Abstract
Objective:
Obesity and its consequences are among the biggest challenges facing the healthcare system. Uterine leiomyomas are the most common gynecologic tumors. The risk of leiomyoma increases with obesity, but the underlying mechanisms of this association remain unclear. The aim of the present study to determine the cellular and molecular mechanisms by which adipocyte contributes to both leiomyoma tumor initiation and promotion.
Methods:
Primary myometrium and leiomyoma cells were isolated from patients who underwent a hysterectomy or myomectomy. Pro-inflammatory, fibrotic, and angiogenic factors were measured using a multiplex cytokine array in human primary and immortalized myometrial and leiomyoma cells cocultured with human adipocyte (SW872) cells, or in animal ELT3 leiomyoma cells cocultured with 3T3-L1 adipocytes. The free fatty acids (FFAs) and fatty acid-binding protein 4 (FABP4) levels were measured using immunofluorescence assays. Other protein abundances were determined using western blots. The expression levels of TNF-α, MCP-1, phospho-NF-κB, TGFβ3 and VEGF-A in lean and obese in different leiomyoma patients were determined by immunofluorescence staining.
Results:
Adipocytes promote inflammation, fibrosis, and angiogenesis in uterine leiomyoma cells by upregulating associated factors, such as IL-1β, TNF-α, MCP-1, GM-CSF, TGF-βs, PLGF, VEGF, HB-EGF, G-CSF and FGF2. Coculture led to the transfer of FFAs and FABP4 from adipocytes to leiomyoma cells, suggesting that adipocytes may modulate metabolic activity in these tumor cells. Increased levels of FFA and FABP4 expressions were detected in obese leiomyoma tissue compared to lean. The adipocyte–leiomyoma cell interaction increased the phospho-NF-κB level, which plays a key role in inflammation, restructuring metabolic pathways, and angiogenesis. Obese leiomyoma patients expressed a higher amount of TNF-α, MCP-1, phospho-NF-κB, TGFβ3 and VEGF-A than lean leiomyoma patients, consistent with in vitro findings. Furthermore, we found that adipocyte secretory factors enhance leiomyoma cell proliferation by increasing PCNA abundance. Finally, the inhibition of the inflammatory factors TNF-α, MCP-1, and NF-κB abrogated the adipocyte coculture-induced proliferation of leiomyoma cells.
Conclusions:
Adipocytes release inflammatory, fibrotic, and angiogenic factors, along with FFAs, which contribute to a tumor-friendly microenvironment that may promote leiomyoma growth and can represent a new target for leiomyoma prevention and treatment.
Keywords: Uterine leiomyoma, adipocyte, inflammation, fibrosis, angiogenesis, proliferation
Introduction
Uterine leiomyomas (fibroids or myomas) are the most common tumors of the female reproductive tract, with symptoms including heavy menstruation, pelvic pain, and subfertility. The lifetime incidence of uterine leiomyomas is currently estimated at 70–80%, which causes significant medical and economic burdens [1]. The available pharmaceutical options only provide short-term relief, meaning surgery (myomectomy or hysterectomy) is the ultimate treatment in many cases [2]. The hallmarks of these tumors include genetic mutations, the dysregulation of several signaling pathways, the proliferation of uterine smooth muscle cells, and excessive disordered extracellular matrix (ECM) [3–5], although the exact cellular and molecular mechanisms underlying tumor development are yet to be fully elucidated.
Worldwide, obesity is a leading cause of metabolic dysfunction, resulting in chronic diseases such as diabetes, cardiovascular disease, and cancer [6]. In the United States, the combined prevalence of overweight (BMI over 25) and obesity (BMI over 30) among women of reproductive age is estimated at 30% [7]. The state of obesity is associated with an increased risk of certain tumors, such as breast, liver, lung, prostate, colorectal, and renal cancers. Adipose tissue acts as an endocrine organ that may affect the tumor microenvironment through the secretion of signaling molecules, including adipokines, cytokines, and chemokines; mostly are pro-inflammatory, fibrotic, and angiogenic factors [8–10]. Consequently, the circulating levels of tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein 1 (MCP-1), interleukin-6 (IL-6), IL-1β, transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), and plasminogen activator inhibitor-1 (PAI-1) are higher in overweight and obese individuals relative to lean controls, which may play a role in tumor cell growth regulation, differentiation, and inflammation [8–10].
The literature supports an obesity-associated increase in leiomyoma risk [11–14]. In our recent cross-sectional study of women at the Johns Hopkins Health System, those with leiomyoma were significantly more likely to be obese (Odds Ratio (OR) = 2.56; 95% Confidence Interval (CI) 2.49–2.63) [11]. Additionally, women with leiomyoma were more likely to be overweight (45.5%) or obese (52.9%) when compared without leiomyoma (36.9% and 35.8%, respectively) [11]. Another study showed that obese women are more than twice as likely to develop leiomyoma than normal-weight or underweight women [15]. A recent meta-analysis demonstrated the association between obesity and leiomyoma and suggested it is non-linear, [16]. Several observational studies have examined the association between obesity and uterine leiomyoma risk [11–14, 17, 18], but the findings are inconsistent, likely due to interactions with other risk factors. Moreover, the biological interplay between adipose tissue dysfunction and leiomyomas is poorly understood, and its mechanisms remain unclear. In obese individuals, serum leptin concentrations are significantly higher than those of lean individuals due to increased levels of dysfunctional adipose tissue [19]. Our group recently demonstrated that leptin induces leiomyoma cells proliferation and ECM deposition through JAK2/STAT3 and MAPK/ERK signaling [17]. To better understand how obesity and uterine leiomyoma biologically interact, a coculture system was used to study the interaction between adipocytes and human uterine myometrium and leiomyoma cells. In the present we determine the cellular and molecular mechanisms by which obesity contributes to both leiomyoma tumor initiation and promotion. We examined the effects of adipocytes on myometrial cells to examine the effect on tumor initiation, and the effects on leiomyoma cells to examine the effect on tumor promotion.
Materials and methods
Cells isolation and culture
We isolated primary myometrium and leiomyoma cells from five different human subjects as we previously performed [20, 21]. All subjects gave their informed consent to participate in the study following its approval by the Institutional Review Board (IRB) of Johns Hopkins University. A Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F-12) (Thermo Fisher Scientific, Waltham, Massachusetts, USA) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic–antimycotic in 5% CO2 at 37°C was used for cell culture. Primary myometrium and leiomyoma cells were characterized using proliferation marker (PCNA), ECM (COL1A, FN), and steroid hormone receptors (ERα, PR-A/B) from different patients. We have seen more ECM dysregulation and steroid hormone receptor expression in leiomyoma cell than in myometrium cell (Supplementary Fig. 1), which is the hallmark of leiomyoma development. We received immortalized human uterine smooth muscle (UtSM) and leiomyoma (HuLM) cells from Dr. Dixon as a gift [22] and they have been used by us and others [18, 20, 21]. The UtSM and HuLM cells ware maintained in a medium that contained smooth muscle cell growth medium (Lonza, Walkersville, Maryland, USA), 5% FBS, 0.1% insulin, 0.2% recombinant human fibroblast growth factor B, 0.1% of a mixture of gentamicin sulfate and amphotericin B, and 0.1% human epidermal growth factor in 5% CO2 at 37°C. We also used Eker rat uterine leiomyoma (ELT3) cell lines from Eker rats which was a kind gift from Dr. Cheryl Walker [23]. This cells have a similar histology to human leiomyomas and have been used before [23, 24]. The ELT3 cells were maintained in DF8 medium containing 10% FBS in 5% CO2 at 37°C, as described previously [23, 24].
