Abstract
Background
Few studies on the long-term efficacy of benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, have been conducted for patients with severe eosinophilic asthma (SEA), especially regarding the improvement of pulmonary function and clinical remission in a real-world setting.
Objective
To elucidate the long-term efficacy and clinical remission rate (CRR) in patients with SEA.
Methods
From July 2018 to July 2022, 23 Japanese patients with SEA received benralizumab for two years or more at Jikei University Hospital. We retrospectively evaluated the patients’ characteristics, biomarkers, number of exacerbations, pulmonary function, asthma symptoms, maintenance oral corticosteroid (OCS) dose and CRR.
Results
The mean observation period was 38.3 (24–49) months. Among the 23 patients, 10 patients switched from mepolizumab to benralizumab. After administration of benralizumab, the forced expiratory volume in one second (FEV1) increased and was maintained for two years in the biologic-naïve group and in the switching group (177 ± 404 and 151 ± 236 [mL], respectively, P = 0.80). In all patients, the %FEV1 improved from 76.7 ± 22.9% to 84.3 ± 18.4% (P = 0.016), and the number of annual exacerbations decreased from 2.5 ± 3.3 to 0.74 ± 1.7 (P = 0.014). Furthermore, the Asthma Control Test score significantly improved, and the reduction in OCS dose was maintained for three years. Ultimately, five patients met the clinical remission criteria and exhibited stabilization of pulmonary function, no exacerbation, no OCS use and well-controlled symptoms. The CRR was significantly higher in patients with a blood basophil count (BBC) ≥ 22 than in those with a BBC < 22 (/µL) (38.5% vs 0%, respectively, P = 0.046).
Conclusion
Long-term treatment with benralizumab significantly improved pulmonary function, alleviated asthma symptoms and decreased the number of exacerbations at two years in a real-world setting. The CRR may be associated with the BBC at baseline.
Keywords: benralizumab, severe asthma, clinical remission, pulmonary function, blood basophil count
Introduction
Severe asthma is defined as asthma that cannot be controlled by high doses of inhaled corticosteroids (ICSs) plus at least one other controller based on the published guidelines.1 The prevalence of severe asthma has been reported to be 3 to 10% among adult patients with asthma.2 Recently, several biologics have become available for patients with severe asthma and are effective in reducing exacerbations, improving health-related quality of life and decreasing socioeconomic loss.3–10 Benralizumab, an anti-interleukin-5 receptor α (IL-5RA) antibody, causes antibody-dependent cell-mediated cytotoxicity (ADCC), a process in which natural killer cells induce eosinophil apoptosis, with a rapid and nearly complete depletion of eosinophils in the peripheral blood. This biologic agent has been demonstrated to be an effective therapy for patients with severe eosinophilic asthma (SEA), as it reduces annual exacerbation rates and maintenance oral corticosteroid (OCS) doses and improves pulmonary function.6,7 However, the long-term efficacy of benralizumab with respect to both exacerbation and pulmonary function decline over time remains unestablished, especially in real-world settings11,12 Furthermore, clinical remission of severe asthma has recently been discussed as a therapeutic goal.13,14
We therefore conducted this single-center retrospective study to elucidate the long-term efficacy of benralizumab in a real-life setting. The primary endpoints were long-term pulmonary function measures, and the secondary endpoints were the clinical remission rate (CRR), the number of annual exacerbations, asthma symptoms, the maintenance OCS dose, and the relationships of these endpoints with patient characteristics.
Methods
Patients
From July 2018 to July 2022, 23 adult Japanese patients with SEA received benralizumab injections (30 mg every 4 weeks for the first three injections and every eight weeks for subsequent injections) for 18 months or more at Jikei University Hospital, Tokyo, Japan. All asthma patients were diagnosed by respiratory physicians based on Japanese guidelines or the Global Initiative of Asthma guidelines.1,15 Severe asthma was defined as asthma requiring a high dose of ICSs plus at least one of the following additional control measures: long-acting β2 agonists (LABAs), long-acting muscarinic antagonists (LAMAs), leukotriene receptor antagonists, a xanthine derivative and a daily OCS.1
The present study was approved by the Ethics Committee of Jikei University [34–161 (11312)]. Additionally, this study was conducted in accordance with the Declaration of Helsinki. Based on the ethical guidelines of Jikei University, informed consent was not necessary for this retrospective study, but we obtained opt-out consent on the website of our hospital. The benralizumab regimen was based on the guidelines of the Pharmaceuticals and Medical Devices Agency in Japan. In principle, the dosing schedule after the initial introduction was every 8 weeks; however, a deviation of several weeks was allowed. This retrospective study included patients who were analyzed in our previous studies of biologic treatment for severe asthma.16–18 Approximately 80% of the patients in this study overlapped with a cohort in a previous benralizumab study.17
Data Collection and Evaluations
