Abstract
Sickle cell disease (SCD) is one of the most common inherited blood disorders. Deoxygenated hemoglobin S (HbS) polymerizes and causes anemia and various end organ effects. Voxelotor acts by increasing HbS oxygen affinity, decreasing anemia and hemolysis. Voxelotor is approved for use in individuals with SCD age 4 years and older. Phase 3 trials demonstrated an increase in hemoglobin levels and a decrease in markers of hemolysis; however, data or benefits related to clinical and quality of life outcomes are relatively limited and varied across different studies. This review summarizes the published clinical trials and research studies focused on the use of voxelotor in SCD to provide an evidence-based practical guide for hematology providers on its utilization in clinical settings, including physicians and independent licensed practitioners.
Keywords: sickle cell, sickle cell anemia, sickle cell disease, hemoglobinopathy, pediatric, children, adolescents, young adults, adult, voxelotor, oxbryta, disease-modifying therapy
Introduction
Sickle cell disease (SCD) is one of the most common inherited blood disorders affecting 100,000 individuals in the United States and millions worldwide.1 A mutation in the beta globin chain of the hemoglobin creates hemoglobin S (HbS) and leads to a complex pathophysiology in SCD, including polymerization of HbS in the deoxygenated state. There are several clinical complications in SCD, such as anemia, vaso-occlusive pain episodes, acute chest syndrome, and various end organ damage, leading to decreased life expectancy.2 Given that curative approaches, namely hematopoietic stem cell transplant and gene therapy or editing, though promising, are still not yet widely available, disease modifying therapies are and will likely remain critical for optimizing health outcomes in SCD.3,4
With recent research advancements in the last decade, there are now four Federal Drug Administration (FDA) approved medications for SCD: hydroxyurea, l-glutamine, crizanlizumab, and voxelotor.3 In particular, voxelotor targets anemia and hemolysis in SCD, which plays a key role in the pathogenesis.2 Voxelotor received initial FDA approval in November 2019 for individuals with SCD age 12 years and older, and recently received FDA approved for children with SCD age 4 years and older.5 Voxelotor binds to hemoglobin resulting in a decrease in HbS polymerization.6,7 Clinical trials demonstrated that voxelotor can effectively increase hemoglobin by 1 g/dL in approximately 50% of patients at 24 weeks with improvement in markers of hemolysis.8,9 With the recent expansion of FDA approval, practical guidance for use of voxelotor in SCD is essential to aid SCD providers considering this disease modifying therapy for their patients.
Methods
A literature search was conducted January 2017 to June 2022 through the PubMed database using key search terms: voxelotor, oxbryta, and GBT 440. Inclusion criteria were phase 3 clinical trials, cross-sectional studies, retrospective studies, and case series focused on the use of voxelotor in children, adolescents, and adults with SCD in English language. Exclusion criteria included non-English language, and phase 1 clinical trials or pre-clinical studies.
