Abstract
The chemokine interleukin-8 (IL-8) has chemoattractant activity for neutrophils and is able to activate and degranulate these cells. We investigated whether IL-8 may exert these effects in children with dengue virus infection. Circulating levels of IL-8, neutrophilic elastase (a constituent of the azurophilic granula of neutrophils), and lactoferrin, released from specific granula, were measured in 186 children with dengue virus infection, 33 healthy children as negative controls and 11 children with bacterial infections as positive controls. Levels of IL-8 on admission were elevated in 71% of the dengue patients, while the elastase and lactoferrin levels were increased in 68 and 17% of patients, respectively. These levels were significantly higher than in healthy children (P < 0.05) for IL-8 and elastase but not for lactoferrin (by the Wilcoxon-Mann-Whitney [WMW] U test). Similar levels of IL-8 were found in patients with bacterial infections. Levels of IL-8 and elastase in patients with shock were significantly higher than in patients without shock (P = 0.02; WMW), but those of lactoferrin were not. IL-8 correlated with elastase and lactoferrin (r = 0.19 and P = 0.009 versus r = 0.24 and P = 0.001, respectively; two-tailed Spearman rank correlation). Thus, IL-8 levels are increased in most patients with dengue virus infection and correlate with degranulation of neutrophils as well as with some clinical and hemodynamic variables. These findings suggest a role for IL-8 in the pathogenesis of dengue virus infection.
Dengue fever (DF) is an acute infectious disease of viral etiology characterized by biphasic fever, headache, pain in various parts of the body, prostration, rash, lymphadenopathy, and leukopenia. Severe cases designated as dengue hemorrhagic fever (DHF) are characterized by abnormalities of hemostasis and by increased vascular permeability. In some patients the dengue shock syndrome (DSS) develops, which has a high mortality rate (17, 18).
The pathogenesis of the shock syndrome in dengue virus infection is still under debate. The primary targets of dengue virus infection are monocytes (19). On histologic examination, swelling and hyaline necrosis of Kupffer cells are commonly seen (5). Also, by using electron microscopy, crystalline arrays of spherical dengue virus-like particles have been observed in the cytoplasma of monocytes of children with DHF-DSS (6). With immunofluorescence techniques, dengue virus antigen has been found to be localized in the cytoplasm of mononuclear cells (7). The dengue virus-infected monocytes presumably become activated to produce various factors (inflammatory mediators), which result in rash, shock, and hemorrhages (19). Among the mediators involved are neutrophils, plasma cascade systems (such as the complement system), and cytokines (4, 19). In particular, the latter are considered to play an important role in the pathogenesis of dengue virus infection. Dengue virus-infected fibroblasts produce interleukin-6 (IL-6) and granulocyte-macrophage colony-stimulating factor (30). In addition, elevated plasma levels of cytokines such as tumor necrosis factor (TNF), IL-6, IL-8, and alpha interferon have been found in patients with severe dengue virus infection (3, 23, 29, 37, 49, 53).
IL-8, a cytokine with potential proinflammatory effects (1, 46, 51), has chemoattractant activity (9, 10, 42, 45, 54) and is able to activate and degranulate neutrophils (11, 36, 42). In vitro, IL-8 is produced by a variety of cells, including monocytes, macrophages, and endothelial cells (1, 35). In vivo, IL-8 is an important regulator of neutrophil activation and migration (22).
Neutrophils are equipped to destroy ingested microorganisms by the generation of a variety of toxic products such as oxygen radicals and by the release of proteinases such as elastase and cathepsin G from primary or azurophilic granules and proteins such as lactoferrin from secondary or specific granules (2, 12–14, 26). In addition, elevated plasma levels of elastase and lactoferrin reflect degranulation of neutrophils (34). Neutrophil elastase is considered to be important for neutrophil-mediated endothelial injury (44, 47, 50). Neutrophil elastase may also facilitate activation of complement, coagulation, and the fibrinolytic system by inactivating the major inhibitors of these systems (25, 41).
In this study we investigated the possible role of activation of neutrophils as assessed by circulating elastase and lactoferrin levels and its relation to IL-8 in the pathogenesis of dengue virus infection.
MATERIALS AND METHODS
Patients.
The children who were included in this study were admitted to the Pediatric Department of Dr. Sardjito Hospital, Yogyakarta, Indonesia, with a clinical diagnosis of fever lasting 2 to 7 days. The diagnosis of DF and DHF was assigned to children according to World Health Organization (WHO) criteria (52), and the severity of DHF was graded as follows: grade I, fever accompanied by nonspecific constitutional symptoms with a positive tourniquet test as the only hemorrhagic manifestation; grade II, same as grade I, but with spontaneous hemorrhagic manifestations; grade III, circulatory failure manifested by a rapid, weak pulse with narrowing of the pulse pressure (<20 mm Hg) or hypotension; grade IV, profound shock with undetectable blood pressure and pulse (52). The definitive diagnosis of dengue virus infection was made when patients had elevated levels of immunoglobulin M (IgM) antibodies with or without detectable IgG antibodies against a dengue virus (52).