We retrospectively examined the following characteristics: sex; age; duration of asthma; comorbid eosinophilic diseases; smoking status; body mass index (BMI); and baseline treatments, including biologics. We examined and evaluated the following parameters at baseline and after 6 (± 3), 12 (± 3), 24 (± 6), 36 (± 6), and 48 (± 6) months: the peripheral blood eosinophil count (BEC), blood basophil count (BBC), serum IgE, fractional exhaled nitric oxide (FeNO), Asthma Control Test (ACT) score, pulmonary function test results (forced vital capacity [FVC], forced expiratory volume in one second [FEV1], FEV1/FVC, %FEV1 and % peak expiratory flow [%PEF]), number of exacerbations and daily OCS maintenance dose as prednisone equivalents (mg). We extrapolated several missing data values using the last-observation-carried-forward approach. The FeNO level was measured using a NIOX VEROTM device (CIRCASSIA, Oxford, UK). The pulmonary function test was performed using a SpiroshiftTM SP-790COPD spirometer (FUKUDA DENSHI, Tokyo, Japan) without discontinuing bronchodilators as a controller. The number of annual exacerbations of asthma symptoms requiring systemic corticosteroid (CS) treatment was defined as the total number of exacerbations x 12/the total duration of the observation period (months). To evaluate asthma symptoms, we utilized the ACT score; the ACT score is clinically useful as a simple scoring system, and scores of 20–25 indicate well-controlled asthma. The minimal clinically important difference (MCID) was an ACT score of three points and a change in FEV1 of 100 (mL).20 Clinical remission was defined according to the following criteria at the last follow-up period: no exacerbation requiring OCS treatment for 12 months, no maintenance OCS use, well-controlled asthma symptoms with an ACT score ≥ 20, and a %FEV1 ≥ 80%.13,14 Furthermore, we evaluated the yearly adherence to regular ICS/LABA treatment using the medication possession ratio (MPR).21 We determined factors that affected clinical remission by evaluating BMI, MPR, disease duration, and comorbidities such as chronic rhinosinusitis with nasal polyp (CRSwNP)/eosinophilic chronic rhinosinusitis (ECRS), BEC, BBC, and FeNO at baseline. Among the predictive factors, BEC ≥ 300 (/µL) and FeNO ≥ 50 (ppb) are clinically important levels, and we adopted them as the cutoff values in the analysis. However, the standard of the BBC is unclear, and we set its value by analyzing the receiver operating characteristic (ROC) curve to determine patients who achieved clinical remission.
Statistical Analysis
All statistical analyses were performed using StatView version 5 (SAS Institute, Inc., Cary, NC, USA). All values are expressed as the means ± standard deviations or standard errors. A P value < 0.05 was considered to indicate statistical significance. The factors associated with patient characteristics were analyzed using the Mann‒Whitney U-test, Fisher’s exact test, or the Wilcoxon signed-rank test (univariate model). ROC curves were analyzed using EZR (version 1.55, Saitama Medical Center, Jichi University, Saitama, Japan) to determine the cutoff value for the BBC for classifying clinical remission.22
Results
Patient Characteristics and Changes in the Parameters
The patient characteristics are shown in Table 1. Of 23 patients with SEA who received benralizumab treatment, 10 had received mepolizumab for 19.7 months (mean, range 3–35 months) prior to benralizumab (switching group), and three of these 10 patients had received omalizumab for 39.7 months (mean) prior to mepolizumab. The other 13 patients were treated with benralizumab as their first biologic agent (bio-naïve group). The most common comorbidity was CRSwNP/ECRS, which occurred in 19 patients (83%), but only three patients suffered from aspirin-exacerbated respiratory disease as a comorbidity (Table 1). Before the administration of benralizumab, nine patients (39%) with SEA had received maintenance OCS at a mean dose of 6.0 mg/day prednisone equivalents. The mean benralizumab treatment period was 38.3 (24–49) months.
Table 1.
Patient Characteristics at Baseline (n=23)
| All Patients (n=23) | Biologics-Naïve (n=13) | Previous Mepolizumab (+) (n=10) | p value Between Two Groups | |
|---|---|---|---|---|
| Male, n (%) | 10 (43) | 5 (38) | 5 (50) | 0.69* |
| Age (years), mean (SD) (range) | 59.8 (10.3) (38–75) | 60.6 (9.8) (43–75) | 58.8 (11.4) (38–72) | 0.73† |
| Duration of disease (years), mean (SD) (range) | 21.9 (12.7) (6–54) | 23.6 (13.9) (6–54) | 19.4 (11.2) (6–36) | 0.57† |
| Body mass index (kg/m2), mean (SD) | 23.1 (4.6) | 24.6 (4.8) | 21.2 (3.6) | 0.07† |
| Smoking (never / former / current), n | 15 / 7 / 1 | 7 / 5 / 1 | 8 / 2 / 0 | 0.37‡ |
| Initial treatments use | ||||
| ―ICS/LABA, n (%) | 23 (100) | 13 (100) | 10 (100) | ― |
| ―ICS dose (µg), mean (SD) | 1503 (547) | 1491 (628) | 1520 (454) | 0.66† |
| ―LAMA, n (%) | 14 (61) | 8 (62) | 6 (60) | >0.99* |
| ―LTRA, n (%) | 19 (83) | 10 (77) | 9 (90) | 0.60* |
| ―xanthine derivative, n (%) | 15 (65) | 8 (62) | 7 (70) | >0.99* |
| ―maintenance therapy of OCS, n (%) | 9 (39) | 6 (46) | 3 (30) | 0.67* |
| ―daily dose of OCS (mg), mean (range) | 6.0 (2.5–15) (n=9) | 6.8 (4–15) (n=6) | 4.5 (2.5–6) (n=3) | 0.50† |
| Comorbidities | ||||
| ―ECRS, n (%) | 19 (83) | 11 (85) | 8 (80) | >0.99* |
| ―EGPA, n (%) | 4 (17) | 1 (8) | 3 (30) | 0.28* |
| ―AERD, n (%) | 3 (13) | 3 (23) | 0 (0) | 0.23* |
| ―chronic eosinophilic pneumonia, n (%) | 3 (13) | 0 (0) | 3 (30) | 0.07* |
| Previous biologics | ||||
| ―omalizumab, n (%) / mean (range) (month) | 3 (13) / 39.7 (11–88) | ― | 3 (30) / 39.7 (11–88) | ― |
| ―mepolizumab, n (%) / mean (range) (month) | 10 (43) / 19.7 (3–35) | ― | 10 (100) / 19.7 (3–35) | ― |
| Observation period (months), mean (range) | 38.3 (24–49) | 37.9 (24–47) | 38.7 (24–49) | 0.76† |
Notes: Data are presented as n (%) or mean (standard deviation), unless otherwise stated. *Fisher’s exact test, †Mann–Whitney U-test, ‡Chi-square test.
