Abstract
The HUGO Gene Nomenclature Committee assigns unique symbols and names to human genes. The use of approved nomenclature enables effective communication between researchers, and there are multiple examples of how the usage of unapproved alias symbols can lead to confusion. We discuss here a recent nomenclature update (May 2022) for a set of genes that encode proteins with a shared repeating β-groove domain. Some of the proteins encoded by genes in this group have already been shown to function as lipid transporters. By working with researchers in the field, we have been able to introduce a new root symbol (BLTP, which stands for “bridge-like lipid transfer protein”) for this domain-based gene group. This new nomenclature not only reflects the shared domain in these proteins, but also takes into consideration the mounting evidence of a shared lipid transport function.
Introduction
For over 40 years the HUGO (Human Genome Organisation) Gene Nomenclature Committee (HGNC) has been assigning standardised nomenclature to human genes [1, 2]. As genomics is being increasingly used in a clinical setting, the need to utilise a common language when referencing genes has become ever more important [3]. Stabilising gene symbols is now a key priority for the HGNC, but there are still some cases where an update to a more functionally informative nomenclature, when supported by the community working on the gene or genes in question, can be justified. In particular, we are still working to update uninformative “placeholder” symbols such as C#orfs, KIAAs and FAMs as long as they have not become entrenched in the literature [1]. As research reveals functional information about the proteins encoded by previously uncharacterised genes, this enables us to assign a new and informative nomenclature, which can then be stabilised.
The initial nomenclature update consultation: KIAA1109
The HGNC initiated a consultation in February 2022 about a potential nomenclature update for the gene previously approved as KIAA1109 (HGNC:26953). When we propose an update for an uninformative placeholder symbol, we write to authors who have published on the gene in question, wherever possible [1]. A previous consultation in 2020 had failed to reach a consensus agreement on new nomenclature, as some researchers felt that at that point there was not yet enough direct evidence to name KIAA1109 based on the possible role of its protein product in endocytosis [4, 5].
The first ortholog of KIAA1109 identified was Csf1p in budding yeast, named for the cold-sensitive fermentation (CSF) phenotype arising from gene disruption [6]; a molecular explanation for this phenotype was lacking for over 20 years [7]. A metazoan ortholog of KIAA1109 was first characterised in Drosophila, and its encoded protein was referred to as “Tweek”. This name originated from the fly neurological phenotype that was associated with seizures and was reminiscent to researchers of a jittery cartoon character. The fly protein Tweek was found to be involved in endocytosis and was required for synaptic vesicle recycling, affecting the availability of phosphoinositide lipids at synapses [5]. Tweek was reported to play a role in regulating a WASp (Wiskott-Aldrich Syndrome Protein)/PI(4,5)P2-dependent pathway, and a separate signalling pathway involving the fly proteins encoded by nwk (nervous wreck) and wit (wishful thinking), both of which are regulators of synaptic growth [8]. These genes are conserved in humans (with FCHSD2 (FCH and double SH3 domains 2) and BMPR2 (bone morphogenetic protein receptor type 2) being the orthologs of nwk and wit, respectively), and FCHSD2 has been shown to play a role in endocytosis [9], suggesting that the pathway in which Tweek functions may be conserved.
Variants in the human KIAA1109 gene have been associated with the congenital neurological malformation disorder Alkuraya-Kucinskas syndrome (MIM number 617822) [8]. To characterise cellular phenotypes upon mutation of KIAA1109, primary dermal fibroblasts from a patient and a control subject were taken, and immunofluorescence techniques and endocytosis assays were used to examine the endosomal, cytoskeletal, and ciliary cellular phenotypes associated with this condition. The findings of Kane et al. suggested that this syndrome is associated with pleiotropic defects in the endocytic pathway, the actin cytoskeleton and primary cilia [10].