In all patients, clinical signs were monitored during their stay in the hospital. All patients were treated with supportive therapy, including the infusion with cristalloids, as well as plasma or fresh whole blood when necessary as a standard therapy. The protocol was approved by the Medical Ethics Committee of the Faculty of Medicine Gadjah Mada University, Dr. Sardjito Hospital. Informed consent was obtained from the parents of each patient included in the study.
Blood sampling.
Blood samples were obtained from patients via the vena mediana cubiti. Blood was collected on admission and in some patients on subsequent days during their hospital stay in tubes containing SBTI (100 pg/ml), benzamidine (10 mmol), and EDTA (10 mmol/liter [final concentration]) to prevent in vitro activation of complement and the contact system of coagulation. The tubes were centrifuged for 10 min at 1,300 × g, and plasma was stored at −70°C until tests were performed.
Blood samples from 33 apparently healthy children in the outpatients' department and from 11 children with a diagnosis of a bacterial infection such as sepsis, bacterial meningitis, and typhoid fever, who served as negative and positive controls, respectively, and who were treated according to the same procedure. In all blood samples the total leukocyte count, platelet count, total protein concentration, and IL-8, elastase, and lactoferrin levels were measured.
Laboratory investigations.
Antibodies against dengue virus were measured by enzyme-linked immunosorbent assay (ELISA). For the detection of dengue virus-specific IgM antibodies, an ELISA was used (48). Dengue virus-specific IgG antibodies were measured by using the indirect ELISA system (48).
IL-8 was measured with an ELISA obtained from the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, according to the manufacturer's instructions (16). Elastase (complexed to α1-antitrypsin) and lactoferrin were determined with radioimmunoassays as described earlier (34). Leukocyte and platelet numbers were assessed according to standard techniques. Plasma protein was measured with a microhematocrit method. Heparin-blood was centrifuged for 10 min at 10,000 to 12,000 rpm (31). The supernatant was analyzed for protein content with a refractometer (Atago SPRN; Atago Co., Ltd.).
Analysis of data.
Differences in age between groups were analyzed by the analysis-of-variance test, and the gender distribution was analyzed by the chi-square test. Differences between patient subgroups or between patient groups and controls and differences between patients with shock or without shock, as well as between the levels of IL-8, elastase, and lactoferrin in patients with or without abnormal clinical parameters were evaluated with the Wilcoxon-Mann-Whitney U mean rank test. Correlations between variables were evaluated with the Spearman correlation test. Statistical significance was accepted at a two-tailed P of <0.05. All calculations were done with SPSS 6.0 software for Windows 95.
RESULTS
Patients.
During September 1995 to May 1996, 235 children were admitted to the hospital with fevers lasting 2 to 7 days, suggesting a dengue virus infection, and they were included in the study. In 186 patients the diagnosis of dengue virus infection could be serologically confirmed (IgM with or without IgG antibodies). A total of 71 patients fulfilled the WHO criteria for DHF: 22 cases for DHF1 (11.8%), 20 cases for DHF2 (10.8%), 18 cases for DHF3 (9.7%), and 11 cases for DHF4 (5.9%). The other patients (n = 115) with positive serology were considered to suffer from DF (61.8%). The mean ages (in years) of patients were as follows: with DF, 8.15 ± 3.15; with DHF1, 9.73 ± 3.15; with DHF2, 8.50 ± 3.28; with DHF3, 8.39 ± 3.58; and with DHF4, 7.40 ± 2.32. The distribution of gender was as follows: DF, 51.8% male, 48.2% female; DHF1, 68.2% male, 31.8% female; DHF2, 40% male, 60% female; DHF3, 33.3% male, 66.7% female; and DHF4, 54.5% male, 45.5% female. There was no statistically significant difference in age and gender distribution between the groups (analysis of variance, P = 0.24; chi-square, P = 0.2 [respectively]).
IL-8 levels.
Levels of IL-8 in plasma samples obtained from 33 healthy children ranged from <20 to 45 pg/ml (median, <20 pg/ml) (Table 1; Fig. 1). Elevated levels, i.e., ≥20 pg/ml, were found in 30.3% of these endemic negative controls.
TABLE 1.
