Abstract
Background: Blood sampling is expensive, time-consuming, invasive, and requires technical facilities, which can be replaced by more convenient samples such as saliva. C-reactive protein (CRP) is a widely used biomarker in the management of many disorders and plasma CRP (pCRP) is suggested to be replaced by salivary CRP (sCRP). This study aimed to systematically review all available literature on the sCRP levels in systemic and oral disorders and how sCRP and pCRP levels correlate among these patients and healthy individuals.
Methods: In this systematic review, a PubMed, Embase, Scopus, and Google Scholar search was conducted on October-2021 to identify all research investigating sCRP levels in systemic and oral disorders.
Results: A total of 130 publications were analyzed in the review. Most of the studies reported that sCRP and pCRP levels are correlated, and sCRP is a reliable alternative for pCRP level for the diagnosis and management of medical conditions. sCRP has been measured in many different medical and oral disorders and significantly correlated with disease activity in most cases.
Conclusion: Salivary CRP is a good alternative for Plasma CRP levels in most cases.
Keywords: C-reactive protein, Oral disorders, Saliva, Systemic disorders
↑What is “already known” in this topic:
Plasma biomarkers are important in the diagnosis and management of disorders which is painful, invasive, and expensive. Therefore, new sampling methods such as saliva are introduced. CRP is a widely used biomarker, which can be measured in saliva instead of plasma.
→What this article adds:
- Salivary CRP in diagnosis and management of disorders
- Correlation between salivary and plasma CRP levels
- Suggestions for further research
Introduction
Blood sampling is one of the most widely used methods for the diagnosis and management of many different medical disorders (1, 2). This is because the levels of most of the biomarkers in the human body are directly represented in blood and plasma samples and correlate well with their total body levels (1, 2). Although any of the body fluids or tissues can be used for this purpose, plasma is yet the most widely accepted and used sample in this regard (2). Taking a plasma sample is invasive, painful, expensive, and needs expertise, professional staff, and technical facilities (1). Therefore, the replacement of this sampling method with other less-invasive, lower-priced, easier, more accessible, and more accepted patients samples have been of specific interest to researchers and clinicians in recent years (1). Saliva is one of the body fluids which is a great replacement for a blood sample in many cases (1, 2). A great number of plasma proteins and biomarkers are secreted in saliva in a considerable amount, with a significant correlation with their plasma and total body concentrations, though their salivary levels can greatly reflect their plasma levels and greatly inform us about the different aspect of a disorder (1, 3, 4).
One of the biomarkers which is highly potential to be measured in saliva instead of plasma in order to diagnose, and monitor many different systemic and oral disorders is C-Reactive Protein (CRP) (1, 2, 4-7). This inflammatory cytokine is being widely measured in plasma for diagnostic and therapeutic purposes in many different medical conditions (1, 4, 6). Measuring the salivary CRP levels instead of plasma levels is an innovative and applicable method because this biomarker is directly transferred from blood to saliva in most cases and salivary concentrations of this biomarker have well correlated with the plasma levels in many studies (1, 2, 4-7). While its salivary and plasma levels are highly correlated, measuring it in saliva samples is a novel method for the evaluation and management of these conditions in a less invasive and more convenient way and many studies have investigated and approved its applicability (1, 4). This protein significantly increases in plasma and saliva during various medical conditions such as systemic inflammation (8), infection (9), myocardial infarction (1, 2, 4), autoimmune disorders (10), oral inflammation (11), periodontitis (11), and sepsis (8). Studies have also presented interesting findings regarding salivary CRP levels among healthy individuals and some of them have investigated salivary CRP levels among patients of different ages (1, 2, 12).
These findings and the expansion of such easy and accessible methods are expandable to other biomarkers and many other medical conditions. This can also be widely used by other disciplines such as Forensic Medicine (13). CRP measurements in human tissue samples are of specific importance because, besides being easy, measurable, viable and inexpensive in a forensic setting, in cases of doubt, CRP level displays the natural mode of death, or in cases of trauma, indicates vital reaction .This is also useful for a pre-autopsy screening and a more extensive search for diseases not easily diagnosed, such as sepsis or ketoacidosis . Therefore, measurement of sCRP levels in forensic settings is more accessible, applicable, easier, and useful than any other human sample (13).
To the best of our knowledge, this is the first study to comprehensively review all performed research evaluating salivary CRP levels in different medical conditions and oral disorders to provide conclusions and suggest further comments for future studies. Further research in this area can greatly help science and open a newer door to the development of less invasive, more acceptable, and faster diagnostic methods for the diagnosis and management of systemic disorders, especially important conditions such as acute MI (1, 2, 4, 6).
This study reviews all publications assessing CRP in human saliva and reports their findings regarding sCRP levels in different systemic and oral disorders, indications of the assessment of sCRP levels in the evaluation, diagnosis, and management of medical conditions, and the relationship between salivary and plasma CRP levels, besides providing a general overview of what has been done in this area and what needs to be done by the future scientists, as well as suggestions for future researchers.
Methods
This systematic review was performed according to thePreferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines (14).
Eligibility Criteria
Inclusion criteria for search and review were case-control and cross-sectional studies that directly investigated salivary CRP levels in association with medical conditions and/or oral disorders. There was no study exclusion based on publication year. Studies with animal subjects, case reports, reviews, and studies in languages other than English were excluded.
Search strategy
A PubMed search using the terms (“Saliva”[Mesh] OR salivary) AND (“C reactive protein”[Mesh] OR CRP) AND (“disease”[Mesh] OR diseases OR systemic disease[Mesh] OR systemic disorders OR oral disease OR oral disorders) and an Embase search using the terms ((saliva) OR (salivary)) AND ((C reactive protein) OR (C-reactive protein) OR (CRP)) AND( disease OR diseases OR systemic disease OR systemic disorders OR oral disease OR oral disorders) were conducted on October 2021. Moreover, a Scopus search using the terms was performed ((saliva) OR (salivary)) AND ((C reactive protein) OR (C-reactive protein) OR (CRP)) AND (disease OR diseases OR systemic disease OR systemic disorders OR oral disease OR oral disorders) for records published before or on October 2021. Besides, A Google Scholar search was performed (“Saliva” OR salivary) AND (“C reactive protein” OR CRP) AND (“disease” OR diseases OR systemic disease OR systemic disorders OR oral disease OR oral disorders) for records published before or on October 2021.
Quality of selected studies and assessment of the risk of bias
Since we aimed to investigate all performed studies in this regard from two valid sources PubMed and Embase and look into what has been done so far, the quality assessment process was time-consuming. We agreed not to perform this process and report the existing literature with as detailed and possible data as possible and gather a comprehensive overview of the literature in this regard. We mainly aimed to report the existing research and ideas in this field and wouldn’t want to exclude any potential report which could be a great lead and potential idea for future researchers.
Data
Data extracted from included records are summarized and presented in tables for reference and simplicity. Tables were created according to the number of disorders investigated in relation to sCRP levels and then were arranged temporally based on publication dates (Table 1).
Table 1. Conclusion and summary of studied articles.
| Category of study | Number of the studies in each category | The number of studies reporting a significant correlation between sCRP and pCRP levels | The number of studies reporting a non-significant correlation between sCRP and pCRP levels | The number of studies reporting no correlation between sCRP and pCRP levels |
|---|---|---|---|---|
| 1. Oral Disorders | 29 | 18 | 6 | 5 |
| 2. Psychological factors | 19 | 19 | - | - |
| 3. Stress | 14 | 14 | - | - |
| 4. Cardiovascular Disorders | 12 | 12 | - | - |
| 5. Obesity | 10 | 10 | - | - |
| 6. Physical Activity | 8 | 6 | 1 | 1 |
| 7. Sleep and Circadian Rhythm | 6 | 6 | - | - |
| 8. Diet | 5 | 2 | - | 3 |
| 9. Sepsis | 4 | 4 | - | - |
| 10. Healthy individuals | 4 | - | - | 4 |
| 11. Physical health | 3 | 3 | - | - |
| 12. Diabetes Mellitus | 3 | 2 | - | 1 |
| 13. Tuberculosis | 2 | 2 | - | - |
| 14. Systemic inflammation | 2 | 2 | - | - |
| 15. Pneumonia | 2 | 2 | - | - |
| 16. Metabolic syndrome | 2 | 2 | - | - |
| 17. COPD | 2 | 2 | - | - |
| 18. Age | 2 | 1 | - | 1 |
| 19. Smoking | 1 | 1 | - | - |
| 20. Sickle Cell Anemia | 1 | 1 | - | - |
| 21. Sexual Activity | 1 | 1 | - | - |
| 22. Neonatal Disorders | 1 | 1 | - | - |
| 23. Renal Disorder | 1 | 1 | - | - |
| 24. Rheumatic Disease | 1 | 1 | - | - |
| 25. Osteoporosis | 1 | - | - | 1 |
| 26. Using Electronic Devices | 1 | - | 1 | - |
| 27. HIV/AIDS | 1 | - | 1 | - |
| 28. Headache | 1 | - | 1 | - |
| 29. Covid 19 | 1 | 1 | - | - |
| 30. Asthma and Allergy | 1 | - | 1 | - |
| 31. Acute Respiratory Illness | 1 | 1 | - | - |
| 32. Dermatologic disorders | 1 | 1 | - | - |
| 33. Polycystic Ovarian Syndrome | 1 | 1 | - | - |
Results
Literature search results
A total of 440 records were identified through a primary PubMed search. After title and abstract analysis, a total of 117 records were included. Moreover, a total of 1107 records were identified through a primary Embase search, and after the title and abstract analysis, a total of 203 records were included in the study. Besides, a total of 767 records were identified through Scopus search, from which a total of 201 studies were included after abstract and full-text assessment, and a total of 744 studies were identified through Google Scholar search, from which a total of 116 records were included. Excluded records were either irrelevant to the review question, case reports, animal subjects, irrelevant systematic review, or were inaccessible even after contacting the authors.
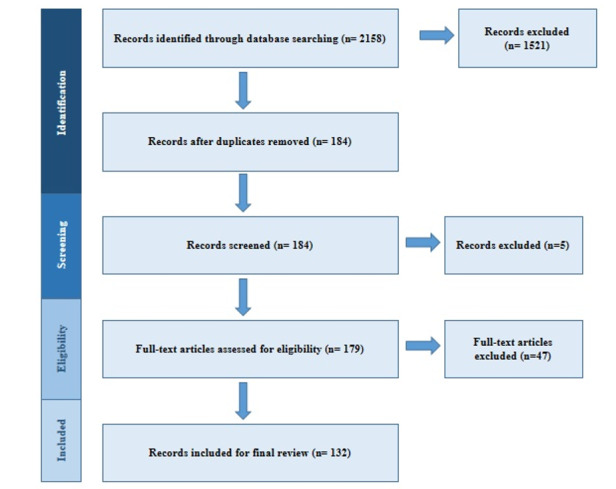
After duplicate removal, a total of 184 full-text records were analyzed, and a total of 132 records that met all inclusion criteria were included for final review. No studies were excluded based on study type (e.g. cross-sectional versus randomized). Studies that didn’t assess and report salivary CRP levels in relation to any medical or oral condition or health status were excluded (Figure 1).
Figure 1.
Search and Study Selection Process
1. Oral Disorders
Twenty-nine studies investigating salivary CRP among patients with oral disorders went under full-text analysis.
1.1. Periodontal Inflammation and Disorders
A total of 25 studies investigated sCRP levels in relation to periodontal inflammation and periodontal disorders. Fourteen of them concluded that there is a significantly positive relationship between oral inflammation and periodontitis with sCRP levels, 6 studies revealed non-significant associations between these variables, and 5 studies didn’t find any relationship between them.