The human liposarcoma SW872 cell line has previously been used as a model for mature adipocytes due to its unique physiological characteristics [25]. This cell line was obtained from the American Type Culture Collection (ATCC; Manassas, Virginia, USA) and maintained in DMEM/F12 with 10% FBS and 1% antibiotic–antimycotic in 5% CO2 at 37°C. We also used differentiated 3T3-L1 mouse cells (purchased from ATCC), a broadly used in vitro model for the study of adipocyte biology [26]. The 3T3-L1 cells were grown for two days in pre-adipocyte expansion medium (DMEM, 10% bovine calf serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin). After two days, when the cells had reached 100% confluence (Day 0), the differentiation process was initiated using differentiation medium (DMEM, 10% FBA, 100 IU/mL penicillin, 100 μg/mL streptomycin, 0.5 mM IBMX, 1 μg/mL dexamethasone, and 1 μg/mL insulin). After another 48 h, this medium was replaced with a maintenance medium (DMEM, 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 1 μg/mL insulin), which was changed every 48 h until Day 7.
Coculture and treatment
Human primary or immortalized myometrium and leiomyoma cells were cocultured with human adipocyte (SW872) cells in a Transwell system using 0.4-mm porous membranes from Corning (Corning, New York, USA) with their respective media. We observed a non-significant inter-individual variance in adipocyte responses in primary myometrium and leiomyoma cells from different subjects. We have not pooled cells from different patients together. Similarly, rat leiomyoma cells (ELT3) and differentiated mouse 3T3-L1 cells were cocultured in a Transwell system. The Transwell system was cultured for eight days, with 50% of the medium being changed every 48 h. In other sets of experiments, the cultured (single cell type) and cocultured (with adipocytes) cells were treated with a TNF-α inhibitor (SPD-304), an MCP-1 inhibitor (bindarit), and a nuclear factor kappa B (NF-κB) inhibitor (withaferin A) on day 7 then left for 24 h. After the incubation, the myometrium and leiomyoma media were collected for the multiplex array, and the cells were harvested for protein quantification and analysis.
The use of coculture systems may better elucidate the interaction between myometrium/leiomyoma cells and adipocytes. This system also allows us to examine the aggregated impact on the cellular secretome (Fig. 1).
Figure 1: Adipocyte, uterine myometrium, and leiomyoma cell coculture model.
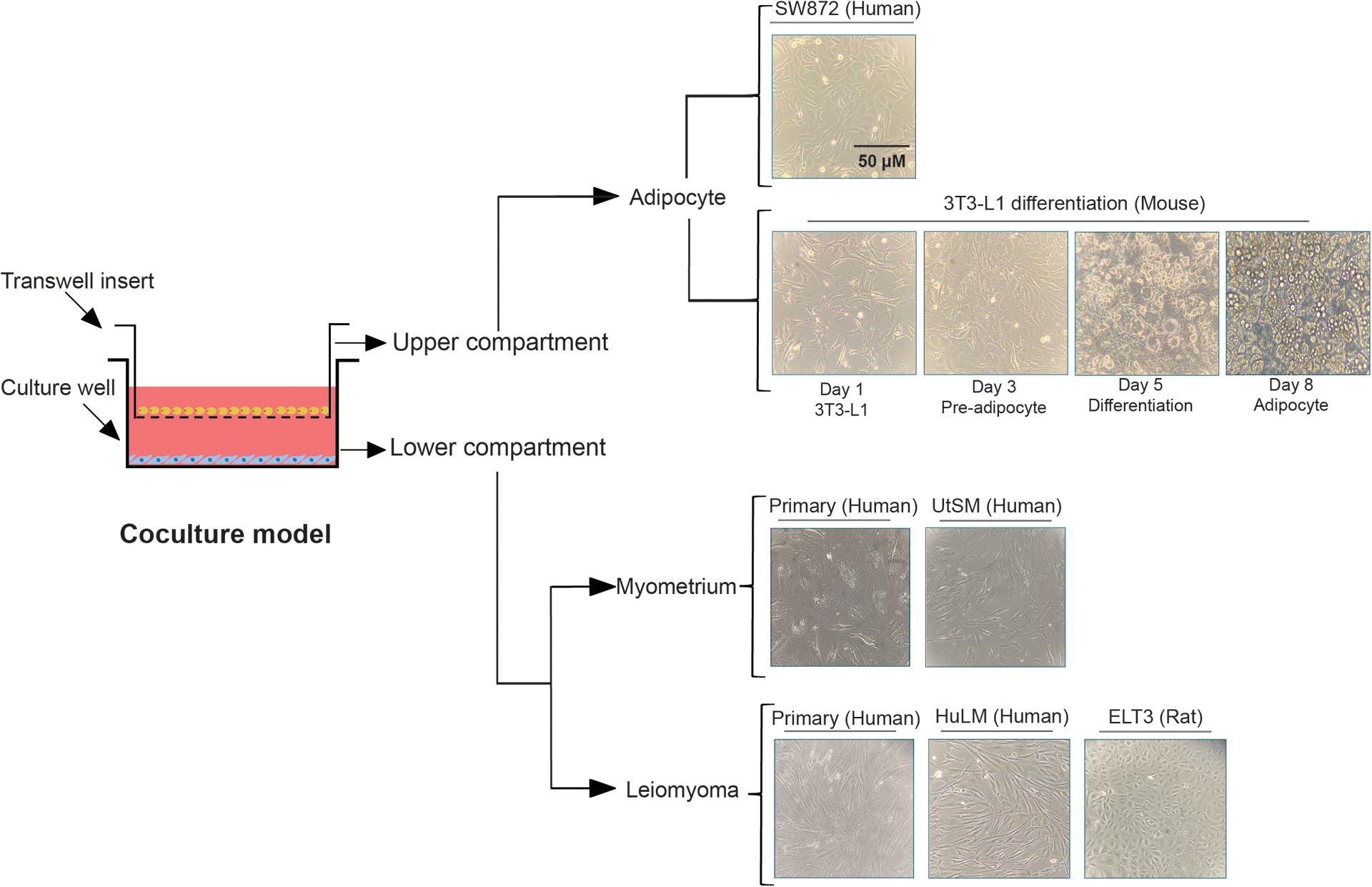
Diagrammatic representation of the cell interactions in the coculture system. All cells were imaged under a light microscope. For the Transwell insert or upper compartment, human liposarcoma SW872 cell lines and the morphological changes of the mouse 3T3-L1 cells differentiating into an adipocyte are displayed. For the culture well or lower compartment, the primary human myometrium or leiomyoma cells and immortalized human uterine smooth muscle (UtSM) or human leiomyoma (HuLM) cells are displayed. Scale bar, 50 μm.
Multiplex cytokine array
We collected, centrifuged, and stored the media collected from the different experiments at −80°C. The media were shipped to Eve Technologies Corporation (Calgary, Alberta, Canada) for analysis. Pro-inflammatory, fibrosis, and angiogenesis markers were measured using a Multiplex Immunoassay (BioPlex 200; Bio-Rad Laboratories, Hercules, California, USA). An ELISA’s sensitivity limit is 2.2 pg/mL, and coefficient of variation were 6.3% and 8.1% intra- and inter-assays, respectively.
