 4.50 | 206.00 | 3.00 | 202.00 | 1.00 |
| 11 | 175.00 | −12.50 | 176.00 | −12.00 | 190.00 | −5.00 | 196.00 | −2.00 | 193.00 | −3.50 |
| 12 | 15.50 | −92.25 | 168.00 | −16.00 | 198.00 | −1.00 | 184.00 | −8.00 | 186.00 | −7.00 |
| 13 | 153.00 | −23.50 | 175.00 | −12.50 | 189.00 | −5.50 | 190.00 | −5.00 | 154.00 | −23.00 |
| 14 | 186.00 | −7.00 | 180.00 | −10.00 | 191.00 | −4.50 | 182.00 | −9.00 | 181.00 | −9.50 |
| 15 | 165.00 | −17.50 | 191.00 | −4.50 | 210.00 | 5.00 | 203.00 | 1.50 | 218.00 | 9.00 |
| 16 | 201.00 | 0.50 | 195.00 | −2.50 | 196.00 | −2.00 | 193.00 | −3.50 | 197.00 | −1.50 |
| Mean | 188.67 | −5.67 | 187.56 | −6.22 | 196.88 | −1.56 | 193.50 | −3.25 | 192.75 | −3.63 |
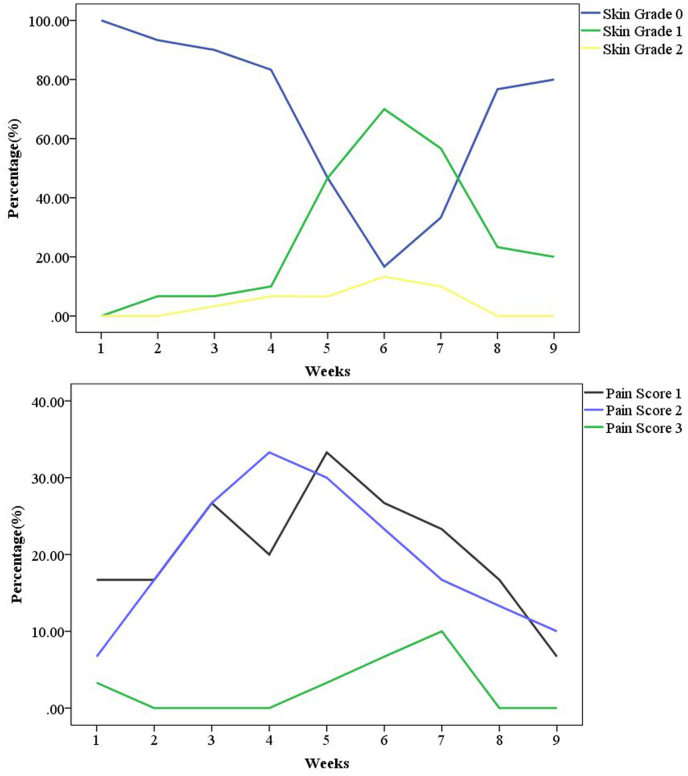
Among the 32 patients during radiotherapy, two patients had an incomplete assessment. As a result, we collected 30 patients for analysis. Three patients (10.0%) had grade 1 skin toxicity in the fourth week, and 14 (46.7%) had grade 1 skin toxicity in the fifth week. The presence of grade 1 and 2 skin toxicity peaked in the sixth week (70.0% and 13.3%) (Fig. 3). Two patients (6.6%) experienced moist desquamation (grade 2 skin toxicity) in the skin folder of the axillary in the fifth and seventh week (Fig. 2D and Fig. 3). At the same time, these two patients experienced pain score 2 and 3 (grade 1) and had interruptions of 3 and 5 days, respectively. The incidence of pain score 1, 2, and 3 peaked in the fifth (33.3%), fourth (33.3%), and seventh (10.0%) week, respectively (Fig. 3). No patients experienced grade 3 or 4 skin toxicity and severe pain.
Fig. 3.