Gene nomenclature in fly can be very different to that of vertebrates; it is often based on phenotypes and may sometimes aim to be humorous or to reference pop culture or literature, e.g., flies with a variant of the tinman gene have cardiac deformities [11, 12], referencing the tinman from the Wizard of Oz who lacks a heart. Many such names are unsuitable for transfer across to their human orthologs, as they could be potentially perceived as pejorative if associated with a phenotype. Understandably, “TWEEK” is among this group, and after discussion with researchers about the nomenclature of KIAA1109, we felt that they appreciated why we could not approve “TWEEK” as the symbol for this gene in human and across vertebrates, although we were able to add “Tweek” to the gene record as a published alias.
A new root symbol proposal: BLTP
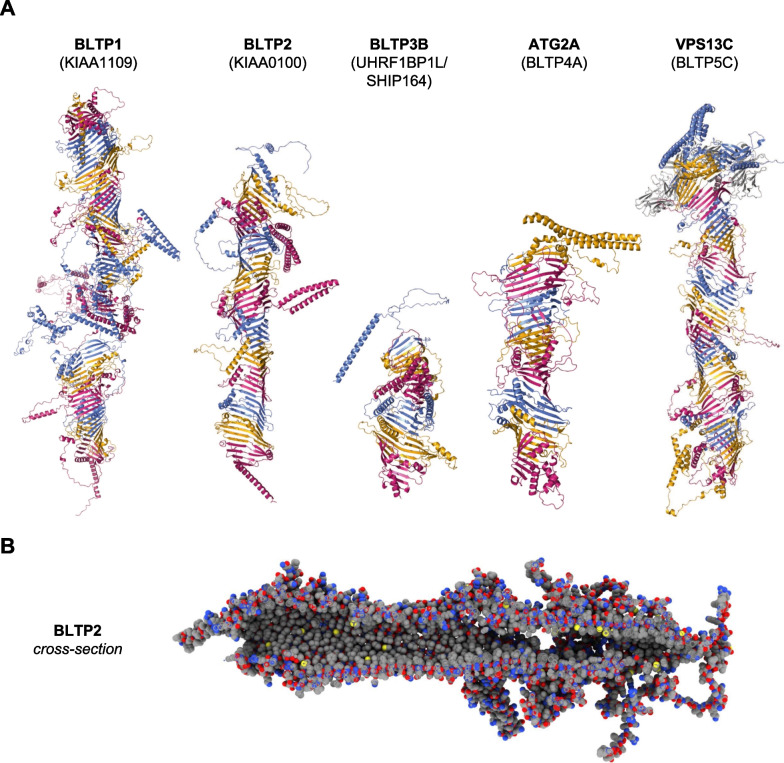
The release of protein structures in the AlphaFold database [13] and modelling with trRosetta [14] highlighted key structural features of KIAA1109 that enhanced understanding of the putative function of this protein [15, 16]. Additionally, analysis of predicted structures led to the identification and classification of the superfamily of proteins of which KIAA1109 is a member: the bridge-like lipid transfer proteins (BLTPs) (Fig. 1).
Fig. 1.
Structures of the human bridge-like lipid transfer protein (BLTP) superfamily members. A Ribbon models of the predicted structure of representative members of the human bridge-like lipid transfer protein (BLTP) superfamily. Repeating β-groove (RBG) domains are alternately labelled in pink, orange, and blue; the VAB domain of VPS13C [17–19] is shown in grey, and the ATG_C and PH domains [13] are shown in teal. Note that the positioning of these domains in VPS13C relative to the hydrophobic groove has not been experimentally determined. Large unstructured loops have been omitted from BLTP1, BLTP3B and ATG2A for visual clarity. HGNC approved symbols (in bold) are used for the encoded proteins; previous symbols/aliases are in parentheses. B Sphere model showing a cross-section of BLTP2 with carbon atoms (grey), oxygen (red), nitrogen (blue), and sulphur (yellow). Note the presence of an inner hydrophobic groove ideal for transport of lipids. The predicted structures of BLTP2, BLTP3B and ATG2A were downloaded from the AlphaFold database [12]. The VPS13C structure was generated using trRosetta [14] following the protocol described in [17]. The BLTP1 structure was generated by using trRosetta [14] to fold seven ~ 1500 amino acid overlapping fragments of the protein; these fragments were then assembled using the matchmaker command with Needleman-Wunsch global alignment in ChimeraX v.1.4 [20]. All ribbon models were coloured and rendered using PyMOL v.2.4.0; sphere model rendered using ChimeraX v.1.4 [20]
The genes currently approved as VPS13A (vacuolar protein sorting 13 homolog A) and ATG2A (autophagy related 2A) are the most well characterised and therefore “founding members” of this newly defined BLTP superfamily. Crystal and cryo-EM structures of fragments of VPS13A and ATG2A [21–24] and full-length ATG2A [25] showed that these proteins fold to form a long rod-like structure with an internal hydrophobic groove ideal for transport of lipids; importantly, purified VPS13A and ATG2A are both capable of transporting lipids between liposomes in vitro [21, 22, 26–28].