Abbreviations: AERD, aspirin-exacerbated respiratory disease; ECRS, eosinophilic chronic rhinosinusitis; EGPA, eosinophilic granulomatosis with polyangiitis; ICS, inhaled corticosteroid; LABA, long-acting β-2 agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroids; SD, standard deviation.
Regarding the changes in parameters between baseline and time points after two years in all patients, benralizumab treatment significantly decreased BEC, BBC, serum IgE levels and the number of annual exacerbations, while it significantly improved FEV1 and the ACT score. The maintenance OCS dose was significantly lower than the pretreatment dose only at the last follow-up; it was not significantly different after two years (Table 2 and footnote). However, FeNO levels did not decrease. In the comparison between the bio-naïve group and the switching group, significant differences were observed only in BEC and FEV1 at baseline (Table 2).
Table 2.
Change from Baseline After 2 Years in Asthma Patients with or Without Previous Biologics
| All Patients (n=23) | Biologics-Naïve (n=13) | Previous Mepolizumab (+) (n=10) | P value Between Two Groups At Baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 2 Years | P value | Baseline | After 2 Years | P value | Baseline | After 2 Years | P value | P value | |
| Peripheral blood eosinophil counts (/µL) | 605 (1354) | 43 (200) | <0.0001 | 971 (1736) | 74 (266) | 0.002 | 130 (137) | 3 (7) | 0.008 | <0.001 |
| Peripheral blood basophil counts (/µL) | 53 (56) | 16 (19) | 0.0002 | 62 (65) | 13 (14) | 0.002 | 41 (42) | 19 (25) | 0.037 | 0.40 |
| Serum IgE (IU/mL) | 249 (233) | 174 (181) | 0.033 | 301 (242) | 202 (207) | 0.11 | 181 (213) | 140 (149) | 0.14 | 0.15 |
| FeNO (ppb) | 71 (52) | 68 (46) | 0.60 | 73 (52) | 73 (48) | 0.66 | 69 (56) | 62 (44) | 0.09 | 0.80 |
| %FVC (%) | 92.3 (14.7) | 101.5 (14.0) | 0.01 | 94.1 (18.1) | 106.0 (15.6) | 0.02 | 90.0 (9.1) | 95.2 (8.9) | 0.24 | 0.60 |
| %FEV1 (%) | 76.7 (22.9) | 84.3 (18.4) | 0.016 | 77.4 (28.4) | 86.8 (22.0) | 0.11 | 75.7 (14.2) | 80.9 (12.6) | 0.042 | 0.95 |
| FEV1/FVC (%) | 67.0 (11.5) | 67.8 (11.5) | 0.47 | 65.5 (12.3) | 66.4 (12.9) | 0.48 | 69.0 (10.6) | 69.7 (9.6) | 0.12 | 0.58 |
| FEV1 (mL) | 1923 (556) | 2101 (528) | 0.045 | 1828 (716) | 2007 (657) | 0.18 | 2048 (205) | 2230 (259) | 0.12 | <0.01 |
| %PEF (%) | 84.7 (25.7) | 85.7 (23.0) | 0.43 | 86.9 (30.4) | 91.7 (24.9) | 0.93 | 81.8 (19.0) | 77.4 (18.4) | 0.26 | 0.95 |
| ACT (pts) | 18.8 (5.2) | 21.7 (3.9) | 0.0007 | 18.0 (5.4) | 22.1 (3.5) | 0.009 | 19.8 (5.1) | 21.2 (4.4) | 0.027 | 0.51 |
| Number of annual exacerbations | 2.5 (3.3) | 0.74 (1.7) | 0.014 | 3.2 (4.0) | 0.92 (1.9) | 0.049 | 1.5 (2.1) | 0.5 (1.3) | 0.17 | 0.44 |
| Prednisolone equivalent dose (mg/day) (n=9) | 6.0 (3.6) | 2.9 (2.4) | 0.07* | 6.8 (4.1) | 2.2 (2.4) | 0.07 | 4.5 (1.8) | 4.5 (1.8) | ― | 0.50 |
Notes: Data are presented as the mean (standard deviation) and were analyzed using the Wilcoxon signed rank test. *Prednisolone equivalent dose significantly decreased to 2.6 (2.1) mg/day at last follow-up (P = 0.042).