The curves above show the probability of 3 grades of skin toxicity for different weeks, the presence of grade 1 and 2 skin toxicity reached its peak (70.0% and 13.3%) in the sixth week; the curves below show the probability of 3 levels of pain score for different weeks, the incidence of pain score 1, 2 and 3 peaked in the fifth (33.3%), fourth (33.3%) and seventh (10.0%) week.
According to the screening value of DT ≥ 4, the number and incidence of distress in 1–5 weeks were 2 cases (6.67%), 4 cases (13.3%), 6 cases (20.0%), and 6 cases (20.0%), 8 cases (26.7%), respectively. Screening with PHQ-9 (score≥10), no patients had significant depression in the first week, and two patients had significant depression in the third and fifth weeks (Table 3). However, when screened with GAD-7 (score≥10), only one patient developed significant anxiety in the first, third, and fifth weeks.
Table 3.
The score of DT, GAD-7 and PHQ-9.
| Patients | DT |
GAD-7 |
PHQ-9 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 1st | 3rd | 5th | 1st | 3rd | 5th | |
| 1 | 0 | 4 | 4 | 7 | 4 | 2 | 1 | 2 | 1 | 1 | 1 |
| 2 | 0 | 1 | 3 | 3 | 4 | 0 | 5 | 5 | 0 | 6 | 3 |
| 3 | 0 | 1 | 2 | 2 | 1 | 5 | 6 | 4 | 7 | 5 | 5 |
| 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 6 | 0 | 1 | 5 | 4 | 4 | 0 | 7 | 0 | 0 | 5 | 0 |
| 7 | 0 | 3 | 5 | 5 | 5 | 3 | 7 | 6 | 5 | 10 | 10 |
| 8 | 2 | 5 | 4 | 4 | 5 | 3 | 5 | 0 | 4 | 6 | 0 |
| 9 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 2 |
| 11 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 |
| 12 | 0 | 3 | 3 | 0 | 0 | 1 | 3 | 0 | 4 | 0 | 0 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 1 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 | 5 | 4 | 4 | 5 | 5 | 5 |
| 15 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 4 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | 0 | 7 | 7 | 0 | 7 | 3 | 0 |
| 17 | 2 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 3 | 1 | 0 |
| 18 | 2 | 1 | 0 | 0 | 0 | 1 | 3 | 0 | 2 | 2 | 0 |
| 19 | 1 | 1 | 1 | 1 | 1 | 4 | 0 | 0 | 6 | 0 | 0 |
| 20 | 5 | 7 | 5 | 5 | 6 | 11 | 14 | 12 | 6 | 12 | 10 |
| 21 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 1 |
| 22 | 1 | 0 | 0 | 1 | 1 | 3 | 0 | 0 | 4 | 0 | 0 |
| 23 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 4 | 2 |
| 24 | 0 | 1 | 1 | 1 | 1 | 7 | 7 | 7 | 4 | 8 | 8 |
| 25 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 11 |
| 26 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| 27 | 5 | 5 | 5 | 6 | 5 | 4 | 3 | 3 | 5 | 2 | 1 |
| 28 | 0 | 1 | 1 | 3 | 4 | 1 | 7 | 5 | 0 | 6 | 8 |
| 29 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 1 | 1 |
| 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Notes: DT, Distress Thermometer; GAD-7, Generalized Anxiety Disorder 7-item Scale; PHQ-9, Patient Health Questionnaire-9. 1st, 2nd, 3rd, 4th, and 5th represent the first to the fifth week of treatment.
4. Discussion
The dose to the chest wall is usually 40–72% without a bolus, and using a bolus can significantly increase the chest wall dose [15]. All patients in this study used TPE-bolus CT simulation and planning, and the D98, D95, D2, CI, and HI of PTV were 4879.13 cGy, 4997.06 cGy, 5381.75 cGy, 0.75, and 0.10, respectively. The CI of the entire PTV was 0.83 ± 0.02 using a 3D printing bolus, which was higher than the result in our study. However, the HI of PTV in our research is better than that of a 3D printing bolus (0.10 ± 0.01) [4]. The mean dose of heart was 348.69 cGy, which was significantly lower than 800.00 cGy in the report [4].