Results
Efficacy
HOPE and HOPE KIDS-1 Trials
Expected clinical efficacy is a key consideration in prescribing voxelotor for SCD. Clinical trials demonstrated statistically significant benefits related to hemoglobin levels and markers of hemolysis, while impact on clinical outcomes varied. The HOPE trial was a multicenter phase 3 randomized clinical trial of individuals with SCD age 12–64 years, mostly HbSS or HbSβ0Thal. In the HOPE trial, voxelotor demonstrated a significant increase in hemoglobin by 1 g/dL in 51% of patients along with a decrease in markers of hemolysis, such as indirect bilirubin and reticulocyte.9,10 Similarly, the HOPE KIDS-1 trial showed a significant increase in hemoglobin by 1 g/dL in 47% of patients as well as decreased markers of hemolysis among children with HbSS or HbSβ0Thal.8 Neither trial showed significant reduction in vaso-occlusive pain episodes or improvement in health-related quality of life outcomes.8–10 However, long-term follow up of the HOPE trial at 72 weeks showed less frequent vaso-occlusive episodes among individuals with SCD on voxelotor with higher hemoglobin response above 10 g/dL with an annualized incidence rate of vaso-occlusive crisis of 2.8 in the voxelotor 1500 mg group compared to 3.2 in placebo.11 Post hoc analysis of the HOPE trial demonstrated voxelotor 1500 mg caused improvement or resolution of leg ulcers in all (5/5, 100%) patients with active leg ulcers.12 Data for efficacy are primarily for individuals with severe phenotypes (ie, HbSS and HbSβ0Thal), while other genotypes, such as HbSC and HbSβ+Thal, were present in small numbers (combined approximately 10% of participants) in the HOPE trial and excluded in the HOPE KIDS-1 trial.8,10
Real-World Evidence
A retrospective study of national claims data of 3128 individuals with SCD age 12 years and older demonstrated voxelotor impacted clinical outcomes, though these potential clinical benefits must be interpreted within the context of the inherent methodological limitations in retrospective studies.13,14 Voxelotor significantly decreased the mean annualized vaso-occlusive episode rate from 10.9 to 8.4 (p<0.001).13 The mean annualized rate of VOC hospitalizations and all-cause hospitalizations decreased from 10.9 to 8.4 (p<0.001), and from 7.4 to 4.6 (p<0.001), respectively. Voxelotor decreased blood transfusion requirements with a decrease in the mean annualized transfusion rate from 7.0 to 3.3 (p<0.001).13,14 The study also demonstrated a decrease in mean number of outpatient visits for individuals on voxelotor from 24.8 to 22.5 visits (p=0.006), and a decrease in the mean days supply of prescribed opioids from 312 to 272 days (p<0.001).13 This study suggested possible real-world evidence for clinical benefits related to the use of voxelotor beyond increasing hemoglobin levels.
Other reported beneficial effects of voxelotor include improvements in well-being, and symptoms of depression.15,16 A single center review of 77 individuals with SCD age 12 years and older demonstrated 67% of pediatric patients and 54% of adult patients on voxelotor had improved quality of life measured by the Patient Global Impression of Change and Clinical Global Impression of Change questionnaires.16 A case series of compassionate use of voxelotor in 7 individuals with SCD demonstrated that 2 of 2 patients with depression had improved affect measured by the Patient Health Questionnaire-9.15 Potential concerns about impact on exercise capacity due to decreased oxygen delivery to tissues was not seen in a pilot study of 9 individuals with SCD ages 12–20 years who had no significant difference in cardiopulmonary exercise testing.18 Research studies evaluating the impact of voxelotor on transcranial Doppler ultrasound velocities (HOPE KIDS-2) and other quality of life outcomes are ongoing.17
Adverse Events
HOPE and HOPE KIDS-1 Trials
Overall, voxelotor is well tolerated, and most side effects are minor, grade 1, or grade 2 as defined by the Common Terminology Criteria for Adverse Events (CTCAE). In the HOPE trial in individuals age 12–64 years, 34/88 (39%) of participants receiving voxelotor 1500 mg had 1 or more treatment related adverse events. Headache and diarrhea were the most common adverse events, occurring in at least 20% of patients receiving voxelotor 1500 mg daily.10 Other common side effects included nausea, abdominal pain, and rash.10,19 Dose modifications occurred in 41% (36/88) of individuals receiving voxelotor. Per protocol, dose reduction was indicated for grade 2 or higher adverse event. The dose was reduced by one 300 mg capsule/tablet and could be resumed to the original dose once the grade 2 adverse event resolved to ≤grade 1. The dose was held for up to five days for grade 3 or higher adverse event and could be resumed at the original dose when adverse event ≤grade 1.10
The HOPE KIDS-1 trial demonstrated similar findings with the most common drug related adverse events including diarrhea, rash, and vomiting with grade 1 adverse events most common.8 Four patients required dose modification due to drug related adverse events. Dose reduction occurred in 1/45 (2%) of patients and was due to a grade 2 rash. Dose was reduced by one 300 mg tablet. Dose interruption without dose reduction occurred in 3/45 (7%) of patients in the HOPE KIDS-1 trial, one for grade 2 hypersensitivity, one for grade 2 liver enzyme elevation, and one for grade 3 liver enzyme elevation and bilirubin increase.10
Real-World Evidence
Case series of use of voxelotor in individuals with SCD demonstrate adverse events similar to those reported in the HOPE and HOPE KIDS-1 trials. A single center review of 77 individuals with SCD age 12 years and older demonstrated that 4 of 77 (5%) of individuals had adverse events that resulted in dose modification, including 2 diarrhea, 1 rash, and 1 fever.16 A case study reported rebound hemolysis and multi-organ dysfunction after abrupt discontinuation of voxelotor in a HbSS patient with pulmonary hypertension and renal disease.17 Although a rare complication, there are limited data on implications of abrupt cessation of voxelotor. This is relevant given voxelotor is not currently widely available in hospital formularies and medication continuation during hospital admission relies on patient supply of the medication from home. There are limited long-term follow up data as well as limited publications on use of voxelotor in certain subpopulations of individuals with SCD including those with multi-organ dysfunction or pregnant.
