Abstract
Background
With burn injuries involving a large total body surface area (TBSA), the body can enter a state of breakdown, resulting in a condition similar to that seen with severe lack of proper nutrition. In addition, destruction of the effective skin barrier leads to loss of normal body temperature regulation and increased risk of infection and fluid loss. Nutritional support is common in the management of severe burn injury, and the approach of altering immune system activity with specific nutrients is termed immunonutrition. Three potential targets have been identified for immunonutrition: mucosal barrier function, cellular defence and local or systemic inflammation. The nutrients most often used for immunonutrition are glutamine, arginine, branched‐chain amino acids (BCAAs), omega‐3 (n‐3) fatty acids and nucleotides.
Objectives
To assess the effects of a diet with added immunonutrients (glutamine, arginine, BCAAs, n‐3 fatty acids (fish oil), combined immunonutrients or precursors to known immunonutrients) versus an isonitrogenous diet (a diet wherein the overall protein content is held constant, but individual constituents may be changed) on clinical outcomes in patients with severe burn injury.
Search methods
The search was run on 12 August 2012. We searched the Cochrane Injuries Group's Specialised Register, The Cochrane Library, MEDLINE (OvidSP), Embase (OvidSP), ISI WOS SCI‐EXPANDED & CPCI‐S and four other databases. We handsearched relevant journals and conference proceedings, screened reference lists and contacted pharmaceutical companies. We updated this search in October 2014, but the results of this updated search have not yet been incorporated.
Selection criteria
Randomised controlled trials comparing the addition of immunonutrients to a standard nutritional regimen versus an isonitrogenated diet or another immunonutrient agent.
Data collection and analysis
Two review authors were responsible for handsearching, reviewing electronic search results and identifying potentially eligible studies. Three review authors retrieved and reviewed independently full reports of these studies for inclusion. They resolved differences by discussion. Two review authors independently extracted and entered data from the included studies. A third review author checked these data. Two review authors independently assessed the risk of bias of each included study and resolved disagreements through discussion or consultation with the third and fourth review authors. Outcome measures of interest were mortality, hospital length of stay, rate of burn wound infection and rate of non‐wound infection (bacteraemia, pneumonia and urinary tract infection).
Main results
We identified 16 trials involving 678 people that met the inclusion criteria. A total of 16 trials contributed data to the analysis. Of note, most studies failed to report on randomisation methods and intention‐to‐treat principles; therefore study results should be interpreted with caution. Glutamine was the most common immunonutrient and was given in seven of the 16 included studies. Use of glutamine compared with an isonitrogenous control led to a reduction in length of hospital stay (mean stay ‐5.65 days, 95% confidence interval (CI) ‐8.09 to ‐3.22) and reduced mortality (pooled risk ratio (RR) 0.25, 95% CI 0.08 to 0.78). However, because of the small sample size, it is likely that these results reflect a false‐positive effect. No study findings suggest that glutamine has an effect on burn wound infection or on non‐wound infection. All other agents investigated showed no evidence of an effect on mortality, length of stay or burn wound infection or non‐wound infection rates.
Authors' conclusions
Although we found evidence of an effect of glutamine on mortality reduction, this finding should be taken with care. The number of study participants analysed in this systematic review was not sufficient to permit conclusions that recommend or refute the use of glutamine. Glutamine may be effective in reducing mortality, but larger studies are needed to determine the overall effects of glutamine and other immunonutrition agents.
Keywords: Humans; Amino Acids, Branched‐Chain; Amino Acids, Branched‐Chain/therapeutic use; Burns; Burns/immunology; Burns/mortality; Burns/therapy; Fatty Acids, Omega‐3; Fatty Acids, Omega‐3/therapeutic use; Glutamine; Glutamine/therapeutic use; Length of Stay; Malnutrition; Malnutrition/immunology; Malnutrition/therapy; Nutrition Therapy; Nutrition Therapy/methods; Ornithine; Ornithine/analogs & derivatives; Ornithine/therapeutic use; Randomized Controlled Trials as Topic; Soybean Proteins; Soybean Proteins/therapeutic use; Vitamins; Vitamins/therapeutic use; Wound Infection; Wound Infection/etiology
Plain language summary
Immunonutrition as an adjuvant therapy for burns
With burn injuries involving a large total body surface area, damage and breakdown of tissues can result in a condition similar to that seen with severe malnourishment. In addition, destruction of the effective skin barrier leads to body temperature dysregulation and increased susceptibility to infection and fluid loss. Previous studies have investigated specific naturally occurring additives to nutritional support, which may lead to an increase in immune system function and therefore a reduction in infection, hospital length of stay and chance of death. These additives are termed immunonutrients and include glutamine, arginine, branched‐chain amino acids (BCAAs) and omega‐3 fatty acids (fish oil). The authors of this review searched for randomised controlled trials assessing the effects of immunonutrients in patients with severe burn injury.
Results of this review show that only glutamine could potentially reduce risk of death. However, the total number of patients within the combined studies is too small; therefore conclusions may be imprecise. More studies are needed to determine the efficacy of immunonutrition.
Background
Description of the condition
Burns are injuries to the skin and underlying structures caused by exposure to various injurious agents, including sources of heat, extreme cold, electricity and chemicals. Globally the World Health Organization suggests that around 265,000 deaths annually are attributable to fire‐related injuries (WHO 2014a), and this is thought to be a conservative estimate. The burden of mortality from fire‐related injuries falls disproportionately in low‐income countries, which report 10.2 deaths per 100,000, compared with 0.8 per 100,000 in higher‐income countries. Regional differences mean that peak mortality is even higher at 12.1 per 100,000 in Africa (WHO 2014b). In addition to mortality, significant physical and psychological morbidity is associated with burn injuries, and optimal patient outcomes require a multi‐disciplinary team approach involving multiple medical and surgical specialities and allied healthcare professionals. The severity of burn injury depends on both the depth of skin damage and the percentage of total body surface area (TBSA) affected. In cases in which more than 20% TBSA is involved, a severe catabolic state can occur, resulting in whole‐body protein breakdown, negative nitrogen balance and reduced body mass akin to acute severe malnourishment (Prelack 2007; Yurt 2001). This impaired metabolic state may become life threatening; therefore nutritional support commonly plays an important role in the management of severe burns. This support can be provided in the form of fortified oral diets, supplementary drinks, enteral tube feedings or parenteral (intravenous) support. The decision to begin nutritional support may depend on general energy requirements or barriers to normal feeding such as invasive airway support or dysphagia (swallowing difficulties) and risk of aspiration.
In addition to inducing a hypermetabolic state, loss of an effective skin barrier impairs the patient’s ability to control body temperature and maintain fluid balance, leaving open a portal for infection. Other factors such as immobility due to pain or postoperative requirements also contribute to high risk of infective complications. Therefore, enhancement of immune function to lower infection risk through specific nutritional support is worthy of investigation.
Description of the intervention
The idea of modulating immune system activity by providing specific nutrients is termed immunonutrition (Calder 2003). This intervention has been used for medicinal purposes since ancient times and was applied in Ayurvedic medicine for over 3000 years (Inaba 2005; Seth 2004). Three potential targets for immunonutrition are known: (1) mucosal barrier function; (2) cellular defence; and (3) local or systemic inflammation. The nutrients most often used for immunonutrition are arginine, glutamine, branched‐chain amino acids (BCAAs), omega‐3 (n‐3) fatty acids and nucleotides (Calder 2003).
These nutrients act in several ways. Glutamine, a non‐essential amino acid synthesised from glutamic acid and ammonia, is abundant throughout the body and is involved in many metabolic processes. It is the principal carrier of nitrogen in the body and is an important energy source for cells. Glutamine appears to be a "conditionally essential" amino acid at times of serious illness or injury (Wischmeyer 2006). Immunonutrient precursors such as ornithine α‐ketoglutarate (OKG) have shown significant functional overlap with glutamine. One of the key working mechanisms of OKG consists of building up tissue glutamine concentrations, thereby improving nitrogen carriage. For several decades, OKG has been considered for use in burn injury; it appears to have anticatabolic actions that are mediated through insulin and human growth hormone modulation (Cynober 1984; Schneid 2003). Branched‐chain amino acids are endogenous precursors of other amino acids such as glutamine and have been shown in cell cultures and animal studies to be necessary for the support of efficient immune function. Reduced availability of BCAAs impairs some aspects of immune function, including killer cell activity and lymphocyte proliferation. However, the role of BCAAs in producing different immunoglobulins and cytokines and in permitting antigen processing and presentation to occur is unknown (Calder 2006). The n‐3 fatty acids have shown potential benefit in their ability to reduce levels of inflammatory prostanoids and endogenous immunosuppressive mediators (Mayer 2008). Dietary nucleotides are important substrates for immune cells and function, particularly in times of stress, as well as for gastric mucosal function (Schloerb 2001). Combinations of the above strategies have been utilised.
How the intervention might work
In patients with burn injuries, sepsis and subsequent multiple organ failure (MOF) are the leading causes of death (Li 1995; Merrell 1989; Sharma 2006; Tang 1999). Loss of the skin barrier and suppression of normal host defences due to intense hypermetabolism in patients with burn injuries suggest that they are likely to benefit from immunonutrition. Several studies in animal models and in clinical settings have shown that immunonutrition can improve immune function (Choudhry 2003; Preiser 2003; Windle 2006; Wischmeyer 2006), but its effects on mortality and on other clinically relevant outcomes remain unknown. Five previous meta‐analyses have assessed the use of immunonutrition in critically ill patients (Beale 1999; Heyland 2001; Jiang 2002; Montejo 2003; Peter 2005), all of which failed to demonstrate a reduction in mortality rates. Moreover, trials with high methodological quality have shown a trend towards increased mortality rates in patients, along with heterogeneous results for length of hospital stay, intensive care unit stay and days on mechanical ventilation. A more recent review (Kurmis 2010) recommended only the use of glutamine in patients with severe burns, as data were insufficient to support the routine use of other immunonutrient therapies such as fish oil, arginine and combination immunonutrition after burn injury.
Why it is important to do this review
Patients with severe burn injuries present a major clinical problem and represent a significant economic and social burden for the community. Treatment of individuals with this condition to reduce mortality while minimising the impact of long‐term physical, cognitive and behavioural effects is an ongoing challenge, and many therapeutic regimens have been proposed with limited success. Immunonutritonal therapies have shown promising results; however, it is unclear whether immunonutrition should be used routinely in the treatment of patients with burns, and questions of dose, route and duration remain unanswered. A systematic review of clinical outcomes may assist practitioners in assessing the use of such strategies in severely burned patients, and the results of such work may serve as a guide for future researchers who seek to better define optimal therapy.
Objectives
To assess the effects of a diet with added immunonutrients (glutamine, arginine, BCAAs, n‐3 fatty acids (fish oil), combined immunonutrients or precursors to known immunonutrients) versus an isonitrogenous diet (a diet wherein the overall protein content is held constant, but individual constituents may be changed) on clinical outcomes in patients with severe burn injury.
Methods
Criteria for considering studies for this review
Types of studies
We considered any randomised clinical trials that compared the addition of immunonutrients to a standard nutritional regimen versus an isonitrogenated diet or another immunonutrient agent.
Types of participants
We focused on all patients of any age with burn injuries of any severity who were admitted to a specialised burn unit, a general intensive care unit or a surgery unit for treatment. We did not use a cutoff for burn TBSA, although we acknowledge that these interventions are more likely to be used for severely burned patients.
Types of interventions
We included all studies with at least one of the following immunonutrients: glutamine, BCAAs, n‐3 fatty acids (fish oil), combined immunonutrients or immunonutrient precursors. We also considered studies that employed standard immunonutrient therapy as the comparator, but administered by a different route or dosage, and those that compared immunonutrients versus no treatment or placebo. Immunonutrient interventions were provided by an enteral (tube feeding into the stomach or small bowel, including percutaneous endoscopic gastrostomy) or parenteral (intravenous) route.
Types of outcome measures
Studies were eligible for inclusion if investigators reported any of the following outcome measures.
Primary outcomes
All‐cause mortality.
Secondary outcomes
Length of hospital stay (LOS) defined as time between admission and wound healing.
Burn wound infection.
Non‐wound infection, including pneumonia, urinary tract infection and bacteraemia.
Following publication of the review protocol, several of these secondary outcomes were not reported on in this review, as they were addressed by a small number, or none, of the included studies.
Numbers of transfusions and quality of life were not reported by any of the screened articles.
Weight loss was removed as a secondary outcome measure, as it was considered not clinically relevant by the review authors; however it was reported by three included studies (Coudray‐Lucas 2000; De Bandt 1998; Zhou 2003). We did not report cost, as this is not a clinical measure, although it was reported by two included studies (Saffle 1997; Zhou 2003). Aesthetic outcomes related to scar tissue were reported by only one study (Donati 1999).
Overall rates of sepsis and nosocomial infection were removed as secondary outcomes, as they were not clearly defined by any of the included studies.
Surrogate endpoints and biochemical markers of nutrition (e.g. serum levels of glutamine, albumin and prealbumin; nitrogen balance; net protein loss) were not included in this review because they were not considered to be clinical outcomes.
Search methods for identification of studies
To reduce publication and retrieval bias, we did not restrict our search by language, date or publication status.
Electronic searches
The Cochrane Injuries Group's Trials Search Co‐ordinator searched the following:
Cochrane Injuries Group Specialised Register (14 August 2012);
Cochrane Central Register of Controlled Trials (CENTRAL) (2012, issue 7 of 12);
MEDLINE (OvidSP) (1946 to 2012 August week 1);
PubMed (14 August 2012);
Embase (OvidSP) (1980 to 2012 week 32);
LILACS (Literatura Latino‐Americana e do Caribe em Ciências da Saúde) (1982 to August 2008);
CINAHL Plus (EBSCO) (1982 to August 2012);
ISI WOS: Science Citation Index‐Expanded (SCI‐EXPANDED) (1970 to August 2012);
ISI WOS: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to August 2012);
Zetoc (1993 to August 2012).
The search strategies applied are listed in Appendix 1.
We combined the Ovid MEDLINE strategy with the Cochrane Highly Sensitive Search Strategy (CHSSS) to identify randomised trials in MEDLINE (Lefebvre 2011). We combined the EMBASE (Ovid SP) and CINAHL (EBSCO) strategies with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2010).
We performed a further search in October 2014 and added 21 studies to Studies awaiting classification; we will incorporate these studies into the review at the next update.
Searching other resources
We handsearched the following journals.
Burns (Journal of the International Society for Burn Injuries).
Journal of Burn Care and Research (Journal of the American Burn Association).
Annals of Burns and Fire Disasters (Journal of the Mediterranean Council for Burns and Fire Disasters).
Plastic and Reconstructive Surgery (Journal of the American Society of Plastic Surgeons).
Journal of Trauma.
Journal of Critical Care Medicine.
American Journal of Clinical Nutrition.
Journal of Nutrition.
British Journal of Nutrition.
We searched the websites of the International Society for Burn Injuries and the American Burn Association for abstracts presented at meetings, congresses or symposia. We also searched the reference lists of relevant studies and contacted specialists in the field, including the authors of included trials. We contacted pharmaceutical manufacturers to verify data and to obtain additional unpublished data.
Data collection and analysis
Selection of studies
Two review authors (SD, RS) were responsible for handsearching and identifying eligible studies. Two review authors (SD, RS) examined the electronic search results and identified potentially eligible studies. Three review authors (JW, HT, TH) retrieved and reviewed independently full reports of these studies for inclusion in the review. Differences were resolved by discussion. Review authors recorded data using the data extraction form developed for this review. All languages were considered. We contacted study authors for clarification of trial methods and for additional data when required. Individual patient data were not available for calculation of means and standard deviations.
Data extraction and management
Two review authors (SD, RS) independently extracted the following data from the included studies and entered the data into RevMan 5. A third review author (LP) checked these data. Data variables extracted included the following.
Design, size and location of the trial.
Characteristics of the study population including age and gender, and any reported exclusion criteria.
Description of the intervention including types and doses of immunonutrients used, route of administration and timing and duration of treatment.
Methodological characteristics as outlined below in the Assessment of risk of bias in included studies section.
Outcome measures: numbers of events for dichotomous outcomes; means and standard deviations for continuous outcomes.
We asked the corresponding authors of included trials to provide missing data. If we received no response, or if the study author was unable to provide the necessary data, the article was excluded from the analysis of the missing outcome.
We performed analyses using RevMan 5.2 software. We conducted intention‐to‐treat analyses when possible. We calculated risk ratios (RRs) and 95% confidence intervals (95% CIs) for dichotomous outcomes, and mean differences (MDs) and 95% CIs for continuous outcomes. We pooled results of comparable groups of trials using the fixed‐effect model.
Assessment of risk of bias in included studies
The same two review authors (SD, RS) independently assessed the risk of bias of each included study and resolved disagreements through discussion or consultation with the third and fourth review authors (JW, HT). We assessed the following methodological domains, as recommended by The Cochrane Collaboration (Higgins 2011a).
Sequence generation.
Allocation concealment.
Blinding of participants, personnel and outcome assessors.
Incomplete outcome data.
Selective outcome reporting.
Other potential threats to validity (intention‐to‐treat analysis, calculation of sample size a priori).
Each of these criteria was explicitly judged as having low risk of bias, high risk of bias or unclear risk of bias (lack of information or uncertainty over the potential for bias). As part of the updating process, we completed the risk of bias assessment for the nine included studies.
Assessment of heterogeneity
We used a fixed‐effect model when no evidence showed significant heterogeneity between studies (I2 < 50%) and employed a random‐effects model when heterogeneity was likely (I2 > 50%) (DerSimonian 1986; Higgins 2003).
Assessment of reporting biases
We considered available data to be insufficient for a meaningful assessment of publication bias through a funnel plot. The largest number of trials in any single analysis was seven (for glutamine, length of stay); this is fewer than the 10 studies suggested for assessment of funnel plot asymmetry by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). However, our search of 'grey literature' combined with our dogged pursuit of trials listed in clinical trial registers and communications with trial authors should have helped to avoid publication bias.
Subgroup analysis and investigation of heterogeneity
We gave consideration to the appropriateness of subgroup analyses based on minor versus major burns, children (0 to 18 years of age) versus adults (19 years of age or older), early versus delayed nutrition, different types of immunonutrients in the experimental group, different types of nutrition in the control group and different doses of immunonutrients. Many studies did not report this level of detail; therefore it was not appropriate to perform the analysis. Data were adequate for performance of a subgroup analysis based on enteral versus parenteral administration. This was performed through calculation of RR or MD in each subgroup with examination of 95% CIs. We regarded non‐overlap of intervals to indicate a statistically significant difference between subgroups.