The surface dose of OSLD (optically stimulated luminescence dosimeters) was within 3% for both standard sheet and 3D printed bolus [2]. Fiedler DA [16] used the brass mesh bolus (BMB) and the transparent polymer-gel bolus (PGB), the measurements of EBT3 were all greater than 90%. In general, the above results in reports were better than ours (ranging from −6.22% to −1.56%). However, our results of MOSFET were better than Dias AG reported that 80% of all measurements were within the range of ±20% [10], which is consistent with the low dose (−4.3 to −9.2%) reported by Qi et al. [17] using MOSFETs. Therefore, using TPE bolus significantly improved the surface dose to more than 90%.
The 3D printing bolus was made according to the contour information extracted from the CT image, which can accurately fit the patient's contour. A decrease in the frequency of air gaps ≥5 mm from 30% with sheet bolus to 13% for 3D printed bolus was observed [2]. For all patients, the maximum mean air gap was 3.9 ± 1.4 mm for the conventional bolus and only 1.9 ± 0.9 mm for the 3D printing bolus [4]. Because the TPE bolus has a certain viscosity, it can fit the chest well even if the patient's contour changes to a certain extent. Therefore, our study didn't find a gap ≥5 mm, which is much better than the traditional bolus. It is worth noting that, with a 3D printing bolus, which extracts the contour from the CT simulation image, local posture and anatomical changes may result in an air gap between the skin and the bolus [2].
However, using bolus, 26 of 53 patients had grade 2 and 3 skin toxicity [13]. The incidence rates of grade 1, grade 2, and grade 3 skin toxicity with bolus were 24.5%, 60.4%, and 9.4%, and those without bolus were 50.9%, 40.5%, and 0.0% [13]. The use of bolus led to higher rates of acute grade 1/2 (75% vs 57%) and grade 3 radiation dermatitis (pooled rates of 9.6% with bolus vs 1.2% without) [1]. There had around 35% of patients developed Grade 2 skin toxicity (RTOG) when the cumulated target dose was around 40–46 Gy [15]. For 16 patients with bolus, one had grade 3 (moist desquamation), two had grade 2 (marked erythema), and 13 had mild or no dermatitis [2]. When no skin bolus was used during radiation therapy only 4.2% of the patients (2 of 48) experienced severe pain, none (0 of 48) had moist desquamation, and 2.1% (1 of 48) had grade 3 skin toxicity [12]. When skin bolus was used on alternate days, the frequency increased to 15% for pain, 22% for moist desquamation, and 26% for grade 3 skin toxicity [12].
In the fifth and sixth weeks, 46.7% and 70.0% of patients had grade 1 skin toxicity. The presence of grade 1 and 2 skin toxicity reached its peak (70.0% and 13.3%), which was lower than (13.3% vs 40.5%) the incidence reported in the literature without bolus [13]. Pignol J-P et al. reported that 28.4% of patients presented with severe moist desquamation at least at 1 assessment, and 32.7% of patients had CTCAE skin toxicity grade 3 [12]. Dahn HM et al. reported that when the bolus was used daily, grade 3 skin toxicity incidence ranged from 45% to 88% [1]. However, in our study, no patients experienced grade 3 or 4 skin toxicity. Similar to the report, skin toxicity usually increases with the treatment dose and reaches a peak 1 week after treatment (sixth week) [10]. Two patients (6.6%) experienced moist desquamation (grade 2 skin toxicity) in the skin folder of the axillary in the fifth and seventh week. These two patients had 25 fractions with bolus in the skin folder of the axillary, and the skin dose reached 5000 cGy. So, the bolus in the skin folder of the axillary is a risk factor for moist desquamation, which should be trimmed in this area.