The predicted structures of VPS13A and ATG2A from AlphaFold [13] showed remarkable similarity to the experimental structures of these proteins [29]. Further analysis of these predicted structures led to the identification of a simple modular unit that contains five antiparallel β-strands curved to form a “U-shape” followed by an unstructured loop that curves back across the β-sheet. This modular unit is called the repeating β-groove (RBG) domain [29]. The inner concave surface of each RBG domain is lined primarily with hydrophobic residues, while the outer convex surface is primarily hydrophilic. Head-to-tail multimerisation of RBG domains leads to the formation of proteins with a long hydrophobic groove (Fig. 1). In cultured cells, VPS13A and ATG2A localise to membrane contact sites [21, 25, 26], where they form bridges that connect two organelle membranes and allow non-vesicular lipid transfer [29, 30], leading to our decision to name this gene family the ‘bridge-like lipid transfer protein’ (BLTP) family.
Notably, the KIAA1109 protein is also composed of RBG domains, and thus is another member of the BLTP protein superfamily [29]. The defects in subcellular PI(4,5)P2 distribution in fly tweek mutant cells [5] are also consistent with a lipid transfer function for this protein.
We briefly considered assigning RBG as a root symbol to this newly identified gene group, in reference to the repeating β-groove domains in their encoded proteins, but this clashed with the nomenclature for two unrelated yeast genes, RBG1 (RiBosome interacting Gtpase 1) and RBG2 (RiBosome interacting Gtpase 2) and would also have been less functionally informative than the BLTP nomenclature. Thus, KIAA1109 has been named BLTP1 (bridge-like lipid transfer protein family member 1).
Additional members of the BLTP superfamily
Alongside KIAA1109, the BLTP family also includes another gene approved with an uninformative placeholder symbol: KIAA0100. This gene has not been extensively published on to date, although its orthologs in yeast and fly have been better studied. The fly ortholog of KIAA0100 is named hobbit, since mutant animals have a substantial reduction in body size [31, 32]. Fly hobbit mutant animals exhibit cell-autonomous defects in regulated exocytosis, and the small body size of the mutant animals is caused by failure to secrete insulin [31]. In flies, insulin is a potent regulator of growth, analogous to IGF1 in vertebrates [33]. There are two paralogs of hobbit in S. cerevisiae: FMP27 (alias HOB1) and HOB2 which are both orthologs of Drosophila HOBbit. The fly and yeast “Hobbit” proteins both localise to endoplasmic reticulum-plasma membrane (ER-PM) contact sites [15, 32], and the subcellular distribution of PI(4,5)P2 is disrupted in fly hobbit mutant cells [32]. Naming this gene in vertebrates after the fly “hobbit” could be viewed as pejorative and hence was not an option. Thus, KIAA0100 has been named BLTP2 (bridge-like lipid transfer protein family member 2).
The final two members of the BLTP family were approved as UHRF1BP1 (UHRF1 binding protein 1) and UHRF1BP1L (UHRF1 binding protein 1 like) in vertebrates. However, the alias symbol “SHIP164,” standing for Syntaxin 6 Habc Interacting Protein of 164 KDa [34, 35], has also been used several times to refer to the protein encoded by UHRF1BP1L. This protein has been shown to localise to endocytic compartments, and purified protein is capable of transferring lipids between liposomes in vitro [35]. We propose to update the nomenclature of these two genes to utilise the BLTP root symbol and have named them as BLTP3A and BLTP3B, respectively.