As investigated by Shojaee et al.(15) in a sample of 90 subjects (30 healthy individuals, 30 gingivitis patients, and 30 chronic periodontitis patients) there were significantly higher sCRP levels among periodontitis patients compared to the controls, and there were significantly positive associations between periodontitis and sCRP levels. Kinney et al. (16) also studied 83 adults (15 healthy(H), 24 gingivitis(G), 24 mild chronic periodontitis(MP), and 20 moderate-severe periodontitis(SP)) over 12 months and showed that sCRP levels significantly decreased within 2 months after treatment among gingivitis patients. Moreover, according to a study by Zambon et al. (17) on 62 women with singleton pregnancies, it was concluded that periodontal pathology in pregnancy is related to significantly higher sCRP levels. Aurer et al. (18) in a sample of 20 veterans with Rapidly Progressive Periodontitis (RPP) with signs and symptoms of Post-Traumatic Stress Disorder (PTSD) and 20 Acute Periodontitis (AP) non-PTSD patients demonstrated that sCRP levels were significantly higher among the RPP group compared to the AP group. Shahidi et al. (19) in a study on 62 subjects (32 OLP, 15 biopsy-confirmed OSCC, 10 healthy matched controls) showed that sCRP levels are significantly higher among OSCC and OLP patients compared to the control group. Then they confirmed that sCRP can be used as a non-invasive predictive tool for dysplastic OLP. As shown by Aurer et al.(20) in a sample of 9 edentulous persons, 10 chronic periodontitis patients, 10 patients with aggressive periodontitis, and 14 periodontally healthy controls showed that there were significant differences between sCRP levels among the groups. The aggressive periodontitis patients had the highest sCRP levels, while Chronic periodontitis and edentulous patients had significantly lower sCRP levels compared to other groups. They concluded that patients with chronic periodontitis generally had lower inflammatory biomarkers including sCRP in their saliva samples. Eebersole et al. (21) in a study on 91 patients with acute Myocardial Infarction (MI), and 111 age and gender-matched non-MI controls, showed that sCRP levels significantly increased only among AMI patients with a greater number of teeth and they also concluded that the relationship between sCRP levels with physical disorders including MI and BMI is directly affected by oral health status. Besides, Altingoz et al. (22) studied a sample of 32 periodontally healthy diabetic patients, 35 diabetic patients with periodontitis, 26 systematically healthy with periodontitis, and 28 periodontally and systematically healthy subjects and revealed that sCRP levels are significantly higher among the periodontitis groups compared to periodontally healthy individuals, and concluded that the effects of oral inflammation on sCRP level are much higher and distinctive than systemic disorders such as diabetes. Moreover, Kalkan et al.(23) studied 54 subjects (18 gingivitis patients, 18 chronic periodontitis patients, and 18 periodontally healthy) and showed that sCRP levels are significantly higher among the patients with chronic periodontitis compared to the other two groups, and sCRP levels are also significantly higher among gingivitis samples compared with healthy subjects. They also concluded that sCRP level is a reliable and non-invasive method for determining the severity of periodontal disease. Pederson et al. (24) in a study on 45 adults showed that there are significant differences between sCRP levels among participants with gingivitis, periodontitis, edentulous, and healthy subjects. They concluded that as the oral health status gets worsened, sCRP levels increase accordingly, and there are significantly higher sCRP levels among patients with chronic periodontitis than in the other groups. Shiva et al. (25)in a sample of 22 OLP patients and 22 healthy controls revealed that there were statistically significant CRP levels in both serum and saliva samples of OLP patients compared to healthy controls, and Buzatu et al. (26) in a sample of 18 patients undergoing fixed orthodontic appliances revealed that wearing the appliance can significantly increase local oral inflammation and increase sCRP levels.
Six studies revealed non-significant associations between sCRP levels with oral inflammation, including a study by Haug et al.(27) on a sample of 42 patients with pulpal or peri-apical inflammation, who suffered from pain, and 39 healthy controls without dental pain or problem, indicating that sCRP levels were higher among patients compared to controls, but the difference wasn’t significant. In a study by Savitha & Shasmitha (28) on a small sample of 10 periodontally infected patients and 5 healthy controls, it was revealed that sCRP levels are higher among patients compared with controls. Moreover, Zogakis et al.(29), in a study on 21 orthodontic patients, showed that sCRP levels slightly increased 6 weeks after bonding the orthodontic appliance compared to one hour after bonding the appliance, but the difference wasn’t significant, and Stratul et al.(30) in a study on 16 patients with chronic periodontitis who were treated with non-surgical periodontal therapy and adjunctive systemic antibiotic therapy in two groups including a test group (n=8, scaling & root planning + systemic antibiotic) and a control group (n=8, scaling & root planning + placebo) showed that sCRP levels decreased among the test group more than the control group by the difference wasn’t significant. Lee et al. (31) also investigated a sample of 34 severe chronic periodontitis patients and 20 participants without periodontal destruction and showed that sCRP levels are higher among the periodontitis patients compared to controls but the difference wasn’t significant. Besides, according to Bosnjak et al. (32) a sample of 16 patients with advanced periodontal disease and otherwise healthy showed that sCRP levels increase in oral inflammation and decrease with healing, but the differences weren’t significant.
A total of 5 studies also revealed that there are no associations between sCRP levels with oral inflammation and periodontitis. Accordingly, Yang et al.(33) in a study on 34 African-American women in their third trimester of pregnancy (22 healthy gingivae and 12 gingivitis) showed that sCRP levels didn’t differ significantly between the groups. In a study by Lee et al.(34) on 121 subjects (28 periodontally healthy subjects, 24 Stage I periodontitis, 24 Stage II, 23 Stage III, and 22 Stage IV) there were no significant associations between the groups in terms of sCRP levels and the authors attributed it to the low sensitivity of the detective capacity of ELISA and suggested to use more sensitive techniques. Moreover, Gawron-Skarbek et al. (35) in a study on 60 older adults (>60 years old) showed that there were no relationships between sCRP levels and any of the oral health status variables, including a number of decayed, missing and filled Teeth, plaque index, dental treatment needs, and periodontal health status, and Redman et al.(36) in a sample of 83 old adults with rheumatic disease and osteoporosis reported that sCRP levels weren’t significantly different between the patients with and without periodontitis. Wu et al. (37) also studied a sample of 57 community adults (30 with periodontitis and 27 without periodontitis) and reported that there was no significant difference between sCRP levels among the groups observed.
1.2. Oral Premalignant and Malignant Disorders
Four studies investigated sCRP levels in oral malignant or pre-malignant disorders. All of them concluded that sCRP levels are suitable non-invasive tools for the prediction of oral malignant changes.
Metgud & Bajaj (38) performed a study on 20 normal individuals, 20 patients with oral premalignant lesions (OPML), and 20 patients with oral squamous cell carcinoma (OSCC) and concluded that sCRP levels were significantly higher among patients with OSCC than OPML and healthy individuals, and significantly higher sCRP levels among OPML group than healthy individuals. Besides, elevated sCRP levels among OSCC patients were associated with advanced tumor stage. Moreover, no association was found between sCRP levels with oral sub-mucous fibrosis (OSMF) and leukoplakia in this study. Similarly, in a study by Uppal et al. (39) on a sample of 10 leukoplakia patients, 10 Oral Lichen Planus patients, 10 OSMF patients, and 30 healthy age and gender-matched controls it was revealed that sCRP levels are significantly higher among the patients than healthy controls. moreover, sCRP and pCRP levels were significantly correlated among all subjects.As shown by Honarmand et al. (40) in a study on 55 cases (15 patients with OSCC, 20 patients with OLP, and 20 healthy controls), sCRP levels were significantly higher in OSCC patients than OLP patients and were higher in OLP subjects than in the control group, but the difference was not significant. Additionally, Tvarijonaviciute et al. (41) in a sample of 20 patients with OLP,19 with burning mouth syndrome (BMS), and 31 control subjects found that sCRP levels are significantly higher among the OLP patients than in BMS and healthy controls (42, 43).
2. Psychological factors
Nineteen studies investigated sCRP levels in relation to several psychological factors, including stress, anxiety, depression, emotions, and early life experiences. Almost all of them reported associations between sCRP and psychological factors, especially emotional domain, among both patients and healthy individuals, of all ages.
Nelson et al. (44) in their study on a sample of 37 male adolescents, showed that temperament is correlated with sCRP levels. They reported that effortful control was significantly associated with lower sCRP levels, while higher levels of negative emotionality were associated with higher levels of sCRP. Condon et al. (45) in a study on 54 maternal-child dyads in a low-income neighborhood of the USA measured the relationship between sCRP and maternal experiences of discrimination and didn’t find any significant correlations. In a study by Boss et al. (46) on a sample of 88 old adults investigating the relationship between three psychosocial factors (loneliness, stress, depression) and sCRP, they found that greater loneliness significantly predicted higher sCRP levels. Moreover, Cicchetti et al. (47) reported that in a sample of 276 maltreated and 222 non-maltreated children of low-income areas, there was a significant relationship between sCRP with child maltreatment and internalizing symptoms. They also revealed that CRP gene variations significantly affect this relationship. Keller et al. (48) studied several factors in relation to sCRP among a sample of 20 children being reared by their grandparents, aged between 5 to 18 years old. They showed a significantly positive relationship between sCRP and child sibling-related stress and a significantly negative relationship between sCRP levels and grandparent age. They also revealed that there are no significant relationships between sCRP with the children’s depression, anxiety, overall stress, grandparent-related stress, sibling-related stress, reactive aggression, physical health, grandparent age, and female gender. They also revealed that there are no significant relationships between sCRP with the grandparents’ number of Children <18, formal custody, education, poor perceived financial status, religious service attendance, private religious activity, physical health, mental health, depression, parenting stress, social support, emotional/informational, tangibility, affectionate positive social interaction, and positive parenting. Measelle and Ablow (49) in a study on 49 children, who aged 17 months old, showed that familial stress, maternal depression, and security of attachment were directly associated with higher salivary inflammation especially sCRP levels. According to Yennurajalingam et al. (50), who investigated the effects of Cranial Electrotherapy Stimulation (CES) Among 33 patients with advanced cancer for the management of depression, anxiety, sleep disturbance, and pain, sCRP didn’t change significantly after a period of 4 week CES therapy. Furthermore, Pace et al. (51) conducted a study on 71 adolescents (cases and controls) with early life adversity, to investigate the effect of Cognitively Based Compassion Training (CBCT) on their sCRP levels after 6 weeks. They found reductions in sCRP levels after this period. In a study on 49 mother-infant dyads, performed when children were 17 months old, Measelle et al. (52) showed that early life adversity and caregiving relationships affect children’s lifelong physical development and salivary inflammatory profile. They showed that children’s histories of attachment with their mothers correlate with their sCRP levels, and disorganized and avoidant regulatory behaviors were associated with significantly higher levels of salivary CRP when facing a stressor. Grasser et al. (53) conducted a study on 36 incoming Iraqi and Syrian refugees and found that greater symptom severity of anxiety, depression, and PTSD were associated with higher levels of sCRP. The relationship between the variables weren’t significant. In a different study by Simpson et al. (54), sCRP moderated the associations between several bacterial component with depression and anxiety among a sample of 66 young participants, who aged 14-18. According to a study by Slavish et al. (55), on a sample of 108 young adults, it was revealed that positive affect was associated with lower levels of sCRP, while negative affect wasn’t associated with any variations in sCRP levels. Moreover, Ross et al. (56) conducted a study on a total of 15 grandmothers who were their grandchildren’s caregivers and 15 grandmothers who weren’t caregivers. They concluded that sCRP had clearly increased from morning to bedtime, more prominently among caregiver grandmothers. They also found a negative association between sCRP and anxiety and a positive association between sCRP and subjunctive demand burden, while none of them reached to statistical significance. Moreover, there were no significant relationships found between sCRP with anxiety, stress, depression, and ways of coping in this study. As Rubin et al. (57) investigated in a sample of 65 patients with HIV, sCRP levels were higher among those with Major Depressive Disorder (MDD) than those without this disorder. This study also indicated that sCRP relates to cognition among the patients with HIV. Additionally, Lopez-Jornet et al. (58) reported that among a sample of 51 patients with burning mouth syndrome and 31 controls, they found a negative correlation between hospital-acquired anxiety and depression with sCRP.According to Landau et al. (59) sCRP was temporally stable among a sample of 86 adolescents at risk for depression. As Nelson et al. (60) revealed in their study, a secure attachment was associated with lower sCRP levels among 12 to 18 months old infants. Interestingly, pace et al. (61) studied 22 dyads of Latina breast cancer survivors and their informal caregivers and found weak correlations between sCRP with fatigue in the physical domain and positive and negative affect in the psychological domain. In contrary with their preliminary hypothesis, they found a strong association between emotional support and sCRP concentrations among the caregivers. In a study by Cullen et al. (62) on 107 children aged between 11 to 14 years, recruited from community, elevated sCRP was associated with poorer cognitive functioning in early life, but this association wasn’t moderated with their concurrent psychopathology.