Human tissue preparation and immunolabeling
Human leiomyoma tissues were obtained from lean (BMI 20–25) and obese (BMI 30–42) patients who underwent a hysterectomy or myomectomy at the Johns Hopkins University, and were confirmed by histopathologic diagnosis. All subjects gave their informed consent to participate in the study following its approval by the IRB of Johns Hopkins University. Tissue processing and immunolabeling was performed as previously described [27]. An autostainer (Roche Diagnostics) was used to immunolabel formalin-fixed, paraffin-embedded sections for TNF-α, MCP1, transforming growth factor beta-3 (TGFβ3), VEGF-A, phospho nuclear factor kappa B (pNFκB) and fatty acid–binding protein 4 (FABP4). The antigens were retrieved with antigen unmasking solution (Vector laboratories #H3300) with a hot steamer for 10 minutes, followed by a cooldown at room temperature for 30 minutes. A humid chamber was used to block tissue sections for 60 minutes with blocking buffer (1x PBS/5% Normal goat serum/0.3% Triton X-100). After fixing the slides, they were incubated at 4°C in a humid box with primary antibody, anti- TNF-α (R&D, #MAB610–100), MCP1 (R&D, #MAB679–100), TGFβ3 (Invitrogen, #PA5–32630), VEGF-A (Abcam, #ab1316), pNFκB (Cell Signaling Technology, #3033) and FABP4 (Thermo Fisher Scientific, #PA5–30591), diluted in antibody dilution buffer (PBS/1 % BSA/0.3% Triton X-100). A goat anti-rabbit IgG (Alexa Fluor 488, Invitrogen, #A11030) and anti-mouse IgG (Alexa Fluor 546, Invitrogen, #A11030) highly cross-adsorbed secondary antibodies were applied for 2 h at room temperature in a humid box. We mounted sections using ProLong Gold Antifade Reagent with DAPI (Cell Signaling, #8961), applied coverslips, and allowed them to dry overnight. Using fluorescent microscopy (Zeiss AxioPlan 2 microscope system, Jena, Germany), images were analyzed. More than ten random fields were used to identify and validate the stained areas on each slide. Using ImageJ (version 1.52r; National Institutes of Health, USA), we analyzed the data by counting the number of cells to normalize the staining intensity.
BODIPY staining
FFAs levels were determined by BODIPY staining. After coculturing, the primary and immortalized myometrium and leiomyoma cells were stained with 2 μM BODIPY staining solution in phosphate-buffered saline (PBS) for 15 min at 37°C. After two washes with PBS, the cells were fixed in 4% formaldehyde for 30 min at room temperature. Again, three washes were performed using PBS, after which ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific) was added to the samples and incubated overnight at room temperature to fix the slide. Pictures were taken at 20× magnification using a Zeiss AxioPlan 2 microscope system (Carl Zeiss, Jena, Germany).
Immunocytochemistry
After coculture, the primary and immortalized myometrium and leiomyoma cells were fixed in 4% formaldehyde. The cells were then incubated for 1 h at room temperature with a blocking solution (1 × PBS, 5% normal goat serum (Cell Signaling Technology, Danvers, Massachusetts, USA), and 0.3% Triton X-100 (MilliporeSigma, Burlington, Massachusetts, USA), after which they were treated with primary antibodies targeting FABP4 and incubated overnight at 4°C. The cells were then treated with secondary antibodies conjugated with anti-rabbit Alexa 488 (Thermo Fisher Scientific, #A11034) and incubated for 1 h in the dark at room temperature. We used ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific) to fix the slide overnight at room temperature. Pictures were taken at 20× magnification with a Zeiss AxioPlan 2 microscope system.
Western blot analyses
After coculture, the primary and immortalized myometrium and leiomyoma cells and the ELT3 cells were harvested and lysed in a lysis buffer (radioimmunoprecipitation assay buffer; MilliporeSigma) containing a protease and phosphatase inhibitor cocktail (MilliporeSigma). We resolved the same amounts of protein lysates with 4–12% Bis-Tris gradient gels (Thermo Fisher Scientific) and transferred them onto nitrocellulose membranes (Thermo Fisher Scientific). The membranes were blocked by soaking in 5% nonfat milk in Tris-buffered Saline with 0.1% Tween-20 (TBST; Thermo Fisher Scientific) for 1 h at room temperature, and then incubated overnight with antibodies targeting IL-1R (Thermo Fisher Scientific; PA5–21776), TNF-R1 (Cell Signaling Technology; #3736S), toll-like receptor 4 (TLR4; Thermo Fisher Scientific; PA5–26689), PCNA (Cell Signaling Technology; #13110), pNF-κB (Cell Signaling Technology; #3033), NF-κB (Cell Signaling Technology; #8242), or β-actin (MilliporeSigma; A3854) at 4°C on a rocker. After washing with TBST, the membranes were incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature, after which they were visualized using an Azure Imager c300 (Azure Biosystems, Dublin, California, USA). The NIH ImageJ software (version 1.52r) was used to quantitate the protein band signals [28].
Statistical analysis
The statistical analysis was performed using Prism 6.01 for Windows (GraphPad Software, San Diego, California, USA). Student’s t-test was used for comparing two groups. We compared multiple groups using a one-way ANOVA with Tukey’s post-hoc test. Differences at P < 0.05 were considered statistically significant. Three independent experiments were performed in duplicate, and the results were expressed as the mean ± standard error of the mean (SEM).
Results
Uterine myometrium and leiomyoma cells respond to adipocyte coculture by secreting pro-inflammatory cytokines
The tumor microenvironment is modulated by obesity through the release of several inflammatory mediators, resulting in a poor prognosis [29]. There is evidence that chronically active inflammatory immune systems can cause leiomyomas [30]. To identify the obesity-induced pro-inflammatory factors responsible for creating an inflammatory environment in leiomyoma, we performed a cytokine array in adipocyte, primary myometrium and leiomyoma cells alone and cocultured with adipocytes. Among the 32 cytokines tested, six cytokines (IL-1β, TNF-α, GM-CSF, IFNγ, IL-8 and MCP-1) were the most markedly increased in adipocyte media compared to culture and cocultured alone. However, in the presence of adipocytes, it increased more than in primary myometrium and leiomyoma cultures alone (Fig. 2).
Figure 2: Adipocyte coculture induces pro-inflammatory cytokine secretion in uterine myometrium and leiomyoma cells.

Primary human myometrium and leiomyoma cells were cocultured with SW872 cells for eight days. Every two days, 50% of the medium was changed for fresh medium. On day eight, the media were collected and used to measure the secretion levels of (A) 1L-1β, (B) TNF-α, (C) GM-CSF, (D) IL-8, (E) MCP-1 and (F) IFNγ in the culture (single cell type) and coculture (with adipocytes) wells. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05; **, p < 0.01 versus the single–cell type culture.
TNF-α and MCP1 levels in lean and obese leiomyoma tissue
According to many studies, obese and overweight individuals have more inflammatory cytokines in their serum than healthy people. To see if these effects are also evaluated in lean and obese leiomyoma tissue, we have checked the immunofluorescence staining of TNF-α and MCP1. Results showed that obese leiomyoma tissue expresses higher levels of TNF-α and MCP1 than the tissue from lean leiomyoma patients (Fig. 3).
Figure 3: Obesity induces more TNF-α and MCP1 expression in leiomyoma tissue.

Human leiomyoma tissues were obtained from lean (BMI 20–25) and obese (BMI 30–42) patients who underwent a hysterectomy or myomectomy. The tissues were fixed with a 10 % buffered formalin solution for 24 h and kept in 70 % ethanol at 4 °C. The tissues were then stained with TNF-α and MCP1 antibodies. Representative immunofluorescence images of the stained TNF-α (A) and MCP1 (B) antibodies from six different patients. Scale bars, 50 μm. The optical density was measured and quantified in 20 images using ImageJ, using 10 histologically similar fields randomly selected from each slide. * P < 0.05.
Coculture of adipocytes with myometrium and leiomyomas cells induces free acid uptake
Inflamed adipocytes release their stored free fatty acids (FFAs), providing tumor cells with an additional source of energy to support rapid growth [31]. To examine whether this occurs in leiomyoma cells, we examined the FFA droplets in the adipocyte-only culture and the coculture of adipocytes with immortalized myometrium and leiomyoma cells. An intense BODIPY staining confirmed the presence of FFAs in all adipocytes. The coculture of myometrium and leiomyoma cells with adipocytes led to them accumulating more FFAs than when cultured without adipocytes (Fig. 4A). The effect was less prominent in primary myometrium cells.
Figure 4: Adipocyte coculture induces free fatty acid and FABP4 uptake by uterine myometrium and leiomyoma cells.

Immortalized human myometrium (UtSM) or leiomyoma (HuLM) cells were cocultured with SW872 cells for eight days. Every two days, 50% of the medium was changed for fresh medium. (A) BODIPY staining was performed to detect the free fatty acid (green fluorescence) droplets and DAPI (blue fluorescence). (B) Immunocytochemistry staining was performed to confirm the presence of FABP4 (green fluorescence) and DAPI (blue fluorescence). All images were captured with same time exposure using a Zeiss AxioPlan 2 microscope system (20× magnification). Scale bar, 50 μm.