Initiating Voxelotor
Eligibility
Individuals with SCD age 4 years and older with severe phenotypes (HbSS and HbSβ0Thal) are eligible for use of voxelotor. It can be considered as an added therapy to hydroxyurea or alternative if hydroxyurea is ineffective, not tolerated, or preferred by patients or parents. Individuals age 12 years and older with milder genotypes, such as HbSC or HbSβ+Thal with baseline hemoglobin <10 g/dL and/or episodes of vaso-occlusive episodes may be considered for voxelotor, consistent with the HOPE trial, but data are limited.10 Patients on concomitant hydroxyurea may be started on voxelotor with data supporting use in patients on stable hydroxyurea dose for at least 3 months.8,10 Combination therapy with voxelotor and other SCD disease modifying agents may provide the ability to target the complex pathophysiology of SCD via different pathways, but data on its use with crizanlizumab and l-glutamate are limited.13 Further, patients’ pill or medication burden should be considered to ensure optimal adherence level over time to one or more therapies, which can be a challenge in clinical practice. In a retrospective study of 3128 individuals with SCD age 12 years and older on voxelotor, patients were also treated with other therapies, including hydroxyurea (57%), 7% l-glutamine (7%), and crizanlizumab (1%).13
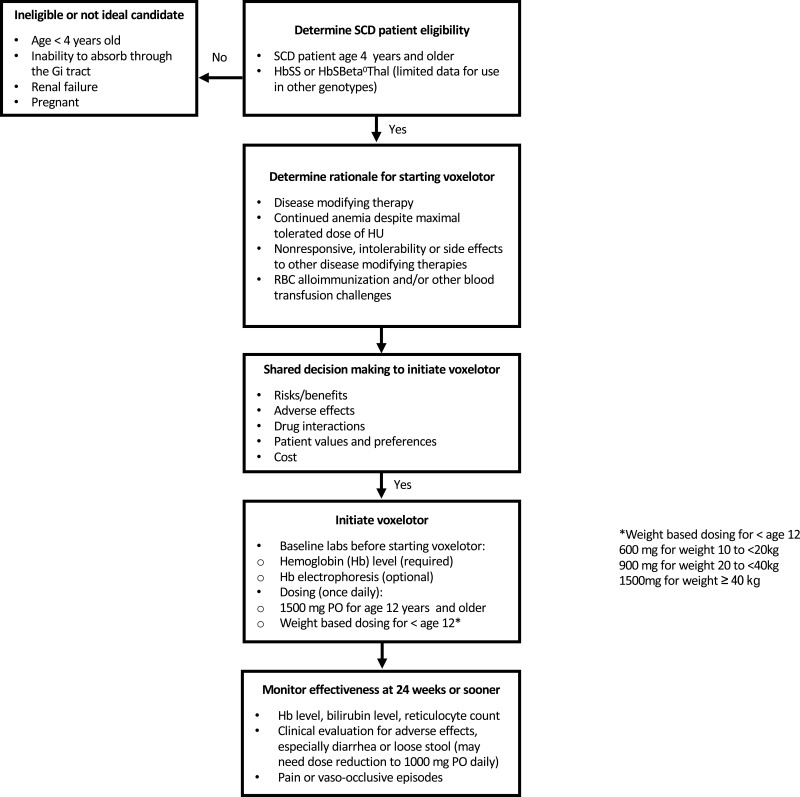
Figure 1 illustrates a suggested algorithm for practical considerations for use of voxelotor in SCD. Potential candidates for voxelotor include SCD individuals with hemoglobin ≤10.5 g/dL and those with continued anemia despite maximum tolerated dose of HU, especially those with symptomatic anemia.8,10 Individuals who are non-responsive or have adverse side effects to hydroxyurea or other disease modifying sickle cell therapy may also be considered. SCD patients with Hb <10.5 g/dL and red blood cell alloimmunization may also be considered as an increase in hemoglobin may be desired, particularly if there is difficulty locating compatible blood for transfusion needs.
