Abstract
Background
Honey is a viscous, supersaturated sugar solution derived from nectar gathered and modified by the honeybee, Apis mellifera. Honey has been used since ancient times as a remedy in wound care. Evidence from animal studies and some trials has suggested that honey may accelerate wound healing.
Objectives
The objective of this review was to assess the effects of honey compared with alternative wound dressings and topical treatments on the of healing of acute (e.g. burns, lacerations) and/or chronic (e.g. venous ulcers) wounds.
Search methods
For this update of the review we searched the Cochrane Wounds Group Specialised Register (searched 15 October 2014); The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 9); Ovid MEDLINE (1946 to October Week 1 2014); Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations 13 October 2014); Ovid EMBASE (1974 to 13 October 2014); and EBSCO CINAHL (1982 to 15 October 2014).
Selection criteria
Randomised and quasi‐randomised trials that evaluated honey as a treatment for any sort of acute or chronic wound were sought. There was no restriction in terms of source, date of publication or language. Wound healing was the primary endpoint.
Data collection and analysis
Data from eligible trials were extracted and summarised by one review author, using a data extraction sheet, and independently verified by a second review author. All data have been subsequently checked by two more authors.
Main results
We identified 26 eligible trials (total of 3011 participants). Three trials evaluated the effects of honey in minor acute wounds, 11 trials evaluated honey in burns, 10 trials recruited people with different chronic wounds including two in people with venous leg ulcers, two trials in people with diabetic foot ulcers and single trials in infected post‐operative wounds, pressure injuries, cutaneous Leishmaniasis and Fournier's gangrene. Two trials recruited a mixed population of people with acute and chronic wounds. The quality of the evidence varied between different comparisons and outcomes. We mainly downgraded the quality of evidence for risk of bias, imprecision and, in a few cases, inconsistency.
There is high quality evidence (2 trials, n=992) that honey dressings heal partial thickness burns more quickly than conventional dressings (WMD ‐4.68 days, 95%CI ‐5.09 to ‐4.28) but it is unclear if there is a difference in rates of adverse events (very low quality evidence) or infection (low quality evidence).
There is very low quality evidence (4 trials, n=332) that burns treated with honey heal more quickly than those treated with silver sulfadiazine (SSD) (WMD ‐5.12 days, 95%CI ‐9.51 to ‐0.73) and high quality evidence from 6 trials (n=462) that there is no difference in overall risk of healing within 6 weeks for honey compared with SSD (RR 1.00, 95% CI 0.98 to 1.02) but a reduction in the overall risk of adverse events with honey relative to SSD. There is low quality evidence (1 trial, n=50) that early excision and grafting heals partial and full thickness burns more quickly than honey followed by grafting as necessary (WMD 13.6 days, 95%CI 9.82 to 17.38).
There is low quality evidence (2 trials, different comparators, n=140) that honey heals a mixed population of acute and chronic wounds more quickly than SSD or sugar dressings.
Honey healed infected post‐operative wounds more quickly than antiseptic washes followed by gauze and was associated with fewer adverse events (1 trial, n=50, moderate quality evidence, RR of healing 1.69, 95%CI 1.10 to 2.61); healed pressure ulcers more quickly than saline soaks (1 trial, n= 40, very low quality evidence, RR 1.41, 95%CI 1.05 to 1.90), and healed Fournier’s gangrene more quickly than Eusol soaks (1 trial, n=30, very low quality evidence, WMD ‐8.00 days, 95%CI ‐6.08 to ‐9.92 days).
The effects of honey relative to comparators are unclear for: venous leg ulcers (2 trials, n= 476, low quality evidence); minor acute wounds (3 trials, n=213, very low quality evidence); diabetic foot ulcers (2 trials, n=93, low quality evidence); Leishmaniasis (1 trial, n=100, low quality evidence); mixed chronic wounds (2 trials, n=150, low quality evidence).
Authors' conclusions
It is difficult to draw overall conclusions regarding the effects of honey as a topical treatment for wounds due to the heterogeneous nature of the patient populations and comparators studied and the mostly low quality of the evidence. The quality of the evidence was mainly downgraded for risk of bias and imprecision. Honey appears to heal partial thickness burns more quickly than conventional treatment (which included polyurethane film, paraffin gauze, soframycin‐impregnated gauze, sterile linen and leaving the burns exposed) and infected post‐operative wounds more quickly than antiseptics and gauze. Beyond these comparisons any evidence for differences in the effects of honey and comparators is of low or very low quality and does not form a robust basis for decision making.
Keywords: Humans; Honey; Wound Healing; Administration, Topical; Apitherapy; Apitherapy/methods; Burns; Burns/therapy; Leg Ulcer; Leg Ulcer/therapy; Pressure Ulcer; Pressure Ulcer/therapy; Randomized Controlled Trials as Topic; Surgical Wound Infection; Surgical Wound Infection/therapy; Varicose Ulcer; Varicose Ulcer/therapy; Wounds and Injuries; Wounds and Injuries/therapy
Plain language summary
Honey as a topical treatment for acute and chronic wounds
We reviewed the evidence about the effects of applying honey on the healing of any kind of wound. We found 26 studies involving 3011 people with many different kinds of wounds. Honey was compared with many different treatments in the included studies.
The differences in wound types and comparators make it impossible to draw overall conclusions about the effects of honey on wound healing. The evidence for most comparisons is low or very low quality. This was largely because we thought that problems with the design of some of the studies made their results unreliable and for many outcomes there was only a small amount of information available. In some cases the results of the studies varied considerably.
There is high quality evidence that honey heals partial thickness burns around 4 to 5 days more quickly than conventional dressings. There is moderate quality evidence that honey is more effective than antiseptic followed by gauze for healing wounds infected after surgical operations.
It is not clear if honey is better or worse than other treatments for burns, mixed acute and chronic wounds, pressure ulcers, Fournier's gangrene, venous leg ulcers, minor acute wounds, diabetic foot ulcers and Leishmaniasis as most of the evidence that exists is of low or very low quality.
This evidence is current up to October 2014.
Summary of findings
Summary of findings for the main comparison. Honey compared with conventional dressings for minor acute wounds.
| Honey compared with conventional dressings for minor acute wounds | ||||||
| Patient or population: patients with Minor acute wounds Settings: Any Intervention: Honey Comparison: Conventional dressings | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional dressings | Honey | |||||
| Complete healing (time to healing)(days) | The mean complete healing (time to healing) in the intervention groups was 2.26 higher (3.09 lower to 7.61 higher) | 213 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | |||
| Adverse events | Study population | RR 1.19 (0.69 to 2.05) | 82 (1 study) | ⊕⊝⊝⊝ very low1,2,4 | ||
| 357 per 1000 | 425 per 1000 (246 to 732) | |||||
| Infection | Study population | RR 0.91 (0.13 to 6.37) | 151 (3 studies) | ⊕⊝⊝⊝ very low1,2,5 | ||
| 14 per 1000 | 13 per 1000 (2 to 88) | |||||
| Costs Average dressing cost per patient | The mean cost of dressing materials per patient was 0.49 ZAR in the honey group and 12.06 ZAR in the control (hydrogel) group |
82 (1 study) | ⊕⊝⊝⊝ very low7,8,9 | |||
| Quality of Life6 | Not reported | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to risk of bias (one level): High risk of attrition bias in all three included studies 2 Downgraded due to inconsistency (one level): the patient populations and comparator interventions differed between the studies 3 Downgraded due to imprecision (two levels): The plausible range of effects extends from a three day reduction in healing time with honey up to a more than seven day extension in healing time 4 Downgraded due to imprecision (two levels): The 95% confidence interval ranges from 0.69 and 2.05 5 Downgraded due to imprecision (two levels): The relative risk of infection for honey‐treated wounds compared with conventional dressings lies somewhere between 0.13 and 6.37 6 None of the studies reported quality of life 7 ITT analysis not done; cost data from withdrawn patients not included 8 Only report cost of dressing material not other related costs e.g., nursing care, other treatments 9 Only one small study reported costs: honey a non‐proprietary product in this study
Summary of findings 2. Honey compared with conventional dressings for burns.
| Honey compared with conventional dressings for burns | ||||||
| Patient or population: patients with Burns Settings: Any Intervention: Honey Comparison: conventional dressings | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional dressings | Honey | |||||
| Complete healing (time to healing)(days) Mean time to healing Follow‐up: median 4 weeks | The mean complete healing (time to healing) in the intervention groups was 4.68 lower (5.09 to 4.28 lower) | 992 (2 studies) | ⊕⊕⊕⊕ high | |||
| Adverse events Follow‐up: median 4 weeks | Study population | RR 0.56 (0.15 to 2.06) | 992 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 206 per 1000 | 115 per 1000 (31 to 424) | |||||
| Negative wound swab Follow‐up: median 8 days | Study population | RR 1.31 (1.01 to 1.7) | 92 (1 study) | ⊕⊝⊝⊝ very low3,4 | ||
| 630 per 1000 | 826 per 1000 (637 to 1000) | |||||
| Costs5 | Not reported | Not estimable5 | N/A | N/A | ||
| Quality of life5 | Not reported | Not estimable5 | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to inconsistency (one level): High heterogeneity was detected with an I‐squared of 70% 2 Downgraded due to imprecision (two levels): The 95% confidence interval ranges from 0.15 to 2.06 3 Downgraded due to indirectness (one level). The outcome of a negative wound swab at 8 days is only a proxy for clinical infection and difficult to interpret 4 Downgraded for imprecision (two levels): this outcome is only reported for one study involving 92 participants 5 Neither study reported costs or quality of life
Summary of findings 3. Honey compared with silver sulfadiazine for burns.
| Honey compared with silver sulfadiazine for burns | ||||||
| Patient or population: patients with Burns Settings: Any Intervention: Honey Comparison: Silver sulfadiazine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silver sulfadiazine | Honey | |||||
| Complete healing Follow‐up: 4‐6 weeks | Study population | RR 1.00 (0.98 to 1.02) | 462 (6 studies) | ⊕⊕⊕⊕ high | ||
| 1000 per 1000 | 1000 per 1000 (980 to 1000) | |||||
| Mean time to complete healing (days) Follow‐up: 21‐60 days | The mean time to complete healing in the intervention groups was 5.12 lower (9.51 to 0.73 lower) | 332 (4 studies) | ⊕⊝⊝⊝ very low1,2 | |||
| Adverse events Follow‐up: 4‐6 weeks | Study population | RR 0.29 (0.2 to 0.42) | 412 (6 studies) | ⊕⊕⊕⊕ high | ||
| 413 per 1000 | 120 per 1000 (83 to 174) | |||||
| Negative wound swab Follow‐up: median 7 days | Study population | RR 3.92 (1.32 to 11.63) | 412 (5 studies) | ⊕⊝⊝⊝ very low3,4,5 | ||
| 236 per 1000 | 923 per 1000 (311 to 1000) | |||||
| Costs Cost of dressing per percent TBSA affected | The cost of dressing treatment per % TBSA affected was 0.75 PKR for honey and 10 PKR for silver sulfadiazine. | 50 (1 study) | ⊕⊕⊝⊝ low7,8 | |||
| Quality of Life6 | Not reported | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to inconsistency (two levels): Very high level of statistical heterogeneity (I squared of 93%) 2 Downgraded due to imprecision (one level): Although the direction of effect is consistently in favour of honey, the confidence interval around the mean difference ranges from a reduction in healing time of less than one day up to nearly 10 days 3 Downgraded two levels due to inconsistency: Very high level of statistical heterogeneity (I squared of 94%) 4 Downgraded due to indirectness (one level) since the outcome of a negative wound swab at 7 days is only an indirect measure of wound infection. 5 Downgraded due to imprecision (one level): The risk of a negative swab at 7 days favour honey however the confidence interval is extremely wide 6 Quality of life not reported in any of the studies 7 Only cost of dressing materials reported not other associated health care costs 8 Only one small study reported cost of materials
Summary of findings 4. Honey for venous leg ulcers.
| Honey for venous leg ulcers | ||||||
| Patient or population: patients with Venous leg ulcers Settings: Any Intervention: Honey | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Honey | |||||
| Complete healing (time to healing) Follow‐up: 12 weeks | Study population | HR 1.1 (0.8 to 1.5) | 368 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 497 per 1000 | 531 per 1000 (423 to 644) | |||||
| Complete healing (proportion wounds healed) | Study population | RR 1.15 (0.96 to 1.38) | 476 (2 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 460 per 1000 | 529 per 1000 (441 to 634) | |||||
| Adverse events | Study population | RR 1.28 (1.05 to 1.56) | 368 (1 study) | ⊕⊕⊝⊝ low1,5 | ||
| 464 per 1000 | 594 per 1000 (487 to 724) | |||||
| Infection Follow‐up: 12 weeks | Study population | RR 0.71 (0.49 to 1.04) | 476 (2 studies) | ⊕⊕⊝⊝ low6 | ||
| 221 per 1000 | 157 per 1000 (108 to 230) | |||||
| Costs Incremental cost effectiveness ratio Follow‐up: 12 weeks | The mean cost in the intervention group was 9.45 NZD lower (95%CI 39.63 NZD lower to 16.07 NZD higher)7 | 368 (1 study) | ⊕⊝⊝⊝ very low8,9 | The ICER was sensitive to the inclusion of hospitalisation costs. Hospitalisation unlikely related to treatment and when these were excluded the ICER was in favour of control. |
||
| Quality of Life SF‐36 PCS Follow‐up: 12 weeks | The mean PCS in the intervention group was 1.1 higher (95% CI 0.8 lower to 3 higher) | 368 (1 study) | ⊕⊕⊕⊝ moderate10 | |||
| Quality of Life SF‐36 MCS Follow‐up: 12 weeks | The mean MCS in the intervention groups was 0.7 higher (95% CI 1.1 lower to 2.4 higher) | 368 (1 study) | ⊕⊕⊕⊝ moderate10 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to risk of bias (one level): Unblinded outcome assessment 2 Downgraded due to imprecision (one level): The confidence interval around the estimate of the hazard ratio ranges from a 20% reduction to a 50% increase in the hazard for healing with honey 3 Downgraded due to risk of bias (one level): Neither study used blinded outcome assessment 4 Downgraded due to imprecision (one level): The result is consistent with there being no important difference between the dressings up to honey increasing the risk of healing by just over a third 5 Downgraded due to imprecision (one level): Wide confidence intervals; only one study 6 Downgraded due to risk of bias (two levels): The diagnosis of infection is partly subjective: both trials were open label 7 Including hospitalisation costs (and $11.34 (‐$2.24 to $26.25) in favour of usual care when hospitalisation costs excluded. 8 Large difference in rates of hospitalisations and therefore associated costs between arms unlikely to be related to treatments. ICER sensitive to inclusion/exclusion of hospitalisation costs 9 Large uncertainty on cost data 10 Patients not blinded to treatment
Background
Description of the condition
Acute and chronic wounds are terms in regular use in clinical practice, yet definition of these terms has received little attention. Lazarus 1994 suggested acute wounds proceed through to healing "in an orderly and timely reparative process". Orderliness refers to the healing sequence of inflammation, angiogenesis, matrix deposition, wound contraction, epithelialisation, and scar remodelling. Timeliness is subjective, but refers to a healing time that could be reasonably expected. A chronic wound is, therefore, a wound where the orderly biological progression to healing has been disrupted and healing is delayed.
Description of the intervention
Honey is a viscous, supersaturated sugar solution derived from nectar gathered and modified by the honeybee, Apis mellifera. Honey contains approximately 30% glucose, 40% fructose, 5% sucrose, and 20% water, as well as many other substances, such as amino acids, vitamins, minerals and enzymes (Sato 2000). Honey has been used in wound care since ancient times and is frequently mentioned in early pharmacopeia, although more usually as an ingredient or carrier vehicle rather than a specific treatment. Dioscorides (40‐80 CE) often mentioned honey as a vehicle for carrying therapeutic agents in de materia medicis (Riddle 1985), and Hippocrates (460‐377 BCE), who is often cited as advocating honey for wound care, simply listed it as one of many ingredients in a multitude of unguents (Adams 1939). Probably the first deliberate advocacy of honey as a wound treatment was by the anonymous author of the Edwin Smith papyrus, an Egyptian surgical text written between 2600‐2200 BCE (Breasted 1930). A dressing made from honey and plant material was also recommended for treating burns in the London Medical Papyrus written around 1325 BCE (Trevisanato 2006). Other early medical customs, including Ayurvedic (Johnson 1992), Chinese (Fu 2001) and Roman traditions (Hajar 2002), also used honey in wound care.
How the intervention might work
Between 1996 and 2006 there was a surge in interest about honey as a wound treatment, with 40 case reports or series in 875 patients published (Jull 2008). Recent research has tended to concentrate on the antibacterial activity of the many different types of honey, rather than its effect on wound healing (Molan 1999). Manuka honey, a monofloral honey derived from the Leptospermum tree in New Zealand and Australia, has been of particular interest, as it has antibacterial activity independent of the effect of honey's peroxide activity and osmolarity (Molan 2001). The substance (or substances) responsible for this non‐peroxide activity has not been definitively identified, but has been termed Unique Manuka Factor (UMF). Manuka honey with a UMF rating has an antibacterial activity equivalent to a similar percentage of phenolic acid in solution. Recent research suggests methylglyoxal is the substance responsible for the non‐peroxide activity (Mavric 2008).
There is evidence from different animal models that honey may accelerate healing (Bergman 1983; Oryan 1998; Postmes 1997). Fifteen of the 16 controlled trials in five different animal models (mice, rat, rabbit, pig, and buffalo calf) found that honey‐treated incisional and excisional wounds, and standard burns, healed faster than control wounds (Jull 2008). In addition, a systematic review of honey as a wound dressing found seven randomised trials in humans, six in burns patients and one in infected post‐operative wounds (Moore 2001). Although the poor quality of the trial reports prevented any recommendations, the findings did suggest an effect in favour of honey.
Honey may exert multiple microscopic actions on wounds. It appears to draw fluid from the underlying circulation, providing both a moist environment and topical nutrition that may enhance tissue growth (Molan 1999). Histologically, honey appears to stimulate tissue growth in animal and human controlled trials, with earlier tissue repair noted (Bergman 1983; Subrahmanyam 1998), fewer inflammatory changes (Oryan 1998; Postmes 1997), and improved epithelialisation (Oryan 1998). Macroscopically, reports have also noted the debriding action of honey (Blomfield 1973; Efem 1988; Ndayisaba 1993; Subrahmanyam 1991).
Why it is important to do this review
Honey dressings are widely available and promoted as effective wound treatments. A systematic review of the evidence is therefore warranted as a basis for clinical and policy decision making. This version comprises a substantive update.
Objectives
To assess the effects of honey compared with alternative wound dressings and topical treatments on the healing of acute (e.g. burns, lacerations) and/or chronic (e.g. venous ulcers) wounds.
The publication of the first version of this review (Jull 2008a) was preceded by a published protocol (Jull 2004).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised controlled trials were included. Quasi‐randomised controlled trials are trials which use a quasi‐random allocation strategy, such as alternate days, date of birth, or hospital number.
Types of participants
Trials involving participants of any age with an acute or chronic wound were included. For the purposes of this review an acute wound was considered to be any of the following: burns, lacerations or other skin injuries resulting from minor trauma, and minor surgical wounds healing by primary or secondary intention. Chronic wounds were considered to be: skin ulcers of any type, pressure ulcers and infected wounds healing by secondary intention.
Types of interventions
The primary intervention was any formulation of honey topically applied by any means, alone or in combination with other dressings or components, to an acute or chronic wound. Eligible comparison interventions were dressings or other topical agents applied to the wound.
Types of outcome measures
Trials had to provide data on one of the primary outcomes listed below, and the unit of analysis had to be by participant (See "Differences between protocol and review" for further information about unit of analysis issues).
Primary outcomes
Time to complete wound healing;
proportion of participants with completely healed wounds.
Secondary outcomes
Incidence of adverse events;
length of hospital stay;
change in wound size:
incidence of infection;
cost;
quality of life.
Search methods for identification of studies
Electronic searches
For the search methods used in the original version of this review see Appendix 1.
For this second update we searched the following databases for reports of eligible randomised controlled trials:
The Cochrane Wounds Group Specialised Register (searched 15 October 2014);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 9);
Ovid MEDLINE (1946 to October Week 1 2014);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations 13 October, 2014);
Ovid EMBASE (1974 to 13 October, 2014);
EBSCO CINAHL (1982 to 15 October, 2014).
The following search strategy was used in CENTRAL and adapted appropriately for other databases:
#1 MeSH descriptor Skin Ulcer explode all trees #2 MeSH descriptor Pilonidal Sinus explode all trees #3 MeSH descriptor Wounds, Penetrating explode all trees #4 MeSH descriptor Lacerations explode all trees #5 MeSH descriptor Burns explode all trees #6 MeSH descriptor Wound Infection explode all trees #7 MeSH descriptor Surgical Wound Dehiscence explode all trees #8 MeSH descriptor Bites and Stings explode all trees #9 MeSH descriptor Cicatrix explode all trees #10 ((plantar or diabetic or heel* or foot or feet or ischaemic or ischemic or venous or varicose or stasis or arterial or decubitus or pressure or skin or leg or mixed or tropical or rheumatoid or sickle cell) NEAR/5 (wound* or ulcer*)):ti,ab,kw #11 (bedsore* or (bed NEXT sore*)):ti,ab,kw #12 (pilonidal sinus* or pilonidal cyst*):ti,ab,kw #13 (cavity wound* or sinus wound*):ti,ab,kw #14 (laceration* or gunshot stab or stabbing or stabbed or bite*):ti,ab,kw #15 ("burn" or "burns" or "burned" or scald*):ti,ab,kw #16 (surg* NEAR/5 infection*):ti,ab,kw #17 (surg* NEAR/5 wound*):ti,ab,kw #18 (wound* NEAR/5 infection*):ti,ab,kw #19 (malignant wound* or experimental wound* or traumatic wound*):ti,ab,kw #20 (infusion site* or donor site* or wound site* or surgical site*):ti,ab,kw #21 (skin abscess* or skin abcess*):ti,ab,kw #22 (hypertrophic scar* or keloid*):ti,ab,kw #23 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22) #24 MeSH descriptor Honey explode all trees #25 honey:ti,ab,kw #26 (#24 OR #25) #27 (#23 AND #26)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2008). There were no restrictions with respect to language, date of publication (taking into account searches from the original review) or study setting.
Searching other resources
For the initial review we contacted experts in the field, authors of the included trials and manufacturers of honey products for wound care (Comvita NZ Ltd and MediHoney Australia Pty Ltd), but did not repeat this for updates. The bibliographies of all obtained studies and review articles were searched for potentially eligible trials for both the initial review and the first update. No language or date restrictions were applied to the trials and both published and unpublished trials were sought.
Data collection and analysis
Selection of studies
Two authors (either AJ and NW, or for this update JD and NC) independently examined titles and abstracts of potentially relevant trials. Full text copies of all relevant trials, or trials that might be relevant to the review were obtained. The two review authors independently selected the trials using the inclusion criteria. Disagreements were resolved by discussion.
Data extraction and management
Data were extracted from included trials by one review author and recorded on a standardised form. The extracted data were independently reviewed for accuracy by a second review author and disagreements resolved by discussion. All data have subsequently been verified by a third (MW) and fourth author (NC). If the data from the trial report were inadequate, or ambiguous, additional information was sought from the trial authors. We collected data on the topics listed below:
Author; title; source of reference.
Study setting.
Study design.
A priori sample size calculation; sample size.
Inclusion/exclusion criteria.
Age of participants; sex of participants.
Wound type.
Intervention and comparison.
Outcomes.
Withdrawals and reason for withdrawal.
Funding source.
Co‐interventions
Assessment of risk of bias in included studies
For the first update, one review author (SD) assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011), and this assessment was checked by a second (AJ) review author. For this second update two more pairs of review authors/editors checked risk of bias (either MW and JD or JD and NC) and reviewed by the lead review author (AJ). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues, such as extreme baseline imbalance (see Appendix 3 for details of criteria on which the judgement was based). Blinding and completeness of outcome data were assessed for each outcome separately. We completed a risk of bias table for each eligible study and discussed any disagreement amongst all review authors to achieve a consensus.
We present an assessment of risk of bias using a 'risk of bias summary figure', which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.
Data synthesis
Where trials were sufficiently alike in terms of population and comparison interventions, their results were combined. Mean differences (MD) and 95% confidence intervals (95% CI) were reported for continuous outcomes, and risk ratio (RR) and 95% confidence intervals (95% CI) were reported for dichotomous variables. Statistical heterogeneity was tested by comparing Cochran's Q statistic and the chi‐squared distribution. Heterogeneity was assumed with P values of less than 0.1 (Higgins 2011). In addition, the I2 statistic was used to determine the percentage of variation due to heterogeneity rather than chance (Higgins 2003), and any sources of heterogeneity were explored. Where significant statistical heterogeneity was present, a random‐effects model was used when combining trials (Ioannidis 2008).
Summary of findings
The evidence was summarised in summary of findings tables using the approach of the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) (Langendam 2013). This approach assesses the quality of the body of evidence per comparison and outcome, taking into account five factors: risk of bias across all studies reporting that outcome; indirectness of population, interventions and outcomes, across all studies reporting the outcome; inconsistency amongst studies; imprecision (taking into account the optimum information size and the confidence intervals) and publication bias. The results are reported below according to condition, comparison and outcome and then the different outcomes are brought together in summary of findings tables (Table 1; Table 2; Table 3; Table 4), which are discussed in the final section (Discussion).
Results
Description of studies
Included studies
Twenty six trials met the inclusion criteria (please see the Characteristics of included studies) and were available for analysis; eight trials were added in updates (Baghel 2009; Gulati 2014; Kamaratos 2014; Mashood 2006; Memon 2005; Nilforoushzadeh 2007; Robson 2009; Shukrimi 2008), including one trial that was mistakenly excluded in the previous review, but was found in the first update to meet the inclusion criteria on re‐screening (Mashood 2006). Another trial was previously wrongly included and has now been excluded in this update (Subrahmanyam 1996c). Three trials are awaiting assessment while attempts are made to contact the authors to request further data (Askarpour 2009; Jan 2012; Maghsoudi 2011).
Ten separate trials were conducted by the same investigator (Subrahmanyam 1991; Subrahmanyam 1993a; Subrahmanyam 1993b; Subrahmanyam 1994; Subrahmanyam 1996a; Subrahmanyam 1996b; Subrahmanyam 1998; Subrahmanyam 1999; Subrahmanyam 2001a; Subrahmanyam 2004) based in India. Important information was missing from the published reports of these studies however some was provided on request.
Fourteen trials recruited participants with acute wounds: 11 with burns (Baghel 2009; Mashood 2006; Memon 2005; Subrahmanyam 1991; Subrahmanyam 1993b; Subrahmanyam 1994; Subrahmanyam 1996a; Subrahmanyam 1996b; Subrahmanyam 1998; Subrahmanyam 1999; Subrahmanyam 2001a), two with minor surgical excisions (Marshall 2005; McIntosh 2006), and one with minor trauma (Ingle 2006). Ten trials recruited participants with chronic wounds including venous leg ulcers (Jull 2008; Gethin 2007), infected surgical wounds (Al Waili 1999), pressure injuries (Weheida 1991), Fournier's gangrene (Subrahmanyam 2004), cutaneous Leishmaniasis (ulcers caused by protozoans injected by sandfly bite) (Nilforoushzadeh 2007), diabetic foot ulcers (Kamaratos 2014; Shukrimi 2008), and a variety of chronic wounds healing by secondary intention though mainly venous leg ulcers (Gulati 2014; Robson 2009). Two trials recruited participants with either chronic or acute wounds (Mphande 2007; Subrahmanyam 1993a).
Eight trials were conducted in community settings or outpatient clinics (Gulati 2014; Kamaratos 2014; Gethin 2007; Ingle 2006; Jull 2008; Marshall 2005; McIntosh 2006; Nilforoushzadeh 2007). The remaining trials were conducted in hospital settings, or a mixed inpatient and outpatient setting (Robson 2009). Eight trials reported recruiting only adults (Al Waili 1999; Gulati 2014; Gethin 2007; Ingle 2006; Jull 2008; Kamaratos 2014; Shukrimi 2008; Subrahmanyam 2004). The remaining trials did not specify an age range (Marshall 2005; McIntosh 2006; Robson 2009), or recruited both children and adults.
Monofloral honey (aloe, jarrah, jamun, jambhul or manuka) was used in ten trials (Gethin 2007; Gulati 2014; Ingle 2006; Jull 2008; Kamaratos 2014; Marshall 2005; McIntosh 2006; Robson 2009; Subrahmanyam 2001a; Subrahmanyam 2004); the type of honey used was not specified in the remaining trials. Honey was delivered as a honey impregnated gauze dressing in six trials (Kamaratos 2014; Mphande 2007; Nilforoushzadeh 2007; Subrahmanyam 1993a; Subrahmanyam 1994; Subrahmanyam 2004); as a honey impregnated alginate dressing in two (Jull 2008; McIntosh 2006); as honey spread between gauze in one trial (Memon 2005) and as topical honey covered with either a film dressing (Ingle 2006; Gulati 2014) or with gauze in the remaining trials. Six trials investigated honey as an adjunct treatment: four included people with venous leg ulcers, and, for these, honey was used as an adjunct to compression (Gethin 2007; Gulati 2014; Jull 2008; Robson 2009). One trial in people with Leishmaniasis gave honey alongside an injection of glucantamine (Nilforoushzadeh 2007). In a further trial, 50% patients receiving honey also received delayed autologous skin grafting, as necessary (Subrahmanyam 1999).