Although ATG2A, ATG2B, VPS13A, VPS13B, VPS13C and VPS13D are all members of the BLTP gene group, their approved nomenclature has not been updated. This is because the existing nomenclature is already functionally informative, well published in the literature and in line with the yeast orthologs of these genes. In addition, variants in VPS13A, VPS13B VPS13C and VPS13D have all been associated with human phenotypes (chorea-acanthocytosis (MIM: 200150), Cohen syndrome (MIM: 216550), early onset Parkinson disease (MIM: 616840) and spinocerebellar ataxia (MIM: 607317), respectively). The HGNC now strives to stabilise the nomenclature of symbols associated with disease phenotypes where possible, especially when they have already been used in clinical publications. However, we were able to assign these genes BLTP# alias symbols (see Table 1).
Table 1.
A summary of the HGNC approved nomenclature of genes in the BLTP superfamily in human
| HGNC id | Approved Symbol | Previous Symbol(s) | Aliases |
|---|---|---|---|
| 26953 | BLTP1 | KIAA1109 | FLJ21404, FSA, KIAA1371, Tweek |
| 28960 | BLTP2 | KIAA0100 | DKFZp686M0843, MGC111488, BCOX1, CT101, BCOX, FMP27, Hob |
| 21216 | BLTP3A | C6orf107, UHRF1BP1 | FLJ20302, dJ349A12.1 |
| 29102 | BLTP3B | UHRF1BP1L | KIAA0701, SHIP164 |
| 20928 | ATG2A | KIAA0404, BLTP4A | |
| 20187 | ATG2B | C14orf103 | FLJ10242, BLTP4B |
| 1908 | VPS13A | CHAC | KIAA0986, BLTP5A |
| 2183 | VPS13B | CHS1, COH1 | BLTP5B |
| 23594 | VPS13C | FLJ20136, FLJ10381, KIAA1421, BLTP5C | |
| 23595 | VPS13D | FLJ10619, KIAA0453, BLTP5D |
BLTP-approved symbols and aliases are highlighted in bold
It is a fairly common issue in nomenclature that one or more very highly published symbols for members of a larger gene group need to be retained, while there is still a great benefit to assigning the rest of the set new, functionally informative nomenclature. In these cases, the genes getting new approved symbols are given the first numbers in the series and the genes that are retaining their current nomenclature but are being assigned group aliases come later in the series; this convention was followed for the BLTP family members. If the genes changing to a new root symbol were given the later numbers, for example if BLTP3 was an approved gene symbol but BLTP1 and BLTP2 were assigned as aliases, it could be confusing, perhaps leading researchers to mistakenly assume that the first genes in the series might be named in other species with no human orthologs.
We wrote to all authors who had previously published on KIAA1109, KIAA0100, UHRF1BP1 and UHRF1BP1L about the proposed BLTP update to gauge their support for this new nomenclature. The majority of respondents to our nomenclature proposal consultation were in favour of this update and so the changes were made as detailed in Table 1. We also proposed assigning BLTP aliases to ATG2A, ATG2B, VPS13A, VPS13B, VPS13C and VPS13D.
An HGNC gene group for the ten human genes encoding “bridge like lipid transfer proteins” can be seen on our website (https://www.genenames.org/data/genegroup/#!/group/2141).
This new BLTP nomenclature is functionally informative, and we hope that as researchers in the field continue to characterise this set of lipid transporters that they will adopt it in their publications.