Figure 1.
Suggested decision algorithm for considering initiation of voxelotor in SCD.
Ineligible patients for voxelotor include individuals with SCD less than age 4 years. Some clinical conditions may represent a challenge, such as short gut syndrome or other complex gastrointestinal history, leading to potential inability to absorb the medication, given the medication is given orally and a parental method of administration is not available. Individuals with liver or kidney impairment require special consideration and may require dose adjustments given the role of the kidneys in drug excretion and potential for liver toxicity.
Medication Administration, Dosage, and Monitoring
Voxelotor is a once daily oral medication available as tablets and tablets for oral suspension. The starting dose for individuals age 12 years and older is 1500 mg once daily. Children age 4–12 years use weight based dosing including: 600 mg for weight 10 to <20 kg, 900 mg for weight 20 to <40 kg and 1500 mg for weight ≥40 kg.8,20 For oral suspension, the tablet can be mixed with a room temperature clear drink as detailed by the manufacturer.20 Medication can be taken with or without food. Dose adjustments are indicated for patients with diarrhea or loose stools, severe hepatic impairment, or those on concomitant CYP3A4 inducers. Voxelotor has a half-life of 35.5 hours and reaches steady state in eight days.21,22 Time to response in the phase 3 clinical trials was 24 weeks but response may be observed earlier and response has been documented as early as 2 weeks in clinical trials.8
Dosage information for certain medical conditions and comorbidities in SCD is limited. Voxelotor has not been extensively studied in individuals with SCD and renal failure. The HOPE trial excluded patients with severe renal impairment and individuals on dialysis.10 A small retrospective study showed a decrease in albuminuria in individuals with SCD and chronic kidney disease stage 1–3 but was not statistically significant.21 Voxelotor has not been studied in pregnant women and there are no dosage recommendations in this population.
Laboratory evaluation of hemoglobin, reticulocyte count, and liver function are recommended to assess for treatment response and adverse effects.23 Voxelotor may interfere with hemoglobin electrophoresis and discussion with a hematopathologist about the potential impact on hemoglobin electrophoresis results in these patients is recommended.24,25 Supporting laboratory results for voxelotor efficacy include improved hemoglobin and a decrease in markers of hemolysis, such as indirect bilirubin and reticulocyte count.9 Moreover, evaluating hemoglobin response or increase with trend over time can be helpful for patients to reinforce adherence behavior and for insurance to maintain coverage.
Dose modification is generally recommended for treatment related adverse event ≥grade 2 per the HOPE and HOPE KIDS-1 trial as well as recommendations from GBT.8,10 In individuals age 12 years and older, there is temporary dose reduction by one 500 mg tablet to 1000 mg daily or dose interruption based on the severity of the adverse effect. Dose reduction in the HOPE KIDS-1 trial was a decrease in dose by one 300 mg tablet.
Special Considerations
There are reported concerns that voxelotor may limit oxygen delivery due to the increase in oxygen affinity. A theoretical model by Hebbel and Hedlund details how increased oxygen affinity may decrease oxygen delivery and functional oxygen content in the brain. Decreased cerebral oxygen delivery may increase risk for adverse cerebrovascular consequences in SCD.26 This model has not been replicated in vivo and there were no reported cerebrovascular events in the clinical trials. Further research on the impact on cerebral hemodynamics and oxygen delivery is warranted.