Next, we evaluated the abundance of FABP4. FABP4 binds FFA, and high levels can be detected at the adipocyte–tumor cell interface [31–33]. To explore its context-dependent role, the FABP4 protein was quantified in the adipocytes alone and in immortalized myometrium and leiomyoma cells cocultured with adipocytes. We detected a strong FABP4 signal in the adipocytes, while more FABP4 was detected in the immortalized leiomyoma cells cocultured with adipocytes than in those cultured without adipocytes (Fig. 4B). No differences were observed between the myometrium cells cultured with or without the adipocytes.
To determine whether the altered regulation of the pro-inflammatory cytokine TNF-α detected in the leiomyoma cell media after their coculture with adipocytes had any impact on FFA uptake in leiomyoma cell, we treated immortalized myometrium and leiomyoma cells cultured with or without adipocytes using a TNF-α inhibitor, SPD-304 for 24 h. We choose to use TNF-α inhibitors based on our prior findings that this cytokine was increased (Fig. 2). These results show that the adipocyte coculture fails to increase FFA levels in the presence of the TNF-α inhibitor (Supplementary Fig. 2) in both cell types.
Free fatty acid levels in lean and obese leiomyoma tissue
In obesity, plasma FFA levels are usually elevated due to an enlarged mass, resulting in decreased FFA clearance. Further, high plasma FFA levels inhibit insulin’s anti-lipolytic action, thus increasing FFA release into circulation [34]. Our cellular results show that adipocyte cocultured induced more FFA levels in leiomyoma than in culture alone. To see if these effects are also recapitulated in humans, we used lean and obese leiomyoma tissue. Based on immunofluorescence staining, obese leiomyoma patients expressed a higher amount of FFA and FABP4 than lean leiomyoma patients, consistent with in vitro findings (Fig. 5).
Figure 5: Obesity induces more free fatty acid and FABP4 uptake in leiomyoma tissue.

Human leiomyoma tissues were obtained from lean (BMI 20–25) and obese (BMI 30–42) patients who underwent a hysterectomy or myomectomy. The tissues were fixed with a 10 % buffered formalin solution for 24 h and kept in 70 % ethanol at 4 °C. A) BODIPY staining was performed to detect the free fatty acid (green fluorescence) droplets and DAPI (blue fluorescence) in the tissue from six different patients. (B) Immunocytochemistry staining was performed to confirm the presence of FABP4 (green fluorescence) and DAPI (blue fluorescence) in the tissue from six different patients. Scale bars, 50 μm. The optical density was measured and quantified in 20 images using ImageJ, using 10 histologically similar fields randomly selected from each slide. * P < 0.05.
Coculture of adipocytes with myometrium and leiomyoma cells induces NF-κB phosphorylation
Pro-inflammatory cytokines activate NF-κB in adipocytes by binding to their respective receptors, resulting in the transduction of the inflammatory signal that drives the expression of several genes involved in tumorigenesis [29, 35]. In addition, there is evidence that TLR4 expression is elevated in obese individuals, and that increased TLR4 contributes to inflammatory responses that lead to insulin resistance by activating NF-kB [36]. To elucidate whether adipocytes induce the production of pro-inflammatory cytokines in myometrium and leiomyoma cells and to explore whether FFAs have any impact on NF-κB activation, we examined the abundance of the IL-1R, TNF-R, and TLR4 receptors in both myometrium and leiomyoma cells. All the receptors were notably more abundant in the primary myometrium and leiomyoma cells compared with the immortalized cell lines (Fig. 6A). Notably, pNF-κB/NF-κB was significantly upregulated in the primary myometrium and leiomyoma cells cultured with adipocytes compared with those cultured alone and the immortalized leiomyoma cells (Fig. 6B, 6C).
Figure 6: Adipocyte coculture induces NF-κB activation in myometrium and leiomyoma cells.

(A) Baseline cytokine receptor expression in primary and immortalized human myometrium (UtSM) or leiomyoma (HuLM) cells. IL-1R, TNF-R, and TLR4 protein levels were analyzed by western blotting. β-actin was used as a loading control. The primary and immortalized human myometrium or leiomyoma cells were cocultured with SW872 cells for eight days. Every two days, 50% of the medium was changed for fresh medium. On day eight, cell lysates were prepared from the cells cultured in the presence or absence of adipocytes. Phosphorylated NF-κB/NF-κB protein ratios were analyzed using western blotting in (B) primary and (C) immortalized cells. β-actin was used as a loading control. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05; **, p < 0.01 versus the single–cell type culture; ns, non-significant.
Phospho-NF-κB levels in lean and obese leiomyoma tissue
To check if obesity has any impacts on the activation of NF-κB in lean and obese leiomyoma patients, we examined the pNF-κB expression in tissue. Leiomyoma patients with obese individuals showed a statistically significant difference from those with lean individuals (Fig. 7).
Figure 7: Obesity induces more phospho-NF-κB expression in leiomyoma tissue.
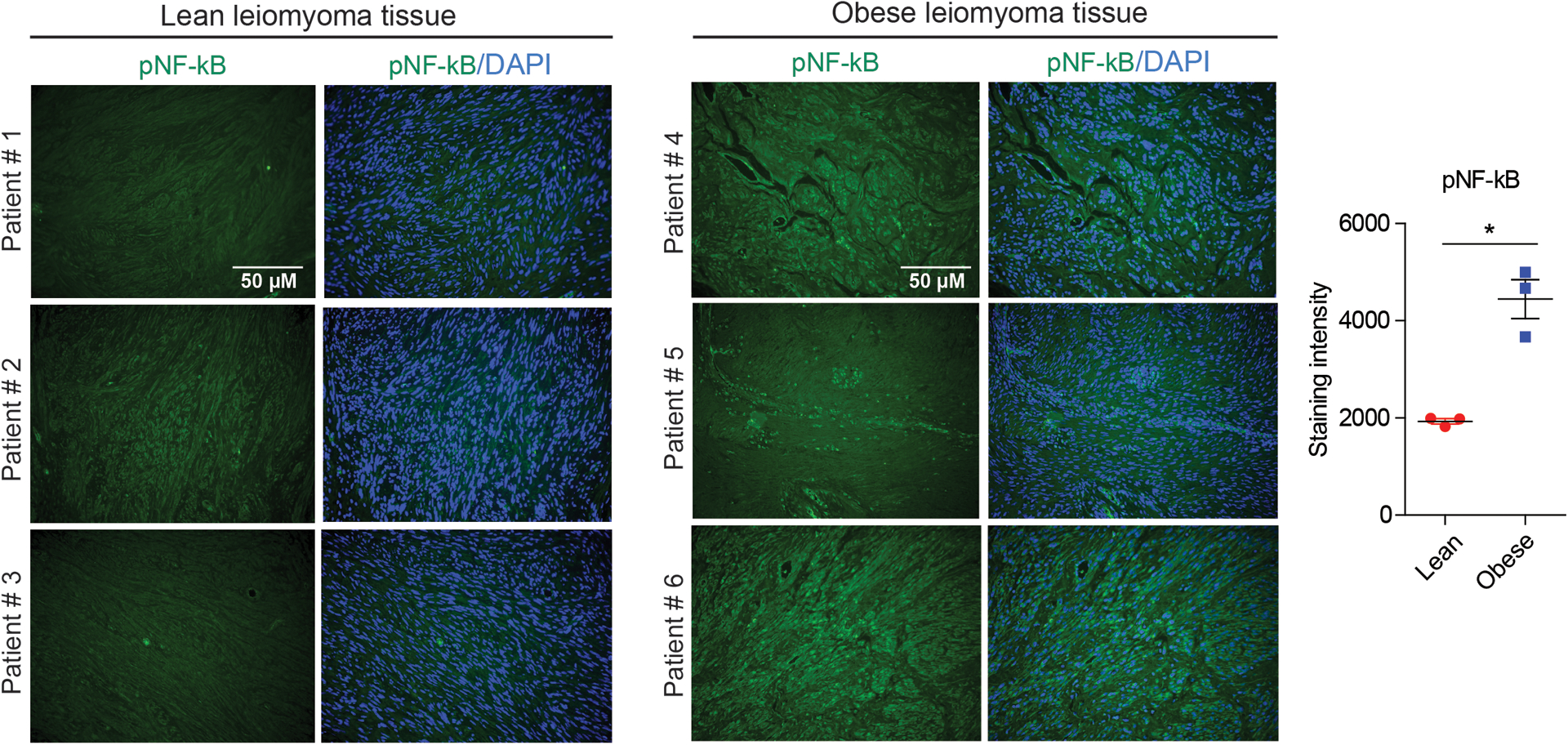
Human leiomyoma tissues were obtained from lean (BMI 20–25) and obese (BMI 30–42) patients who underwent a hysterectomy or myomectomy. The tissues were fixed with a 10 % buffered formalin solution for 24 h and kept in 70 % ethanol at 4 °C. Representative immunofluorescence images of the stained pNF-κB antibody from six different patients. Scale bars, 50 μm. The optical density was measured and quantified in 20 images using ImageJ, using 10 histologically similar fields randomly selected from each slide. * P < 0.05.