There was a range of comparison treatments in this review, which have been grouped under the broad categories of conventional dressings, silver sulfadiazine (SSD), antiseptics, early excision and atypical dressings. Seven trials compared honey with SSD, and of these, the comparator was a SSD impregnated dressing in four trials (Subrahmanyam 1991; Subrahmanyam 1993b; Subrahmanyam 1998; Subrahmanyam 2001a) and SSD cream in three (Baghel 2009; Mashood 2006; Memon 2005). Two studies compared honey with hydrogel (Gethin 2007; Ingle 2006). In the adjunct trials, the comparators were either other dressings plus compression (Gethin 2007; Gulati 2014; Jull 2008; Robson 2009) or glucantamine injection alone (Nilforoushzadeh 2007). The comparator for the trial giving honey plus delayed skin grafting was early tangential excision and skin grafting (Subrahmanyam 1999).
In view of the clinical diversity of the evidence, this review is organised by wound type, and then by comparison type.
There was a great deal of variation in the outcomes reported by the included studies which makes the drawing of overall conclusions very difficult. Many of the included studies report "mean time to healing" but it was frequently not clear whether every participant's wound had healed during follow up (in which case the mean time with associated SD or 95% confidence interval would be acceptable). However if all wounds in a study do not heal during the period of observation, simple calculation of the mean (or median) time to healing without accounting for censoring is inappropriate since, by definition, this approach excludes people who did not heal during follow up (as they cannot contribute to the numerator). Importantly excluding people who failed to heal from the data excludes treatment failures and will over‐estimate treatment success. Time to healing is a type of "time to event" outcome and should be analysed as such using a survival approach which allows people who did not heal to contribute data to the analysis for the period for which they were observed. Only three studies (Jull 2008; Nilforoushzadeh 2007; Robson 2009) analysed time to healing as time to event data. One study (Shukrimi 2008) reported time to readiness for wound closure surgery.
The multiplicity of time points at which healing was reported was a further problem which had not been anticipated in the protocol. We opted to analyse the outcomes for the longest point of follow up shared by several trials of the same comparison (since to extract and analyse all time points risks a Type I error).
Excluded studies
For details on the excluded studies please see Characteristics of excluded studies table. Of the 57 excluded studies, 24 were not RCTs or quasi‐RCTs (Abdelatif 2008; Ahmed 2003; Al Waili 2004c; Al Waili 2005; Bose 1982; Dunford 2004; Freeman 2010; Gethin 2005; Lusby 2002; Marshall 2002; Mayer 2014; Misirligou 2003; Moghazy 2010; Molan 2002; Molan 2006; Mwipatayi 2004; Nagane 2004; Robson 2002; Schumacher 2004; Subrahmanyam 1993; Thurnheer 1983; Tostes 1994; Vijaya 2012; Visscher 1996). Seven of the excluded studies were not in wounds (Al Waili 2003; Albietz 2006; Biswal 2003; Johnson 2005; Quadri 1998; Quadri 1999; Somaratne 2012). Three were studies in animal models (Al Waili 2004a; Al Waili 2004b; Subrahmanyam 2001b). Thirteen studies did not report sufficient information on healing (Bangroo 2005; Chokotho 2005; Gad 1988; Heidari 2013; Jeffery 2008; Lund‐Nielsen 2011; Mat Lazim 2013; Robson 2012; Rogers 2010; Rucigaj 2006; Saha 2012; Subrahmanyam 2003; Ur‐Rehman 2013). Three studies did not evaluate honey (Berchtold 1992; Muller 1985), one evaluated the effect of adding vitamins and polyethylene glycol to honey (Subrahmanyam 1996c) and a further two could not be obtained for assessment (Calderon Espina 1989; Rivero Varona 1999). Four studies had unit of analysis issues, they had randomised, or reported, by wound rather than by the participant. Such unit of randomisation or analysis issues were not considered in the protocol for the review, but we considered that such studies could not contribute usefully to this review. For further discussion on the rationale for this decision see "Differences between protocol and review" (Malik 2010; Okeniyi 2005; Oluwatosin 2000; Yapucu Gunes 2007).
Risk of bias in included studies
Risk of bias is summarised in the figures (Figure 1; Figure 2) with judgements explained in the Characteristics of included studies. Risk of bias was a key consideration in assessing the quality of the evidence and used (and justified) in the Summary of Findings Tables to downgrade the evidence where appropriate. Overall the quality of reporting was poor and it was frequently not possible to determine whether allocation was fully concealed. Two studies (Mphande 2007; Kamaratos 2014) used quasi‐random methods of allocation and these are at high risk of selection bias as a consequence. Most of the included studies were at risk of performance bias as neither participants nor health care providers were blinded to treatment allocation. The main outcomes of complete healing, adverse events and infection have, to varying extents, an element of subjectivity inherent in them and 14 out of 26 included studies reported having used blinded outcome assessment.
1.
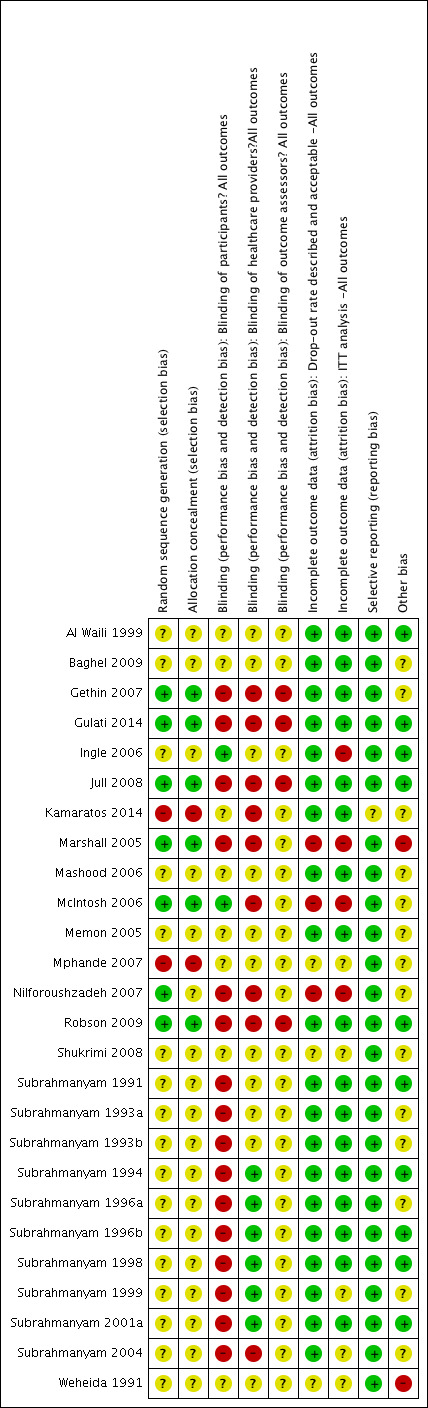
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.
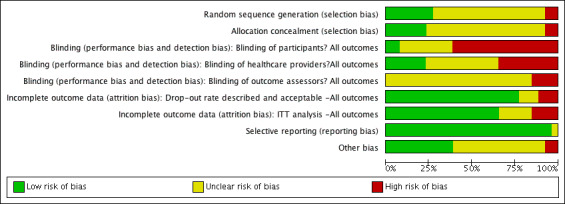
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
The 26 trials included 3011 participants. The trials were generally small (median sample size 83.5, range 30 to 900), and there was very obvious clinical and methodological heterogeneity. It was not appropriate, therefore, to combine the trials in a single meta‐analysis to produce a summary statistic for honey overall, or even subgroup summary statistics for acute, chronic, or mixed wounds. Within the subgroups (acute, mixed acute and chronic, and chronic wounds) trials have been combined in meta‐analysis where appropriate. Otherwise the trials have been summarised narratively.
In common with wounds research in general, adverse event reporting was variable in nature and often poor in quality. Five trials did not report adverse events (Baghel 2009; Kamaratos 2014; Subrahmanyam 1996a; Subrahmanyam 1998; Weheida 1991), and five trials stated that there were no adverse events or no adverse events related to treatment (Gethin 2007; Gulati 2014; Marshall 2005; McIntosh 2006; Subrahmanyam 1996b). One trial reported the number of people with any adverse event (Jull 2008), as well as itemising specific types of events. The remaining trials appear to have limited reporting of events to specific types of events, rather than encouraging reports of any event. Adverse events are presented by wound type, and the comparators indicated in footnotes and any meta‐analysis uses a random‐effects model due to the heterogeneity. Although only one trial explicitly reported frequency of events by participant (Jull 2008), it is assumed one event equals one participant in all other trials (this may be an erroneous assumption).
The "infection" data in these studies are not well reported and impossible to analyse in a robust manner and interpret reliably across all wound types. One of the main problems is the lack of a definition of infection within most trial reports and an inconsistency between trials. Most of the burns trials reported "positive" swab cultures at baseline and then the proportion rendered "sterile" at subsequent time points (usually 7 days). However a positive swab culture is NOT the same as a clinical infection (the diagnosis of the latter being dependent on signs and symptoms as well as culture). Consequently we cannot draw conclusions about the treatment effects of honey dressings and comparators from these data. We have confined our analysis of the burns studies to the outcome of "proportion of burns with negative swabs at 7 days" however this is a less clinically relevant outcome than healing, or clinical infection.
1. Acute wounds
1.1 Minor acute wounds
1.1.1 Honey compared with conventional dressings
Three trials (213 participants) recruited participants with minor acute wounds (Ingle 2006; Marshall 2005; McIntosh 2006). In two trials, the wounds were surgical wounds created after partial or total toenail avulsions (Marshall 2005; McIntosh 2006), with the control group treated with paraffin gauze in one trial and an iodophor dressing in the other. The remaining trial recruited mine workers with lacerations or shallow abrasions and treated control participants with a hydrogel (Ingle 2006).
Outcome: healing
We combined the results of the three trials using a random effects model. It is unclear whether there is a difference in time to healing between honey and control (difference in mean days to healing was 2.26 days longer with honey, 95% CI ‐3.09 to 7.61 days (Analysis 1.1). Moderate heterogeneity (I² = 47%). Very low quality evidence (downgraded for risk of bias, inconsistency and imprecision) (Table 1).
1.1. Analysis.
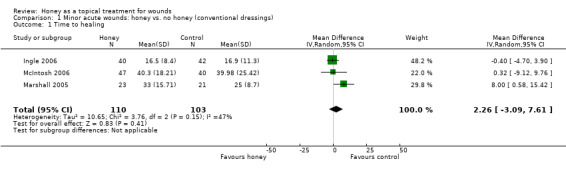
Comparison 1 Minor acute wounds: honey vs. no honey (conventional dressings), Outcome 1 Time to healing.
Outcome: adverse events
Ingle and colleagues reported the frequency of itching, burning and pain (Ingle 2006). There was no significant difference in the rates of these events between honey and hydrogel RR 1.19, 95% CI 0.69 to 2.05) (Analysis 1.2). No patient stopped treatment due to these sensations. It remains unclear as to whether there are more or fewer adverse events with honey dressings compared with non‐honey dressings in people with minor acute wounds. Very low quality evidence (downgraded for risk of bias, serious inconsistency and imprecision) (Table 1).
1.2. Analysis.
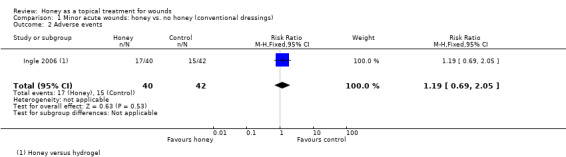
Comparison 1 Minor acute wounds: honey vs. no honey (conventional dressings), Outcome 2 Adverse events.
Outcome: infection
Infection was not reported by Ingle 2006. There was only one instance of infection reported in each of the other two trials (Marshall 2005; McIntosh 2006): in 1/27 participants in the honey group compared with 0/24 in the iodine group of Marshall 2005, and in 1/48 participants of the iodine group compared with 0/52 in the honey group of McIntosh 2006 (pooled RR of infection 0.91, 95% CI 0.13 to 6.37, fixed effect, Analysis 1.3). It is therefore unclear if honey affects rates of wound infection in minor acute wounds. Very low quality evidence (downgraded for the reasons outlined above) (Table 1).
1.3. Analysis.
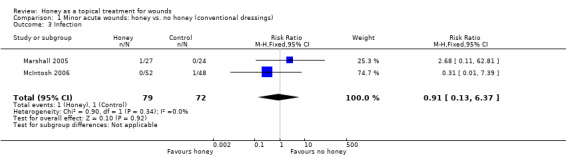
Comparison 1 Minor acute wounds: honey vs. no honey (conventional dressings), Outcome 3 Infection.
Outcome: costs
Only Ingle 2006 reported on costs, and then only in terms of average costs of dressing materials per patient in each group. These were lower in the honey group (average cost per patient 0.49 Rand) than the hydrogel group (average cost per patient 12.06 Rand) however this does not appear to have been a commercial honey preparation (hence low cost).
Outcome: quality of life
Not reported.
1.2 Burns
There were six comparison treatments, which have been grouped under the four broad categories of conventional dressings, early excision, silver sulfadiazine (SSD) and atypical dressings for this review.
1.2.1 Honey compared with conventional dressings
Two trials (992 participants) compared honey with conventional dressings for the treatment of partial‐thickness burns (Subrahmanyam 1993a; Subrahmanyam 1996a). In one trial (Subrahmanyam 1993a) the comparison was a polyurethane film dressing and in the other trial (Subrahmanyam 1996a) the control participants were treated with a range of interventions: polyurethane film (OpSite, n = 90), paraffin gauze (n = 90), sterile linen dressings (n = 90), antimicrobial impregnated gauze (Soframycin, n = 90) or left exposed (n = 90).
Outcome: healing
Mean days to healing were reported but not the standard deviations, though this information was later provided by the author (personal communication: M Subrahmanyam). The two trials were pooled using a fixed effect model (I2 = 0%). Burns treated with honey healed more quickly (WMD ‐4.68 days, 95% CI ‐5.09 to ‐4.28 days, Analysis 2.1). High quality evidence (Table 2).
2.1. Analysis.
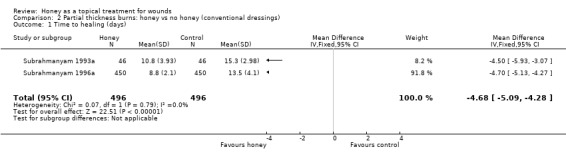
Comparison 2 Partial thickness burns: honey vs no honey (conventional dressings), Outcome 1 Time to healing (days).
Outcome: adverse events
In Subrahmanyam 1993a there were two cases of over‐granulation and two of contracture in the honey group compared with two of over‐granulation and one of contracture in the control group. In Subrahmanyam 1996a there were five cases of hypergranulation and 28 of scarring with honey and 12 of hypergranulation and 87 of scarring with conventional dressings. These data were pooled (random effects). Overall there was no clear difference in risk of adverse events (RR 0.56, 95% CI 0.15 to 2.06) (Analysis 2.2).Very low quality evidence (downgraded for inconsistency i.e., high heterogeneity, and imprecision) (Table 2).
2.2. Analysis.
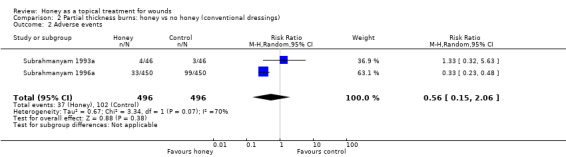
Comparison 2 Partial thickness burns: honey vs no honey (conventional dressings), Outcome 2 Adverse events.
Outcome: infection
Subrahmanyam 1996a did not report infection rates. Subrahmanyam 1993a reported a greater proportion of honey participants having a negative swab at Day 8 (38/46 participants in the honey group cf. 29/46 in the polyurethane film group), (RR of a negative swab 1.31, 95%CI 1.01 to 1.70), Analysis 2.3. Low quality evidence (downgraded for imprecision and indirectness, in that a negative swab on a particular day is not a measure of infection per se)(Table 2).
2.3. Analysis.
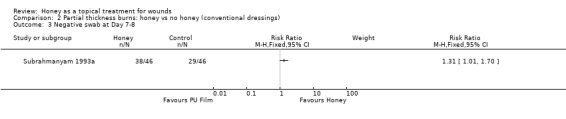
Comparison 2 Partial thickness burns: honey vs no honey (conventional dressings), Outcome 3 Negative swab at Day 7‐8.
Outcomes: costs and quality of life
Not reported.
1.2.2 Honey plus delayed grafting compared with early excision and grafting (no honey)
One trial (50 participants) compared early tangential excision and skin grafting with honey dressings plus delayed skin grafting where needed, for the treatment of mixed partial‐ and full‐thickness burns (Subrahmanyam 1999).
Outcome: healing
Mean time to healing was not published, but was later provided by the author (personal communication: M Subrahmanyam). Burns healed more slowly when treated with honey (followed by delayed grafting where needed) than with early excision and grafting (no honey) (WMD 13.6 days, 95% CI 9.82 to 17.38 days, Analysis 3.1). The quality of this evidence was downgraded for imprecision on the basis that there is only one trial with a total of 50 participants and whilst the difference in time to healing was statistically significant and clinically important, this is a small single study. We also downgraded the quality of the evidence for indirectness, since honey is not the only systematic difference in the treatments for this comparison as the burn excision and grafting interventions were also different (therefore the trial does not directly address the question of whether honey dressings are effective for burns). Low quality evidence.
3.1. Analysis.
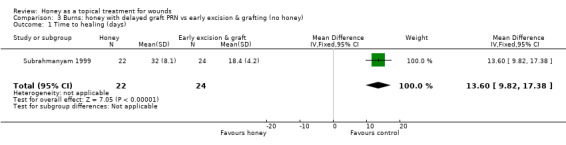
Comparison 3 Burns: honey with delayed graft PRN vs early excision & grafting (no honey), Outcome 1 Time to healing (days).
Outcome: adverse events
In Subrahmanyam 1999 there was a total of 6 adverse events in the honey group (3 deaths, 3 contractures), compared with one death in the early excision group. Low quality evidence (see above).
Outcome: infection
Infection rates per se were not reported for Subrahmanyam 1999 however days of antibiotic therapy (which is a proxy for infection), were. Participants in the honey group received a mean of 32 (SD 18) days of antibiotics compared with 16 (SD 3) in the early excision and graft group (difference in mean days of antibiotic therapy 16.00, 95% CI 8.85 to 23.15) (Analysis 3.2). Low quality evidence (downgraded two levels for indirectness since honey is not the only systematic difference between treatments and the outcome "days of antibiotic therapy" is only a proxy for infection).
3.2. Analysis.
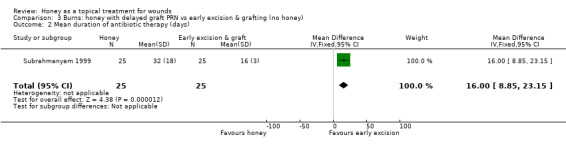
Comparison 3 Burns: honey with delayed graft PRN vs early excision & grafting (no honey), Outcome 2 Mean duration of antibiotic therapy (days).
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
1.2.3 Honey compared with silver sulfadiazine
Six trials (462 participants) compared honey with SSD (Baghel 2009; Mashood 2006; Memon 2005; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001a). The trials used different burn grading systems to report the depth of burns, which reflected the age of the trials and the lack of a clinical consensus on reporting burn depth. The burns, however, did tend towards the less severe end of the spectrum, although early staging systems (e.g. first‐, second‐ and third‐degree burns, or superficial, partial‐thickness and full‐thickness) do not have the sensitivity of more recent systems (i.e. epidermal, superficial dermal, mid‐dermal, deep dermal and full‐thickness), and participants with deep partial‐thickness and deep dermal burns are likely to require skin grafting (NZGG 2007).
Trials recruited participants with superficial and partial‐thickness burns (Baghel 2009), superficial burns (Subrahmanyam 1991; Subrahmanyam 1998), superficial, partial‐ and deep partial‐thickness burns (Mashood 2006), and one recruited participants with superficial, dermal, mid and deep dermal burns (Memon 2005). The remaining trial did not report their eligibility criteria but some of the participants had full thickness burns (Subrahmanyam 2001a).
The studies used different treatment regimens. All applied honey topically, covered with gauze. Three studies gave SSD as a topical cream covered by gauze (Baghel 2009; Mashood 2006; Memon 2005) and the rest as SSD impregnated gauze. Two studies changed both types of dressings daily (Mashood 2006; Subrahmanyam 1991); two changed both types of dressing on alternate days (Memon 2005; Subrahmanyam 2001a) and the other study did not report the frequency of dressing change (Baghel 2009). The study by Subrahmanyam 1998 changed the SSD dressings daily and the honey dressings on alternate days.
Outcome: healing
One trial reported mean time to healing and the risk of healing at different times (Subrahmanyam 2001a), and five trials reported either mean time to healing without standard deviations (Baghel 2009; Memon 2005), or risk of complete healing (Mashood 2006; Subrahmanyam 1991; Subrahmanyam 1998), but at different time points i.e., two, four, and six weeks (Mashood 2006), and seven, 10, 15, 21 and 30 days (Subrahmanyam 1998).
Additional information was sought from authors and provided by one author (personal communication: M Subrahmanyam). Baghel 2009 reported an exact p‐value for the comparison of time to healing and this was used to calculate the standard error for mean time to healing. Thus mean time to healing was used as the outcome, with the result that four trials out of six could be pooled for the outcome of mean time to healing using a random effects model (heterogeneity was extremely high, I² = 93%). In these four trials, people whose burns were treated with honey experienced an average reduction in healing time of 5 days (WMD ‐5.12 days, 95% CI ‐9.51 to ‐0.73) (Analysis 4.1). This was very low quality evidence (downgraded for inconsistency and imprecision) (Table 3).
4.1. Analysis.
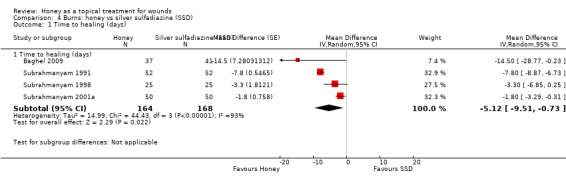
Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 1 Time to healing (days).
In the trial that did not report standard deviations with mean time to healing (Memon 2005), the honey‐treated group had a mean time to healing of 15.3 days and it was 20.0 days in the SSD group (Memon 2005). In the remaining trial (Mashood 2006), all 25 participants in the honey‐treated group were healed by four weeks, while all patients in the SSD group were healed by six weeks.
All six trials reported the risk of complete healing at either 4 or six weeks (or both) as well as several earlier time points. We wished to reduce the risk of Type I errors inherent in multiple endpoint analysis. We therefore report the pooled risk of complete healing for all six trials at 4 to 6 weeks (Mashood 2006; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001a; Baghel 2009; Memon 2005) using a random effects model because although the I2 was 0% these trials are clearly different in terms of frequency of dressing changes, types of burns and duration of follow up. There was no difference in the risk of burns healing by 4 to 6 weeks when treated with honey compared with SSD (RR 1.00 95% CI 0.98 to 1.02). High quality evidence (Table 3).
Outcome: adverse events
Burns trials tended to report the frequencies of hypergranulation, contracture, hypertrophic scarring, minor scarring, itching and burning as adverse events however it was generally unclear if events were reported by individual without double counting, or (more likely) some individuals experienced more than one adverse event. Consequently we are not confident of the accuracy of the adverse events data. The adverse event data across the five trials that reported them (Baghel 2009; Mashood 2006; Memon 2005; Subrahmanyam 1991; Subrahmanyam 2001a) were pooled (random effects); importantly Mashood 2006 stated that there was "no difference" between groups in rates of contracture and hypertrophic scarring but only reported rates of itching and burning. Overall there were significantly fewer adverse events with honey than SSD (RR 0.29 95% CI 0.20 to 0.42) (Analysis 4.3). High quality evidence (Table 3).
4.3. Analysis.
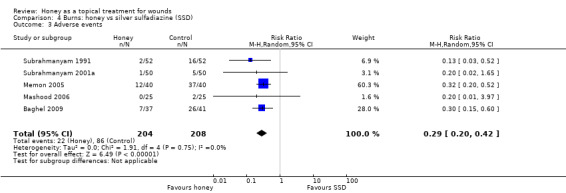
Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 3 Adverse events.
Outcome: infection
Infection related outcomes were reported in a variety of ways: most consistently five out of six trials reported the proportion of burns yielding negative swabs at Day 7 (Baghel 2009; Memon 2005; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001a). The report of Mashood 2006 merely stated the time to sterility (with no SD or other measure of variance) in each group. There was very high statistical heterogeneity when pooling the five studies (I2=94%). Overall wound swabs from honey treated burns were more likely to be negative at 7 days than were swabs from SSD treated burns (RR 3.92 95% CI 1.32 to 11.63) (Analysis 4.4) however this is very low quality evidence (downgraded for inconsistency, imprecision and indirectness) (Table 3).
4.4. Analysis.
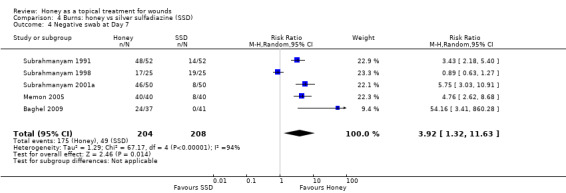
Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 4 Negative swab at Day 7.
Outcomes: costs
Only Mashood 2006 reported any cost data and then only as standardised unit costs (per percentage of (total body surface area) TBSA) of dressing treatments. They reported that the honey cost 0.75 Rupees per percentage TBSA compared with SSD which costs 10 Rupees per percentage TBSA.
Outcomes: quality of life
Not reported.
1.2.4 Honey compared with atypical dressings
Two trials (164 participants) by the same investigator compared honey with atypical dressings or materials (Subrahmanyam 1994; Subrahmanyam 1996b). The first trial recruited 64 participants with partial‐thickness burns and compared honey‐impregnated gauze with treatment with amniotic membranes (Subrahmanyam 1994) and the second recruited 100 people with partial thickness burns and compared honey with boiled potato peel dressings (Subrahmanyam 1996b).
Outcome: healing
The mean time to healing was reported for both trials without SDs which were subsequently supplied by the author (personal communication: M Subrahmanyam). Burns treated with honey healed approximately 8 days more quickly than those treated with amniotic membranes (WMD ‐8.10 days, 95% CI ‐10.88 to ‐5.32). In the second trial, burns treated with honey healed approximately 6 days more quickly than those treated with boiled potato peel (WMD ‐5.80 days, 95% CI ‐6.68 to ‐4.92).
The comparator treatments for these two trials are too different to pool them. The evidence for both these comparisons should be downgraded for imprecision (due to the relative lack of evidence) and therefore this constitutes moderate quality evidence.
Outcome: adverse events
Subrahmanyam 1994 reported that 4/40 and 3/40 people in the honey group experienced scarring/contractures and severe pain respectively compared with 5/24 and 6/24 in the amniotic membrane group (we cannot assume that the people experiencing pain were separate from the people having scarring or contractures). It was stated that there were no allergies or side effects in either group in the other study (Subrahmanyam 1996b).
Outcome: infection
Both studies reported the outcome of a negative swab at Day 7. In Subrahmanyam 1994, 36/40 (90%) of honey treated burns had negative swabs at Day 7 compared with 18/24 (75%) of burns treated with amniotic membrane. In Subrahmanyam 1996b, 36/50 (72%) of burns treated with honey had negative swabs at Day 7 compared with 8/50 (16%) treated with potato peel.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
2. Mixed acute and chronic wounds
Two trials (140 participants) each recruited participants with a range of different acute and chronic wounds. Subrahmanyam 1993b recruited 100 participants with burns (50% of the study population), lower limb ulcers caused by trauma, pressure, diabetes, and venous disease, or trophic ulcers and compared honey with SSD. In a quasi‐randomised study, Mphande 2007 recruited 40 participants with ulcers, chronic osteomyelitis, abscesses, post‐surgical or traumatic wounds; the comparison treatment was sugar dressings.
Outcome: healing
For the Subrahmanyam 1993b study, information on overall mean time to healing was provided by the author (personal communication: M Subrahmanyam). Wounds treated with honey healed more quickly than those treated with SSD (difference in mean days to healing ‐13.0 days, 95% CI ‐10.76 to ‐15.24) (Analysis 6.1)
6.1. Analysis.
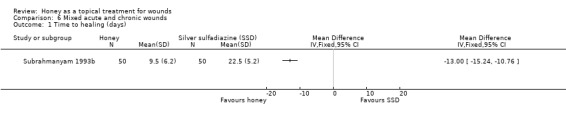
Comparison 6 Mixed acute and chronic wounds, Outcome 1 Time to healing (days).
In the Mphande 2007 study, median time to complete healing was 31.5 days in the honey‐treated group and 56.0 days in the sugar‐treated group.
Outcome: adverse events
Subrahmanyam 1993b reported hypergranulation, hypertrophic scarring, contractures and irritation as adverse events: 2/50 (4%) in the honey group experienced these compared with 14/50 (28%) in the SSD group. Mphande 2007 did not refer to adverse events generally but did report on pain in terms of being pain free during dressing changes at 3 weeks and also pain during mobilisation at 3 weeks. 19/22 participants (86.4%) in the honey group were pain free during dressing changes at 3 weeks compared with 13/18 (72.2%) in the sugar dressing group. 20/22 participants (90.9%) in the honey group and 13/18 (72.2%) in the sugar group were pain free during mobilisation.
Outcome: infection
Both Mphande 2007 and Subrahmanyam 1993b reported the outcome of negative swabs at 7 days however these results cannot be pooled as the comparator treatments are so different. It is unclear whether honey is associated with more negative swabs at 7 days than either SSD or sugar dressings due to imprecision and risk of (performance) bias (Analysis 6.2).
6.2. Analysis.
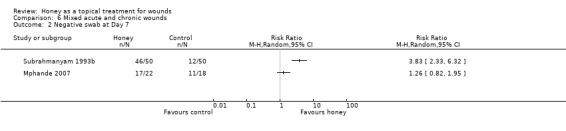
Comparison 6 Mixed acute and chronic wounds, Outcome 2 Negative swab at Day 7.