Our study's low incidence of skin toxicity (13.3% grade 2 and no grade 3 or 4 skin toxicity) is presumably related to the weekly skin care recommendation (e.g. advise the patient to keep the treatment area dry, not scratch, not use any makeup, not touch water even if showering, etc.). In order to reduce the patient's skin toxicity, the treatment fractions with bolus can be reduced [2,18]. Robar JLet.al reported that 42.4 Gy in 16 fractions or 50 Gy in 25 fractions treatment 8 and 12 fractions with bolus [2]. Andic F et al. reported that using a 1-cm thick bolus in up to 15 of the total 25 fractions increased minimum skin doses with a tolerable increase in maximum doses [18]. Use 5 mm bolus common alternate-day [1,19], or discontinue bolus use when patients experience a grade 2 skin toxicity (cumulated target dose was around 40–46 Gy) were reported [15]. According to these findings, we can conclude that the skin toxicity of 20 fractions with TPE bolus is similar (or lower) to those without bolus (grade 2 skin toxicity 13.3% vs 10% [20] or 40.5% [13]). Consistent with the report [21], the incidence of pain in patients increased as the radiotherapy fractions increased. Pignol J-P et al. [12] reported that the pain score was significantly correlated to moist desquamation (P < 0.001) but not to skin dryness (P = 0.76), erythema (P = 0.17), or edema (P = 0.49). In our study, two patients with moist desquamation simultaneously experienced a pain score of 3. Therefore, there was a statistical correlation between moist desquamation and pain. If we used 20 fractions bolus, no moist desquamation and grade 3 or 4 skin toxicity occurred.
A recent study pointed out no statistically significant difference in local recurrence (LR) and breast cancer mortality with and without bolus (10-year LR was 1.9% vs 0.9%) [19]. Therefore, using bolus without large randomized controlled trials remains controversial [22]. However, the bolus is still recommended for patients with skin at risk of recurrence [1,19]. It has been reported that local recurrence is associated with interruption of radiotherapy, with a high rate of local recurrence with a mean interruption time of more than 14.45 days [13]. The treatment interruption rate ranged from 4% to 38% using the bolus, and the rate was 6.0% in the group without the bolus [1]. The patients in our study used TPE bolus, the incidence of treatment interruption (6.6%) was similar to that without bolus (6.0%) reported in the literature, and the maximum interruption was five days. Regarding skin toxicity, interruption, and clinical workload (3D printing bolus need a second CT scan for simulation and about 10 h for segmentation and printing), 20 fractions of TPE bolus is a good choice for patients with a high risk of skin recurrence. Therefore, the TPE bolus can be a better alternative to the 3D printing bolus for PMRT.
Patients who receive postmastectomy radiotherapy understand that the bolus increase the skin dose, which may increase skin toxicity and, therefore, may increase the patient's psychological distress. In our study, the incidence of psychological distress when screened with DT was higher than with GAD-7 or PHQ-9, and patients tended to give higher scores when using DT. GAD-7 screened one patient with anxiety (simultaneous with depression), and PHQ-9 screened two patients with depression (one patient simultaneously experienced depression and anxiety), which were consistent with the results of DT, reaffirming the effectiveness of DT as a primary screening tool. The three patients with obvious psychological distress were mainly related to emotion, not the use of bolus and skin toxicity. 2 of 3 patients experienced psychological distress at the beginning of radiotherapy. Their psychological distress level was maintained at the same level during radiotherapy, indicating the importance of screening and patient care for the first radiotherapy [23]. The overall incidence of psychological distress among the 30 patients is lower than the 31% reported in the literature [24], which is estimated to be related to the fact that the therapists will give more skin care recommendations and encouragement when the patients receive a questionnaire every week.
The limitation of this study is that we used the MOSFET in vivo measurement. Since the MOSFET has a certain volume and is a rigid structure, the fit with the skin is not perfect. The next step may consider using the OSLD or EBT3 film for better fit skin. Second, we did not evaluate late skin toxicity. Thirdly, the enrolled patients were all postmastectomy patients, the chest wall was relatively flat, and the TPE fit well with the skin. The next step will be to evaluate the TPE bolus in post-mastectomy breast reconstruction patients.
5. Conclusion
The TPE bolus can accurately fit skin and improve the surface dose to more than 90%. Twenty fractions with TPE bolus had similar skin toxicity and pain to those without bolus, and did not increase patients' distress and clinical workload compared with the literature's data, which is a better alternative to the 3D printing bolus and a good choice for patients with a high risk of skin recurrence for PMRT.