Uterine myometrium and leiomyoma cells respond to adipocyte coculture by secreting pro-fibrotic and angiogenic mediators
The circulating levels of pro-fibrotic and angiogenic mediators, which play an important role in the regulation of leiomyoma cell growth, differentiation, and inflammation [37–39], are higher in overweight and obese individuals relative to lean controls [8, 29, 40, 41]. As such, we screened for changes in the ability of myometrium and leiomyoma cells to release pro-fibrotic and angiogenic mediators when cocultured with adipocytes. Adipocyte released more TGF-β1, TGF-β2, and TGF-β3 compared with those cultured alone. Both primary myometrium and leiomyoma cells released a significant amount of TGF-β1, TGF-β2, and TGF-β3 when cocultured with adipocytes (Fig. 8A–8C). We also screened for the release of several angiogenic factors and found that FGF-1, PLGF, VEGF-A, endoglin, FGF-2, and HB-EGF were significantly increased in both the primary human myometrium and leiomyoma cell media when these cells were cocultured with adipocytes compared to those cultured alone (Fig. 8D–8I).
Figure 8: Adipocyte coculture increases pro-fibrotic and angiogenic mediators in uterine myometrium and leiomyoma cells.

Primary human myometrium and leiomyoma cells were cocultured with SW872 adipocyte cells, respectively, for eight days. Every two days, 50% of the medium was changed for fresh medium. On day eight, the media were collected and used to measure the levels of (A) TGF-β1, (B) TGF-β2, (C) TGF-β3, (D) VEGF-A, (E) PLGF, (F) G-CSF, (G) HB-EGF, (H) FGF-1 and (I) FGF-2 in culture (single cell type) and coculture (with adipocytes) wells. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05; **, p < 0.01 versus the single–cell type culture; ns, non-significant.
TGF-β3 and VEGF-A levels in lean and obese leiomyoma tissue
To investigate whether obesity has any impact on the expression of TGF-β3 and VEGF-A levels, we obtained leiomyoma tissue from lean and obese individuals. As shown in Fig. 9, increased levels of TGF-β3 and VEGF-A expressions were detected in obese leiomyoma tissue compared to lean (Fig. 9)
Figure 9: Obesity induces more TGF-β3 and VEGF-A expression in leiomyoma tissue.

Human leiomyoma tissues were obtained from lean (BMI 20–25) and obese (BMI 30–42) patients who underwent a hysterectomy or myomectomy. The tissues were fixed with a 10 % buffered formalin solution for 24 h and kept in 70 % ethanol at 4 °C. The tissues were then stained with TGF-β3 and VEGF-A antibodies. Representative immunofluorescence images of the stained TGF-β3 (A) and VEGF-A (B) antibodies from six different patients. Scale bars, 50 μm. The optical density was measured and quantified in 20 images using ImageJ, using 10 histologically similar fields randomly selected from each slide. * P < 0.05.
Coculture of adipocytes with myometrium and leiomyoma cells induces PCNA production
We next addressed whether the adipocyte-produced factors have any impact on myometrium and leiomyoma cell growth by examining PCNA abundance. We found a strongly upregulated PCNA level in both primary and immortalized leiomyoma cells cocultured with adipocytes compared with those cultured alone (Fig. 10A, 10B). These results were further confirmed by coculturing fully differentiated adipocytes (3T3-L1) with leiomyoma cells of animal origin (Fig. 10C).
Figure 10: Adipocyte coculture increases PCNA levels in uterine myometrium and leiomyoma cells.

Primary and immortalized human myometrium (UtSM) and leiomyoma (HuLM) cells, and mouse leiomyoma ELT3 cells were cocultured with SW872 and 3T3-L1 adipocyte cells, respectively, for eight days. Every two days, 50% of the medium was changed for fresh medium. On day eight, cell lysates were prepared from cells cultured alone or with adipocytes. The PCNA protein levels were analyzed using western blotting for (A) primary, (B) immortalized, and (C) mouse culture (single cell type) and coculture (with adipocytes) cells. β-actin was used as the loading control. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05; **, p < 0.01 versus the single–cell type culture; ns, non-significant.
Effects of inhibiting TNF-α and MCP-1, and NF-κB signaling on PCNA production
To determine whether the altered regulation of the pro-inflammatory cytokines TNF-α and MCP-1 detected in the leiomyoma cell media after their coculture with adipocytes had any impact on leiomyoma cell proliferation, we treated primary and immortalized leiomyoma cells cultured with or without adipocytes using a TNF-α inhibitor, SPD-304, and the MCP-1 inhibitor bindarit for 24 h. We choose to use TNF-α and MCP-1 inhibitors based on our prior findings that these cytokines were increased (Fig. 2). These results show that the adipocyte coculture fails to induce PCNA production in the presence of the TNF-α (Fig. 11A) or MCP-1 inhibitor (Fig. 11B) in both cell types. These results indicate that adipocytes promote TNF-α- and MCP-1-induced leiomyoma cell growth. The inhibitors also inhibited the pro-inflammatory marker in myometrial cells (Supplementary Fig. 3). This is in addition to the observation that coculture increased PCNA expression in both leiomyoma and myometrial cells. Therefore, we believe that obesity may contribute to both leiomyoma tumor initiation (via its effect on myometrial cells) and tumor promotion (via its effect on leiomyoma cells).
Figure 11: Effect of TNF-α and MCP-1, and NF-κB signaling inhibitor treatment on PCNA levels in uterine leiomyoma cells.

Primary and immortalized human leiomyoma (HuLM) cells were cocultured with SW872 adipocyte cells, for seven days. On day seven, primary and immortalized leiomyoma cells in both culture conditions were treated with a TNF-α inhibitor (SPD-304; A), an MCP-1 inhibitor (bindarit; B), and a NF-κB inhibitor (withaferin A; C) for 24 h before a western blot analysis of PCNA abundance. β-actin was used as the loading control. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05; **, p < 0.01 versus the single–cell type culture; #, p < 0.05; ##, p < 0.01 versus coculture.
Based on the observed induction of NF-κB signaling, we next sought to investigate whether the adipocyte coculture–induced proliferative effects could be mitigated by NF-κB signaling. Primary and immortalized leiomyoma cells cultured with or without adipocytes were incubated with a NF-κB inhibitor, withaferin A, for 24 h. We observed that the inhibition of NF-κB signaling reduced most of the proliferative effects in the adipocyte-cocultured leiomyoma cells (Fig. 11C).