Overall there is low quality evidence (downgraded for imprecision on account of the two small studies, and indirectness on account of the mixed patient population and difficulties of interpretation) that, on average, honey heals a heterogeneous population of acute and chronic wounds more quickly than SSD or sugar dressings though the comparative rates of adverse events and infection are unclear.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3. Chronic Wounds
Ten trials (819 participants) evaluated the effects of honey in chronic wounds. Two trials recruited people with venous leg ulcers (Gethin 2007; Jull 2008); one study (Robson 2009) recruited participants with any type of wound healing by secondary intention but most of these were leg ulcers (70%); one study recruited people with a range of chronic wounds though most were venous leg ulcers (Gulati 2014). Two studies recruited people with diabetic foot ulcers (Kamaratos 2014; Shukrimi 2008) and one study for each of the following: ulcers caused by Leishmaniasis (Nilforoushzadeh 2007); pressure injuries (Weheida 1991); infected post‐operative wounds (Al Waili 1999), and Fournier's gangrene (Subrahmanyam 2004). Five of these ten studies were added at the first or second update (Gulati 2014; Kamaratos 2014; Nilforoushzadeh 2007; Robson 2009; Shukrimi 2008),
Three trials reported either the mean or median time to healing (Al Waili 1999; Jull 2008), or the mean time to surgical closure (Shukrimi 2008); seven trials reported the proportion of participants with completely healed wounds (Gethin 2007; Gulati 2014; Jull 2008; Kamaratos 2014; Nilforoushzadeh 2007; Robson 2009; Weheida 1991). One trial only reported the outcome, "mean hospital stay", but data on mean time to healing were provided by the author (Subrahmanyam 2004). Given the clinical and methodological heterogeneity between the trials, it was not possible to combine the trials to produce an overall summary statistic for the effect of honey on chronic wounds and instead we consider the evidence by wound type below.
3.1 Infected post‐operative wounds
One trial (50 participants) randomly allocated participants with infected caesarean or hysterectomy wounds to twice daily applications of honey or antiseptic washes of 70% ethanol and povidone‐iodine, followed by gauze dressings (Al Waili 1999), in addition to systemic antibiotics. There was very limited information on baseline comparability and no real indication of the duration of treatment or length of follow‐up.
Outcome: healing
More people healed with honey (84.6%) than with antiseptic washes followed by gauze dressings (50.0%); RR 1.69 (95% CI 1.10 to 2.61) (Analysis 7.1) (moderate quality evidence; downgraded for imprecision). This equates to an increase in the absolute risk of healing of 35% (95%CI 8.7% to 55.4%).
7.1. Analysis.
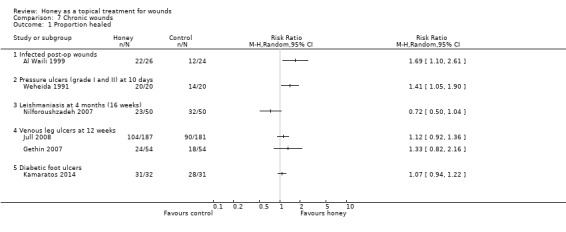
Comparison 7 Chronic wounds, Outcome 1 Proportion healed.
Outcome: adverse events
People were less likely to be recorded as having experienced adverse events with honey: 4/26 honey treated wounds (15.3%) compared with 12/24 (50%) of control wounds dehisced; 6/24 (25%) of control wounds needed resuturing compared with none in the honey group (moderate quality evidence; downgraded for imprecision).
Outcome: infection
Al Waili 1999 reported the mean time (days) to a negative swab as a measure of infection; this was 6 days (SD 1.9) for honey compared with 14.8 days (SD 4.2) for antiseptics and gauze (moderate quality evidence, downgraded for imprecision).
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.2 Pressure injuries
One trial (40 participants) randomly allocated participants with uninfected grade I or grade II pressure injuries greater than 2 cm in diameter to daily applications of honey or saline‐soaked gauze dressings for 10 days treatment (Weheida 1991). There was limited information on baseline comparability.
Outcome: healing
More people treated with honey were healed at 10 days (100%) than those treated with saline soaks (70%); RR 1.41 (95% CI 1.05 to 1.90) Analysis 7.1 (very low quality evidence due to imprecision and potential selection bias as assessed by baseline imbalance).
Outcome: adverse events
Not reported.
Outcome: infection
Not reported.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.3 Fournier's gangrene
One trial in which 30 men with Fournier's gangrene (23 of whom were chronic alcoholics) were randomly allocated to be treated with monofloral (jamun) honey‐soaked gauze dressings or antiseptic EUSOL‐soaked gauze dressings (Subrahmanyam 2004). Fournier's gangrene is an infection of the scrotum that can also involve the perineum and abdominal wall.
Outcome: healing
Secondary suturing and skin grafting were required in 9/14 (64.3%) of the honey group and 9/16 (56.3%) of the EUSOL group. Only mean length of hospital stay was reported in the paper, but mean time to healing was supplied by the author (personal communication M Subrahmanyam). Mean time to healing was shorter in the honey‐treated group (MD ‐8.00 days, 95% CI ‐6.08 to ‐9.92 days, Analysis 7.2), but we note this was a very small sample size and the high rates of further surgical intervention (secondary suturing and skin grafting) required by participants in both groups is worth noting (very low quality evidence, on account of the very small sample size and single study).
7.2. Analysis.
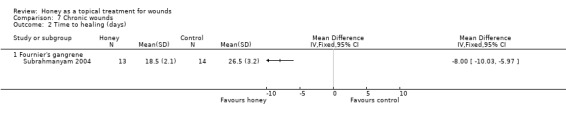
Comparison 7 Chronic wounds, Outcome 2 Time to healing (days).
Outcome: adverse events
One participant in the honey‐treated group and two in the EUSOL group died.
Outcome: infection
The primary condition (Fournier's gangrene) is itself the result of infection; rates of secondary infection were not reported.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.4 Cutaneous Leishmaniasis
One trial (100 participants) randomly allocated participants with ulcers caused by Leishmaniasis to treatment with intralesional injections of meglumine antimoniate (glucantamine) plus honey‐soaked gauze dressings or intralesional injections alone (Nilforoushzadeh 2007).
Outcome: healing
Thirteen participants withdrew from the honey dressings arm due to treatment failure (n=12) or contact dermatitis (n=1) (and therefore remain in this analysis in the denominator) whilst 10 withdrew from the meglumine antimoniate alone group due to treatment failure (similarly remaining in the denominator). Fewer people treated with injections plus honey had healed lesions compared with those not receiving honey at 4 months although this difference was not statistically significant (51.1% versus 71.1%) (RR 0.72; 95% CI 0.50 to 1.04) (Analysis 7.1) (low quality evidence due to imprecision and high risk of bias).
Outcome: adverse events
The study by Nilforoushzadeh 2007 reported one withdrawal due to sensitivity in the honey group and none in the control group.
Outcome: infection
Not reported.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.5 Venous leg ulcers
Two trials recruited only participants with venous leg ulcers. One trial (368 participants) recruited patients presenting to community‐based nursing services for assessment and treatment of their venous ulcers (Jull 2008). Participants were allocated to receive either manuka honey‐impregnated calcium alginate dressings or usual care. Participants allocated usual care could receive any dressing that was clinically indicated from the wide range normally available to community nurses (non‐adherent, alginate, hydrogel, hydrofibre, hydrocolloid, silver or iodophor dressings). Both groups received compression bandaging as a standard background treatment. Participants were treated for 12 weeks. The second trial recruited 108 participants with uninfected venous ulcers, the surfaces of which were at least 50% covered by slough (Gethin 2007). Participants were allocated to receive either manuka honey dressings or hydrogel dressings for four weeks and then standard care (the nature of which was individually determined by the clinician) for the remaining eight weeks of the 12 week follow‐up. Both groups received compression bandaging as a standard background treatment. A third trial (Robson 2009) compared honey with usual care in a population that included approximately 70% people with venous leg ulcers. Although it was not possible to separate out the results for people with venous leg ulcers that study also found no difference in risk of healing between honey and usual care (see section 3.6 below).
Outcome: healing
The Jull 2008 trial appropriately analysed healing as a time to event outcome using a survival approach. There was no difference in healing between groups treated with honey and usual (non‐honey) care (adjusted hazard ratio, HR, 1.1, 95% CI 0.8 to 1.5, P=0.451). There was no difference in the risk of healing at 12 weeks with or without honey (RR 1.12, 95% CI 0.92 to 1.37).
The Gethin 2007 study had change in area of slough at four weeks as the primary outcome and proportion of ulcers healed at 12 weeks as a secondary outcome. In this study 24/54 (44.4%) participants in the honey group had healed at 12 weeks compared with 18/54 (33.3%) in the control group (RR of healing 1.33, 95% CI 0.82 to 2.16).
Although the duration of treatment was dissimilar across the two trials, they were considered sufficiently alike to be able to provide meaningful information when combined (I² 0%). Overall it is not clear whether honey increases the healing of venous leg ulcers compared with no honey (RR 1.15, 95% CI 0.96 to 1.38) (Analysis 7.3) (low quality evidence; downgraded for risk of bias due to unblinded outcome assessment and imprecision) (Table 4).
7.3. Analysis.
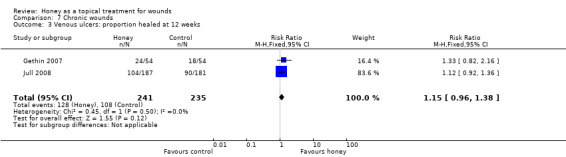
Comparison 7 Chronic wounds, Outcome 3 Venous ulcers: proportion healed at 12 weeks.
Outcome: adverse events
The Jull 2008 trial (368 participants) reported all adverse events, whether or not the event was believed to be related to the treatment, whereas the Gethin 2007 trial (108 participants) only referred to events that were attributable to the wound agent, of which there were none (and this approach is subject to ascertainment bias in an open label study). In the Jull 2008 trial there were significantly more adverse events (including deterioration of the ulcer) reported in the honey‐treated group than the control group (RR 1.28, 95% CI 1.05 to 1.56). The frequency of the different adverse events is presented in Table 5. It is notable that there were high frequencies of pain (47/187 versus 18/181) and of ulcer deterioration (19/187 versus 9/181) with honey (low quality evidence, downgraded for risk of bias and imprecision, Table 4).
1. Frequency of adverse events reported in venous ulcer trial (Jull 2008).
| Adverse event | Honey treatment | Control treatment |
| Ulcer pain | 47/187 | 18/181 |
| Bleeding | 3/187 | 3/181 |
| Dermatitis | 8/187 | 8/181 |
| Deterioration of ulcer | 19/187 | 9/181 |
| Erythema | 6/187 | 4/181 |
| Oedema | 4/187 | 1/181 |
| Increased exudate | 5/187 | 1/181 |
| Deterioration of surrounding skin | 5/187 | 3/181 |
| New ulceration | 16/187 | 15/181 |
| Other | 6/187 | 3/181 |
| Cardiovascular | 4/187 | 3/181 |
| Cancer | 2/187 | 2/181 |
| Neurological | 4/187 | 1/181 |
| Gastrointestinal | 4/187 | 2/181 |
| Injury | 10/187 | 9/181 |
| Musculoskeletal | 13/187 | 9/181 |
| Respiratory | 6/187 | 3/181 |
| Other | 3/187 | 8/181 |
Outcome: infection
The Jull 2008 trial reported risk of infection whilst the Gethin 2007 study reported withdrawals due to infection. Infection was operationally defined as clinical signs of infection or a positive swab result, and treatment with antibiotics in Jull 2008.These data were pooled (fixed effect) and it remains unclear if honey reduces leg ulcer infection rates relative to no honey (RR 0.71, 95% CI 0.49 to 1.04) (Analysis 7.4) (low quality evidence, downgraded for risk of bias, Table 4).
7.4. Analysis.
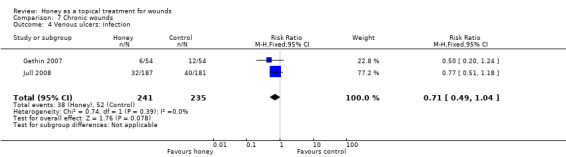
Comparison 7 Chronic wounds, Outcome 4 Venous ulcers: infection.
Outcome: cost
Jull 2008 and colleagues conducted a full cost‐effectiveness analysis using a New Zealand health service perspective. Information was collected on dressings and related products, district nursing time, general practitioner and laboratory time, outpatient consultations, antibiotic use, and hospitalisation. In the base case analysis, the average cost of treatment with honey was NZD 917.00 per participant compared with NZD 972.68 per participant for usual care. This cost was driven by a small difference in hospitalisations that was considered likely to be due to chance variation (three participants in the honey group were hospitalised for ulcer‐related reasons for 10 days, compared to six participants hospitalised for 40 days). A sensitivity analysis excluding the hospitalisations found the average cost of treatment was reversed with usual care being cheaper (NZD 811.12 per participant) than treatment with honey (NZD 877.90 per participant).
Outcome: quality of life
The trial by Jull 2008 reported quality of life. Two generic instruments (SF‐36, EQ5D) and one disease‐specific instrument (Charing Cross Venous Ulcer Questionnaire) were used. There was little difference between the groups, with narrow confidence intervals, for both the physical summary component of SF‐36 (mean difference 1.1, 95% CI ‐0.8 to 3.0; scale 0 to 100, high is better, for a control group mean of 37.9) and the mental component summary score (mean difference 0.7, 95% CI ‐1.1 to 2.4, for a control group mean of 50.4). There was also little difference on EQ‐5D (mean difference 1.6, 95% CI ‐1.5 to 4.7; scale 0 to 100, high is better, for a control group mean of 73.5) or the Charing Cross Venous Ulcer Questionnaire.
3.5 Diabetic foot ulcers
Two trials (93 participants) recruited people with diabetes and foot ulcers of Wagner grade I or II (Kamaratos 2014) or Wagner grade II (Shukrimi 2008) and compared the effects of honey with either saline soaks (Kamaratos 2014) or povidone‐iodine gauze (Shukrimi 2008). In both studies participants also received initial debridement and antibiotics as necessary. Each trial measured and reported healing in a different way which precluded meta‐analysis.
Outcome: healing
There was no difference in healing between honey and saline gauze in the trial by Kamaratos 2014 (31/32 people completed healed by 16 weeks (97%) in the honey‐treated group compared with 28/31 (90%) in the saline‐gauze group). This equate to a RR for healing with honey of 1.07 (95% CI 0.94 to 1.22) (Analysis 7.1).
In Shukrimi 2008 the mean time to surgical closure was 14.4 days in the honey‐treated group and 15.4 days in the povidine‐iodine group, but it was unclear whether all wounds healed. The study did not report standard deviations or the numbers analysed, but stated the difference was not statistically significant.
Overall the evidence suggests there is little difference in the healing of diabetic foot ulcers between honey and saline‐soaked gauze or povidone iodine however this is low quality evidence (downgraded for high risk of bias and imprecision).
Outcome: adverse events
The study by Shukrimi 2008 reported subjective, impressions of pain and exudate only. Kamaratos 2014 did not mention adverse events or side effects.
Outcome: infection
Kamaratos 2014 reported negative wound swabs at 4 weeks: there was no difference between honey and saline dressings (100% of swabs in the honey group were negative at 4 weeks compared with 87% in the saline group; RR 1.07, 95%CI 0.94 to 1.22). Shukrimi 2008 did not report infection.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.6 Mixed chronic wounds
One trial (105 participants) recruited participants with different chronic wounds, of whom approximately 70% had venous leg ulcers (Robson 2009). Participants were allocated to receive either manuka honey or a usual care dressing. If slough was present, control participants were to be treated in the first instance with a hydrogel. Compression treatments were given as appropriate.
A second trial (45 participants) also recruited people with a range of chronic wounds, of whom 47% had venous leg ulcers (Gulati 2014). Participants received either sterilized honey followed by a film dressing or povidone iodine covered with a film dressing. People with venous ulcers also received elastic compression.
In Robson 2009 the reported healing rates at 12 weeks were 46.2% for honey compared with 34.0% for usual care (RR 1.36, 95% CI 0.84 to 2.19) and at 24 weeks, 72.7% versus 63.3% (RR 1.14, 95% CI 0.88 to 1.48) (Analysis 7.5). Robson 2009 also reported an unadjusted hazard ratio for healing of 1.30 (95% CI 0.77 to 2.19), with an adjusted analysis (for wound type, age, sex, wound area) of HR 1.51 (95% CI 0.88 to 2.58), Analysis 7.5.
7.5. Analysis.
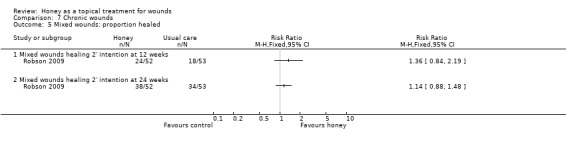
Comparison 7 Chronic wounds, Outcome 5 Mixed wounds: proportion healed.
In Gulati 2014 7/23 (30.4%) in the honey group completely healed at 6 weeks, compared with 0/22 in the povidone iodine group. We decided it was inappropriate to pool the healing data from Gulati 2014 and Robson 2009 due to the different patient populations, comparator interventions and durations of follow up.
Overall it is unclear whether honey speeds the healing of a mixed population of chronic wounds relative to usual care or povidone iodine; this evidence is of low quality (downgraded for risk of bias and imprecision).
Outcome: adverse events
In the Robson 2009 study there were 7 adverse events in the honey group (1 death, 1 pain, 2 cases of ulcer deterioration and 3 people discontinuing treatment due to other concomitant treatment) compared with 5 in the usual care group (1 death, 1 deterioration of the ulcer, 3 discontinuation due to other treatment). In Gulati 2014 there were no reported adverse events.
Outcome: infection
Not reported in either study.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
Discussion
This is a complex review addressing a diverse range of wound types and with many trials at high or unclear risk of bias. Generally, the evidence, when assessing using GRADE, is of low or very low quality. This means that new, better quality research is highly likely to change the overall conclusions of this review. The findings are discussed below with respect to specific wound types and using a GRADE approach to addressing the quality of the evidence.
Summary of main results
1. Acute wounds
1.1 Minor acute wounds
It is unclear, on the basis of the very low quality evidence from three small trials (two in toenail bed avulsion and one in minor traumatic wounds) whether honey affects time to wound healing in minor acute wounds compared with conventional dressings. It is also unclear if honey and conventional dressings have different effects on adverse events or rates of infection in people with minor acute wounds (Table 1).
1.2 Burns
The 11 trials of honey for burns used a wide range of comparator treatments, viz. various conventional dressings (Subrahmanyam 1993a; Subrahmanyam 1996a), early burn excision and grafting (Subrahmanyam 1999), silver sulfadiazine (SSD) (Baghel 2009; Mashood 2006; Memon 2005; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001a), amniotic membrane (Subrahmanyam 1994) and potato peelings (Subrahmanyam 1996b).
The evidence for the effects of honey relative to these comparators is mixed, and is generally of low or very low quality. The strongest (high quality) evidence suggests that honey dressings heal partial thickness burns, on average, between 4 and 5 days more quickly than conventional dressings, however it is not clear if there is a difference in the rates of adverse events or infection (Table 2).
One trial (low quality evidence) suggests that early excision and grafting heals burns on average 13.6 days more quickly (95% CI 9.82 days to 17.3 days) than honey followed by grafting as necessary, however the relative effects on adverse events and infection rates are unclear (Subrahmanyam 1999).
There is very low quality evidence that honey reduces the time for burns to heal by between 0.73 and 9.51 days (average difference was 5.12 days) compared with SSD dressings/cream and high quality evidence of a reduction in adverse events with honey and more negative wound swabs at day 7. There was no difference for the outcome of risk of complete healing by 4 to 6 weeks (high quality evidence) (Table 3).
Perhaps unsurprisingly, the current evidence suggests that burns heal more quickly with honey than with amniotic membranes (by approximately 8 days) (Subrahmanyam 1994) and approximately 6 days more quickly than with potato peelings (Subrahmanyam 1996b) (single small studies, moderate quality evidence). The relative effects of these interventions on adverse events and infection are unclear.
2. Mixed acute and chronic wounds
The rationale for conducting trials in which the participants have either burns or a mix of chronic wounds is unclear. The aetiologies are so different that no matter what the results, the findings will be difficult to interpret and unlikely to influence clinical practice. Overall we found low quality evidence from two studies Mphande 2007; Subrahmanyam 1993b), that on average honey heals a heterogeneous population of acute and chronic wounds more quickly than sugar dressings or SSD. The comparative rates of adverse events and infection are unclear.
3. Chronic wounds
Most of the evidence for the effects of honey on chronic wounds is of low or very low quality. Most trials were small, with comparators that may not be relevant to current practice and at high or unclear risk of bias.
3.1 Infected post‐operative wounds
There is moderate quality evidence that honey increases the absolute risk of healing by 35% (95%CI 8.7% to 55.4%) relative to antiseptic (povidone iodine) washes followed by gauze. The single trial (Al Waili 1999) was small (50 participants) and the report lacked sufficient detail to permit the risk of bias to be determined accurately for most domains. The comparator was an antiseptic which has been proposed to impair wound healing although clinical evidence for such an effect is lacking (Leaper 1986). In the same study there were fewer adverse events and a shorter time to a negative wounds swab with honey.
3.2 Pressure ulcers
There is very low quality evidence that honey increases the relative risk of healing of pressure ulcers by 41% (95% CI 5% to 90%) relative to saline soaked gauze (Weheida 1991). This equates to an increase in the absolute risk of healing with honey of 30% (95%CI 7.7% to 51.9%). The quality of the evidence for the effect on healing from this trial was downgraded to very low quality for imprecision and possible selection bias. There were no data on adverse events or infection.
3.3 Fournier's gangrene
There is very low quality evidence that honey healed Fournier’s gangrene, on average, 8 days more quickly than Eusol soaks (1 trial, WMD ‐8.00 days, 95% CI ‐6.08 to ‐9.92 days). In this small study (30 participants) Subrahmanyam 2004), 64% (honey group) and 69% (EUSOL group) of participants also required secondary suturing and grafting. In addition, the comparator was EUSOL, an antiseptic that has been shown to impair wound healing in animal model studies (Brennan 1985).
3.4 Cutaneous Leishmaniasis
It is not clear whether honey influences the healing of lesions caused by Leishmaniasis when used as an adjuvant to meglumine antimoniate. The low quality evidence from a single small trial suggests that honey may impair healing compared with meglumine antimoniate alone (RR 0.72; 95% CI 0.50 to 1.04) (Nilforoushzadeh 2007) however the quality of this evidence for an effect on healing was downgraded for imprecision and high risk of bias. In terms of adverse events, there was one reported withdrawal from the honey group for sensitivity and none in the control group. Infection rates were not reported.
3.5 Venous leg ulcers
There is currently no clear evidence that honey improves the rate of healing of venous ulcers. Such evidence that there is, from two trials (Gethin 2007; Jull 2008) is low quality and finds no overall difference in healing (RR 1.15, 95% CI 0.96 to 1.38) (Table 4). The larger of the two studies (Jull 2008) analysed healing (appropriately) as a time‐to‐event outcome which is more informative (and uses more of the information on healing) than crudely analysing the proportions of participants healed at a particular (arbitrary) time point. This study found no difference in the hazard of healing between honey and usual care (hazard ratio 1.1, 95%CI 0.8 to 1.5). These trials reported adverse events differently with Gethin 2007 only reporting those they "attributed" to the wound treatment (there were none). This approach has an inherently high risk of ascertainment bias in an open label study. By contrast Jull 2008 documented and reported all adverse events and there were more in the honey group than the control group, with pain and ulcer deterioration being frequent (absolute increase in risk of adverse events with honey was 12.9%, 95%CI 2.7% to 22.8%).
3.6 Diabetic foot ulcers
The effect of honey on diabetic foot ulcers cannot be determined. The two included studies (Kamaratos 2014; Shukrimi 2008) were small and the evidence for honey in this patient group was low quality.
Overall completeness and applicability of evidence
There are significant weaknesses in the completeness and applicability of the evidence overall. Most of the studies in burns have been conducted by one team in India (10 out of 26 included studies; Subrahmanyam 1991; Subrahmanyam 1993a; Subrahmanyam 1993b; Subrahmanyam 1994; Subrahmanyam 1996a; Subrahmanyam 1996b; Subrahmanyam 1998; Subrahmanyam 1999; Subrahmanyam 2001a; Subrahmanyam 2004) and we relied on further information supplied by the authors to supplement an absence of detail in the original published trial reports. This review is therefore disproportionately reliant on evidence from one single research team from one part of the world and this evidence may not be applicable elsewhere (particularly since the prevailing microbiological environment, health care facilities and climate are likely to have strong effects on burn outcomes and infection rates).
Only one study (Jull 2008) reported costs and quality of life. Clearly any impact of treatments on patients' quality of life is invaluable information for all decision makers but particularly patients. The relative cost‐effectiveness of competing treatments is essential information for health care funders and providers. We therefore urge that future research in this field uses contemporary methodologies to measure these outcomes.
There is relatively little replication of studies, with single, small studies for most comparisons. This weak evidence base makes it impossible to draw firm conclusions with confidence.
Some of the comparators chosen have little or no relevance to current clinical decision making (e.g., amniotic membrane, potato peelings). Other comparators will have relevance in some parts of the world but not others. For example antiseptics such as EUSOL and povidone iodine are used less frequently in open wounds than they used to be, in the developed world at least, because in vitro studies were interpreted as evidence that they may impair wound healing (Leaper 1986). Silver sulfadiazine, which is commonly used in burns and was the comparator in several of the studies included here, has been shown in a related review (Wasiak 2013) to impair the healing of burns relative to several comparator dressings.
Quality of the evidence
In common with most wounds‐related topic areas, the quality of the evidence in this review was generally low or very low ‐ mainly due to imprecision, risk of bias and inconsistency. Imprecision of effect estimates was usually low because there are only one or two small studies for each comparison. Risk of bias was generally high due to unblinded outcome assessment. Most studies did not guard against performance bias (by blinding participants and health care professionals) however the importance of performance bias in wound care studies is not clear. Blinded outcome assessment is however a substantial threat to validity (Hróbjartsson 2012). We downgraded the quality of the evidence for inconsistency where there was obvious clinical or statistical heterogeneity. Poor reporting is a major issue and the majority of studies were unclear for one or more important bias domains in the risk of bias assessment. We carefully considered downgrading the evidence for risk of bias where trial reports were unclear (most studies, see Figure 1). We decided against downgrading for unclear risk of bias (because it does not appear to be the norm) however this means that, if anything, the evidence is lower quality than we have rated it. The importance of following international standards for trial reporting (i.e., CONSORT, Schulz 2010), which most of the studies in this review did not adhere to, cannot be over‐emphasised .
The reporting of adverse events was poor in most trials, and non‐existent in a few trials. This makes accurate assessment of the risk of adverse events associated with honey dressings compared with comparators, difficult. The International Conference on Harmonization's Guideline for Good Clinical Practice (ICH GCP) defines an adverse event as any untoward medical occurrence in a trial subject who has been administered an intervention, whether related to the intervention or not. With the exception of Jull 2008, it was not clear if trials reported all adverse events as required by ICH GCP. Therefore the adverse event findings should be interpreted very cautiously, as the full adverse event profile of honey vs. comparators in different wounds is unknown.
The evidence regarding the effect of honey on wound infection rates was poor quality and difficult to interpret. The accurate identification of wound infection is problematic and since wound infection is dependent to some extent on the host response to the micro‐organism (e.g., manifesting as inflammation and pain), and the assessment of this is partly subjective, there is no "gold standard" diagnostic tool . Clinical presentation is an important indicator, but presentation may vary with wound type (Cutting 2005), therefore, trialists should strive for unambiguous definitions of infection to ensure that study results are interpretable and their meta‐analyses both feasible and sensible.
Infection is a significant and threatening consequence of burns. Wound sterility after a burn is maintained by careful attention to asepsis during wound care and the use of preventive agents. Honey dressings appear to increase the probability that a cultured swab from a burn will remain negative compared with a range of control treatments but the clinical importance of negative swabs at day 7 is unclear. Future trialists should identify and use reliable and valid measures of infection that can be applied in a range of settings.
Only one study conducted a full cost‐effectiveness analysis using a health services perspective (Jull 2008). As the effectiveness of honey was not established by the trial, honey cannot be considered the dominant strategy. In the same vein, this was also the only study that reported health related quality of life.
Potential biases in the review process
This review has a number of limitations, driven largely by the nature of the included studies. Firstly one of the included studies was led by one of the authors of this review (Jull 2008). This situation is not uncommon in Cochrane reviews and there are robust policies in place to reduce or eliminate any bias this may bring. Specifically data extraction, risk of bias assessment and analysis were checked by three further authors or editors (MW, NC, JD).
Secondly, several studies report and analyse mean time to healing as the main effect measure. Time to healing should be treated as a type of time‐to‐event outcome rather than a continuous measure, as this enables all participants to contribute data to the analysis irrespective of whether they experienced the outcome or remained in the study (only people who healed can contribute data to an analysis of mean time to healing as a continuous outcome). However, we were limited to using a common means of measurement wherever possible. Third, we attempted to contact authors where the original publication did not provide sufficient data, and then incorporate that data into the review. Where authors did not respond, we excluded the studies that did not report sufficient data even to be included in a narrative analysis. Finally it was not possible to evaluate the overall possibility of publication bias, as not all trials reported the same outcomes and overall the trials were too heterogeneous to combine.