Author contributions
Pan Gong collected data and drafted the manuscript. Guyu Dai measured the dose in vivo and analyzed the data. Xiaoyu Wu and Shuni Xu helped collect the data and skin care. Li Xie delineated the contour, designed the dose prescription and reviewed the image registration. Xuetao Wang designed treatment plans and analyzed the data. Renming Zhong designed the study, revised and finally approved the manuscript. All authors read and confirmed the manuscript.
Funding
This work was supported by the 1.3.5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (No: 2021HXFH029) and the Science and Technology Support Program of Sichuan province, China (No.2021JDKP0070, 2021YFQ0065).
6. Ethics
The Ethics Committee on Biomedical Research, West China Hospital of Sichuan University, approved this study (Number:2020674).
Declaration of competing interest
The authors declare that they have no competing interests.
References
- 1.Dahn H.M., Boersma L.J., Ruysscher D de, Meattini I., Offersen B.V., Pignol J.-P., et al. The use of bolus in postmastectomy radiation therapy for breast cancer: a systematic review. Crit Rev Oncol Hematol. 2021;163 doi: 10.1016/j.critrevonc.2021.103391. [DOI] [PubMed] [Google Scholar]
- 2.Robar J.L., Moran K., Allan J., Clancey J., Joseph T., Chytyk-Praznik K., et al. Intrapatient study comparing 3D printed bolus versus standard vinyl gel sheet bolus for postmastectomy chest wall radiation therapy. Pract Radiat Oncol. 2018;8(4):221–229. doi: 10.1016/j.prro.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Park S.-Y., Choi C.H., Park J.M., Chun M., Han J.H., Kim J.-I. A patient-specific polylactic acid bolus made by a 3D printer for breast cancer radiation therapy. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0168063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Huang Y., Ding S., Liang J., Kuang J., Mao Q., et al. A clinical trial to compare a 3D-printed bolus with a conventional bolus with the aim of reducing cardiopulmonary exposure in postmastectomy patients with volumetric modulated arc therapy. Cancer Med. 2022;11(4):1037–1047. doi: 10.1002/cam4.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamoto T., Shikama N., Kurokawa C., Hara N., Oshima M., Sasai K. Dosimetric assessment of bolus for postmastectomy radiotherapy. Med Dosim. 2021;46(1):e1–e4. doi: 10.1016/j.meddos.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Dipasquale G., Poirier A., Sprunger Y., Uiterwijk J.W.E., Miralbell R. Improving 3D-printing of megavoltage X-rays radiotherapy bolus with surface-scanner. Radiat Oncol. 2018;13(1):203. doi: 10.1186/s13014-018-1148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehler E., Sterling D., Dusenbery K., Lawrence J. Workload implications for clinic workflow with implementation of three-dimensional printed customized bolus for radiation therapy: a pilot study. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0204944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arenas M., Sabater S., Sintas A., Arguís M., Hernández V., Árquez M., et al. Individualized 3D scanning and printing for non-melanoma skin cancer brachytherapy: a financial study for its integration into clinical workflow. J Contemp Brachytherapy. 2017;9(3):270–276. doi: 10.5114/jcb.2017.68134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen K., Xiong J., Wang Z., Wang W., Li W., Zhou J., et al. Design of a new breast vacuum bag to reduce the global and local setup errors and to reduce PTV margin in post-mastectomy radiation therapy. J Radiat Res. 2020;61(6):985–992. doi: 10.1093/jrr/rraa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias A.G., Pinto D.F.S., Borges M.F., Pereira M.H., Santos J.A.M., Cunha L.T., et al. Optimization of skin dose using in-vivo MOSFET dose measurements in bolus/non-bolus fraction ratio: a VMAT and a 3DCRT study. J Appl Clin Med Phys. 2019;20(2):63–70. doi: 10.1002/acm2.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C.-J., Hou M.-F., Luo K.-H., Wei S.-Y., Huang M.-Y., Su S.-J., et al. RTOG, CTCAE and WHO criteria for acute radiation dermatitis correlate with cutaneous blood flow measurements. Breast. 