Abbreviations: ACT, Asthma Control Test; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in one second; FVC, forced volume capacity; PEF, peak expiratory flow.
Change in FEV1 Over a Maximum of 4 Years and Predictive Factors for Efficacy
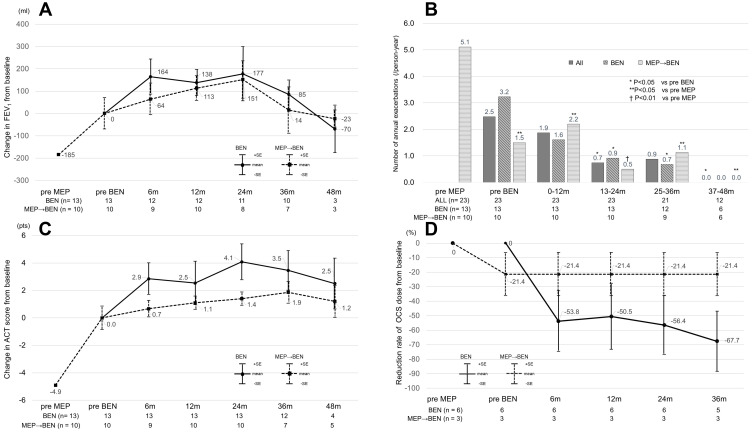
The mean changes in FEV1 from baseline to the various time points of benralizumab administration in the bio-naïve/switching groups (Figure 1A) were as follows: +164 (mL)/+64 (mL) at six months, +138 (mL)/+113 (mL) at 12 months, +177 (mL)/+151 (mL) at 24 months, +94 (mL)/+14 (mL) at 36 months, and −140 (mL)/-23 (mL) at 48 months. Although the change in FEV1 at 36 months had been maintained in the bio-naïve group, FEV1 in the switching group had decreased to the baseline level by 36 months.
Figure 1.
Changes in clinical parameters before and after benralizumab treatment (A) Change in FEV1 from baseline in the bio-naïve group and switching group (B) Number of annual exacerbations in all patients, the bio-naïve group and the switching group (C) Change in ACT score from baseline in the bio-naïve group and switching group (D) Reduction rate of the OCS dose from baseline in the bio-naïve group and switching group. All data are presented as the mean. In (A), (B) and (C), the data were analyzed using the Wilcoxon signed-rank test. The upper and lower bars represent the standard errors in (A) and (C). *P < 0.05 compared with pre-BEN. **P < 0.05 compared with pre-MEP. †P <0.01 compared with pre-MEP.
Abbreviations: ACT, Asthma Control Test; FEV1, forced expiratory volume in one second; BEN, benralizumab; MEP, mepolizumab; pre-BEN or pre-MEP, pretreatment with BEN or MEP; m, month(s); n, number of patients for whom clinical data were available; OCS, oral corticosteroid.
Numbers of Annual Exacerbations Over a Maximum of 4 Years
The numbers of annual exacerbations requiring OCS are shown in Figure 1B. The numbers significantly decreased over a maximum of four years in the two groups. The rate of reduction in the number of annual exacerbations was −72% at 24 months (from 2.5 to 0.7/person-year) and −64% at 36 months (from 2.5 to 0.9/person-year) in all patients. On the other hand, a significant difference was not observed between the two groups. The most common cause of exacerbation was respiratory tract infection; however, only one patient suffered from coronavirus disease 2019 (COVID-19) during the observational period in the present study (data not shown).
Change in the ACT Score Over a Maximum of 4 Years
We analyzed the changes in ACT scores in the bio-naïve group and the switching group (Figure 1C). The ACT scores in the bio-naïve group significantly improved with an MCID (≥ 3) for three years, and the efficacy was maintained for approximately four years. In the switching group, the ACT scores further increased without an MCID after switching to benralizumab and were maintained for four years.
Reductions in Maintenance OCS Doses in the Two Groups
In the switching group, the patients who received maintenance OCS continued mepolizumab treatment for 16.0 (12–23) months and the rate of reduction in the maintenance OCS dose was −21.4% (Figure 1D). However, no further reduction in the maintenance OCS dose was observed in the switching group after switching to benralizumab. On the other hand, the maintenance OCS dose in the bio-naïve group was reduced after benralizumab administration, without a significant difference at two years. The reduction rates were −50.5 to −67.7%, and the reductions were maintained for three years in the bio-naïve group (Figure 1D). Among the nine patients prescribed maintenance OCS at baseline, three patients in the biologic-naïve group could cease OCS as a consequence of the benralizumab treatment.