Agreements and disagreements with other studies or reviews
Two relevant systematic reviews have been published since the last update of this review. In 2013 Vandamme 2013 concluded that honey stimulates wound healing in human burns, ulcers and other wounds, and that it debrides, removes odour, has anti‐inflammatory and pain reducing properties. There are key methodological differences between our review and the review by Vandamme 2013. They did not restrict inclusion to study designs at lowest risk of bias (but included published controlled trials, clinical trials and case reports as well as RCTs). Secondly there appears to have been no systematic assessment of study quality in the review by Vandamme 2013, but rather allusion to strengths and weaknesses of the included studies in an ad hoc way. Thirdly GRADE was not used to assess the quality of the evidence in the Vandamme 2013 review and conclusions were reached by vote counting rather than meta‐analysis. There is a great deal of overlap of study inclusion however (though we had excluded several studies included in their review e.g., Malik 2010, Yapucu Gunes 2007, Oluwatosin 2000, Lund‐Nielsen 2011, Misirligou 2003). Elsewhere the explanation for excluding studies from our review that were included in Vandamme 2013 was the different eligibility criteria for study population (they included studies of oral mucositis and radiation damage to skin). Notwithstanding these differences there was some agreement in the conclusions reached, viz. their conclusion that "Many of the included studies have methodological problems, and the quality of certain studies is low, making it difficult to formulate conclusive guidelines" (Vandamme 2013). Another systematic review was also published in 2013 (Rűttermann 2013). This review was prepared to support new German wound care guidelines and used the GRADE approach in summarising the quality of the evidence. Again the patient population differed slightly from ours in that they excluded studies in people with burns and other acute wounds. The review by Rűttermann 2013 concluded that honey does not accelerate wound healing and increases pain (a conclusion based solely on the studies of Gethin 2007 and Jull 2008 included here). There is much agreement therefore between our conclusions represented in this review update and those of other recent systematic reviews.
Authors' conclusions
Implications for practice.
The main challenge to practitioners in considering this evidence is deciding whether the patient populations and comparator interventions are clinically relevant to their practice.
There is high quality evidence from two trials that honey dressings heal partial thickness burns more quickly than conventional dressings by around five days however practitioners must consider whether the comparator dressings in these trials are clinically meaningful to them. In one of the two trials the control dressing was a polyurethane film dressing whilst in the other the control group received a range of interventions (polyurethane film, paraffin gauze, sterile linen, framyecetin‐impregnated tulle, left exposed). It is unclear whether there is a difference in rates of adverse events (very low quality evidence) or infection (low quality evidence) between honey and conventional dressings for partial thickness burns.
It is unclear whether there is a difference in the effect on the healing of burns between honey and SSD, since the result is very sensitive to the outcome measure used. Furthermore, SSD has been shown to slow the healing of burns relative to other comparators in a related Cochrane review (Wasiak 2013).
There is low quality evidence from one small trial that early excision and grafting of partial and full thickness burns may be more effective than honey followed by later grafting as necessary.
Low quality evidence from two small trials with different comparators suggests that honey may heal a mixed population of acute and chronic wounds more quickly than SSD or sugar dressings. Again practitioners will need to consider the relevance of this evidence to current practice.
Moderate quality evidence from one trial suggests that honey heals infected post‐operative wounds more quickly than povidone iodine washes followed by gauze and is associated with fewer adverse events.
Very low quality evidence from one small trial suggests that honey may heal pressure ulcers more quickly than saline soaks.
Very low quality evidence from one trial suggests that honey may heal Fournier’s gangrene more quickly than Eusol soaks.
The effect of honey relative to comparators is unclear for: venous leg ulcers (2 trials, low quality evidence); minor acute wounds (3 trials, very low quality evidence); diabetic foot ulcers (2 trials, low quality evidence); Leishmaniasis (1 trial, low quality evidence); mixed chronic wounds (2 trials, low quality evidence).
Implications for research.
The implications for further research arising from this review fall into two categories: important research questions and improving the conduct and reporting of future research.
An important research question may well be whether honey dressings heal partial thickness burns more quickly than relevant, current comparator dressings, since currently the only evidence compares honey with film dressings or with a range of very different dressings. It is consequently impossible to determine the effectiveness of honey relative to competing dressings. Evidence from this and a related review (Wasiak 2013) is beginning to accumulate that suggests SSD may not be an effective treatment for burns and yet it is still widely used. Given the quality of the existing evidence, substantial doubt remains and therefore there may be an argument for a definitive, high quality three‐arm trial comparing honey dressings with SSD and another widely used conventional dressing.
People with surgical wounds that are infected or have broken down may be another patient group in which to evaluate honey dressings since there is some evidence from one study in this review that they may be effective; again the comparator dressing used in this trial (povidone iodine wash followed by gauze) is no longer relevant in some parts of the world.
For those wounds that are a consequence of underlying systemic disease (chronic wounds such as pressure ulcers, venous ulcers and diabetic foot ulcers, and Leishmaniasis) it seems likely that management of the underlying health problem (or reduction of the applied pressure in the case of diabetic foot and pressure ulcers) will make more difference to healing than the type of dressing and we would suggest that these trials are of lower priority.
In terms of improving the quality of future research we would make the following recommendations:
Where trials are measuring a time to event outcome such as time to healing, they should employ survival approaches which account for censoring.
Trials should focus on populations that share a single wound aetiology rather people with wounds of different underlying causes.
Trials should be appropriately powered based on identifying a clinically important difference for a pre‐specified primary outcome.
Trials should apply properly random and concealed allocation strategies and these should be clearly reported.
Future studies should use either blinded outcome assessment or some form of masked, remote adjudication of outcomes e.g., photography.
It is important to follow up as great a proportion of randomised participants for clinical outcomes as possible, even when they withdraw from trial treatments.
Analysis should use the intention‐to‐treat principle and include all participants in the denominator. Where participants have been lost to follow‐up, appropriate and valid methods of imputation should be used and reported.
The patient should be the unit of randomisation and analysis, rather than individual wounds.
Future trials should measure and report health‐related quality of life using valid and reliable measures (both generic and wound specific). Similarly future trials should measure the costs of alternative treatments and ideally assess cost‐effectiveness.
Trialists should ensure that the above elements of trial quality are adequately reported, and journals should require that trial reporting is consistent with the Consolidated Statement on Reporting of Trials. Data for outcomes relevant to wound healing should be reported to support full evaluation and reuse.
All trials should be registered with a trials register that meets the WHO criteria, and principal investigators should keep their contact details on the register up to date.
Feedback
Authors' conclusions, 14 June 2013
Summary
Email received from Barry Wolfenson on behalf of Derma Sciences expressing concern at the wording of the Authors' conclusions ‐ as follows: “There is insufficient evidence to guide clinical practice in other types of wounds, and purchasers should refrain from providing honey dressings for routine use until sufficient evidence of effect is available.”
Reply
Andrew Jull, Author; Nicky Cullum, Coordinating Editor CWG; Sally Bell‐Syer Managing Editor CWG.
Thank you for your feedback. this statement has been present in the Authors' conclusions since the review was first published in 2008. However this is a valid point and does contravene Cochrane guidance from the Handbook which states " The primary purpose of the review should be to present information, rather than to offer advice". As a result we have modified this section to read: "There is insufficient evidence to guide clinical practice in other areas, health services may wish to consider avoiding routine use of honey dressings until sufficient evidence of effect is available"
Contributors
Barry J. Wolfenson, Group President, Advanced Wound Care & Drug Development, Derma Sciences. Andrew Jull, Author; Nicky Cullum, Coordinating Editor CWG; Sally Bell‐Syer Managing Editor CWG.
Feedback questions from Derma Sciences, 4 September 2013
Summary
Email received from Barry Wolfenson, Group President, Advanced Wound Care & Drug Development, Derma Sciences.
Q1. Can you please let me know, regarding the 132 Cochrane Reviews on the topic of Wounds, aside from the one titled “Honey as a topical treatment for wounds”, how many include studies within the review that are authored by authors of the Cochrane Review itself?
Authors response: This is a very general question; you have not identified the 132 reviews you are referring to and we do not routinely record that information outside of the review. However, any included trial which has a trialist who is also an author of the Cochrane review must declare this in the Declaration of Interests section of the review.
Q2. Given that one of your stated core principles is to minimize bias, can you please let me know your organizational guidelines regarding authorship of reviews and whether or not authors should be able to review their own studies? To a lay person, there seems to be quite a potential for conflict here.
Authors response: In the Cochrane Wounds Group we adhere strictly to the policy that if an author of a Cochrane review is also a trialist of an included trial in that same review they must declare this in the Declaration of Interests section of the review. This declaration is also made in the online Declaration of Interests form which has to be completed by all authors before a protocol or a review can be published on the Cochrane Library.
Q3. As reviewers of the Jull et al study (Randomized clinical trial of honey‐impregnated dressings for venous leg ulcers), how did you judge the relative bias of the study author’s ad hoc decision to remove incidence of infections from the adverse events table?
Authors response: This question is directly with respect to a trial we conducted, not the systematic review. We did not remove incidence of infections from adverse events. The incidence of infection was analysed separately as specified a priori in the protocol and statistical analysis plan in response to interest in whether honey may prevent infections in venous leg ulcers.
Q4. With regard to the above, the removal of infections from the adverse events section somehow allowed the study authors to also remove the costs associated with hospitalizations associated with those infections from their overall cost of care analysis. However, the other costs associated with hospitalization (not having to do with infection) remained. When the costs associated with hospitalizations due to infection were included in the analysis, the honey arm was less expensive. Only after the costs associated with the additional hospitalizations associated with infection were removed (ad hoc) was the honey arm more expensive. Given that the decision to remove infections was ad hoc, how did you judge the relative bias of the statement by the study authors that the honey arm was likely more expensive?
Authors response: This question is directly with respect to a trial we conducted, not the systematic review. Incidence of infection was included in all the cost analyses. An analysis for sensitivity of the base case (all costs for ulcer treatment) to the small number of patients hospitalized for treatment related to their ulcer was undertaken. We do not know what the exact purpose of the hospitalization was i.e. whether it was related to infection or not, only that it was self‐reported by the patients as related to their ulcer. As explained in the paper, the difference was likely due to random variability rather than use of honey or not. The decision to sensitivity test the base case was not ad hoc.
Q5. As reviewers of the Jull et al study, how did you judge the relative bias of the study regarding the author’s conclusion that honey dressings should not be considered for venous leg ulcer healing given that all the primary and secondary endpoints favored the honey arm (although not statistically)? It would seem, based on the results of the study, that honey should be included into the control group of alginate, hydrofiber, hydrocolloid, foam, non‐adherent, iodine, and silver‐based dressings as dressings that have demonstrated efficacy as adjuvants to compression therapy. Again, given the positive nature of the evidence derived in this study, how did you judge the potential bias within the authors’ conclusion?
Authors response: This question is directly with respect to a trial we conducted, not the systematic review. There were no statistically significant differences between the groups on the primary outcome, nor on any of the secondary outcomes. In fact the results for the blinded verification of healing suggest barely any difference between groups (absolute difference 0.5%, 95%CI ‐10.6% to 11.3%) on the primary outcome. Routine use of honey dressings is therefore not superior to other dressings for promoting healing compared to usual care. This conclusion does not preclude non‐routine use of honey.
Q6. You included a second study in your Cochrane Review to address the management of venous leg ulcers. This second study, by Gethin et al, allowed only those wounds with greater than 50% slough coverage. In the larger Jull et al study of potentially likely to heal venous leg ulcers, the authors stated that the positive results (not statistically significant) favoring honey should be “generalizable” to all venous leg ulcers, regardless of wound bed appearance, unless new data shows otherwise. In the Gethin study, the wounds in both arms had average slough coverage ranging from 78% ‐ 86%, and had average wound areas of 9.9cm2 to 10.5cm2. However, in the Jull study, there is no notation of slough coverage and the wounds ranged in average area from only 2.6cm2 to 2.7cm2. Thus, the wound bed appearance was dramatically different between these two studies. If the authors of the Jull study thought it was wise to view different wound bed appearance as indicative of a different patient set, why did you chose to combine results of these two vastly different studies? It should be noted that in the Gethin study on harder‐to‐heal wounds, even a much smaller number of patients resulted in the honey arm providing statistically significant healing benefit over control. Based on what the authors of the Jull et al study stated, shouldn’t your conclusions from these two studies have been: Honey provides a non‐significant healing benefit for routine venous leg ulcers, and provides a significant healing benefit for non‐routine venous leg ulcers?
Authors response: The commentator states two studies were “vastly different” but mistakenly compares median ulcer size reported in Jull et al to mean ulcer size reported in Gethin & Cowman, two statistics that are not comparable. The median ulcer area reported in Gethin & Cowman was 5.4 and 4.2 cm2. Ulcer duration is also an important consideration when considering likelihood of healing. The mean duration of ulceration prior to enrolment in the Gethin and Cowman study was 39 weeks and 30 weeks, which was similar to the means in Jull et al (39 and 48 weeks). To suggest therefore that Gethin & Cowman recruited harder‐to‐heal wounds compared to Jull et al is not accurate. These studies were not dissimilar in terms of populations and there is no evidence to suggest that they were different in terms of wound bed appearance – one trial provided information on this factor, another did not. In the Gethin & Cowman study, the statistically significant difference only emerged in the adjusted analysis. The unadjusted analysis for Gethin and Cowman is presented in the systematic review and shows no significant difference (RR 1.33, 95%CI 0.82 – 2.16).
Q7. Regarding honey versus SSD, why should the results of these studies be disregarded as you suggest due to other studies having shown benefit of hydrocolloids and silicone based dressings over SSD? Does this mean that you recommend that no other studies be done comparing one arm to SSD in the treatment of partial thickness burns?
Authors response: There is evidence that suggests SSD cream applied from time treatment initiated until healing may delay healing. (Thomas SS et al. J Wound Care 1995;4:218‐20. Wyatt D et al. J Trauma 1990;30:857‐65. Bugmann P et al. Burns 1998;24:609‐12.) It appears the issue may be how SSD cream is used, not whether it is used. Our focus in the review was in trying to understand whether honey conferred a benefit. Using an appropriate comparator is pertinent to such an analysis and it is not clear that SSD applied daily from treatment initiation to healing is an appropriate comparator.
Q8. Regarding the above, which do you think is the more commonly used product on partial thickness burns, SSD or hydrocolloids? Are you aware of the commonplace usage on SSD in burn centers? If so, why would you suggest disregarding the results of studies which show a benefit over SSD? If not, and you find this to be true (that SSD is still commonly used in burn centers), would you change your decision to disregard the evidence derived from studies utilizing SSD as a control arm?
Authors response: See above
Q9. Also regarding the honey vs SSD studies, can you provide a more thorough clarification on why the CHIT method employed by the investigator disqualified these studies from your analysis?
Authors response: The use of the chit method did not disqualify any trial from consideration. Authors of trials that did not describe the method for generating the random sequence were approached for further information. The author of 11 trials replied stating the sequence was “manually generated random numbers by the chit method”, without providing any further information about what was meant by this approach with respect to sequence generation. We sought the advice of broadly experienced senior biostatistician who could not infer from this response what method of sequence generation was used. We therefore stated in the Risk of bias tables “method of generation of random sequence not reported. The author informed us that the sequence was generated by the ‘chit method’, but it is not clear what this method is.”
Q10. Of the 6 additional studies cited as rationale for this “updated” Cochrane Review, 5 out of the 6 are positive in favor of honey. Why is only the one negative study included in your updated conclusion? How did the biases of this study, as well as the overall structure of the study, compare with the other 5 studies which were not included in your updated conclusion? Were these differences the reason you only cited the negative study?
Authors response: When a review is updated we run the searches again and assess the resulting output. For this update we identified six additional studies which met the inclusion criteria published in the review, and all six studies were included in the review with the results of those studies presented.
Q11. Would someone be wrong if they stated it appears as though your inclusion of only the one negative study, and lack of inclusion of the 5 positive studies, appears to be “cherry picking”?
Authors response: A systematic review is conducted according to a defined, peer reviewed and published protocol to minimize bias. Any studies which are excluded have to be identified in the Table of Excluded studies and the reasons for the exclusion made clear. I am assuming you are referring to the six additional studies which were included in the last update of this review. All six studies are “included studies”.
Q12. Regarding the one negative study, which was on the treatment of cutaneous Leishmaniasis (a wound resulting from a bite from a sand fly indigenous to the Middle East), the study authors note that further studies should be done using standardized medical grade honeys, such as Leptospermum Scoparium (Manuka Honey). They acknowledge the fact that they used plain local honey could have been the reason for not achieving positive results. Why did you not include this in your conclusions or anywhere in your description of the study?
Authors response: The purpose of the review was to summarise the available evidence, not to restate authors’ interpretations.
Q13. Regarding the negative study on honey vs early excision and grafting of mixed partial and full thickness ulcers, to the best of your knowledge, how many studies do you know that compare one dressing vs this surgical standard and show improved results? Given the rest of the consistently positive results of the other honey studies cited for management of partial thickness burns, do you think the results of the early excision and grafting study suggest that honey would in any way be dangerous to use on partial thickness wounds that do not require early excision and grafting? If so, why?
Authors response: This review does not set out to present results of any dressing when compared with early excision and grafting of mixed partial and full thickness ulcers. Clearly there was sufficient equipoise for a trial to be conducted comparing early excision and grafting versus treatment with honey and delayed grafting as necessary. This trial was therefore included in the review.
Q14. Can you please provide the total numbers of the following: How many studies cited in your review had a statistically significantly positive outcome for the honey arm? How many studies cited in your review had a positive outcome (although not significantly) for the honey arm? How many studies had a negative outcome (although not significantly) for the honey arm? How many studies had a statistically significantly negative outcome for the honey arm?
Authors response: The data extracted from the trials and presented in the results section is provided in the review. Counting the number of statistically significant studies is not a sensible approach to summarising evidence when meta‐analysis is possible.
Q15. Given the numbers above, why did you feel that it was important to include in your updated conclusion the following statement; “…purchasers should refrain from providing honey dressings for routine use until sufficient evidence of effect is available”? This statement seems cautionary in its advice. What is the caution that you believe purchasing agents should take into account when considering routine use of honey based dressings?
Authors response: In the light of insufficient evidence a cautionary approach is warranted. Routine use of honey dressings refers to use of honey as a first line dressing.
Q16. Given that Andrew Jull is not only is the author of the key study cited in the Cochrane Review, but that he is also on the editorial board for the wounds group reviewing all Cochrane Reviews on wound care, this question is directed specifically to him in his role as an editor: Due to the lack of well controlled RCTs, the vast majority of Cochrane Reviews on wound dressings / technologies (with the exception of total contact casts) have negative conclusions. The majority of these conclusions all seem to have a “boiler plate” type of statement, basically stating “there is not enough evidence to suggest the use of one product over another.” Given the recent updated conclusion in the review of honey dressings, do you think, if any of the other reviews are similarly “updated” (including those on negative pressure, silver based dressings, alginates, hydrocolloids, foams, etc.), without any meaningful positive results from well controlled studies, do you think that clinicians should similarly be advised to refrain from routine use of those dressings/technologies for routine use until sufficient evidence of effect is available? If not, then why?
Authors response: Andrew Jull is an Editor of the Cochrane Wounds Group; he does not review all Cochrane reviews in wound care and reviews are distributed amongst all the editors and each review is peer reviewed by a number of editors and reviewers. It is not appropriate for the authors of this review to suggest how other authors should write their reviews.
Q17. Why was the statement, “purchasers should refrain from providing honey dressings for routine use until sufficient evidence of effect is available” subsequently changed?
Authors response: This change was in response to feedback received from Barry Wolfenson on behalf of Derma Sciences (14th June 2013) expressing concern at the wording of the Authors' conclusions ‐ as follows: “There is insufficient evidence to guide clinical practice in other types of wounds, and purchasers should refrain from providing honey dressings for routine use until sufficient evidence of effect is available.” This statement had been present in the Authors' conclusions since the review was first published in 2008. Cochrane guidance from the Handbook states "The primary purpose of the review should be to present information, rather than to offer advice". Having considered the feedback and received the advice of the Cochrane Collaboration, we have modified this section to read: "…There is insufficient evidence to guide clinical practice in other areas, and health services may wish to consider avoiding routine use of honey dressings until sufficient evidence of effect is available". Although the primary purpose of a review is to present information, rather than to offer advice, review authors are invited to interpret the findings of their review in the "implications for practice" section. This section is clearly labelled as "authors' conclusions".
Q18. Do you believe the following statement is intended to provide advice; “…Health services may wish to consider avoiding routine use of honey dressings until sufficient evidence of effect is available”? If not, how do you interpret this statement to not be providing advice on usage?
Authors response: Review authors are invited to interpret the findings of their review in the "implications for practice" section and these are clearly labelled as "authors' conclusions". It seems perfectly reasonable where no compelling evidence of benefit has been identified to suggest that decision makers consider this finding. The use of the terms "consider" and "routine" clearly indicate that this is not intended to be a didactic recommendation, simply something for consideration by decision makers.
Q19. How many times is this type of advice given in any of the other Cochrane Reviews regarding wound dressings? Please only limit your response to those Cochrane Reviews that had negative conclusions, such as on alginates, foams, silver dressings, negative pressure, etc… If the answer is 0 (zero), what do you think is the distinction between the evidence presented for honey‐based dressings and the evidence presented for these other dressings/technologies?
Authors response: There is no blueprint for the precise wording of this section, and it is not unusual for Cochrane Reviews to present text that provides guidance that decision makers should consider the absence of high quality evidence of benefit when making treatment decisions.
Q20. Specifically regarding your statement in the Cochrane Review that clinicians may consider refraining from routine use on honey‐based dressings, can you please clarify:
20.1 For routine venous leg ulcers: Should clinicians refrain from using honey‐based dressings? If so, why? Authors response: It is reasonable to highlight the apparent failure of routine use honey dressings to deliver such an important outcome as healing rate.
20.2 For chronic/stalled venous leg ulcers: Should clinicians refrain from using honey‐based dressings? If so, why? Authors response: All venous leg ulcers are chronic. The evidence from the trials does not address “stalled” venous ulcers.
20.3 For other routine ulcers such as pressure ulcers: Should clinicians refrain from using honey‐based dressings? If so, why? Authors response: The results of the review demonstrate clear uncertainty in respect of this outcome.
20.4 For other chronic/stalled ulcers such as pressure ulcers: Should clinicians refrain from using honey‐based dressings? If so, why? Authors response: The evidence from the single available trial does not address “stalled” pressure ulcers.
20.5 For partial thickness burns that do not require early excision and grafting: Should clinicians refrain from using honey‐based dressings? If so, why?Authors response: The results of the review demonstrate clear uncertainty in respect of this outcome.
20.6 For any challenging wounds where a clinician would typically use negative pressure, silver‐based dressings, iodine‐based dressings, alginates, or foams: Should clinicians refrain from using honey‐based dressings? If so, why? Authors response: There were no trials where the wounds were defined as ‘challenging’.
20.7 Do you consider chronic wounds (whether they be venous leg ulcers, diabetic foot ulcers, pressure ulcers, or dehisced surgical wounds) to be “routine”? Authors response: It is not clear what is meant by “routine” in this context – none of the conditions are uncommon.
20.8 Do you consider partial thickness burns that are treated in a burn center to be “routine”? Authors response: It is not clear what is meant by “routine” in this context.
Reply
The authors of the review have responded to this feedback giving a point by point response to the questions above.
Contributors
Barry Wolfenson, Group President, Advanced Wound Care & Drug Development, Derma Sciences. Declaration of interest: I am the President of Advanced Wound Care at Derma Sciences. Thus, I clearly have involvement with an organization with a financial interest in the subject matter of my feedback.
Andrew Jull, Author; Nicky Cullum, Coordinating Editor CWG; Sally Bell‐Syer Managing Editor CWG.
Feedback questions from Laura Bolton, 27 September 2013
Summary
Comment: I strongly support evidence‐based practice and was trained as a Cochrane reviewer in 1995. Am concerned that this SR is eroding Cochrane credibility as so many wound care professionals have asked me to clarify issues in it. Please help correct the following errors as quickly and thoroughly as possible: 1. Errors in describing the studies (omission of important facts or perspective), 2. Arbitrary emphasis on non‐statistically significant or irrelevant or inappropriate findings in abstract and conclusions 3. Omitting statistically significant RCT healing results favorable to honey in abstract and conclusions 4. Statements in the Authors conclusion and Abstract do not seem to reflect the results reported in the body of the SR 5. Inappropriate recommendations to avoid honey use that usurp clinician's point of care decisions rather than inform them of level of evidence supporting honey use for various indications, which appears no less sufficient than that for many other wound care interventions. My Cochrane trainers insisted we avoid recommending and simply describe levels of evidence to empower clinical decision making. Has this changed? To clarify the issues Dr. Janice Beitz and I presented a webinar with more detail on each issue accessible at: http://www.dermasciences.com/pdf/Scientific‐Review‐of‐Cochrane‐Review‐Honey‐as‐Topical‐Treatment‐for‐Wounds‐Dr.‐Bolton‐and‐Dr.%20Beitz.pdf Thank you and the hard working Cochrane Wounds Group for your immediate attention to help uphold Cochrane credibility!
Reply
The authors of the review have considered the feedback and are pleased to report that the review has been fully updated, a new search conducted with two additional studies included. All the data have been checked and the evidence assessed using the GRADE approach and four Summary of Findings Tables added. The Results section has been restructured to bring all outcomes together for each comparison and the conclusions of the review have been slightly amended. This updated review has been peer reviewed.
Contributors
Laura Bolton, Adjunct Associate Professor of Surgery, Rutgers University Medical School. Declaration of interest: I certify that I consult on evidence‐based product development and study design for EuroMed, Derma Sciences and Systagenix, but have never received any corporate funding for honey‐related consulting or speaking nor for my part in presenting the Webinar described.
Andrew Jull, Author; Nicky Cullum, Coordinating Editor CWG; Sally Bell‐Syer Managing Editor CWG.
Comment from Joy Schank, 26 October 2013
Summary
I was concerned to learn of the recent negative review regarding honey and treating wounds. The review did not seem to provide a balance of the available literature. Thank you for considering this comment.
Reply
The authors have carefully considered and incorporated the observations and items of feedback submitted through the “Submit Comments” facility on the Cochrane Library for this review. These comments and the replies from the authors have been retained in this version of the review. This is to enable readers to follow the exchange and to form their own interpretation of the evidence that is now available.
Contributors
Joy Schank, Private Practice, Nurse Practitioner. I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Andrew Jull, Author; Nicky Cullum, Coordinating Editor CWG; Sally Bell‐Syer Managing Editor CWG.
Comment from Jennifer Gardner, 27 March 2014
Summary
Dear Editor: As a Certified Wound Specialist, a Doctor of Physical Therapy with a Masters in Healthcare Administration, and a director of both inpatient and outpatient wound care services at Inspira Health Network in New Jersey I have recently read the Cochrane Review “Honey as a Topical Treatment for Wounds” by Jull et al, 2013. A sales representative that does not sell honey products pointed out the conclusion of the Cochrane review to me stating “health services may wish to consider avoiding routine use of honey dressing until sufficient evidence is available”. For many years I have been using honey dressings on a variety of wounds including pressure ulcers, venous leg ulcers, arterial ulcers, surgical wounds and diabetic foot ulcers. I have authored and presented multiple case series at international wound congresses demonstrating my success with the use of these dressings on a variety of wounds. I have treated hundreds of wounds with honey and have found these dressings to be helpful in the healing of these wounds and have never noted any severe adverse reactions. I was extremely surprised to see that the conclusion to “avoid” usage and question how this recommendation can be made when I have not been able to find evidence nor have experienced any negative issues. Upon my concern with the conclusion I then read with great interest the entire published review. Pertaining to the author’s recommendation to “avoid” usage seems to indicate a safety or efficacy concern. In the AE table published in the review, “ulcer pain” is the only difference in the number of AEs. However, in the review authors’ own study incorporated into the review (HALT study by Jull et al 2007), the authors note that while pain on dressing changes was more frequent in the honey arm, the pain was not severe enough to cause patients to exit the study, suggesting it was well tolerated. This is similar to my own experience, where if a patient does feel pain upon dressing changes, it is short in duration and well tolerated. Additionally, it has been my experience that application of honey helps to reduce the chronic wound pain commonly suffered by venous leg ulcer patients, potentially due to a reduction in inflammation. Thus, I do not see how this could possibly be an issue with regard to use of honey dressings. Can you please clarify what is the reason for authors’ recommendation to avoid usage? There were only four articles out of 25 reviewed that did not favor honey so please clarify what specific concern the authors have that led to that recommendation. I would like to commend the Editors of the Cochrane Review on providing the health care community with a balanced review of clinical information that is helpful in making decisions regarding the treatment of our patients with new procedures and medical products. Unfortunately, this review is inconsistent with my clinical experience as well as the long established excellence of the Cochrane Review. I believe the authors’ conclusion needs to be made clearer. I did agree with the vast majority of the studies having more favorable results in the honey arms of the study but I would also note that the recommendation itself seems out of place in a Cochrane review, and I was very surprised to find it in the review at all.
Reply
The authors of the review have considered the feedback and are pleased to report that the review has been fully updated, a new search conducted with two additional studies included. All the data have been checked and the evidence assessed using the GRADE approach and four Summary of Findings Tables added. The Results section has been restructured to bring all outcomes together for each comparison and the conclusions of the review have been slightly amended. This updated review has been peer reviewed.
Contributors
Jennifer A. Gardner, PT DPT MHA CWS. Manager, Wound Care Services. Inspira Medical Center Woodbury. I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Andrew Jull, Author; Nicky Cullum, Coordinating Editor CWG; Sally Bell‐Syer Managing Editor CWG.
Comment from Kevin Foster, 26 March 2014
Summary
Dear Editor in Chief, I am Burn Surgeon and the Director of one of the largest burn centers in the US, which treats thousands of patients annually. I recently had the pleasure of reviewing your 2013 publication Honey as a topical treatment for wounds (Review) written by Drs. Jull, Walker and Deshpande. While I did enjoy reading the review, I would like to express my concern about the Authors’ Conclusions. As a clinician who regularly treats patients who have suffered from partial‐thickness and full‐thickness burns, I do not share the sentiment expressed by the authors that “health services may wish to consider avoiding routine use of honey dressings until sufficient evidence of effect is available.” In my personal experience, communication with other physicians and reviews of available literature, I have found that honey dressings are quite effective, especially where previous treatments have failed to provide benefit. Additionally, I believe that the authors have put significant weight in their conclusion on one study (Subrahmanyam 1999) which evaluated the use of honey dressings compared to the industry standard for moderate to severe partial‐thickness and full‐thickness burns. It is my opinion, given the vast amount of supportive literature from this author as well as others, that although the results of this study were not in favor of the use of honey dressings, results should not be used to discredit their use. As early excision and grafting is an established practice, it is my opinion that the author was attempting to evaluate honey as an alternative remedy for more troublesome burn injuries. The fact that the study failed to show positive results in this setting informs an upper limit for defining honey dressings as a standard of care treatment in burns and validates the practice of grafting rather than discrediting the use of honey dressings in less severe clinical scenarios. It is evident that honey dressings are not a panacea for all wound types and severity, however, the recommendation that the medical community avoid the use of such dressings is confusing as it is not founded by the clinical safety and efficacy evidence (or lack thereof) provided in the review. Therefore, I would ask that you consider revising the aforementioned conclusion. Thank you for your time and consideration.