2.1. Depression
A total of 3 studies investigated sCRP levels in relation to depression. Two of them reported no associations, and one reported significant associations between the variables. According to Byrne et al. (63) in a study on 17 depressed adolescents and 18 non-depressed controls, there were significant positive associations between sCRP and pCRP levels, while there were no associations between depressive symptoms and sCRP levels. As shown by Cubala & Jerzy Landowski (64) in a sample of 20 patients with major depressive disorder (MDD) and 20 non-MDD controls, there were significant positive associations between sCRP and pCRP levels, while there were no associations between depressive symptoms and sCRP levels. On the other hand, Delany et al. in a sample of 103 community adolescents and children aged between 8 to 11 years old, showed that sCRP is significantly and positively associated with negative mood/ physical symptoms.
In another study on 500 female high school students, it was revealed that sCRP levels significantly increased among depressed individuals (65).
3. Stress
A total of 14 studies investigated the associations between sCRP levels with stress and stress-related factors, and almost all of them indicated positive associations between HPA-Axis activity, sympathetic activity, cortisol response to stress, and psychosocial stress with sCRP levels.
According to Lucas et al. (66), a sample of 118 healthy African American adults showed that individual-level and contextual justice factors affect inflammatory biomarkers, including sCRP in response to psychological stress. In other words, participants with a strong belief in justice had higher sCRP and stress levels when justice was low. Besides, Campisi et al. (67) in a sample of 15 undergraduate college students, showed that although exposure to acute psychosocial stressors significantly activates sympathetic activity, sCRP showed no change over this one-day experiment of acute stress. In a small sample of 10 ICU admitted old (>60 years old) adults, Branson et al. (68) revealed that animal-assisted activity did not significantly affect the participants’ bio-behavioral stress. According to David et al. (69) in a sample of 49 mother-infant dyads, it was concluded that the experiences of early life adversity, maternal psychosocial stress, and socioeconomic disadvantage were associated with higher infant CRP levels. As shown in a study by Cicchetti et al. (47) among 267 maltreated and 222 non-maltreated children, sCRP levels were associated with HPA axis activity in response to stress, while this relationship showed temporal variations. In other words, the timing and chronicity of the stress due to maltreatment affected the levels of sCRP. Accordingly, Laurent et al. (70) in a sample of 115 healthy African-American adults, showed that HPA axis activity in response to social stress is associated with an inflammatory response. They also revealed that negative affect predicted greater alignment with sCRP levels. Cubala et al. (64) in a sample of 20 major depressive patients and 20 healthy controls, showed a significant positive correlation between the stress marker of cortisol and sCRP levels among subjects and controls, both. Additionally, Goetz and Lucas (71) in a sample of 118 community African Americans, showed that sCRP and dried blood spot CRP positively associated with each other at baseline, while only sCRP increased in response to an acute stressor task. Moreover, according to Halpern et al. (72) intimate partner violence as a stressor was significantly associated with increasing levels of sCRP among a sample of 37 community African-American women. Moreover, Berndt et al. (73) showed that in a sample of 20 ballroom dancers chronic stress was associated with more inflammatory response including sCRP levels which is highly dependent on the timing of chronic stress. In other words, the sCRP levels increase more in response to more chronic stress. According to Nelson et al. (12) in a sample of 48 mother-infant dyads, it was concluded that although there were no cross-sectional associations between maternal factors of stress and infant sCRP, maternal parenting stress and social support when infants were 12 months old predicted infant sCRP levels at 18 months old. Therefore, maternal stress could predict infant sCRP changes between 12 to 18 months of age. Hellewell and Cernak (74) also showed that sCRP significantly correlates with maladaptive stress response among a sample of 116 military personnel exposed to stress. Furthermore, Lucas et al. (75) showed that stress response was associated with variations in sCRP levels in a sample of 118 African-American women. Besides, there were significant positive associations between perceived discrimination and sCRP and significantly negative associations between racial identity and sCRP at baseline. Furthermore, as shown in a study by ESQUIRE et al. (76) in a small sample of 5 subjects, sCRP levels are significantly correlated with plasma CRP levels, and in response to acute environmental-auditory- stressor, concomitant declines in sCRP and plasma CRP levels while cortisol increased were observed. A potential role for sCRP was suggested in this study to signal catecholamine release.
4. Cardiovascular Disorders
A total of 12 studies investigated salivary CRP levels in patients with cardiovascular disorders (CVD), and generally concluded that sCRP significantly correlates with plasma CRP levels and can be used for the management of cardiovascular events.
According to Out et al. (77), sCRP correlates with plasma CRP concentrations and can be used for the screening of cardiovascular risk status. Their sample consisted of 107 women exposed to domestic violence, who were at high risk of cardiovascular diseases. In a study by Justino et al. (2) on 15 healthy subjects and 15 patients with a recent history of CVD, sCRP was highly correlated with pCRP levels, and sCRP was highly potential to be measured instead of pCRP for the management of CVD risk. Moreover, Hirasaki et al. (78) showed that sCRP was significantly higher among patients with heart diseases than healthy controls among a sample of 38 patients with heart disorders and 10 healthy controls. Naidoo et al. (79) showed that poor cardio-respiratory fitness was independently associated with higher sCRP levels and was an independent predictor of sCRP levels. As shown by Labat et al. (80) in a sample of 2059 community individuals, in an age-adjusted and sex-adjusted analysis, sCRP was significantly and positively correlated with mean arterial blood pressure, pulse pressure, pulse wave velocity, and intima-media thickness. In a study by Punyadeera et al. (81) among a sample of 55 healthy individuals and 28 cardiac patients, they showed that sCRP is significantly correlated with pCRP and sCRP is adequately capable of distinguishing between healthy individuals and CVD patients. Moreover, in a sample of 91 acute myocardial infarction (MI) patients and 111 non-MI controls, Ebersole et al. (21) showed that sCRP and pCRP are significantly and positively correlated and sCRP showed significant increases among MI patients rather than healthy controls. According to Foley et al. (82) in a study on 21 patients undergoing Alcohol Septal Ablation (ASA) and 97 healthy controls, sCRP was significantly and positively correlated with pCRP. There was a significant elevation of sCRP levels among the patients than in controls, and this study suggested that sCRP reflects the changes that occur during and after myocardial necrosis following ASA. In a study by Dekker et al. (83) on 75 hospitalized patients with heart failure, a significantly positive correlation between sCRP and pCRP levels was found. Furthermore, Jabber and Ahmed (84) showed that in a sample of 22 patients with heart diseases taking warfarin and 20 healthy controls, the mean sCRP was significantly higher among the patients than in the control group. As shown by Jamshidpour et al. (85), in a sample of 40 volunteer male patients with CAD, phase III cardiac rehabilitation could effectively improve sCRP and pCRP levels independent of anthropometric measures. In these cases, the measurement of sCRP is a reliable replacement for pCRP assessment. Interestingly, Miller et al. (86) analyzed several salivary biomarkers including troponin I, B-type natriuretic peptide (BNP), and creatine kinase-MB (CK-MB), and CRP in saliva and serum of 92 acute MI patients and 105 asymptomatic healthy controls.
4.1. Blood pressure
Two studies have investigated the relationship between sCRP levels and blood pressure among children, one of them indicating significant associations and the other finding no relationships between them. According to Rice et al. (87) in a sample of 27 Black children and their mothers, it was revealed that there was a significantly positive relationship between child sCRP and child diastolic blood pressure. Jones et al. also showed that in a sample of 151 community children and their parents (57 children, 57 mothers, 37 fathers), there were no significant associations between sCRP level and hypertension risk.
5. Obesity
A total of 10 studies investigated the relationship between overweight/obesity with sCRP levels and found a significant relationship. Almost all of them concluded that overweight/obesity and BMI are associated with higher sCRP levels.
According to a study by Shi & Goodson (88) in a sample of 726 Kuwait children, it was revealed that obesity, as measured using Wrist Circumference (WC) and Body Mass Index (BMI) was associated with higher sCRP levels. Moreover, Selvaraju et al. (89) in a study on 40 normal children and 36 overweight/obese children, showed that sCRP was significantly high among overweight/obese children than in normal children and had good discrimination power among the groups. Besides, there were significant associations between BMI, WC, and waist-to-height ratio with sCRP, and the authors concluded that increasing levels of sCRP may be an indicator of obesity/overweight and risk of metabolic dysregulation. In a study by Naidoo et al. (79) on 170 South African children, it was revealed that overweight/obesity is an independent predictor of high sCRP. Zambon et al. (17) in their study on 62 singleton pregnant women, showed that sCRP was significantly higher among obese participants especially in association with Gestational Diabetes Mellitus. As shown by Labat et al. (80), in a sample of 2059 community individuals, sCRP was significantly and positively correlated with BMI, metabolic syndrome, and waist-to-hip ratio. Moreover, Goodson et al. (90) showed that in a sample of 744 children aged 11 years old, sCRP was significantly higher among obese subjects compared to normal subjects. According to a study by Tvarijonaviciute et al. (91) in a sample of 129 children aged between 8 to 12 years old, there was a significantly positive correlation between sCRP and PCRP levels, and obesity and diet composition were both positively associated with sCRP levels. Furthermore, Ebersole et al. (21) in a study on 92 patients with acute MI and 111 age and gender-matched controls, concluded that sCRP levels are related to the participants' BMI.
6. Physical Activity
In total, 8 studies have investigated the sCRP levels in relation to physical activity. Six of them concluded that physical activity is significantly associated with sCRP variations and one study didn’t find any relationship.
According to Qian et al. (92) rest-activity rhythms were significantly associated with sCRP levels in a sample of 411 healthy children who wore a wrist accelerometer for 7 days consecutively. Moreover, as shown by Tauler et al. (93) in a sample of 107 participants participating in two ultra-endurance exercises, sCRP significantly increased after the exercise, while the time to complete the exercise was not related to the sCRP levels. In a study by Gawron-Skarbek et al. (94) on 42 subjects who were middle and older aged adult patients with acute MI, it was revealed that a single cardiac rehabilitation exercise did not change sCRP levels within the 4 weeks of the program. As shown by Izawa et al. (95) in a sample of 1042 male police officers engaged in a system of 24-hours working shifts, more effort was significantly associated with higher sCRP levels. Moreover, in a study by Harnett et al. (96) a sample of 38 athlete rugby players was assessed over 17 weeks when they were encompassing competitions. The results indicated that more muscle soreness due to physical activity was associated with significantly higher levels of sCRP levels. Tvarijonaviciute et al. (91) also in a sample of 129 healthy children aged between 8 to 12 years, investigated the relationship between sCRP and physical activity and found no significant relationships between them. Furthermore, Willoughby et al. (97) studied 19 healthy females aged between 40 to 60, categorized into two groups with either increased or decreased activity within 10 days of the study. This study showed that short-term changes in daily movement behavior could significantly affect sCRP levels in a way that increased steps per day can decrease sCRP levels and vice versa. Moreover, as shown by Roca et al. (98) in a sample of 41 male marathon runners sCRP levels significantly increased 48 hours after marathon.
7. Sleep and Circadian Rhythm
A total of 6 studies investigated the variations of sCRP levels in relation to sleep patterns and circadian rhythm.
