Abstract
The process of chlamydial cell division has not been thoroughly investigated. The lack of detectable peptidoglycan and the absence of an FtsZ homolog within chlamydiae suggest an unusual mechanism for the division process. Our laboratory has identified an antigen (SEP antigen) localized to a ring-like structure at the apparent septum within dividing chlamydial reticulate bodies (RB). Antisera directed against SEP show similar patterns of antigen distribution in Chlamydia trachomatis and Chlamydia psittaci RB. In contrast to localization in RB, SEP in elementary bodies appears diffuse and irregular, suggesting that the distribution of the antigen is developmental-stage specific. Treatment of chlamydiae with inhibitors of peptidoglycan synthesis or culture of chlamydiae in medium lacking tryptophan leads to the formation of nondividing, aberrant RB. Staining of aberrant RB with anti-SEP reveals a marked redistribution of the antigen. Within C. trachomatis-infected cells, ampicillin treatment leads to high levels of SEP accumulation at the periphery of aberrant RB, while in C. psittaci, treatment causes SEP to localize to distinct punctate sites within the bacteria. Aberrancy produced via tryptophan depletion results in a different pattern of SEP distribution. In either case, the reversal of aberrant formation results in the production of normal RB and a redistribution of SEP to the apparent plane of bacterial division. Collectively these studies identify a unique chlamydial-genus-common and developmental-stage-specific antigen that may be associated with RB division.
Chlamydiae are obligately intracellular pathogens that cause significant disease in both humans and animals. Chlamydia trachomatis causes one of the most commonly reported sexually transmitted infections, with untreated cases leading to pelvic inflammatory disease, salpingitis, and ectopic pregnancy (14). Other serovars of C. trachomatis cause trachoma, the leading cause of preventable blindness worldwide (30). Chlamydia psittaci is a pathogen primarily of veterinary concern but also provides a representative animal model for studying chlamydial infections within humans (22). Within the host, chlamydiae exist inside a nonacidified vacuole (the inclusion) where the bacteria sustain a unique intracellular developmental cycle. Shortly after entry, the infectious elementary bodies (EB) differentiate to reticulate bodies (RB) and undergo several rounds of multiplication before redifferentiating back to EB. While these events have been documented carefully at the ultrastructural level, the molecular events associated with chlamydial division are not well understood. The recent availability of three chlamydial genome sequences provides information about some aspects of the division process (R. S. Stephens [http://chlamydia-www.berkeley.edu:4231/]).
Cell division in virtually all prokaryotic systems is facilitated by a series of Fts (filamentation temperature sensitive) proteins that participate in septum formation. Of these proteins, FtsZ plays a major role in septation (10). FtsZ, as well as other Fts proteins, localizes to a ring-like structure at the plane of division (5, 18, 24). In Escherichia coli, inactivation of FtsZ results in filamentous cells that lack any evidence of a septal ring. Genes encoding FtsZ homologs have been identified in all prokaryotic organisms thus far examined, including mycoplasmas (2, 10, 32). Curiously, chlamydiae do not encode a protein sharing significant identity with FtsZ (27), which suggests a unique mechanism for the chlamydial division process.
A major paradox of chlamydial biology is the apparent absence of peptidoglycan (PG) within the bacterial cell envelope. The chlamydial genome contains all genes necessary to encode proteins to carry out PG synthesis, assembly, and degradation (8, 27). However, mass spectrometry and labeling with anti-PG antisera have failed to provide significant evidence of PG or of its structural precursors (11, 15). PG has multiple structural roles within most walled bacteria. In addition to its involvement in osmotic stability and rigidity, PG also plays a major role in bacterial division by forming an invagination between separating daughter cells during cytokinesis (20, 24). The absence of detectable PG in chlamydiae is surprising in light of the remarkable stability of the EB cell wall. EB are quite resistant to physical disruption—moderate sonication steps are included in EB purification protocols (7). As a substitute for PG, EB cell wall integrity is maintained by a series of outer membrane proteins linked through disulfide bonds (13). In contrast, the fragile RB lacks the proteins involved in cell wall stability.
Although chlamydiae do not accumulate detectable amounts of PG, there is metabolic evidence that PG synthesis occurs in the cell. The production of infectious EB is highly sensitive to inhibitors of PG synthesis, including β-lactam antibiotics and d-cycloserine (21, 28, 34). Treatment of infected cells with these agents inhibits cell division and leads to the formation of large, aberrant RB that cannot differentiate to EB. These studies indicate that chlamydial PG synthesis may be required for chlamydial cell division and proper differentiation.
