Abstract
Background
Accumulating evidence shows that homocysteine (Hcy) is implicated in the pathophysiology of schizophrenia, and plays an important role in clinical characteristics. This study evaluated the relationships between Hcy levels and clinical features in first-episode, Chinese Han, drug-naïve (FEDN) patients with schizophrenia.
Methods
FEDN individuals (119 with schizophrenia and 81 healthy controls matched for age, sex, education, and body mass index (BMI)) were enrolled. The serum Hcy levels were determined by enzyme cycle assay experiments. Severities of clinical symptoms were rated on the Positive and Negative Syndrome Scale (PANSS).
Results
FEDN individuals with schizophrenia had higher Hcy levels compared with healthy controls (F = 46.865, P < 0.001). Correlation analysis and multiple stepwise regression analyses showed that serum Hcy levels in FEDN schizophrenia individuals were positively correlated with PANSS general psychopathology subscale (r = 0.294, P = 0.001) and PANSS total score (r = 0.273, P = 0.003). No significant association was found between Hcy and age, BMI, PANSS positive subscale, and the PANSS negative subscale (all, P > 0.05). Male individuals had significantly higher serum Hcy levels than female individuals (F = 7.717, P = 0.006) after controlling for confounding factors (F = 0.759, P = 0.011).
Conclusions
Serum Hcy levels were increased in FEDN individuals with schizophrenia, and Hcy levels may be involved in pathophysiological mechanisms. Sex differences in Hcy levels were observed, with higher levels in male FEDN individuals compared to females.
Keywords: Schizophrenia, Homocysteine, Sex difference, Pathophysiology
Introduction
Schizophrenia has an insidious onset, and is a chronic and highly disabling mental disorder, which affects approximately 1% of the worldwide population [1]. The manifestations of schizophrenia are heterogeneous in term of positive symptoms, negative symptoms, and cognitive impairments, which mostly occur in early adulthood [2, 3]. The availability of atypical antipsychotics has alleviated or mitigated some symptoms of schizophrenia, but characteristics of the disorder still constitute a major social burden [4, 5]. However, the pathophysiological mechanism of schizophrenia still remains multifactorial and unclear.
Homocysteine (Hcy) is a non-protein and sulfur-containing amino acid originating from the essential amino acid, methionine, which is derived from dietary proteins and involved in many pathophysiological processes [6]. Hcy is a pro-oxidant, which contributes to oxidative stress in nerve cells [7]. Previous studies have reported that Hcy interacted with N-methyl-D-aspartate receptors or participated in oxidative stress, nitrosative stress, the transsulfuration pathways, pro-inflammatory states, mitochondrial dysfunction, and DNA methylation [8–10]. A mouse model has also revealed a close relationship between hyperhomocysteinemia and inflammatory cytokines activated via NF-κB and microglial cells [11]. These studies partially explain the mechanisms involved in Hcy abnormalities leading to schizophrenia. A Mendelian randomization study reported that increased plasma Hcy levels increased the risk of schizophrenia and bipolar disorders [12]. A systematic review and meta-analysis reported that levels of Hcy were higher in first-episode psychosis compared to controls, suggesting that an imbalance of antioxidant status may be relevant to first-episode psychosis [13]. In addition, the relationships between abnormal Hcy levels and schizophrenia and clinical symptoms have been reported in different countries such as United States [14], Tunisia [15], Republic of Korea [16], Turkey [17], and India [18]; however, the results are controversial and even some negative results have been reported [19, 20].
Moreover, stress, poor lifestyle, age, catabolic dysregulation, and genetics can lead to abnormalities in Hcy, while abnormal Hcy levels may be considered as an etiological or risk factor in neurodegenerative diseases such as dementias, Alzheimer’s disease, Parkinson’s disease, cardiovascular diseases, and metabolic disease [21–23], but individuals in northern Italy with severe mental illness may not be at higher risk of cardiovascular disease than the general population, especially in the relatively wealthy areas and with traditional healthy dietary habits such as the Mediterranean diet [24]. A recent Mendelian Randomization study reported that in the general population, higher Hcy concentration was a risk factor for metabolic syndrome, but not for body mass index (BMI) [25], while another study reported that higher Hcy levels correlated with lower BMIs in schizophrenia patients [26]. In addition, several studies have reported that sex may also be a factor affecting Hcy in the general population [27], in bipolar disorders [28], and even in rat animal models [29]. First-episode drug-naïve (FEDN) individuals with schizophrenia are the optimal population to evaluate the relationships between Hcy and schizophrenia and clinical features, because of the absence of confounding factors such as medication, BMI, diet, and the stage of illness.
The present study therefore aimed to assess Hcy levels and its associations with clinical features in FEDN schizophrenia individuals of Chinese Han ethnicity. We hypothesized that Hcy levels may be altered and may be correlated with schizophrenia clinical features. The results showed (1) whether serum Hcy levels differed between FEDN individuals with schizophrenia and healthy controls and (2) whether there were associations between serum Hcy levels, sex, and BMI, and the severities of clinical symptoms.