Accordingly, Dolsen & Harvey (99) in a sample of 165 adolescents, showed interactions between short sleeping time and emotional problems in the context of higher sCRP levels and suggested that these two factors are related to each other and this relationship is more significant among the adolescents with higher sCRP levels. In a study by Nag & Pradhan (100) in a sample of 2105 community population, it was revealed that although higher sCRP levels were observed among evening types, but morningness/eveningness didn’t have any significant relationship with sCRP levels. Moreover, Andrianome et al. (101) showed that in a sample of 30 electro-hypersensitive individuals and 25 matched control groups aged between 22 and 66, sCRP levels significantly increased in the morning for both groups. Harnett et al. (96) found that there are associations between decreased sleep quality and sleep quantity with increased sCRP levels among a sample of 19 rugby player athletes. As shown by Nag & Pradhan (102) in a sample of 2105 community individuals, significantly higher sCRP levels were observed among sleep-deprived individuals and those with excessive sleepiness during day time. According to Izawa et al. (103) in a study on 27 students who provided saliva samples 8 times a day for two consecutive days, it was revealed that sCRP levels peak at awakening and are lower during the daytime. Besides, sCRP levels showed moderate to high stability over 2 days of sample collection.
8. Diet
Regarding dietary intake, 3 studies showed that there are no short-term changes in sCRP levels after using dietary supplements or oral methadone, while 2 studies showed significant associations between long-term dietary change with sCRP levels.
A study by Gawron-Skarbek et al. (104) on a sample of 80 old adults revealed that dietary vitamins C, E and Beta Carotene intake didn’t have any relationship with sCRP levels. Moreover, as shown by Roca et al. (98) in a sample of 41 male marathon runners sCRP levels didn’t change after usage of a standardized polysaccharide-based multi-ingredient supplement.
According to Akbari et al. (105), in a sample of 40 heroin abusers who underwent Maintenance Methadone Therapy, sCRP levels didn’t change significantly one month after withdrawal compared to the time before the therapy.
On the contrary, Tvarijonaviciute et al. (91) studied a sample of 129 children aged between 8 to 12 years and showed that dietary composition is positively associated with sCRP levels. According to their results, energy intake (kcal), fat intake, protein intake, Monounsaturated fatty acids intake, and Saturated fatty acid intake are positively and significantly related to sCRP level, while there are no significant relationships between Cholesterol intake, Carbohydrate intake, Polyunsaturated fatty acid intake, and Fibre intake with sCRP.
In a study by Brett et al. (106) on a total of 262 school children, it was revealed that regular probiotic intake is significantly associated with lower sCRP levels.
Another study assessed and compared sCRP levels among a total of 15 vegans and 15 omnivore adults and indicated that there is no significant difference between these groups in terms of sCRP levels (107).
9. Sepsis
A total of 4 studies investigated sCRP levels among sepsis patients and compared them with non-septic controls, and all of them concluded that sCRP levels are directly related to pCRP levels and sCRP is a great representative of sepsis.
According to Datla et al. (108) in a sample of 135 neonates ≤28 days of life who were suspected to sepsis or with perinatal risk factors for sepsis, it was revealed that sCRP significantly and positively correlates with pCRP levels. Besides, CRP was detectable in the saliva of neonates with sepsis and showed significantly elevation compared to those with only suspicion of sepsis but with non-elevated biomarkers. Moreover, Lin et al. (109) showed that among a sample of 18 septic neonates and 22 healthy controls, a significantly positive correlation was found between sCRP and pCRP levels and sCRP is a reliable indicator of neonatal sepsis. As shown by Galhardo et al. (9) in a sample of 26 hospitalized patients in an intensive care unit with the diagnosis of sepsis and 26 controls without sepsis, it was revealed that sCRP levels are higher among sepsis patients but not in a significant way. Additionally, according to Omran et al. (110) in a study on 35 septic neonates and 35 controls, sCRP levels significantly increase among septic neonates. They concluded that sCRP has a good positive accuracy for predicting elevated pCRP levels among septic neonates and can be used as a diagnostic marker for neonatal sepsis.
10. Healthy individuals
Variations in sCRP level have also been considered and investigated among healthy individuals in 4 studies. They showed that sCRP levels didn’t change significantly over time among healthy individuals, and there were no correlations between sCRP levels and pCRP levels in all of the studies.
According to Dillon et al. (111), in a sample of 55 healthy adults, there was no significant correlation between sCRP and pCRP levels. While sCRP levels were measurable, similarly, as shown by Wetterö et al. (112) in a sample of 107 middle-aged individuals from the general population, sCRP levels didn’t straightforwardly reflect pCRP levels. Moreover, Nam et al. (113) showed that sCRP levels didn’t significantly correlate with pCRP levels in a sample of 37 healthy young male participants over 3 days, and Idris et al. (114) showed that in a sample of 20 healthy young males, sCRP levels remained stable throughout 4 times sampling over a day.
11. Physical health
Three studies have reported that sCRP levels are related to physical health status.
According to Dolsen et al. (99) in a sample of 165 adolescents, a lower baseline sCRP level was related to reduced physical health risk. In a study by pace et al. (61) on 22 Latina survivor-caregiver, it was revealed that there is a weak positive correlation between sCRP and physical fatigue. Additionally, Siavoshi et al. (115) in a sample of 20 subjects, found a significantly negative relationship between sCRP concentrations and fitness exercises.
12. Diabetes Mellitus
Three studies have investigated sCRP levels in diabetes patients and compared them with healthy controls. Accordingly, 2 studies found significant relationships between sCRP levels and diabetes mellitus, while one didn’t find any associations between them.
In a study by Agho et al. (116) on 39 community individuals with type 2 diabetes mellitus and 36 healthy controls, sCRP level was significantly higher among diabetic subjects, and Dezayee & Al-Nimer (117) in a sample of 50 Type 2 diabetes patients, 25 Type 1 diabetes patients, and 25 healthy subjects showed that sCRP was related to the glycemic index. On the contrary, Valle et al. (118) in a sample of 25 children with type1 diabetes and 25 non-diabetic controls, showed that sCRP levels didn’t vary significantly among the groups (119).
13. Tuberculosis
As shown by Loxton et al. (120), in a sample of 38 individuals with pulmonary tuberculosis (TB) symptoms that 11 of them were TB positive after sputum culture. It was revealed that the sCRP level was significantly higher among TB patients and this is a potential diagnostic biomarker.
According to Jacobs et al. (121) in a sample of 104 patients with pulmonary TB symptoms that 32 cases were pulmonary TB positive, and there were significantly higher sCRP levels among TB-positive patients. they also provided a biosensor including sCRP and 6 other biomarkers that could positively and validly predict pulmonary TB.
14. Systemic inflammation
Two studies reported a significant positive relationship between sCRP levels and systemic inflammation.
According to Out et al. (77) in a sample of 107 women exposed to domestic violence, systemic inflammation as indicated by higher pCRP levels was moderately associated with higher sCRP levels.
Naidoo et al. (79) showed that in a sample of 170 Black South-African children, there was a significantly positive association between inflammatory status and sCRP levels.
15. Pneumonia
Two publications investigated and revealed that there is a significantly positive association between pneumonia and sCRP levels.
According to Omran et al. (110), among a sample of 70 full-term neonates including 35 with late-onset neonatal pneumonia and 35 healthy controls, sCRP could accurately predict elevated pCRP levels in neonates with pneumonia and was suggested as a suitable diagnostic marker for late-onset neonatal pneumonia. Additionally, Tsai et al. (122) in a sample of 106 children with pneumonia and 60 healthy controls aged between 2-17 years, showed that sCRP levels significantly and positively correlated with pCRP levels among these groups and sCRP level could be a valid alternative for pCRP especially among pediatric patients with pneumonia. Interestingly, in this study, as pneumonia improved both sCRP and pCRP levels decreased as well.
16. Metabolic syndrome
Two studies investigated and found a significant positive relationship between metabolic syndrome measures and sCRP levels.
According to Labat et al. (80) in a sample of 2059 community individuals, sCRP and pCRP levels were significantly and positively correlated, and there was a significantly positive correlation between sCRP level and mean arterial blood pressure, pulse pressure, pulse wave velocity, BMI, metabolic syndrome, waist-to-hip ratio and intima-media thickness. Dezayee & Al-Nimer (117), in a sample of 50 Type 2 diabetes patients, 25 Type 1 diabetes patients, and 25 healthy subjects, showed that sCRP was related to anthropometric measurements, blood pressure, and glycemic index and it can be used as an indicator of metabolic syndrome.
17. COPD
Two studies investigated sCRP levels among COPD patients and both of them found a significant relationship between patient symptoms and sCRP levels.
Patel et al. (123), in a sample of 143 subjects (including 98 COPD patients, 20 never-smokers, and 25 smokers with normal spirometry) showed that there was a significant positive correlation between sCRP and pCRP levels. They also concluded that in COPD patients, sCRP levels positively correlated with breathing scores. Moreover, according to Bhavsar et al. (124) in a sample of 100 COPD patients and 100 age and sex-matched non-COPD controls, there was a significant positive correlation between sCRP and pCRP levels, and sCRP level was significantly higher among COPD patients than controls.
18. Age
Two studies have investigated and reported sCRP levels in relation to age.
Andrianome et al. (101), in a sample of 55 adults aged between 22 and 66, showed that sCRP level was negatively correlated with age. Moreover, Jones et al. (125) in a sample of 151 community children and adults, didn’t find any associations between sCRP level with age.
19. Smoking
According to Azar and Richard (126), in a sample of 45 healthy youth (including 10 active smokers, 22 passive smokers, and 13 non-smokers), there was a dose-response relationship between tobacco smoke exposure and sCRP levels, while sCRP and cotinine level weren’t correlated. Interestingly, they concluded that smokers and passive smokers both had significantly higher sCRP levels than non-smokers, while sCRP levels didn’t differ significantly among smokers and passive smokers.
20. Sickle Cell Anemia
According to Pradhan et al. (127), among 30 subjects (10 sickle cell disease patients, 10 trait sickle cell individuals, and 10 healthy controls), the sCRP level is significantly higher among sickle cell patients compared to healthy individuals. Moreover, sCRP levels significantly fluctuated among traits of sickle cell individuals in the absence of chronic inflammation.
21. Sexual Activity
According to a study by Lorenz et al. (128) on 32 healthy premenstrual women, it was revealed that higher intercourse frequency predicted greater mid-cycle decreases in CRP among sexually active women.
22. Neonatal Disorders
According to Iyengar et al. (129), among a total of 40 neonates admitted to NICU for different reasons, it was revealed that sCRP levels significantly correlated with pCRP levels and sCRP is a feasible tool for screening pCRP level among this population.
23. Renal Disorder
According to a study by Pallos et al. (130) on 119 subjects (38 Chronic Renal Failure (CRF) patients undergoing hemodialysis, 34 CRF non-hemodialysis patients, and 47 non-CRF non-hemodialysis controls), sCRP levels among the CRF with hemodialysis group was significantly higher than CRF non-hemodialysis and non-CRF non-hemodialysis group. Besides, there were no significant differences between the sCRP levels of the CRF non-hemodialysis group and the non-CRF group. Therefore, they concluded that hemodialysis of CRF patients significantly affects sCRP levels and salivary inflammatory components.
24. Rheumatic Disease
According to Sikorska et al. (10), in a sample of 19 patients with rheumatic disease, who underwent anti TNF-α therapy sCRP levels were measured before and 12 weeks after therapy. This study showed that sCRP levels significantly correlated with pCRP levels and decreased significantly after successful treatment. Accordingly, sCRP is a potential indicator of rheumatic disease activity.
25. Osteoporosis
Twardowski et al. (131), in a study on 567 community women, investigated the association between sCRP levels and plasma 25(OH)D and found no significant associations.
26. Using Electronic Devices
Hasehmipour et al. (132) in a sample of 80 healthy community individuals using Samsung mobile phones showed that long-term use of the mobile phone was associated with higher sCRP levels but the association wasn’t significant.
27. HIV/AIDS
Rogers et al. (133) in a study on 103 HIV/AIDS participants, showed that generally, sCRP levels were higher among these patients than general healthy population, but this relationship wasn’t significant.