Cost
Medication cost is important to consider when initiating voxelotor. As of November 2022, the cost is $138.89 USD per 500 mg tablet and $208.34 USD per 300 mg soluble tablet. A cost effective analysis by the Institute for Economic and Clinical Review found that voxelotor was not cost effective at the current price.27 Further research regarding cost effectiveness of voxelotor is needed. Insurance coverage and financial assistance from the manufacturer Global Blood Therapeutics (GBT) may help address financial barriers.
Counseling Patients and Shared Decision Making
Shared decision making (SDM) can aid discussions on the initiation of voxelotor. Key components of SDM include a collaborative approach between the patient and clinicians resulting in a partnership to make medical decisions supported by evidence and aligned with the patient’s preferences, values, and treatment goals.28,29 Published strategies on the use of SDM for voxelotor in SCD are limited. Clinical teams may consider referencing shared decision making practices for other sickle cell disease modifying therapies, such as hydroxyurea when utilizing SDM for voxelotor.30 Adapting these strategies for voxelotor has not been validated but may help facilitate open communication about the decision to initiate therapy.29 A well informed discussion can be facilitated by a clinical team that is familiar with the clinical evidence as well as the expected benefit and risks of voxelotor. Key topics that should be discussed in SCD SDM aids include clinical efficacy, side effects, costs, and overall benefits while weighing the pros and cons of different potential disease modifying therapies.29
Conclusion
Voxelotor is a well-tolerated oral agent in the era of multiple disease modifying agents for SCD. It can be considered as a component of multimodal treatment strategy to optimize outcomes in SCD. There are limited data to support its use as monotherapy. Randomized controlled trials demonstrated efficacy in raising the hemoglobin level and improving markers of hemolysis as well as a favorable safety profile. Data on the impact of voxelotor on clinical outcomes, such as pain episodes and quality of life, are less robust but suggest potential benefits related to reduction in pain episodes, hospitalizations, and blood transfusion requirements. Most data on the impact on clinical outcomes are limited to retrospective studies or case series which have limitations and are at risk for bias including confounding and selection bias. Further research is needed to assess the impact on clinical outcomes. Theoretical risks to oxygen delivery exist and warrant consideration and further research.31 Cost of the medication, insurance coverage, and limited current availability on hospital formularies warrant consideration.
Several clinical questions related to voxelotor remain unanswered. Efficacy in children with SCD less than 4 years old is currently being studied. Long-term data on the impact on end organ dysfunction are not yet available, and efficacy on neurovascular outcomes, including TCD velocities, is currently being studied. Further research on clinical outcomes is essential, needed to better support counseling of patients and families on voxelotor. Additional research on SDM tools and resources for voxelotor in SCD may aid practical guidance and facilitate discussion with patients and families as they make their decisions among different disease modifying therapies.
Acknowledgment
This project was supported by grant (K23HL150232, PI: Badawy) from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the National Institutes of Health.
Abbreviations
CTCAE, Common Terminology Criteria for Adverse Events; GBT, Global Blood Therapeutics; SCD, Sickle cell disease; SDM, Shared decision making; FDA, Food and Drug Administration; HbSβ0Thal, Hemoglobin S Beta Zero thalassemia; HbSβ+Thal, Hemoglobin S Beta Plus thalassemia.
Disclosure
The authors report no relevant conflict or competing interests for this work.
References
- 1.Centers for Disease Control and Prevention. Data and statistics on sickle cell disease. Available from: https://www.cdc.gov/ncbddd/sicklecell/data.html. Accessed June 28, 2022.