Plasma levels of IL-8, elastase, and lactoferrin on admission in the various groups of patientsa
| Group | IL-8
|
Elastase
|
Lactoferrin
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median (range) (pg/ml) | % Elevated | n | Median (range) (ng/ml) | % Elevated | n | Median (range) (ng/ml) | % Elevated | |
| DF | 113 | 28* (<20–482) | 66.4 | 114 | 128° (36–5,080) | 59.6 | 114 | 160† (25–3,000) | 16.7 |
| DHF1 | 21 | 34* (<20–137) | 81 | 22 | 146.5° (64–1,564) | 72.7 | 22 | 187.5† (49–895) | 27.3 |
| DHF2 | 20 | 44* (<20–366) | 80 | 20 | 129.5° (43–806) | 85 | 20 | 127.5† (29–521) | 10 |
| DHF3 | 18 | 30.5* (<20–268) | 61.1 | 18 | 173° (47–908) | 83.3 | 18 | 174.5† (49–440) | 5.6 |
| DHF4 | 11 | 80* (<20–288) | 90.9 | 11 | 270° (80–920) | 90.9 | 11 | 222† (56–1,132) | 36.4 |
| Dengue virus infection | 183 | 30* (<20–482) | 70.5 | 185 | 137° (36–5,080) | 68.1 | 185 | 163† (25–3,000) | 17.3 |
| Negative control | 33 | <20 (<20–45) | 30.3 | 33 | 89 (45–1,961) | 39.4 | 33 | 144 (35–785) | 12.1 |
| Positive control | 11 | 51 (<20–839) | 90.9 | 11 | 355 (73–1,330) | 81.8 | 11 | 1,048 (86–2,208) | 54.5 |
Significance values: *, P < 0.05 compared to negative control and P > 0.05 compared to positive control except in groups DF and DHF1; °, P < 0.05 compared to negative control and P > 0.05 compared to positive control except in the total dengue group, DF, and DHF2; †, P > 0.05 compared to negative control and P < 0.05 compared to positive control except in group DHF4.
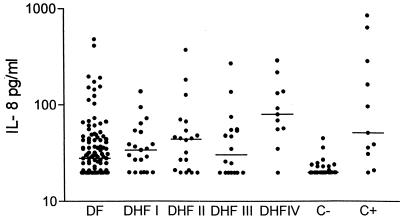
FIG. 1.
Levels of IL-8 in healthy children, in the dengue virus infection group, and in bacterial infections. DHFI to -IV, DHF1 to -4; C−, negative control; C+, positive control. The line indicates the median. In dengue patients IL-8 was measured in plasma obtained upon admission; in the bacterial infection group it was measured within 48 h of diagnosis.
In the plasma samples obtained on admission from the dengue virus-infected patients, IL-8 levels were elevated in 70.5% (Table 1), with a range of <20 to 482 pg/ml and a median of 30 pg/ml. In three patients not enough plasma was available for IL-8 measurement: two in the DF group and one in the DHF1 group. The highest proportion of elevated IL-8 levels was found in the DHF4 group (90.9%), with a range of <20 to 288 pg/ml and a median of 80 pg/ml, followed by the DHF1 (81%), DHF2 (80%), DF (66.4%), and DHF3 (61.1%; see Table 1) groups. In the group with bacterial infection (positive control), the median plasma level of IL-8 was 51 pg/ml, with a range of <20 to 839. Levels were elevated in most (90.9%) patients (Table 1; Fig. 1).
Plasma levels of IL-8 in the dengue virus-infected patient groups were significantly higher than those in the healthy child group (Table 1), whereas levels were not different between the dengue virus infection patient groups and the bacterial infection patient group, except for the levels in patients with DHF1 and DF (Table 1). In 82 patients blood samples for IL-8 measurement were available on the day after admission (day 2), and in 34 patients they were available on day 3. In the dengue patients in whom serial measurements were available, the highest levels of IL-8 occurred in almost all patients on admission and typically decreased in the following days.
Elastase and lactoferrin levels.
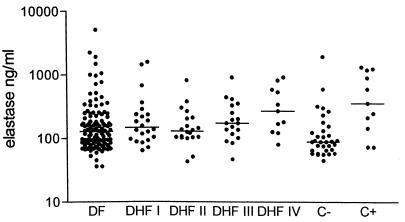
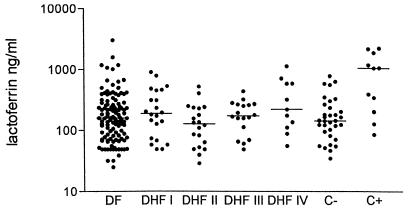
In all patients except one (in the DF group) elastase and lactoferrin were measured in plasma obtained upon admission. Plasma levels of elastase were elevated in 68.1% and those of lactoferrin were elevated in 17.3% of patients with dengue virus infection (Table 1; Fig. 2 and 3). Elastase levels in the patients with dengue virus infection were significantly higher than in healthy children, whereas elastase levels were similar to those in patients with bacterial infections (Table 1; Fig. 2). Plasma levels of lactoferrin were comparable to those in the healthy children (Table 1; Fig. 3).
FIG. 2.
Levels of elastase in healthy children, in the dengue virus infection groups, and in those with bacterial infections. DHF I to IV, DHF1 to -4; C−, negative control; C+, positive control. The line indicates the median. In dengue patients IL-8 was measured in plasma obtained on admission; in the bacterial infection group it was measured within 48 h of diagnosis.
FIG. 3.