2015;24(3):230–236. doi: 10.1016/j.breast.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Pignol J.-P., Vu T.T.T., Mitera G., Bosnic S., Verkooijen H.M., Truong P. Prospective evaluation of severe skin toxicity and pain during postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 2015;91(1):157–164. doi: 10.1016/j.ijrobp.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Abel S., Renz P., Trombetta M., Cowher M., Day Werts E., Julian T.B., et al. Local failure and acute radiodermatological toxicity in patients undergoing radiation therapy with and without postmastectomy chest wall bolus: is bolus ever necessary? Pract Radiat Oncol. 2017;7(3):167–172. doi: 10.1016/j.prro.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Ullrich A., Ascherfeld L., Marx G., Bokemeyer C., Bergelt C., Oechsle K. Quality of life, psychological burden, needs, and satisfaction during specialized inpatient palliative care in family caregivers of advanced cancer patients. BMC Palliat Care. 2017;16(1):31. doi: 10.1186/s12904-017-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobo D., Banerjee S., Srinivas C., Athiyamaan M.S., Reddy S., Sunny J., et al. Surface dose measurements in chest wall postmastectomy radiotherapy to achieve optimal dose delivery with 6 MV photon beam. J Med Phys. 2021;46(4):324–333. doi: 10.4103/jmp.jmp_59_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiedler D.A., Hoffman S., Roeske J.C., Hentz C.L., Small W., Kang H. Dosimetric assessment of brass mesh bolus and transparent polymer-gel type bolus for commonly used breast treatment delivery techniques. Med Dosim. 2021;46(3):e10–e14. doi: 10.1016/j.meddos.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Qi Z.-Y., Deng X.-W., Huang S.-M., Zhang L., He Z.-C., Li X.A., et al. In vivo verification of superficial dose for head and neck treatments using intensity-modulated techniques. Med Phys. 2009;36(1):59–70. doi: 10.1118/1.3030951. [DOI] [PubMed] [Google Scholar]
- 18.Andic F., Ors Y., Davutoglu R., Baz Cifci S., Ispir E.B., Erturk M.E. Evaluation of skin dose associated with different frequencies of bolus applications in post-mastectomy three-dimensional conformal radiotherapy. J Exp Clin Cancer Res. 2009;28:41. doi: 10.1186/1756-9966-28-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichol A., Narinesingh D., Raman S., Germain F., Chan E.K., Tran E., et al. The effect of bolus on local control for patients treated with mastectomy and radiation therapy. Int J Radiat Oncol Biol Phys. 2021;110(5):1360–1369. doi: 10.1016/j.ijrobp.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Shiba S., Okamoto M., Kiyohara H., Okano N., Yoshimoto Y., Murata H., et al. Clinical advantage of chest-wall post-mastectomy radiation therapy without bolus. In Vivo (Attiki) 2018;32(4):961–965. doi: 10.21873/invivo.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam E., Wong G., Zhang L., Drost L., Karam I., Yee C., et al. Self-reported pain in breast cancer patients receiving adjuvant radiotherapy. Support Care Cancer. 2021;29(1):155–167. doi: 10.1007/s00520-020-05462-5. [DOI] [PubMed] [Google Scholar]
- 22.Kaidar-Person O., Dahn H.M., Nichol A.M., Boersma L.J., Ruysscher D de, Meattini I., et al. A Delphi study and International Consensus Recommendations: the use of bolus in the setting of postmastectomy radiation therapy for early breast cancer. Radiother Oncol. 2021;164:115–121. doi: 10.1016/j.radonc.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Grilo A.M., Gomes A.I., Monsanto F., Albino D., Augusto C., Pragana C. First day of radiotherapy for women with breast cancer: predictors of anxiety. Support Care Cancer. 2020;28(3):1241–1248. doi: 10.1007/s00520-019-04902-1. [DOI] [PubMed] [Google Scholar]
- 24.Antoni D., Vigneron C., Clavier J.-B., Guihard S., Velten M., Noel G. Anxiety during radiation therapy: a prospective randomized controlled trial evaluating a specific one-on-one procedure announcement provided by a radiation therapist. Cancers. 2021;13(11) doi: 10.3390/cancers13112572. [DOI] [PMC free article] [PubMed] [Google Scholar]