Discussion
In this study, we used a coculture system as a model to examine intercellular communication between adipocytes and uterine leiomyoma cells. We discovered that adipocyte-derived mediators lead to alterations in the inflammation, fibrosis, angiogenesis, and proliferation in leiomyoma cells (Fig. 12). Moreover, we found that adipocyte coculture with leiomyoma cells increased NF-κB activation and PCNA expression. Blocking the pro-inflammatory factors TNF-α, MCP-1, and NF-κB revoked the adipocyte coculture-associated proliferative effects in leiomyoma cells.
Figure 12: Adipocyte-derived factors contribute to leiomyoma initiation and progression.

Coculture with adipocytes contributes to leiomyoma cell proliferation via the induction of the expression and secretion of pro-inflammatory, proliferative, fibrotic, and angiogenic markers. IL, interleukin; TNF-α, tumor necrosis factor-alpha; MCP-1, monocyte chemoattractant protein 1; TGF-β1,2,3, transforming growth factor β; PLGF, placental growth factor; VEGF, vascular endothelial growth factor; TLR4, toll-like receptor 4; IL-1R, interleukin 1 receptor; TNF-R, tumor necrosis factor receptor; NF-κB, nuclear factor kappa B; IκB, inhibitor of nuclear factor kappa B.
Adipocytes and immune cells (primarily lymphocytes and macrophages) located in adipose tissue are hypertrophied in obese individuals, leading to higher levels of pro-inflammatory cytokines and chemokines (collectively called adipokines) [8–10, 42]. Among the pro-inflammatory cytokines released by obesity adipose tissue are TNF-α, IL-6, leptin, visfatin, resistin, angiotensin II, and plasminogen activator inhibitor 1, which in turn induce the NF-κB and JNK pathway activation and leading to metabolic disturbances [42]. Uterine leiomyoma development is known to be linked to inflammation [43]. A histopathologic study showed that IL-1β, TNF-α, COX-2, and iNOS levels are higher in uterine leiomyoma cells than in adjacent myometrium [44]. In our study, we found that baseline secretion of the pro-inflammatory cytokines IL-1β, TNF-α, and GM-CSF is higher in primary leiomyoma cells compared to myometrium cells. Furthermore, we identified a significant increase in the IL-1β, IL-13, TNF-α, GM-CSF, and MCP-1 levels in the media of leiomyoma cells cocultured with adipocyte from only leiomyoma cells. We observed more TNF-α and MCP-1 expression in obese leiomyoma tissue compared to lean individuals. These findings suggest that transitioning from normal myometrium to leiomyoma phenotype may be associated with a shift towards a more pro-inflammatory microenvironment.
In obese people, plasma FFAs are elevated [34]. The plasma FFAs activated the inflammatory nuclear factor NF-κB pathway. They resulted in increased pro-inflammatory cytokines, including TNF-α and IL-1β, as well as an increase in circulating MCP-1, supporting the notion that FFAs are the primary link between obesity and inflammation [45]. Using a coculture model, Nieman et al. showed ovarian cancer cells could take up and utilize FFAs from surrounding fat cells [31]. Here, our results indicate that adipocytes provide a proliferative advantage to leiomyoma cells and transfer fatty acids. High levels of FABP4, a protein that binds long-chain fatty acids, are produced by adipocytes [32]. Several studies suggest that FABP4 modulates the import and metabolism of fatty acids and that the upregulated expression of the FABP4 gene in tumor cells increases their lipid accumulation [31, 33, 46]. We found that the FABP4 levels were low in leiomyoma cells cultured in the absence of adipocytes but elevated in the adipocyte and leiomyoma cocultures. A higher amount of FFA and FABP4 levels were detected in obese leiomyoma patients when stained with immunofluorescence, which is consistent with in vitro results.
Several studies have demonstrated that the inhibitor of nuclear factor-kappa B kinase activates NF-κB-regulated gene expression with TLRs and cytokine receptors as downstream targets [29, 35, 36]. NF-κB mediates inflammation by regulating target genes related to tumorigenesis in a pleiotropic manner, restructuring metabolic pathways [29, 35, 36]. Our results showed that adipocyte coculture with leiomyoma cells increased pNF-κB production, indicating that adipocytes enhance the inflammation-induced pro-inflammatory cytokine environment in leiomyoma. Similar results were also obtained in obese than lean leiomyoma tissue from patients.
There is evidence that the pro-inflammatory and fibrotic cytokine TGF-β subfamily plays an important role in numerous aspects of metabolic syndrome [47]. We found that the TGF-β1, TGF-β2, and TGF-β3 levels were increased in the leiomyoma cells cocultured with adipocytes, indicating that adipocytes contribute to maintaining the fibrotic environment. The growth and remodeling of adipose tissue vasculature are regulated by angiogenesis modulators, such as PLGF, VEGFA, endoglin, FGF-2, and EGF, which influence fat mass expansion and metabolism [48]. Several angiogenesis-related factors also influence leiomyoma growth [39]. In the present study, we observed an increase in FGF, PLGF, endoglin, VEGF-A, and HB-EGF in leiomyoma cells cocultured with adipocytes. The TGF-β3 and VEGF-A expressions were higher in obese leiomyoma than in lean tissue.
Using a coculture model, we saw that adipocyte secretory factors significantly enhance leiomyoma cell proliferation, consistent with previous results [18]. In this context, the inhibition of TNF-α, MCP-1, or NF-κB inflammatory factors reduced the proliferation of leiomyoma cells, verifying the role of the secreted factors released by adipocytes in stimulating leiomyoma cell growth. Using JAK2/STAT3 and MAPK/ERK signaling pathways, we have recently demonstrated that leptin stimulates leiomyoma cells proliferation and ECM deposition [17].
Developing obesity and its complications are closely linked to the imbalance between white adipose tissue, the body’s main store of energy, and brown adipose tissue, which produces energy via nonshivering thermogenesis [49]. In response to chronic exposure to obesogenic environments, brown adipocytes may become progressively whiter, indicating a significant role in obesity-related inflammation, thus suggesting a novel explanation for the prevalence of visceral fat in determining the adverse metabolic and cardiovascular consequences of obesity [50].
Statin is an FDA-approved drug widely used for lowering cholesterol levels in cardiovascular patients. From our group, several studies have demonstrated that statin reduces tumor growth in a patient-derived leiomyoma xenograft model [24] and induces calcium-dependent and caspase-dependent apoptosis [51], reduces the expression of extracellular matrix proteins as well as alters the mechanotransduction [20] and inhibits estrogen and Wnt/β-catenin signaling [21, 52] in human leiomyoma cells. Several studies highlight the anti-inflammatory aspects of statin action in adipocytes [53–55]. It is possible that lowering the inflammation in adipocytes can have a protective effect on leiomyoma growth remains to be further investigated.
Furthermore, macrophages are important in integrating innate and adaptive immunity and are responsible for adipose tissue inflammation in obesity [56]. In a chronic inflammatory state, macrophage accumulation in adipose tissue may impair adipocyte function and lead to insulin resistance [57]. In addition, obesity-associated phenotypic changes in macrophage polarization also trigger an increase in pro-inflammatory cytokines, mainly TNF-α, IL1β and MCP-1 [56]. Future studies should investigate the relationship between adipose tissue macrophages and obesity-related leiomyomas. In addition, we may also examine the comparisons of hypertrophic versus non-hypertrophic or insulin-resistant versus insulin-sensitive adipocytes with leiomyoma cells.
In summary, our findings suggest that the inflammatory, fibrotic, and angiogenic factors secreted in obesity and adipose tissue dysfunction, along with lipids and other metabolites, may contribute to a tumor-friendly microenvironment that could facilitate leiomyoma growth. Understanding the mechanisms by which dysfunctional adipose tissue contributes to leiomyoma pathology may elucidate the epidemiological association between obesity and leiomyoma and uncover new molecular therapeutic targets.
Supplementary Material
Supplementary Figure 1: Characterization of the human primary myometrium and leiomyoma cells. Primary myometrium (PM) and leiomyoma (PL) cells were isolated from different human subjects. (A) PCNA, COL1A, FN, ERα and PR-A/B protein expression levels were detected by Western blotting, and β- actin was used as a loading control. (B) The data were quantified by using fold change.