Methods
Subjects and clinical assessments
FEDN schizophrenia individuals were enrolled at Beijing Hui-Long-Guan Hospital. The inclusion criteria included (1) age of 18—45 years, (2) Han Chinese ethnicity, (3) meeting the criteria of schizophrenia according to the Structured Clinical Interview of the Diagnostic and Statistical Manual-IV by two psychiatrists, and (4) patients were never treated with antipsychotics. Their demographic data included sex, age, years of education, smoking, BMI, duration of illness, and age at onset, using a questionnaire. The Positive and Negative Syndrome Scale (PANSS) [30] was used by two experienced psychiatrists to assess the psychopathological status of FEDN schizophrenia individuals. The PANSS score of interrater correlation was 0.8.
Healthy controls were enrolled after responding to advertisements in Beijing. They were matched for sex, age, years of education, and BMI. Individuals were also evaluated for mental status using Axis I disorders. Candidates who met psychiatric disorders or a family history for psychiatric disorders were excluded.
The health status of each participant was identified by physical examinations and laboratory tests. Individuals with the following conditions were excluded: mental retardation, epilepsy, traumatic or chronic brain injury, diabetes, thyroid diseases, infections, cardiovascular and cerebrovascular diseases, pregnancy, and drug or alcohol abuse/dependence.
All subjects or their guardians gave informed written consent, and the research protocol was approved by the Ethics and Research Committee of Beijing Hui-Long-Guan Hospital.
Hcy determination
WE used an EDTA tube to collect venous blood samples from patients and healthy controls between 08:00 and 09:00 after overnight fasting. The collected blood samples were immediately centrifuged (3000 rpm) for collection of serum for Hcy concentrations, which were determined by enzyme cycle assay experiments using commercially available kit instructions (Beijing Leadman Biotechnology, Beijing, China). Sample quality was observed before detection to exclude hemolysis, celiac blood, etc. All blood samples were measured by standard blood biochemistry assays on a AU5800 automated biochemical analyzer (Beckman Coulter, Tokyo, Japan). The parameters were set before the assay: R1 reagent, sample and R2 reagent were added at 0, 18 and 198 s, respectively, and the first measurement time point was 396 s, the second measurement time point was 486 s, the primary wavelength was 340 nm and the secondary wavelength was 700 nm. The same investigator who was blinded to the clinical status tested all samples.
Statistical analysis
All data were analyzed using the Statistical Package for Social Sciences (SPSS), version 19.0 (SPSS, Chicago, IL, USA). If data were nonparametric according to the Kolmogorov–Smirnov test, they were transformed into normally distributed data using a logarithm. The transformed values were used for comparisons, while the original data were used for descriptions and were expressed as the median (25th and 75th quartiles). As Hcy levels was not normally distributed, we log-transformed the Hcy values. Continuous variables between groups were analyzed using analysis of variance, and expressed as mean ± standard deviation, and categorical variables were analyzed using the chi-squared test. Analysis of covariance was used to analyze potential confounding variables including sex, age, years of education, and BMI. Pearson’s correlation coefficients were used to evaluate the associations between variables. The Bonferroni correction was used to control for multiple tests. Multiple stepwise regression analysis was used to assess the relationship of Hcy levels with demographic and clinical characteristics. P < 0.05 was considered statistically significant.
Results
Sociodemographic characteristics
A total of 119 FEDN schizophrenia subjects were enrolled including 60 males and 59 females, with average age of 27.73 ± 7.54 years, mean years of education of 12.23 ± 2.00 years, mean duration of untreated illness of 1.99 ± 1.83 years, average age at onset of 25.75 ± 6.56 years, and BMI of 24.62 ± 2.96 kg/m2. A total of 81 healthy controls were enrolled, including 42 males and 39 females. Table 1 shows the demographics and clinical profiles of FEDN schizophrenia individuals and healthy controls. There was no significant difference in sex, age, years of education, smoking, and BMI (all P > 0.05).
Table 1.
Demographics and clinical characteristics of FEDN patients with schizophrenia and healthy controls
| FEDN (n = 119) | HC (n = 81) | F/χ2 | P | |
|---|---|---|---|---|
| Age (years)a | 27.73 ± 7.54 | 25.95 ± 6.56 | 2.979 | 0.086 |
| Sex (M/F) | 60/59 | 42/39 | 0.040 | 0.842 |
| Education (years)a | 12.23 ± 2.00 | 12.57 ± 2.31 | 1.235 | 0.268 |
| Smoking (Yes/No) | 25/94 | 13/68 | 0.770 | 0.380 |
| BMI (kg/m2)a | 24.62 ± 2.96 | 24.30 ± 2.39 | 0.609 | 0.436 |
| Duration of untreated illness (years)a | 1.99 ± 1.83 | |||
| Age at onset (years)a | 25.75 ± 6.56 | |||
| PANSS positive subscorea | 27.91 ± 7.33 | |||
| PANSS negative subscorea | 16.09 ± 7.58 | |||
| PANSS general subscorea | 25.18 ± 9.29 | |||
| PANSS total scorea | 69.18 ± 14.81 | |||
| Hcy (μmol/L)b | 19.80 (15.51, 16.40) | 13.01 (11.65, 16.55) | 46.865 | < 0.001 |
FEDN first-episode drug-naïve, HC healthy control, BMI body mass index, PANSS Positive and Negative Syndrome Scale, Hcy homocysteine
aThe data were expressed as the mean ± standard deviation
bThe data were expressed as the median (25th quartile and 75th quartile). Significant difference (P < 0.05) is indicated in bold
Serum Hcy concentrations in FEDN individuals and healthy controls
Because the serum Hcy concentrations were nonparametrically distributed both in FEDN individuals and healthy controls according to the Kolmogorov–Smirnov test (P < 0.05), we first transformed the data into normal distributions using logarithmic transformation. The log-transformed values for individuals with schizophrenia and controls groups were 1.32 ± 0.21 vs 1.15 ± 0.13 μmol/L, and analysis of variance showed that serum Hcy concentrations were significantly higher in FEDN individuals with schizophrenia than in healthy controls (P < 0.001) (Table 1, Fig. 1). Analysis of variance also showed that there was significant difference after controlling for sex, age, years of education, smoking, and BMI (F = 44.49, P < 0.001).