Sensitivity analysis
We intended to perform sensitivity analyses for missing data and study quality, but because of the small number of included studies, we were not able to do so. If appropriate, we had also planned to conduct a sensitivity analysis by study quality based on the presence or absence of a reliable random allocation method, concealment of allocation and blinding of participants or outcome assessors. If appropriate data were found, we planned to consider subgroup analysis based on burn aetiology and trial funding (pharmaceutical and/or nutrition industry or other). We performed no sensitivity analyses.
Results
Description of studies
Independent scrutiny of titles and abstracts from all searches conducted to date revealed that a total of 16 studies met the inclusion criteria (Characteristics of included studies). A total of 95 studies did not meet the inclusion criteria and were excluded from the review (Characteristics of excluded studies).
Results of the search
Results of the search are shown in Figure 1.
1.
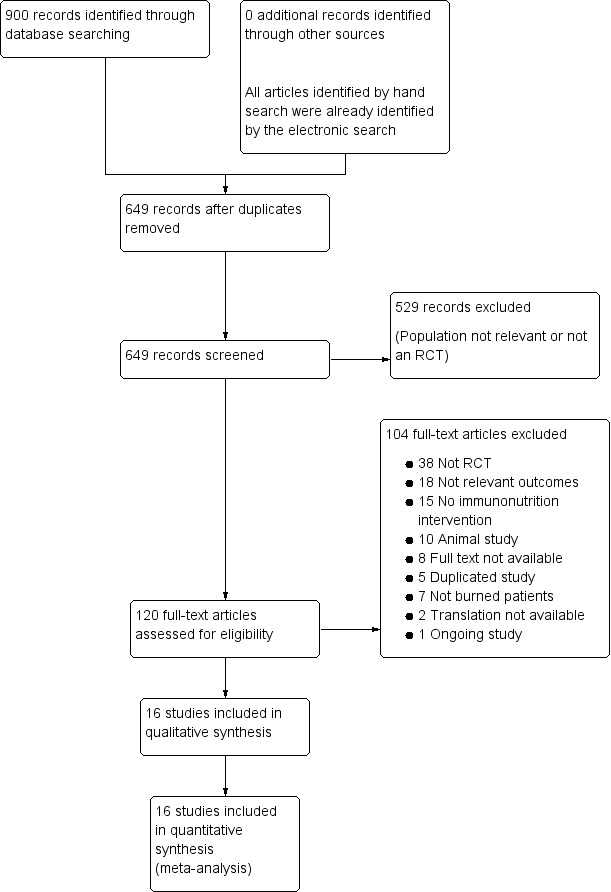
Study flow diagram.
This review includes results from the search run in August 2012. A further six possible eligible studies were identified by a recent search run In January 2014; these studies have not yet been incorporated into the review but will be assessed in the next update. Details are provided under Characteristics of studies awaiting classification.
Included studies
See Characteristics of included studies.
Design
All of the included studies were randomised. One study was nine‐armed (De Bandt 1998), three studies were three‐armed (Garrel 1995; Gottschlich 1990; Lu 2004) and the remaining 12 studies were two‐armed. Two studies used stratification to produce balanced groups according to burn size (Garrel 2003; Saffle 1997); one trial used burn size, age and institution (Gottschlich 1990).
Setting
Studies were set in hospitals and were conducted in North America, South America, Europe or Asia: one each in Italy and Chile (Donati 1999; Toledo 2007), two each in France and Canada (Coudray‐Lucas 2000; De Bandt 1998; Garrel 1995; Garrel 2003), five in China (Lu 2004; Peng 2004b; Zhou 2002; Zhou 2003; Zhou 2004) and five in the USA (Brown 1990; Gottschlich 1990; Saffle 1997; Wibbenmeyer 2006; Wischmeyer 2001). Except for one (Gottschlich 1990), all studies were conducted at a single site; one study did not report the number of sites involved (Donati 1999).
Participants
A total of 678 participants were included in this review: 45 participants in a study of BCAAs; 43 in a study of fish oil; 163 in studies of immunonutrient precursors; 164 in studies of combined immunonutrients and 285 in studies of glutamine.
Studies included both adults and children, and the mean age of participants ranged from 19.2 to 41 years. The mean size of burns across studies ranged from 32.3% to 67.5% TBSA. The mechanism or cause of injury was unexplained in all studies. In three studies (Zhou 2002; Zhou 2003; Zhou 2004), data on the gender of participants were not available. In the remaining studies, most participants were men. In all, 12 studies detailed the depth of the burn (Brown 1990; Coudray‐Lucas 2000; De Bandt 1998; Garrel 1995; Garrel 2003; Gottschlich 1990; Lu 2004; Peng 2004; Wischmeyer 2001; Zhou 2002; Zhou 2003; Zhou 2004). Only two trials did not report burn size as an inclusion or exclusion criterion (Peng 2004b; Saffle 1997). Many studies excluded people with a wide range of medical conditions such as pregnancy, chronic illness and respiratory, cardiac or renal insufficiency.
Interventions
Various types of immunonutrient interventions were tested in the included studies (for further details, see the Characteristics of included studies table); however no included studies examined arginine only. Interventions examined included BCAAs (Brown 1990), fish oil (Garrel 1995), OKG (Coudray‐Lucas 2000; De Bandt 1998; Donati 1999), combined immunonutrients (Gottschlich 1990; Saffle 1997; Toledo 2007; Wibbenmeyer 2006) and glutamine (Garrel 2003; Lu 2004; Peng 2004b; Wischmeyer 2001; Zhou 2002; Zhou 2003; Zhou 2004). Of the 16 included studies, three studies administered the immunonutrient through the parenteral route (Brown 1990; Wischmeyer 2001; Zhou 2004) and the other 13 studies used the enteral route.
The comparisons under test fell into four categories: various isonitrogenous mixtures containing standard amino acids (Brown 1990; Garrel 2003; Lu 2004; Wischmeyer 2001; Zhou 2003; Zhou 2004), soy protein (Coudray‐Lucas 2000; De Bandt 1998) and multi‐nutrient supplements made up of protein, vitamins and minerals (Garrel 1995; Gottschlich 1990; Saffle 1997; Wibbenmeyer 2006) or placebo (Donati 1999; Peng 2004b; Toledo 2007; Zhou 2002).
Outcomes
Overall mortality rate was reported in 13 studies but was not reported in three (Lu 2004; Zhou 2002; Zhou 2004). Fourteen studies reported hospital length of stay, but two did not (Coudray‐Lucas 2000; Donati 1999). One study reported only hospital LOS as “Length of stay/%burn” (Gottschlich 1990). Five studies reported on rate of burn wound infection (Garrel 2003; Gottschlich 1990; Saffle 1997; Wibbenmeyer 2006; Zhou 2003). Rates of other non‐wound infections such as pneumonia, urinary tract infection and bacteraemia were reported in four studies (Garrel 1995; Gottschlich 1990; Saffle 1997; Wibbenmeyer 2006).
Excluded studies
The reasons for excluding 95 studies are given in the Characteristics of excluded studies section. A total of 37 studies were not randomised controlled clinical trials. A further 14 studies did not compare an immunonutrient intervention versus a control, and 13 studies did not report on the primary outcome or any secondary outcomes as outlined in the methodology section of this review. Six studies contained data duplicated from another included study. Ten trials were animal studies, and in six studies participants were not solely patients with burns.
Studies awaiting classification
A total of 11 studies could not be classified for various reasons, as is shown under Characteristics of studies awaiting classification. Two trials were reported in non‐English publications and a translator was not available to assist with data extraction. The full text or the abstract was not available in seven studies. One study was published as an abstract only. One study was extracted from a clinical trials database and has concluded, but data are not yet available. Studies awaiting classification were excluded from data aggregation and analysis.
Ongoing studies
Details of one ongoing trial are given under Characteristics of ongoing studies.
Risk of bias in included studies
Two figures show our assessment of risk of bias for all included trials (Figure 2 and Figure 3).
2.
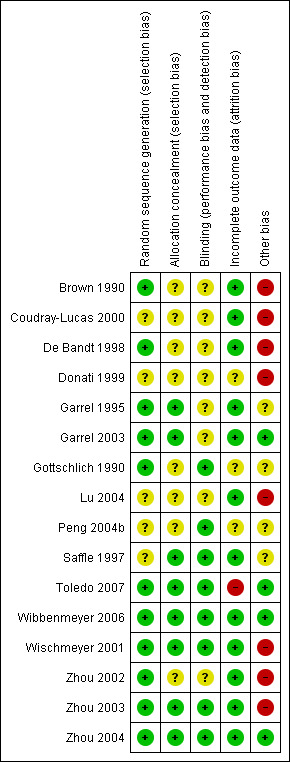
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Seven studies were assessed as having low risk of selection bias because investigators reported adequate random sequence generation and allocation concealment (Garrel 1995; Garrel 2003; Toledo 2007; Wibbenmeyer 2006; Wischmeyer 2001; Zhou 2003; Zhou 2004). Risk of selection bias was assessed as unclear in four studies, as methods of neither random sequence generation nor treatment allocation were specified (Coudray‐Lucas 2000; Donati 1999; Lu 2004; Peng 2004b). A further five studies were assessed as having unclear risk of selection bias, as researchers adequately reported only random sequence generation (Brown 1990; De Bandt 1998; Gottschlich 1990; Zhou 2002) or only treatment allocation (Saffle 1997), but not both.
Blinding
Eight studies were assessed as having low risk of bias, as they were described as double‐blinded and reported adequate blinding methods (Gottschlich 1990; Peng 2004b; Saffle 1997; Toledo 2007; Wibbenmeyer 2006; Wischmeyer 2001; Zhou 2003; Zhou 2004). Two studies were assessed as having unclear risk of bias, as they were described as double‐blinded but blinding methods were not specified (Coudray‐Lucas 2000; Garrel 2003).
In one study by Garrel 1995, blinding of medical staff and investigators was described, but the method was unclear. Another trial briefly implied the process of blinding but did not adequately describe the extent or method (Donati 1999). These two studies therefore were assessed as having unclear risk of bias. Four trials did not address blinding and were assessed as having unclear risk of bias (Brown 1990; De Bandt 1998; Lu 2004; Zhou 2002).
Incomplete outcome data
A total of 12 studies were assessed as having low risk of bias, as incomplete outcome data were adequately addressed or the study author specifically stated that there were no dropouts or withdrawals (Brown 1990; Coudray‐Lucas 2000; De Bandt 1998; Garrel 1995; Garrel 2003; Lu 2004; Saffle 1997; Wibbenmeyer 2006; Wischmeyer 2001; Zhou 2002; Zhou 2003; Zhou 2004). Risk of bias was uncertain in three studies (Donati 1999; Gottschlich 1990; Peng 2004b), as reports inferred there were no dropouts or withdrawals, but this was not specifically stated. One study did not inadequately address the numbers or reasons for dropouts and withdrawals and therefore was assessed as having high risk of incomplete data (Toledo 2007).
Other potential sources of bias
We found that only four studies had low risk of other bias (Garrel 2003; Toledo 2007; Wibbenmeyer 2006; Zhou 2004). For all of these studies, concerns about other bias were related to lack of information on, or known lack of, an intention‐to‐treat approach to results analysis.
Effects of interventions
Glutamine
We found seven studies that compared glutamine versus an isonitrogenous mixture containing standard amino acids (Garrel 2003; Lu 2004; Wischmeyer 2001; Zhou 2003; Zhou 2004) or placebo (Peng 2004b; Zhou 2002).
All‐cause mortality
See Analysis 1.1. Three studies (Garrel 2003; Wischmeyer 2001; Zhou 2003) examined a total of 111 participants. The pooled RR of death was 0.25 (95% CI 0.08 to 0.78; P value 0.02).
1.1. Analysis.

Comparison 1 All‐cause mortality, Outcome 1 Glutamine vs control.
Length of stay
See Analysis 2.1. All seven studies (Garrel 2003; Lu 2004; Peng 2004b; Wischmeyer 2001; Zhou 2002; Zhou 2003; Zhou 2004) reported hospital length of stay. This analysis included a total of 255 participants. The pooled RR was ‐5.65 (95% CI ‐8.09 to ‐3.22; P value < 0.0001).
2.1. Analysis.
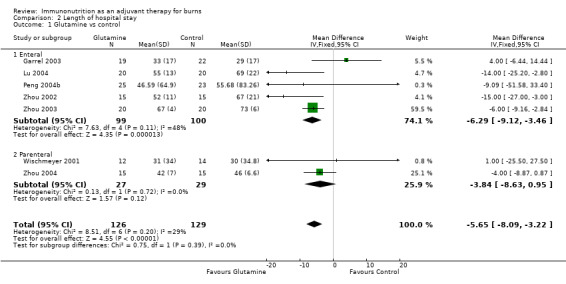
Comparison 2 Length of hospital stay, Outcome 1 Glutamine vs control.
Burn wound infection
See Analysis 3.1. Two studies by Garrel 2003 and Zhou 2003 looked at a total of 81 participants. The pooled RR was 0.42 (95% CI 0.16 to 1.06; P value 0.07).
3.1. Analysis.
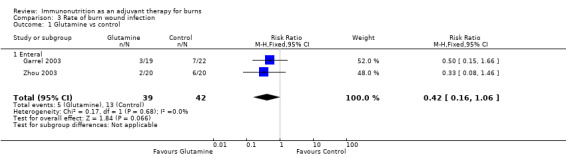
Comparison 3 Rate of burn wound infection, Outcome 1 Glutamine vs control.
Non‐wound infection
See Analysis 4.1. Two studies by Garrel 2003 and Wischmeyer 2001 looked at a total of 67 participants. The pooled RR was 0.73 (95% CI 0.27 to 1.95; P value 0.53).
4.1. Analysis.

Comparison 4 Rate of non‐wound infection, Outcome 1 Glutamine vs control.
Immunonutrient precursors (ornithine α‐ketoglutarate)
We found three studies that compared ornithine α‐ketoglutarate versus soy protein (Coudray‐Lucas 2000; De Bandt 1998) or placebo (Donati 1999).
All‐cause mortality
See Analysis 1.2. The three studies (Coudray‐Lucas 2000; De Bandt 1998; Donati 1999) looked at a total of 155 participants. The pooled RR of death was 0.93 (95% CI 0.37 to 2.36; P value 0.88).
1.2. Analysis.
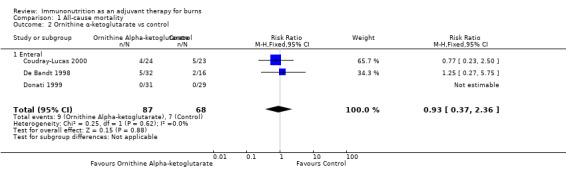
Comparison 1 All‐cause mortality, Outcome 2 Ornithine α‐ketoglutarate vs control.
Length of stay
See Analysis 2.2. Only one study (De Bandt 1998) looked at length of stay and reported on 48 participants. The pooled RR was ‐4.21 (95% CI ‐18.87 to 10.45; P value 0.57).
2.2. Analysis.
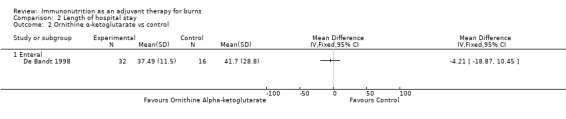
Comparison 2 Length of hospital stay, Outcome 2 Ornithine α‐ketoglutarate vs control.
Branched‐chain amino acids (BCAAs)
We assessed one study (Brown 1990) that compared BCAAs versus an isonitrogenous mixture containing standard amino acids.
All‐cause mortality
See Analysis 1.3. This study examined all‐cause mortality in 20 participants. The RR was 2.50 (95% CI 0.63 to 10.00; P value 0.20).
1.3. Analysis.

Comparison 1 All‐cause mortality, Outcome 3 Branched‐chain amino acids vs control.
Length of stay
See Analysis 2.3. This study reported hospital length of stay in 20 participants. The RR was 4.00 (95% CI ‐27.63 to 35.63; P value 0.80).
2.3. Analysis.
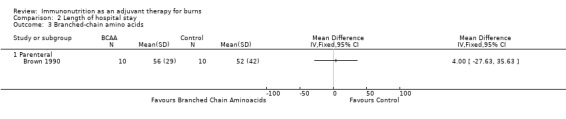
Comparison 2 Length of hospital stay, Outcome 3 Branched‐chain amino acids.
N‐3 fatty acids (fish oil)
We found one study (Garrel 1995) that compared fish oil versus multi‐vitamins.
All‐cause mortality
See Analysis 1.4. This single study examined all‐cause mortality in 25 participants. The RR was 0.41 (95% CI 0.05 to 3.49; P value 0.41).
1.4. Analysis.

Comparison 1 All‐cause mortality, Outcome 4 Fish oil vs control.
Length of stay
See Analysis 2.4. This single study reported hospital length of stay in 25 participants. The RR was ‐21.0 (95% CI ‐41.03 to ‐0.97; P value 0.04).
2.4. Analysis.
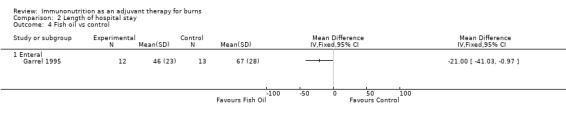
Comparison 2 Length of hospital stay, Outcome 4 Fish oil vs control.
Non‐wound infection
See Analysis 4.2. This single study reported pneumonia as a non‐wound outcome in 25 participants. The RR was 0.31 (95% CI 0.08 to 1.21; P value 0.09).
4.2. Analysis.

Comparison 4 Rate of non‐wound infection, Outcome 2 Fish oil vs control.
Combined immunonutrients
We found four studies that compared combined immunonutrients versus multi‐nutrient supplementation (Gottschlich 1990; Saffle 1997; Wibbenmeyer 2006) or placebo (Toledo 2007).
All‐cause mortality
See Analysis 1.5. These four studies (Gottschlich 1990; Saffle 1997; Toledo 2007; Wibbenmeyer 2006) looked at a total of 163 participants. The pooled RR was 1.10 (95% CI 0.47 to 2.60; P value 0.83).
1.5. Analysis.
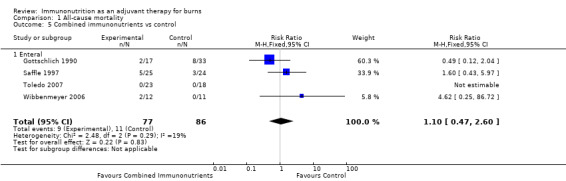
Comparison 1 All‐cause mortality, Outcome 5 Combined immunonutrients vs control.
Length of stay
See Analysis 2.5. Three studies (Saffle 1997; Toledo 2007; Wibbenmeyer 2006) reported hospital length of stay in 113 participants. The pooled RR was 1.93 (95% CI ‐4.41 to 8.28; P value 0.55).