Adherence as Evaluated by MPR
The mean MPRs of ICS/LABA adherence were maintained at more than 80% in the bio-naïve and switching groups throughout the observation period. Specifically, the values for the bio-naïve/switching groups were 86.0 ± 18.8%/84.4 ± 25.1% prior to benralizumab treatment, 90.4 ± 23.2%/87.6 ± 11.9% at 12 months, 84.4 ± 23.3%/87.1 ± 13.6% at 24 months, 92.3 ± 14.2%/81.0 ± 20.7% at 36 months, and 83.9 ± 32.2%/87.9 ± 26.8% at 48 months, respectively.
Clinical Remission and Four Criteria
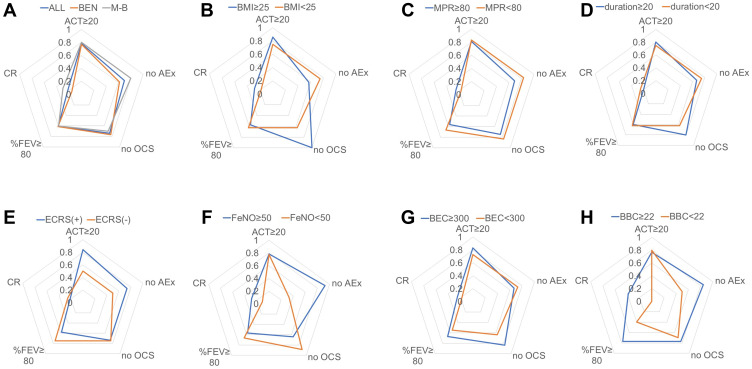
We also analyzed the achievement rates for the following four criteria of clinical remission based on the patients’ characteristics and biomarkers (Figure 2A–H and Supplementary Table 1): an ACT score ≥ 20, no exacerbation, no maintenance OCS use and a %FEV1 ≥ 80%. There were no differences between the bio-naïve and switching groups. The achievement rate of each criterion was approximately 60 to 80%, and the percentage of patients who met all four criteria was 21.7% (n=5). There was a significant difference in the “no exacerbation” rate between patients with an FeNO ≥ 50 (ppb) and those with an FeNO < 50 (ppb) (Figure 2F, P=0.0049, Fisher’s exact test). Furthermore, based on the BBC cutoff value at baseline for clinical remission, which we determined by analyzing the ROC curve, we observed significant differences in the CRR between patients with a BBC ≥ 22 and those with a BBC < 22 (/µL) (P = 0.046) (Figure 2H and Supplementary Figure 1), but a significant difference in the percentages of patients with a %FEV1 ≥ 80% was not observed (P = 0.10).
Figure 2.
Achievement rates of clinical remission and the four criteria. This radar chart is composed of an ACT ≥ 20, no exacerbation, no maintenance OCS, a %FEV1 ≥ 80 and clinical remission. The analyses were conducted for (A) all patients, bio-naïve (BEN) patients and switching (M-B) patients; (B) patients with a BMI ≥ 25 and patients with a BMI < 25 (kg/m2); (C) patients with an MPR ≥ 80 and patients with an MPR < 80 (%); (D) patients with a disease duration ≥ 20 and patients with a disease duration < 20 (years); (E) ECRS (+) and ECRS (-) patients; (F) patients with a BEC ≥ 300 and patients with a BEC < 300 (/µL); (G) patients with a BBC ≥ 22 and patients with a BBC < 22 (/µL); and (H) patients with FeNO ≥ 50 and patients with FeNO < 50 (ppb).
Abbreviations: ACT, Asthma Control Test; OCS, oral corticosteroid; FEV1, forced expiratory volume in one second; BEN, benralizumab; M-B, switching from mepolizumab to benralizumab; BMI, body mass index; MPR, medication possession ratio; ECRS, eosinophilic chronic rhinosinusitis; BEC, blood eosinophil count; BBC, blood basophil count; FeNO, fractional exhaled nitric oxide.