Fig. 1.
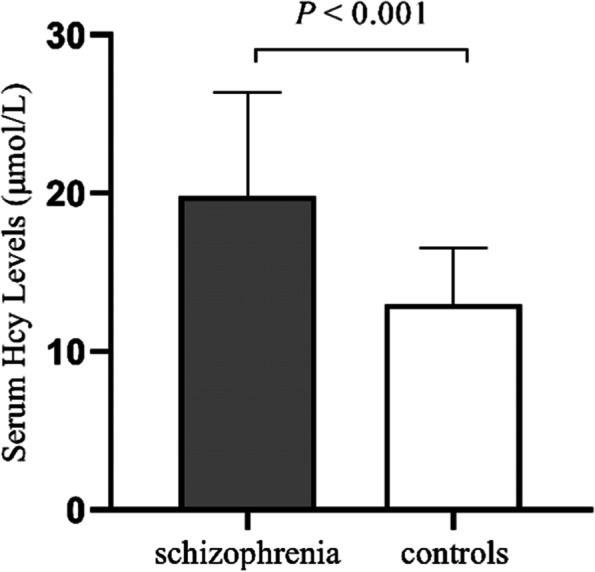
Serum levels of Hcy in FEDN patients with schizophrenia and in healthy controls
Associations between serum Hcy concentrations and clinical symptoms
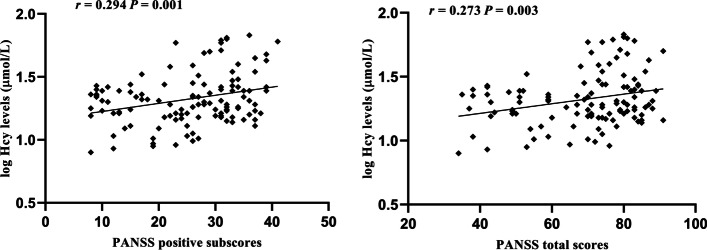
Pearson’s correlation analysis showed that serum Hcy concentration was positively associated with PANSS general subscore (r = 0.294, df = 119, P = 0.001) and PANSS total score (r = 0.273, df = 119, P = 0.003) in FEDN schizophrenia individuals (Fig. 2). There were also significant differences after Bonferroni corrections (all P < 0.05), but no significant association between levels of Hcy and the PANSS positive subscale, PANSS negative subscale, and BMI (all P > 0.05).
Fig. 2.
Correlations between Hcy levels and PANSS positive subscores and total scores
Multiple stepwise regression showed that sex (beta = 0.694, t = 10.625, P < 0.001), duration of illness (beta = -0.168, t = -2.636, P = 0.010), and Hcy (beta = 0.144, t = 2.200, P = 0.030) were influencing factors for the PANSS general psychopathology subscale (R2 = 0.544), and sex (beta = 0.554, t = 7.335, P < 0.001), duration of illness (beta = -0.228, t = -3.087, P = 0.003), and Hcy (beta = 0.166, t = 2.197, P = 0.030) were correlated with PANSS total scores (R2 = 0.390) after controlling for age, years of education, age at onset, smoking, and BMI.
Sex differences in Hcy levels with clinical symptoms
Table 2 shows that serum Hcy levels, PANSS positive symptoms, negative symptoms, general psychopathology, and PANSS total scores were significantly different between male and female patients (all P < 0.05) except for age, years of education, BMI, duration of illness, and age at onset (all P > 0.05).
Table 2.
Characteristics of male and female FEDN patients with schizophrenia
| Male | Female | F | P | |
|---|---|---|---|---|
| Age (years)a | 28.62 ± 8.05 | 26.83 ± 6.95 | 1.677 | 0.198 |
| Education (years)a | 12.18 ± 2.00 | 12.27 ± 2.02 | 0.057 | 0.812 |
| BMI (kg/m2)a | 24.52 ± 3.48 | 24.71 ± 2.34 | 0.115 | 0.735 |
| Duration of illness (years)a | 2.22 ± 2.18 | 1.75 ± 1.35 | 1.999 | 0.160 |
| Age at onset (years)a | 26.40 ± 6.72 | 25.08 ± 6.37 | 1.198 | 0.276 |
| PANSS positive subscalea | 25.67 ± 7.81 | 30.19 ± 6.06 | 12.421 | 0.001 |
| PANSS negative subscalea | 20.12 ± 7.65 | 12.00 ± 4.85 | 47.550 | < 0.001 |
| PANSS general subscalea | 31.68 ± 6.21 | 18.58 ± 6.95 | 117.764 | < 0.001 |
| PANSS total scorea | 77.47 ± 9.60 | 60.76 ± 14.48 | 55.211 | < 0.001 |
| Hcy (μmol/L)b | 21.95 (16.25, 29.03) | 19.10 (14.90, 23.80) | 7.717 | 0.006 |
BMI body mass index, PANSS Positive and Negative Syndrome Scale, Hcy homocysteine
aThe data are expressed as the mean ± standard deviation
b The data are expressed as the median (25th quartile and 75th quartile). Significant differences (P < 0.05) are indicated in bold
After adjusting for age, years of education, smoking, BMI, duration of illness, and age at onset, we found using analysis of variance that Hcy levels were significantly higher in male FEND schizophrenia patients than in females (F = 6.759, P = 0.011). No significant difference in Hcy level was found between male and female healthy controls (P > 0.05).