2.5. Analysis.

Comparison 2 Length of hospital stay, Outcome 5 Combined immunonutrients vs control.
Burn wound infection
See Analysis 3.3. Three studies (Gottschlich 1990; Saffle 1997; Wibbenmeyer 2006) reported on burn wound infection in 122 participants. The pooled RR was 0.79 (95% CI 0.51 to 1.20; P value 0.26).
3.3. Analysis.

Comparison 3 Rate of burn wound infection, Outcome 3 Combined immunonutrients vs control.
Non‐wound infection
See Analysis 4.3. Three studies (Gottschlich 1990; Saffle 1997; Wibbenmeyer 2006) examined rates of pneumonia in a total of 122 participants. The pooled RR was 0.83 (95% CI 0.59 to 1.15; P value 0.26). Two studies (Saffle 1997; Wibbenmeyer 2006) looked at urinary tract infection (RR 2.46, 95% CI 0.98 to 6.20; P value 0.06) and bacteraemia (RR 1.77, 95% CI 0.81 to 3.88; P value 0.16).
4.3. Analysis.
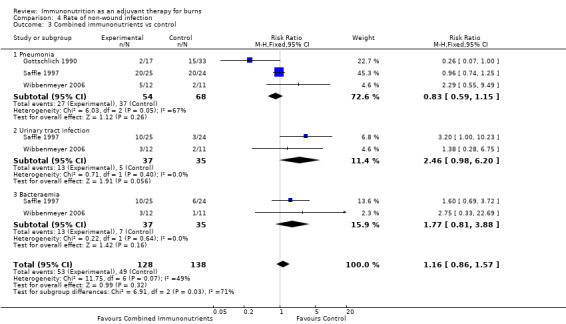
Comparison 4 Rate of non‐wound infection, Outcome 3 Combined immunonutrients vs control.
Discussion
Patients with major burn injuries are uniquely different from other trauma or critical care patients. Their hypermetabolic state often persists beyond regeneration or replacement of burned skin, with rapid depletion of key nutrients, trace elements and vitamins. Early nutritional intervention (within 24 hours post injury) is now considered essential, and the enteral route of administration is preferred to the parenteral route. Its benefits are well documented in terms of attenuating the hypermetabolic response and stress hormone levels (Mochizuki 1984), reducing gastric ulceration and malnutrition (Chiarelli 1990; Venter 2007) and increasing immunoglobulin production (Lam 2008). Mechanisms that lead to immunosuppression in patients with major burns include a characteristic counter anti‐inflammatory response syndrome (CARS). This mediator‐driven reaction is aimed at minimising inflammation‐induced tissue injury and is initiated after the acute inflammatory response subsides. Vulnerability to infective and wound complications, as well as risk of death, is therefore increased (Bone 1996; Bone 1996a; Jeschke 2008).
Summary of main results
We found that glutamine when given in enteral feeds led to a reduction in length of stay (P value < 0.0001) and mortality (P value 0.002) when compared with an isonitrogenous control. No other agents were found to have evidence of an impact on mortality, length of stay or burn wound infection or non‐wound infection rates.
Glutamine
Glutamine is the principal nitrogen carrier in the body and is a conditionally essential amino acid. It acts as a fuel for lymphocytes and enterocytes and is a precursor for glutathione, a powerful antioxidant. Glutamine is the most thoroughly investigated of all immunomodulating agents in burns, accounting for 285 participants in seven studies (Garrel 2003; Lu 2004; Peng 2004b; Zhou 2002; Zhou 2003; Wischmeyer 2001; Zhou 2004). However not all studies examined all of the outcomes.
Only three studies reported on mortality (n = 111 participants; Garrel 2003; Zhou 2003; Wischmeyer 2001), for which evidence of benefit has been found (RR 0.25, 95% CI 0.08 to 0.78). In the same studies, we noted a statistically significant reduction in length of hospital stay by 5.6 days (n = 255 participants; 95% CI 3.2 to 8.1) compared with isonitrogenous control.
Statistical reasoning shows that it is likely that the positive effects of glutamine on mortality and length of stay are spurious, but this finding should not be considered conclusive. Additional trials would be required to confirm this result.
The same three studies (Garrel 2003; Wischmeyer 2001; Zhou 2003) reported on infection rates and found no clear evidence of a change in rates of wound or non‐wound infection (RR 0.42, 95% CI 0.16 to 1.06, and RR 0.73, 95% CI 0.27 to 1.95; P value 0.68 and P value 0.92, respectively).
Administration of glutamine in split boluses separate from enteral feeds (≥ 0.3 g/kg/d) is described as preferable in a number of studies, although this has not been shown to have a significant impact on outcomes. This dosing regimen is thought to provide a preferentially systemic rather than a gut fuel source, with increased delivery to the burn wound. Included studies on use of adjuvant glutamine in major burns would suggest that it is safe. However, no clear evidence has indicated optimal dosage or duration of treatment. It is already clear that enteral regimens across the board are effective. Two studies on parenteral use of glutamine neither raised concerns about its use (from a safety point of view) nor showed clear evidence of benefit for length of stay; however these studies included only 56 participants, and further assessment would be warranted (MD ‐3.84 days, 95% CI ‐8.63 to +0.95; Wischmeyer 2001; Zhou 2004).
The latest recommendations from the European Society for Clinical Nutrition and Metabolism (ESPEN) on nutrition in major burns suggest strong consideration of glutamine supplementation, although no comments are provided on dose and route and duration of administration. The basis for this recommendation is described as ‘weak evidence’ (Rousseau 2013). Kurmis and colleagues refer to the enteral route as the most thoroughly investigated and supported route (Kurmis 2010).
Immunonutrient precursor (ornithine α‐ketoglutarate (OKG))
OKG is a precursor of both glutamine and arginine. It has been shown to trigger release of insulin and growth hormone, both of which induce trophic changes. The three included studies of OKG (Coudray‐Lucas 2000; De Bandt 1998; Donati 1999) report that its effects—versus those of isonitrogenous controls—were not statistically significant in terms of mortality and length of stay (RR 0.93, 95% CI 0.37 to 2.36, and RR ‐4.21, 95% CI ‐18.87 to 10.45, respectively). One study reported enhanced wound healing with ornithine α‐ketoglutarate supplementation (Coudray‐Lucas 2000); this study was prospective and double‐blinded, and blinding methods were not specified. This study gave participants two boluses each of 10 grams of OKG per day for three weeks. This precursor is available only in France and is used as a 30 gram daily dose divided into two or three doses. Its use as an immunomodulator is strongly suggested by the latest ESPEN recommendations (Rousseau 2013). For this agent, no clear evidence of its impact on patient outcomes or of its optimal dose duration has been found. If larger studies were to find benefits with OKG, more complex trials would be needed to ascertain whether better outcomes could be attained by supplementation of both arginine and glutamine compared with their common precursor.
Branched‐chain amino acids
Branched‐chain amino acids (leucine, isoleucine and valine) are among the nine essential amino acids. Early reports (Bower 1986) described the critical care and trauma setting of improvements in nitrogen balance when compared with use of standard amino acids, particularly via the parenteral route. One included study (Brown 1990; n = 20) showed no improved outcomes for mortality and length of stay versus isonitrogenous control (RR 2.5, 95% CI 0.63 to 10.0, and RR 4, 95% CI ‐27.63 to 35.63, respectively). Visceral protein concentrations, including prealbumin and retinol‐binding protein, were raised in the branched‐chain amino acid group compared with the standard group, but this was not reflected in outcomes. This highlights that serum markers used to measure response to interventions are not always predictive of outcome or of true nutritional status. Both Kurmis and colleagues and ESPEN have not recommended the use of BCAAs.
N‐3 fatty acids (fish oil)
Fish oils contain the omega‐3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—precursors of certain eicosanoids that are known to reduce inflammation and reduce immunosuppressive metabolites throughout the body. Despite ease of administration because fish oil is liquid, this has not been investigated as an individual agent. One included study (Garrel 1995; n = 25) looked at a low‐fat nutritional regimen with and without fish oil, demonstrating no clear evidence of a difference in mortality or in the presence of non–wound‐related infection (RR 0.41, 95% CI 0.05 to 3.49, and RR 0.31, 95% CI 0.08 to 1.21, respectively). However, a reduction in overall length of hospital stay was noted. Therefore this agent has no proven role as a sole immunonutrient. In addition, according to the latest ESPEN recommendations, lipid contents of nutrition regimens should be kept to less than 35% of energy requirements (Rousseau 2013). Therefore particular care must be taken when n‐3 fatty acids are administered in conjunction with high‐lipid drugs (e.g. propofol). However, again, this is supported only by ‘weak’ evidence.
Combined immunonutrients
Second to glutamine, combined immunonutrient regimens have been the next most thoroughly investigated. This may reflect their commercial availability, with the rationale for their use resting on providing muscle fuel and reducing sepsis. Four included studies (Gottschlich 1990; Saffle 1997; Toledo 2007; Wibbenmeyer 2006) report no clear impact on mortality or length of stay (RR 1.1, 95% CI 0.47 to 2.60, and RR 1.93, 95% CI ‐4.41 to 8.28, respectively) versus isonitrogenous controls. No effects on burn wound infection rate (RR 0.79, 95% CI 0.51 to 1.2) or non‐wound infection rate (RR 1.16, 95% CI 0.86 to 1.57) were noted. For urinary tract infection, a trend towards increased events was noted in the experimental group (RR 2.46, 95% CI 0.98 to 6.2). The concern associated with these comparative studies is that the control formula was often immunonutritive itself. One study formula showed promise (Gottschlich 1990), with reasonable impact on burn wound infection rates (RR 0.24, 95% CI 0.06 to 0.93) and length of stay, expressed as days per % TBSA (P value < 0.02). This formula—with high‐protein and low‐fat and low‐linoleic acid content—was supplemented with omega‐3 fatty acids, arginine, cysteine, histidine, vitamin A, zinc and ascorbic acid. As there is no possibility of determining which agent and what dose of that agent led to improved outcomes, the study poses more questions than answers.
Kurmis and associates do not support use of combination immunonutrition regimens, and ESPEN has issued no statement regarding combination therapy.
Overall completeness and applicability of evidence
A large number of studies examining immunonutrition were excluded from this review. These trials were not randomised controlled clinical trials, or they did not compare an immunonutrient intervention versus a control. Also, many articles were excluded because they reported only biochemical markers of immune activity—not clinically significant outcomes. Future studies should focus on study outcomes such as mortality, MOF rate and rates of sepsis and wound infection in severely burned patients with more than 20% of body surface area burned, or in patients with more than 40% of TBSA, to target the most labile population. Other studies were excluded because they lacked an available English translation, they contained duplicated data, they were published in abstract format only or they were animal trials. Only 16 studies met the inclusion criteria; these trials provide evidence for use of the various immunonutrients (summarized in Characteristics of included studies).
Quality of the evidence
Most studies failed in reporting the randomisation methods used and in specifically reporting how they concealed the randomisation sequence and the outcome assessment. Many studies were unclear about or failed to use intention‐to‐treat principles in their analyses. In addition, the starting framework for future studies should be based on multi‐centre single agent versus control studies focused on optimal dosing strategies. This is similar to the methodology used in phase 1 and 2 trials for new cancer agents, whereby the dose that achieves the best outcome before signs of toxicity appear is then used in a larger population.
Potential biases in the review process
Although no conflict of interest was declared in any of the included studies, potential biases include availability of products (i.e. some agents will be available or licenced only in certain parts of the world). Along with this, some of the outcome measures used are subject to a high degree of variability (e.g. time to healing). It is for this reason that only four included outcomes were used in this review, with the greatest quantity of evidence found for mortality and length of stay.
The review authors acknowledge that there is lag time between the most recent literature search and the review publication date. Future updates will incorporate any new studies that may be suitable for inclusion.
Agreements and disagreements with other studies or reviews
Nutritional intervention in itself is immune‐enhancing and is essential for improved mortality and infection‐related outcomes (Prelack 2007). This review critically appraises existing evidence for use of specific immunomodulating agents in burns. Despite extensive research in this field, unanswered questions remain in terms of which agent/s, which route, what dose, for how long and for what gain, if any. Nutritional assessment and outcome measures need to be clinically and economically valid to ensure that potential detrimental or beneficial effects of these interventions are clear. Two other recent review articles have addressed this topic (Kurmis 2010; Rousseau 2013), one of which provides the updated ESPEN recommendations for 2013. A key conclusion of these reviews is that larger, multi‐centre studies are required in the field of immunonutrition, with a focus on the addition of single agents at varying doses to determine their true impact on outcomes. Kurmis 2010 supports the addition of glutamine to enteral nutrition, despite lack of evidence to support dose, timing and duration of supplementation. The latest ESPEN recommendations suggest that glutamine or OKG supplementation should be strongly considered, although again, no clear instruction is provided on dosage or timing, and no safe starting point is given (Rousseau 2013). Neither review recommends key outcome measures for the future, which are of course essential for clinical intervention and economic validity.
Authors' conclusions
Implications for practice.
Although glutamine may provide some beneficial effect for mortality, its routine administration to patients with severe burns cannot be recommended as standard practise, nor can we pronounce against its use. It is likely that the number of participants studied for this intervention it is too small to permit robust statistical conclusions.
No other intervention shows evidence of any effect on mortality or secondary outcomes. Therefore evidence on all of the immunonutrient agents studied is insufficient.
Implications for research.
More studies on the benefits of immunonutrients are required, especially for the purpose of testing the effect of glutamine on mortality. The total sample size needed to permit reliable conclusions on a 30% relative reduction in mortality risk is 977 participants; this is almost nine‐fold the total number of participants in whom glutamine has been studied in the whole body of literature. This number of burned persons greatly surpasses the admission rate of one decade of most burn units worldwide; thus the only way to obtain an answer to the clinical question is to perform a large‐scale, multi‐continent, multi‐centre randomised controlled trial; given the fact that the largest RCT ever published included 344 participants (Danilla 2009), it is very unlikely that this trial will ever occur.
Future studies need to be directed, starting with active single nutrients, to determine ideal and safe doses free from metabolic or drug interactions and adverse events. Combination regimens can then include single‐agent safe doses with proven successful outcomes that are then tested as part of polytherapy. This reflects standard pharmacological dosing principles. It is crucial that studies of burn patients are separated from critical care and trauma patient studies, as other recent work has also noted (Kurmis 2010; Rousseau 2013). We know that these patient groups are very different in terms of physiological demands and have different nutritional requirements. Specific and accurate markers of adequate nutrition are essential for monitoring the success of new regimens alongside clinical outcomes. Future trials and reviews should consider adverse effects of immunonutrition therapy.
Furthermore, we advise study authors to strictly follow CONSORT guidelines when reporting their findings to minimise bias in reporting.
The studies listed under Characteristics of studies awaiting classification may alter the conclusions of this review once assessed.
History
Protocol first published: Issue 2, 2008 Review first published: Issue 12, 2014
| Date | Event | Description |
|---|---|---|
| 14 May 2008 | Amended | Converted to new review format |
| 19 February 2008 | New citation required and major changes | Protocol first published |
Acknowledgements
Sincere thanks to Dr Zhaowei Zhou (Department of Cardiothoracic Surgery, Hammersmith Hospital, London, UK) for translating Chinese articles, to Dr María Teresa Valenzuela for sending letters to the authors of selected articles and to Frances Phillips (Head of Dietetics and Nutrition, Royal Perth Hospital, Perth, AUS) for providing insights into clinical content.