Discussion
In the present study, benralizumab treatment increased FEV1 and the ACT score and reduced the number of annual exacerbations; moreover, these effects were maintained for approximately three years. We showed that the change in FEV1 was +177 mL in the bio-naïve group at 24 months, which is similar to the findings of previous studies.11,23 On the other hand, the change in FEV1 after mepolizumab treatment in the switching group further increased to +151 mL at 24 months, and this clinically significant improvement in pulmonary function corresponded to that in previous real-world studies conducted over two years.24,25 However, FEV1 in both the bio-naïve and switching groups tended to decrease after 36 months in the present study. The extension study of mepolizumab (COLUMBA study) showed that the initially improved FEV1 gradually declined to the baseline level by four years (228 weeks) despite the maintained efficacy with respect to asthmatic exacerbation.19 However, no previous study of adult patients has examined the changes in pulmonary function more than two years after benralizumab administration. Based on clinical records, patients with asthma who receive ICSs/LABAs, especially budesonide/formoterol or fluticasone propionate/formoterol, inadequately self-adjusted their treatments without following their physicians’ instructions. In addition, physicians stopped LAMA treatment in several patients after an improvement in asthma control was obtained using benralizumab treatment, resulting in a decrease in FEV1. Actually, a decrease in inhalation adherence is reported to occur in more than 40% of all patients treated with biologics, and thus a routine examination of pulmonary function is important for evaluating adherence and for adjusting treatment, and appropriate guidance is needed in cases of long-term treatment and improvement.26 However, reduced adherence to inhalers, as indicated by parameters such as an MPR < 80%, was not obviously associated with worsening of factors related to clinical remission (Figure 2C). Accordingly, we speculate that the mechanisms underlying the decrease in FEV1 after long-term benralizumab treatment are likely attributed to several factors, including a chronological decline in pulmonary function, decreased adherence to inhalants,19 and the potential existence of a mechanism for airway obstruction induced by long-term use of a single biologic, which should be examined in future studies using combinations of biologics or switching usage to different biologics, including dupilumab. Previous studies showed the effectiveness of switching and combination biologic treatment, including biologics targeting IL-5 and IL-4.24,27–30 Intriguingly, the present study showed a similar trajectory for the change in FEV1 from baseline after benralizumab treatment in both the bio-naïve and switching groups (Figure 1A), and these results may support the hypothesis that the switch to different biologics may be efficient for preventing the decline in pulmonary function after long-term usage of single biologic. The improvement in pulmonary function in patients treated with benralizumab may have been due not only to a decrease in BEC but also to decreases in tissue eosinophil counts, including in the airway wall. Moreover, a BBC cutoff of ≥ 22 was selected in this study as a predictive factor for clinical remission. Basophils are an important source of IL-4 and are involved in the activation of group 2 innate lymphoid cells (ILC2s), which produce IL-5 and IL-13.31,32 It has been reported that blood basophil counts correlate with asthma symptoms and a decline in pulmonary function and that the expression of CD203c, a surface marker of degranulation from basophils, negatively correlates with pulmonary function.33–36 Since IL-13 affects the thickness of the basement membrane and airway remodeling, the reductions in both eosinophil and basophil numbers due to the ADCC activity of benralizumab may have contributed to clinical remission, including the improvement in pulmonary function, in a complex manner.37–39 Accordingly, we speculate that blood basophil counts can be a promising biomarker for predicting CRR by benralizumab treatment, which should be examined in future studies.
A previous study reported that the annual decrease in FEV1 of patients with high FeNO levels was significantly greater than that of patients with low FeNO levels.40 Although there was no significant reduction in FeNO after the administration of benralizumab in the present study, the improvement in FEV1 was maintained for three years. This finding may support the hypothesis that FeNO levels may not affect the decrease in FEV1 if biologics efficiently reduce the number of annual exacerbations requiring OCS treatment. Although IL-13 is involved in the production of FeNO, which may be controlled by dupilumab treatment, the effect of anti-IL-5/IL-5RA antibody treatment on the decrease in FeNO is controversial.16,17,41 Thus, FeNO levels are related to eosinophilic airway inflammation, but the changes in FeNO levels may not reflect the efficacy of anti-IL-5/IL-5RA antibody treatment, indicating that the interpretation of FeNO levels should be considered carefully during benralizumab treatment.
Because the number of patients was small in the present study, the maintenance OCS dose showed nonsignificant reductions over two years but decreased significantly over the maximum of four years in response to benralizumab treatment. The maintenance OCS dose required in the bio-naïve group decreased for the first six months and was maintained for three years. In a study on maintenance OCS elimination using an algorithm in patients treated with benralizumab, namely, the PONENTE trial, the median time to reach the final dose was 19.7–25.0 weeks, which is similar to the time frame in the present study.42 On the other hand, in the group switching from mepolizumab treatment, no further reduction in the maintenance OCS doses was achieved with benralizumab treatment. The SIRIUS and COSMOS trials of the reduction in the maintenance OCS dose of mepolizumab indicated that the time to reach this optimal dose was approximately 24 weeks.43,44 In the present study, the mean duration of mepolizumab treatment was 16.0 months in the switching group, suggesting that the maximum reduction in the OCS dose was already achieved at the time of switching to benralizumab.
Recently, clinical remission has become a new goal for patients with severe asthma, especially in the setting of using biologic agents. As criteria for clinical remission, we used no exacerbations, no OCS use, well-controlled symptoms and stabilization of the %FEV1; however, these criteria are not definitive and have some controversies.13,14 A previous pooled post hoc analysis of benralizumab reported a CRR of approximately 15%, which is lower than that in the present study. One possible explanation for this difference is selection bias of participants. Both patients who switched from mepolizumab and biologically naïve patients were included in the present study. In addition, 83% of patients suffered from ECRS, which is one of the predictive factors for a favorable response to benralizumab treatment.45,46 Another possible explanation is that the pooled post hoc analysis used different criterion of FEV1 for clinical remission, namely, an improvement in prebronchodilator FEV1 ≥ 100 (mL). Because we did not examine prebronchodilator FEV1, %FEV1 ≥ 80% was used in the present study. A third explanation might be that the number of asthmatic exacerbations decreased due to infection prevention measures such as universal “masking” against COVID-19 during our observation period.47,48
The present study had several limitations. First, this single-center retrospective study was conducted using a small number of patients. Thus, selection bias could not be excluded in this study. However, this study first reported long-term benralizumab data up to four years in patients in both bio-naïve and switching groups and proposed several real-world clinical issues, such as a decrease in pulmonary function or adherence.