Discussion
IN the present study, we found that (1) FEDN individuals with schizophrenia had higher serum Hcy levels than healthy controls, (2) serum concentrations of Hcy were positively correlated with the PANSS general psychopathology subscale and PANSS total scores for patients, and (3) male patients had significantly higher Hcy levels than female patients. To the best of our knowledge, few studies have reported the sex difference in serum Hcy levels in FEDN individuals with schizophrenia of Chinese ethnicity [31, 32].
The present study showed that serum levels of Hcy were significantly increased in FEDN schizophrenia individuals, when compared to healthy controls, which is consistent with other reports [26, 33]. A cross-sectional study reported that Hcy levels were increased in schizophrenia, when compared with bipolar disorder patients [34]. Another study reported that common polygenic variants, such as methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism, correlated with plasma total Hcy levels, which had a cumulative effect on schizophrenia and may be a risk factor for this disorder [35]. There is also increasing evidence that Hcy is directly or indirectly involved in multiple pathways in pathophysiological mechanisms of schizophrenia. Methylation pathway defects (including catecholamine methylation) due to folate and cobalamin deficiencies, and impaired re-methylation of methionine by Hcy, can cause hyperhomocysteinemia [36]. Another impaired pathway is transsulfuration pathway, which is a metabolic pathway from Hcy to L-cysteine to glutathione (GSH), resulting in reduced GSH levels and oxidative phosphorylation [37]. In addition, Hcy induces immune responses, and in a mouse model, elevated Hcy activates nuclear translocation of transcription factor NF-κB and increased expressions of interleukins-1β and tumor necrosis factor-α [11]. However, the exact etiology and pathways of Hcy in schizophrenia remain ambiguous and warrant further study.
Another finding of the study was that serum Hcy levels were positively correlated with psychopathological symptoms of FEDN schizophrenia individuals. Similar results have been reported in several previous studies. Trześniowska-Drukała et al. [38] reported that increased Hcy concentrations resulted in worse cognitive functions and higher PANSS scores in patients with schizophrenia, suggesting that Hcy blood levels were related to the severity of schizophrenia. Song et al. [39] reported that increased serum Hcy levels positively correlated with PANSS total scores in FEDN schizophrenia patients after controlling for influencing factors. Petronijević et al. [40] reported that plasma Hcy levels were positively correlated with PANSS negative subscores both in the exacerbation and remission phases of young male schizophrenia patients. Gao et al. [41] reported that MTHFR C677T polymorphism was correlated with PANSS negative symptoms and cognitive deficits in chronic schizophrenia patients. Furthermore, models fed Hcy were found to have significantly impaired cognitive functions during the reversal phase of the Morris water maze test [42]. These findings suggested that increased Hcy levels were closely related to the severity of clinical symptoms and cognitive symptoms of schizophrenia in different phases of pathology. The exact pathological mechanisms underlying the correlation between Hcy and the severity of clinical symptoms are not known. However, a review reported that Hcy may regulate dopamine, serotonin, acetylcholine, glutamate function, and the interaction between Hcy and oxidative stress and neuronal apoptosis, and that aberrant DAN methylation were related to schizophrenia [43]. Together, these results suggested that elevated Hcy may play a role in the disease process of schizophrenia, possibly as a consequence of pathological processes involved in schizophrenia or as a causative factor of schizophrenia, so the relationship between elevated Hcy levels and the clinical symptoms of schizophrenia needs further study.
Previous studies have proposed there are several factors influencing Hcy levels such as age, sex, BMI, smoking, coffee drinking, poor nutrition, vitamin intake, folate, and ultraviolet radiation [44, 45]. Previous studies have reported differences in Hcy levels between male and female schizophrenia patients, although the results have been inconsistent. Meta-analyses suggested that plasma total Hcy levels were higher in both male and female schizophrenia patients [46]. A cross sectional study also reported no significant disparity in hyperhomocysteinemia between male and female schizophrenia patients [34]. In the present study, we found that the serum Hcy levels were higher in male patients with FEDN schizophrenia, when compared with female patients, which was consistent with the findings of an earlier study [42]. Another study reported that male sex and older age were risk factors for developing hyperhomocysteinemia in schizophrenia patients [31]. These inconsistencies in the results may be due to different antipsychotic medications, methods of Hcy measurement, different phases of the disease, nutrient status, and ethnicity. Notably, sex differences in Hcy levels were found in patients with schizophrenia, and also in patients with bipolar disorders [47], and Alzheimer’s dementia [48]. However, the exact etiological mechanism underlying the discrepancy in Hcy levels between males and females remains unclear. A large cross-sectional study in the general population reported that the ratio of increased plasma Hcy concentrations, defined as abnormal Hcy results at above 15 μmol/L, were higher in males than in females [49]. The study reported that a possible explanation for the differences in Hcy between males and females was that Hcy metabolism was different, which involved the different pathways of trans-sulfuration and methylation. A review stated that high dose testosterone administration increased total Hcy levels in female-to-male transsexuals [50]. Two other studies reported that hormone treatment, oral ethinyl estradiol and transdermal 17beta estradiol treatments for male-to-female transsexuals reduced Hcy levels [51, 52]. Moreover, one study reported a significant sex disparity in Hcy levels for MTHFR C677T polymorphism [46]. Consequently, we postulate that hormone levels and genetics may be causative factors contributing to sex differences in Hcy levels.