Appendices
Appendix 1. Search strategies
Cochrane Injuries Group Specialised Register ("Fatty Acid" or "amino acid" or Arginin* or glutamin* or nutrition or immunonutr* or nucleoside* or nucleotide*) and (burn*) Cochrane Central Register of Controlled Trials #1immunonutrient* or immunonutrition #2MeSH descriptor Nutritional Support explode all trees #3glutamine or L‐glutamine or L Glutamine or D‐glutamine or D Glutamine #4MeSH descriptor Glutamine explode all trees #5(arginin*) or (Arg* next 15) #6branched chain amino acid* or (n‐3 near acid*) or (Omega near Acid*) or (Fatty near Acid*) #7MeSH descriptor Amino Acids, Branched‐Chain explode all trees #8nucleoside* or nucleotide* #9MeSH descriptor Nucleosides explode all trees #10MeSH descriptor Nucleotides explode all trees #11MeSH descriptor Arginine explode all trees #12(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) #13burn* #14thermal near2 injur* #15MeSH descriptor Burns explode all trees #16(#13 OR #14 OR #15) #17(#12 AND #16) MEDLINE (OvidSP) 1.(immuno?nutrient* or immuno?nutrition* or immunonutrient* or immunonutrition*).ab,ti. 2.exp Nutritional Support/ 3.(glutamin* or L?glutamin* or LGlutamin* or D?glutamin*or DGlutamin*).ab,ti. 4.exp Glutamine/ 5.(arginin* or Arg 15?aprotinin* or arginin* 15?aprotinin* or arginin* 15?bovine pancreatic trypsin inhibitor* or arginine?15?kallikrein?trypsin inactivator* or Arg?15?PTI or arginin* l?isomer or dl?arginin* acetate* monohydrate or l?arginin*).ab,ti. 6.exp Arginine/ 7.(Branched?Chain Amino Acid* or n?3 fatty acid* or Omega?3 Fatty Acid* or Omega3 Fatty Acid* or n3 Fatty Acid* or n?3 Polyunsaturated Fatty Acid* or n3 Polyunsaturated Fatty Acid*).ab,ti. 8.exp Amino Acids, Branched‐Chain/ 9.(nucleoside* or nucleotide*).ab,ti. 10.exp Nucleosides/ 11.exp Nucleotides/ 12.or/1‐11 13.exp Burns/ 14.burn*.ab,ti. 15.(thermal adj2 injur*).ab,ti. 16.or/13‐15 17.12 and 16 18.randomi?ed.ab,ti. 19.randomized controlled trial.pt. 20.controlled clinical trial.pt. 21.placebo.ab. 22.clinical trials as topic.sh. 23.randomly.ab. 24.trial.ti. 25.18 or 19 or 20 or 21 or 22 or 23 or 24 26.(animals not (humans and animals)).sh. 27.25 not 26 28.17 and 27 Embase (OvidSP) 1.(immuno?nutrient* or immuno?nutrition* or immunonutrient* or immunonutrition*).ab,ti. 2.exp Nutritional Support/ 3.(glutamin* or L?glutamin* or LGlutamin* or D?glutamin*or DGlutamin*).ab,ti. 4.exp Glutamine/ 5.(arginin* or Arg 15?aprotinin* or arginin* 15?aprotinin* or arginin* 15?bovine pancreatic trypsin inhibitor* or arginine?15?kallikrein?trypsin inactivator* or Arg?15?PTI or arginin* l?isomer or dl?arginin* acetate* monohydrate or l?arginin*).ab,ti. 6.exp Arginine/ 7.(Branched?Chain Amino Acid* or n?3 fatty acid* or Omega?3 Fatty Acid* or Omega3 Fatty Acid* or n3 Fatty Acid* or n?3 Polyunsaturated Fatty Acid* or n3 Polyunsaturated Fatty Acid*).ab,ti. 8.Branched Chain Amino Acid/ 9.(nucleoside* or nucleotide*).ab,ti. 10.exp Nucleoside/ 11.exp Nucleotide/ 12.or/1‐11 13.exp Burn/ 14.burn*.ab,ti. 15.(thermal adj2 injur*).ab,ti. 16.or/13‐15 17.12 and 16 18.exp Randomized Controlled Trial/ 19.exp controlled clinical trial/ 20.randomi?ed.ab,ti. 21.placebo.ab. 22.*Clinical Trial/ 23.randomly.ab. 24.trial.ti. 25.18 or 19 or 20 or 21 or 22 or 23 or 24 26.exp animal/ not (exp human/ and exp animal/) 27.25 not 26 28.17 and 27 ISI Web of Science: Science Citation Index‐Expanded (SCI‐EXPANDED) and Conference Proceedings Citation Index‐Science (CPCI‐S) #1Topic=(Burn* or (thermal near/3 injur*)) AND Topic=(immuno?nutrient* or immuno?nutrition* or immunonutrient* or immunonutrition* or nutrition* or glutamin* or arginin* or aprotinin* or Amino Acid* or fatty acid* or nucleoside* or nucleotide*) #2Topic=((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)) OR Topic=((clinical OR control* OR placebo OR random*) NEAR/3 (trial* or group* or study or studies or placebo or controlled)) NOT Title=(Animal* or rat or rats or rodent* or mouse or mice or murine or dog or dogs or canine* or cat or cats or feline* or rabbit or rabbits or pig or pigs or porcine or swine or sheep or ovine* or guinea pig*) #3#1 AND #2 PubMed #1Search (Burn* OR (thermal AND injur*)) AND (immuno?nutrient* or immuno?nutrition* or immunonutrient* or immunonutrition* or nutrition* or glutamin* or arginin* or aprotinin* or Amino Acid* or fatty acid* or nucleoside* or nucleotide*) #2(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh]) NOT ((models, animal[mh] OR Animals[mh] OR Animal Experimentation[mh] OR Disease Models, Animal[mh] OR Animals, Laboratory[mh]) NOT (Humans[mh])) #3#1 and #2
CINAHL
S28 S16 AND S27 S27 S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 S26 MH quantitative studies S25 TX random* N3 allocat* S24 (MH "Random Assignment") S22 (MH "Placebos") S21 TX randomi?ed N3 control* N3 trial* S20 TI ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) or (tripl* N3 blind*) ) or TI ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) ) or AB ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) ) or AB ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) ) S19 TX clinical N3 trial* S18 PT clinical trial* S17 (MH "Clinical Trials") S16 S11 AND S15 S15 S12 OR S13 OR S14 S14 (MH "Burns+") S13 thermal N2 injur* S12 burn* S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 S10 (MH "Arginine") S9 (MH "Nucleotides+") S8 (MH "Nucleosides+") S7 nucleoside* or nucleotide* S6 ((branched chain amino acid*) or (n‐3 near acid*) or (Omega near Acid*) or (Fatty near Acid*)) S5 (arginin*) or (Arg* N 15) S4 (MH "Glutamine") S3 glutamine or L‐glutamine or L Glutamine or D‐glutamine or D Glutamine S2 (MH "Nutritional Support+") S1 immunonutrient* or immunonutrition
LILACS
(cancer$ or tumor$ or tumour$ or neoplas$ or malignan$ or carcinoma$ or adenocarcinoma$ or choricarcinoma$ or leukemia$ or leukaemia$ or metastat$ or sarcoma$ or teratoma$) [Words] and (cachexia or cachexic) or (weight or underweight or malnutrition or wasting) and (fit* or activ* or movement* or exercis* or aerobic* or resistance* or strength* or walk* or endurance*) [Words] and ((PT randomized controlled trial OR PT controlled clinical trial OR PT multicenter study OR MH randomized controlled trials as topic OR MH controlled clinical trials as topic OR MH multicenter study as topic OR MH random allocation OR MH double‐blind method OR MH single‐blind method ) OR (( ensaio $ OR ensayo $ OR trial $) AND ( azar OR acaso OR placebo OR control $ OR aleat $ OR random $ OR enmascarado $ OR simpleciego OR (( simple $ OR single OR duplo $ OR doble $ OR double $) AND ( cego OR ciego OR blind OR mask ))) AND clinic $)) AND NOT (MH animals)) [Words]
Data and analyses
Comparison 1. All‐cause mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Glutamine vs control | 3 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.78] |
| 1.1 Enteral | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.06, 0.93] |
| 1.2 Parenteral | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.04, 2.27] |
| 2 Ornithine α‐ketoglutarate vs control | 3 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.36] |
| 2.1 Enteral | 3 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.36] |
| 3 Branched‐chain amino acids vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Parenteral | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fish oil vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Enteral | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined immunonutrients vs control | 4 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.60] |
| 5.1 Enteral | 4 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.60] |
Comparison 2. Length of hospital stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Glutamine vs control | 7 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐5.65 [‐8.09, ‐3.22] |
| 1.1 Enteral | 5 | 199 | Mean Difference (IV, Fixed, 95% CI) | ‐6.29 [‐9.12, ‐3.46] |
| 1.2 Parenteral | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐3.84 [‐8.63, 0.95] |
| 2 Ornithine α‐ketoglutarate vs control | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Enteral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Branched‐chain amino acids | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Parenteral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fish oil vs control | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Enteral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined immunonutrients vs control | 3 | 113 | Mean Difference (IV, Fixed, 95% CI) | 1.93 [‐4.41, 8.28] |
| 5.1 Enteral | 3 | 113 | Mean Difference (IV, Fixed, 95% CI) | 1.93 [‐4.41, 8.28] |
Comparison 3. Rate of burn wound infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Glutamine vs control | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.06] |
| 1.1 Enteral | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.06] |
| 2 Ornithine α‐ketoglutarate vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Enteral | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Combined immunonutrients vs control | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.20] |
| 3.1 Enteral | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.20] |
3.2. Analysis.

Comparison 3 Rate of burn wound infection, Outcome 2 Ornithine α‐ketoglutarate vs control.
Comparison 4. Rate of non‐wound infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Glutamine vs control | 2 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.27, 1.95] |
| 1.1 Bacteraemia | 2 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.27, 1.95] |
| 2 Fish oil vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Pneumonia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Combined immunonutrients vs control | 3 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.86, 1.57] |
| 3.1 Pneumonia | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.59, 1.15] |
| 3.2 Urinary tract infection | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.98, 6.20] |
| 3.3 Bacteraemia | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.81, 3.88] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 1990.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: not reported. Sample size calculation: not reported. Generation of allocation: random numbers table. Allocation concealment: not reported. Blinded assessment of treatment allocation: not reported. Withdrawals: 3 recruited participants withdrawn (2 deaths, 1 transferred to another unit on day 2). Intention‐to‐treat analysis: not reported. Follow‐up: not reported | |
| Participants |
How many enter the study on each arm? 23 participants, 20 analysed (BCAA 4 women and 6 men; control 1 woman and 9 men) How many finish the study on each arm? BCAA group 7; control group 2 Mean age: BCAA group 43 years; control group 33 years (overall 38 years) Mean total burn surface area (TBSA): BCAA 45%; control 49% Inclusion criteria: consecutively thermally injured patients who required PN for > 7 days and > 10% TBSA Exclusion criteria: TBSA < 10%, acute renal failure, liver failure and insulin‐dependent diabetes mellitus; patients who received steroids within 24 hours of the start of parenteral nutrition |
|
| Interventions | All participants had parenteral nutrition―isocaloric, isonitrogenous and isovolumic (n = 20): Experimental/BCAA group (n = 10): modified amino acid solution with 45% BCAA Control (n = 10): standard amino acid solution with 19% BCAA |
|
| Outcomes | Death, LOS | |
| Notes | Other outcomes (biochemical): C‐reactive protein, albumin, prealbumin, fibronectin, etc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by random numbers sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not address blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants excluded after randomisation 3 recruited participants withdrawn (2 deaths, 1 transferred to another unit on day 2) |
| Other bias | High risk | No intention‐to‐treat principle applied |
Coudray‐Lucas 2000.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: not reported. Sample size calculation: based on previous study (De Bandt 1998). Generation of allocation: randomisation in blocks of 10, not clear how the blocks were generated. Allocation concealment: not reported. Blinded assessment of treatment allocation: The term 'double blinded' is used in the study, but it is unclear how it was applied. Investigators and participants were blind to product identity, although no further details were given.Withdrawals: 2 died of sepsis (1 from each group) and were excluded in week 1. Intention‐to‐treat analysis: not reported. Follow‐up: Until 95% of the wound healed, time was not reported | |
| Participants |
How many enter the study on each arm? 49 participants, 2 died; therefore 47 included. n = 24 in treatment OKG group; 23 in control group How many finish the study on each arm? all participants in both groups Mean age: 37 years OKG; 34 years control Mean total burn surface area (TBSA): 50% OKG; 48.4% control Inclusion criteria: age 15 to 60 years, minimum TBSA 25%; on low flow rate continuous enteral nutrition Exclusion criteria: admission after third postburn day, non‐thermal burn, severe associated trauma, hepatic or renal disease, pregnancy |
|
| Interventions | All participants received polymeric diet aiming for 50 kcal/kg body weight and 0.4 g nitrogen/kg body weight, then were prospectively assigned to: Experimental OKG group (n = 24): received 20 grams ornithine α‐ketoglutarate as 2 boluses of 10 g twice daily Control (n = 23): received isonitrogenous control/soy protein mixture (Protil‐1; Jacquemaire) |
|
| Outcomes | Mortality | |
| Notes | Wound healing, LOS on ICU, survival rate, duration of therapy LOS not reported in Results section, days to 95% healing reported in a non‐reliable format for data extraction Other outcomes (biochemical): serum transthyretin, plasma phenylalanine, urinary 3MH/Cr |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how blocks of 10 were generated |
| Allocation concealment (selection bias) | Unclear risk | Not clear whether the person who enrolled the participant was unaware of the allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The term "double blinded" is used in text, but it is unclear how this was applied. It is not stated for example whether labels were inserted on similar bags or vials |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 49 (4.08%) dropped out 2 died of sepsis (1 from each group) and were excluded in week 1 |
| Other bias | High risk | 2 participants died early after randomisation and were not included |
De Bandt 1998.
| Methods | RCT, 9 arms: 3 bolus OKG, 3 infusion OKG, 3 control Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: not reported. Sample size calculation: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinded assessment of treatment allocation: not reported. Withdrawals: 6 excluded (death/severe complication/logistical problem). Intention‐to‐treat analysis: not reported. Follow‐up: 21 days |
|
| Participants |
How many enter the study on each arm? 48 analysed (started with 54); OKG group n = 32: 6 women and 26 men; control group n = 16: 2 women and 14 men. OKG group divided into bolus (n = 15) and continuous (n = 17) How many finish the study on each arm? all participants Mean age: 33.5 years Mean total burn surface area (TBSA): 34% Inclusion criteria: admitted for thermal injury with TBSA 20% to 50% Exclusion criteria: admission 24 hours after injury, renal or hepatic failure, age < 15 or > 60 years, no enteral nutritional support |
|
| Interventions | All participants received enteral nutrition with Osmolite for 24 to 48 hours post injury followed by commercial polymeric NG infusion Experimental group (n = 32): supplemented with OKG (10, 20 or 30 gram bolus per day) or OKG as infusion (10, 20 or 30 g/d) Control group (n = 16): received isonitrogenous control/soy protein mixture (Protil‐1; Jacquemaire) |
|
| Outcomes | Mortality, LOS, sepsis: defined as 2 consecutive blood cultures yielding the same organism | |
| Notes | Intervention group arms were merged for analysis into OKG and control groups Weighted means and SDs used Other outcomes (biochemical): weight loss, nitrogen balance, urinary excretion of 3MH and hydroxyproline, plasma glutamine and ornithine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not address blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
6 (11.1%) participants excluded after randomisation: 1 intestinal haemorrhage 1 cardiac arrest 2 deaths (respiratory failure and renal failure) 2 enteral diet not available (Dripsol 81) |
| Other bias | High risk | No ITT analysis |
Donati 1999.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: not reported. Sample size calculation: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinded assessment of treatment allocation: not reported. Withdrawals: not specifically reported but all 60 included. Intention‐to‐treat analysis: not reported. Follow‐up: 21 days | |
| Participants |
How many enter the study on each arm? 60 adults: n = 31 randomly assigned to OKG group (treatment with ornithine α‐ketoglutarate) and n = 29 to placebo/control group (isocaloric, maltodextrins) How many finish the study on each arm? all participants in both groups Mean age: 37 years in treatment group, 39 years in control group Mean total burn surface area (TBSA): 32% Inclusion criteria: 20% to 60% TBSA Exclusion criteria: pulmonary burns; septicaemia; hepatic, renal or cardiac failure; diabetes; pregnancy |
|
| Interventions | After shock resuscitation and when digestive capacity had recovered (intestinal sounds present) All participants received NG (n = 23 treated and n = 22 placebo) or oral enteral feed (n = 8 treated and n = 7 placebo) Experimental OKG group (n = 31): received 20 grams ornithine α‐ketoglutarate as 2 boluses of 10 g twice daily Control (n = 29): received isocaloric placebo as 2 × 10 grams maltodextrins |
|
| Outcomes | Infection incidence: Local infectious Systemic infectious |
|
| Notes | Graft quality and wound healing measures not reliable, no data extracted Other outcomes (biochemical): nitrogenated balance, plasmatic TTR, RBP, graft quality, weight, quality of wound healing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported: "after blindness removal" mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. Not clear whether there were exclusions after randomisation |
| Other bias | High risk | Not reported |
Garrel 1995.