28. Headache
Bougea et al. (134) in a sample of 30 migraineurs, 30 tension-type headache patients, and 30 age-matched healthy controls, showed that sCRP levels didn’t vary significantly among tension-type headache patients and migraine headache patients. Moreover, higher sCRP levels were correlated with lower symptom scores of anxiety and depression prior to or immediately after the participants’ headache period.
29. Environmental Factors
In a study by Zhu et al. (135) on 40 healthy nonsmoking adults, it was revealed that exposure to fine particle matter (PM2.5, particulate matter with aerodynamic diameter _ 2.5 mm) was associated with higher sCRP levels.
30. COVID 19
According to Azzi et al. (136) in a study on 25 Covid19 patients, it was revealed that there is an inverse tendency between viral load and sCRP levels.
Asthma and Allergy
According to Krasteva et al. (5), in a sample of 32 children with allergic asthma and 20 control children, it was concluded that there were significantly higher sCRP levels among children with asthma/allergy compared to healthy controls. Besides, there were elevated sCRP levels among allergic children treated with corticosteroids and sCRP levels were significantly higher among children treated with antihistamines compared to controls.
31. Acute Respiratory Illness
Gofin et al. (137) showed that in a sample of 104 children aged between 2 and 18 diagnosed with acute respiratory illness, sCRP level was highly specific for predicting high pCRP level. Therefore, sCRP is a potential alternative for pCRP in acute pediatric settings.
32. Dermatologic disorders (Acne Vulgaris)
Monib et al. (138) in a study on 84 acne vulgaris patients and 105 healthy controls, showed that acne vulgaris patients had significantly higher sCRP levels than non-acne Vulgaris controls. moreover, sCRP and pCRP levels were significantly correlated in this study (139).
33. Polycystic Ovarian Syndrome
In a sample of women with PCOS and 26 healthy women, it was revealed that PCOS women had a significant increase in Salivary CRP levels compared with the control group (140).
Discussion
Sialochemistry as an alternative for blood biochemical analysis offers many advantages like Non-invasive nature, the feasibility of collection, good patient cooperation, cost-effectiveness, and low risk of infection. (141) CRP is a very useful and important biomarker in the diagnosis and management of several medical conditions, which is secreted in saliva in considerable and measurable amounts.
According to the results, sCRP levels have been measured among patients with several different disorders. The results and the existing literature indicate that many of the systemic and oral inflammatory processes are remarkably represented in saliva, like sCRP concentrations.
To date, most of the studies have investigated sCRP levels among patients with oral inflammation, psychological disorders, and cardiovascular disorders, and a limited number of studies have provided evidence that sCRP levels are reliably correlated with the status of many other medical conditions such as oral malignancies, infections, diabetes, renal failure, pulmonary disorders including asthma and COPD, blood pressure disorders, metabolic syndrome, rheumatic disease, headache, Covid19, and dermatologic disorders.
Moreover, results revealed that sCRP levels are remarkably affected by several factors, including demographic features, environmental factors, health status, physical activity, dietary habits, smoking, sleep patterns, and mobile phone usage. Therefore, future studies are recommended to control these associating factors as much as possible to reach more accurate results. Although saliva is proven to be a useful specimen in forensic medicine for drug testing, sCRP levels in this context were not investigated thoroughly (142).
This review also provided evidence that sCRP levels significantly correlate with pCRP levels in most cases with a good temporal relation, and further investigation of the conditions that didn’t have correlated levels of sCRP and pCRP can answer many questions and fill the gap of controversies in this regard. Hence, sCRP is a potential alternative for invasive pCRP sampling. Therefore, this is suggested that future researchers investigate this relationship in larger samples and meticulously controlled design. The temporal relationship between variations in pCRP levels and the subsequent changes in sCRP levels is also another interesting gap that needs further investigation. Moreover, a limited number of studies mentioned that sCRP levels and salivary components vary among different sampling sites of the mucosa. Therefore, this is another interesting finding which may affect the results and requires careful consideration in future studies.
Results of the search for the existing literature about the level and role of sCRP in systemic and oral disorders are briefly presented in Table 1. As shown in this table, most of the studies have found a correlation between sCRP and pCRP levels and have suggested sCRP as an alternative for pCRP levels.
Conclusion
Measurement of salivary CRP levels can be a promising novel method for assessment, diagnosis, monitoring, and therapeutic purposes of various medical and oral conditions. Although inconsistent, various studies suggest that salivary CRP levels can well demonstrate the plasma CRP concentrations. Therefore, as a less-invasive, inexpensive, easier, and faster method of sampling, salivary CRP can be used instead of plasma CRP samples for most cases in the future.
Limitations and Suggestions
Since most of the previous studies in this area were not controlled or didn’t have a satisfactory sample size for obtaining a general conclusion, further research in this area is suggested to be controlled and have a satisfactory sample size and cases. For most of the disorders, there were a limited number of studies performed in this regard, which makes it difficult to obtain a reliable conclusion.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgments
We appreciate all people who helped us in providing this manuscript.
Ethical approval
All content of this research adheres to the ethical guidelines developed by the Committee on Publication Ethics (COPE) during the 2nd World Conference on Research Integrity in Singapore in 2010. All parts of this study meet the American Psychological Association's (APA) Ethical Principles of Psychologists and Code of Conduct (the Ethics Code) and adhere to the legal requirements of the study country, Iran.
Cite this article as: Babaei M, Rezaei S, Saghafi Khadem Sh, Shirinbak I, Basir Shabestari S. The Role of Salivary C-Reactive Protein in Systemic and Oral Disorders: A Systematic Review. Med J Islam Repub Iran. 2022 (19 Nov);36:138. https://doi.org/10.47176/mjiri.36.138
References
- 1.Gohel V, Jones JA, Wehler CJ. Salivary biomarkers and cardiovascular disease: a systematic review. Clin Chem Lab Med. 2018 Aug 28:1432. doi: 10.1515/cclm-2017-1018. [DOI] [PubMed]
- 2.Justino CI, Duarte K, Lucas S, Chaves P, Bettencourt P, Freitas AC. et al. Assessment of cardiovascular disease risk using immunosensors for determination of C-reactive protein levels in serum and saliva: a pilot study. Bioanalysis. 2014 Jun:1459. doi: 10.4155/bio.14.12. [DOI] [PubMed]
- 3.Ouellet-Morin I, Danese A, Williams B, Arseneault L. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain Behav Immun. 2011 May:640. doi: 10.1016/j.bbi.2010.12.020. [DOI] [PubMed]
- 4.Yao Z, Zhang Y, Wu H. Regulation of C-reactive protein conformation in inflammation. Inflamm Res. 2019 Oct:815. doi: 10.1007/s00011-019-01269-1. [DOI] [PubMed]
- 5.Krasteva PP, Ivanova I, Altankova T, Bocheva, Kisselova. Alteration in Salivary Components of Children with Allergic Asthma. Biotechnol Biotechnol Equip. 2010:1866.
- 6.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004 Nov 19:48487. doi: 10.1074/jbc.R400025200. [DOI] [PubMed]
- 7.Saghafi Khadem, Zeidabadi F, Moghiman T, Basir Shabestari. High-Sensitivity C-Reactive Protein and Irisin: Comparison of Serum and Saliva Levels in MI patients. Clin Surg. 2020
- 8.Galhardo LF, Ruivo GF, de Oliveira, Parize G, Santos SSFD, Pallos D. et al. Inflammatory markers in saliva for diagnosis of sepsis of hospitalizes patients. Eur J Clin Invest. 2020 May:e13219. doi: 10.1111/eci.13219. [DOI] [PubMed]
- 9.Sikorska D, Orzechowska Z, Rutkowski R, Prymas A, Mrall-Wechta M, Bednarek-Hatlińska D. et al. Diagnostic value of salivary CRP and IL-6 in patients undergoing anti-TNF-alpha therapy for rheumatic disease. Inflammopharmacology. 2018 Oct:1183. doi: 10.1007/s10787-018-0515-8. [DOI] [PMC free article] [PubMed]
- 10.Almasi S, Sabbagh MK, Barzi D, Atyabi H, Shabestari SB. Relationship between clinical and laboratory findings of rheumatoid arthritis patients with their oral status and disease activity. Caspian J Intern Med. 2021:22. doi: 10.22088/cjim.12.1.22. [DOI] [PMC free article] [PubMed]
- 11.Gaetano Isola, Alessandro P, Angela A, Paolo M, Francesco I. Identification of the different salivary Interleukin-6 profiles in patients with periodontitis: A cross-sectional study. Arch Oral Biol. 2021 doi: 10.1016/j.archoralbio.2020.104997. [DOI] [PubMed]
- 12.Benjamin W, Nelsona D, Nicholas B, Allena N, Heidemarie K, Laurenta K. Maternal stress and social support prospectively predict infant inflammation. Brain, Behavior, and Immunity. 2020:14-21 doi: 10.1016/j.bbi.2019.05.010. [DOI] [PubMed]
- 13.Thomsen A. The routine use of C-reactive protein in forensic investigations. Forensic Sci Int. 2007 doi: 10.1016/j.forsciint.2006.10.021. [DOI] [PubMed]
- 14.Page, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed]
- 15.Shojaee M, Fereydooni GM, Maliji G, Bijani A, Aghajanpour Mir, Mousavi Kani. C - reactive protein levels in patients with periodontal disease and normal subjects. Int J Mol Cell Med. 2013 Summer:151. [PMC free article] [PubMed]
- 16.Janet S, Kinney TM, Min OH, Thomas M, Braun C, Ramseier A. et al. Giannobile. Crevicular Fluid Biomarkers and Periodontal Disease Progression. J Clin Periodontol doi: 10.1111/jcpe.12194. [DOI] [PMC free article] [PubMed]
- 17.Zambon CM, Lissoni A, Anelli GM, Novielli C, Cardellicchio M, Leone M. et al. Inflammatory and Oxidative Responses in Pregnancies With Obesity and Periodontal Disease. Reprod Sci. 2018:1. doi: 10.1177/1933719117749758. [DOI] [PubMed]
- 18.Aurer A, Aurer-Kozelj J, Stavljenić-Rukavina A, Kalenić S, Ivić-Kardum M, Haban V. Inflammatory mediators in saliva of patients with rapidly progressive periodontitis during war stress induced incidence increase. Coll Antropol. 1999 Jun:117. [PubMed]
- 19.Shahidi M, Jafari S, Barati M, Mahdipour M, Gholami MS. Predictive value of salivary microRNA-320a, vascular endothelial growth factor receptor 2, CRP and IL-6 in Oral lichen planus progression. Inflammopharmacology. 2017 May 13 doi: 10.1007/s10787-017-0352-1. [DOI] [PubMed]
- 20.Aurer A, Jorgić-Srdjak K, Plancak D, Stavljenić-Rukavina A, Aurer-Kozelj J. Proinflammatory factors in saliva as possible markers for periodontal disease. Coll Antropol. 2005 Dec:435. [PubMed]
- 21.Ebersole JL, Kryscio RJ, Campbell C, Kinane DF, McDevitt J, Christodoulides N. et al. Salivary and serum adiponectin and C-reactive protein levels in acute myocardial infarction related to body mass index and oral health. J Periodontal Res. 2017 Jun:419. doi: 10.1111/jre.12406. [DOI] [PMC free article] [PubMed]
- 22.Altıngöz M, Önder C, Serdar M, Ünlütürk U, Uyanık M, Başkal N. et al. Salivary and Serum Oxidative Stress Biomarkers and Advanced Glycation End Products in Periodontitis Patients with or without Diabetes: A Cross-Sectional Study. J Periodontol. 2020 doi: 10.1002/JPER.20-0406. [DOI] [PubMed]
- 23.Kalkan RE, Gökmenoğlu C, Kara C. Salivary fetuin-A, S100A12 and High-Sensitivity C-reactive protein levels in periodontal diseases. Oral Dis. 2018 doi: 10.1111/odi.12927. [DOI] [PubMed]
- 24.Pederson ED, Stanke SR, Whitener SJ, Sebastiani PT, Lamberts BL, Turner DW. Salivary levels of alpha 2-macroglobulin, alpha 1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Arch Oral Biol. 1995 Dec:1151. doi: 10.1016/0003-9969(95)00089-5. [DOI] [PubMed]
- 25.Shiva A, Mousavi J, Zamanian A, Maboudi A. Serum and Salivary Level of Nitric Oxide (NOx) and CRP in Oral Lichen Planus (OLP) Patients. J Dent Shiraz Univ Med Sci. 2020:6. doi: 10.30476/DENTJODS.2019.77842. [DOI] [PMC free article] [PubMed]
- 26.Buzatu R, Valceanu AS, Scrobota I, Onisei D, Szuhanek C. The Dynamics of Salivary Parameters in Patients Undergoing Orthodontic Treatment. Revchim. 2017
- 27.Haug M. Acute Dental Pain and Salivary Biomarkers for Stress and Inflammation in Patients with Pulpal or Periapical Inflammation. J Oral Facial Pain Head. 2018:1-8 doi: 10.11607/ofph.2007. [DOI] [PubMed]
- 28.Shasmitha S. Comparision of Salivary CRP Level in Chronic Periodontitis and Healthy Individual. J Pharm Sci Res. 2015:729.