In the present study, we found that severities of clinical symptoms were significantly different between male and female FEDN individuals with schizophrenia, which were consistent with the results of other studies [53, 54]. However, some discrepant results were reported due to different stages of illness, duration of illness, representation of research subjects, and administration of medications. In addition, there was no significant relationship of Hcy concentrations for BMI in our study. Although the results are the same as previously reported [55], there are nevertheless many reports of varying results, suggesting that Hcy concentrations correlated with BMI in patients with schizophrenia [26, 56]. The Hcy levels were closely associated with elderly males, obesity, uric acid content, glycolipid metabolism, and metabolic syndromes [31, 48, 57], whereas our study subjects were on average young and unmedicated, mitigating the effects of confounding factors on Hcy levels, so the results may be more accurate.
There were several limitations in this study. First, based on a cross-sectional study, a causal interaction between Hcy and clinical profile could not be determined. Second, the study subjects were recruited from only one region, and it is possible that different dietary habits and nutrition may have had an impact on the results, so future studies will need to be conducted in a more general area. Third, we did not collect markers related to B vitamins, folate, and glycolipid metabolism, which previous studies suggested may have had an effect on Hcy levels. Fourth, in our study, the number of control sample was less than the patient sample, and will need to be matched in further studies.
In summary, our findings confirmed that serum Hcy levels were higher in FEDN individuals with schizophrenia, when compared with healthy controls, and that higher Hcy levels were correlated with severe clinical symptoms. Importantly, male FEDN schizophrenia individuals had higher Hcy levels than female individuals. Considering the limitations, the present study preliminarily suggested that Hcy played an important role in the pathophysiology of schizophrenia, which requires further validation in a longitudinal study.
Acknowledgements
We would like to thank the participants in the study.
Authors’ contributions
Xu Yang and Haidong Yang wrote the manuscript; Xiaobin Zhang and Weiye Liang were responsible for study design; Haidong Yang performed the statistical analysis; Xu Yang, Na Li, and Chunyu Li were responsible for recruiting the patients, performing the clinical rating and collecting the samples. All authors have contributed to and have approved the final manuscript.
Funding
The study was supported by Suzhou Clinical Medical Center for Mood Disorders (Szlcyxzx202109), The Capital Health Research and Development of Special (2020–1-2131), Suzhou Clinical Key disciplines for Geriatric Psychiatry (SZXK202116), General Program of Lianyungang Health Committee (NO.202130), and Suzhou Key Technology Research (SKY2021063). The finding sources of this study had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All experimental protocols were approved by the Ethics Committee of Beijing Hui Long Guan Hospital. Informed consent was obtained from all subjects. All methods were carried out in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xu Yang and Haidong Yang contributed equally to this study. They should be regarded as joint first authors.
Contributor Information
Xu Yang, Email: qyyangxu1983@163.com.
Haidong Yang, Email: yanghaidonglyg@163.com.
Na Li, Email: yxln1983@sina.com.
Chunyu Li, Email: rainnotears@163.com.
Weiye Liang, Email: lwy_00@126.com.
Xiaobin Zhang, Email: zhangxiaobim@163.com.