Levels of lactoferrin in healthy children, in dengue virus infection groups, and in those with bacterial infections. DHFI to IV, DHF1 to -4; C−, negative control; C+, positive control. The line indicates the median. In dengue patients IL-8 was measured in plasma obtained on admission; in the bacterial infection group it was measured within 48 h of diagnosis.
Relationship of elevated plasma levels of IL-8 and the presence of shock.
Of the 186 patients with dengue virus infection, 29 fulfilled the WHO criteria for shock (DHF3 plus DHF4). Plasma levels of IL-8 and elastase on admission were significantly higher in patients who developed shock during their hospital stay than those in patients without shock (Table 2), whereas plasma levels of lactoferrin were comparable between patients with or without shock (Table 2). Thus, elevated plasma levels of IL-8 and elastase were significantly associated with the development of shock, although there was considerable overlap of these variables between patients with and those without shock.
TABLE 2.
Plasma levels of IL-8, elastase, and lactoferrin on admission in patients with shock versus normotensive patients with dengue virus infectiona
| Group | IL-8
|
Elastase
|
Lactoferrin
|
|||
|---|---|---|---|---|---|---|
| n | Median (range) (pg/ml) | n | Median (range) (ng/ml) | n | Median (range) (ng/ml) | |
| Shock | 29 | 54 (<20–288) | 29 | 181 (47–920) | 29 | 180 (49–1,132) |
| Normotensive | 154 | 29 (<20–482) | 156 | 131 (36–5,080) | 156 | 160 (25–3,000) |
P = 0.03, 0.009, and 0.23 for IL-8, elastase, and lactoferrin, respectively.
Relationship of IL-8 to neutrophil elastase and lactoferrin.
Plasma levels of IL-8 correlated significantly, although weakly, with those of elastase (r = 0.19, P = 0.009), as well as with those of lactoferrin (r = 0.24, P = 0.001).
Relationship of plasma levels of IL-8 elastase, lactoferrin, and clinical and laboratory variables.
We tried to correlate admission levels of IL-8, elastase, and lactoferrin with highest or lowest values of some continuous laboratory and clinical variables measured during hospital stay (Table 3). The levels of these mediators were also compared between patients who developed clinical complications such as ascites, pleural effusion, or extremely cold extremities, as well as abnormal clotting parameters during their hospital stay and patients who did not show these abnormalities (Table 4).
TABLE 3.
Relation of admission IL-8, elastase, and lactoferrin levels to laboratory and hemodynamic parameters
| Parametersa | IL-8
|
Elastase
|
Lactoferrin
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | r | P | n | r | P | n | r | P | |
| Hematocrit (max) | 180 | 0.02 | 0.7 | 182 | 0.13 | 0.07 | 182 | −0.02 | 0.7 |
| Leukocyte count (max) | 181 | 0.02 | 0.7 | 183 | 0.15 | 0.04 | 183 | 0.15 | 0.04 |
| Platelet count (min) | 181 | −0.15 | 0.04 | 183 | −0.27 | <0.01 | 183 | −0.04 | 0.6 |
| Plasma protein (min) | 177 | 0.03 | 0.7 | 179 | −0.42 | <0.01 | 179 | −0.09 | 0.2 |
| Heart rate (max) | 178 | 0.15 | 0.05 | 180 | 0.14 | 0.06 | 180 | 0.12 | 0.1 |
| Temp (max) | 177 | 0.12 | 0.1 | 179 | 0.14 | 0.06 | 179 | 0.17 | 0.02 |
max, maximal value during hospital stay; min, minimal value during hospital stay.
TABLE 4.
Relationships between levels of IL-8, elastase, and lactoferrin on admission with clinical and coagulation complications
| Parametera | IL-8
|
Elastase
|
Lactoferrin
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median (range) (pg/ml) | P | n | Median (range) (ng/ml) | P | n | Median (range) (ng/ml) | P | |
| Ascites | |||||||||
| Positive | 40 | 54 (<20–366) | 40 | 173 (53–1,564) | 40 | 170 (39–1,132) | |||
| Negative | 129 | 28 (<20–412) | <0.0001 | 131 | 127 (36–5,080) | 0.003 | 131 | 163 (25–1,568) | 0.9 |
| Pleural effusion | |||||||||
| Positive | 27 | 54 (<20–366) | 27 | 173 (47–920) | 27 | 169 (29–715) | |||
| Negative | 142 | 28.5 (<20–412) | 0.01 | 144 | 126 (36–5,080) | 0.02 | 144 | 161 (25–1,568) | 0.7 |
| Cold extremities | |||||||||
| Positive | 19 | 55 (<20–268) | 19 | 252 (85–5,080) | 19 | 195 (49–1,568) | |||
| Negative | 159 | 29 (<20–482) | 0.04 | 161 | 131 (36–2,248) | 0.002 | 161 | 159 (25–3,000) | 0.07 |
| Fibrinogen | |||||||||
| Decreased | 17 | 92 (<20–366) | 17 | 186 (53–806) | 17 | 236 (56–895) | |||
| Normal | 9 | <20 (<20–52) | 0.001 | 9 | 95 (45–354) | 0.04 | 9 | 190 (49–499) | 0.5 |
| APTT | |||||||||
| Prolonged | 12 | <20 (<20–152) | 12 | 174.5 (45–908) | 12 | 180 (49–444) | |||
| Normal | 107 | 32 (<20–412) | 0.0009 | 107 | 138 (43–5,080) | 0.6 | 107 | 169 (25–1,568) | 0.6 |
| PTT | |||||||||
| Prolonged | 47 | 29 (<20–412) | 47 | 125 (45–1,564) | 47 | 125 (45–1,564) | |||
| Normal | 67 | 36 (<20–366) | 0.1 | 67 | 155 (43–5,080) | 0.2 | 67 | 155 (43–5,080) | 0.01 |
| Clotting time | |||||||||
| Prolonged | 39 | 39 (<20–366) | 39 | 149 (45–1,424) | 39 | 156 (29–586) | |||
| Normal | 68 | 27 (<20–412) | 0.02 | 70 | 133 (43–2,248) | 0.2 | 70 | 169 (25–1,180) | 0.5 |
APTT, activated partial thromboplastin time; PTT, partial thromboplastin time.