Reply
The authors of the review have considered the feedback and are pleased to report that the review has been fully updated, a new search conducted with two additional studies included. All the data have been checked and the evidence assessed using the GRADE approach and four Summary of Findings Tables added. The Results section has been restructured to bring all outcomes together for each comparison and the conclusions of the review have been slightly amended. This updated review has been peer reviewed.
Contributors
Kevin Foster, MD, MBA, FACS. Director Burn Services. The Arizona Burn Center at Maricopa Burn Center Phoenix, Arizona. I certify that I have the affiliations/involvement as described. I have spoken to the sales force for Medihoney, describing my experience with its use. I received an honorarium and travel expenses.
Andrew Jull, Author; Nicky Cullum, Coordinating Editor CWG; Sally Bell‐Syer Managing Editor CWG.
Comment from Dimitrios Lintzeris, 27 March 2014
Summary
To the Editors of the Cochrane Collaboration, After reviewing one of your recent publications, Honey as a topical treatment for wounds (Review), I have become very concerned about some of the language used in the Authors’ Conclusions regarding “avoiding use” of honey dressings. I am a practicing doctor of osteopathy and Medical Director at Wayne Memorial Wound Care Center in North Carolina. Over 120 patients are seen in our clinic on a weekly basis. I regularly use impregnated honey dressings and honey gel for diabetic, venous and pressure ulcers as well as acute and chronic wounds. I have personally experienced positive patient outcomes and no safety or efficacy issues with this intervention. Additionally I have found honey dressings to be cost efficient to go along with a high patient compliance rate. In reading the review, the data presented seems to point towards a positive effect of honey, which correlates with my personal experience. For example, in the pressure ulcer study cited (Weheida 1991), the mean time to healing favored honey with statistical significance. Also in the Diabetic foot ulcer study cited (Shukrimi 2008) the endpoint of time to healing also favored honey. Therefore, it is confusing to see this conclusion and recommendation that “There is insufficient evidence to guide clinical practice in other areas, and health services may wish to consider avoiding routine use of honey dressings until sufficient evidence of effect is available.” It inaccurately gives the reader ‐ especially those who do not read the full review ‐ the impression that this intervention is not safe and efficacious; an impression which is not supported by the data presented or my experience in clinical practice. It would like to suggest that you consider revising the current conclusion to help readers have a more balanced perspective of the body of evidence supporting honey base wound care. I appreciate your consideration of this matter.
Reply
The authors of the review have considered the feedback and are pleased to report that the review has been fully updated, a new search conducted with two additional studies included. All the data have been checked and the evidence assessed using the GRADE approach and four Summary of Findings Tables added. The Results section has been restructured to bring all outcomes together for each comparison and the conclusions of the review have been slightly amended. This updated review has been peer reviewed.
Contributors
Dr. Dimitrios Lintzeris, DO, CWS. Medical Director. Wayne Memorial Wound Care Center. I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Andrew Jull, Author; Nicky Cullum, Coordinating Editor CWG; Sally Bell‐Syer Managing Editor CWG.
Comment from Margarita Simon, 8 October 2013
Summary
Comment: I was handed a copy of the Cochrane Report on medihoney by the Santyl rep in our area. He of course espoused the report's statement that clinicians should cease use of the product as stated in the report. This prompted me to assess carefully tow of the patients which I was overseeing their complex wounds and using medihoney. I can tell you that the wound made outstanding progress with medihoney ‐ in fact remarkable considering both patients were complex and compromised ‐ one with active cancer undergoing radiation and chemontherapy and the other had breast cancer several years before with reconstructive surgery. At no time did they have any failure of their wound care and I have the photos to prove their excellent results. I am appalled at the campaign Smith & Nephew/Healthpoint has directed at medihoney based on the Cochrane Report ‐ a report that is riddled with inconsistencies.
Reply
The authors of the review have considered the feedback and are pleased to report that the review has been fully updated, a new search conducted with two additional studies included. All the data have been checked and the evidence assessed using the GRADE approach and four Summary of Findings Tables added. The Results section has been restructured to bring all outcomes together for each comparison and the conclusions of the review have been slightly amended. This updated review has been peer reviewed.
Contributors
Margarita Simon, MS, FNP‐BC, CWCN. wound care consultant. Simon WOund Consulting, PLLC. I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Andrew Jull, Author; Nicky Cullum, Coordinating Editor CWG; Sally Bell‐Syer Managing Editor CWG.
9. Comment from Herbert B. Slade, 29 May 2015
Summary
Comment: The March 2015 update includes a paper by Gethin (2009, J Clin Nursing) which was retracted in July 2014, and thus it should not be included. The unpublished thesis by Gethin (2007) is only available by visiting the library at the Royal College of Surgeons in Dublin. It should be read and the data carefully evaluated if it is to be included, as readers of the review won't easily be able to draw their own independent conclusions.
Reply
Thank you for your comment. We were aware of the article’s retraction and the reason for the retraction. As noted in the review history, review notes, and the references, we used Gethin’s 2007 PhD thesis as a primary source of information for description of the trial, risk of bias assessment, and outcome data on healing rates and infections. It is worth noting that the extracted data for risk of bias assessments, healing rates, and infections is not in dispute. We used Dr Gethin's data as an input into a meta‐analysis that produced a summary statistic for the two trials being pooled. Our interpretations are based on our meta‐analysis, not any other analyses from primary sources.
Contributors
Herbert B. Slade MD (Email Address: bert.slade@smith‐nephew.com). Affiliation: Univ of North Texas Health Sciences Center, Smith & Nephew. Role: Assoc Clin Prof Pediatrics (UNT), Chief Medical Officer (S&N). I have modified the conflict of interest statement below to declare my interests: I am the chief scientific and medical officer of a medical devices company which markets products for wounds.
Andrew Jull, Author.
What's new
| Date | Event | Description |
|---|---|---|
| 9 June 2015 | Feedback has been incorporated | Feedback submitted and review author response added to the review. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 17 February 2015 | Feedback has been incorporated | The authors have carefully considered and incorporated the observations and items of feedback submitted through the “Submit Comments” facility on the Cochrane Library for this review. These comments and the replies from the authors have been retained in this version of the review. This is to enable readers to follow the exchange and to form their own interpretation of the evidence that is now available. |
| 17 February 2015 | New search has been performed | New search: two new studies included (Gulati 2014; Kamaratos 2014), all evidence assessed using the GRADE approach and four Summary of Findings Tables added. Results section restructured to bring all outcomes together for each comparison. |
| 17 February 2015 | New citation required and conclusions have changed | Conclusions slightly amended. |
| 4 February 2014 | Feedback has been incorporated | Response to feedback |
| 3 October 2013 | Feedback has been incorporated | Feedback received, author team to respond |
| 19 June 2013 | Feedback has been incorporated | Text edits in response to feedback |
| 13 June 2012 | New citation required and conclusions have changed | Six additional studies included (Baghel 2009; Mashood 2006; Memon 2005; Nilforoushzadeh 2007; Robson 2009; Shukrimi 2008), conclusions updated. New author contributed to the review update (Sohan Deshpande) and Anthony Rodgers who contributed to the original review has not contributed to the update. |
| 13 June 2012 | New search has been performed | New search, risk of bias assessment completed on all included studies; primary reference for Gethin 2007 changed to Gethin 2007. |
| 11 August 2009 | Amended | Contact details updated. |
| 13 May 2009 | Amended | Contact details updated. |
| 28 March 2008 | Amended | Converted to new review format. |
Notes
The authors have carefully considered and incorporated the observations and items of feedback submitted through the “Submit Comments” facility on the Cochrane Library for this review. These comments and the replies from the authors have been retained in this version of the review. This is to enable readers to follow the exchange and to form their own interpretation of the evidence that is now available.
During the updating of this review the review authors became aware that the publication Gethin G, Cowman S. Manuka honey vs. hydrogel ‐ a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. Journal of Clinical Nursing 2009;18(3):466‐74 (http://onlinelibrary.wiley.com/doi/10.1111/jocn.12652/abstract) has been retracted by agreement between the journal Editor‐in‐Chief, the authors and John Wiley & Sons, Ltd. The retraction has been agreed due to errors in the data analysis which affect the article's findings. The review authors would like to confirm that the data in this updated review is taken from the source: Gethin G. Manuka honey versus hydrogel ‐ a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. Unpublished PhD thesis 2007.
Acknowledgements
The review authors would like to thank the following referees for their comments on the review, Wounds Group Editors: Mieke Flour, Andrea Nelson and Gill Worthy, referees: Margaret Harrison, Lois Orton, Consumer referee: Durhane Wong‐Rieger. Anthony Rodgers contributed to the original review but was not involved in the updating of the review, we would like to acknowledge his contribution. Elizabeth Royle copy edited the updated review.
The review authors would also like to thank David Tovey and Toby Lasserson of the Cochrane Editorial Unit for their advice and review of this updated review.
Dr Walker is supported by a Heart Foundation Douglas Senior Fellowship in Heart Health (Prevention).
Appendices
Appendix 1. Search Methods for Original Review ‐ 2008
Electronic searches
Searches of the following electronic databases were undertaken:
Cochrane Wounds Group Specialised Register (Searched 27/5/08) The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library Issue 2 2008 Ovid MEDLINE ‐ 1950 to May Week 2 2008 Ovid EMBASE ‐ 1980 to 2008 Week 21 Ovid CINAHL ‐ 1982 to May Week 4 2008
The following search strategy was used in the CENTRAL and adapted where appropriate for other databases :
#1 MeSH descriptor Skin Ulcer explode all trees #2 MeSH descriptor Pilonidal Sinus explode all trees #3 MeSH descriptor Wounds, Penetrating explode all trees #4 MeSH descriptor Lacerations explode all trees #5 MeSH descriptor Burns explode all trees #6 MeSH descriptor Wound Infection explode all trees #7 MeSH descriptor Surgical Wound Dehiscence explode all trees #8 MeSH descriptor Bites and Stings explode all trees #9 MeSH descriptor Cicatrix explode all trees #10 ((plantar or diabetic or heel* or foot or feet or ischaemic or ischemic or venous or varicose or stasis or arterial or decubitus or pressure or skin or leg or mixed or tropical or rheumatoid or sickle cell) NEAR/5 (wound* or ulcer*)):ti,ab,kw #11 (bedsore* or (bed NEXT sore*)):ti,ab,kw #12 (pilonidal sinus* or pilonidal cyst*):ti,ab,kw #13 (cavity wound* or sinus wound*):ti,ab,kw #14 (laceration* or gunshot stab or stabbing or stabbed or bite*):ti,ab,kw #15 ("burn" or "burns" or "burned" or scald*):ti,ab,kw #16 (surg* NEAR/5 infection*):ti,ab,kw #17 (surg* NEAR/5 wound*):ti,ab,kw #18 (wound* NEAR/5 infection*):ti,ab,kw #19 (malignant wound* or experimental wound* or traumatic wound*):ti,ab,kw #20 (infusion site* or donor site* or wound site* or surgical site*):ti,ab,kw #21 (skin abscess* or skin abcess*):ti,ab,kw #22 (hypertrophic scar* or keloid*):ti,ab,kw #23 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22) #24 MeSH descriptor Honey explode all trees #25 honey:ti,ab,kw #26 (#24 OR #25) #27 (#23 AND #26)
The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format. The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network. Additionally, LILACS (1982 to October 2006), AMED (1985 to October 2006) and Google Scholar were searched using the text word "honey".
Searching other resources
Contact was made with experts in the field, authors of the included trials and manufacturers of honey products for wound care (Comvita NZ Ltd and MediHoney Australia Pty Ltd). The bibliographies of all obtained studies and review articles were searched for potentially eligible trials. No language or date restrictions were applied to the trials and both published and unpublished trials were sought.
Appendix 2. Search strategies for Medline, Embase and CINAHL
Ovid Medline
1 exp Skin Ulcer/ (18230) 2 exp Pilonidal Sinus/ (543) 3 exp Wounds, Penetrating/ (14308) 4 exp Lacerations/ (1423) 5 exp Burns/ (17087) 6 exp Wound Infection/ (14097) 7 exp Surgical Wound Dehiscence/ (2829) 8 exp "Bites and Stings"/ (7739) 9 exp Cicatrix/ (13623) 10 ((plantar or diabetic or heel$ or foot or feet or ischaemic or ischemic or venous or varicose or stasis or arterial or decubitus or pressure or skin or leg or mixed or tropical or rheumatoid or sickle cell) adj5 (wound$ or ulcer$)).ti,ab. (18800) 11 (bedsore$ or bed sore$).ti,ab. (239) 12 (pilonidal sinus$ or pilonidal cyst$).ti,ab. (437) 13 (cavity wound$ or sinus wound$).ti,ab. (37) 14 (laceration$ or gunshot or stab or stabbing or stabbed or bite$).ti,ab. (19976) 15 (burn or burns or burned or scald$).ti,ab. (19171) 16 (surg$ adj5 wound$).ti,ab. (5431) 17 (surg$ adj5 infection$).ti,ab. (8723) 18 (wound adj5 infection$).ti,ab. (10745) 19 (malignant wound$ or experimental wound$ or traumatic wound$).ti,ab. (428) 20 (infusion site$ or donor site$ or wound site$).ti,ab. (7460) 21 (skin abscess$ or skin abcess$).ti,ab. (162) 22 (hypertrophic scar$ or keloid scar$).ti,ab. (1595) 23 or/1‐22 (130992) 24 exp Honey/ (1328) 25 honey.ti,ab. (3036) 26 or/24‐25 (3160) 27 23 and 26 (249)
Ovid Embase
1 exp Skin Ulcer/ (30058) 2 exp Pilonidal Sinus/ (883) 3 exp Penetrating Trauma/ (5303) 4 exp Laceration/ (4283) 5 exp Skin Abrasion/ (2135) 6 exp Burns/ (25289) 7 exp Wound Infection/ (17999) 8 exp Surgical Wound/ (3060) 9 exp Wound Dehiscence/ (6273) 10 exp Bite Wound/ (340) 11 exp Scar/ (28923) 12 ((plantar or diabetic or heel$ or foot or feet or ischaemic or ischemic or venous or varicose or stasis or arterial or decubitus or pressure or skin or leg or mixed or tropical or rheumatoid or sickle cell) adj5 (wound$ or ulcer$)).ti,ab. (26847) 13 (bedsore$ or bed sore$).ti,ab. (397) 14 (pilonidal sinus$ or pilonidal cyst$).ti,ab. (628) 15 (cavity wound$ or sinus wound$).ti,ab. (50) 16 (laceration$ or gunshot or stab or stabbing or stabbed or bite$).ti,ab. (26569) 17 (burn or burns or burned or scald$).ti,ab. (26981) 18 (surg$ adj5 wound$).ti,ab. (7562) 19 (surg$ adj5 infection$).ti,ab. (12748) 20 (wound adj5 infection$).ti,ab. (15320) 21 (malignant wound$ or experimental wound$ or traumatic wound$).ti,ab. (559) 22 (infusion site$ or donor site$ or wound site$).ti,ab. (9548) 23 (skin abscess$ or skin abcess$).ti,ab. (278) 24 (hypertrophic scar$ or keloid scar$).ti,ab. (2338) 25 or/1‐24 (183396) 26 exp Honey/ (2368) 27 honey.ti,ab. (4323) 28 or/26‐27 (4693) 29 25 and 28 (337)
EBSCO CINAHL
S29 S25 and S28 S28 S26 or S27 S27 TI honey or AB honey S26 (MH "Honey") S25 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 S24 TI ( hypertrophic scar* or keloid scar* ) or AB ( hypertrophic scar* or keloid scar* ) S23 TI ( skin abscess* or skin abcess* ) or AB ( skin abscess* or skin abcess* ) S22 TI ( infusion site* or donor site* or wound site* ) or AB ( infusion site* or donor site* or wound site* ) S21 TI ( malignant wound* or experimental wound* or traumatic wound* ) or AB ( malignant wound* or experimental wound* or traumatic wound* ) S20 TI wound* N5 infect* or AB wound* N5 infect* S19 TI surg* N5 infection* or AB surg* N5 infection* S18 TI surg* N5 wound* or AB surg* N5 wound* S17 TI ( burn or burns or burned or scald* ) or AB ( burn or burns or burned or scald* ) S16 TI (laceration* or gunshot or stab or stabbing or stabbed or bite*) or AB (laceration* or gunshot or stab or stabbing or stabbed or bite*) S15 TI ( cavity wound* or sinus wound* ) or AB ( cavity wound* or sinus wound* ) S14 TI ( pilonidal sinus* or pilonidal cyst* ) or AB ( pilonidal sinus* or pilonidal cyst*) S13 TI ( bedsore* or bed sore* ) or AB ( bedsore* or bed sore* ) S12 AB ( plantar or diabetic or heel* or foot or feet or ischemia or ischemic or venous or varicose or stasis or arterial or crural or decubitus or pressure or skin or leg or mixed or tropical or rheumatoid or sickle cell ) and AB ulcer* S11 TI ( plantar or diabetic or heel* or foot or feet or ischemia or ischemic or venous or varicose or stasis or arterial or crural or decubitus or pressure or skin or leg or mixed or tropical or rheumatoid or sickle cell ) and TI ulcer* S10 (MH "Cicatrix+") S9 (MH "Bites and Stings+") S8 (MH "Surgical Wound Dehiscence") S7 (MH "Surgical Wound") S6 (MH "Wound Infection+") S5 (MH "Burns+") S4 (MH "Tears and Lacerations") S3 (MH "Wounds, Penetrating+") S2 (MH "Diabetic Foot") S1 (MH "Skin Ulcer+")
Appendix 3. Risk of bias assessment criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of Yes or No, as above.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes that were unsealed, non‐opaque or not numbered sequentially); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of Yes or No, as above. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially‐numbered, opaque and sealed.
3. Blinding was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following:
No blinding, or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Either of the following:
Insufficient information to permit judgement of Yes or No, as above.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not high enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is high enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
As‐treated analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit judgement of Yes or No, as above (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
Not all of the study's pre‐specified primary outcomes have been reported.
One or more primary outcomes reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of Yes or No, as above. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias:
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
stopped early due to some data‐dependent process (including a formal‐stopping rule); or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Minor acute wounds: honey vs. no honey (conventional dressings).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to healing | 3 | 213 | Mean Difference (IV, Random, 95% CI) | 2.26 [‐3.09, 7.61] |
| 2 Adverse events | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.69, 2.05] |
| 3 Infection | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.13, 6.37] |
Comparison 2. Partial thickness burns: honey vs no honey (conventional dressings).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to healing (days) | 2 | 992 | Mean Difference (IV, Fixed, 95% CI) | ‐4.68 [‐5.09, ‐4.28] |
| 2 Adverse events | 2 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.15, 2.06] |
| 3 Negative swab at Day 7‐8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. Burns: honey with delayed graft PRN vs early excision & grafting (no honey).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to healing (days) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 13.60 [9.82, 17.38] |
| 2 Mean duration of antibiotic therapy (days) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [8.85, 23.15] |
Comparison 4. Burns: honey vs silver sulfadiazine (SSD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to healing (days) | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Time to healing (days) | 4 | 332 | Mean Difference (Random, 95% CI) | ‐5.12 [‐9.51, ‐0.73] |
| 2 Proportion burns completely healed | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Burns: honey vs SSD at 4 to 6 weeks | 6 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.98, 1.02] |
| 3 Adverse events | 5 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.20, 0.42] |
| 4 Negative swab at Day 7 | 5 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 3.92 [1.32, 11.63] |
4.2. Analysis.
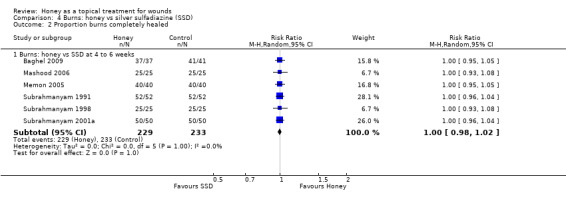
Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 2 Proportion burns completely healed.
Comparison 5. Burns: honey vs. no honey (atypical dressings).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to healing (days) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
5.1. Analysis.
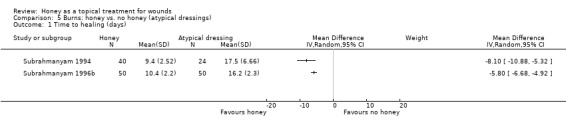
Comparison 5 Burns: honey vs. no honey (atypical dressings), Outcome 1 Time to healing (days).
Comparison 6. Mixed acute and chronic wounds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to healing (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Negative swab at Day 7 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Chronic wounds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion healed | 6 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Infected post‐op wounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pressure ulcers (grade I and II) at 10 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Leishmaniasis at 4 months (16 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Venous leg ulcers at 12 weeks | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Diabetic foot ulcers | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Time to healing (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Fournier's gangrene | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Venous ulcers: proportion healed at 12 weeks | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 4 Venous ulcers: infection | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.49, 1.04] |
| 5 Mixed wounds: proportion healed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Mixed wounds healing 2' intention at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mixed wounds healing 2' intention at 24 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al Waili 1999.
| Methods | Single‐centred, 2‐armed parallel group RCT. | |
| Participants | 50 participants who had had Caesarean sections or hysterectomies. Setting: hospital. Country: United Arab Emirates. Inclusion criteria: acute post‐operative bacterial wound infections confirmed by culture and sensitivity. Exclusion criteria: not reported. | |
| Interventions | Group 1 (n = 26): Yemeni honey covered with dry gauze.
Group 2 (n = 24): 70% ethanol with povidone‐iodine covered with dry gauze.
Treatment duration: not reported, dressing changed 12‐hourly. All participants received systemic antibiotics. |
|
| Outcomes | Complete healing: Group 1: 22/26 (84.6%); definition of complete wound healing included freedom from dehiscence Group 2: 12/24 (50.0%). | |
| Notes | The authors have not responded to requests for additional information. Funding source: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After informed consent the patients were allocated randomly into two groups”. Comment: method of generating the random schedule not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Table 6 shows that all the randomised participants completed follow‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was assumed to have been done and to be acceptable, since there were no drop‐outs reported, and all the randomised participants completed the study. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Low risk | Quote: “Both groups were comparable with regards to age, sex, and duration of symptoms, type and severity of bacterial infections, clinical signs and symptoms and use of systematic antibiotics” Comment: there was no obvious imbalance in the baseline characteristics, and the study seemed to be free from other forms of bias. |
Baghel 2009.
| Methods | Single‐centered, 2‐arm parallel group RCT. | |
| Participants | 78 participants admitted to burn unit of MY hospital Indore over a period of 2 years (June 2006‐June 2008). 1st degree (57%) and 2nd degree burns, Positive swabs at admission: 26/37 (70%) and 34/41 (83%).Setting: hospital. Country: India. Inclusion criteria: 10‐50 years of age, with 1st‐ and 2nd‐degree burns, burn area < 50% of TBSA. Exclusion criteria: patients on chemotherapy, with renal and/or liver failure, immuno‐compromised state and those with bronchial asthma. | |
| Interventions | Group 1 (n = 37): honey dressing (honey covered with gauze) applied daily. Group 2 (n = 41): SSD (cream covered with gauze) applied daily. Treatment duration: not reported, duration of follow‐up 2 months. | |
| Outcomes | Complete recovery which was defined as 'complete healing without scar or contracture' (30/37 in the honey group and 15/41 in the SSD group). Numbers of patients whose burns completely healed, whilst not explicitly reported, can be inferred from Table 3 in the paper (viz. all patients). The duration of follow up was 42 days. Complete healing at 42 days: Group 1: 37/37 ; Group 2: 41/41 Mean time to healing: Group 1: 18.1 days (No SD); Group 2: 32.6 days (No SD). p value for the difference 0.05, allows calculation of standard error: mean difference ‐14.5 (SE 7.28) |
|
| Notes | Complete recovery included healing without scarring or contractures. Formation of soft scar, hypertrophic scar and/or contracture was counted as incomplete recovery. The authors have not responded to requests for additional information. Funding source: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After taking consent from the patients/parents or guardians, patients were randomly attributed into two study groups; Honey group and SSD group . . .”. Comment: method of generation of the random sequence not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Tables 3 and 5 showed there were no drop‐outs, all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since no drop‐outs were reported, and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: although the baseline characteristics were broadly similar, a greater proportion of patients treated with honey were admitted to hospital within 1‐8 h of the burn (65%) compared to the SSD group (11%). At baseline, 70% and 82% respectively had positive swabs for bacteria. The study seemed to be free from other forms of bias. |
Gethin 2007.
| Methods | Multi‐centred, 2‐armed, open label RCT. | |
| Participants | 108 participants recruited February 2003‐January 2006. People with uninfected venous leg ulcers of duration 39.5 and 29.9 weeks for honey and hydrogel respectively; median area 5.4 and 4.2 cm2; Margolis index 0,1,2 = 33%, 30%, 37% for honey group and 46%, 31%, 22% for hydrogel group; 86% and 78% covered in slough. Setting: hospital and community leg ulcer clinics. Country: Ireland. Inclusion criteria: > 18 years, wound area < 100 cm2, > 50% of wound covered by slough, able to provide written informed consent. Exclusion criteria: current wound infection, medicated with antibiotics or steroids for any reason, cavity or malignant lesion. | |
| Interventions | Group 1 (n = 54): monofloral (manuka) honey (Woundcare 18+) at dose of 5 g/20 cm2 plus compression applied weekly.
Group 2 (n = 54): hydrogel (IntraSite) at dose of 3 g/20 cm2 plus compression applied weekly. Treatment duration: 4 weeks, dressing changed with compression. Then both groups received follow‐on treatment according to clinical assessment |
|
| Outcomes | Complete healing at 12 weeks:
Group 1: 24/54 (44.4%);
Group 2: 18/54 (33.3%). The authors' regression analysis was adjusted for Margolis score: they reported an OR 3.1 (95%CI 1.15 to 8.35) and a RR of 1.38 (95%CI 1.02 to 1.88). |
|
| Notes | Healing was a secondary outcome. The primary outcome was change in area of slough at 4 weeks. Funding source: Research and Education Foundation in Sligo General Hospital, European Wound Management Association and the Health Research Board of Ireland. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "78 pieces of both green and yellow card were counted, checked, and shuffled by a person independent of the trial. The card was then inserted into opaque brown envelopes, counted, sealed, and shuffled by another person independent of the trial. The envelopes were then, sequentially numbered. This process represented
generation of an unpredictable allocation sequence. Yellow indicated allocation to the Manuka honey treatment and green allocation to the IntraSite treatment." Comment: shuffling is an adequate method of sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: “Envelopes were given to a person independent of participant enrolment at a remote location. This process ensured allocation concealment. When a person agreed to participate and having met the inclusion criteria and signed the consent form, the recruiting nurse phoned the remote number, gave details of the patient including, name, gender, trial centre, ABPI, percentage of wound covered in slough and ulcer size. The external person then, using the pre‐generated allocation sequence, allocated the trial number for the patient and the treatment allocation." Comment: centrally randomised via remote phone allocation. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Quote: "The consequence of this for the writers RCT is that patients cannot be blinded to the treatment as the intervention is an orange/brown ointment, while the comparator IntraSite GelTM is a clear gel, thus the difference between treatments is obvious." Comment: Blinding of participants not done. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | High risk | Quote: Comment: Blinding of healthcare providers not done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | High risk | Quote: "Blinded outcome assessment is not possible for two reasons. As stated, honey leaves an orange staining on the periwound skin (photo 3.1) which would identify which treatment the patient is receiving and secondly, within the main study centre clinic and in community leg ulcer clinics, a person trained in wound management would not be available toassess wounds. Photographs would not be used for blinded outcome assessment, as the ability to achieve high quality photos from each centre for each patient was not possible. Therefore, the trial would be classified as open label." Comment: primary outcome assessors were not blinded. Quote: “However, to add some element of blinding, the laboratory would not be made aware of which treatment the patient was receiving and the statistician would not be aware of the identity of each group." Comment: Secondary outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Figure 5.2 (PhD p243) shows that no patients were lost to follow‐up, but there were 9/54 and 17/54 withdrawals; 6 and 12 because of infection in the wound. This is small compared with the event rate, so acceptable |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Quote: “Data was analysed on an intention to treat (ITT) basis.”. Comment: ITT analysis had been done, as all the randomised participants were included in the final results. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk |
Quote: “Analysis using independent t‐test determined there was no statistically significant difference between treatment groups for any of the baseline continuous variables such as wound duration, size, patient age, and slough. Chi‐square analysis did not report any statistically significant differences between groups for baseline categorical."