Acknowledgements
This work was supported in part by the National Institutes of Health, USA (GM123204 to A.B.). The work of the HGNC is supported by the National Human Genome Research Institute (Grant U24HG003345) and the Wellcome Trust (Grant 208349/Z/17/Z). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author contributions
B.B wrote the first draft of the manuscript. E.A.B, S.D.N and A.B edited the manuscript. A.T.C prepared Fig. 1. All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bruford EA, Braschi B, Denny P, Jones TEM, Seal RL, Tweedie S. Guidelines for human gene nomenclature. Nat Genet. 2020;52:754–758. doi: 10.1038/s41588-020-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tweedie S, Braschi B, Gray K, Jones TEM, Seal RL, Yates B, et al. Genenames.org: the HGNC and VGNC resources in 2021. Nucleic Acids Res. 2021;49:D939–D946. doi: 10.1093/nar/gkaa980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braschi B, Seal RL, Tweedie S, Jones TEM, Bruford EA. The risks of using unapproved gene symbols. Am J Hum Genet. 2021;108:1813–1816. doi: 10.1016/j.ajhg.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeng EE, Bhadkamkar V, Ibe NU, Gause H, Jiang L, Chan J, et al. Systematic identification of host cell regulators of legionella pneumophila pathogenesis using a genome-wide CRISPR screen. Cell Host Microbe. 2019;26:551–63.e6. doi: 10.1016/j.chom.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verstreken P, Ohyama T, Haueter C, Habets RLP, Lin YQ, Swan LE, et al. Tweek, an evolutionarily conserved protein, is required for synaptic vesicle recycling. Neuron. 2009;63:203–215. doi: 10.1016/j.neuron.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokai M, Kawasaki H, Kikuchi Y, Ouchi K. Cloning and characterization of the CSF1 gene of Saccharomyces cerevisiae, which is required for nutrient uptake at low temperature. J Bacteriol. 2000;182:2865–2868. doi: 10.1128/JB.182.10.2865-2868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John Peter AT, van Schie SNS, Cheung NJ, Michel AH, Peter M, Kornmann B. Rewiring phospholipid biosynthesis reveals resilience to membrane perturbations and uncovers regulators of lipid homeostasis. EMBO J. 2022;41:e109998. doi: 10.15252/embj.2021109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gueneau L, Fish RJ, Shamseldin HE, Voisin N, Tran Mau-Them F, Preiksaitiene E, et al. KIAA1109 variants are associated with a severe disorder of brain development and arthrogryposis. Am J Hum Genet. 2018;102:116–132. doi: 10.1016/j.ajhg.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao G-Y, Schmid SL. FCHSD2 controls oncogenic ERK1/2 signaling outcome by regulating endocytic trafficking. PLoS Biol. 2020;18:e3000778. doi: 10.1371/journal.pbio.3000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane MS, Diamonstein CJ, Hauser N, Deeken JF, Niederhuber JE, Vilboux T. Endosomal trafficking defects in patient cells with KIAA1109 biallelic variants. Genes Dis. 2019;6:56–67. doi: 10.1016/j.gendis.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- 12.Stutt N, Song M, Wilson MD, Scott IC. Cardiac specification during gastrulation–The Yellow Brick Road leading to Tinman. Semin Cell Dev Biol. 2022;127:46–58. doi: 10.1016/j.semcdb.2021.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Z, Su H, Wang W, Ye L, Wei H, Peng Z, et al. The trRosetta server for fast and accurate protein structure prediction. Nat Protoc. 2021;16:5634–5651. doi: 10.1038/s41596-021-00628-9. [DOI] [PubMed] [Google Scholar]
- 15.Toulmay A, Whittle FB, Yang J, Bai X, Diarra J, Banerjee S, et al. Vps13-like proteins provide phosphatidylethanolamine for GPI anchor synthesis in the ER. J Cell Biol. 2022 doi: 10.1083/jcb.202111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John Peter AT, Cheung NJ, Kornmann B. Csf1: a putative lipid transport protein required for homeoviscous adaptation of the lipidome. Contact. SAGE Publications Inc. 2022;5:25152564221101974. [DOI] [PMC free article] [PubMed]