| Methods | Design: randomised controlled trial, 3 arms: control, low fat (not used on this SR), low fat with fish oil. Multi‐centre or single‐centre: single‐centre. Period: September 1990 to September 1994. Sample size calculation: not reported. Generation of allocation: random numbers table. Allocation concealment: individual responsible for participant's inclusion was not aware in advance to which group the participant would be assigned. Blinded assessment of treatment allocation: Nursing staff and surgeons were not aware of the types of nutritional support received by participants, nor did they know whether or not a participant was receiving the study intervention. Not stated whether different treatments were identical. Withdrawals: 6 of 43 participants died before completion of the trial and were excluded from analysis. Intention‐to‐treat analysis: not done. Follow‐up: unclear; apparently 25 days | |
| Participants |
How many enter the study on each arm? 43 participants: control 16; LF1 (not omega‐3) 14; LF2 (fish oil) 13 How many finish the study on each arm? 37 participants: control 13 (11 M, 2 F); LF1 12 (9 M,3 F); LF2 12 (9 M, 3 F) Mean age: control 39.8 years; experimental (LF2) 36.3 years Mean TBSA: control 39%; experimental (LF2) 39% Inclusion criteria: thermal burns > 20% TBSA (not including 1st‐degree burn), admitted within 24 hours after injury Exclusion criteria: age > 65 years old, BMI > 30, diabetes, chronic visceral insufficiency, long‐term use of alcohol or cocaine |
|
| Interventions |
Experimental (LF2) (n = 12): 15% fat (50% fish oil); 60% carbohydrate; 25% protein Control (n = 13): 35% fat; 40% carbohydrate; 25% protein |
|
| Outcomes |
Pneumonia: positive sputum culture, use of ABx and radiological findings LOS: “length of care”: time required for participants' wounds to heal (grafted and ungrafted) Mortality |
|
| Notes | Other outcomes: biochemical changes, WCC, sepsis score, amount of insulin received, ARDS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Individual responsible for participants' inclusion was not aware in advance to which group participants would be assigned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Nursing staff and surgeons were not aware of the types of nutritional support received by participants, nor did they know whether or not participants were receiving the study intervention. Not stated whether different treatments were identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants died and were not evaluated |
| Other bias | Unclear risk | Unclear whether ITT was applied |
Garrel 2003.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: July 1998 to May 2001. Sample size calculation: not reported. Generation of allocation: randomisation by random numbers tables stratified by TBSA: 2% to 40%/40% to 60%/60% to 80%. Allocation concealment: Investigator was blinded to randomisation sequence. Blinded assessment of treatment allocation: not reported. Withdrawals: 45 participants were randomly assigned; 3 died in the first 72 hours and 1 could not receive enteral feedings so did not receive glutamine. These 4 participants were included only in the mortality analysis and were not included for other outcomes. Intention‐to‐treat analysis: used for mortality analysis but not for other outcomes. Follow‐up: not reported | |
| Participants | 45 recruited; 45 analysed for mortality data and 41 for other outcomes How many enter the study on each arm? 45 participants: 4 excluded (2 control, 2 glutamine); control 22 (21 M,1 F); glutamine 19 (16 M, 3 F) How many finish the study on each arm? 41 participants: control 22 (21 M,1 F); glutamine 19 (16 M,3 F) Mean age: control 38 years; glutamine 39 years Mean total burn surface area (TBSA): control 42%; glutamine 40% Inclusion criteria: 45 adult patients admitted to the burn centre within 24 hours of thermal injury who sustained 2nd‐ or 3rd‐degree burns to at least 20% but less than 80% of total body surface area Exclusion criteria: age > 65; total body surface area burn > 80%; pregnancy; chronic illness: respiratory, cardiac or renal insufficiency; cancer |
|
| Interventions | Before 48 hours of admission by nasoenteral tube: Experimental (n = 19): glutamine (Cambridge Neutraceutical, Boston, MA) 4.3 grams 4 times a day (26 grams/d) Control (n = 22): isonitrogenous mixture of aspartic acid, asparagine and glycine All participants received nasoenteral feeding by the following formula: 15% fat, 20% protein, 65% carbohydrate (Sandosource, Novartis, Minneapolis, MN) with no immunonutrient. Total energy requirements were calculated using the Curreri formula and were adjusted for measurement of energy expenditure by indirect calorimetry |
|
| Outcomes |
Overall mortality: ITT, per‐protocol LOS: “length of care”: time required for participant's wounds to heal (grafted and ungrafted) Positive blood culture: unspecified definition Number of participants with gram‐negative bacteria Wound infection: wound swab |
|
| Notes | Other outcomes (biochemical): phagocytosis by blood polymorphonuclear cells, IL‐6, IL2Ra, serum glutamine Erratum (2004 reference) taken into count. Data extracted from 2003 article Number of ventilated participants, number of days with mechanical ventilation, number of participants with LIS < 5, number of days with LIS < 5, LOC days, LOC days/TBSA, number of deaths (ITT), number of blood cultures per participant, number of participants with PBC, number of participants with PBC + Pseudomonas aeruginosa, number of participants with > 2 days of PBC, number of PBC days/participant, % of study time with > 3 antibiotics, number of participants with wound infection |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers tables stratified by burn severity |
| Allocation concealment (selection bias) | Low risk | Randomization sequence concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not stated in Methods section |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis when possible |
| Other bias | Low risk | |
Gottschlich 1990.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: multi‐centre (Cincinnati Shriners Burns Institute or University Hospital). Period: April 6, 1986, to May 20, 1988. Sample size calculation: not specified. Generation of allocation: random numbers table stratified for age, institution and burn size. Allocation concealment: not reported. Blinded assessment of treatment allocation: Tube feeding was blinded in such a fashion that all physicians, nurses, laboratory technicians and other clinical and research personnel were not aware to which group participants were assigned. Withdrawals: none reported. Intention‐to‐treat analysis: none reported (no withdrawals). Follow‐up: not reported | |
| Participants | 50 participants Group 1 control: 14 (11 male, 1 female); group 2 experimental: 17 (14 male, 3 female); group 3 control: 19 (12 male, 7 female) Mean age: group 1, 15.1 years; group 2, 21.3 years; group 3, 21.3 years Mean TBSA: group 1, 38.3%; group 2, 45.0%; group 3, 38.6% Inclusion criteria: acutely burned patients (> 3 years old), burns > 10% TBSA, admitted within 5 days of burn injury |
|
| Interventions |
Control 1 (group 1): Enteral Osmolite/Promix + Nutrisource Vitamins Experimental (group 2): modular tube feeding recipe; linoleic acid–restricted formulation Protein: Whey 87%; arginine 9%; cysteine 2%; histidine 2% Fats: 50% fish oil (omega‐3 fatty acids); 50% safflower oil Control 2 (group 3): Traumacal (1 kcal/mL) + Nutrisource Vitamins All groups: additional 5000 IU of vitamin A per 1000 mL. All participants also received daily oral supplements of 1 gram vitamin C and 220 mg heptahydrous zinc sulfate (46 mg elemental zinc) |
|
| Outcomes |
Overall mortality and causes Hospital LOS (days/%burn) Total infectious episodes: bacteraemia + wound infection + pneumonia Clinical sepsis: presence of 3 of the following events: positive blood culture, disorientation, ileus, hypotension, increased fluid requirement, decreased urine output, leukopaenia, hypothermia, metabolic acidosis, thrombocytopenia, decreased progression of wound healing, pulmonary failure, renal failure, hepatic dysfunction, stress ulceration, tachypnoea or tachycardia Bacteraemia: required positive blood culture and clinical need for antibiotic therapy Wound infection: positive wound culture greater than 105 CFU/g tissue, systemic antibiotic treatment or significant graft loss Pneumonia: positive sputum culture with consistent radiographic changes upon chest x‐ray and systemic antibiotic therapy |
|
| Notes | Other outcomes: tolerance of tube feeding, days requiring antibiotics, number of ventilator days, number of surgeries, days requiring albumin infusion, biochemical measures, weight | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table stratified for age, institution and burn size |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Tube feeding was blinded in such a fashion that all physicians, nurses, laboratory technicians and other clinical and research personnel were not aware to which group participants were assigned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals from study. 50 participants included and analysed but not clearly specified |
| Other bias | Unclear risk | Sample size calculation not reported, ITT not reported, follow‐up not reported |
Lu 2004.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: not reported. Sample size calculation: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinded assessment of treatment allocation: not reported. Withdrawals: no withdrawals. Intention‐to‐treat analysis: not reported. Follow‐up: not reported | |
| Participants |
How many enter the study on each arm? all together 60 participants (36 men + 24 women): glutamine alone 20, glutamine + rhGH 20, control 20 How many finish the study on each arm? all finished on each arm Mean age: glutamine alone 39.2 ± 12.9 years; glutamine + rhGH 38.2 ± 10.8 years; control 38.4 ± 11.4 years Mean total burn surface area (TBSA): Glutamine alone 62.9 ± 12.7% with 33.6 ± 7.6% TBSA, 3rd‐degree burns Glutamine + rhGH 61.2 ± 14.9% with 32.9 ± 7.9% TBSA, 3rd‐degree burns Control 60.3 ± 14.8% with 32.9 ± 8.3% TBSA, 3rd‐degree burns Inclusion criteria: admission to burn unit within 2 hours post injury, no severe inhalational injury, TBSA 30% to 80%, 3rd‐degree burn > 20% TBSA, age 19 years to 65 years Exclusion criteria: See above |
|
| Interventions |
Experimental: glutamine alone (n = 20): glutamine granules 0.5 g/kg/d (oral with warm water). Treatment started on day 1 post injury and ended on day 14. 2nd‐degree and 3rd‐degree burn wounds received SD‐Ag. Most 3rd‐degree burn wounds received escharotomy and skin grafting between days 3 and 5. Participants in shock received fluid resuscitation. Venous blood samples were taken on days 1, 7, 14 and 21 post injury Glutamine + rhGH (n = 20): same as glutamine alone group but plus subcutaneous rhGH injections (0.2 U/kg/d) from day 7 to day 14 post injury Control (n = 20): Same amount of placebo (glycine) was given according to same instructions as for glutamine alone group. All other interventions were the same |
|
| Outcomes | ||
| Notes | Only glutamine alone group was used for pooled analysis as experimental group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All together 60 participants were scrutinised. All were assessed for final analysis |
| Other bias | High risk | No ITT reported; no sample size calculation reported |
Peng 2004b.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: April 2001 to January 2003. Sample size calculation: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinded assessment of treatment allocation: yes. Withdrawals: not clear. Intention‐to‐treat analysis: not reported. Follow‐up: not reported | |
| Participants | 48 adults, 30% to 75% TBSA, mean 50.95 ± 10.51% (glutamine group: 11 women and 14 men; control group: 8 women and 15 men) Mean age: 36.5 years Inclusion criteria: not reported Exclusion criteria: not clear; patients with inhalation injury or need for mechanical ventilation and any history of gastrointestinal tract, cardiovascular, liver or renal disease were excluded, apparently after they had been recruited |
|
| Interventions | All received 180 kcal/kg/d and 2 g/kg/d proteins, with non‐protein energy (kcal)‐to‐N (g) ratio 150:1 Active (n = 25): glutamine granules 0.5 g/kg/d Control (n = 23): placebo 0.5 g/kg/d |
|
| Outcomes | Clinical: LOS | |
| Notes | Other outcomes (biochemical): plasma glutamine concentration, plasma diamine oxidase activity, intestinal mucosal permeability, plasma endotoxin level | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Test and control solutions were packed at the same size, and packaging materials had the same appearance. Neither participants nor investigators knew whether the applied enteral nutrition regimen was with or without glutamine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Other bias | Unclear risk | Not stated |
Saffle 1997.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: November 1993, finish date not reported. Sample size calculation: not reported. Generation of allocation: randomisation stratified by burn size, but generation of the sequence not reported. Allocation concealment: concealed. Blinded assessment of treatment allocation: yes. Withdrawals: 1 participant. Intention‐to‐treat analysis: not reported. Follow‐up: followed until discharge; all participants completed the study; mean follow‐up 20 days | |
| Participants | 50 recruited, 49 analysed Experimental: 5 women and 20 men Control: 3 women and 21 men Mean age: 37 years Mean TBSA: 35% Inclusion criteria: patients 4 years of age or older admitted to Intermountain Burn Center for treatment of acute burns if they were predicted to require enteral nutritional support for at least 7 days, and if feedings could be started within 48 hours of injury Exclusion criteria: patients not anticipated to survive, or who had evidence of concomitant renal or liver disease or diabetes; those recently treated with cancer chemotherapy or corticosteroids; patients for whom informed consent could not be obtained. Consent was sought only after enteral feeding tubes had been placed |
|
| Interventions | Early enteral feeding Control (n = 24): Replete (Clintec, Deerfield, IL) Experimental (n = 25): Impact: omega‐3 FFAs, arginine and RNA (Sandoz Nutrition, Minneapolis, MN) |
|
| Outcomes | Clinical: mortality, LOS changes | |
| Notes | Other outcomes: biochemical changes, blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma‐glutamyltransferase, total bilirubin, serum albumin and serum transferrin, total urinary creatinine, urinary nitrogen, nitrogen balance and total nitrogen intake on the day preceding injury | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stratified by burn size: 0% to 20%, 21% to 40% and > 40% TBSA. Generation of the sequence not clear |
| Allocation concealment (selection bias) | Low risk | Randomising and dispensing of both products was controlled by the Research Pharmacy at the University of Utah Medical Center |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unlabelled bags containing appropriate products were delivered to each participant daily so that investigators, participants and burn centre staff were blinded to randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed, sensitivity analysis performed |
| Other bias | Unclear risk | Not clear whether ITT was used |
Toledo 2007.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: April 2003 to December 2004. Sample size calculation: yes. Generation of allocation: random numbers tables in blocks of 4 generated by computer. Allocation concealment: concealed to all study participants. Blinded assessment of treatment allocation: placebo for all interventions identical to active formulas. Withdrawals: no. Intention‐to‐treat analysis: yes. Follow‐up: All participants were followed until discharge from the hospital | |
| Participants | 41 recruited, 41 analysed Experimental: 2 women and 21 men Control: 1 woman and 17 men Mean age: 32.3 years Mean TBSA: 7.3% Inclusion criteria: work‐related injury caused by flame, hot liquids, steam, contact or chemical; burn area > 2% of deep partial‐thickness or full‐thickness or > 15% TBSA; between 18 and 65 years of age Exclusion criteria: electrical burn, smoke inhalation injury, refractory shock at admission, mechanical ventilation requirement at admission, serious psychiatric illness, chronic liver and kidney failure, immunodeficency, diabetes mellitus, previously malnourished, morbid obesity, > 12 hours from injury to admission, pregnancy |
|
| Interventions | All participants received standard intake with 80 g proteins and 2500 kcal Experimental (n = 23): glutamine 30 grams TID + calcium caseinate 30 grams TID + zinc 200 mg/d + multi‐vitaminic complex Control (n = 18): placebo |
|
| Outcomes | Main outcomes: LOS, weight loss, sepsis, need for enteral support, death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated in blocks of 6 |
| Allocation concealment (selection bias) | Low risk | Sealed boxes of identical appearance with non‐identifiable numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo for all interventions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All participants who entered were analysed |
| Other bias | Low risk | Intention‐to‐treat analysis performed, outcomes clearly defined |
Wibbenmeyer 2006.
| Methods |
Design: double‐blind randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: July 2002 to August 2004. Sample size calculation: not reported. Generation of allocation: not reported. Allocation concealment: consecutively numbered sealed envelopes. Blinded assessment of treatment allocation: yes, by identical products dispensed by nutrition department. Withdrawals: 3 participants. Intention‐to‐treat analysis: not reported. Follow‐up: 6 months (mortality) |
|
| Participants | 23 recruited, not clear how many were analysed for primary outcome Experimental: 3 women and 9 men Control: 2 women and 9 men Mean age: ˜ 35.6 years Mean TBSA: 35.6% Inclusion criteria: older than 18 years of age, burns > 20% TBSA, admitted to our burn unit within 12 hours of injury Exclusion criteria: participation in another clinical study, not expected to need enteral nutrition, pregnancy or lactation, allergy to seafood |
|
| Interventions | All isonitrogenous enteral intragastric feeding within 48 hours of admission Experimental (n = 12): arginine 10 + omega‐3 3.5 (Crucial, Nestle Nutrition, Glendale, CA; 1.5 kcal/mL, 94 g protein/L) Control (n = 11): arginine 1.1 + omega‐3 1.7 (ProBalance with Promix, Probalance from Nestle, Glendale, CA; ProMix R.D., Promix from Navaco Laboratories, Phoenix, AZ; to equal 1.4 kcal/mL, 94 g protein/L) Because of differences in vitamin C content, control group received supplemental vitamin C elixir through nasogastric tube. Intervention group received placebo Study formula continued until > 50% calorie requirement taken orally |
|
| Outcomes |
Time to donor site healing: by visual clinical assessment Infection: primary (detected on blood cultures with no specific source), secondary (UTI, pneumonia), wound infection (diagnosis based on Peck’s guidelines) Mortality: at 3 months and 6 months post discharge Other: weight maintenance |
|
| Notes | Other outcomes: (biochemical) C‐reactive protein, creatinine, prealbumin Supported by Nestle Study authors contacted, data for pooled analysis provided by study authors in a letter |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation for the Crucial study was done using the method of permuted blocks, with blocks of size n = 4 and n = 6. This method ensured approximately equal numbers of participants in each arm during the course of the study |
| Allocation concealment (selection bias) | Low risk | Allocation was assigned in consecutively numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only the dietician was aware of the blinding. The formula was premixed in the dietary area and was marked with participants' names and the words 'Study formula.' The study formula was placed in the unit refrigerator by dietary staff. Our unit nutritionist was the only person aware of the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants withdrawn from the study after randomisation not analysed. Study flow chart not presented; 3 participants excluded after randomisation; not clear whether the 23 included participants were analysed Note from study authors: "We looked at 26 patients for the study. One patient was withdrawn after 12 hours by the PI secondary to impending renal failure. One patient withdrew from the study (perceived dietary intolerance). One patient was removed prior to study start by the IRB because the consent had the wrong date on it. The final group was composed of 23 patients. One patient not included in wound healing analysis (FAD/intervention group) due to herpes infection at donor sites" |
| Other bias | Low risk | ITT performed, sample size calculated a priori |
Wischmeyer 2001.
| Methods | Design: prospective double‐blind randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: February 1998 to August 1999. Sample size calculation: not done. Generation of allocation: computer generated. Allocation concealment: yes. Blinded assessment of treatment allocation: yes. Withdrawals: adequately described. Intention‐to‐treat analysis: vulnerate because exclusion criteria applied after randomisation. Follow‐up: all included participants followed up for 30 days or until discharge from ICU | |
| Participants | 31 recruited, 26 analysed Glutamine group: 1 woman and 11 men Control group: 2 women and 12 men Mean age: 34.5 years Inclusion criteria: age > 2 years, admission within 72 hours of burn injury, total burn surface area > 25% Mean TBSA: ˜ 50% Exclusion criteria: severe hepatic or renal disease, pregnancy, death within 72 hours of admission, inability to obtain informed consent from patient or suitable proxy |
|
| Interventions | Both groups received standard care; feeds started within 48 hours of admission; Pro‐Balance (Nestle, IL) was standard enteral diet with no added immunonutrients. TPN available as alternative. Treatment continued for duration of ICU stay Experimental (n = 12): IV L‐glutamine (0.57 g/kg/d) given as continuous infusion over 24 hours Control (n = 14): IV isonitrogenous amino acid solution |
|
| Outcomes |
Positive blood cultures: within 30 days of admission; decision of whether to take cultures made by intensivist Antibiotic usage: within 30 days Mortality: during hospital stay Other clinical: date of first surgery, number of surgeries, LOS on ICU |
|
| Notes | Other outcomes (biochemical): transferrin, prealbumin, C‐reactive protein Around half of participants had inhalational lung injury All participants had areas of full‐thickness burn |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Randomisation generated by the pharmacist, who did not participate in participant care |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unlabelled bags containing the appropriate solution were delivered to each participant daily so that investigators, participants and burn centre staff were blinded to randomisation. The 2 solutions were identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 of 31 (16.1%) excluded after randomisation (application of exclusion criteria after randomisation) |
| Other bias | High risk | Intention‐to‐treat analysis not applied Supported in part by grant from IVONYX Home Care, Plano, TX, who also provided IV amino acid solutions |
Zhou 2002.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre.Period: not reported. Sample size calculation: not reported. Generation of allocation: random numbers table. Allocation concealment: not reported.Blinded assessment of treatment allocation: not reportedWithdrawals: no withdrawals. Intention‐to‐treat analysis: not reported.Follow‐up: not reported | |
| Participants | 30 recruited: 15 in each group Mean age: treatment group 42 ± 3.4 years; control group 36.8 ± 4.6 years Mean total burn surface area (TBSA): treatment group 66.1 ± 5.2% with 38.1 ± 6.2% TBSA, 3rd‐degree burns; control group 62.6 ± 8.1% with 36.6 ± 4.8% TBSA, 3rd‐degree burns Inclusion criteria: admission to the burn unit within 6 hours post injury, TBSA 30% to 60%, 3rd‐degree burn > 20% TBSA, age 18 to 60 years Exclusion criteria: inhalation injury |
|
| Interventions |
Experimental (n = 15): glutamine dipeptide powder 0.5 g/kg/d (oral with warm water). Treatment lasted 12 days. 2nd‐degree and 3rd‐degree burn wounds received SD‐Ag. Most 3rd‐degree burn wounds received escharotomy and skin grafting on day 5 post injury. Participants in shock received fluid resuscitation Control (n = 15): Same amount of placebo was given according to the same instructions as for the treatment group. All other interventions were the same |
|
| Outcomes | Length of stay Wound healing |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Other bias | High risk | ITT not reported, sample size calculation not reported |
Zhou 2003.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: not stated. Sample size calculation: not stated. Generation of allocation: computer generated. Allocation concealment: concealed. Blinded assessment of treatment allocation: yes. Withdrawals: 1/41 (2.4%). Intention‐to‐treat analysis: no. Follow‐up: not clearly stated | |
| Participants | 41 recruited, 40 analysed (no data on gender) Mean age: 41 years Mean TBSA: 67.5% Inclusion criteria: 18 to 50 years old, TBSA 50% to 80%, 3rd‐degree 20% to 40% Exclusion criteria: smoke inhalation injury/other respiratory injury |
|
| Interventions | Both groups received standard enteral formula Experimental (n = 20): 0.35 grams L‐glutamine/kg/d (enteral) Control (n = 20): isocaloric, isonitrogenous balanced aminoacid mix Participants had no oral food intake until PBD + 12, then received unsupplemented formula with additional food intake permitted |
|
| Outcomes |
Wound infection: clinical examination of skin graft site on PBD + 12, wound swabs Wound healing: % of wound assessed as being healed on PBD + 30 |
|
| Notes | Other outcomes: costs, plasma amino acids, gut permeability, plasma endotoxin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Feeds prepared by pharmacist, who did not take care of participants |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All study participants blinded; enteral feeding identical in colour, texture, smell and taste |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 of 41 participants not analysed (developed gastric stress ulcer PBD + 2) |
| Other bias | High risk | Intention‐to‐treat analysis not performed. Study supported in part by a grant provided by company that manufactured the glutamine |
Zhou 2004.