References
- 1.McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-An Overview. JAMA Psychiat. 2020;77(2):201–210. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- 2.Carra G, Crocamo C, Angermeyer M, Brugha T, Toumi M, Bebbington P. Positive and negative symptoms in schizophrenia: A longitudinal analysis using latent variable structural equation modelling. Schizophr Res. 2019;204:58–64. doi: 10.1016/j.schres.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Mihaljević-Peleš A, BajsJanović M, Šagud M, Živković M, Janović Š, Jevtović S. Cognitive deficit in schizophrenia: an overview. Psychiatr Danub. 2019;31(Suppl 2):139–142. [PubMed] [Google Scholar]
- 4.Zhong Q, Tan Y, Chen W, Huang H, Huang J, Li S, Teng Z, Shen M, Wu C, Wang L, et al. Disease burden of schizophrenia patients visiting a Chinese regional mental health centre. J Comp Eff Res. 2020;9(7):469–481. doi: 10.2217/cer-2019-0129. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Liou TH, Miao NF, Chang KH, Yen CF, Liao HF, Chi WC, Chou KR. Using World Health Organization Disability Assessment Schedule 2.0 in people with schizophrenia: a 4-year follow-up. Eur Arch Psychiatry Clin Neurosci. 2020;270(3):301–310. doi: 10.1007/s00406-019-01000-5. [DOI] [PubMed] [Google Scholar]
- 6.Esse R, Barroso M, Tavares de Almeida I, Castro R. The Contribution of homocysteine metabolism disruption to endothelial dysfunction: state-of-the-art. Int J Mol Sci. 2019;20(4):867. doi: 10.3390/ijms20040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tchantchou F, Goodfellow M, Li F, Ramsue L, Miller C, Puche A, Fiskum G. Hyperhomocysteinemia-Induced Oxidative Stress Exacerbates Cortical Traumatic Brain Injury Outcomes in Rats. Cell Mol Neurobiol. 2021;41(3):487–503. doi: 10.1007/s10571-020-00866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharjee N, Borah A. Oxidative stress and mitochondrial dysfunction are the underlying events of dopaminergic neurodegeneration in homocysteine rat model of Parkinson's disease. Neurochem Int. 2016;101:48–55. doi: 10.1016/j.neuint.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Gorny M, Wnuk A, Kaminska A, Kaminska K, Chwatko G, Bilska-Wilkosz A, Iciek M, Kajta M, Rogoz Z, Lorenc-Koci E. Glutathione Deficiency and alterations in the sulfur amino acid homeostasis during early postnatal development as potential triggering factors for schizophrenia-like behavior in adult rats. Molecules. 2019;24(23):4253. doi: 10.3390/molecules24234253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canever L, Alves CSV, Mastella G, Damazio L, Polla JV, Citadin S, De Luca LA, Barcellos AS, Garcez ML, Quevedo J, et al. The evaluation of folic acid-deficient or folic acid-supplemented diet in the gestational phase of female rats and in their adult offspring subjected to an animal model of schizophrenia. Mol Neurobiol. 2018;55(3):2301–2319. doi: 10.1007/s12035-017-0493-7. [DOI] [PubMed] [Google Scholar]
- 11.Elsherbiny NM, Sharma I, Kira D, Alhusban S, Samra YA, Jadeja R, Martin P, Al-Shabrawey M, Tawfik A. Homocysteine Induces Inflammation in Retina and Brain. Biomolecules. 2020;10(3):393. doi: 10.3390/biom10030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Xue R, Wang Q, Yu H, Liu X. The effects of plasma homocysteine level on the risk of three major psychiatric disorders: a Mendelian randomization study. Front Psychiatry. 2022;13:841429. doi: 10.3389/fpsyt.2022.841429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraguas D, Diaz-Caneja CM, Ayora M, Hernandez-Alvarez F, Rodriguez-Quiroga A, Recio S, Leza JC, Arango C. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. 2019;45(4):742–751. doi: 10.1093/schbul/sby125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordaro M, Siracusa R, Fusco R, Cuzzocrea S, Di Paola R, Impellizzeri D. Involvements of Hyperhomocysteinemia in neurological disorders. Metabolites. 2021;11(1):37. doi: 10.3390/metabo11010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouaziz N, Ayedi I, Sidhom O, Kallel A, Rafrafi R, Jomaa R, Melki W, Feki M, Kaabechi N, El Hechmi Z. Plasma homocysteine in schizophrenia: determinants and clinical correlations in Tunisian patients free from antipsychotics. Psychiatry Res. 2010;179(1):24–29. doi: 10.1016/j.psychres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Kim TH, Moon SW. Serum homocysteine and folate levels in Korean schizophrenic patients. Psychiatry Investig. 2011;8(2):134–140. doi: 10.4306/pi.2011.8.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazici E, MutuPek T, Guzel D, Yazici AB, AkcayCiner O, Erol A. Klotho, vitamin D and homocysteine levels during acute episode and remission periods in schizophrenia patients. Nord J Psychiatry. 2019;73(3):178–184. doi: 10.1080/08039488.2019.1582697. [DOI] [PubMed] [Google Scholar]
- 18.Narayan SK, Verman A, Kattimani S, Ananthanarayanan PH, Adithan C. Plasma homocysteine levels in depression and schizophrenia in South Indian Tamilian population. Indian journal of psychiatry. 2014;56(1):46–53. doi: 10.4103/0019-5545.124746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayesa-Arriola R, Perez-Iglesias R, Rodriguez-Sanchez JM, Mata I, Gomez-Ruiz E, Garcia-Unzueta M, Martinez-Garcia O, Tabares-Seisdedos R, Vazquez-Barquero JL, Crespo-Facorro B. Homocysteine and cognition in first-episode psychosis patients. Eur Arch Psychiatry Clin Neurosci. 2012;262(7):557–564. doi: 10.1007/s00406-012-0302-2. [DOI] [PubMed] [Google Scholar]