- 2.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. doi: 10.1038/nrdp.2018.10 [DOI] [PubMed] [Google Scholar]
- 3.Leibovitch JN, Tambe AV, Cimpeanu E, et al. l-glutamine, crizanlizumab, voxelotor, and cell-based therapy for adult sickle cell disease: hype or hope? Blood Rev. 2022;53:100925. doi: 10.1016/j.blre.2021.100925 [DOI] [PubMed] [Google Scholar]
- 4.Williams DA, Esrick E. Investigational curative gene therapy approaches to sickle cell disease. Blood Adv. 2021;5(23):5452. doi: 10.1182/bloodadvances.2021005567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Food & Drug Administration. FDA approves drug to treat sickle cell disease in patients aged 4 up to 11 years; 2021. Available from: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-sickle-cell-disease-patients-aged-4-11-years#:~:text=Action,older%20with%20sickle%20cell%20disease. Accessed May 19, 2022.
- 6.Metcalf B, Chuang C, Dufu K, et al. Discovery of GBT440, an Orally Bioavailable R-State Stabilizer of Sickle Cell Hemoglobin. ACS Med Chem Lett. 2017;8(3):321–326. doi: 10.1021/acsmedchemlett.6b00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaros AK, Razvi R, Shah N, Zaidi AU. Voxelotor: alteration of sickle cell disease pathophysiology by a first-in-class polymerization inhibitor. Ther Adv Hematol. 2021;12:20406207211001136. doi: 10.1177/20406207211001136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estepp JH, Kalpatthi R, Woods G, et al. Safety and efficacy of voxelotor in pediatric patients with sickle cell disease aged 4 to 11 years. Pediatr Blood Cancer. 2022;69(8):e29716. doi: 10.1002/pbc.29716 [DOI] [PubMed] [Google Scholar]
- 9.Howard J, Ataga KI, Brown RC, et al. Voxelotor in adolescents and adults with sickle cell disease (HOPE): long-term follow-up results of an international, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. 2021;8(5):e323–e333. doi: 10.1016/s2352-3026(21)00059-4 [DOI] [PubMed] [Google Scholar]
- 10.Vichinsky E, Hoppe CC, Ataga KI, et al. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N Engl J Med. 2019;381(6):509–519. doi: 10.1056/NEJMoa1903212 [DOI] [PubMed] [Google Scholar]
- 11.Vichinsky E, Gordeuk VR, Telfer P, et al. Higher hemoglobin levels achieved with voxelotor are associated with lower vaso-occlusive crisis incidence: 72-week analysis from the HOPE study. Blood. 2020;136:31–32. doi: 10.1182/blood-2020-140863 [DOI] [Google Scholar]
- 12.Minniti CP, Knight-Madden J, Tonda M, Gray S, Lehrer-Graiwer J, Biemond BJ. The impact of voxelotor treatment on leg ulcers in patients with sickle cell disease. Am J Hematol. 2021;96(4):E126–e128. doi: 10.1002/ajh.26101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah N, Lipato T, Alvarez O, et al. Real-world effectiveness of voxelotor for treating sickle cell disease in the US: a large claims data analysis. Expert Rev Hematol. 2022;15(2):167–173. doi: 10.1080/17474086.2022.2031967 [DOI] [PubMed] [Google Scholar]
- 14.Zaidi AU, Lipato T, Alvarez OA, et al. Real-World Effectiveness of Voxelotor for Treating Sickle Cell Disease in the US. Blood. 2020;136:25. doi: 10.1182/blood-2020-140525 [DOI] [PubMed] [Google Scholar]
- 15.Blyden G, Bridges KR, Bronte L. Case series of patients with severe sickle cell disease treated with voxelotor (GBT440) by compassionate access. Am J Hematol. 2018;93(8):E188–E190. doi: 10.1002/ajh.25139 [DOI] [PubMed] [Google Scholar]
- 16.Muschick K, Fuqua T, Stoker-Postier C, Anderson AR. Real-World Data On Voxelotor To Treat Patients With Sickle Cell Disease. Eur J Haematol. 2022;109(2):154–161. doi: 10.1111/ejh.13782 [DOI] [PubMed] [Google Scholar]