Supplementary Figure 3: Effect of TNF-α and MCP-1, and NF-κB signaling inhibitor treatment on PCNA levels in uterine myometrium cells. Primary human myometrium cells were cocultured with SW872 adipocyte cells, for seven days. On day seven, cells in both culture conditions were treated with a TNF-α inhibitor (SPD-304; A), an MCP-1 inhibitor (bindarit; B), and a NF-κB inhibitor (withaferin A; C) for 24 h before a western blot analysis of PCNA abundance. β-actin was used as the loading control. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05 versus the single–cell type culture; #, p < 0.05 versus coculture.
Supplementary Figure 2: Effect of TNF-α inhibitor treatment on free fatty acid uptake by uterine myometrium and leiomyoma cells. Immortalized human myometrium (UtSM) or leiomyoma (HuLM) cells were cocultured with SW872 cells for seven days. On day seven, cells in both culture conditions were treated with a TNF-α inhibitor (SPD-304; A) for 24 h. (A) BODIPY staining was performed to detect the free fatty acid (green fluorescence) droplets and DAPI (blue fluorescence). All images were captured with same time exposure using a Zeiss AxioPlan 2 microscope system (20× magnification). Scale bar, 50 μm. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05 versus the single–cell type culture; #, p < 0.05 versus coculture.
Acknowledgments:
This work was supported, in part, by National Institutes of Health grant 1R01HD094380.
We are grateful to all women who provided the leiomyoma tissues used in this study.
Funding:
This work was supported, in part, by NIH grant 1R01HD094380.
Footnotes
Disclosure summary: The authors have nothing to disclose.
Competing interests: The authors declare no conflict of interest.
Data and materials availability:
All data associated with this study are presented in the paper and supplementary parts.
References
- [1].Okolo S, Incidence, aetiology and epidemiology of uterine fibroids, Best practice & research Clinical obstetrics & gynaecology, 22 (2008) 571–588. [DOI] [PubMed] [Google Scholar]
- [2].Fritton K, Borahay MA, New and emerging therapies for uterine fibroids, in: Seminars in Reproductive Medicine, vol. 35, Thieme Medical Publishers, 2017, pp. 549–559. [DOI] [PubMed] [Google Scholar]
- [3].Walker CL, Stewart EA, Uterine fibroids: the elephant in the room, Science, 308 (2005) 1589–1592. [DOI] [PubMed] [Google Scholar]
- [4].Borahay MA, Al-Hendy A, Kilic GS, Boehning D, Signaling pathways in leiomyoma: understanding pathobiology and implications for therapy, Molecular medicine, 21 (2015) 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Borahay MA, Asoglu MR, Mas A, Adam S, Kilic GS, Al-Hendy A, Estrogen receptors and signaling in fibroids: role in pathobiology and therapeutic implications, Reproductive sciences, 24 (2017) 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M, Health and economic burden of the projected obesity trends in the USA and the UK, The Lancet, 378 (2011) 815–825. [DOI] [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention. Obesity among women of child-bearing age: United States, 2008–2018. Behavioral risk factor surveillance system: march of dimes. Available at: www.marchofdimes.org/peristats. Accessed September 15, 2020 [Google Scholar]
- [8].Van Kruijsdijk RC, Van Der Wall E, Visseren FL, Obesity and cancer: the role of dysfunctional adipose tissue, Cancer Epidemiology and Prevention Biomarkers, 18 (2009) 2569–2578. [DOI] [PubMed] [Google Scholar]
- [9].Park J, Morley TS, Kim M, Clegg DJ, Scherer PE, Obesity and cancer—mechanisms underlying tumour progression and recurrence, Nature Reviews Endocrinology, 10 (2014) 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Quail DF, Dannenberg AJ, The obese adipose tissue microenvironment in cancer development and progression, Nature Reviews Endocrinology, 15 (2019) 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alashqar A, El Ouweini H, Gornet M, Yenokyan G, Borahay MA, Cardiometabolic profile of women with uterine leiomyoma: a cross-sectional study, Minerva obstetrics and gynecology, (2022), 10.23736/S2724-606X.22.04952-1 [DOI] [PubMed] [Google Scholar]
- [12].Sato F, Nishi M, Kudo R, Miyake H, Body fat distribution and uterine leiomyomas, Journal of epidemiology, 8 (1998) 176–180. [DOI] [PubMed] [Google Scholar]
- [13].Afrin S, AlAshqar A, El Sabeh M, Miyashita-Ishiwata M, Reschke L, Brennan JT, Fader A, Borahay MA, Diet and nutrition in gynecological disorders: A focus on clinical studies, Nutrients, 13 (2021) 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].AlAshqar A, Patzkowsky K, Afrin S, Wild R, Taylor HS, Borahay MA, Cardiometabolic risk factors and benign gynecologic disorders, Obstetrical & gynecological survey, 74 (2019) 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT, Risk factors for uterine fibroids: reduced risk associated with oral contraceptives, Br Med J (Clin Res Ed), 293 (1986) 359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Qin H, Lin Z, Vásquez E, Luan X, Guo F, Xu L, Association between obesity and the risk of uterine fibroids: a systematic review and meta-analysis, J Epidemiol Community Health, 75 (2021) 197–204. [DOI] [PubMed] [Google Scholar]
- [17].Reschke L, Afrin S, El Sabah M, Charewycz N, Miyashita-Ishiwata M, Borahay MA, Leptin induces leiomyoma cell proliferation and extracellular matrix deposition via JAK2/STAT3 and MAPK/ERK pathways, F&S Science, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nair S, Al-Hendy A, Adipocytes enhance the proliferation of human leiomyoma cells via TNF-α proinflammatory cytokine, Reproductive sciences, 18 (2011) 1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW, Obesity and leptin resistance: distinguishing cause from effect, Trends in Endocrinology & Metabolism, 21 (2010) 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Afrin S, Islam MS, Patzkowsky K, Malik M, Catherino WH, Segars JH, Borahay MA, Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells, Am. J. Obstet. Gynecol, 223 (2020) 733.e731–733.e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Afrin S, El Sabeh M, Islam MS, Miyashita-Ishiwata M, Malik M, Catherino WH, Akimzhanov AM, Boehning D, Yang Q, Al-Hendy A, Simvastatin modulates estrogen signaling in uterine leiomyoma via regulating receptor palmitoylation, trafficking and degradation, Pharmacol Res, 172 (2021) 105856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Carney SA, Tahara H, Swartz CD, Risinger JI, He H, Moore AB, Haseman JK, Barrett JC, Dixon D, Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics, Laboratory investigation, 82 (2002) 719–728. [DOI] [PubMed] [Google Scholar]
- [23].Howe SR, Gottardis MM, Everitt JI, Goldsworthy TL, Wolf DC, Walker C, Rodent model of reproductive tract leiomyomata. Establishment and characterization of tumor-derived cell lines, The American journal of pathology, 146 (1995) 1568. [PMC free article] [PubMed] [Google Scholar]
- [24].Borahay MA, Vincent K, Motamedi M, Sbrana E, Kilic GS, Al-Hendy A, Boehning D, Novel effects of simvastatin on uterine fibroid tumors: in vitro and patient-derived xenograft mouse model study, Am. J. Obstet. Gynecol, 213 (2015) 196. e191–196. e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Richardson M, Berg D, Johnston P, McClure D, Grinnell B, Human liposarcoma cell line, SW872, secretes cholesteryl ester transfer protein in response to cholesterol, Journal of lipid research, 37 (1996) 1162–1166. [PubMed] [Google Scholar]
- [26].Morrison S, McGee SL, 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages, Adipocyte, 4 (2015) 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Afrin S, El Sabeh M, Miyashita-Ishiwata M, Charewycz N, Singh B, Borahay MA, Simvastatin reduces plasma membrane caveolae and caveolin-1 in uterine leiomyomas, Life Sciences, 304 (2022) 120708. [DOI] [PubMed] [Google Scholar]