Second, since the number of patients was small, we could not perform multivariate logistic regression analysis for predictors. Further validation of the present findings based on long-term efficacy evaluations, including the value of the BBC cutoff as a biomarker and CRR, should be performed in future studies with increased numbers of participants.
Third, we previously reported the efficacy of benralizumab treatment for a median of 11.5 (4–17) months, and the primary endpoint was the GETE score.17 However, we believe that this study is the first report demonstrating the trajectory of pulmonary function and CRR in patients with long-term benralizumab treatment up to four years in the real-world setting.
In conclusion, benralizumab treatment in patients with SEA showed real-world effectiveness for a maximum of three years with regard to pulmonary function, asthma symptoms, annual exacerbations and maintenance OCS dose. However, FEV1 may decrease after long-term benralizumab treatment without an appropriate explanation, but decreased adherence to inhalation therapy should be carefully monitored as a potential risk factor for decreasing pulmonary function.
Funding Statement
There is no funding to report.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Due to the retrospective nature of this study, we provided an opt-out consent on the website of our hospital. This study was approved by the Ethical Committee of the Jikei University School of Medicine [34-161 (11312)] on Sep 21, 2022. The director/administer of Jikei University Hospital granted us permission to access the medical records. The data used in this study were anonymized before use.
Author Contributions
All authors made significant contributions to the work reported, whether to the study conception, design, and execution; data acquisition, analysis, and interpretation; or all these areas. All authors contributed to drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention; 2021. Available from: www.ginasthma.org. Accessed November 24, 2022.
- 2.Hekking PPW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi: 10.1111/j.1398-9995.2004.00772.x [DOI] [PubMed] [Google Scholar]
- 4.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi: 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 5.Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi: 10.1016/S2213-2600(17)30125-X [DOI] [PubMed] [Google Scholar]
- 6.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 7.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 8.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 9.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 10.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi: 10.1056/NEJMoa1804093 [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald JM, Bleecker ER, Bourdin A, et al. Two-year integrated efficacy and safety analysis of benralizumab in severe asthma. J Asthma Allergy. 2019;12:401–413. doi: 10.2147/JAA.S227170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korn S, Bourdin A, Chupp G, et al. Integrated safety and efficacy among patients receiving benralizumab for up to 5 years. J Allergy Clin Immunol Pract. 2021;9(12):4381–4392.e4. doi: 10.1016/j.jaip.2021.07.058 [DOI] [PubMed] [Google Scholar]
- 13.Menzies-Gow A, Bafadhel M, Busse WW, et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol. 2020;145(3):757–765. doi: 10.1016/j.jaci.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 14.Thomas D, McDonald VM, Pavord ID, Gibson PG. Asthma remission- what is it and how can it be achieved? Eur Respir J. 2022;60:2102583. doi: 10.1183/13993003.02583-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura Y, Tamaoki J, Nagase H, et al. Japanese guidelines for adult asthma 2020. Allergol Int. 2020;69(4):519–548. doi: 10.1016/j.alit.2020.08.001 [DOI] [PubMed] [Google Scholar]
- 16.Numata T, Nakayama K, Utsumi H, et al. Efficacy of mepolizumab for patients with severe asthma and eosinophilic chronic rhinosinusitis. BMC Pulm Med. 2019;19:176. doi: 10.1186/s12890-019-0952-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Numata T, Miyagawa H, Nishioka S, et al. Efficacy of benralizumab for patients with severe eosinophilic asthma: a retrospective, real-life study. BMC Pulm Med. 2020;20(1):207. doi: 10.1186/s12890-020-01248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Numata T, Araya J, Miyagawa H, et al. Effectiveness of switching biologics for severe asthma patients in Japan: a single-center retrospective study. J Asthma Allergy. 2021;14:609–618. doi: 10.2147/JAA.S311975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatri S, Moore W, Gibson PG, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143(5):1742–1751. doi: 10.1016/j.jaci.2018.09.033 [DOI] [PubMed] [Google Scholar]
- 20.Schatz M, Kosinski M, Yarlas AS, et al. The minimally important difference of the asthma control test. J Allergy Clin Immunol. 2009;124(4):719–723. doi: 10.1016/j.jaci.2009.06.053 [DOI] [PubMed] [Google Scholar]
- 21.D’Ancona G, Kavanagh JE, Dhariwal J, et al. Adherence to inhaled corticosteroids and clinical outcomes following a year of benralizumab therapy for severe eosinophilic asthma. Allergy. 2021;76(7):2238–2241. doi: 10.1111/all.14737 [DOI] [PubMed] [Google Scholar]
- 22.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kavanagh JE, Hearn AP, Dhariwal J, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2021;159(2):496–506. doi: 10.1016/j.chest.2020.08.2083 [DOI] [PubMed] [Google Scholar]