- 20.Reif A, Schneider MF, Kamolz S, Pfuhlmann B. Homocysteinemia in psychiatric disorders: association with dementia and depression, but not schizophrenia in female patients. J Neural Transm (Vienna) 2003;110(12):1401–1411. doi: 10.1007/s00702-003-0061-3. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan P, Tatarkova Z, Sivonova MK, Racay P, Lehotsky J. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. Int J Mol Sci. 2020;21(20):7698. doi: 10.3390/ijms21207698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul R, Phukan BC, Justin Thenmozhi A, Manivasagam T, Bhattacharya P, Borah A. Melatonin protects against behavioral deficits, dopamine loss and oxidative stress in homocysteine model of Parkinson's disease. Life Sci. 2018;192:238–245. doi: 10.1016/j.lfs.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Shen W, Gao C, Cueto R, Liu L, Fu H, Shao Y, Yang WY, Fang P, Choi ET, Wu Q, et al. Homocysteine-methionine cycle is a metabolic sensor system controlling methylation-regulated pathological signaling. Redox Biol. 2020;28:101322. doi: 10.1016/j.redox.2019.101322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clerici M, Bartoli F, Carretta D, Crocamo C, Bebbington P, Carra G. Cardiovascular risk factors among people with severe mental illness in Italy: a cross-sectional comparative study. Gen Hosp Psychiatry. 2014;36(6):698–702. doi: 10.1016/j.genhosppsych.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Lee HS, In S, Park T. The homocysteine and metabolic syndrome: a Mendelian randomization study. Nutrients. 2021;13(7):2440. doi: 10.3390/nu13072440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Wu K, Li H, Zhou J, Xiong D, Huang X, Li J, Liu Y, Pan Z, Mitchell DT, et al. Homocysteine level, body mass index and clinical correlates in Chinese Han patients with schizophrenia. Sci Rep. 2020;10(1):16119. doi: 10.1038/s41598-020-72934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu R, Huang F, Wang Y, Liu Q, Lv Y, Zhang Q. Gender- and age-related differences in homocysteine concentration: a cross-sectional study of the general population of China. Sci Rep. 2020;10(1):17401. doi: 10.1038/s41598-020-74596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu L, Lin Y, Huang X, Ning Y, Wu F, Zhang XY. Sex differences in the prevalence and clinical correlates of hyperhomocysteinemia in patients with bipolar disorder. Hum Psychopharmacol. 2020;35(2):e2724. doi: 10.1002/hup.2724. [DOI] [PubMed] [Google Scholar]
- 29.de Souza FG, Rodrigues MD, Tufik S, Nobrega JN, D'Almeida V. Acute stressor-selective effects on homocysteine metabolism and oxidative stress parameters in female rats. Pharmacol Biochem Behav. 2006;85(2):400–407. doi: 10.1016/j.pbb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Wang J, Xiong Z, Yao X, Zhang Y, Ning X, Zhong Y, Liu Z, Zhang Y, Zhao T, et al. Prevalence and clinical demography of hyperhomocysteinemia in Han Chinese patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2021;271(4):759–765. doi: 10.1007/s00406-020-01150-x. [DOI] [PubMed] [Google Scholar]
- 32.Zhou S, Huang Y, Kuang Q, Yan S, Feng Y, Li H, Wu K, Wu F, Huang X. Gender differences in associations of cognitive impairments with homocysteine in schizophrenia. Asian J Psychiatr. 2022;75:103214. doi: 10.1016/j.ajp.2022.103214. [DOI] [PubMed] [Google Scholar]
- 33.Lyu N, Xing G, Yang J, Zhu X, Zhao X, Zhang L, Wang G. Comparison of inflammatory, nutrient, and neurohormonal indicators in patients with schizophrenia, bipolar disorder and major depressive disorder. J Psychiatr Res. 2021;137:401–408. doi: 10.1016/j.jpsychires.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Fe'li SN, YassiniArdekani SM, Dehghani A. Relationship between serum homocysteine and metabolic syndrome among patients with schizophrenia and bipolar disorder: a cross sectional study. Iran J Psychiatry. 2020;15(4):266–273. doi: 10.18502/ijps.v15i4.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinoshita M, Numata S, Tajima A, Nishi A, Muraki S, Tsuchiya A, Umehara H, Watanabe SY, Imoto I, Ohmori T. Cumulative effect of the plasma total homocysteine-related genetic variants on schizophrenia risk. Psychiatry Res. 2016;246:833–837. doi: 10.1016/j.psychres.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Zhilyaeva TV, Piatoikina AS, Bavrina AP, Kostina OV, Zhukova ES, Shcherbatyuk TG, Blagonravova AS, Dubinina EE, Mazo GE. Homocysteine in Schizophrenia: independent pathogenetic factor with prooxidant activity or integral marker of other biochemical disturbances? Schizophr Res Treatment. 2021;2021:7721760. doi: 10.1155/2021/7721760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry T, Abohamza E, Moustafa AA. Treatment-resistant schizophrenia: focus on the transsulfuration pathway. Rev Neurosci. 2020;31(2):219–232. doi: 10.1515/revneuro-2019-0057. [DOI] [PubMed] [Google Scholar]
- 38.Trzesniowska-Drukala B, Kalinowska S, Safranow K, Kloda K, Misiak B, Samochowiec J. Evaluation of hyperhomocysteinemia prevalence and its influence on the selected cognitive functions in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2019;95:109679. doi: 10.1016/j.pnpbp.2019.109679. [DOI] [PubMed] [Google Scholar]