| Methods | Design: randomised controlled trial. Multi‐centre or single‐centre: single‐centre. Period: not stated. Sample size calculation: not stated. Generation of allocation: random numbers table. Allocation concealment: envelope. Blinded assessment of treatment allocation: yes. Withdrawals: none. Intention‐to‐treat analysis: NA. Follow‐up: to 12th postoperative day | |
| Participants |
30 recruited: 15 in each group Mean age: treatment group 34.6 ± 7.8 years; control 33.4 ± 8.1 years Mean total burn surface area (TBSA): treatment group 40.7 ± 6.8% with 19.3 ± 4.2% TBSA, 3rd‐degree burns Control: 39.1 ± 6.3% with 20.9 ± 3.9% TBSA, 3rd‐degree burns Inclusion criteria: patients with severe burns (total body surface area burns 30% to 50%; 3rd‐degree burns 15% to 25%) following major escharotomy Exclusion criteria: respiratory injury or smoke inhalation, multi‐trauma, septicaemia, diabetes, chronic renal disease, liver disease |
|
| Interventions | Participants received isocaloric and isonitrogenous parental nutrition (PN) solution for 12 days
Control participants received standard amino acid solution (Novamine, 18AA‐II, SSPC, Wuxi, China), infused at a rate of 0.2570.05 gN/kg bw/d. Total energy supply was 3575 kcal/kg bw/d. Ratio of non‐protein calories to nitrogen was 150:1 Gln group received alanyl‐glutamine dipeptide (ala‐gln) solution (Dipeptiven, Fresenius Kabi, Bad Homburg, Germany) at a dose of 0.5 g/kg bw/d corresponding to 0.35 g L‐glutamine/kg bw/d. Isonitrogenous regimen in the control group was achieved by adding corresponding amounts of nitrogen in the form of Novamine |
|
| Outcomes | Length of stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random tables |
| Allocation concealment (selection bias) | Low risk | Envelope for randomisation. Pharmacist performed randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outside look of the 3 L bags was similar; physicians, nursing staff and laboratory workers were not aware of the procedure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed. No dropouts reported |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abribat 2000 | No immunonutrition intervention; no clinical outcomes |
| Alexander 1980 | No immunonutrition intervention |
| Alpers 2006 | Not an RCT |
| Anisimova 2005 | Not an RCT |
| Barbosa 2006 | Animal study |
| Berger 1996 | No immunonutrition intervention; trace elements only (Cu, Se, Zn) |
| Berger 1998 | No immunonutrition intervention; trace elements only (Ca, Se, and Zn); not included in protocolised interventions in the systematic review |
| Berger 2003 | Not an RCT; narrative review |
| Berger 2006 | Not an RCT; narrative review |
| Berger 2007 | No immunonutrition intervention; trace elements only (Cu, Se, Zn) |
| Berger 2007a | No immunonutrition intervention |
| Bernier 1998 | Data duplicated from Garrel 2005 |
| Cai 2006 | Animal study |
| Cen 1999 | No relevant outcomes |
| Chen 1999 | Animal study |
| Chen 2001 | No relevant outcomes |
| Chen 2006 | No relevant outcomes |
| Chuntrasakul 1998a | Not an RCT; mixed population |
| Chuntrasakul 2003 | Not an RCT; mixed population |
| Cynober 1984 | Not an RCT; no outcomes |
| De Bandt 2006 | Not an RCT; narrative review |
| Demling 2000 | Not an RCT; narrative review |
| Dhanraj 1997 | No immunonutrition intervention |
| Donati 1983 | Not an RCT; narrative review |
| Dunn 2008 | No immunonutrition intervention |
| Furukawa 1997 | No burned participants |
| Garcia‐De‐Lorenzo 2005 | No immunonutrition intervention (omega‐6 fatty acid) |
| Garrel 2004 | Not an RCT; letter to editor |
| Garrel 2004a | Erratum to Garrel 2003 |
| Ge 2003 | No relevant outcomes |
| Gore 2005 | Not an RCT; not an immunonutrition study |
| Griffiths 2001 | Not an RCT; narrative review |
| Guo 2007 | Animal study |
| Hansbrough 1995 | No immunonutrition intervention |
| Herndon 1987 | No immunonutrition intervention |
| Horton 1998 | Animal study |
| Jacobs 2004 | Not an RCT; clinical practice guideline |
| Jaffin 1994 | Not an RCT; narrative review |
| Jenkins 1994 | No immunonutrition intervention |
| Jiang 2002a | Not an RCT |
| Juang 2007 | Not an RCT; retrospective case control study |
| King 1990 | No relevant outcomes |
| Krenitsky 2006 | Not an RCT; narrative review |
| Kreymann 2006 | Not an RCT |
| L‐arginine | Not an RCT |
| Le 1997 | No relevant outcomes |
| Le 1999 | Animal study |
| Leboucher 1997 | Animal study |
| Liljedahl 1982 | Not an RCT; no outcomes |
| Lu 1993 | Not an RCT |
| Lu 2003 | Animal study |
| Lu 2005 | No relevant outcomes |
| Lu 2006 | No relevant outcomes |
| Lu 2006a | Animal study |
| Manelli 1984 | No relevant outcomes |
| Manelli 1984c | Not available |
| Marin 2002 | Congress abstract; complete study published and included |
| Marin 2003 | Congress abstract; complete study published and included |
| Marin 2006 | No relevant outcomes |
| Mayes 2008 | Not an RCT |
| Melis 2004 | Not an RCT |
| Montejo 2003a | Not an RCT |
| Murakami 2007 | Animal study |
| NCT00181753 | Outcomes not relevant |
| NCT00216970 | Outcomes not relevant |
| NCT00216983 | Outcomes not relevant |
| NCT00216996 | Outcomes not relevant |
| NCT00217035 | Outcomes not relevant |
| NCT00536276 | No immunonutrition intervention |
| Ogle 1994 | Not an RCT |
| Pacifico 2005 | Not an RCT; narrative review |
| Peck 2004 | No immunonutrition intervention |
| Peng 2004 | Participants not solely burn patients |
| Peng 2004a | Participants not solely burn patients |
| Peng 2004c | No relevant outcomes |
| Peng 2005 | Participants not solely burn patients |
| Peng 2005a | Study duplicate of Peng 2004a |
| Peng 2005b | Participants not solely burn patients |
| Peng 2006 | Participants not solely burn patients |
| Peng 2006a | Study duplicate of Peng 2004a Volume 32, Issue 5, August 2006, pp 589–593 |
| Purdue 2007 | Not an RCT; narrative review |
| Rimdeika 2006 | No immunonutrition intervention |
| Saffle 2003 | Not an RCT; narrative review |
| Sheridan 2004 | Not an RCT; cross‐over study with 9 participants |
| Taylor 1999 | Not an RCT |
| Wilmore 2001 | Not an RCT; narrative review |
| Windle 2006a | Not an RCT (systematic review); references extracted |
| Wischmeyer 2005 | Not an RCT |
| Yan 2005 | No relevant outcomes |
| Yan 2007 | No relevant outcomes |
| Yu 1988 | Not an RCT; cross‐over study |
| Yu 1995 | Not an RCT; cross‐over study |
| Yu 2001 | Not an RCT |
Characteristics of studies awaiting assessment [ordered by study ID]
Badetti 1994.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | In French; translation being sought |
Blass 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Data from repeat search (2014) not yet fully analysed and incorporated into the review |
Chakravarty 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only; data on intervention and control groups not available; attempts made to contact study author (April 2014) will be followed up |
Chuntrasakul 1998b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full text not available; attempts made to contact study author (April 2014) will be followed up |
Chuntrasakul 1999.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full text not available; attempts made to contact study author (April 2014) will be followed up |
Cucereanu‐Badica 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Data from repeat search (2014) not yet fully analysed and incorporated into the review |
Hartman 1994.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full text not available |
Heyland 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Data from repeat search (2014) not yet fully analysed and incorporated into the review |
Kesey 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Data from repeat search (2014) not yet fully analysed and incorporated into the review |
Lekmanov 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Data from repeat search (2014) not yet fully analysed and incorporated into the review |
Liljedahl 1972.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full text not available |
Najmi 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Will be incorporated into next update of the review |
NCT00561210.
| Methods | Study type: interventional Study design: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Inclusion criteria: thermic burn from 20% to 80%, 15 < age < 70 years, written informed consent Exclusion criteria: diabetes mellitus, corticoid or immunosuppressive therapy, HIV, evolutive cancers, pregnancy, abdominal lesion, hepatic or renal failure |
| Interventions | Arm 1: total enteral tube feeding with Crucial for at least 14 days and at most 6 months; Apports depend on method of Curreri Arm 2: total enteral tube feeding with Sondalis HP for at least 14 days and at most 6 months. Apports depend on method of Curreri |
| Outcomes | Primary outcome measures: number of infections, number of multiple organ failures (time frame: 6 months) Secondary outcome measures: digestive tolerance, healing (time frame: 6 months) |
| Notes | clinicaltrials.gov register NCT00561210 Trial completed but results outstanding; attempts made to contact study author (April 2014) will be followed up |
Pattanshetti 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Data from repeat search (2014) not yet fully analysed and incorporated into the review |
Peng 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full text not available/foreign language study; attempts made to contact study author (April 2014) will be followed up |
Petrovskii 1969.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full text not available |
Pérez‐Bárcena 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Data from repeat search (2014) not yet fully analysed and incorporated into the review |
Sun 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Data from repeat search (2014) not yet fully analysed and incorporated into the review |
Vivic 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Data from repeat search (2014) not yet fully analysed and incorporated into the review |
Zhou 1999.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | In Chinese; attempts made to contact study author (April 2014) will be followed up |
Zhou 2002a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full text not available; attempts made to contact study author (April 2014) will be followed up |
Differences between protocol and review
Performed trial sequential analysis for mortality on glutamine intervention post hoc according to the peer review process.
Changed population of interest from “patients with severe burn injuries” to "patients of any age with a burn of any severity" because of the heterogeneity of the definition of "severe" burn injury.
Removed the following secondary outcomes: mortality due to sepsis, rates of multiple organ failure (MOF).
Combined the following secondary outcomes into "Rate of non‐wound infection": pneumonia, urinary tract infection, burn wound sepsis, central venous catheter–associated bloodstream infection.
-
Did not perform the following subgroup analyses.
Minor versus major burns (major burns are defined as burns to at least 20% of the total body surface).
Early versus delayed nutrition.
Children (birth to 18 years of age) versus adults (19 years of age or older).
Different types of immunonutrients in the experimental group.
Different types of nutrition in the control group.
Different doses of immunonutrients.
Contributions of authors
Hannah Beatrix Tan: trial screening, manuscript drafting and editing, assessing quality.
Stefan Danilla: manuscript drafting, trial screening, extracting data, assessing quality, performing statistical analysis.
Alexandra Murray: trial screening, manuscript drafting and editing.
Ramon Serra: searching grey literature, manuscript drafting, extracting data, assessing quality.
Regina El Dib: manuscript drafting, trial screening.
Tom Henderson: trial screening, manuscript drafting.
Jason Wasiak: trial screening, manuscript drafting and editing, assessing quality.
Declarations of interest
All authors: none known.
New
References
References to studies included in this review
Brown 1990 {published data only}
- Brown RO, Buonpane EA, Vehe KL, Hickerson WL, Luther RW. Comparison of modified amino acids and standard amino acids in parenteral nutrition support of thermally injured patients. Critical Care Medicine 1990;18(10):1096‐101. [DOI] [PubMed] [Google Scholar]
Coudray‐Lucas 2000 {published data only}
- Coudray‐Lucas C, Bever H, Cynober L, Bandt JP, Carsin H, Coudray‐Lucas C, et al. Ornithine alpha‐ketoglutarate improves wound healing in severe burn patients: a prospective randomized double‐blind trial versus isonitrogenous controls. Critical Care Medicine 2000;28(6):1772‐6. [DOI] [PubMed] [Google Scholar]
De Bandt 1998 {published data only}
- Bandt JP, Coudray‐Lucas C, Lioret N, Lim SK, Saizy R, Giboudeau J, et al. A randomized controlled trial of the influence of the mode of enteral ornithine alpha‐ketoglutarate administration in burn patients. The Journal of Nutrition 1998;128(3):563‐9. [DOI] [PubMed] [Google Scholar]
Donati 1999 {published data only}
- Donati L, Ziegler F, Pongelli G, Signorini MS. Nutritional and clinical efficacy of ornithine alpha‐ketoglutarate in severe burn patients. Clinical Nutrition 1999;18(5):307‐11. [DOI] [PubMed] [Google Scholar]
Garrel 1995 {published data only}
- Garrel DR, Razi M, Lariviere F, Jobin N, Naman N, Emptozbonneton A, et al. Improved clinical status and length of care with low‐fat nutrition support in burn patients. Journal of Parenteral and Enteral Nutrition 1995;19(6):482‐91. [DOI] [PubMed] [Google Scholar]
Garrel 2003 {published data only}
- Garrel D, Patenaude J, Nedelec B, Samson L, Dorais J, Champoux J, et al. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial. Critical Care Medicine 2003;31(10):2444‐9. [DOI] [PubMed] [Google Scholar]
Gottschlich 1990 {published data only}
- Gottschlich MM, Jenkins M, Warden GD, Baumer T, Havens P, Snook JT, et al. Differential effects of three enteral dietary regimens on selected outcome variables in burn patients.[see comment]. Journal of Parenteral & Enteral Nutrition 1990;14(3):225‐36. [DOI] [PubMed] [Google Scholar]
Lu 2004 {published data only}
- Lu CJ, Lin C, Xu JJ, Zhang P, Cao GZ, Hong BS. [The influence of combined supplementation of glutamine and recombinant human growth hormone on the protein metabolism in severely burned patients]. [Chinese]. Zhonghua Shao Shang Za Zhi 2004;20(4):220‐2. [PubMed] [Google Scholar]
Peng 2004b {published data only}
- Peng X, Yan H, You HY, Wang P, Wang SL. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 2004;30(2):135‐9. [DOI] [PubMed] [Google Scholar]
Saffle 1997 {published data only}
- Saffle JR, Wiebke G, Jennings K, Morris SE, Barton RG, Saffle JR, et al. Randomized trial of immune‐enhancing enteral nutrition in burn patients. Journal of Trauma‐Injury Infection & Critical Care 1997;42(5):793‐802. [DOI] [PubMed] [Google Scholar]
Toledo 2007 {published data only}
- Toledo J, Danilla S, Leniz P, Pineros J, Fernandez A, Roldan P, et al. Early immunonutrition in moderately burned patients: a prospective, double masked, randomized controlled trial. Burns 2007;33(1 Suppl):s61‐s62. [ZETOC ID: RN200522896] [Google Scholar]
Wibbenmeyer 2006 {published data only}
- Wibbenmeyer LA, Mitchell MA, Newel IM, Faucher LD, Amelon MJ, Ruffin TO, et al. Effect of a fish oil and arginine‐fortified diet in thermally injured patients. Journal of Burn Care & Research 2006;27(5):694‐702. [DOI] [PubMed] [Google Scholar]
Wischmeyer 2001 {published data only}
- Wischmeyer PE, Lynch J, Liedel J, Wolfson R, Riehm J, Gottlieb L, et al. Glutamine administration reduces gram‐negative bacteremia in severely burned patients: a prospective, randomized, double‐blind trial versus isonitrogenous control. Critical Care Medicine 2001;29(11):2075‐80. [DOI] [PubMed] [Google Scholar]
Zhou 2002 {published data only}
- Zhou Y, Sun Y, Jiang Z, He G, Yang N. [The effects of glutamine dipeptide on the improvement of endotoxemia in severely burned patients]. Zhonghua Shao Shang Za Zhi = Zhonghua Shaoshang Zazhi = Chinese Journal of Burns 2002;18(6):343‐5. [PubMed] [Google Scholar]
Zhou 2003 {published data only}
- Zhou YP, Jiang ZM, Sun YH, Wang XR, Ma EL, Wilmore D, et al. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double‐blind, controlled clinical trial. Journal of Parenteral & Enteral Nutrition 2003;27(4):241‐5. [DOI] [PubMed] [Google Scholar]
Zhou 2004 {published data only}
- Zhou YP, Jiang ZM, Sun YH, He GZ, Hong S. The effects of supplemental glutamine dipeptide on gut integrity and clinical outcome after major escharectomy in severe burns: a randomized, double‐blind, controlled clinical trial. Clinical Nutrition 2004;(Suppl 1):55‐60. [Google Scholar]
References to studies excluded from this review
Abribat 2000 {published data only}
- Abribat T, Nedelec B, Jobin N, Garrel DR, Abribat T, Nedelec B, et al. Decreased serum insulin‐like growth factor‐I in burn patients: relationship with serum insulin‐like growth factor binding protein‐3 proteolysis and the influence of lipid composition in nutritional support. Critical Care Medicine 2000;28(7):2366‐72. [DOI] [PubMed] [Google Scholar]
Alexander 1980 {published data only}
- Alexander JWM. Beneficial effects of aggressive protein feeding in severely burned children. Annals of Surgery 1980; Vol. 192, issue 4:505‐17. [DOI] [PMC free article] [PubMed]
Alpers 2006 {published data only}
Anisimova 2005 {published data only}
- Anisimova IUN, Borovskii VR, Osadchaia OI, Bychkova NG, Ratushniak VV, Klimenko LA, et al. [Effect of additional enteral feeding based on soy suspension on burn wound healing]. [Russian]. Likarska Sprava 2005;5‐6:53‐8. [PubMed] [Google Scholar]
Barbosa 2006 {published data only}
- Barbosa RCC, Guimaraes SB, Vasconcelos PRC, Chaves CR, Vasconcelos PRL. Metabolic effects of L‐alanyl‐glutamine in burned rats. Burns 2006;32(6):721‐7. [DOI] [PubMed] [Google Scholar]
Berger 1996 {published data only}
- Berger MM, Spertini F, Shenkin A, Reymond MJ, Schindler C, Tappy L, et al. Clinical, immune and metabolic effects of trace element supplement in burns: a double‐blind placebo‐controlled trial. Clinical Nutrition 1996;15(2):94‐6. [ZETOC ID: RN005778384] [DOI] [PubMed] [Google Scholar]
Berger 1998 {published data only}
- Berger MM, Spertini F, Shenkin A, Wardle C, Wiesner L, Schindler C, et al. Trace element supplementation modulates pulmonary infection rates after major burns: a double‐blind, placebo‐controlled trial. American Journal of Clinical Nutrition 1998;68(2):365‐71. [DOI] [PubMed] [Google Scholar]
Berger 2003 {published data only}
- Berger MM. Nutrients as antioxidants—effect of antioxidative trace elements and vitamins on outcome of critically ill burn and trauma patients. Aktuelle Ernahrungsmedizin 2003; Vol. 28, issue 6:376‐9.