A negative weak correlation was found between plasma levels of IL-8 with platelet count, and a positive weak correlation was found with heart rate (Table 3). Plasma levels of IL-8 in patients with ascites, pleural effusion, cold extremities, and decreased fibrinogen levels were higher than those in whom these abnormalities were not present (Table 4). Also, IL-8 levels were higher in patients with a prolonged activated partial thromboplastin time and clotting time than in patients in whom these variables were normal. We found a negative correlation between plasma levels of elastase with plasma protein levels and with platelet count and a weak positive correlation with leukocyte count as well (Table 3). Patients with ascites, pleural effusion, cold extremities, and decreased fibrinogen levels had higher plasma levels of elastase than patients without these abnormalities (Table 4). Body temperature and leukocyte count were significantly correlated with plasma levels of lactoferrin (Table 3), but no differences were found in lactoferrin levels between patients with and those without clinical abnormalities and abnormal coagulation variables (except partial thromboplastin time), as shown in Table 4.
Relationship of IL-8, elastase, and lactoferrin and sequential infection.
A total of 53 patients had suspected primary dengue virus infection (IgM positive, IgG negative), whereas 131 patients had suspected secondary infection (IgM positive, IgG positive). Two patients could not be classified because of missing IgG results in the presence of a positive IgM result. Levels of IL-8 and of lactoferrin were comparable in patients with primary or secondary infection. On the other hand, plasma levels of elastase were significantly higher in patients with secondary infections than in patients with primary infections (P = 0.01).
DISCUSSION
In this study we found elevated plasma levels of the chemokine IL-8 and the neutrophil degranulation products elastase and lactoferrin in patients with dengue virus infection. In ca. 70% of these patients, IL-8 and elastase levels were increased, whereas only 17% had increased plasma concentrations of lactoferrin. Levels of these mediators significantly correlated with each other. Moreover, levels of IL-8 and elastase measured on admission were higher in patients who developed shock and in patients with ascites or pleural effusion, which was not the case for lactoferrin.
In several diseases, such as bacterial meningitis, sepsis, or typhoid fever, the plasma levels of IL-8 are markedly increased in the majority of patients (16, 27). In agreement with those findings, we found increased levels in 90.9% of patients with a bacterial infection tested as positive controls. Levels in these patients were similar to those in patients with dengue virus infection. Occasionally, IL-8 is increased in patients with sepsis by more than 1,000-fold relative to values in healthy controls (16). In the present study, circulating IL-8 was only moderately elevated in about 70% of the patients, though patients with a more severe form of DF more often had elevated IL-8 levels. In sepsis IL-8 levels are usually the highest on admission (16). Since patients with dengue virus infection usually are not admitted to the hospital before the third or fourth day after onset of fever or even later, we cannot exclude the possibility that we missed high levels occurring in the early stages of the disease.
The plasma levels of IL-8 in the patients with shock were higher than in normotensive patients (Table 2), although the difference was of borderline statistical significance. In addition, we found a correlation between IL-8 levels and the clinical signs of ascites, pleural effusion, and cold extremities. Thus, it is conceivable that high levels of IL-8 in patients with dengue virus infection may correlate with plasma leakage, which is a mechanism contributing to the development of shock in severe DHF. Our data are somewhat at variance with data from a recent study of DHF involving 188 children with shock on admission and 71 children who developed it later (3). This study showed that in shock patients who survived there was no increase of plasma levels of IL-8 whatsoever, but in patients with fatal shock (n = 6) the levels of IL-8 were markedly increased (median, 200 pg/ml; range, 20 to 550 pg/ml) (3). Only one of our patients died, and the difference between our results and those described in the cited report (3) could result from different assays and the limited number of shock patients in our study. On the other hand, another recent study with 73 DHF patients showed an association of elevated IL-8 levels with severity of illness and fatal outcome, a finding similar to our results (37). Among patients with DHF1 10% had elevated IL-8 levels, a percentage progressively increasing (together with mean values for IL-8) with the severity of illness up to 61% in DHF4 (37). All six nonsurviving patients had IL-8 plasma levels higher than 200 pg/ml.