Comment: ulcers in the honey group were larger (median ulcer areas 5.4 cm2 vs 4.2 cm2; mean ulcer areas 10.52 cm2 vs. 9.87 cm2), present for longer (median durations 18 weeks vs 14 weeks; mean durations 39.4 weeks vs 29.9 weeks) and had a greater area covered by slough (85.5% vs 78.2%), however, these factors would affect the likelihood of healing in the honey group. The Margolis index had a higher proportion of score 2 patients in the honey group (37% versus 22%). The study seems to be free from other forms of bias. In addition, the randomised treatments were for only 4 weeks and then treatment was given according to clinical assessment; this was not reported, so no information on comparability of treatments. |
Gulati 2014.
| Methods | Single centre, 2‐armed, parallel group RCT. | |
| Participants | Setting: surgical outpatients, India. Inclusion criteria: people aged ≥ 18 years with chronic wounds of duration ≥ 6 weeks Exclusion criteria: people with wounds with signs of acute inflammation or infection, postoperative wounds, burns, skin graft donor sites, wounds >5cm max diameter, known allergy to honey, povidone iodine or Tegaderm dressing. Osteomyelitis ruled out. Types of wound: 21/42 venous ulcer (50%); 1/42 arterial ulcer (2.4%); 8/42 diabetic ulcer (19%); 2/42 pressure ulcer (4.8%); 9/42 traumatic wound (21.4%); 1/42 not stated (2.4%). |
|
| Interventions | Group I: 23 people. Honey applied to wounds sufficient to fill any cavity. Wounds then covered with film dressing (Tegaderm). Dressings changed on alternate days for 6 weeks. People with venous leg ulcers wore compression “garments”. Group II: 22 people. 10% povidone iodine applied to wounds sufficient to fill any cavity. Wounds then covered with film dressing (Tegaderm). Dressings changed on alternate days for 6 weeks. People with venous leg ulcers wore compression “garments”. |
|
| Outcomes | Wound size (tracings); complete healing; adverse reactions noted. Primary outcome was complete healing at 6 weeks (healing not defined). Secondary outcome was reduction in wound surface area, pain during dressing change (VAS 0 to 10 where 0 no pain), and overall comfort. The latter two are not outcomes for this review. Results only provided for those completing trial (complete case analysis) i.e., 1 lost from honey group and 2 from control group. Group I: 7/22 healed at 6 wks Group II: 0/20 healed at 6 wks Group I: final median area 0.55 (0‐12.1) cm2 Group II: final median area 1.95 (0‐7.8) cm2 Explicitly stated no adverse events with honey; did not state for povidone iodine. |
|
| Notes | Honey from beehive on a neem tree, sterilized by gamma irradiation. Wounds observed at two weekly intervals. Wounds swabbed each visit. They seem to have tested for within not between group differences for 2’ outcomes (change scores within groups). Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “computer generated random numbers using block randomization were used to develop the randomization schedule” |
| Allocation concealment (selection bias) | Low risk | Quote: “The subjects were randomized into two groups – the honey dressing group and the povidone iodine dressing group – with the help of numbered opaque sealed envelopes” |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Quote: "There was no blinding" |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | High risk | Quote: "There was no blinding" |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | High risk | Quote: "There was no blinding" |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Three people (total sample size 45) were excluded from the analysis (1 from the honey group and 2 from the povidone iodine group) (7% of those randomised). This is unlikely to impact on the findings. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: Three people were excluded from the final analysis as above. No ITT analysis but unlikely to have had a substantial impact on results. |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Low risk | Comment: The two groups seemed comparable for important prognostic factors at baseline. |
Ingle 2006.
| Methods | Single‐centred, 2‐armed, double‐blind, parallel group RCT. | |
| Participants | 87 participants with uninfected shallow wounds and abrasions recruited September 1995‐July 1996. Setting: community. Country: South Africa. Inclusion criteria: patients with wounds < 2 cm deep, abrasions 10 cm2‐100 cm2 (including donor sites for skin grafting and partial‐thickness burns). Exclusion criteria: patients with wounds > 100 cm2; unwilling to have an HIV test; infected wound; genital or malignant ulcers; wounds on legs, perineum, fingers or toes that would make measurement difficult; systemic disease; chronic alcoholism. | |
| Interventions | Group 1 (n = 40; 25 shallow wounds and 15 abrasions, stratified randomisation): monofloral (Aloe vera) honey, covered with OpSite dressing, applied daily. Group 2 (n = 42; 25 shallow wounds 17 abrasions, stratified randomisation): hydrogel (IntraSite), covered with OpSite dressing, applied daily. Treatment duration: until complete healing (abrasion) or wound < 3 cm2 (shallow wound). | |
| Outcomes | Mean time to healing (all wounds) ‐ information supplied by authors:
Group 1: 16.48 days (SD 8.40); all wounds healed
Group 2: 16.88 days (SD 11.31); all wounds healed Costs (average costs of dressing materials only): Group 1: average cost per patient 0.49 Rand Group 2: average cost per patient 12.06 Rand |
|
| Notes | Diet supplemented with zinc sulphate and vitamins A, B and C for all participants. 5 participants excluded from analysis after randomisation. Funding source: Not reported although it is noted that research presented was one of the author's degree dissertation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “then randomised (using random permuted blocks of size 10) to treatment with either honey or IntraSite Gel”. Comment: method of generation of random schedule not clearly reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Low risk | Quote: “A prospective, randomised, double‐blind controlled trial was carried out by authors . . . Patients did not know which agent was being used”. Comment: the study is described by the authors as double blind although quite how blinding was maintained is not reported. Nevertheless we have rated this as low risk for performance bias on the basis of the blinding. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Quote: “A prospective, randomised, double‐blind controlled trial was carried out by authors . . . Patients did not know which agent was being used”. Comment: whilst reported as double‐blind, the information given was insufficient to permit judgement as it did not state whether the healthcare providers were blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Quote: “A prospective, randomised, double‐blind controlled trial was carried out by authors . . . When the healing endpoint was approaching [KP] measured the surface area daily, still blinded, the applied agent from the previous day having been washed off with normal saline”. Comment: Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as either high or unclear risk for blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Quote: “Of 87 patients enrolled, 5 were excluded from the analysis . . . ”. Comment: the loss to follow‐up was less than 10%, and judged to be acceptable. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | High risk | Quote: “Of 87 patients enrolled, 5 were excluded from the analysis . . .”. Comment: ITT analysis was not done, as 5 of the 87 patients defaulted, and were not included in the results. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Low risk | Quote: “The composition of the groups did not differ significantly in terms of recorded characteristics”. Comment: there was no imbalance in the baseline characteristics and the study seemed to be free of other forms of bias. |
Jull 2008.
| Methods | Multi‐centred, 2‐armed, parallel group RCT. | |
| Participants | 368 participants with venous (97% honey and 98% control) or mixed venous/arterial leg ulcers recruited May 2004‐September 2005. Infection levels at baseline were not reported. The median wound size was 2.7 and 2.6 cm2 for honey and control groups respectively, and the median duration of ulcer was 20 and 16 weeks; the Margolis index was 0,1,2 = 46%, 37%, 17 % for both groups. Setting: community nursing services. Country: New Zealand. Inclusion criteria: venous ulcer (clinical presentation + AB I > 0.8) or mixed venous/arterial ulcer (clinical presentation + ABI > 0.7), receiving compression, able to provide informed consent, residing in one of 4 study regions. Exclusion criteria: diagnosis of diabetes, rheumatoid arthritis or significant peripheral arterial disease, allergy to honey or calcium alginate, currently using honey treatment. | |
| Interventions | Group 1 (n = 187): monofloral (manuka) honey‐impregnated calcium alginate dressing (ApiNate) + compression bandaging system normally available at study centre.
Group 2 (n = 181): usual care: choice of any dressing clinically indicated + compression system normally available at study centre. Treatment duration: until healing or 12 weeks, dressing changed with compression. |
|
| Outcomes | Complete healing at 12 weeks:
Group 1: 104/187 (55.6%);
Group 2: 90/181 (49.7%). Hazard Ratio (unadjusted) 1·1 (95% CI 0·8 to 1·5); P = 0·451 Adverse events: Group 1: 111/187 Group 2: 84/181 Incidence of infection: Group 1: 32/187 (17.1%) Group 2: 40/181 (22.1%) HRQoL (SF‐36, Charing Cross Venous Ulcer Questionnaire (CXVUQ), EQ‐5D): SF‐36 Physical Component Summary (PCS) Mean difference 1.1 (‐0.8 to 3.0); p=0.0256 SF‐36 Mental Component Summary (MCS) Mean difference 0.7 (‐1.1 to 2.4); p=0.0437 CXVUQ (overall) Mean difference ‐1.6 (‐4.2 to 0.9); p=0.204 EQ‐5D VAS Mean difference 1.6 (‐1.5 to 4.7); p=0.313 Cost effectiveness: ICER (NZD) ‐$9.45 ($39.63 to $16.07) in favour of honey (including hospitalizations ‐ 3 in Group 1 for total of 10 days vs. 6 in Group 2 for total of 40 days) ICER (NZD) $11.34 ($‐$2.24 to $26.25) in favour of usual care (excluding hospitalizations) |
|
| Notes | No difference between groups, change in ulcer area, or health‐related quality of life. Funding source: Health Research Council of New Zealand and Comvita New Zealand. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The allocation sequence was stratified by study centre and Margolis index using minimization”. Comment: sequence generated using minimization technique. |
| Allocation concealment (selection bias) | Low risk | Quote: “Participants were randomly assigned to one of two groups by an independent central telephone service”. Comment: allocation concealed using an independent central telephone service. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Quote: “open‐label, multicentre randomised controlled trial was conducted”. Comment: open label RCT, so blinding of participants not done. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | High risk | Quote: “open‐label, multicentre randomised controlled trial was conducted”. Comment: open label RCT, so blinding of healthcare providers not done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | High risk |
Quote: “The primary outcome measure was the proportion of participants with completely healed reference ulcers at 12 weeks, as determined by the research nurse. The research nurse was not blind to allocation”. Comment: primary outcome assessor not blinded. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Quote: “Three hundred and sixty two (98.4%) participants were followed up at 12 weeks. Six participants were lost to follow up, all in the usual care group. Two participants died (both for reasons unrelated to treatment), three participants moved out of the area (one to Samoa, one to England and one within New Zealand, but could not be traced) and one participant could not be contacted. All participants who withdrew from treatment with honey were followed up at 12 weeks.”. (NB this quote comes from p.90 of Jull's PhD thesis). Comment: The overall loss to follow‐up was < 10% and judged to be acceptable. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Quote: “The primary analysis was by intention to treat, with all participants included, and participants lost to follow‐up deemed treatment failures”. Comment: ITT analysis was done, as all the randomised participants were included in the final results. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. The trial was registered in a publicly available trials register (ISRCTN06161544). |
| Other bias | Low risk | Quote: “Baseline data were similar for both study groups”. Comment: there was no imbalance in the baseline characteristics and the study seemed to be free from other forms of bias. |
Kamaratos 2014.
| Methods | Single centre, two‐armed trial (alternate allocation) with 16 weeks follow up. | |
| Participants | Setting: Diabetic foot ulcer clinics (outpatients) in tertiary hospital, Greece. Inclusion criteria: people with Type II diabetes and neuropathic foot ulcers of Wagner grade I and II. Exclusion criteria: allergy to honey/bee products; end stage renal failure; serious medical illness; chronic steroid treatment; ABPI of less than 0.9 Note – all participants had a positive swab culture at initial visit. |
|
| Interventions | Debridement on initial visit and when necessary thereafter. Group I: 32 people Medihoney tulle dressing. Group II: 31 people allocated saline soaked gauze. Wounds dressed on daily basis initially then reducing frequency as necessary. All patients received off loading. |
|
| Outcomes | Wound area (max length x max width by two independent observers in duplicate). Swabs cultured on weekly basis. PEDIS system for assessment of wound infection (Perfusion, Extent, Depth tissue loss, Infection, Sensation). Use of antibiotics. Report number of wounds healed (healing not defined). Results: Group I: 31/32 completely healed Group II: 28/31 completely healed Negative wound swabs at 4 weeks: Group I: 32/32 Group II: 27/31 No mention of adverse events or side effects. |
|
| Notes | The mean duration of healing data have been ignored as not everyone healed and survival methods we e not used (therefore disregarded non healers). Healing was not defined. Funding source: Diabetes Center Research Fund. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: “The patients were randomly assigned to two groups…The first patient was enrolled in group I and the subsequent patients were enrolled between groups II and I in alternating fashion” Comment: Although in principle alternate allocation of patients to groups may result in randomised groups, the sequence is entirely predictable and therefore open to manipulation. |
| Allocation concealment (selection bias) | High risk |
Quote: “The patients were randomly assigned to two groups…The first patient was enrolled in group I and the subsequent patients were enrolled between groups II and I in alternating fashion” Comment: The allocation was not concealed and therefore at high risk of selection bias. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Unclear risk | Comment: Not stated. Study described as “double blind” but treating staff were clearly aware of which dressings were being used they were merely unaware of the protocol. Not clear the extent to which patients were aware as the dressings may have appeared similar to them however they had provided “written informed consent”. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | High risk |
Quote: “Preparation and application of dressings were performed by qualified nurses unaware of the study protocol”. Comment: This implies they could see what the dressings were but were unaware of the detail of the study therefore the potential for performance bias is there. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk |
Quote: “Patients were followed up in the outpatient diabetic foot clinic by a distinct research team unaware of the study protocol”. Comment: This implies that those measuring wound progress did not see the dressings removed or reapplied but not sufficiently clear. Furthermore both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as either unclear or high risk for blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | The report implies that all patients remained in follow up those this is not explicitly stated. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | The report does not specifically state that ITT analysis was undertaken however there do not appear to have been dropouts and it is implied that people were analysed in the groups to which they had been allocated. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. Whilst most of the outcomes stated in the methods section were reported in the results, the PEDIS data were not reported. |
| Other bias | Unclear risk | Study report thin on detail; only baseline comparability data are for mean ages, HbA1c of patients in each group which were similar. |
Marshall 2005.
| Methods | Single‐centred, 2‐armed, single‐blind, parallel group RCT. | |
| Participants | 51 participants. Setting: outpatient clinic. Country: England. Inclusion criteria: patients suitable for toenail removal (unilateral or bilateral, partial or total) with matrix phenolisation. Exclusion criteria: unable to give informed consent, unable to attend follow‐up clinics, peripheral vascular disease, peripheral neuropathy. | |
| Interventions | Group 1 (n = 27): monofloral (jarrah) honey dressing (honey covered with gauze) daily. Group 2 (n = 24): povidone iodine (Inadine) dressing daily. Treatment duration: until complete epithelialisation of nail bed. | |
| Outcomes | Mean time to healing: Group 1: 33 days (SD 15.71); 23 patients Group 2: 25days (SD 8.70); 21 patients | |
| Notes | Imbalance in numbers of diabetics in honey group compared to comparison treatment (9 vs 4) and in total avulsions (16 vs 7) both of which favoured the comparison treatment. Funding source: Not reported ‐ appears that a company provided some trial material. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “random tables were used to determine group allocation”. Comment: method of generation of random schedule adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: “Those given written informed consent were randomly assigned to the intervention groups by telephone randomisation. This involved a phone call to an independent assistant located outside of the clinical settings with no prior knowledge of the participants”. Comment: allocation concealed using an independent central telephone service. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Quote: “The study was single blind trial. While the operating clinician and the patients could not be blinded to the intervention . . .”. Comment: blinding of participants not done. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | High risk | Quote: “The study was single blind trial. While the operating clinician and the patients could not be blinded to the intervention . . .”. Comment: blinding of healthcare providers not done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Quote: “ . . . the outcome assessor was unaware of group allocation”. Comment: although outcome assessors were described as blinded to treatment, at least two trialists (Gethin and Jull) have reported that discolouration of the peri‐ulcer skin by honey unblinds the outcome assessors so we have graded as unclear risk. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | High risk | Quote: “A total of 7/51 participants withdrew from the trial: 4/27 in the honey group, of which 2/4 were lost to follow up and 2/4 were withdrawn due to non‐compliance. In the iodine group, 3/24 withdrew from the trial; 1/3 lost to follow up, 1/3 withdrawn for non‐compliance, and 1/3 required further surgical intervention.” Comment: reasons for drop‐outs were given, but the rate was > 10%, and judged to be unacceptable. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | High risk | Quote: “All the seven participants were excluded from the primary analysis.” Comment: ITT analysis not done, as the randomised participants were not all included in the final analyses. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | High risk | Quote: “In respect of prognostic factors randomisation allocated more patients who smoked (7 vs 3), and more patients with diabetes (9 vs 4) to the honey group”. Comment: there was baseline imbalance with respect to demographics, as more participants who smoked and had diabetes were allocated to the honey group and there were more total avulsions in the honey group (16 vs 7). |
Mashood 2006.
| Methods | Single‐centered, 2‐armed, parallel group RCT. | |
| Participants | 50 participants recruited September 2002‐August 2003. Setting: hospital. Country: Pakistan. Inclusion criteria: patients of all ages who sustained superficial and partial‐thickness burns of < 15% TBSA, and with no co‐morbidities present. Exclusion criteria: patients with deep burns and those who sustained burns of > 15% TBSA, whether was superficial or deep. | |
| Interventions | Group 1 (n = 25): honey (pure, undiluted, unprocessed; covered with gauze), applied daily. Group 2 (n = 25): 1% SSD (cream covered with gauze), applied daily. Treatment until healed. Duration of follow‐up: 6 months. | |
| Outcomes | Complete healing in weeks:
Group 1: 2 weeks = 13/25, 4 weeks = 25/25;
Group 2: 2 weeks = 5/25, 4 weeks = 15/25, 6 weeks = 25/25. Cost of treatment/% TBSA: Group 1: 0.75 Rupees (5ml honey) Group 2: 10 Rupees (2g SSD) |
|
| Notes | Patients with deep partial‐thickness burns were included. The authors have not responded to requests for additional information. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “They were randomly assigned to two groups. Each group contained 25 patients”. Comment: method of generating the random sequence not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Tables 1 and 2 show that there were no drop‐outs; all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since no drop‐outs were reported, and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: the baseline characteristics were not reported, so there was insufficient information to judge whether any other important form of bias existed. |
McIntosh 2006.
| Methods | Single‐centred, 2‐armed, double‐blind, parallel group RCT. | |
| Participants | 100 participants. Setting: outpatient clinic. Country: England. Inclusion criteria: patients suitable for toenail surgery (unilateral or bilateral, partial or total) with matrix phenolisation. Exclusion criteria: age < 16 years, unable to give informed consent, unable to attend follow‐up clinics, communication barriers, unsuitable for toenail surgery (patients with peripheral vascular disease, unstable diabetes, or where local anaesthetic was contra‐indicated). | |
| Interventions | Group 1 (n = 52): monofloral (manuka) honey‐impregnated calcium alginate dressing (ApiNate) twice weekly.
Group 2 (n = 48): paraffin‐impregnated gauze (Jelonet), twice weekly. Treatment duration: until healed. |
|
| Outcomes | Mean time to healing: Group 1: 40.30 days (SD 18.21); 47 patients analysed Group 2: 39.98 days (SD 25.42); 40 patients analysed | |
| Notes | Funding source: not reported but declarations of interest are noted as 'none'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Random tables were used to determine intervention allocation”. Comment: method of generation of random schedule adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: “Participants were assigned to intervention groups by remote randomisation. This involved a telephone call to an independent assistant located outside of the study setting who had no prior knowledge of the participants”. Comment: allocation concealed using an independent central telephone service. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Low risk | Quote: “This was a double blind study. Both the outcomes assessors and participants were blind to the intervention throughout. Removal and application of all dressings were performed in a treatment group with only the investigator and participant present; a screen concealed the participant’s feet during dressing removal and application”. Comment: blinding of participants was done. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | High risk | Quote: “Removal and application of all dressings were performed in a treatment group with only the investigator and participant present”. Comment: blinding of healthcare providers not done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Quote: “This was a double blind study. Both the outcomes assessors and participants were blind to the intervention throughout ... All evidence of the intervention was removed and wounds were irrigated before the outcome assessors entered the room”. Comment: Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as either unclear or high risk for blinding of outcome assessors. . |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | High risk | Quote: “A total of 13/100 participants withdrew from the trial: 5/52 from the honey group (one was lost to follow‐up and four withdrew because of non‐concordance) and 8/48 from the paraffin tulle gras group (five were lost to follow‐up and three withdrew due to non‐concordance)". Comment: Reasons for drop‐outs were given, but the rate was more than 10% and judged to be unacceptable. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | High risk | Quote: “All 13 withdrawals were excluded from primary analyses”. Comment: ITT analysis not done, as not all the randomised participants were included in the final analyses. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Quote: “There were disparities in baseline demographics. Established prognostic factors differed between groups: more smokers were assigned to the paraffin tulle gras group, and more diabetics to the honey group.”. Comment: whilst the study report stated that there were more smokers assigned to the paraffin tulle gras group, the data in Table 5 (baseline demographics) indicates that slightly more smokers were assigned to the honey group but the difference was not great and unlikely to impact on the results (33% of the honey group were smokers in Table 5 vs 27% of the control group). |
Memon 2005.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 80 participants with superficial‐dermal, mid‐dermal or deep‐dermal burns recruited January 2002‐December 2003. Setting: hospital. Country: Pakistan. Inclusion criteria: age 4‐62 years, TBSA burnt 10‐40%. Exclusion criteria: patients with chemical or electrical burns, superficial burns, full‐thickness burns or burns involving > 40% TBSA. | |
| Interventions | Group 1 (n = 40): natural, unprocessed honey‐gauze dressings every other day. Group 2 (n = 40): SSD‐dressings (SSD cream covered with occlusive dressing) every other day. Treatment duration: not reported. Follow‐up duration: until healed. |
|
| Outcomes | Number healed: Group 1: by day 16 (n = 20), by day 26 (n = 12), by day 30 (n = 8). Mean 15.3 days (no SD). Group 2: by day 20 (n = 16), by day 36 (n = 18), by day 46 (n = 6). Mean 20.0 days (no SD). | |
| Notes | The authors have not responded to requests for additional information. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were allotted at random in two different groups”. Comment: in addition, it was reported in the abstract that the design was "a quasi‐experimental study". The method for generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Tables 4 and 5 showed there were no drop‐outs; all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since no drop‐outs were reported and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: there was some baseline imbalance in baseline characteristics. More participants with burns to 16‐25% TBSA were randomised to the SSD group (20 (50%) vs 14 (35%)). The honey group had more participants with burns 10‐15% TBSA (18 (45%) vs 12 (30%). More participants in the SSD group had deep‐dermal burns (20 (50%) vs 16 (40%)), whereas more participants in the honey group had superficial‐dermal burns (18 (45%) vs 12 (30%)). |
Mphande 2007.
| Methods | Single‐centred, 2‐armed, quasi‐randomised controlled trial. | |
| Participants | 40 participants with open or infected wounds (chronic osteomyelitis n = 7, post‐surgical n = 14, ulcer n = 8, trauma n = 9, abscess n = 2) recruited February‐November 2005. Setting: hospital with outpatient follow‐up. Country: Malawi. Inclusion criteria: not reported. Exclusion criteria: lived too far from hospital for follow‐up. | |
| Interventions | Group 1 (n = 22): honey‐soaked gauze daily; frequency reduced after 1 week if wound healing progressing. Group 2 (n = 18): sugar covered with gauze dressing; frequency reduced after 1 week if wound healing progressing. Treatment duration: not reported. Follow up duration: not reported. | |
| Outcomes | Median time to complete healing: Group 1: 31.5 days (no SD); Group 2: 56.0 days (no SD). | |
| Notes | Funding source: not reported but declarations of interest are noted as 'none'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Patients were randomised to receive a honey or sugar dressing. They were allocated to one of the two groups on an alternating basis at admission”. Comment: method of generating sequence was not random and, therefore, not adequate. |
| Allocation concealment (selection bias) | High risk | Quote: “They were allocated to one of the two groups on an alternating basis at admission”. Comment: allocation was judged to have been inadequately concealed, as alternation was used. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Unclear risk | Comment: the study did not state whether there were any drop‐outs, or whether all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Unclear risk | Comment: no drop outs or withdrawals were reported. The total numbers of participants assessed were also not reported. We cannot judge whether an ITT analysis was conducted. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Quote: “The honey group comprised 22 patients (13 males and nine females) with a mean age of 12.7 years (range 1–39). The sugar group comprised 18 patients (12 males and six females) with a mean age of 13.8 years (range 3–53).There was a range of causes of wounds, but their distribution was similar between the two groups.”. Comment: age, sex and types of wounds similar between the two groups, but no information was reported on other baseline characteristics. |
Nilforoushzadeh 2007.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 100 participants with confirmed cutaneous Leishmaniasis. Setting: skin disease and Leishmaniasis research centre. Country: Iran. Inclusion criteria: patients with confirmed cutaneous Leishmaniasis with direct smear, no history of systemic or topical therapy for cutaneous Leishmaniasis, absence of malnutrition or severe predisposing disease such as cardiac, renal or hepatic disease and other contraindication for glucantime. Exclusion criteria: pregnant and lactating women, lesions < 3 months old, and patients treated with drugs that interact with glucantime. | |
| Interventions | Group 1 (n = 45): intralesional injection of meglumine antimoniate (glucantamine) once weekly and dressed with honey‐soaked gauze twice daily. Group 2 (n = 45): intralesional injection of meglumine antimoniate (glucantamine) once weekly. Treatment duration:until complete healing of wounds, or maximum 6 weeks. | |
| Outcomes | Complete healing:
Group 1: 23/45 (51.1%);
Group 2: 32/45 (71.1%). Mean time to healing in days: Group 1: 7.04 (± 3.09); Group 2 : 6.30 (± 2.29) However because not all participants healed, the mean time to healing data cannot be used. |
|
| Notes | This RCT reported that, initially, 100 patients were confirmed with cutaneous Leishmaniasis and were randomised into two groups. Further on, in the results section of the abstract, the authors report that each group had 45 patients and stated that 10 patients left the study. They also reported that 23 patients in the honey‐treated group achieved complete cure, whilst in the glucantime alone group 32 patients achieved complete cure. 13 patients from the honey‐treated group left the study, as did 10 patients from the glucantime group. Funding source: Skin Disease and Leishmaniasis Research Center. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomised into 2 groups, using Random allocation software. (version 1.0, may 2004; Saghaei)”. Comment: method of generation of random sequence adequate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated. However the difference in the interventions means that blinding unlikely. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | High risk | Comment: not stated. However the difference in interventions means that blinding unlikely. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Quote: "If the patients had not achieved complete healing after 6 weeks of the treatment, direct smear and culture were performed again. Diameter of the lesion and size of the erythema, induration and ulcer were measured by use of the millimeter papers. These evaluation performed by the investigators who were blinded to the type of treatment". Comment: The paper is ambiguously written and could mean that outcome assessors were only blinded after 6 weeks. Furthermore both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as either unclear or high risk for blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | High risk | Quote: "Overall, in the topical honey treated group, 13 [out of 45] patients left out [sic] the study. One patient (7.7%) left out the study because of contact dermatitis to honey and 12 patients left out of the study because of progression of their lesions. In the glucantime treated group, 10 patients [out of 45] left out [sic] the study because of progression of their lesions". Comment: reasons for drop‐outs provided, but the rate was > 10%, and judged to be unacceptable. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | High risk | Comment: the study stated that 100 participants were randomised, but there were only 45 included in each arm (see table 1 baseline demography). Percentages were inaccurate because the denominators used were 45. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: age, sex, number and location of lesions similar between the two groups, but no information reported on potentially prognostic baseline characteristics, such as size of lesions. |
Robson 2009.
| Methods | Single‐centred, parallel group, open‐label RCT. | |
| Participants | 105 participants recruited September 2004‐May 2007. Patients comprised: 73/105 with leg ulcers (69.5%); 15/105 with breast wounds (14.3%); 17/105 with "other" types of wound including donor sites, foot ulcers and surgical wounds (16.2%). Setting: large district hospital. Country: United Kingdom. Inclusion criteria: patients with a wound healing by secondary intention. Exclusion criteria: patients with: diabetes, history of neuroses, psychoses or dementia, known allergy to bee/honey products, venous ulcers of < 12 week duration, Grade 1 or Grade 4 pressure ulcers (EPUAP grades), wounds containing exposed tendon, muscle or bone, or wounds where malignancy was present or suspected; patients with an existing wound infection requiring systemic antibiotics and those who had received antibiotic therapy in the preceding 2 weeks. |
|
| Interventions | Group 1 (n = 52): manuka honey covered with low adherent dressing plus compression where clinically indicated. Group 2 (n = 53): conventional treatment; if wounds had slough or necrosis, they were treated with hydrogel; compression when indicated Duration of treatment 24 weeks. | |
| Outcomes | Healing rate at 24 weeks: Group 1: 38/52 (72.7%); Group 2: 34/53 (63.3%). Healing rate at 12 weeks: Group 1: 24/52 (46.2%); Group 2: 18/53 (34.0%). Unadjusted hazard ratio for healing (HR) 1.30 (0.77 to 2.19) Adjusted for sex, wound type, age and wound area at start of treatment: HR 1.51 (95%CI 0.88 to 2.58) |
|
| Notes | 52 patients were randomized to receive honey and 53 to receive conventional treatment. 2 patients randomized to the honey group (3.8%) did not receive honey (1 as a result of the patient’s decision, 1 as a result of a clinical decision), and 6 allocated to the conventional treatment group (11.3%) received honey (all except 1 as a result of a clinician’s decision). Funding source: Aintree University Hospitals NHS Foundation Trust,The Florence Nightingale Trust and Huntleigh. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Allocation to treatment was determined using blocked randomisation (with sequence produced using computer software (STATA version 8.2; StataCorp, College \station, TX, USA) with randomly varying block size), stratified by two factors, age (< 40 and ≥ 40 year old) and size of wound (< 10 and ≥ 10 cm2)”. Comment: method of generation of random schedule adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: “Sealed, opaque, serially numbered envelopes were produced from the randomisation sequence for each stratum separately, and an independent third party with access to the envelopes was contacted by telephone to determine treatment allocation as patients were recruited”. Comment: allocation concealed using sealed, opaque, serially numbered envelopes and an independent central telephone service. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Quote: “The study was designed as a single centre, open‐label, randomized controlled trial”. Comment: open label RCT, so blinding of participants not done. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | High risk | Quote: “The study was designed as a single centre, open‐label, randomized controlled trial”. Comment: open label RCT, so blinding of healthcare providers not done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | High risk | Quote: “The study was designed as a single centre, open‐label, randomized controlled trial”. Comment: open label RCT, so blinding of outcome assessors not done. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Quote: “One patient in the honey group (1.9%) was lost to follow up (as they moved to an alternative hospital) and one patient died in each group (1.9% in each group)”. Comment: The three patients who were lost to follow up were included in the analysis until they were lost. The small numbers are unlikely to impact on the results. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Quote: “Statistical analysis was carried out on an intention to treat (ITT) basis, retaining patients in their randomised treatment groups regardless of the actual treatment received and including protocol violators and ineligible patients”. Comment: ITT analysis was done, as all the randomised participants were included in the final results. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Low risk | Comment: there was no imbalance in the baseline characteristics, and the study seemed to be free from other forms of bias. |
Shukrimi 2008.
| Methods | Single‐centered, 2‐armed, parallel group RCT. | |
| Participants | 30 participants. Setting: hospital. Country: Malaysia. Inclusion criteria: all non‐Insulin dependent diabetes mellitus patients (NIDDM) with Wagner grade II ulcers who were admitted for surgery were enrolled if the following parameters were met: age 35‐65, transcutaneous oxygen tension > 30 mmHg and serum albumin level of > 35 g/dl. Exclusion criteria: multiple medical co‐morbidity, steroid therapy, neutrophil count < 2000/mm3. | |
| Interventions | Group 1: clean non‐sterile pure honey (for food), covered with gauze, applied daily. Group 2: povidone‐iodine soaked gauze applied daily. Duration: until wound closure. | |
| Outcomes | Mean time to readiness for surgical closure or further debridement: Group 1: 14.4 (no SD; range 7 to 26 days); Group 2: 15.4 (no SD; range 9 to 36 days). | |
| Notes | The study did not state the number of participants randomised to each arm, or the percentage of wounds healed in each group. Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomised to two dressing arms; honey dressing group and standard dressing group”. Comment: method of generating the random schedule not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Quote: “All the wounds were assessed every other day by a surgeon blinded to the material of dressing”. Comment: Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as either unclear or high risk for blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Unclear risk | Comment: the study did not report the numbers allocated to each treatment, drop‐outs, or state whether all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Unclear risk | Comment: the study did not report the numbers allocated to each treatment, drop‐outs or withdrawals. The total numbers of participants assessed were not reported either. We cannot judge whether an ITT analysis was conducted. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: the baseline characteristics were not reported, so there was insufficient information to judge whether any important form of bias existed. |
Subrahmanyam 1991.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 104 participants with burns < 40% TBSA (mean 26.5 and 27.2%) recruited July 1988‐December 1989. 43/50 and 41/52 had positive swab cultures at baseline Setting: hospital. Country: India. Inclusion criteria: superficial burns. Exclusion criteria: not reported. | |
| Interventions | Group 1 (n = 52): unprocessed, undiluted honey dressings (pure undiluted unprocessed honey covered with gauze); applied daily. Group 2 (n = 52): SSD‐impregnated gauze daily. Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 9.4 days (SD 2.3); Group 2: 17.2 days (SD 3.2). | |
| Notes | Information on allocation method, allocation concealment, blinding, mean TBSA, mean time to healing and standard deviation for mean time to healing were provided by the author. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The cases were allocated at random to two groups”. Comment: method of generating random sequence not reported. Author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was by means of sequentially‐numbered, sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated in study report, but author responded to request for further information by stating the patients were not blinded. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all as unclear risk for blinding of outcome assessors. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response. However both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as at either unclear or high risk for blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Table 2 showed there were no drop‐outs, and that all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since no drop‐outs were reported, and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Low risk | Comment: there was no imbalance in baseline characteristics, and the study seemed to be free from other forms of bias. |
Subrahmanyam 1993a.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 92 participants with burns < 40% TBSA (mean 22.8 and 22,6%) recruited January 1990‐January 1991. 10/46 and 9/46 had positive swab cultures at baseline.