- 17.Nagalapuram V, Kanter J. Multi‐organ dysfunction secondary to abrupt discontinuation of voxelotor in a patient with severe sickle cell disease. Am J Hematol. 2022;97(8):E318–E320. doi: 10.1002/ajh.26631 [DOI] [PubMed] [Google Scholar]
- 18.Vissa M, Vichinsky E. Voxelotor for the treatment of sickle cell disease. Expert Rev Hematol. 2021;14(3):253–262. doi: 10.1080/17474086.2021.1893688 [DOI] [PubMed] [Google Scholar]
- 19.Phan V, Caldarera L, Cortez AL, et al. The Effect of Voxelotor on Exercise Capacity of Youths with Sickle Cell Anemia. Blood. 2021;138:2045. doi: 10.1182/blood-2021-149163 [DOI] [Google Scholar]
- 20.Herity LB, Vaughan DM, Rodriguez LR, Lowe DK. Voxelotor: a Novel Treatment for Sickle Cell Disease. Ann Pharmacother. 2021;55(2):240–245. doi: 10.1177/1060028020943059 [DOI] [PubMed] [Google Scholar]
- 21.Han J, Molokie RE, Hussain F, Njoku F, Gordeuk VR, Saraf SL. Voxelotor and albuminuria in adults with sickle cell anaemia. Br J Haematol. 2022;197(5). doi: 10.1111/bjh.18076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Global Blood Therapuetics. Oxbryta [prescribing information]; 2022. Available from: https://www.oxbryta.com/taking-oxbryta. Accessed May 24, 2022.
- 23.Han J, Saraf SL, Gordeuk VR. Systematic Review of Voxelotor: a First-in-Class Sickle Hemoglobin Polymerization Inhibitor for Management of Sickle Cell Disease. Pharmacotherapy. 2020;40(6):525–534. doi: 10.1002/phar.2405 [DOI] [PubMed] [Google Scholar]
- 24.Rutherford-Parker NJ, Campbell ST, Colby JM. Voxelotor Treatment Interferes With Quantitative and Qualitative Hemoglobin Variant Analysis in Multiple Sickle Cell Disease Genotypes. Am J Clin Pathol. 2020;154(5):627–634. doi: 10.1093/ajcp/aqaa067 [DOI] [PubMed] [Google Scholar]
- 25.Tsitsikas DA, Kamal M, Braimoh A, Benson S, Abukar J. Hb S (HBB: c.20A>T) Characteristics by High Performance Liquid Chromatography in Patients with Sickle Cell Disease Receiving the Novel Agent Voxelotor. Hemoglobin. 2021;45:1–3. doi: 10.1080/03630269.2020.1788074 [DOI] [PubMed] [Google Scholar]
- 26.Hebbel RP, Hedlund BE. Sickle hemoglobin oxygen affinity‐shifting strategies have unequal cerebrovascular risks. Am J Hematol. 2018;93(3):321–325. doi: 10.1002/ajh.24975 [DOI] [PubMed] [Google Scholar]
- 27.Bradt P, Spackman E, Synnott P, et al. Crizanlizumab, voxelotor, and L-glutamine for sickle cell disease: effectiveness and value. Ins Clin Economic Rev. 2020;1:58. [Google Scholar]
- 28.Crosby LE, Walton A, Shook LM, et al. Development of a Hydroxyurea Decision Aid for Parents of Children With Sickle Cell Anemia. J Pediatr Hematol Oncol. 2019;41(1):56–63. doi: 10.1097/mph.0000000000001257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyatt KD, List B, Brinkman WB, et al. Shared Decision Making in Pediatrics: a Systematic Review and Meta-analysis. Acad Pediatr. 2015;15(6):573–583. doi: 10.1016/j.acap.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 30.Hood AM, Strong H, Nwankwo C, et al. Engaging Caregivers and Providers of Children With Sickle Cell Anemia in Shared Decision Making for Hydroxyurea: protocol for a Multicenter Randomized Controlled Trial. JMIR Res Protoc. 2021;10(5):e27650. doi: 10.2196/27650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry ER, Metaferia B, Li Q, et al. Treatment of sickle cell disease by increasing oxygen affinity of hemoglobin. Blood J Am Soc Hematol. 2021;138(13):1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]