Our study also provides evidence for neutrophil activation in dengue virus infection as neutrophil degranulation products (e.g., elastase and lactoferrin) in the circulation were increased. The statistical-significance correlation between IL-8 and these degranulation products suggests that IL-8 is involved in neutrophil activation in dengue virus infection, although other agonists likely are involved as well. A significantly higher level of elastase in patients with shock than in normotensive patients suggests that this degranulation product may be involved in the pathogenesis of shock. This is supported by the fact that there was a correlation between elastase and some clinical parameters (Table 4). Elastase as a neutrophil degranulation product may facilitate activation of the complement, thereby contributing to vasodilatation and increased capillary permeability in dengue virus infection. Remarkably, the high levels of IL-8 and elastase were not associated with high levels of lactoferrin, indicating predominant release of azurophilic granules.
In vitro studies have indicated that many cell types, including monocytes, macrophages, and endothelial cells, can produce IL-8 in response to stimulation with endotoxin, IL-1 or TNF-α (1, 35). The latter agents also have been shown to induce IL-8 release in vivo. In dengue virus infection, monocytes are the predominant primary cells to be infected (19). In addition, pathological studies have shown the swelling of endothelial cells of small vessels in dengue virus infection (32, 38, 40). Thus, mononuclear cells, and possibly also endothelial cells, stimulated during dengue virus infection may well be responsible for the IL-8 release.
IL-8 is a chemoattractant, induces neutrophil degranulation, promotes adherence of neutrophils to endothelium by increasing integrin expression on neutrophils, and regulates transendothelial migration of these cells (8, 24). High local concentrations of IL-8 induce neutrophil infiltration, edema formation due to neutrophil mediated endothelial damage, and subsequent plasma leakage (21). These proinflammatory effects of IL-8, and the fact that levels of IL-8 were correlated with the presence of ascites and pleural effusion, suggest a role for IL-8 in the pathogenesis of increased microvascular permeability which may explain the association of IL-8 levels with the development of shock (Table 3).
Results from previous studies have suggested that individuals who have a secondary infection are at significant risk for developing DHF or Dengue shock syndrome (DSS) (20, 28, 39). However, cases of DHF or DSS have been documented in patients experiencing dengue virus infections for the first time (15, 33, 43). In this study only 5.8% of the patients with a primary infection developed shock. This may be a reason why in this study no significant difference in plasma levels of IL-8 between primary and secondary infections was found.
In conclusion, we show that circulating levels of IL-8 and of neutrophilic degranulation products are increased in a number of patients with dengue virus infection and correlate with several important biochemical and clinical parameters. These data suggest a role for IL-8 and neutrophils as inflammatory mediators in the pathogenesis of dengue virus infection.
ACKNOWLEDGMENTS
We thank J. Groen, Laboratory for Exotic Viral Infections, Department of Virology, Erasmus Medical Center Rotterdam, for providing serologic dengue virus measurements. The assistance of A. J. M. Eerenberg-Belmer and G. van Mierlo, Central Laboratory for the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands, is also greatly acknowledged.
This study was completed as part of The Pathophysiology of Dengue Fever project by The Dutch-Indonesian Study Group, supported by grant 94-BTM-01 of The Royal Dutch Academy of Sciences.