Setting: hospital. Country: India. Inclusion criteria: treated within 6 h of injury, partial‐thickness burns. Exclusion criteria: not reported. |
|
| Interventions | Group 1 (n = 46): unprocessed, undiluted, honey‐impregnated gauze, changed on day 2 and then alternate days. Group 2 (n = 46): polyurethane film (OpSite) left intact until day 8, unless evidence of infection, excessive exudate or leakage. Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 10.8 days (SD 3.93); Group 2: 15.3 days (SD 2.98). | |
| Notes | Information on allocation method, allocation concealment, blinding, mean TBSA, mean time to healing and standard deviation for mean time to healing provided by author. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After initial management, patients were allotted at random to two groups”. Comment: method of generation of random sequence not reported. Author informed us that the sequence was generated by the "chit method", which is a method of drawing lots however the detail provided by the authors was minimal and not sufficient to reassure us that the sequence was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was via sequentially‐numbered sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded, but not patients. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all as unclear risk for blinding of outcome assessors. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. However both Gethin and Jull have reported how honey stained the peri‐ulcer skin making blinding of outcome assessment difficult and we have therefore rated all studies as at either unclear or high risk of detection bias. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment:Table 5 showed that there were no drop‐outs, and that all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since no drop‐outs were reported, and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: few baseline characteristics were reported, so there was insufficient information available to judge whether any important form of bias existed. |
Subrahmanyam 1993b.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 100 participants with burns or ulcers (27 old burns, 23 fresh burns, 20 traumatic ulcers, 14 bed sores, 8 diabetic ulcers, 6 varicose ulcers, 2 trophic ulcers), recruited January 1989‐January 1990. 43/50 and 41/50 had positive swabs at baseline Setting: hospital. Country: India. Inclusion criteria: not reported. Exclusion criteria: not reported. | |
| Interventions | Group 1 (n = 50): unprocessed, undiluted, honey, covered with gauze daily. Group 2 (n = 50): SSD‐impregnated gauze daily. Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 9.5 days (SD 6.2); Group 2: 22.5 days (SD 5.2). | |
| Notes | Information on allocation method, allocation concealment, blinding, mean time to healing and standard deviation for mean time to healing provided by author. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were divided into two groups and they were distributed at random”. Comment: method of generating random sequence was not reported. Author provided information that the sequence was generated by the "chit method", which is a method of drawing lots, however the information supplied by the author lacked detail and was insufficient for us to judge whether the sequence was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was by means of sequentially‐numbered, sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded, but not patients. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as at either unclear or high risk of detection bias. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Table 3 showed there were no drop‐outs, and that all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since no drop‐outs were reported, and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: there was no baseline imbalance with respect to patients' age between two groups, however, other baseline characteristics were not reported, so there was insufficient information to judge whether any important risk of bias existed. |
Subrahmanyam 1994.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 100 participants with partial‐thickness burns; TBSA mean 18.5 and 19.4%; treated within 6h of burn; recruited June 1991‐July 1992. 28/40 (70%) and 19/24 (79%) had positive swabs at baseline Setting: hospital. Country: India. Inclusion criteria: treated within 6 h of injury, TBSA burnt < 40%. Exclusion criteria: not reported. | |
| Interventions | Group 1 (n = 40): unprocessed, undiluted, honey‐impregnated gauze changed every 2nd day. Group 2 (n = 24): amniotic membrane left intact until day 8, and then changed every 2nd day. Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 9.4 days (SD 2.52); Group 2: 17.5 days (SD 6.66). | |
| Notes | Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by author. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After initial treatment, patients were allotted to two groups at random”. Comment: method of generating the random sequence not reported. Author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was by means of sequentially‐numbered, sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated in study report, but author responded to request for further information by stating the patients were not blinded. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Low risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as at either unclear or high risk of detection bias. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Tables 2 and 3 show that there were no drop‐outs, all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since no drop‐outs were reported, and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Low risk | Comment: there was no imbalance in the baseline characteristics, and the study seemed to be free from other risk of bias. |
Subrahmanyam 1996a.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 900 participants with partial‐thickness burns recruited July 1987‐December 1993, 90% within 6h of burn. TBSA mean 24.5 and 26.2%; wound infections at baseline not stated. Setting: hospital. Country: India. Inclusion criteria: TBSA burnt < 40%. Exclusion criteria: not reported. | |
| Interventions | Group 1 (n = 450): pure, unprocessed, undiluted, honey, covered with gauze, changed every 2nd day. Group 2 (n = 450): Soframycin (90 participants), Vaseline‐impregnated gauze (90 participants), OpSite (90 participants), sterile gauze (90 participants) or left exposed (90 participants). "Dressings were replaced on alternative days, except in the case of OpSite, which was continued until the wounds healed... sterile linen changed at frequent intervals." Frequency of dressing change is not mentioned with respect to the sterile gauze group. Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 8.8 days (SD 2.1); Group 2: 13.5 days (SD 4.1). | |
| Notes | Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by author. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After initial treatment, the cases were divided at random into a study group treated with honey dressing and a control group treated with conventional dressing”. Comment: method of generating the random sequence not reported. Author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was by means of sequentially‐numbered, sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated in study report, but author responded to request for further information by stating the patients were not blinded. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Low risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as at either unclear or high risk of detection bias. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment:Table 5 showed that there were no drop‐outs; all the randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since were no drop‐outs were reported, and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: the baseline characteristics were not reported, so there was insufficient information to judge whether any important form of bias existed. |
Subrahmanyam 1996b.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 100 participants with partial‐thickness burns recruited July 1992‐December 1993. TBSA mean 16.5 and 17.2%; 40/50 and 42/50 had positive swabs at baseline.
Setting: hospital. Country: India. Inclusion criteria: treated within 6 h of injury, TBSA burnt < 40%. Exclusion criteria: not reported. |
|
| Interventions | Group 1 (n = 50): pure, unprocessed, undiluted honey, covered with gauze every 2nd day. Group 2 (n = 50): potato peel bandages every 2nd day. Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 10.4 days (SD 2.2); Group 2: 16.2 days (SD 2.3). | |
| Notes | Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by author. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After initial management, patients were allotted at random to two groups”. Comment: method of generating the random sequence not reported. Author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was by means of sequentially‐numbered sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated in study report, but author responded to request for further information by stating the patients were not blinded. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Low risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as at either unclear or high risk of detection bias. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment:Table 5 showed that there were no drop‐outs; all randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since no drop‐outs were reported, and all randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Low risk | Comment: there was no imbalance in the baseline characteristics, and the study seemed to be free from other forms of bias. |
Subrahmanyam 1998.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 50 participants with superficial thermal burns recruited June 1995‐December 1996. TBSA mean 14.5 and 15.6%; treated within 6h of burn; 23/25 and 22/25 had positive swabs at baseline
Setting: hospital. Country: India. Inclusion criteria: present within 6 h of injury, TBSA burnt < 40%. Exclusion criteria: not reported. |
|
| Interventions | Group 1 (n = 25): pure, unprocessed, undiluted honey,covered with pads and bandages; applied every 2nd day. Group 2 (n = 25): SSD‐impregnated gauze, applied daily (c.f. alternate day application of honey). Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 4.92 days (SD 3.61); Group 2: 8.22 days (SD 8.31). | |
| Notes | Information about allocation method, allocation concealment, blinding, mean time to healing and standard deviation for mean time to healing provided by author. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After initial management, patients were allotted at random to two groups”. Comment: method of generation of random sequence not reported. Author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was by means of sequentially‐numbered sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated in study report, but author responded to request for further information by stating the patients were not blinded. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Low risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as at either unclear or high risk of detection bias. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: Table 2 showed that there were no drop‐outs; all randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis not reported, but since no drop‐outs were reported, and all randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Low risk | Comment: there was no imbalance in the baseline characteristics, and the study seemed to be free from other forms of bias. |
Subrahmanyam 1999.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 50 participants with mixed‐depth (partial‐ and full‐thickness) burns recruited January 1996‐December 1997.TBSA mean 24 and 23%; full thickness TBSA 13 and 12%. 41/123 swabs (not patients) and 7/71 were positive at baseline.
Setting: hospital. Country: India. Inclusion criteria: aged 10‐40 years, haemodynamically stable, no systemic illness or smoke inhalation injury, TBSA burnt < 30%. Exclusion criteria: not reported. |
|
| Interventions | Group 1 (n = 25): unprocessed honey, covered with gauze every 2nd day, then (delayed) autologous skin grafting as required (in 11/22 patients; 3 died). Group 2 (n = 25): early tangential excision and skin grafting 3‐6 days after admission. Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 32.0 days (SD 8.1); Group 2: 18.4 days (SD 4.2). | |
| Notes | Information about allocation method, allocation concealment, blinding, mean time to healing and standard deviation for mean time to healing provided by author.
3 participants in the honey‐treated group died, and 1 in the early tangential excision group. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Twenty five patients were randomly assigned to the TE group”. Comment: method of generation of the random sequence was not reported. Author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was by means of sequentially‐numbered sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated in study report, but author responded to request for further information by stating the patients were not blinded. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Low risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as at either unclear or high risk of detection bias. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Quote: “One TE patient died, from status asthaticus, while 3 HT patients died with septicaemia”. Comment: overall the number of drop‐outs was < 10%, however, drop‐outs were due to death, which is of some concern given that the burns were described as “moderate”. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Unclear risk | Comment: the study did not state whether all the randomised participants were followed‐up and included in the final analysis, hence, we cannot judge if an ITT analysis was conducted. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: few baseline characteristics were reported, so there was insufficient information available to judge whether any important form of bias existed. |
Subrahmanyam 2001a.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 100 participants with mixed‐depth (partial‐ and full‐thickness) burns recruited June 1998‐December 1999. TBSA mean 22.5 and 23.5%; mean full thickness TBSA 3.2 and 4.7%. 44/50 and 42/50 had positive swabs at baseline.
Setting: hospital. Country: India. Inclusion criteria: treated within 6 h of injury, TBSA burnt < 40%. Exclusion criteria: not reported. |
|
| Interventions | Group 1 (n = 50): unprocessed, undiluted, monofloral (Jambhul) honey, covered with gauze, every 2nd day (skin grafting required in 4 patients) Group 2 (n = 50): SSD‐impregnated gauze every 2nd day (skin grafting required in 11 patients). Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 15.4 days (SD 3.2) Group 2: 17.2 days (SD 4.3). | |
| Notes | Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by author. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were allocated at random to two groups, the initial management being the same”. Comment: method of generation of random sequence not reported. Author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was by means of sequentially‐numbered sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Comment: not stated in study report, but author responded to request for further information by stating the patients were not blinded. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Low risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated in study report, but author responded to request for further information by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as at either unclear or high risk of detection bias. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Quote: “Thus, all the patients in this group, the wound healed by day 21” (patients treated with honey). Quote: “In the group treated with sulphur sulphadiazine, the wounds healed in 4 patients by day 7, in 22 patients by 14 day, and in 24 patients by day 21”. Comment: no drop‐outs, as all the participants randomised were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Low risk | Comment: ITT analysis was not reported, but since no drop‐outs were reported, and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Low risk | Comment: there was no imbalance in the baseline characteristics, and the study seemed to be free from other forms of bias. |
Subrahmanyam 2004.
| Methods | Single‐centred, 2‐armed, blinded (outcome assessor), parallel group RCT. | |
| Participants | 30 consecutive males with Fournier's gangrene recruited April 2001‐May 2003. All patients had infected wounds at baseline.
Setting: hospital. Country: India. Inclusion criteria: not reported. Exclusion criteria: not reported. |
|
| Interventions | Group 1 (n = 14): unprocessed, undiluted, monofloral (Jamun) honey (gauze dipped in honey), daily. Group 2 (n = 16): Edinburgh Solution of Lime‐ (EUSOL) soaked gauze daily. Treatment duration: until healed. | |
| Outcomes | Mean time to healing: Group 1: 18.5 days (SD 2.1); Group 2: 26.5 days (SD 3.2). | |
| Notes | Information about allocation method, allocation concealment, blinding, mean time to healing and standard deviation for mean time to healing provided by author.
3 participants died: 1 in the honey‐treated group and 2 in the EUSOL‐treated group. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “For assessing the beneficial effects of local dressings, the patients were divided into two groups by randomisation”. Comment: method of generating the random sequence not reported. Author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but author provided information that allocation concealment was by means of sequentially numbered envelopes, although it is not clear whether they were sealed or opaque. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | High risk | Quote: “The assessor was not aware of the treatment given (single blind)”. Comment: blinding of participants was not done. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | High risk | Quote: “The assessor was not aware of the treatment given (single blind)”. Comment: blinding of providers was not done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Quote: “The assessor was not aware of the treatment given (single blind)”. Comment: blinding of outcome assessors was done and it was unlikely that the blinding could have been broken. Both Gethin and Jull have reported how it was difficult or impossible to blind outcome assessment due to discolouration of peri‐ulcer skin by honey. We have therefore classed all studies as at either unclear or high risk of detection bias. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Low risk | Comment: 3 deaths reported: 1 in the honey‐treated group and 2 in the EUSOL‐treated group. The causes of death were not mentioned, but the drop‐out rate was < 10%, which was acceptable. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Unclear risk | Comment: the information available was not sufficient to judge whether an ITT analysis had been conducted. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Comment: the baseline characteristics were not reported, so there was insufficient information available to judge whether any important form of bias existed. |
Weheida 1991.
| Methods | Single‐centred, 2‐armed, parallel group RCT. | |
| Participants | 40 participants with grade I or II pressure ulcers; honey 20/20 (100%) grade I; saline 16/20 (80%) grade I
Setting: hospital. Country: Egypt. Inclusion criteria: orthopaedic patients aged ≥ 21 years, ulcer ≥ 2 cm in diameter, ulcer uninfected, haemoglobin ≥ 10 g/dL, oral temperature ≤ 37.5 oC, restricted to bed or wheelchair for at least 2 weeks Exclusion criteria: debilitant co‐morbidities e.g. diabetes, cancer. |
|
| Interventions | Group 1 (n = 20): honey dressing changed daily. Group 2 (n = 20): saline‐soaked gauze changed daily. Treatment duration: 10 days. Follow‐up duration: three months. |
|
| Outcomes | Healing rate at 10 days: Group 1: 20/20 (100%); Group 2: 14/20 (70%). |
|
| Notes | Grade I ulcer defined as moist irregular partial‐thickness ulcer confined to epidermis and dermis. Grade II ulcer defined as full‐thickness ulcer descending into subcutaneous tissue. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects of the study were randomly recruited to one of the two treatment groups”. Comment: method of generation of randomisation schedule not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of participants? All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of healthcare providers?All outcomes | Unclear risk | Comment: not stated. |
| Blinding (performance bias and detection bias) Blinding of outcome assessors? All outcomes | Unclear risk | Comment: not stated. |
| Incomplete outcome data (attrition bias) Drop‐out rate described and acceptable ‐All outcomes | Unclear risk | Comment: the study did not state whether there were any drop‐outs or whether all randomised participants were followed‐up. |
| Incomplete outcome data (attrition bias) ITT analysis ‐All outcomes | Unclear risk | Comment: the study did not state whether all randomised participants were followed‐up and included in the final analysis, hence, we cannot judge whether an ITT analysis was conducted. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | High risk | Quote: “Eighty percent of group I [saline] had ulcers grade I before treatment ... For group II [honey], all the subjects had ulcers grade I, and all of which were completely healed after treatment [sic]”. Comment: there was a baseline imbalance in ulcer grades between 2 groups; all grade II ulcers were in the saline dressing group, while all ulcers in the honey dressing group were grade I ulcers. Therefore, the ulcers in the saline dressing group were more severe. In addition, there were fewer males and pressure ulcers developed at later times from admission in the honey group |
Abbreviations
> = greater/more than ≥ = greater than or equal to < = less than ≤ = less than or equal to ABI = ankle‐brachial index EUSOL = Edinburgh solution of lime h = hour(s) ITT = intention‐to‐treat analysis RCT = randomised controlled trial TBSA = total body surface area SSD = silver sulfadiazine vs = versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelatif 2008 | Not a randomised or controlled clinical trial. |
| Ahmed 2003 | Not a randomised or controlled clinical trial. |
| Al Waili 2003 | Participants did not have wounds; trial of honey mixture for atopic dermatitis or psoriasis. |
| Al Waili 2004a | Animal‐model study. |
| Al Waili 2004b | Animal‐model study. |
| Al Waili 2004c | Not a randomised or controlled clinical trial. |
| Al Waili 2005 | Not a randomised or controlled clinical trial. |
| Albietz 2006 | Participants did not have wounds. |
| Bangroo 2005 | Insufficient information on healing ‐ no response to attempts to contact corresponding author. |
| Berchtold 1992 | Did not use honey. |
| Biswal 2003 | Participants did not have wounds; trial of honey for radiation‐induced mucositis. |
| Bose 1982 | Not a randomised or controlled clinical trial. |
| Calderon Espina 1989 | Could not be obtained for assessment. |
| Chokotho 2005 | No information on healing. No response to attempts to contact investigator. |
| Dunford 2004 | Not a randomised or controlled clinical trial. |
| Freeman 2010 | Not a randomised or controlled clinical trial. |
| Gad 1988 | No information on healing. No response to attempts to contact investigator. |
| Gethin 2005 | Not a randomised or quasi‐randomised controlled trial. |
| Heidari 2013 | No healing data reported. |
| Jeffery 2008 | No information on healing. |
| Johnson 2005 | Participants did not have wounds; trial of honey to prevent catheter‐associated infections in haemodialysis patients. |
| Lund‐Nielsen 2011 | No information on healing. |
| Lusby 2002 | Not a randomised or quasi‐randomised controlled trial. |
| Malik 2010 | Unit of analysis issue ‐ wounds randomised, not patients. |
| Marshall 2002 | Not a randomised or quasi‐randomised controlled trial. |
| Mat Lazim 2013 | No healing data (as per specified primary outcomes) reported. |
| Mayer 2014 | Not a randomised or controlled trial; no control group. |
| Misirligou 2003 | Not a randomised or controlled clinical trial. |
| Moghazy 2010 | Not a randomised or controlled trial; no control group. |
| Molan 2002 | Not a randomised or controlled clinical trial. |
| Molan 2006 | Not a randomised or controlled clinical trial. |
| Muller 1985 | Did not use honey. |
| Mwipatayi 2004 | Not a randomised or controlled clinical trial. |
| Nagane 2004 | Not a randomised or controlled clinical trial. |
| Okeniyi 2005 | Unit of analysis issue ‐ wounds randomised, not participants. 32 participants had 43 wounds and individual participants may have been treated by both honey and the comparator (EUSOL). Healing rate provided by wound, not by participant. |
| Oluwatosin 2000 | Unit of analysis issue. No information on healing. |
| Pascoe 2013 | Protocol for RCT of study on prevention of catheter associated infection in peritoneal dialysis (not a wound healing study). |
| Quadri 1998 | Participants did not have wounds; trial of honey to prevent catheter‐associated infections in haemodialysis patients. Duplicate study. |
| Quadri 1999 | Participants did not have wounds; trial of honey to prevent catheter‐associated infections in haemodialysis patients. |
| Rivero Varona 1999 | Could not be obtained. |
| Robson 2002 | Not a randomised or controlled clinical trial. |
| Robson 2012 | Neither of healing primary outcomes reported (focus is on MRSA). |
| Rogers 2010 | Insufficient information on healing. |
| Rucigaj 2006 | Insufficient information on healing ‐ corresponding author unwilling to provide information until study published. |
| Saha 2012 | Neither of healing primary outcomes reported. |
| Schumacher 2004 | Not a randomised or controlled clinical trial. |
| Somaratne 2012 | No method of allocation stated so unlikely to be RCT; no data on wound healing (only swabs for MRSA). |
| Subrahmanyam 1993 | Not a randomised or controlled clinical trial. |
| Subrahmanyam 1996c | This RCT was a comparison of honey with honey plus vitamins A, C and polyethyline glycol. As the systematic difference between study arms was neither the presence of honey nor different types of honey, this study was ineligible. |
| Subrahmanyam 2001b | Animal‐model study. |
| Subrahmanyam 2003 | No data on healing ‐ biochemical data only. |
| Thurnheer 1983 | Not a randomised or controlled clinical trial. |
| Tostes 1994 | Not a randomised or controlled clinical trial. |
| Ur‐Rehman 2013 | Neither of healing primary outcomes reported, only change in area. |
| Vijaya 2012 | Not a randomised or controlled clinical trial. |
| Visscher 1996 | Not a randomised or controlled clinical trial. |
| Yapucu Gunes 2007 | Unit of analysis issue ‐ outcomes reported by pressure injury, not by participant. |
Characteristics of studies awaiting assessment [ordered by study ID]
Askarpour 2009.
| Methods | Unclear how participants were allocated. |
| Participants | People undergoing laparotomy. |
| Interventions | Honey vs no honey |
| Outcomes | Granulation tissue formation; appears neither of primary outcomes reported. |
| Notes | Awaiting clarification from authors. |
Jan 2012.
| Methods | Unclear how participants were allocated. |
| Participants | People with diabetic foot ulcers (Wagner's Grade I to IV) |
| Interventions | Daily honey dressings vs. pyodine soaked surgical gauze dressings |
| Outcomes | Ulcer healing and amputation. |
| Notes | Awaiting clarification from authors. |
Maghsoudi 2011.
| Methods | Single‐centred, 2‐armed, parallel group RCT |
| Participants | 100 participants with partial‐thickness burns recruited March 2010‐March 2011. Setting: hospital. Country: Iran. Inclusion criteria: TBSA burnt < 40%. Exclusion criteria: not reported. |
| Interventions | Group 1 (n = 50): 16‐30 ml unprocessed, undiluted honey dressings changed on alternate days. Group 2 (n = 50): mafenide acetate‐impregnated gauze changed daily. Treatment duration: until healed. |
| Outcomes | Mean time to healing and standard deviation not available. |
| Notes | Attempting to contact authors. |
Differences between protocol and review
In the first version of the review, two trials that compared active interventions allocated wounds to the interventions rather than participants (Oluwatosin 2000; Okeniyi 2005). The participants had multiple wounds in many cases, and some participants would have received both interventions. In this update, a trial that required participants to have two burns (Malik 2010), and a trial that may have randomised participants but appears to have reported healing by pressure injury rather than by participant (Yapucu Gunes 2007), were excluded. The data in these trials were presented by wound and thus could not be combined (if possible) with trials where data were presented by participant in both the first version of the review and this update. Such methods were not foreseen in the protocol, where it was assumed that data would be presented by participant. Randomising by wound breaches the assumption of independence that underpins inferential testing, increases the weight of a study inappropriately if included in a meta‐analysis (by doubling the denominator) and thereby artificially improves the precision of the confidence interval for the summary statistic. Additionally, a trial requiring participants to have two wounds that randomises one wound to each treatment is not clinically generalisable as it has reduced between‐patient variability. Between‐patient variability in pragmatic trials drives external validity. The inclusion criteria have been adjusted in this update to reflect this change.
Contributions of authors
Andrew Jull: designed and co‐ordinated the review. Extracted data (first review), reviewed risk of bias assessments and data extraction (this update). Analysed or interpreted data and performed statistical analysis, wrote to study author/experts/companies, completed or reviewed the drafts, revisions, and the final review (first review and this update). He is guarantor of the review.
Nicky Cullum: checked others' data extraction and extracted data (this update); checked others' risk of bias assessment and conducted risk of bias assessments (this update). Analysed and interpreted data; constructed summary of findings tables (this update); completed drafts and revisions of the review and approved the final version of this update prior to publication.
Jo Dumville: checked others' data extraction and extracted data (this update); checked others' risk of bias assessment and conducted risk of bias assessments (this update). Analysed and interpreted data; constructed summary of findings tables (this update); completed drafts and revisions of the review and approved the final version of this update prior to publication.
Maggie Westby: checked others' data extraction and extracted data (this update); checked others' risk of bias assessment and conducted risk of bias assessments (this update). Analysed and interpreted data; constructed summary of findings tables (this update); completed drafts and revisions of the review and approved the final version of this update prior to publication.
Natalie Walker: designed the review and checked studies to be included, checked risk of bias assessment and the quality of statistical analysis (first review), performed part of writing or editing of the review (first review and first update). Approved final review prior to submission (first review and all updates).
Sohan Deshpande: checked studies to be included, extracted data, performed risk of bias assessments and contributed to writing (first update).
Contributions of editorial base:
Nicky Cullum: for the original review and first update ‐ edited the review, advised on methodology, interpretation and review content; approved the final review prior to submission. Liz McInnes, Editor: approved the first review update prior to submission. Sally Bell‐Syer: co‐ordinated the editorial process; advised on methodology, interpretation and content; edited the review and the updated versions of the review. Ruth Foxlee: designed the search strategy and edited the search methods section.
David Tovey (Editor in Chief) approved the final version of this updated review (second update). Toby Lasserson (Senior Editor) of the Cochrane Editorial Unit advised on the Summary of Findings Tables and approved the final version of this updated review (second update).
Sources of support
Internal sources
Senior Health Research Scholarship, University of Auckland, New Zealand.
School of Nursing, Midwifery and Social Work, University of Manchester, UK.
External sources
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
The Douglas Senior Fellowship in Heart Health (Prevention), New Zealand.