- 29.Shalish I. Effect of fixed orthodontic appliances on nonmicrobial salivary parametersEffect of fixed orthodontic appliances on nonmicrobial salivary parameters. Angle Orthodontist. 2018:806. doi: 10.2319/111317-773.1. [DOI] [PMC free article] [PubMed]
- 30.Boia S, Boariu M, Ursoniu S, Goţia Sl, Boia Er, Borza C. Evaluation of antioxidant capacity and clinical assessment of patients with chronic periodontitis treated with nonsurgical periodontal therapy and adjunctive systemic antibiotherapy. Rom J Morphol Embryol. 2018:1107. [PubMed]
- 31.Lee C, Tu W, Chang A. The potential of salivary biomarkers for predicting the sensitivity and monitoring the response to nonsurgical periodontal therapy: A preliminary assessment. J Periodont Res. 2018:1-10 doi: 10.1111/jre.12544. [DOI] [PubMed]
- 32.Bosnjak B, Vucicevic B, Vucicevic B, Brozović S. Whole Saliva Levels of Some Inflammatory Mediators in Patients with Previous Evidence of Periodontitis: a Pilot Study. Acta Stomatol Croat. 2009
- 33.Yang I, Knight AK, Dunlop AL, Corwin EJ. Characterizing the Subgingival Microbiome of Pregnant African American Women. J Obstet Gynecol Neonatal Nurs. 2019 Mar:140. doi: 10.1016/j.jogn.2018.12.003. [DOI] [PMC free article] [PubMed]
- 34.Lee J, Song HY, Son MJ, Li L, Rhyu IC, Lee YM. et al. Diagnostic Models for Screening of Periodontitis with Inflammatory Mediators and Microbial Profiles in Saliva. Diagnostics. 2020 doi: 10.3390/diagnostics10100820. [DOI] [PMC free article] [PubMed]
- 35.Gawron-Skarbeka A, Dynowska B, Guligowska A, Prymont-Przymińska A, Nowak D, Kostka T. Salivary and plasma native and non-urate total antioxidant capacity versus oral health status in older non-smoking adults. Arch Oral Biol. 2019 doi: 10.1016/j.archoralbio.2019.104515. [DOI] [PubMed]
- 36.Redman GK, Payne JB, Mikuls TR, Huang J, Sayles HR, Becker KL. et al. Salivary and serum procalcitonin and C-reactive protein as biomarkers of periodontitis in United States veterans with osteoarthritis or rheumatoid arthritis. Biotechnic Histochem. 2016:77. doi: 10.3109/10520295.2015.1082625. [DOI] [PMC free article] [PubMed]
- 37.Wu YC, Ning L, Tu YK, Huang CP, Huang NT, Chen YF. et al. Salivary biomarker combination prediction model for the diagnosis of periodontitis in a Taiwanese population. J Formos Med Assoc. 2018 Sep:841. doi: 10.1016/j.jfma.2017.10.004. [DOI] [PubMed]
- 38.Metgud R, Bajaj S. Altered serum and salivary C-reactive protein levels in patients with oral premalignant lesions and oral squamous cell carcinoma. Biotech Histochem. 2016:96. doi: 10.3109/10520295.2015.1077393. [DOI] [PubMed]
- 39.Kaur Uppal, Subash BV, Patil S, Sharma M, Thakar S. Estimation and Correlation of Serum and Salivary C‑Reactive Protein in Oral Potentially Malignant Disorders. J Indian Acad Oral Med Radiol. 2021:47-53
- 40.Honarmand M, Farhad-Mollashahi L, Smailpoo RA. Salivary Lactate Dehydrogenase, C-Reactive Protein, and Cancer Antigen 125 Levels in Patients with Oral Lichen Planus and Oral Squamous Cell Carcinoma. Int J Cancer Manag. 2021
- 41.Tvarijonaviciute A, Aznar-Cayuela C, Rubio CP, Ceron JJ, López-Jornet P. Evaluation of salivary oxidate stress biomarkers, nitric oxide and C-reactive protein in patients with oral lichen planus and burning mouth syndrome. J Oral Pathol Med. 2017 May:387. doi: 10.1111/jop.12522. [DOI] [PubMed]
- 42.Shabestari, Shirinbak I, Azadarmaki R. A comprehensive look at oromaxillofacial and laryngopharyngeal cancers: Cancer Genetics and Psychotherapy. Springer. 2017:531-587
- 43.Basir-Shabestari S, Shirinbak I, Saghafi-Khadem S, Eshghyar N. Schwannoma in the posterior hard palate and anterior mandibular gingiva: A report of two cases. J Kerman Univ Med Sci. 2018:375.
- 44.Nelson BW, Byrne ML, Simmons JG, Whittle S, Schwartz OS, O'Brien-Simpson NM. et al. Adolescent temperament dimensions as stable prospective risk and protective factors for salivary C-reactive protein. Br J Health Psychol. 2018 Feb:186. doi: 10.1111/bjhp.12281. [DOI] [PubMed]
- 45.Condon EM, Holland ML, Slade A, Redeker NS, Mayes LC, Sadler LS. Associations Between Maternal Experiences of Discrimination and Biomarkers of Toxic Stress in School-Aged Children. Matern Child Health J. 2019 Sep:1147. doi: 10.1007/s10995-019-02779-4. [DOI] [PMC free article] [PubMed]
- 46.Lisa Boss, Stanley C, Duck-Hee K. Biobehavioral Examination of Religious Coping, Psychosocial Factors, and Executive Function in Homebound Older Adults. Religions. 2016:42.
- 47.Cicchetti D, Handley ED, Rogosch FA. Child Maltreatment, Inflammation, and Internalizing Symptoms: Investigating the Roles of C-Reactive Protein, Gene Variation and Neuroendocrine Regulation. Dev Psychopathol. 2015:553. doi: 10.1017/S0954579415000152. [DOI] [PMC free article] [PubMed]
- 48.Keller PS, Bi S, Schoenberg N. Children being Reared by their Grandparents in Rural Appalachia: A Pilot Study of Relations Between Psychosocial Stress and Changes in Salivary Markers of Inflammation Over Time. J Child Adolesc Trauma. 2018 Jun 8:269. doi: 10.1007/s40653-018-0214-z. [DOI] [PMC free article] [PubMed]
- 49.Measelle JR, Ablow JC. Contributions of early adversity to pro-inflammatory phenotype in infancy: the buffer provided by attachment security. Attach Hum Dev. 2018 Feb:1. doi: 10.1080/14616734.2017.1362657. [DOI] [PubMed]
- 50.Yennurajalingam S, Kang DH, Hwu WJ, Padhye NS, Masino C, Dibaj SS. et al. Cranial Electrotherapy Stimulation for the Management of Depression, Anxiety, Sleep Disturbance, and Pain in Patients With Advanced Cancer: A Preliminary Study. J Pain Symptom Manage. 2018:198. doi: 10.1016/j.jpainsymman.2017.08.027. [DOI] [PubMed]
- 51.Pace TW, Negi LT, Dodson-Lavelle B, Ozawa-de Silva, Reddy SD, Cole SP. et al. Engagement with Cognitively-Based Compassion Training is associated with reduced salivary C-reactive protein from before to after training in foster care program adolescents. Psychoneuroendocrinology. 2013 Feb:294. doi: 10.1016/j.psyneuen.2012.05.019. [DOI] [PubMed]
- 52.Measelle JR, David J, Ablow JC. Increased Levels of Inflammation Among Infants with Disorganized Histories of Attachment. Behav Brain Res. 2017:260. doi: 10.1016/j.bbr.2016.12.001. [DOI] [PubMed]
- 53.Grasser LR, Burghardt P, Daugherty AM, Amirsadri A, Javanbakht A. Inflammation and Trauma-Related Psychopathology in Syrian and Iraqi Refugees. Behav Sci (Basel) 2020 Apr 7:75. doi: 10.3390/bs10040075. [DOI] [PMC free article] [PubMed]
- 54.Simpson CA, Adler C, du Plessis, Landau ER, Dashper SG, Reynolds EC. et al. Oral microbiome composition, but not diversity, is associated with adolescent anxiety and depression symptoms. Physiol Behav. 2020 Nov 1 doi: 10.1016/j.physbeh.2020.113126. [DOI] [PubMed]
- 55.Slavish DC, Jones DR, Smyth JM, Engeland CG, Song S, McCormick NM. et al. Positive and Negative Affect and Salivary Markers of Inflammation Among Young Adults. Int J Behav Med. 2020 Jun:282. doi: 10.1007/s12529-019-09795-2. [DOI] [PMC free article] [PubMed]
- 56.Trail Ross, Kang DH, Cron S. Psychological Profile, Salivary Cortisol, C-Reactive Protein, and Perceived Health of Grandmothers With Childrearing Responsibility. J Fam Issues. 2015:1904.