REFERENCES
- 1.Baggiolini M, Walz A, Kunkel S L. Neutrophil-activating peptide-1/interleukin-8, a novel cytokine that activates neutrophils. J Clin Investig. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainton D F. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J Cell Biol. 1973;58:249–254. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bethell D B, Flobbe K, Thanh Phuong C X, et al. Pathophysiologic and prognostic role of cytokines in dengue hemorrhagic fever. J Infect Dis. 1998;177:778–782. doi: 10.1086/517807. [DOI] [PubMed] [Google Scholar]
- 4.Bhakdi S, Kazatchkine M D. Pathogenesis of dengue: an alternative hypothesis. Southeast Asian J Trop Med Public Health. 1990;21:652–656. [PubMed] [Google Scholar]
- 5.Bhamarapravati N, Tuchinda P, Boonyapaknavik V. Pathology of Thailand hemorrhagic fever: a study of 100 autopsy cases. Ann Trop Med Parasitol. 1967;61:500–510. doi: 10.1080/00034983.1967.11686519. [DOI] [PubMed] [Google Scholar]
- 6.Boonpucknavig V, Bhamarapravati N, Boonpucknavig S, Futrakul P, Tanpaichitr P. Glomerular changes in dengue hemorrhagic fever. Arch Pathol Lab Med. 1976;100:206–212. [PubMed] [Google Scholar]
- 7.Boonpucknavig S, Boonpucknavig V, Bhamarapravati N, Nimmanitya S. Immunofluorescence study of skin rash in patients with dengue hemorrhagic fever. Arch Pathol Lab Med. 1979;103:463–466. [PubMed] [Google Scholar]
- 8.Carveth H J, Bohnsack J F, McIntyre T M, Baggiolini M, Prescott S M, Zimmerman G A. Neutrophil activating factor (NAF) induces polymorphonuclear leukocyte adherence to endothelial cells and to subendothelial matrix proteins. Biochem Biophys Res Commun. 1989;162:387–393. doi: 10.1016/0006-291x(89)92009-3. [DOI] [PubMed] [Google Scholar]
- 9.Colditz I, Zwahlen R, Dewald B, Baggiolini M. In vivo inflammatory activity of neutrophil-activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989;134:755–760. [PMC free article] [PubMed] [Google Scholar]
- 10.Collins P D, Jose P J, Williams T J. The sequential generation of neutrophil chemoattractant proteins in acute inflammation in the rabbit in vivo: relationship between C5a and proteins with the characteristics of IL-8/neutrophil-activating protein 1. J Immunol. 1991;146:677–684. [PubMed] [Google Scholar]
- 11.Downey G P. Mechanism of leukocyte motility and chemotaxis. Curr Opin Immunol. 1994;6:113–124. doi: 10.1016/0952-7915(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 12.Estensen R D, White J G, Holmes B. Specific degranulation of human polymorphonuclear leukocytes. Nature. 1974;248:347–349. doi: 10.1038/248347a0. [DOI] [PubMed] [Google Scholar]
- 13.Goetzl E J, Goldstein I M. Cellular components of inflammation: granulocytes. In: Kelly W N, Harris E D, Ruddy S, Sledge C B, editors. Textbook of rheumatology. Philadelphia, Pa: The W. B. Saunders Co.; 1985. pp. 115–144. [Google Scholar]
- 14.Goldstein I M, Hoffstein S T, Weissmann G. Mechanism of lysosomal enzyme release from human polymorphonuclear leukocytes. Effects of phorbol myristate. J Cell Biol. 1975;66:647–651. doi: 10.1083/jcb.66.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubler D J, Reed D, Rosen L, Hitchcock J C. Epidemiologic, clinical, and virologic observations on dengue in the Kingdom of Tonga. Am J Trop Med Hyg. 1978;27:581–589. doi: 10.4269/ajtmh.1978.27.581. [DOI] [PubMed] [Google Scholar]
- 16.Hack C E, Hart M, Strack van Schijndel R J M, et al. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun. 1992;60:2835–2842. doi: 10.1128/iai.60.7.2835-2842.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halstead S B. Immunological parameters of togavirus disease syndromes. In: Schlesinger R W, editor. The togaviruses. New York, N.Y: Academic Press, Inc.; 1980. pp. 107–173. [Google Scholar]
- 18.Halstead S B. Global epidemiology of dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 1990;21:636–641. [PubMed] [Google Scholar]
- 19.Halstead S B. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11:830–839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 20.Halstead S B. Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med. 1970;42:350–362. [PMC free article] [PubMed] [Google Scholar]
- 21.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 22.Hechtman D H, Cybulsky M I, Fuchs H J, Baker J B, Gimbrone M A., Jr Intravascular IL-8: inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J Immunol. 1991;147:883–892. [PubMed] [Google Scholar]
- 23.Hober D, Poli L, Roblin B, et al. Serum levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in dengue-infected patients. Am J Trop Med Hyg. 1993;48:324–331. doi: 10.4269/ajtmh.1993.48.324. [DOI] [PubMed] [Google Scholar]
- 24.Huber A R, Kunkel S L, Todd III R F, Weiss S J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 25.Johnson U, Ohlsson K, Olsson I. Effects of granulocyte neutral proteases on complement components. Scand J Immunol. 1976;5:421–426. doi: 10.1111/j.1365-3083.1976.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnston R B, Jr, Lehmeyer J E, Guthrie L A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976;143:1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keuter M, Dharmana E, Gasem M H, et al. Pattern of proinflammatory cytokines and inhibitors during typhoid fever. J Infect Dis. 1994;169:1306–1311. doi: 10.1093/infdis/169.6.1306. [DOI] [PubMed] [Google Scholar]