Declarations of interest
Andrew Jull, Natalie Walker and Anthony Rodgers were investigators in the Honey as Adjuvant Leg ulcer Treatment (HALT) trial (ISRCTN 06161544), one of the trials included in this review. The Clinical Trials Research Unit, which employed Andrew Jull, Natalie Walker and Antony Rodgers received a small, unconditional cash contribution from a manufacturer of honey dressings for the conduct of the HALT trial. Dr Walker is supported by a Heart Foundation Douglas Senior Fellowship in Heart Health (Prevention). She has provided consultancy to the manufacturers of smoking cessation medications, received honoraria for speaking at a research meeting and received benefits in kind and travel support from a pharmaceutical company that makes smoking cessation medications. She has also received product in kind from a pharmaceutical company that makes smoking cessation medications, for use in an investigator initiated phase III clinical trial.  She has been contracted by two companies to undertake clinical trials for them ‐ one company wanted her to evaluate a treatment for leg ulcers (but this was not honey) and the second was an asthma trial for a New Zealand Crown entity that decides, on behalf of District Health Boards, which medicines and pharmaceutical products are subsidised for use in the community and public hospitals.Â
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Al Waili 1999 {published data only}
- Al‐Waili NS, Saloom KY. Effects of topical honey on post‐operative wound infections due to gram positive and gram negative bacteria following caesarean sections and hysterectomies. European Journal of Medical Research 1999;4(3):126‐30. [PubMed] [Google Scholar]
Baghel 2009 {published data only}
- Baghel PS, Shukla S, Mathur RK, Randa R. A comparative study to evaluate the effect of honey dressing and silver sulfadiazene dressing on wound healing in burn patients. Indian Journal of Plastic Surgery 2009;42(2):176‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gethin 2007 {published and unpublished data}
- Gethin G. Manuka honey versus hydrogel ‐ a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. Unpublished PhD thesis 2007. [DOI] [PubMed]
- Gethin G, Cowman S. Bacteriological changes in sloughy venous leg ulcers treated with manuka honey or hydrogel: an RCT. Journal of Wound Care 2008;17(6):241‐7. [DOI] [PubMed] [Google Scholar]
- Gethin G, Cowman S. Manuka honey versus hydrogel to deslough venous leg ulcers: a randomised controlled trial. 17th European Wound Management Association conference, Glasgow, 2‐4 May. 2007:29.
- Gethin G, Cowman S. Manuka honey vs. hydrogel ‐ a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. Journal of Clinical Nursing 2009;18(3):466‐74. [DOI] [PubMed] [Google Scholar]
Gulati 2014 {published data only}
- Gulati S, Qureshi A, Srivastava A, Kataria K, Kumar P, Balakrishna Ji A. A prospective randomized study to compare the effectiveness of honey dressings vs. povidone iodine dressing in chronic wound healing. Indian J Surg 2014;76(3):193‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingle 2006 {published and unpublished data}
- Ingle R, Levin J, Polinder K. Wound healing with honey ‐ a randomised controlled trial. South African Medical Journal 2006;96(9):831‐5. [PubMed] [Google Scholar]
Jull 2008 {published and unpublished data}
- Jull A. Honey and venous leg ulceration: a systematic review and randomised controlled trial. Unpublished PhD thesis, University of Auckland 2007.
- Jull A, Walker N, Parag V, Molan P, Rodgers A, on behalf of the Honey as Adjuvant Leg Ulcer Therapy trial collaborators. Randomized clinical trial of honey‐impregnated dressings for venous leg ulcers. British Journal of Surgery 2008;95(2):175‐82. [DOI] [PubMed] [Google Scholar]
- Jull A, Walker N, Rodgers A, Bennett D, Molan P, Arroll B, et al. Honey for leg ulcers ‐ The HALT Trial. [Poster]. 2nd World Union of Wound Healing Societies Meeting, Paris, 8‐13 July. 2004:88.
Kamaratos 2014 {published data only}
- Kamaratos AV, Tzirogiannis KN, Iraklianou SA, Panoutsopoulos GI, Kanellos IE, Melidonis AI. Manuka honey‐impregnated dressings in the treatment of neuropathic diabetic foot ulcers. International Wound Journal 2014;3:259‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2005 {published data only}
- Marshall C, Queen J, Manjooran J. Honey vs povidine iodine following toenail surgery. Wounds UK 2005;1(1):10‐8. [Google Scholar]
Mashood 2006 {published data only}
- Mashhood AA, Khan TA, Sami AN. Honey compared with 1% silver sulfadiazine cream in the treatment of superficial and partial thickness burns. Journal of Pakistan Association of Dermatologists 2006;16(1):14‐9. [Google Scholar]
McIntosh 2006 {published data only}
- Marshall CD, Thomson C. The effect of honey dressings on post‐operative healing following toenail surgery: a randomised controlled study. 2nd World Union of Wound Healing Societies Meeting, Paris, 8‐13 July. 2004:A 012 (page 8 oral).
- McIntosh CD, Thomson CE. Honey dressing versus paraffin tulle gras following toenail surgery. Journal of Wound Care 2006;15(3):133‐6. [DOI] [PubMed] [Google Scholar]
Memon 2005 {published data only}
- Memon AR, Tahir SM, Khushk IA, Ali Memon G. Therapeutic effects of honey versus silver sulfadiazine in the management of burn injuries. Journal of Liaquat University Medicine and Health Sciences 2005;4(3):100‐4. [Google Scholar]
Mphande 2007 {published and unpublished data}
- Mphande ANG, Killow C, Phalira S, Wynn Jones H, Harrison WJ. Effects of honey and sugar dressings on wound healing. Journal of Wound Care 2007;16(7):317‐9. [DOI] [PubMed] [Google Scholar]
Nilforoushzadeh 2007 {published and unpublished data}
- Nilforoushzadeh MA, Jaffary F, Moradi S, Derakhshan R, Haftbaradaran H. Effect of topical honey application along with intralesional injection of glucantime in the treatment of cutaneous leishmaniasis. BMC Complementary and Alternative Medicine 2007;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robson 2009 {published data only}
- Robson V, Dodd S, Thomas S. Standardized antibacterial honey (Medihoney) with standard therapy in wound care: randomized clinical trial. Journal of Advanced Nursing 2009;65(3):565‐75. [DOI] [PubMed] [Google Scholar]
Shukrimi 2008 {published data only}
- Shukrimi A, Sulaiman AR, Halim AY, Azril A. A comparative study between honey and povidone iodine as dressing solution for Wagner type II diabetic foot ulcers. Medical Journal of Malaysia 2008;63(1):44‐6. [PubMed] [Google Scholar]
Subrahmanyam 1991 {published and unpublished data}
- Subrahmanyam M. Topical application of honey in treatment of burns. British Journal of Surgery 1991;78(4):497‐8. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1993a {published and unpublished data}
- Subrahmanyam M. Honey impregnated gauze versus polyurethane film (OpSite) in the treatment of burns ‐ a prospective randomised study. British Journal of Plastic Surgery 1993;46(4):322‐3. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1993b {published data only}
- Subrahmanyam M. Honey as a surgical dressing for burns and ulcers. Indian Journal of Surgery 1993;55(9):468‐73. [Google Scholar]
Subrahmanyam 1994 {published and unpublished data}
- Subrahmanyam M. Honey‐impregnated gauze versus amniotic membrane in the treatment of burns. Burns 1994;20(4):331‐3. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1996a {published and unpublished data}
- Subrahmanyam M. Honey dressing for burns ‐ an appraisal. Annals of Burns and Fire Disasters 1996;IX(1):33‐5. [Google Scholar]
Subrahmanyam 1996b {published and unpublished data}
- Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns 1996;22(6):491‐3. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1998 {published and unpublished data}
- Subrahmanyam M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns 1998;24(2):157‐61. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1999 {published data only}
- Subrahmanyam M. Early tangential excision and skin grafting of moderate burns is superior to honey dressing: a prospective randomised trial. Burns 1999;25(8):729‐31. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 2001a {published and unpublished data}
- Subrahmanyam M, Sahapure AG, Nagane NS, Bhagwat VR, Ganu JV. Effects of topical application of honey on burn wound healing. Annals of Burns and Fire Disasters 2001;XIV(3):143‐5. [Google Scholar]
Subrahmanyam 2004 {published and unpublished data}
- Subrahmanyam M, Ugane SP. Honey dressing beneficial in treatment of Fournier's gangrene. Indian Journal of Surgery 2004;66(2):75‐7. [Google Scholar]
Weheida 1991 {published data only}
- Weheida SM, Nagubib HH, El‐Banna HM, Marzouk S. Comparing the effects of two dressing techniques on healing of low grade pressure ulcers. Journal of the Medical Research Institute 1991;12(2):259‐78. [Google Scholar]
References to studies excluded from this review
Abdelatif 2008 {published data only}
- Abdelatif M, Yakoot M, Etmaan M. Safety and efficacy of a new honey ointment on diabetic foot ulcers: a prospective pilot study. Journal of Wound Care 2008;17(3):108‐10. [DOI] [PubMed] [Google Scholar]
Ahmed 2003 {published data only}
- Ahmed AKJ, Hoekstra MJ, Hage JJ, Karim RB. Honey‐medicated dressing: transformation of an ancient remedy. Annals of Plastic Surgery 2003;50(2):143‐8. [DOI] [PubMed] [Google Scholar]
Albietz 2006 {published data only}
- Albietz JM, Lenton LM. Effect of antibacterial honey on the ocular flora in tear deficiency and Meibomian gland disease. Cornea 2006;25(9):1012‐9. [DOI] [PubMed] [Google Scholar]
Al Waili 2003 {published data only}
- Al Waili NS. Topical application of natural honey, beeswax and olive oil mixture for atopic dermatitis or psoriasis: partially controlled, single‐blinded study. Complementary Therapies in Medicine 2003;11(4):226‐34. [DOI] [PubMed] [Google Scholar]
Al Waili 2004a {published data only}
- Al‐Waili NS. Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. Journal of Medicinal Food 2004;7(2):210‐22. [DOI] [PubMed] [Google Scholar]
Al Waili 2004b {published data only}
- Al‐Waili NS. Effect of honey on antibody production against thymus‐dependent and thymus‐independent antigens in primary and secondary immune responses. Journal of Medicinal Food 2004;7(4):491‐4. [DOI] [PubMed] [Google Scholar]
Al Waili 2004c {published data only}
- Al‐Waili NS. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Medical Science Monitor 2004;10(8):MT94‐8. [PubMed] [Google Scholar]
Al Waili 2005 {published data only}
- Al Waili NS. Clinical and mycological benefits of topical application of honey, olive oil and beeswax in diaper dermatitis. Clinical Microbiology and Infection 2005;11(2):160‐3. [DOI] [PubMed] [Google Scholar]
Bangroo 2005 {published data only}
- Bangroo AK, Ramji K, Smita C. Honey dressing in pediatric burns. Journal of the Indian Association Pediatric Surgeons 2005;10(2):172‐5. [Google Scholar]
Berchtold 1992 {published data only}
- Berchtold E, Maibach R, Muller U. Reduction of side effects from rush‐immunotherapy with honey bee venom by pretreatment with terfenadine. Clinical and Experimental Allergy 1992;22(1):59‐65. [DOI] [PubMed] [Google Scholar]
Biswal 2003 {published data only}
- Biswal BM, Zakaria A, Ahmad NM. Topical application of honey in the management of radiation mucositis. A preliminary study. Support Care Cancer 2003;11:242‐8. [DOI] [PubMed] [Google Scholar]
Bose 1982 {published data only}
- Bose B. Honey or sugar in treatment of infected wounds?. Lancet 1982;1(8278):963. [DOI] [PubMed] [Google Scholar]
Calderon Espina 1989 {published data only (unpublished sought but not used)}
- Calderon Espina LE. Use of honey in surgical wound dehiscence after surgery of obstetric patients [Uso de miel de abeja en heridas operatorias dehiscentes en pacientes post‐cirugía obstétrica]. Universidad de San Carlos de Guatemala 1989.
Chokotho 2005 {published data only}
- Chokotho L, Hasselt E. The use of tannins in the local treatment of burn wounds ‐ a pilot study. Malawi Medical Journal 2005;17:19‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunford 2004 {published data only}
- Dunford CE, Hanano R. Acceptability to patients of a honey dressing for non‐healing venous leg ulcers. Journal of Wound Care 2004;13:193‐7. [DOI] [PubMed] [Google Scholar]
Freeman 2010 {published data only}
- Freeman A, May K, Wraight P. Honey: the bee's knees for diabetic foot ulcers?. Wound Practice and Research 2010;18(3):144‐7. [Google Scholar]
Gad 1988 {published data only}
- Gad ESM, Ali MA, Sherif ME, Hamdy HE. The use of honey in dressing of septic wounds versus cetrimide 15%+ chlorhexidine gluconate 1.5% (Savlon): randomized controlled clinical trial. Medical Journal of Cairo University 1988;56:257‐64. [Google Scholar]
Gethin 2005 {published data only}
- Gethin G, Cowman S. Case series of manuka honey in leg ulceration. International Wound Journal 2005;2:10‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heidari 2013 {published data only}
- Heidari T, Roozbahani N, Amiri Farahani L, Attarha M, Akbari Torkestani N, Jamilian M, Bekhradi R. Does Iranian Astragalus gossypinus honey assist in healing caesarean wounds and scars?. European Journal of Integrative Medicine 2013;5(3):226‐233.. [Google Scholar]
Jeffery 2008 {published data only}
- Jeffery S. A honey‐based dressing for diabetic foot ulcers: A controlled study. Diabetic Foot Journal 2008;11(2):87‐91. [Google Scholar]
Johnson 2005 {published data only}
- Johnson DW, Eps C, Mudge DW, Wiggins KJ, Armstrong K, Hawley CM, et al. Randomized, controlled trial of topical exit‐site application of honey (MediHoney) versus mupirocin for the prevention of catheter‐associated infections in hemodialysis patients. American Journal of Nephrology 2005;16(5):1456‐62. [DOI] [PubMed] [Google Scholar]
Lund‐Nielsen 2011 {published data only}
- Lund‐Nielsen B, Adamsen L, Gottrup F, Rorth M, Tolver A, Kolmos HJ. Qualitative bacteriology in malignant wounds ‐ a prospective, randomized, clinical study to compare the effect of honey and silver dressings. Ostomy Wound Management 2011;57(7):28‐36. [PubMed] [Google Scholar]
- Lund‐Nielsen B, Adamsen L, Kolmos HJ, Rorth M, Tolver A, Gottrup F. The effect of honey‐coated bandages compared with silver‐coated bandages on treatment of malignant wounds‐a randomized study. Wound Repair and Regeneration 2011;19(6):664‐70. [DOI] [PubMed] [Google Scholar]
Lusby 2002 {published data only}
- Lusby PE. Honey: a potent agent for wound healing?. Journal of Wound, Ostomy and Continence Nursing 2002;29(6):295‐300. [DOI] [PubMed] [Google Scholar]
Malik 2010 {published data only}
- Malik KI, Malik MAN, Aslam A. Honey compared with silver sulphadiazine in the treatment of superficial partial‐thickness burns. International Wound Journal 2010;7(5):413‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2002 {published data only}
- Marshall C. The use of honey in wound care: a review article. British Journal of Podiatry 2002;52(2):47‐9. [Google Scholar]
Mat Lazim 2013 {published data only}
- Mat Lazim N, Abdullah B, Salim R. The effect of Tualang honey in enhancing post tonsillectomy healing process. An open labelled prospective clinical trial. International Journal of Pediatric Otorhinolaryngology 2013;77(4):457‐461. [DOI] [PubMed] [Google Scholar]
Mayer 2014 {published data only}
- Mayer A, Slezak V, Takac P, Olejnik J, Majtan J. Treatment of non‐healing leg ulcers with honeydew honey. Journal of Tissue Viability 2014;23(3):94‐97. [DOI] [PubMed] [Google Scholar]
Misirligou 2003 {published data only}
- Misirlioglu A, Eroglu S, Karacaglan N, Akan M, Akoz T, Yildirim S. Use of honey as an adjunct in the healing of split‐thickness skin graft donor site. Dermatologic Surgery 2003;29(2):168‐72. [DOI] [PubMed] [Google Scholar]
Moghazy 2010 {published data only}
- Moghazy AM, Shams ME, Adly OA, Abbas AH, El‐Badawy MA, Elsakka DM, Hassan SA, Abdelmohsen WS, Ali OS, Mohamed BA. The clinical and cost effectiveness of bee honey dressing in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract 2010;29(3):303‐9. [DOI] [PubMed] [Google Scholar]
Molan 2002 {published data only}
- Molan PC. Re‐introducing honey in the management of wounds and ulcers ‐ theory and practice. Ostomy/Wound Management 2002;48(11):28‐40. [PubMed] [Google Scholar]
Molan 2006 {published data only}
- Molan PC. The evidence supporting the use of honey as a wound dressing. International Journal of Lower Extremity Wounds 2006;5(1):40‐54. [DOI] [PubMed] [Google Scholar]
Muller 1985 {published data only}
- Muller U, Lanner A, Schmid P, Bischof M, Dreborg S, Hoigne R. A double blind study on immunotherapy with chemically modified honey bee venom: monomethoxy polyethylene glycol‐coupled versus crude honey bee venom. International Archives of Allergy and Applied Immunology 1985;77(1‐2):201‐3. [DOI] [PubMed] [Google Scholar]
Mwipatayi 2004 {published data only}
- Mwipatayi BP, Angel D, Norrish J, Hamilton MJ, Scott A, Sieunarine K. The use of honey in chronic leg ulcers: a literature review. Primary Intention 2004;12(3):107‐12. [Google Scholar]
Nagane 2004 {published data only}
- Nagane NS, Ganu JV, Bhagwat VR, Subramanium M. Efficacy of topical honey therapy against silver sulphadiazine treatment in burns: a biochemical study. Indian Journal of Clinical Biochemistry 2004;19(2):173‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okeniyi 2005 {published data only}
- Okeniyi JAO, Olubanjo OO, Ogunlesi TA, Oyelami OA. Comparison of healing of incised abscess wounds with honey and EUSOL dressing. Journal of Alternative and Complementary Medicine 2005;11(3):511‐3. [DOI] [PubMed] [Google Scholar]
Oluwatosin 2000 {published data only}
- Oluwatosin OM, Olabanji JK, Oluwatosin OA, Tijani LA, Onyechi HU. A comparison of topical honey and phenytoin in the treatment of chronic leg ulcers. African Journal of Medicine and Medical Science 2000;29:31‐4. [PubMed] [Google Scholar]
Pascoe 2013 {published data only}
- Pascoe EM, Lo S, Scaria A, Badve SV, Beller EM, Cass A, Hawley CM, Johnson DW. The HONEYPOT Randomized Controlled Trial Statistical Analysis Plan. Peritoneal Dialysis International 2013;33(4):426‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quadri 1998 {published data only}
- Quadri KHM, Tanimu DZ, Iqbal A, Bachand R, Al Saggabi AA, Abu Romeh S, et al. A prospective randomized controlled trial of topical honey versus povidine iodine in the prevention of hemodialysis catheter related sepsis. Journal of the American Society of Nephrology 1998;9:180‐1A. [Google Scholar]
Quadri 1999 {published data only}
- Quadri KHM, Huraib SO. Manuka honey for central vein catheter exit site care. Seminars in Dialysis 1999;12:397‐8. [Google Scholar]
Rivero Varona 1999 {published data only (unpublished sought but not used)}
- Rivero Varona T. Therapeutic use of honey in the treatment of alveolitis [Uso terapéutico de la miel en el tratamiento de las alveolitis]. Revista Archivo Médico de Camagüey 1999;3:43‐7. [Google Scholar]
Robson 2002 {published data only}
- Robson V. Leptospermum honey used as a debriding agent. Nurse 2 Nurse 2002;2:66‐8. [Google Scholar]
Robson 2012 {published data only}
- Robson V, Yorke J, Sen RA, Lowe D, Rogers SN. Randomised controlled feasibility trial on the use of medical grade honey following microvascular free tissue transfer to reduce the incidence of wound infection. British Journal of Oral and Maxillofacial Surgery 2012;50(4):321‐327. [DOI] [PubMed] [Google Scholar]
Rogers 2010 {published data only}
- Rogers S, Robson V, Barrow S, Sen R, Lowe D, York J. The feasibility of a randomised control trial on the use of medical grade honey to reduce the incidence of wound infection following free tissue transfer in head and neck cancer. Clinical Oncology 2010;22:900. [Google Scholar]
Rucigaj 2006 {published data only}
- Planinsek Rucigaj T. Comparative effects of honey based and silver/charcoal based dressings on the healing of venous leg ulcers: a randomized clinical study. 17th Conference of the European Wound Management Association; 2007, 2‐4 May; Glasgow, Scotland. 2007.
- Planinsek Rucigaj T. Comparative effects of polyhydrated ionogens/honey based dressings and silver based dressings on the healing of venous leg ulcers. Proceedings of 16th Annual Meeting of the European Tissue Repair Society, Pisa, 13‐16 September. 2006.
Saha 2012 {published data only}
- Saha A, Chattopadhyay S, Azam Md, Sur P. The role of honey in healing of bedsores in cancer patients. South Asian Journal of Cancer 2012;1(2):66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2004 {published data only}
- Schumacher HH. Use of medical honey in patients with chronic venous leg ulcers after split‐skin grafting. Journal of Wound Care 2004;13:451‐2. [DOI] [PubMed] [Google Scholar]
Somaratne 2012 {published data only}
- Somaratne K, Banagala A, Senanayake K, Seneviratne T, Thelikorala S, Tissera W. Controlled trial on the use of bee's honey on wounds where methicillin resistant staphylococcus aureas (MRSA) is isolated. 4th Congress of the World Union of Wound Healing Societies, September 2012, Yokohama, Japan. 2012.
Subrahmanyam 1993 {published data only}
- Subrahmanyam M. Storage of skin grafts in honey. Lancet 1993;341(8836):63‐4. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1996c {published and unpublished data}
- Subrahmanyam M. Addition of antioxidants and polyethylene glycol 4000 enhances the healing property of honey in burns. Annals of Burns and Fire Disasters 1996;IX(2):93‐5. [Google Scholar]
Subrahmanyam 2001b {published data only}
- Subrahmanyam M, Hemmady A, Pawar S. Antibacterial activity of honey on bacteria isolated from wounds. Annals of Burns and Fire Disaster 2001;XIV:22‐4. [Google Scholar]
Subrahmanyam 2003 {published data only}
- Subrahmanyam M, Shahapure AG, Nagane NS, Bhagwat VR, Ganu JV. Free radical control ‐ the main mechanism of the action of honey in burns. Annals of Burns and Fire Disaster 2003;XVI:135‐7. [Google Scholar]
Thurnheer 1983 {published data only}
- Thurnheer U, Muller U, Stoller R, Lanner A, Hoigne R. Venom immunotherapy in hymenoptera sting allergy. Comparison of rush and conventional hyposensitization and observations during long‐term treatment. Allergy 1983;38(7):465‐75. [DOI] [PubMed] [Google Scholar]
Tostes 1994 {published data only}
- Tostes ROG, Leite FE. New insights about topical sugar and honey treatment of wounds [Novas consideraçöes sobre o uso tópico de açúcar e mel em feridas]. Revista Médica de Minas Gerais 1994;4:35‐9. [Google Scholar]
Ur‐Rehman 2013 {published data only}
- Ur‐Rehman E, Afzal MO, Ali A, Qureshi A‐RZ‐U‐R, Rashid M. Comparison between honey and povidone‐iodine/normal saline dressing for management of Wagner's grade I & II diabetic foot ulcers. Pakistan Journal of Medical and Health Sciences 2013;7(4):1082‐1085. [Google Scholar]
Vijaya 2012 {published data only}
- Vijaya KK, Nishteswar K. Wound healing activity of honey: a pilot study. Ayu 2012;33(3):374‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visscher 1996 {published data only}
- Visscher P, Vetter R, Camazine S. Removing bee stings. Lancet 1996;348(9023):301‐2. [DOI] [PubMed] [Google Scholar]
Yapucu Gunes 2007 {published data only}
- Yapucu Gunes U, Eser I. Effectiveness of a honey dressing for healing pressure ulcers. Journal of Wound Ostomy and Continence Nursing 2007;34(2):184‐90. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Askarpour 2009 {published data only}
- Askarpour S, Rashidi I, Daei SS, Mahmoodi M. Effect of honey in wound repair in patients whom underwent laparotomy. Internet Journal of Surgery 2010;23:2. [Google Scholar]
Jan 2012 {published data only}
- Jan WA, Shah H, Khan M, Fayaz M, Ullah N. Comparison of conventional pyodine dressing with honey dressing for the treatment of diabetic foot ulcers. Journal of Postgraduate Medical Institute 2012;26(4):402‐407. [Google Scholar]
Maghsoudi 2011 {published data only}
- Maghsoudi H, Salehi F, Khosrowshahi MK, Baghaei M, Nasirzadeh M, Shams R. Comparison between topical honey and mafenide acetate in treatment of burn wounds. Annals of Burns and Fire Disasters 2011;24(3):132‐7. [PMC free article] [PubMed] [Google Scholar]
Additional references
Adams 1939
- Adams F. The genuine works of Hippocrates. Baltimore: Williams & Wilkins, 1939. [Google Scholar]
Bergman 1983
- Bergman A, Yanai J, Weiss J, Bell D, David MP. Acceleration of wound healing by topical application of honey. An animal model. American Journal of Surgery 1983;145:374‐6. [DOI] [PubMed] [Google Scholar]
Blomfield 1973
- Blomfield R. Honey for decubitis ulcers. JAMA 1973;224(6):905. [DOI] [PubMed] [Google Scholar]
Breasted 1930
- Breasted JH. The Edwin Smith papyrus: published in facsimile and hieroglyphic transliteration. Chicago: University of Chicago Press, 1930. [Google Scholar]
Brennan 1985
- Brennan SS, Leaper DJ. The effect of antiseptics on wound healing: a study using the rabbit ear chamber. British Journal of Surgery 1985;72(10):780‐2. [DOI] [PubMed] [Google Scholar]
Cutting 2005
- Cutting KF, White RJ. Criteria for identifying wound infection. Ostomy/Wound Management 2005;51(1):28‐34. [PubMed] [Google Scholar]
Efem 1988
- Efem SEE. Clinical observations on the wound healing properties of honey. British Journal of Surgery 1988;75:679‐81. [DOI] [PubMed] [Google Scholar]
Fu 2001
- Fu X, Wang Z, Sheng Z. Advances in wound healing research in China: from antiquity to present. Wound Repair and Regeneration 2001;9:2‐10. [DOI] [PubMed] [Google Scholar]
Hajar 2002
- Hajar R. Honey from folklore to medical marvel. Heart Views 2002;3:180‐8. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org, The Cochrane Collaboration, 2011. [Google Scholar]
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, Ravaud P, Brorson S. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ 2012;344:1119. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ioannidis 2008
- Ioannidis J, Patsopoulos N, Rothstein H. Reasons or excuses for avoiding meta‐analysis in forest plots. BMJ 2008;336:1413‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1992
- Johnson A. A short history of wound dressings: from animal grease and lint to hydorcolloids and alginates. Ostomy/Wound Management 1992;38:36‐40. [PubMed] [Google Scholar]
Jull 2004
- Jull AB, Rodgers A, Walker N. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD005083] [DOI] [PubMed] [Google Scholar]
Jull 2008a
- Jull AB, Rodgers A, Walker N. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD005083.pub2] [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Aki EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2(81):doi:10.1186/2046‐4053‐2‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lazarus 1994
- Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Archives of Dermatology 1994;130(4):489‐93. [PubMed] [Google Scholar]
Leaper 1986
- Leaper DJ, Simpson RA. The effect of antiseptics and topical antimicrobials on wound healing. Journal of Antimicrobial Chemotherapy 1986;17(2):135‐7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mavric 2008
- Mavric E, Wittmann S, Barth G, Henle T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Molecular Nutrition and Food Research 2008;52(4):483‐9. [DOI] [PubMed] [Google Scholar]
Molan 1999
- Molan PC. Why honey is effective as a medicine. 1. Its use in modern medicine. Bee World 1999;80:80‐92. [Google Scholar]
Molan 2001
- Molan PC. Why honey is effective as medicine. 2. The scientific explanation of its effects. Bee World 2001;82:22‐40. [Google Scholar]
Moore 2001
- Moore OA, Smith LA, Campbell F, Seers K, McQuay HJ, Moore RA. Systematic review of the use of honey as a wound care dressing. BMC Complementary & Alternative Medicine 2001;1(2):http://www.biomedcentral.com/1472‐6882/1/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ndayisaba 1993
- Ndayisaba G, Bazira L, Habonimana E, Muteganya D. Clinical and bacteriological results in wounds treated with honey. Journal of Orthopaedic Surgery 1993;7(2):202‐4. [PubMed] [Google Scholar]
NZGG 2007
- New Zealand Guidelines Group. Management of burns and scalds in primary care. Evidence‐based practice guideline. Wellington: Accident Compensation Corporation, 2007. [Google Scholar]
Oryan 1998
- Oryan A, Zaker SR. Effects of topical application of honey on cutaneous wound healing in rabbits. Journal of Veterinary Medicine Series A 1998;45(3):181‐3. [DOI] [PubMed] [Google Scholar]
Postmes 1997
- Postmes TJ, Bosch MMC, Dutrieux R, Baare J, Hoekstra MJ. Speeding up healing of burns with honey. An experimental study with histological assessment of wound biopsies. In: Mizrahi A, Lensky Y editor(s). Bee Products: Properties, Applications and Apitherapy. New York: Plenum Press, 1997. [Google Scholar]
Riddle 1985
- Riddle JM. Dioscorides on pharmacy and medicine. Austin: University of Texas Press, 1985. [Google Scholar]
Rűttermann 2013
- Rüttermann M, Maier‐Hasselmann A, Nink‐Grebe B, Burckhardt M. Local treatment of chronic wounds: in patients with peripheral vascular disease,chronic venous insufficiency, and diabetes. Deutsches Ärzteblatt International 2013;110(3):25‐31. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 2000
- Sato T, Miyata G. The nutraceutical benefit, part III: honey. Nutritional Pharmaceuticals 2000;16(6):468‐9. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2008
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 27 May 2008).
Trevisanato 2006
- Trevisanato SI. Treatments for burns in the London Medical Papyrus show the first seven biblical plagues of Egypt are coherent with Santorini's volcanic fallout. Medical Hypotheses 2006;66(1):193‐6. [DOI] [PubMed] [Google Scholar]
Vandamme 2013
- Vandamme L, Heyneman A, Hoeksema H, Verbelen J, Monstrey S. Honey in modern wound care: a systematic review. Burns 2013;39(8):1514‐25. [DOI: 10.1016/j.burns.2013.06.014.] [DOI] [PubMed] [Google Scholar]
Wasiak 2013
- Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for superficial and partial thickness burns. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]