- 57.Rubin LH, Langenecker SA, Phan KL, Keating SM, Neigh GN, Weber KM. et al. Remitted depression and cognition in HIV: The role of cortisol and inflammation. Psychoneuroendocrinology. 2020 Apr doi: 10.1016/j.psyneuen.2020.104609. [DOI] [PMC free article] [PubMed]
- 58.Lopez-Jornet P, Felipe CC, Pardo-Marin L, Ceron JJ, Pons-Fuster E, Tvarijonaviciute A. Salivary Biomarkers and Their Correlation with Pain and Stress in Patients with Burning Mouth Syndrome. J Clin Med. 2020 Mar 28:929. doi: 10.3390/jcm9040929. [DOI] [PMC free article] [PubMed]
- 59.Landau ER, Trinder J, Simmons JG, Raniti M, Blake M, Waloszek JM. et al. Salivary C-reactive protein among at-risk adolescents: A methods investigation of out of range immunoassay data. Psychoneuroendocrinology. 2019 Jan doi: 10.1016/j.psyneuen.2018.08.035. [DOI] [PMC free article] [PubMed]
- 60.Nelson BW, Bernstein R, Allen NB, Laurent HK. The quality of early infant-caregiver relational attachment and longitudinal changes in infant inflammation across 6 months. Dev Psychobiol. 2020 Jul:674. doi: 10.1002/dev.21940. [DOI] [PubMed]
- 61.Pace TWW, Badger TA, Segrin C, Sikorskii A, Crane TE. The Relationship Between Health-Related Quality of Life and Saliva C-Reactive Protein and Diurnal Cortisol Rhythm in Latina Breast Cancer Survivors and Their Informal Caregivers: A Pilot Study. J Transcult Nurs. 2021 Jul:326. doi: 10.1177/1043659620926537. [DOI] [PubMed]
- 62.Cullen AE, Tappin BM, Zunszain PA, Dickson H, Roberts RE, Nikkheslat N. et al. The relationship between salivary C-reactive protein and cognitive function in children aged 11-14years: Does psychopathology have a moderating effect. Brain Behav Immun. 2017 Nov doi: 10.1016/j.bbi.2017.07.002. [DOI] [PMC free article] [PubMed]
- 63.Byrne ML, O'Brien-Simpson NM, Reynolds EC, Walsh KA, Laughton K, Waloszek JM. et al. Acute phase protein and cytokine levels in serum and saliva: a comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain Behav Immun. 2013 Nov doi: 10.1016/j.bbi.2013.08.010. [DOI] [PubMed]
- 64.Cubała WJ, Landowski J. C-reactive protein and cortisol in drug-naïve patients with short-illness-duration first episode major depressive disorder: possible role of cortisol immunomodulatory action at early stage of the disease. J Affect Disord. 2014 Jan doi: 10.1016/j.jad.2013.10.004. [DOI] [PubMed]
- 65.Al-Bazaz NA, MH Radhi. Depression status in relation to dental caries and salivary C-Reactive Protein among 17 years old secondary school female in Baghdad City/Iraq. J Bagh Coll Dent [Internet] 2021 Mar
- 66.Lucas T, Lumley MA, Flack JM, Wegner R, Pierce J, Goetz S. A preliminary experimental examination of worldview verification, perceived racism, and stress reactivity in African Americans. Health Psychol. 2016 Apr:366. doi: 10.1037/hea0000284. [DOI] [PMC free article] [PubMed]
- 67.Campisi J, Bravo Y, Cole J, Gobeil K. Acute psychosocial stress differentially influences salivary endocrine and immune measures in undergraduate students. Physiol Behav. 2012 Oct 10:317. doi: 10.1016/j.physbeh.2012.09.003. [DOI] [PubMed]
- 68.Branson S, Boss L, Hamlin S, Padhye NS. Animal-Assisted Activity in Critically Ill Older Adults: A Randomized Pilot and Feasibility Trial. Biol Res Nurs. 2020 Jul:412. doi: 10.1177/1099800420920719. [DOI] [PubMed]
- 69.David J, Measelle J, Ostlund B, Ablow J. Association between early life adversity and inflammation during infancy. Dev Psychobiol. 2017 Sep:696. doi: 10.1002/dev.21538. [DOI] [PubMed]
- 70.Heidemarie K. Laurenta TL, Jennifer Pierceb, Stefan G, Douglas A. Coordination of cortisol response to social evaluative threat withautonomic and inflammatory responses is moderated by stressappraisals and affect. Biol Psychol doi: 10.1016/j.biopsycho.2016.04.066. [DOI] [PMC free article] [PubMed]
- 71.Lucas SMGT. C-reactive protein in saliva and dried blood spot as markers of stress reactivity in healthy African–Americans. Biomark Med. 2020 doi: 10.2217/bmm-2019-0391. [DOI] [PMC free article] [PubMed]
- 72.Halpern LR, Shealer ML, Cho R, McMichael EB, Rogers J, Ferguson-Young D. et al. Influence of Intimate Partner Violence (IPV) Exposure on Cardiovascular and Salivary Biosensors: Is There a Relationship. J Natl Med Assoc. 2017 Winter:252. doi: 10.1016/j.jnma.2017.08.001. [DOI] [PMC free article] [PubMed]
- 73.Berndt C, Strahler J, Kirschbaum C, Rohleder N. Lower stress system activity and higher peripheral inflammation in competitive ballroom dancers. Biol Psychol. 2012 Dec:357. doi: 10.1016/j.biopsycho.2012.08.006. [DOI] [PubMed]
- 74.Hellewell SC, Cernak I. Measuring Resilience to Operational Stress in Canadian Armed Forces Personnel. J Trauma Stress. 2018 Feb:89. doi: 10.1002/jts.22261. [DOI] [PubMed]
- 75.Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, Granger DA. Perceived Discrimination, Racial Identity, and Multisystem Stress Response to Social Evaluative Threat Among African American Men and Women. Psychosom Med. 2017 Apr:293. doi: 10.1097/PSY.0000000000000406. [DOI] [PMC free article] [PubMed]
- 76.Esquire RG, Pederson ED, Covill PJ, Smith PF. Relationship of parotid saliva C-reactive protein to catecholamine release. Ann N Y Acad Sci. 1993 Sep 20 doi: 10.1111/j.1749-6632.1993.tb18363.x. [DOI] [PubMed]
- 77.Out D, Hall RJ, Granger DA, Page GG, Woods SJ. Assessing salivary C-reactive protein: longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain Behav Immun. 2012 May:543. doi: 10.1016/j.bbi.2012.01.019. [DOI] [PMC free article] [PubMed]
- 78.Hirasaki S, Yamazaki T, Shiba K. Changes in salivary components by drug administration in patients with heart diseases. J Med Dent Sci. 2005 Dec:183. [PubMed]
- 79.Naidoo T, Konkol K, Biccard B, Dudose K, McKune AJ. Elevated salivary C-reactive protein predicted by low cardio-respiratory fitness and being overweight in African children. Cardiovasc J Afr. 2012 Oct:501. doi: 10.5830/CVJA-2012-058. [DOI] [PMC free article] [PubMed]
- 80.Labat C, Temmar M, Nagy E, Bean K, Brink C, Benetos A. et al. Inflammatory mediators in saliva associated with arterial stiffness and subclinical atherosclerosis. J Hypertens. 2013 Nov:2251. doi: 10.1097/HJH.0b013e328363dccc. [DOI] [PMC free article] [PubMed]
- 81.Punyadeera C, Dimeski G, Kostner K, Beyerlein P, Cooper-White J. One-step homogeneous C-reactive protein assay for saliva. J Immunol Methods. 2011 Oct 28:19. doi: 10.1016/j.jim.2011.07.013. [DOI] [PubMed]
- 82.Foley JD, Sneed JD, Steinhubl SR, Kolasa JR, Ebersole JL, Lin Y. et al. Salivary biomarkers associated with myocardial necrosis: results from an alcohol septal ablation model. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012 Nov:616. doi: 10.1016/j.oooo.2012.05.024. [DOI] [PMC free article] [PubMed]
- 83.Dekker RL, Lennie TA, Moser DK, Miller CS, Ebersole JL, Chung ML. et al. Salivary Biomarkers, Oral Inflammation, and Functional Status in Patients With Heart Failure. Biol Res Nurs. 2017 Mar:153. doi: 10.1177/1099800416665197. [DOI] [PMC free article] [PubMed]
- 84.Faisal R, Jabber W. Salivary profile and periodontal condition in patients with heart disease under warfarin treatment. J Int Dent Med Res. 2018:449.
- 85.Jamshidpour B, Moghadam BA, Vasaghi-Gharamaleki B, Mirzaii-Dizgah I, Nejatian M. The effects of phase III cardiac rehabilitation in serum and salivary Hs-CRP and anthropometric measurements in patients with coronary artery disease. J Contemp Dent Pract. 2013 Sep 1:819. doi: 10.5005/jp-journals-10024-1409. [DOI] [PubMed]
- 86.Miller CS, Foley JD, Floriano PN, Christodoulides N, Ebersole JL, Campbell CL. et al. Utility of salivary biomarkers for demonstrating acute myocardial infarction. J Dent Res. 2014 Jul:72S. doi: 10.1177/0022034514537522. [DOI] [PMC free article] [PubMed]
- 87.Rice M, Turner-Henson A, Park NJ, Azuero A, Amiri A, Feeley CA. et al. Child and Maternal Factors That Influence Child Blood Pressure in Preschool Children: An Exploratory Study. Appl Nurs Res. 2016 Aug doi: 10.1016/j.apnr.2016.01.008. [DOI] [PubMed]
- 88.Shi P, Goodson M. A Data Mining Approach Identified Salivary Biomarkers That Discriminate between Two Obesity Measures. J Obes. 2019 doi: 10.1155/2019/9570218. [DOI] [PMC free article] [PubMed]
- 89.Selvaraju V, Babu JR, Geetha T. Association of salivary C-reactive protein with the obesity measures and markers in children. Diabetes Metab Syndr Obes. 2019 Jul 23 doi: 10.2147/DMSO.S211624. [DOI] [PMC free article] [PubMed]
- 90.Goodson JM, Kantarci A, Hartman ML, Denis GV, Stephens D, Hasturk H. et al. Metabolic Disease Risk in Children by Salivary Biomarker Analysis. PLoS One. 2014:e98799. doi: 10.1371/journal.pone.0098799. [DOI] [PMC free article] [PubMed]
- 91.Tvarijonaviciute A, Martinez-Lozano N, Rios R, Marcilla de, Garaulet M, Cerón JJ. Saliva as a non-invasive tool for assessment of metabolic and inflammatory biomarkers in children. Clin Nutr. 2020 Aug:2471. doi: 10.1016/j.clnu.2019.10.034. [DOI] [PubMed]
- 92.Qian J, Martinez-Lozano N, Tvarijonaviciute A, Rios R, Scheer FAJL, Garaulet M. Blunted rest-activity rhythms link to higher body mass index and inflammatory markers in children. Sleep. 2021 May 14:zsaa256. doi: 10.1093/sleep/zsaa256. [DOI] [PMC free article] [PubMed]
- 93.Tauler P, Martinez S, Moreno C, Martínez P, Aguilo A. Changes in salivary hormones, immunoglobulin A, and C-reactive protein in response to ultra-endurance exercises. Appl Physiol Nutr Metab. 2014 May:560. doi: 10.1139/apnm-2013-0466. [DOI] [PubMed]
- 94.Gawron-Skarbek A, Chrzczanowicz J, Nowak D, Gawor R, Kostka T. Effects of two different types of single exercise modes on salivary C-reactive protein concentration, oxidative stress and antioxidant capacity in post-myocardial infarction patients. Redox Rep. 2021 Dec:29. doi: 10.1080/13510002.2021.1890516. [DOI] [PMC free article] [PubMed]
- 95.Izawa S, Tsutsumi A, Ogawa N. Effort-reward imbalance, cortisol secretion, and inflammatory activity in police officers with 24-h work shifts. Int Arch Occup Environ Health. 2016 Oct:1147. doi: 10.1007/s00420-016-1154-2. [DOI] [PubMed]
- 96.Harnett JE, Pyne DB, McKune AJ, Penm J, Pumpa KL. Probiotic supplementation elicits favourable changes in muscle soreness and sleep quality in rugby players. J Sci Med Sport. 2021 Feb:195. doi: 10.1016/j.jsams.2020.08.005. [DOI] [PubMed]
- 97.Truba TN, Doan J, Currie CL, Copeland JL. Short-term changes in daily movement behaviour influence salivary C-reactive protein in healthy women. Appl Physiol Nutr Metab. 2018 Aug:854. doi: 10.1139/apnm-2017-0758. [DOI] [PubMed]
- 98.Roca E, Cantó E, Nescolarde L. Effects of a polysaccharide-based multi-ingredient supplement on salivary immunity in non-elite marathon runners. J Int Soc Sports Nutr. 2019 doi: 10.1186/s12970-019-0281-z. [DOI] [PMC free article] [PubMed]
- 99.Dolsen MR, Harvey AG. IL-6, sTNF-R2, and CRP in the context of sleep, circadian preference, and health in adolescents with eveningness chronotype: Cross-sectional and longitudinal treatment effects. Psychoneuroendocrinology. 2021 Jul doi: 10.1016/j.psyneuen.2021.105241. [DOI] [PubMed]
- 100.Nag C, Pradhan RK. Impact of lifestyle on circadian orientation and sleep behaviour. Sleep Biol Rhythms 2012;10:94–99
- 101.Andrianome S, Hugueville L, de Seze, Selmaoui B. Increasing levels of saliva alpha amylase in electrohypersensitive (EHS) patients. Int J Radiat Biol. 2017 Aug:841. doi: 10.1080/09553002.2017.1325971. [DOI] [PubMed]
- 102.Pradhan C. Sleep deprivation and level of C-reactive protein. Biol. Rhythm Res
- 103.Izawa S, Miki K, Liu X, Ogawa N. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain Behav Immun. 2013 Jan:38. doi: 10.1016/j.bbi.2012.07.001. [DOI] [PubMed]
- 104.Gawron-Skarbek AG, Prymont P, Godala M, Kolmaga A, Nowak D, Szatko F. et al. Dietary Vitamin C, E and -Carotene Intake Does Not Significantly Affect Plasma or Salivary Antioxidant Indices and Salivary C-Reactive Protein in Older Subjects. Nutrients. 2017 doi: 10.3390/nu9070729. [DOI] [PMC free article] [PubMed]
- 105.Akbari M, Afshari R, Sharif M, Hashemy S, Majidinia I. Evaluation of the Effect of Diacetyl Morphine on Salivary Factors and their Changes after Methadone Therapy. J Contempo Dent Pract. 2014:730-5 doi: 10.5005/jp-journals-10024-1607. [DOI] [PubMed]
- 106.Brett BE, Koko BK, Doumbia HOY, Koffi FK, Assa SE, Zahé K. et al. Salivary biomarkers of stress and inflammation in first graders in Côte d'Ivoire: Effects of a probiotic food intervention. Psychoneuroendocrinology. 2021 Jul doi: 10.1016/j.psyneuen.2021.105255. [DOI] [PubMed]
- 107.Kandir G, Thondre P. A comparison of nutritional adequacy, body composition, blood pressure and salivary C-reactive protein between a sample of young adults following vegan and omnivorous diets. Proceed Nutr Soc. 2021:E165.