- 17.Cai S, Wu Y, Guillén-Samander A, Hancock-Cerutti W, Liu J, De Camilli P. In situ architecture of the lipid transport protein VPS13C at ER-lysosome membrane contacts. Proc Natl Acad Sci U S A. 2022;119:e2203769119. doi: 10.1073/pnas.2203769119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bean BDM, Dziurdzik SK, Kolehmainen KL, Fowler CMS, Kwong WK, Grad LI, et al. Competitive organelle-specific adaptors recruit Vps13 to membrane contact sites. J Cell Biol. 2018;217:3593–3607. doi: 10.1083/jcb.201804111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adlakha J, Hong Z, Li P, Reinisch KM. Structural and biochemical insights into lipid transport by VPS13 proteins. J Cell Biol. 2022 doi: 10.1083/jcb.202202030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar N, Leonzino M, Hancock-Cerutti W, Horenkamp FA, Li P, Lees JA, et al. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol. 2018;217:3625–3639. doi: 10.1083/jcb.201807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Lees JA, Lusk CP, Reinisch KM. Cryo-EM reconstruction of a VPS13 fragment reveals a long groove to channel lipids between membranes. J Cell Biol. 2020 doi: 10.1083/jcb.202001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osawa T, Noda NN. Crystal structure of the PE-bound N-terminal domain of Atg2. Worldwide Protein Data Bank. 2019. 10.2210/pdb6a9j/pdb
- 24.Osawa T, Noda NN. Crystal structure of the N-terminal domain of Atg2. Worldwide Protein Data Bank. 2019. 10.2210/pdb6a9e/pdb
- 25.Valverde DP, Yu S, Boggavarapu V, Kumar N, Lees JA, Walz T, et al. ATG2 transports lipids to promote autophagosome biogenesis. J Cell Biol. 2019;218:1787–1798. doi: 10.1083/jcb.201811139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillén-Samander A, Leonzino M, Hanna MG, Tang N, Shen H, De Camilli P. VPS13D bridges the ER to mitochondria and peroxisomes via Miro. J Cell Biol. 2021 doi: 10.1083/jcb.202010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otomo T, Chowdhury S, Lander GC. The rod-shaped ATG2A-WIPI4 complex tethers membranes in vitro. Contact. 2018 doi: 10.1177/2515256418819936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda S, Otomo C, Otomo T. The autophagic membrane tether ATG2A transfers lipids between membranes. Elife. 2019 doi: 10.7554/eLife.45777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuman SD, Levine TP, Bashirullah A. A novel superfamily of bridge-like lipid transfer proteins. Trends Cell Biol. 2022 doi: 10.1016/j.tcb.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melia TJ, Reinisch KM. A possible role for VPS13-family proteins in bulk lipid transfer, membrane expansion and organelle biogenesis. J Cell Sci. 2022 doi: 10.1242/jcs.259357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuman SD, Bashirullah A. Hobbit regulates intracellular trafficking to drive insulin-dependent growth during Drosophila development. Development. 2018 doi: 10.1242/dev.161356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuman SD, Jorgensen JR, Cavanagh AT, Smyth JT, Selegue JE, Emr SD, et al. The Hob proteins are novel and conserved lipid-binding proteins at ER-PM contact sites. J Cell Sci. The Company of Biologists; 2022;135. Available from: https://journals.biologists.com/jcs/article-abstract/135/5/jcs259086/271892. [DOI] [PMC free article] [PubMed]
- 33.Al-Samerria S, Radovick S. The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells. 2021 doi: 10.3390/cells10102664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otto GP, Razi M, Morvan J, Stenner F, Tooze SA. A novel syntaxin 6-interacting protein, SHIP164, regulates syntaxin 6-dependent sorting from early endosomes. Traffic. 2010;11:688–705. doi: 10.1111/j.1600-0854.2010.01049.x. [DOI] [PubMed] [Google Scholar]
- 35.Hanna MG, Suen PH, Wu Y, Reinisch KM, De Camilli P. SHIP164 is a chorein motif lipid transfer protein that controls endosome-Golgi membrane traffic. J Cell Biol. 2022 doi: 10.1083/jcb.202111018. [DOI] [PMC free article] [PubMed] [Google Scholar]