- 24.Drick N, Milger K, Seeliger B, et al. Switch from il-5 to il-5-receptor α antibody treatment in severe eosinophilic asthma. J Asthma Allergy. 2020;13:605–614. doi: 10.2147/JAA.S270298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez-Bastero Fernández A, Medina Gallardo JF, Delgado Romero J, et al. Effectiveness of switching to benralizumab in severe refractory eosinophilic asthma. J Asthma Allergy. 2022;15:727–735. doi: 10.2147/jaa.s358705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corren J, Silver J, Molfino NA, et al. A real-world study of inhaled corticosteroid use in patients with severe eosinophilic asthma treated with mepolizumab. Ann Allergy Asthma Immunol. 2022;128(2):184–192.e1. doi: 10.1016/j.anai.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 27.Mümmler C, Munker D, Barnikel M, et al. Dupilumab improves asthma control and lung function in patients with insufficient outcome during previous antibody therapy. J Allergy Clin Immunol Pract. 2021;9(3):1177–1185.e4. doi: 10.1016/j.jaip.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 28.Hamada S, Ogino E, Yasuba H. Cycling biologic therapy for severe asthma: cycling therapy. Pulmonology. 2022;28(1):65–67. doi: 10.1016/j.pulmoe.2021.07.009 [DOI] [PubMed] [Google Scholar]
- 29.Hamada S, Ogino E, Yasuba H. Cycling therapy with benralizumab and dupilumab for severe eosinophilic asthma with eosinophilic chronic rhinosinusitis and eosinophilic otitis media. Allergol Int. 2021;70(3):389–391. doi: 10.1016/j.alit.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 30.Numata T, Araya J, Miyagawa H, et al. Real-world effectiveness of dupilumab for patients with severe asthma: a retrospective study. J Asthma Allergy. 2022;15:395–405. doi: 10.2147/JAA.S357548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motomura Y, Morita H, Moro K, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40(5):758–771. doi: 10.1016/j.immuni.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 32.Klose CSN, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–774. doi: 10.1038/ni.3489 [DOI] [PubMed] [Google Scholar]
- 33.Winter NA, Qin L, Gibson PG, et al. Sputum mast cell/basophil gene expression relates to inflammatory and clinical features of severe asthma. J Allergy Clin Immunol. 2021;148(2):428–438. doi: 10.1016/j.jaci.2021.01.033 [DOI] [PubMed] [Google Scholar]
- 34.Ono E, Taniguchi M, Higashi N, et al. CD203c expression on human basophils is associated with asthma exacerbation. J Allergy Clin Immunol. 2010;125(2):483–489.e3. doi: 10.1016/j.jaci.2009.10.074 [DOI] [PubMed] [Google Scholar]
- 35.Lewis SA, Pavord ID, Stringer JR, Knox AJ, Weiss ST, Britton JR. The relation between peripheral blood leukocyte counts and respiratory symptoms, atopy, lung function, and airway responsiveness in adults. Chest. 2001;119(1):105–114. doi: 10.1378/chest.119.1.105 [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Wang C, Li H, et al. Circulating white blood cells and lung function impairment: the observational studies and Mendelian randomization analysis. Ann Med. 2021;53(1):1118–1128. doi: 10.1080/07853890.2021.1948603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19(6):676–680. doi: 10.1016/j.coi.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 38.Doherty TA, Broide DH. Airway innate lymphoid cells in the induction and regulation of allergy. Allergol Int. 2019;68(1):9–167. doi: 10.1016/j.alit.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lommatzsch M, Marchewski H, Schwefel G, Stoll P, Virchow JC, Bratke K. Benralizumab strongly reduces blood basophils in severe eosinophilic asthma. Clin Exp Allergy. 2020;50(11):1267–1269. doi: 10.1111/cea.13720 [DOI] [PubMed] [Google Scholar]
- 40.Matsunaga K, Hirano T, Oka A, Ito K, Edakuni N. Persistently high exhaled nitric oxide and loss of lung function in controlled asthma. Allergol Int. 2016;65(3):266–271. doi: 10.1016/j.alit.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 41.Hearn AP, Kavanagh J, D’Ancona G, et al. The relationship between Feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2021;9(5):2093–2096.e1. doi: 10.1016/j.jaip.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 42.Menzies-Gow A, Gurnell M, Heaney LG, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med. 2021;2600(21):1–12. doi: 10.1016/s2213-2600(21)00352-0 [DOI] [PubMed] [Google Scholar]
- 43.Wenzel EHB, Thompson S, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 44.Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38(9):2058–2070.e1. doi: 10.1016/j.clinthera.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 45.FitzGerald JM, Bleecker ER, Menzies-Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6(1):51–64. doi: 10.1016/S2213-2600(17)30344-2 [DOI] [PubMed] [Google Scholar]
- 46.Bagnasco D, Brussino L, Bonavia M, et al. Efficacy of benralizumab in severe asthma in real life and focus on nasal polyposis. Respir Med. 2020;171:106080. doi: 10.1016/j.rmed.2020.106080 [DOI] [PubMed] [Google Scholar]
- 47.Abe K, Miyawaki A, Nakamura M, Ninomiya H, Kobayashi Y. Trends in hospitalizations for asthma during the COVID-19 outbreak in Japan. J Allergy Clin Immunol Pract. 2021;9(1):494–496.e1. doi: 10.1016/j.jaip.2020.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klompas M, Morris CA, Sinclair J, Pearson M, Shenoy ES. Universal masking in hospitals in the covid-19 era. N Engl J Med. 2020;382(21):e63. doi: 10.1056/NEJMp2006372 [DOI] [PubMed] [Google Scholar]