- [28].Schneider CA, Rasband WS, Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis, Nature methods, 9 (2012) 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Crespi E, Bottai G, Santarpia L, Role of inflammation in obesity-related breast cancer, Current Opinion in Pharmacology, 31 (2016) 114–122. [DOI] [PubMed] [Google Scholar]
- [30].Orciani M, Caffarini M, Biagini A, Lucarini G, Delli Carpini G, Berretta A, Di Primio R, Ciavattini A, Chronic inflammation may enhance leiomyoma development by the involvement of progenitor cells, Stem cells international, 2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth, Nature medicine, 17 (2011) 1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Furuhashi M, Hotamisligil GS, Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets, Nature reviews Drug discovery, 7 (2008) 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gyamfi J, Yeo JH, Kwon D, Min BS, Cha YJ, Koo JS, Jeong J, Lee J, Choi J, Interaction between CD36 and FABP4 modulates adipocyte-induced fatty acid import and metabolism in breast cancer, NPJ breast cancer, 7 (2021) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Boden G, Obesity and free fatty acids, Endocrinology and metabolism clinics of North America, 37 (2008) 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shoelson S, Lee J, Yuan M, Inflammation and the IKKβ/IκB/NF-κB axis in obesity-and diet-induced insulin resistance, International journal of obesity, 27 (2003) S49–S52. [DOI] [PubMed] [Google Scholar]
- [36].Reyna SM, Ghosh S, Tantiwong P, Meka CR, Eagan P, Jenkinson CP, Cersosimo E, DeFronzo RA, Coletta DK, Sriwijitkamol A, Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects, Diabetes, 57 (2008) 2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].El Andaloussi A, Chaudhry Z, Al-Hendy A, Ismail N, Uterine fibroids: bridging genomic defects and chronic inflammation, in: Seminars in Reproductive Medicine, vol. 35, Thieme Medical Publishers, 2017, pp. 494–498. [DOI] [PubMed] [Google Scholar]
- [38].Arici A, Sozen I, Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation, Fertil. Steril, 73 (2000) 1006–1011. [DOI] [PubMed] [Google Scholar]
- [39].Fleischer R, Weston GC, Vollenhoven BJ, Rogers PA, Pathophysiology of fibroid disease: angiogenesis and regulation of smooth muscle proliferation, Best Practice & Research Clinical Obstetrics & Gynaecology, 22 (2008) 603–614. [DOI] [PubMed] [Google Scholar]
- [40].Doerstling SS, O’Flanagan CH, Hursting SD, Obesity and cancer metabolism: a perspective on interacting tumor–intrinsic and extrinsic factors, Frontiers in oncology, 7 (2017) 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC, Fazakerley DJ, Grewal T, Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration, Cancer & metabolism, 5 (2017) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Monteiro R, Azevedo I, Chronic inflammation in obesity and the metabolic syndrome, Mediators of inflammation, 2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chegini N, Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder, Semin. Reprod. Med, 28 (2010) 180–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Plewka A, Madej P, Plewka D, Kowalczyk A, Miskiewicz A, Wittek P, Leks T, Bilski R, Immunohistochemical localization of selected pro-inflammatory factors in uterine myomas and myometrium in women of various ages, Folia Histochemica et Cytobiologica, 51 (2013) 73–83. [DOI] [PubMed] [Google Scholar]
- [45].Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid–induced inflammatory changes in the interaction between adipocytes and macrophages, Arteriosclerosis, thrombosis, and vascular biology, 27 (2007) 84–91. [DOI] [PubMed] [Google Scholar]
- [46].Guaita-Esteruelas S, Bosquet A, Saavedra P, Guma J, Girona J, Lam EWF, Amillano K, Borras J, Masana L, Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins, Molecular carcinogenesis, 56 (2017) 208–217. [DOI] [PubMed] [Google Scholar]
- [47].Lee M-J, Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity, Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1864 (2018) 1160–1171. [DOI] [PubMed] [Google Scholar]
- [48].Lijnen HR, Angiogenesis and obesity, Cardiovascular research, 78 (2008) 286–293. [DOI] [PubMed] [Google Scholar]
- [49].Giroud M, Jodeleit H, Prentice KJ, Bartelt A, Adipocyte function and the development of cardiometabolic disease, The Journal of Physiology, 600 (2022) 1189–1208. [DOI] [PubMed] [Google Scholar]
- [50].Rana MN, Neeland IJ, Adipose Tissue Inflammation and Cardiovascular Disease: An Update, Current Diabetes Reports, (2022) 1–11. [DOI] [PubMed] [Google Scholar]
- [51].Borahay MA, Kilic GS, Yallampalli C, Snyder RR, Hankins GDV, Al-Hendy A, Boehning D, Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells, J. Biol. Chem, 289 (2014) 35075–35086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].El Sabeh M, Saha SK, Afrin S, Borahay MA, Simvastatin inhibits Wnt/β-catenin pathway in uterine leiomyoma, Endocrinology, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Van Harmelen V, Skurk T, Röhrig K, Hauner H, HMG-CoA reductase inhibitor cerivastatin inhibits interleukin-6 expression and secretion in human adipocytes, Hormone and metabolic research, 35 (2003) 466–470. [DOI] [PubMed] [Google Scholar]
- [54].Parisi V, Petraglia L, D’Esposito V, Cabaro S, Rengo G, Caruso A, Grimaldi MG, Baldascino F, De Bellis A, Vitale D, Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue, International journal of cardiology, 274 (2019) 326–330. [DOI] [PubMed] [Google Scholar]
- [55].Abe M, Matsuda M, Kobayashi H, Miyata Y, Nakayama Y, Komuro R, Fukuhara A, Shimomura I, Effects of statins on adipose tissue inflammation: their inhibitory effect on MyD88-independent IRF3/IFN-β pathway in macrophages, Arteriosclerosis, thrombosis, and vascular biology, 28 (2008) 871–877. [DOI] [PubMed] [Google Scholar]
- [56].Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Obesity is associated with macrophage accumulation in adipose tissue, The Journal of clinical investigation, 112 (2003) 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Olefsky JM, Glass CK, Macrophages, inflammation, and insulin resistance, Annual review of physiology, 72 (2010) 219–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Characterization of the human primary myometrium and leiomyoma cells. Primary myometrium (PM) and leiomyoma (PL) cells were isolated from different human subjects. (A) PCNA, COL1A, FN, ERα and PR-A/B protein expression levels were detected by Western blotting, and β- actin was used as a loading control. (B) The data were quantified by using fold change.
Supplementary Figure 3: Effect of TNF-α and MCP-1, and NF-κB signaling inhibitor treatment on PCNA levels in uterine myometrium cells. Primary human myometrium cells were cocultured with SW872 adipocyte cells, for seven days. On day seven, cells in both culture conditions were treated with a TNF-α inhibitor (SPD-304; A), an MCP-1 inhibitor (bindarit; B), and a NF-κB inhibitor (withaferin A; C) for 24 h before a western blot analysis of PCNA abundance. β-actin was used as the loading control. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05 versus the single–cell type culture; #, p < 0.05 versus coculture.
Supplementary Figure 2: Effect of TNF-α inhibitor treatment on free fatty acid uptake by uterine myometrium and leiomyoma cells. Immortalized human myometrium (UtSM) or leiomyoma (HuLM) cells were cocultured with SW872 cells for seven days. On day seven, cells in both culture conditions were treated with a TNF-α inhibitor (SPD-304; A) for 24 h. (A) BODIPY staining was performed to detect the free fatty acid (green fluorescence) droplets and DAPI (blue fluorescence). All images were captured with same time exposure using a Zeiss AxioPlan 2 microscope system (20× magnification). Scale bar, 50 μm. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05 versus the single–cell type culture; #, p < 0.05 versus coculture.
Data Availability Statement
All data associated with this study are presented in the paper and supplementary parts.