- 108.Datla S, Kitchanan S, Sethuraman G. Diagnostic Reliability of Salivary C-Reactive Protein as an Alternative Noninvasive Biomarker of Neonatal Sepsis. Indian Pediatr. 2021 Aug 15:745. [PubMed]
- 109.Lin GC, Küng E, Smajlhodzic M, Domazet S, Friedl HP, Angerer J. et al. Directed Transport of CRP Across In Vitro Models of the Blood-Saliva Barrier Strengthens the Feasibility of Salivary CRP as Biomarker for Neonatal Sepsis. Pharmaceutics. 2021 Feb 12:256. doi: 10.3390/pharmaceutics13020256. [DOI] [PMC free article] [PubMed]
- 110.Omran A, Maaroof A, Mohammad MHS, Abdelwahab A. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J Pediatr (Rio J) 2018 Jan-Feb:82. doi: 10.1016/j.jped.2017.03.006. [DOI] [PubMed]
- 111.Dillon M, Opris D, Kopanczyk R, Lickliter J, Cornwell H, Bridges E. et al. Detection of Homocysteine and C-Reactive Protein in the Saliva of Healthy Adults: Comparison with Blood Levels. Biomark Insights. 2010 doi: 10.4137/bmi.s5305. [DOI] [PMC free article] [PubMed]
- 112.Wetterö J, von Löhneysen, Cobar F, Kristenson M, Garvin P, Sjöwall C. Pronounced Diurnal Pattern of Salivary C-Reactive Protein (CRP) With Modest Associations to Circulating CRP Levels. Front Immunol. 2021 Jan 8 doi: 10.3389/fimmu.2020.607166. [DOI] [PMC free article] [PubMed]
- 113.Nam Y, Kim YY, Chang JY, Kho HS. Salivary biomarkers of inflammation and oxidative stress in healthy adults. Arch Oral Biol. 2019 Jan doi: 10.1016/j.archoralbio.2018.10.026. [DOI] [PubMed]
- 114.Idris FP, Wan Y, Zhang X, Punyadeera C. Within-Day Baseline Variation in Salivary Biomarkers in Healthy Men. OMICS. 2017 Feb:74. doi: 10.1089/omi.2016.0168. [DOI] [PubMed]
- 115.Siavoshi H, Kashi A, Samavati Sharif, Helalizadeh M. The Relationships Between Some Physical Fitness Factors and Muscle Damage in People With Intellectual Disabilities. Iran Rehabil J. 2020
- 116.Agho ET, Owotade FJ, Kolawole BA. Salivary inflammatory biomarkers and glycated haemoglobin among patients with type 2 diabetic mellitus. BMC Oral Health. 2021 doi: 10.1186/s12903-021-01453-y. [DOI] [PMC free article] [PubMed]
- 117.Dezayee ZM, Al-Nimer MS. Saliva C-reactive protein as a biomarker of metabolic syndrome in diabetic patients. Indian J Dent Res. 2016 Jul-Aug:388. doi: 10.4103/0970-9290.191887. [DOI] [PubMed]
- 118.López del, Ocasio-López C, Steffen M. Comparison of Levels of Salivary Cytokines in Diabetic and Nondiabetic Puerto Rican Children: A Case-control Pilot Study. Pediatr Dent. 2015 Jan-Feb:30. [PubMed]
- 119.Baghani Z, Basir Shabestari, Karrabi M. Extent of clinical attachment loss as a practical parameter in efficacy of adjunctive photodynamic therapy in stage II - IV grade C Periodontitis (Aggressive periodontitis): A systematic review and meta-analysis. Bosn J Basic Med Sci. 2022 May 29 doi: 10.17305/bjbms.2022.7157. [DOI] [PMC free article] [PubMed]
- 120.Phalane KG, Kriel M, Loxton AG, Menezes A, Stanley K, van der. et al. Differential expression of host biomarkers in saliva and serum samples from individuals with suspected pulmonary tuberculosis. Mediators Inflamm. 2013 doi: 10.1155/2013/981984. [DOI] [PMC free article] [PubMed]
- 121.Jacobs R, Tshehla E, Malherbe S, Kriel M, Loxton AG, Stanley K. et al. Host biomarkers detected in saliva show promise as markers for the diagnosis of pulmonary tuberculosis disease and monitoring of the response to tuberculosis treatment. Cytokine. 2016 May doi: 10.1016/j.cyto.2016.02.004. [DOI] [PubMed]
- 122.Tsai CM, Tang KS, Cheng MC, Liu TY, Huang YH, Chen CC. et al. Use of Saliva Sample to Detect C-Reactive Protein in Children with Pneumonia. Pediatr Pulmonol. 2020:2457. doi: 10.1002/ppul.24947. [DOI] [PubMed]
- 123.Patel N, Belcher J, Thorpe G, Forsyth NR, Spiteri MA. Measurement of C-reactive protein, procalcitonin and neutrophil elastase in saliva of COPD patients and healthy controls: correlation to self-reported wellbeing parameters. Respir Res. 2015 May 28:62. doi: 10.1186/s12931-015-0219-1. [DOI] [PMC free article] [PubMed]
- 124.Bhavsar NV, Dave BD, Brahmbhatt NA, Parekh R. Periodontal status and oral health behavior in hospitalized patients with chronic obstructive pulmonary disease. J Nat Sci Biol Med. 2015 Aug:S93. doi: 10.4103/0976-9668.166097. [DOI] [PMC free article] [PubMed]
- 125.Blake L, Jones SE, Zoe E, Taylor F. Salivary uric acid and C-reactive protein associations with hypertensionin Midwestern Latino preadolescents and their parents. Dev Psychobiol. 2017:1-7 doi: 10.1002/dev.21577. [DOI] [PubMed]
- 126.Azar R, Richard A. Elevated salivary C-reactive protein levels are associated with active and passive smoking in healthy youth: A pilot study. J Inflamm (Lond) 2011 Dec 7:37. doi: 10.1186/1476-9255-8-37. [DOI] [PMC free article] [PubMed]
- 127.Pradhan RK, Mishra R, Nag C. Diurnal variations of C-reactive protein in trait and sickle cell disease patients. Biol Rhythm Res. 2013:277.
- 128.Lorenz TK, Demas GE, Heiman JR. Partnered sexual activity moderates menstrual cycle-related changes in inflammation markers in healthy women: an exploratory observational study. Fertil Steril. 2017 Mar:763. doi: 10.1016/j.fertnstert.2016.11.010. [DOI] [PMC free article] [PubMed]
- 129.Iyengar A, Paulus JK, Gerlanc DJ, Maron JL. Detection and potential utility of C-reactive protein in saliva of neonates. Front Pediatr. 2014 doi: 10.3389/fped.2014.00131. [DOI] [PMC free article] [PubMed]
- 130.Pallos D, Leão MV, Togeiro FC, Alegre L, Ricardo LH, Perozini C. et al. Salivary markers in patients with chronic renal failure. Arch Oral Biol. 2015 Dec:1784. doi: 10.1016/j.archoralbio.2015.09.008. [DOI] [PubMed]
- 131.Twardowski SE, Wactawski-Wende J, Hovey KM, Andrews CA, Banack HR, LaMonte MJ. et al. Plasma 25-Hydroxyvitamin D Concentrations and Serum and Salivary C-Reactive Protein in the Osteoporosis and Periodontal Disease Study. Nutrients. 2021 Mar 31:1148. doi: 10.3390/nu13041148. [DOI] [PMC free article] [PubMed]
- 132.Hashemipour MS, Yarbakht M, Gholamhosseinian A, Famori H. Effect of mobile phone use on salivary concentrations of protein, amylase, lipase, immunoglobulin A, lysozyme, lactoferrin, peroxidase and C-reactive protein of the parotid gland. J Laryngol Otol. 2014 May:454. doi: 10.1017/S0022215114000899. [DOI] [PubMed]
- 133.Rogers BG, Glynn TR, Bainter SA, McCauley T, Antoni MH, Safren SA. Syndemics and salivary inflammation in people living with HIV/AIDS. Psychol Health. 2021 Apr:496. doi: 10.1080/08870446.2020.1763995. [DOI] [PMC free article] [PubMed]
- 134.Bougea A, Spantideas N, Galanis P, Katsika P, Boufidou F, Voskou P. et al. Salivary inflammatory markers in tension type headache and migraine: the SalHead cohort study. Neurol Sci. 2020 Apr:877. doi: 10.1007/s10072-019-04151-4. [DOI] [PubMed]
- 135.Zhu X, Chen C, Zhang B, Ge Y, Wang W, Cai J. et al. Acute effects of personal exposure to fine particulate matter on salivary and urinary biomarkers of inflammation and oxidative stress in healthy adults. Chemosphere. 2021 Jun doi: 10.1016/j.chemosphere.2021.129906. [DOI] [PubMed]
- 136.Azzi L, Carcano G, Gianfagna F, Grossi P, Gasperina DD, Genoni A. et al. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020 Jul:e45. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed]
- 137.Gofin Y, Fanous E, Pasternak Y, Prokocimer Z, Zagoory-Sharon O, Feldman R. et al. Salivary C-reactive protein-a possible predictor of serum levels in pediatric acute respiratory illness. Eur J Pediatr. 2021 Aug:2465. doi: 10.1007/s00431-021-04047-6. [DOI] [PubMed]
- 138.Monib KME, El-Fallah AA, Salem RM. Inflammatory markers in acne vulgaris: Saliva as a novel diagnostic fluid. J Cosmet Dermatol. 2022 Mar:1280. doi: 10.1111/jocd.14236. [DOI] [PubMed]
- 139.Schackert HK, Agha-Hosseini F, Görgens H, Jatzwauk M, von Kannen, Noack B. et al. Complete homozygous deletion of CTSC in an Iranian family with Papillon-Lefèvre syndrome. Int J Dermatol. 2014 Jul:885. doi: 10.1111/j.1365-4632.2012.05769.x. [DOI] [PubMed]
- 140.Jamal Abbas, Al Saree. Elevation Salivary C - Reactive Protein (CRP) Levels in Iraqi Women with Polycystic ovary syndrome (PCOS) and Oral diseases. Mustansiria Dent J. 2014
- 141.Zhang CZ, Cheng XQ, Li JY, Zhang P, Yi P, Xu X. et al. Saliva in the diagnosis of diseases. Int J Oral Sci. 2016:133. doi: 10.1038/ijos.2016.38. [DOI] [PMC free article] [PubMed]
- 142.Kuwayama K, Miyaguchi H, Yamamuro T, Tsujikawa K, Kanamori T, Iwata YT. et al. Effectiveness of saliva and fingerprints as alternative specimens to urine and blood in forensic drug testing. Drug Test Anal. 2016 Jul:644. doi: 10.1002/dta.1831. [DOI] [PubMed]