Berger 2006 {published data only}
Berger 2007 {published data only}
- Berger MM, Baines M, Raffoul W, Benathan M, Chiolero RL, Reeves C, et al. Trace element supplementation after major burns modulates antioxidant status and clinical course by way of increased tissue trace element concentrations. The American Journal of Clinical Nutrition 2007;85(5):1293‐300. [DOI] [PubMed] [Google Scholar]
Berger 2007a {published data only}
- Berger MM, Binnert C, Chiolero RL, Taylor W, Raffoul W, Cayeux MC, et al. Trace element supplementation after major burns increases burned skin trace element concentrations and modulates local protein metabolism but not whole‐body substrate metabolism. American Journal of Clinical Nutrition 2007;85(5):1301‐6. [DOI] [PubMed] [Google Scholar]
Bernier 1998 {published data only}
- Bernier J, Jobin N, Emptoz‐Bonneton A, Pugeat MM, Garrel DR. Decreased corticosteroid‐binding globulin in burn patients: relationship with interleukin‐6 and fat in nutritional support. Critical Care Medicine 1998;26(3):452‐60. [DOI] [PubMed] [Google Scholar]
Cai 2006 {published data only}
- Cai C, Guo GH, Xu QL, Tang YZ, Liu S. [Protective effects of enteral immunonutrition on intestinal mucosa injury in burned rats]. [Chinese]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2006;18(10):609‐12. [PubMed] [Google Scholar]
Cen 1999 {published data only}
- Cen Y, Luo XS, Liu XX, Cen Y, Luo XS, Liu XX. [Effect of L‐arginine supplementation on partial‐thickness burned patients]. [Chinese]. Chung‐Kuo Hsiu Fu Chung Chien Wai Ko Tsa Chih/Chinese Journal of Reparative & Reconstructive Surgery 1999;13(4):227‐31. [PubMed] [Google Scholar]
Chen 1999 {published data only}
- Chen XL, Li YP, Cai XT, Xu WS, Lu SL, Shi JX. Dose‐effect of dietary L‐arginine supplementation on burn wound healing in rats. Chinese Medical Journal 1999;112(9):828‐31. [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen G, Xie W, Jiang H. [Clinical observation of the protective effect of oral feeding of glutamine granules on intestinal mucous membrane]. Zhonghua Shao Shang Za Zhi = Zhonghua Shaoshang Zazhi = Chinese Journal of Burns 2001;17(4):210‐1. [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen G‐X Han. Economic evaluation of early enteral nutrition in severely burned patients. Chinese Journal of Clinical Nutrition 2006; Vol. 14, issue 1:7‐10.
Chuntrasakul 1998a {published data only}
- Chuntrasakul C, Siltharm S, Sarasombath S, Sittapairochana C, Leowattana W, Chockvivatanavanit S, et al. Metabolic and immune effects of dietary arginine, glutamine and omega‐3 fatty acids supplementation in immunocompromised patients. Journal of the Medical Association of Thailand 1998;81(5):334‐43. [PubMed] [Google Scholar]
Chuntrasakul 2003 {published data only}
- Chuntrasakul C, Siltham S, Sarasombath S, Sittapairochana C, Leowattana W, Chockvivatanavanit S, et al. Comparison of a immunonutrition formula enriched arginine, glutamine and omega‐3 fatty acid, with a currently high‐enriched enteral nutrition for trauma patients. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet 2003;86(6):552‐61. [PubMed] [Google Scholar]
Cynober 1984 {published data only}
- Cynober L, Saizy R, Nguyen Dinh F, Lioret N, Giboudeau J, Cynober L, et al. Effect of enterally administered ornithine alpha‐ketoglutarate on plasma and urinary amino acid levels after burn injury. Journal of Trauma‐Injury Infection & Critical Care 1984;24(7):590‐6. [DOI] [PubMed] [Google Scholar]
De Bandt 2006 {published data only}
Demling 2000 {published data only}
Dhanraj 1997 {published data only}
- Dhanraj P, Chacko A, Mammen M, Bharathi R. Hospital‐made diet versus commercial supplement in postburn nutritional support. Burns 1997;23(6):512‐4. [DOI] [PubMed] [Google Scholar]
Donati 1983 {published data only}
- Donati L, Lazzarin A, Signorini M, Candiani P, Klinger M, Moroni M. Preliminary clinical experiences with the use of immunomodulators in burns. Journal of Trauma 1983;23(9):816‐31. [PubMed] [Google Scholar]
Dunn 2008 {published data only}
- Dunn K. The effect of enteral administration of synbiotics upon infection rates in major burns. Current Controlled Trials. [ISRCTN16675260]
Furukawa 1997 {published data only}
Garcia‐De‐Lorenzo 2005 {published data only}
- Garcia‐De‐Lorenzo A, Denia R, Atlan P, Martinez‐Ratero S, Brun A, Evard D, et al. Parenteral nutrition providing a restricted amount of linoleic acid in severely burned patients: a randomised double‐blind study of an olive oil‐based lipid emulsion v. medium/long‐chain triacylglycerols. British Journal of Nutrition 2005;94(2):221‐30. [DOI] [PubMed] [Google Scholar]
Garrel 2004 {published data only}
- Garrel D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns. Journal of Parenteral and Enteral Nutrition 2004;28(2):123. [DOI] [PubMed] [Google Scholar]
Garrel 2004a {published data only}
- Garrel D, Patenaude J, Nedelec B. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial (Vol 31, pp 2444, 2003). Critical Care Medicine 2004;32(2):621. [DOI] [PubMed] [Google Scholar]
Ge 2003 {published data only}
- Ge L, Hou GZ, Huang CB. Observation of the efficacy and safety of L‐glutamine granules in treating severe burn patients. Journal of Chongqing Medical University 2003;28(4):509‐2. [Google Scholar]
Gore 2005 {published data only}
Griffiths 2001 {published data only}
Guo 2007 {published data only}
- Guo GH, Cai C, Fan J, Zhang HY, Li GH. [The influence of early enteral immunonutrition on immunological function of body and intestine in severely scalded rats]. [Chinese]. Zhonghua Shao Shang Za Zhi 2007;23(4):257‐60. [PubMed] [Google Scholar]
Hansbrough 1995 {published data only}
- Hansbrough JF, Herndon DN, Heimbach DM, Solem LD, Gamelli RL, Tompkins RG. Accelerated healing and reduced need for grafting in pediatric patients with burns treated with arginine‐glycine‐aspartic acid peptide matrix. RGD Study Group. The Journal of Burn Care & Rehabilitation 1995;16(4):377‐87. [DOI] [PubMed] [Google Scholar]
Herndon 1987 {published data only}
- Herndon DN, Stein MD, Rutan TC, Abston S, Linares H. Failure of TPN supplementation to improve liver function, immunity, and mortality in thermally injured patients. The Journal of Trauma 1987;27(2):195‐204. [DOI] [PubMed] [Google Scholar]
Horton 1998 {published data only}
- Horton JW, White J, Maass D, Sanders B. Arginine in burn injury improves cardiac performance and prevents bacterial translocation. Journal of Applied Physiology 1998;84(2):695‐702. [DOI] [PubMed] [Google Scholar]
Jacobs 2004 {published data only}
- Jacobs DG, Jacobs DO, Kudsk KA, Moore FA, Oswanski MF, Poole GV, et al. Practice management guidelines for nutritional support of the trauma patient. Journal of Trauma 2004;57(3):660‐78. [DOI] [PubMed] [Google Scholar]
Jaffin 1994 {published data only}
- Jaffin AJW. Nutritional support of the burned patient. Problems in General Surgery 1994; Vol. 11, issue 4:590‐606.
Jenkins 1994 {published data only}
- Jenkins ME, Gottschlich MM, Warden GD. Enteral feeding during operative procedures in thermal injuries. The Journal of Burn Care & Rehabilitation 1994;15(2):199‐205. [DOI] [PubMed] [Google Scholar]
Jiang 2002a {published data only}
- Jiang H, Jiang ZM, Luo B, Shuai X, Li YP. [An appraisal of immunonutrition for clinical nutritional support with a systematic review of English and Chinese documents]. [Chinese]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2002;24(6):552‐8. [PubMed] [Google Scholar]
Juang 2007 {published data only}
- Juang P, Fish DN, Jung R, Maclaren R. Enteral glutamine supplementation in critically ill patients with burn injuries: a retrospective case‐control evaluation. Pharmacotherapy 2007;27(1):11‐9. [DOI] [PubMed] [Google Scholar]
King 1990 {published data only}
- King P, Power DM. Branched chain amino/keto acid supplementation following severe burn injury: a preliminary report. Clinical Nutrition 1990;9(4):226‐30. [DOI] [PubMed] [Google Scholar]
Krenitsky 2006 {published data only}
- Krenitsky J. Immunonutrition—Fact, fancy or folly?. Practical Gastroenterology 2006; Vol. 30, issue 5:47‐68.
Kreymann 2006 {published data only}
L‐arginine {published data only}
- Anonymous. L‐arginine: monograph. Alternative Medicine Review 2005;10(2):139‐48. [PubMed] [Google Scholar]
Le 1997 {published data only}
- Bricon T, Coudray‐Lucas C, Lioret N, Lim SK, Plassart F, Schlegel L, et al. Ornithine alpha‐ketoglutarate metabolism after enteral administration in burn patients: bolus compared with continuous infusion. American Journal of Clinical Nutrition 1997;65(2):512‐8. [DOI] [PubMed] [Google Scholar]
Le 1999 {published data only}
- Boucher J, Farges MC, Minet R, Vasson MP, Cynober L. Modulation of immune response with ornithine alpha‐ketoglutarate in burn injury: an arginine or glutamine dependency?. Nutrition 1999;15(10):773‐7. [DOI] [PubMed] [Google Scholar]
Leboucher 1997 {published data only}
- Leboucher J, Coudraylucas C, Lasnier E, Jardel A, Ekindjian OG, Cynober LA. Enteral administration of ornithine alpha‐ketoglutarate or arginine alpha‐ketoglutarate: a comparative study of their effects on glutamine pools in burn‐injured rats. Critical Care Medicine 1997;25(2):293‐8. [DOI] [PubMed] [Google Scholar]
Liljedahl 1982 {published data only}
- Liljedahl SO, Larsson J, Schildt B, Vinnars E. Metabolic studies in severe burns. Clinical features, routine biochemical analyses, nitrogen balance and metabolic rate. Acta Chirurgica Scandinavica 1982;148(5):393‐400. [PUBMED: 6817568] [PubMed] [Google Scholar]
Lu 1993 {published data only}
- Lu SL, Lu SL. [Effect of arginine supplementation on T‐lymphocyte function in burn patients] [Chinese]. Chung‐Hua Cheng Hsing Shao Shang Wai Ko Tsa Chih/Chinese Journal of Plastic Surgery & Burns 1993;9(5):368‐71. [PubMed] [Google Scholar]
Lu 2003 {published data only}
- Lu SL, Jin SW, Zhang J, Shigeo I, Hideaki S, Liao ZJ, et al. [An experimental study on the effects of postburn dietary supplementation of enhanced nutrients] [Chinese]. Zhonghua Shao Shang Za Zhi 2003;19(4):197‐201. [PubMed] [Google Scholar]
Lu 2005 {published data only}
- Lu SL, Ge K, Xie T, Jin SW, Shi JX. [Influence of L‐arginine supplementation on the plasma amino acid spectrum in burn patients] [Chinese]. Zhonghua Shao Shang Za Zhi 2005;21(4):247‐50. [PubMed] [Google Scholar]
Lu 2006 {published data only}
- Lu C‐J Yang. Effects of glutamine dipeptide on T‐lymphocyte subgroups in severely burned patients. Chinese Journal of Clinical Nutrition 2006; Vol. 14, issue 1:15‐7.
Lu 2006a {published data only}
- Lu SJ, Peng X, Zhang Y, Sun Y, You ZY. [Effects of glutamine given through different avenues on intestine mucosal barrier function in burned rats] [Chinese]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2006;18(10):619‐22. [PubMed] [Google Scholar]
Manelli 1984 {published data only}
- Manelli JC, Garabedian M. Effects on muscle and whole body protein breakdown of a branched chain amino acid enriched solution in burn patients [Effets sur la proteolyse musculaire et generale du brule d'une solution enrichie en acides amines ramifies]. Annales Francaise d'Anesthesie et de Reanimation 1984;3(4):256‐60. [DOI] [PubMed] [Google Scholar]
Manelli 1984c {published data only}
- Manelli JC, Garabedian M, Rose F, Ounis N, Houvenaeghel M. Effects of branched chain amino acids upon nitrogen loss and muscle protein breakdown in burn patients. Clinical Nutrition 1984; Vol. 2, issue (Spec Suppl).
Marin 2002 {published data only}
- Marin VB, Rodriguez‐Osiac L, Schlesinger L, Villegas J, Marcelo L, Castillo‐Duran C. Controlled study of enteral arginine‐supplementation in burned children: immunological and metabolic effect. Pediatric Research 2002;51(4):189A. [Google Scholar]
Marin 2003 {published data only}
- Marin BV, Rodriguez‐Osiac L, Castillo‐Duran C, Schlesinger L, Villegas J, Lopez M, et al. Controlled study of arginine‐supplemented diet in burn pediatric patients: immunological and metabolic effect. Pediatric Research 2003;53(5):872. [Google Scholar]
Marin 2006 {published data only}
- Marin VB, Rodriguez‐Osiac L, Schlessinger L, Villegas J, Lopez M, Castillo‐Duran C. Controlled study of enteral arginine supplementation in burned children: impact on immunologic and metabolic status. Nutrition (Burbank, Los Angeles County, CA) 2006;22(7‐8):705‐12. [DOI] [PubMed] [Google Scholar]
Mayes 2008 {published data only}
- Mayes T, Gottschlich MM, Kagan RJ, Mayes Theresa, Gottschlich Michele M, Kagan Richard J. An evaluation of the safety and efficacy of an anti‐inflammatory, pulmonary enteral formula in the treatment of pediatric burn patients with respiratory failure. Journal of Burn Care & Research 2008;29(1):82‐8. [DOI] [PubMed] [Google Scholar]
Melis 2004 {published data only}
Montejo 2003a {published data only}
Murakami 2007 {published data only}
- Murakami K, Enkhbaatar P, Yu YM, Traber LD, Cox RA, Hawkins HK, et al. L‐arginine attenuates acute lung injury after smoke inhalation and burn injury in sheep. Shock 2007;28(4):477‐83. [DOI] [PubMed] [Google Scholar]
NCT00181753 {published data only}
- NCT00181753. Study of Glutamate and Glutamine Metabolism in Burn Patients Receiving Enteral or Parenteral Nutrition. http://clinicaltrials.gov/show/NCT00181753 2009. [NCT00181753]
NCT00216970 {published data only}
- NCT00216970. Study of Arginine Metabolism and Nitric Oxide Formation in Relation to Glutamine Supply in Severely Burned Patients. http://clinicaltrials.gov/show/NCT00216970 1997. [NCT00216970]
NCT00216983 {published data only}
- NCT00216983. Proline Metabolism in Severely Burned Patients: Effect of Modulated Parenteral Feeding. http://clinicaltrials.gov/show/NCT00216983 1997. [NCT00216983]
NCT00216996 {published data only}
- NCT00216996. Study on the Effect of Glutamine Supplemented Nutrition Support on Protein and Glutamine Metabolism in Severely Burned Patients. http://clinicaltrials.gov/show/NCT00216996 1997. [NCT00216996]
NCT00217035 {published data only}
- NCT00217035. Glutamine Enriched Total Parenteral Feeding and Proline Metabolism in Severely Burned Patients. http://clinicaltrials.gov/show/NCT00217035 1997. [NCT00217035]
NCT00536276 {published data only}
- NCT00536276. Evaluation of Vitamin D Status in Children With Acute Burns (Vitamin D). http://clinicaltrials.gov/show/NCT00536276 2008. [NCT00536276]
Ogle 1994 {published data only}
- Ogle CK, Ogle JD, Mao JX, Simon J, Noel JG, Li BG, et al. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. Journal of Parenteral & Enteral Nutrition 1994 Mar‐Apr;18(2):128‐33. [DOI: ] [DOI] [PubMed] [Google Scholar]
Pacifico 2005 {published data only}
- Pacifico SLL. Glutamine supplementation: is it beneficial to critically ill children?. Revista de Nutricao 2005; Vol. 18, issue 1:95‐104.