- 39.Song X, Fan X, Li X, Kennedy D, Pang L, Quan M, Chen X, Gao J, Zhang W, Zhang J, et al. Serum levels of BDNF, folate and homocysteine: in relation to hippocampal volume and psychopathology in drug naive, first episode schizophrenia. Schizophr Res. 2014;159(1):51–55. doi: 10.1016/j.schres.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 40.Petronijevic ND, Radonjic NV, Ivkovic MD, Marinkovic D, Piperski VD, Duricic BM, Paunovic VR. Plasma homocysteine levels in young male patients in the exacerbation and remission phase of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1921–1926. doi: 10.1016/j.pnpbp.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Gao J, Xiu MH, Liu DY, Wei CW, Zhang X. Interactive effect of MTHFR C677T polymorphism and sex on symptoms and cognitive functions in Chinese patients with chronic schizophrenia. Aging. 2020;12(11):10290–10299. doi: 10.18632/aging.103248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine J, Sela BA, Osher Y, Belmaker RH. High homocysteine serum levels in young male schizophrenia and bipolar patients and in an animal model. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(7):1181–1191. doi: 10.1016/j.pnpbp.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 43.Moustafa AA, Hewedi DH, Eissa AM, Frydecka D, Misiak B. Homocysteine levels in schizophrenia and affective disorders-focus on cognition. Front Behav Neurosci. 2014;8:343. doi: 10.3389/fnbeh.2014.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nwanaji-Enwerem JC, Colicino E, Gao X, Wang C, Vokonas P, Boyer EW, Baccarelli AA, Schwartz J. Associations of Plasma Folate and Vitamin B6 With Blood DNA Methylation Age: An Analysis of One-Carbon Metabolites in the VA Normative Aging Study. J Gerontol A Biol Sci Med Sci. 2021;76(5):760–769. doi: 10.1093/gerona/glaa257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones P, Lucock M, Martin C, Thota R, Garg M, Yates Z, Scarlett CJ, Veysey M, Beckett E. Independent and interactive influences of environmental UVR, Vitamin D levels, and folate variant MTHFD1-rs2236225 on Homocysteine levels. Nutrients. 2020;12(5):1455. doi: 10.3390/nu12051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishi A, Numata S, Tajima A, Kinoshita M, Kikuchi K, Shimodera S, Tomotake M, Ohi K, Hashimoto R, Imoto I, et al. Meta-analyses of blood homocysteine levels for gender and genetic association studies of the MTHFR C677T polymorphism in Schizophrenia. Schizophr Bull. 2014;40(5):1154–1163. doi: 10.1093/schbul/sbt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Autet M, Arranz B, Safont G, Sierra P, Garcia-Blanco A, de la Fuente L, Garriga M, Garcia-Portilla MP. Gender differences in C-reactive protein and homocysteine modulation of cognitive performance and real-world functioning in bipolar disorder. J Affect Disord. 2018;229:95–104. doi: 10.1016/j.jad.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 48.Kim HJ, Sohn IW, Kim YS, Jun JB. The Different Relationship between Homocysteine and Uric Acid Levels with Respect to the MTHFR C677T polymorphism according to gender in patients with cognitive impairment. Nutrients. 2020;12(4):1147. doi: 10.3390/nu12041147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen E, Margalit I, Shochat T, Goldberg E, Krause I. Gender differences in homocysteine concentrations, a population-based cross-sectional study. Nutr Metab Cardiovasc Dis. 2019;29(1):9–14. doi: 10.1016/j.numecd.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Gooren LJG, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008;5(4):765–776. doi: 10.1111/j.1743-6109.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- 51.Giltay EJ, Verhoef P, Gooren LJG, Geleijnse JM, Schouten EG, Stehouwer CDA. Oral and transdermal estrogens both lower plasma total homocysteine in male-to-female transsexuals. Atherosclerosis. 2003;168(1):139–146. doi: 10.1016/S0021-9150(03)00090-X. [DOI] [PubMed] [Google Scholar]
- 52.Lioudaki E, Ganotakis ES, Mikhailidis DP, Nair DR. The estrogenic burden on vascular risk in male-to-female transsexuals. Curr Pharm Des. 2010;16(34):3815–3822. doi: 10.2174/138161210794455049. [DOI] [PubMed] [Google Scholar]
- 53.Bergen SE, O'Dushlaine CT, Lee PH, Fanous AH, Ruderfer DM, Ripke S, International Schizophrenia Consortium SSC. Sullivan PF, Smoller JW, Purcell SM, et al. Genetic modifiers and subtypes in schizophrenia: investigations of age at onset, severity, sex and family history. Schizophr Res. 2014;154(1–3):48–53. doi: 10.1016/j.schres.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gangadhar BN, PannerSelvan C, Subbakrishna DK, Janakiramaiah N. Age-at-onset and schizophrenia: reversed gender effect. Acta Psychiatr Scand. 2002;105(4):317–319. doi: 10.1034/j.1600-0447.2002.1153.x. [DOI] [PubMed] [Google Scholar]
- 55.Nakazato M, Maeda T, Takamura N, Wada M, Yamasaki H, Johnston KE, Tamura T. Relation of body mass index to blood folate and total homocysteine concentrations in Japanese adults. Eur J Nutr. 2011;50(7):581–585. doi: 10.1007/s00394-010-0165-0. [DOI] [PubMed] [Google Scholar]
- 56.Akanji AO, Ohaeri JU, Al-Shammri SA, Fatania HR. Associations of blood homocysteine concentrations in Arab schizophrenic patients. Clin Biochem. 2007;40(13–14):1026–1031. doi: 10.1016/j.clinbiochem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Zhong X, Ao Q, Xing F. Serum Levels of HCY, MIF, and hs-CRP Correlate with Glycolipid Metabolism in Adults with Never-Medicated First-Episode Schizophrenia. Evid Based Complement Alternat Med. 2021;2021:7394699. doi: 10.1155/2021/7394699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.