- 28.Kliks S C, Nisalak A, Brandt W E, Wahl L, Burke D C. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 29.Kurane I, Innis B L, Nimmannitya S, Nisalak A, Meager A, Ennis F A. High levels of interferon alpha in the sera of children with dengue virus infection. Am J Trop Med Hyg. 1993;48:222–229. doi: 10.4269/ajtmh.1993.48.222. [DOI] [PubMed] [Google Scholar]
- 30.Kurane I, Janus J, Ennis F A. Dengue virus infection of human skin fibroblasts in vitro production of IFN-β, IL-6 and GM-CSF. Arch Virol. 1992;124:21–30. doi: 10.1007/BF01314622. [DOI] [PubMed] [Google Scholar]
- 31.Merck E. Hematological laboratory methods. Darmstadt, Germany: GIT Verlag-Earnst Gieblre; 1983. [Google Scholar]
- 32.Monath T P. Flaviviruses. In: Fields B W, Knipe D M, Chanock R M, Malnick J L, Roizman B, Shope R E, editors. Virology. New York, N.Y: Raven Press; 1985. pp. 955–1004. [Google Scholar]
- 33.Morens D M, Sather G E, Gubler D J, Rammohan M, Woodall J P. Dengue shock syndrome in an American traveler with primary dengue 3 infection. Am J Trop Med Hyg. 1987;36:424–426. doi: 10.4269/ajtmh.1987.36.424. [DOI] [PubMed] [Google Scholar]
- 34.Nuijens J H, Abbink J J, Wachtfogel Y T, Coleman R W, Eerenberg A J, Dors D, Kamp A J, Strack van Schindel R J, Thijs L G, Hack C E. Plasma elastase α1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J Lab Clin Med. 1992;119:159–168. [PubMed] [Google Scholar]
- 35.Patterson C E, Barnard J W, Lafuze J E, Hull M T, Baldwin S J, Rhoades R A. The role of activation of neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167:1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1994;167:1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raghupathy R, Chaturvedi U C, Al-Sayer H, et al. Elevated levels of IL-8 in dengue hemorrhagic fever. J Med Virol. 1998;56:280–285. doi: 10.1002/(sici)1096-9071(199811)56:3<280::aid-jmv18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 38.Sabin A B. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 39.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyaspongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halsted S B. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 40.Schlesinger R W. Dengue viruses. New York, N.Y: Springer-Verlag; 1977. [Google Scholar]
- 41.Schmidt W, Egbring R, Havemann K. Effect of elastase-like and chymotrypsin-like neutral proteases from human granulocytes on isolated clotting factors. Thromb Res. 1975;6:326–329. doi: 10.1016/0049-3848(75)90081-x. [DOI] [PubMed] [Google Scholar]
- 42.Schroder J M, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin-1 activity. J Immunol. 1987;139:3473–3483. [PubMed] [Google Scholar]
- 43.Scott R M, Nimmannitya S, Bancroft W H, Mansuwan P. Shock syndrome in primary dengue infections. Am J Trop Med Hyg. 1976;25:866–874. doi: 10.4269/ajtmh.1976.25.866. [DOI] [PubMed] [Google Scholar]
- 44.Smedly L A, Tonnesen M G, Sandhaus R A, Haslett C, Guthrie L A, Johnston R B, Jr, Henson P M, Worthen G S. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essensial role of neutrophil elastase. J Clin Investig. 1986;77:1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Damme J, van Beumen J, Opdenakker G, Billiau A. A novel, NH2-terminal sequence-characterized human monokine possessing neutrophil chemotactic, skin-reactive, and granulocytosis-promoting activity. J Exp Med. 1988;167:1364–1376. doi: 10.1084/jem.167.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Damme J. Interleukin-8 and related molecules. In: Thomson A W, editor. The cytokine handbook. London, England: Academic Press; 1991. pp. 201–214. [Google Scholar]
- 47.Varani J, Ginsburg I, Schuger L, Gibbs D F, Bromberg J, Johnson K J, Ryan J S, Ward P A. Endothelial cell killing by neutrophils. Synergistic interaction of oxygen products and proteases. Am J Pathol. 1989;135:435–438. [PMC free article] [PubMed] [Google Scholar]
- 48.Velzing J, Groen J, Drouet M T, van Amerongen G, Copra C, Osterhaus A D, Deubel V. Induction of protective immunity against dengue virus type 2: comparison of candidate live attenuated and recombinant vaccines. Vaccine. 1999;17:1312–1320. doi: 10.1016/s0264-410x(98)00393-4. [DOI] [PubMed] [Google Scholar]
- 49.Vitarana T, de Silva H, Withana N, Gunasekera C. Elevated tumour necrosis factor in dengue fever and dengue haemorrhagic fever. Ceylon Med J. 1991;36:63–65. [PubMed] [Google Scholar]
- 50.Weiss S J, Young J, LoBuglio A, Slivka A, Nimeh N. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Investig. 1981;68:714–718. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westwick J, Li S W, Camp R D. Novel neutrophil-stimulating peptides. Immunol Today. 1989;10:146–147. doi: 10.1016/0167-5699(89)90164-3. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 53.Yadav M, Kamath K R, Iyngkaran N, Sinniah M. Dengue haemorrhagic fever and dengue shock syndrome: are they tumour necrosis factor-mediated disorders? Microbiol Immunol. 1991;89:45–50. doi: 10.1111/j.1574-6968.1991.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura T, Matsushima K, Tanaka S, et al. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]