Peck 2004 {published data only}
- Peck MD, Kessler M, Cairns BA, Chang YH, Ivanova A, Schooler W, et al. Early enteral nutrition does not decrease hypermetabolism associated with burn injury. Journal of Trauma—Injury Infection & Critical Care 2004;57(6):1143‐8. [DOI] [PubMed] [Google Scholar]
Peng 2004 {published data only}
- Peng X, You ZY, Huang XK, Zhang CQ, He GZ, Xie WG, et al. [Analysis of the therapeutic effect and the safety of glutamine granules per os in patients with severe burns and trauma] [Chinese]. Zhonghua Shao Shang Za Zhi 2004;20(4):206‐9. [PubMed] [Google Scholar]
Peng 2004a {published data only}
- Peng X, You ZY, Huang XK, Zhang SQ, He GZ, Xie WG, et al. [Effects of glutamine granules on protein metabolism in trauma patients]. Zhonghua Wai Ke Za Zhi [Chinese Journal of Surgery] 2004;42(7):406‐9. [PubMed] [Google Scholar]
Peng 2004c {published data only}
- Peng X, Yan H, You Z, Wang P, Zhao Y, Wang S. Analysis of efficacy and safety of glutamine granules in severely burned patients. Annals of Burns and Fire Disasters 2004 June;XVII(2):77‐80. [Google Scholar]
Peng 2005 {published data only}
- Peng X, You ZY, Huang XK, Zhang CQ, He GZ, Quan ZF, et al. [Effects of glutamine granules on immunofunction in trauma patients: a double‐blind randomized controlled, multi‐center clinical trail with 120 patients]. Zhonghua wai ke za zhi [Chinese journal of surgery] 2005;43(17):1123‐6. [PubMed] [Google Scholar]
Peng 2005a {published data only}
- Peng X, Yan H, You Z, Wang P, Wang S. Clinical and protein metabolic efficacy of glutamine granules‐supplemented enteral nutrition in severely burned patients. Burns : journal of the International Society for Burn Injuries 2005;31(3):342‐6. [DOI] [PubMed] [Google Scholar]
Peng 2005b {published data only}
- Peng X, You ZY, Huang XK, Zhang CQ, He GZ, Quan ZF, et al. [Effects of glutamine granules on immunofunction in trauma patients: a double‐blind randomized controlled, multi‐center clinical trail with 120 patients]. [Chinese]. Chung‐Hua Wai Ko Tsa Chih [Chinese Journal of Surgery] 2005;43(17):1123‐6. [PubMed] [Google Scholar]
Peng 2006 {published data only}
- Peng X, Yi D, Fan SZ, Liao ZJ, Yao YZ, Cong TQ, et al. [Analysis of the influence on the prognosis and safety of arginine in patients with severe trauma and burns—a multi‐center randomized double blinded, placebo controlled, clinical trail in 86 patients] [Chinese]. Zhonghua Shao Shang Za Zhi 2006;22(4):243‐6. [PubMed] [Google Scholar]
Peng 2006a {published data only}
- Peng X, Yan H, You Z, Wang P, Wang S, Peng Xi, et al. Glutamine granule‐supplemented enteral nutrition maintains immunological function in severely burned patients. Burns 2006;32(5):589‐93. [DOI] [PubMed] [Google Scholar]
Purdue 2007 {published data only}
- Purdue GF. American Burn Association Presidential Address 2006 on Nutrition: Yesterday, Today, and Tomorrow. Journal of Burn Care & Research 2007;28(1):1‐5. [DOI] [PubMed] [Google Scholar]
Rimdeika 2006 {published data only}
- Rimdeika R, Gudaviciene D, Adamonis K, Barauskas G, Pavalkis D, Endzinas Z, et al. The effectiveness of caloric value of enteral nutrition in patients with major burns. Burns 2006;32(1):83‐6. [DOI] [PubMed] [Google Scholar]
Saffle 2003 {published data only}
Sheridan 2004 {published data only}
- Sheridan RL, Prelack K, Yu YM, Lydon M, Petras L, Young VR, et al. Short‐term enteral glutamine does not enhance protein accretion in burned children: a stable isotope study. Surgery 2004;135(6):671‐8. [DOI] [PubMed] [Google Scholar]
Taylor 1999 {published data only}
- Taylor SJ. Early enhanced enteral nutrition in burned patients is associated with fewer infective complications and shorter hospital stay. Journal of Human Nutrition and Dietetics 1999;12(2):85‐91. [Google Scholar]
Wilmore 2001 {published data only}
- Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. Journal of Nutrition 2001;131(Suppl 9):2543S‐9S. [DOI] [PubMed] [Google Scholar]
Windle 2006a {published data only}
- Windle EM. Glutamine supplementation in critical illness: evidence, recommendations, and implications for clinical practice in burn care. Journal of Burn Care & Research 2006;27(6):764‐72. [DOI] [PubMed] [Google Scholar]
Wischmeyer 2005 {published data only}
Yan 2005 {published data only}
- Yan H, Peng X, Wang P, Huang YS, Wang SL. [Influence of the enteral feeding of levorotatory arginine on severely burned patients during shock stage] [Chinese]. Zhonghua Shao Shang Za Zhi 2005;21(4):251‐4. [PubMed] [Google Scholar]
Yan 2007 {published data only}
- Yan H, Peng X, Huang YS, Zhao MY, Li FY, Wang P. Effects of early enteral arginine supplementation on resuscitation of severe burn patients. Burns 2007;33(2):179‐84. [DOI] [PubMed] [Google Scholar]
Yu 1988 {published data only}
- Yu YM, Wagner DA, Walesreswski JC, Burke JF, Young VR. A kinetic study of leucine metabolism in severely burned patients. Comparison between a conventional and branched‐chain amino acid‐enriched nutritional therapy. Annals of Surgery 1988;207(4):421‐9. [PUBMED: 3128190] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 1995 {published data only}
- Yu YM, Young VR, Castillo L, Chapman TE, Tompkins RG, Ryan CM, et al. Plasma arginine and leucine kinetics and urea production rates in burn patients. Metabolism: Clinical & Experimental 1995;44(5):659‐66. [DOI] [PubMed] [Google Scholar]
Yu 2001 {published data only}
- Yu YM, Ryan CM, Castillo L, Lu XM, Beaumier L, Tompkins RG, et al. Arginine and ornithine kinetics in severely burned patients: increased rate of arginine disposal. American Journal of Physiology Endocrinology and Metabolism 2001;280(3):E509‐17. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Badetti 1994 {published data only}
- Badetti C, Cynober L, Bernini V, Garabedian M, Manelli JC, Badetti C, et al. [Nutrition proteins and muscular catabolism in severely burnt patients. Comparative effects of small peptides or free amino acids] [erratum appears in Annales Francaises d'Anesthesie et de Reanimation 1994;13(6):797] [French]. Annales Francaises d'Anesthesie et de Reanimation 1994;13(5):654‐62. [DOI] [PubMed] [Google Scholar]
Blass 2012 {published data only}
- Blass SC, Goost H, Tolba RH, Stoffel‐Wagner B, Kabir K, Burger C, et al. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: a PRCT. Clinical Nutrition 2012;4:469‐75. [DOI] [PubMed] [Google Scholar]
Chakravarty 2002 {published data only}
- Chakravarty VIM, Markandayalu M, Lakshmi V, Chacko A, Malleshi NG. Glutamine, fish oil and probiotic: anabolic and immunomodulatory nutrients in enteral food for burn patients. The American Journal of Clinical Nutrition 2002;75(2S):374S. [Google Scholar]
Chuntrasakul 1998b {published data only}
- Chuntrasakul C, Sarasonibath S, Siliharm S, Leowaltana W, Chockviwatwanich S, Sitlapairochna C, et al. A randomized controlled study of arginine, glutamine, and omega‐3 fatty acids enriched enteral diet and specialized high stressed enteral diet in immunocompromised burn patients. Thai Journal of Surgery 1998;19(1):40. [Google Scholar]
Chuntrasakul 1999 {published data only}
- Chuntrasakul C. A randomized controlled study of arginine, glutamine, and omega‐3 fatty acids enriched enteral diet and specialized high stressed enteral diet in immunocompromised injured patients. Thai Journal of Surgery 1999;20(1):25‐6. [Google Scholar]
Cucereanu‐Badica 2013 {published data only}
- Cucereanu‐Badica I, Luca I, Negres S, Grintescu I, Lascar I. The effects of intravenous glutamine supplementation in severely burned, multiple traumatized patients. Farmacia 2013;61(1):212‐9. [Google Scholar]
Hartman 1994 {published data only}
- Hartman KR, Cioffi WG, Schoene NW, Drost A, Carrougher GJ, Wright DG. Neutrophil and plasma lipids in patients with severe burns: effects of 3 fatty acid substitution in enteral feeding formulas. Blood 1994;84:136a. [Google Scholar]
Heyland 2013 {published data only}
- Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. The New England Journal of Medicine 2013;16:1489‐97. [DOI] [PubMed] [Google Scholar]
Kesey 2013 {published data only}
- Kesey J, Dissanaike S. A protocol of early aggressive acceleration of tube feeding increases ileus without perceptible benefit in severely burned patients. Journal of Burn Care & Research 2013;34(5):515‐20. [DOI] [PubMed] [Google Scholar]
Lekmanov 2013 {published data only}
- Lekmanov AU, Erpuleva UV, Zolkina IV, Rossaus PA. Study of glutamine solution use efficiency in pediatric patients with heavy thermic burns and concomitant injuries in the intensive care unit. Anesteziologiia i Reanimatologiia 2013;1:49‐51. [PubMed] [Google Scholar]
Liljedahl 1972 {published data only}
- Liljedahl SO. Treatment of burns at the Karolinska Hospital. Int Z Vitam Ernahrungsforsch Beih 1972;12:184‐96. [PUBMED: 4199448] [PubMed] [Google Scholar]
Najmi 2014 {published data only}
NCT00561210 {published data only}
- NCT00561210. Effect of Early Enteral Tube Feeding Nutrition With an Immune Enhancing Diet in Severe Burn Patients. clinicaltrials.gov/show/NCT00561210.
Pattanshetti 2009 {published data only}
- Pattanshetti VM, Powar RS, Godhi AS, Metgud SC. Enteral glutamine supplementation reducing infectious morbidity in burns patients: a randomised controlled trial. Indian Journal of Surgery 2009;71(4):193‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2001 {published data only}
- Peng X, Wang FJ, You ZY, Jie WG. Random pairs and double‐blind study of glutamine on protein metabolism in severe burn. Acta Academiae Medicinae Militaris Tertiae 2001;23(12):1393‐5. [Google Scholar]
Pérez‐Bárcena 2012 {published data only}
- Pérez‐Bárcena J, Marsé P, Cervera M, Frontera G, Llompart‐Pou JA, Raurich JM, et al. Efficacy of the dipeptide N(2)‐L‐Alanyl‐L‐glutamine in traumatic patients admitted to the ICU: a prospective, randomized, double‐blind, multicentre study. Nutrición Hospitalaria 2012;1:116‐22. [DOI] [PubMed] [Google Scholar]
Petrovskii 1969 {published data only}
- Petrovskii KS, Bedulevich TS, Lipatkina LA. Free amino acids in the blood serum of burn patients and changes in their content under the effect of glutamic acid. Vopr Pitan 1969;28(3):12‐7. [PUBMED: 5367258] [PubMed] [Google Scholar]
Sun 2013 {published data only}
- Sun Y, Wang LX, Zhou YF, Sun SG, Mao XF, Deng XD, et al. Effects of glutamine combined with ulinastatin on inflammatory response of patients with severe burn injury. Zhonghua Shao Shang Za Zhi 2013;29(4):349‐54. [PubMed] [Google Scholar]
Vivic 2013 {published data only}
- Vicic VK, Radman M, Kovacic V. Early initiation of enteral nutrition improves outcomes in burn disease. Asia Pacific Journal of Clinical Nutrition 2013;22(4):543‐7. [DOI] [PubMed] [Google Scholar]
Zhou 1999 {published data only}
- Zhou Y, Jiang Z, Sun Y. [Glutamine dipeptide enriched enteral nutrition improving gut permeability in severe burns]. Zhonghua Yi Xue Za Zhi 1999;79(11):825‐7. [PubMed] [Google Scholar]
Zhou 2002a {published data only}
- Zhou YP, Liu W, Jiang ZM, Liu YW. Glutamine dipeptide enriched enteral nutrition on gut barrier function in severe burns. Journal of Parenteral and Enteral Nutrition 2002;23(5):S160. [Google Scholar]
Additional references
Beale 1999
- Beale RJ, Bryg DJ, Bihari DJ. Immunonutrition in the critically ill: a systematic review of clinical outcome. Critical Care Medicine 1999;27(12):2799‐805. [DOI] [PubMed] [Google Scholar]
Bone 1996
- Bone RC. A personal experience with SIRS and MODS. Critical Care Medicine 1996;24(8):1417–8. [DOI] [PubMed] [Google Scholar]
Bone 1996a
- Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Critical Care Medicine 1996;24(7):1125–8. [DOI] [PubMed] [Google Scholar]
Bower 1986
- Bower RH, Muggia‐Sullam M, Vallgren S, Hurst JM, Kern KA, LaFrance R, et al. Branched chain amino acid‐enriched solutions in the septic patient. A randomized, prospective trial. Annals of Surgery 1986;203(1):13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calder 2003
- Calder PC. Immunonutrition. BMJ 2003;327(7407):117‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calder 2006
- Calder PC. Branched‐chain amino acids and immunity. The Journal of Nutrition 2006;136(1):288S‐93S. [DOI] [PubMed] [Google Scholar]
Chiarelli 1990
- Chiarelli A, Enzi G, Casadei A, Baggio B, Valerio A, Mazzoleni F. Very early nutrition supplementation in burned patients. American Journal of Clinical Nutrition 1990;51(6):1035‐9. [DOI] [PubMed] [Google Scholar]
Choudhry 2003
- Choudhry MA, Haque F, Khan M, Fazal N, Al‐Ghoul W, Ravindranath T, et al. Enteral nutritional supplementation prevents mesenteric lymph node T‐cell suppression in burn injury. Critical Care Medicine 2003;31(6):1764‐70. [DOI] [PubMed] [Google Scholar]
Cynober 1984
- Cynober L, Vaubourdolle M, Dore A, Giboudeau J. Kinetics and metabolic effects of orally administered ornithine alpha‐ketoglutarate in healthy subjects fed with a standardized regimen. American Journal of Clinical Nutrition 1984;39(4):514‐9. [DOI] [PubMed] [Google Scholar]
Danilla 2009
- Danilla S, Wasiak J, Searle S, Arriagada C, Pedreros C, Cleland H, et al. Methodological quality of randomised controlled trials in burns care. A systematic review. Burns 2009;35(7):956‐61. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Heyland 2001
- Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001;286(8):944‐53. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. www.cochrane‐handbook.org.
Inaba 2005
- Inaba R, Mirbod SM, Sugiura H. Effects of Maharishi Amrit Kalash 5 as an Ayurvedic herbal food supplement on immune functions in aged mice. BMC Complementary and Alternative Medicine 2005;25:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeschke 2008
- Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Annals of Surgery 2008;248:387‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2002
- Jiang H, Jiang ZM, Luo B, Shuai X, Li YP. [An appraisal of immunonutrition for clinical nutritional support with a systematic review of English and Chinese documents]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2002;24(6):552‐8. [PubMed] [Google Scholar]
Kurmis 2010
- Kurmis R, Parker A, Greenwood J. The use of immunonutrition in burn injury care: where are we?. Journal of Burn Care & Research 2010;31:677‐91. [DOI: 10.1097/BCR.0b013e3181eebf01] [DOI] [PubMed] [Google Scholar]
Lam 2008
- Lam NN, Tien NG, Khoa CM. Early enteral feeding for burned patients: an effective method which should be encouraged in developing countries. Burns 2008;34:192‐6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Li 1995
- Li X, Xiang J, Liao Z. [Statistical analysis of 4,547 burn patients and the mortality in different periods]. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 1995;11(5):335‐8. [PubMed] [Google Scholar]
Mayer 2008
- Mayer K, Seeger W. Fish oil in critical illness. Current Opinion in Clinical Nutrition and Metabolic Care 2008;11:121‐7. [DOI] [PubMed] [Google Scholar]
Merrell 1989
- Merrell SW, Saffle JR, Larson CM, Sullivan JJ. The declining incidence of fatal sepsis following thermal injury. The Journal of Trauma 1989;29(10):1362‐6. [DOI] [PubMed] [Google Scholar]
Mochizuki 1984
- Mochizuki H, Trocki O, Dominioni L, Brackett KA, Joffe SN, Alexander JW. Mechanism of prevention of postburn hypermetabolism an catabolism by early enteral feeding. Annals of Surgery 1984;200:297‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montejo 2003
- Montejo JC, Zarazaga A, Lopez‐Martinez J, Urrutia G, Roque M, Blesa AL, et al. Immunonutrition in the intensive care unit. A systematic review and consensus statement. Clinical Nutrition 2003;22(3):221‐33. [DOI] [PubMed] [Google Scholar]
Peter 2005
- Peter JV, Moran JL, Phillips‐Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Critical Care Medicine 2005;33(1):213‐20. [PUBMED: 15644672] [DOI] [PubMed] [Google Scholar]
Preiser 2003
- Preiser JC, Wernerman J. Glutamine, a life‐saving nutrient, but why?. Critical Care Medicine 2003;31(10):2555‐6. [DOI] [PubMed] [Google Scholar]
Prelack 2007
- Prelack K, Dylewski M, Sheridan RL. Practical guidelines for nutritional management of burn injury and recovery. Burns 2007;33(1):14‐24. [DOI] [PubMed] [Google Scholar]
Rousseau 2013
- Rousseau AF, Losser MR, Ichai C, Berger MM. ESPEN endorsed recommendations: nutritional therapy in major burns. Clinical Nutrition 2013;32(4):497‐502. [DOI] [PubMed] [Google Scholar]
Schloerb 2001
- Schloerb P. Immune‐enhancing diets: products, components and their rationales. Journal of Parenteral and Enteral Nutrition 2001;25(2):S3‐7. [DOI] [PubMed] [Google Scholar]
Schneid 2003
- Schneid C, Bandt JP, Cynober L, Torres E, Reach G, Darquy S. In vivo induction of insulin secretion by ornithine alpha‐ketoglutarate: involvement of nitric oxide and glutamine. Metabolism 2003;52(3):344‐50. [DOI] [PubMed] [Google Scholar]
Seth 2004
- Seth SD, Sharma B. Medicinal plants in India. Indian Journal of Medical Research 2004;120(1):9‐11. [PubMed] [Google Scholar]
Sharma 2006
- Sharma BR, Harish D, Singh VP, Bangar S. Septicemia as a cause of death in burns: an autopsy study. Burns 2006;32(5):545‐9. [DOI] [PubMed] [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random.
Tang 1999
- Tang H, Zhaofan X, Liu S, Chen Y, Ge S. The experience in the treatment of patients with extensive full‐thickness burns. Burns 1999;25(8):757‐9. [DOI] [PubMed] [Google Scholar]
Venter 2007
- Venter M, Rode H, Sive A, Visser M. Enteral resuscitation and early enteral feeding in children with major burns effect on McFarlane response to stress. Burns 2007;33:464‐71. [DOI] [PubMed] [Google Scholar]
WHO 2014a
- World Health Organization. Burns Factsheet. www.who.int/mediacentre/factsheets/fs365/en (updated April 2014).
WHO 2014b
- World Health Organization. Global Health Observatory Data (2011 data). http://apps.who.int/gho/data/node.main.GHECOD?lang=en (accessed 19 April 2014).
Windle 2006
- Windle EM. Glutamine supplementation in critical illness: evidence, recommendations, and implications for clinical practice in burn care. Journal of Burn Care & Research 2006;27(6):764‐72. [DOI] [PubMed] [Google Scholar]
Wischmeyer 2006
- Wischmeyer PE. The glutamine story: where are we now?. Current Opinion in Critical Care 2006;12(2):142‐8. [DOI] [PubMed] [Google Scholar]
Yurt 2001
- Yurt R. Burns. In: Norton JA, Barie PS, Bollinger RR, Chang AE, Lowry SF, Mulvihill SJ, et al. editor(s). Surgery: Basic Science and Clinical Evidence. New York: Springer‐Verlag, 2001:327‐40. [Google Scholar]