Aberrant, persistent chlamydial growth can also be mediated through amino acid starvation (9). A well-characterized example of this occurs in cells in which intracellular tryptophan (Trp) pools are reduced in response to gamma interferon (IFN-γ) (3, 4). Host cell contact with IFN-γ activates indoleamine 2,3-dioxygenase, which degrades intracellular Trp, starving intracellular pathogens of their Trp supply (29). Under these conditions, chlamydiae also develop into large, nondividing, aberrant RB.
In this study, we characterize an antigen localized to a ring-like structure at or near the plane of chlamydial division, which we have termed the SEP (septum) antigen. Fluorescent-antibody labeling of SEP reveals a unique localization pattern, different from any other seen in chlamydiae and resembling the distribution of FtsZ to the septum in other bacterial species (5). The distribution of SEP is developmentally regulated; it localizes to the septum only in actively dividing RB, not in EB or aberrant forms. These findings indicate that SEP may be associated with the chlamydial division process.
MATERIALS AND METHODS
Cell culture and bacterial infection.
HeLa cells were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) (MEM-10; Gibco, Grand Island, N.Y.) at 37°C under 5% CO2. Monolayers were cultured on sterile glass coverslips to approximately 30 to 50% confluency. Cells were washed with Hanks balanced salt solution (HBSS; Gibco) and infected with the guinea pig inclusion conjunctivitis (GPIC) strain of C. psittaci or with C. trachomatis serovar L2 strain 434/Bu. All chlamydiae were purified by density gradient centrifugation as described previously (7) and were diluted in HBSS prior to inoculation of cells. Inocula were incubated on cells for 1 h at room temperature (RT) and were then replaced with the appropriate culture medium. E. coli BM2711 containing pGB2inv (12) was grown in Luria-Bertani (LB) medium at 34°C and diluted in MEM-10 lacking gentamicin prior to inoculation of cells. Inocula remained on cells for 3 h at RT and were then replaced with MEM-10 containing gentamicin for 4 h prior to methanol fixation.
Production of anti-SEP antisera.
Hartley strain guinea pigs (500 g) were immunized with Ribi trivalent adjuvant (Ribi Chemical Co., Hamilton, Mont.) three times over the course of 3 months. The adjuvant consists of a mixture of monophosphoryl lipid A (MPL), corynebacterial trehalose dimycolate (TDM), and mycobacterial cell wall skeleton (MCWS). The antigen used for injection consisted solely of the adjuvant mixed with phosphate-buffered saline (PBS). Anesthetized animals were injected intramuscularly, subcutaneously, and intraperitoneally with a total of 0.5 ml of adjuvant-PBS. Twenty-one days after the final immunization, sera were collected and tested for antichlamydial antibody activity by fluorescence microscopy. Control sera were collected from uninjected Hartley strain guinea pigs.
Immunofluorescence labeling.
Infected cells, cultured for times indicated for each experiment, were fixed in 100% methanol for 5 min, rinsed with HBSS, and incubated in fluorescent-antibody (FA) block (2% bovine serum albumin [BSA] in PBS) for 20 min. Monolayers were then incubated for 1 h with the appropriate primary antibody diluted in FA block and subsequently rinsed three times with PBS. The appropriate secondary antibody was added, and after a second 1-h incubation, the cells were again washed three times with PBS. Coverslips were rinsed with distilled H2O and inverted onto a drop of nonphotobleaching agent (Vector Laboratories, Burlingame, Calif.) on a microscope slide. Stained slides were observed under a Zeiss fluorescent microscope. Photographs were taken under a 100× oil immersion objective with a Zeiss camera using Kodak CN400 film.
Production and reversion of aberrant RB.
Aberrant RB were produced in both C. psittaci- and C. trachomatis-infected cells by two methods. First, infected cells were cultured in MEM-10 containing 1 μg of cycloheximide/ml plus ampicillin for the times indicated in the figure legends. Cells were then fixed with methanol and prepared for microscopy. Initial experiments used ampicillin concentrations of 100 μg/ml, which resulted in aberrant forms incapable of reverting to functional, replicative RB within the time limits of the experiment. Optimal ampicillin concentrations for production of aberrant RB capable of fully reverting to typical developmental forms were determined by culturing C. psittaci- and C. trachomatis-infected HeLa cells in MEM-10 containing a range of ampicillin concentrations (0.0125 to 10 μg/ml). Infected cells were cultured until aberrant RB were clearly visible by light microscopy (approximately 24 h postinfection [hpi]). The medium was then removed, and the cells were washed twice before MEM-10 without ampicillin was added. Optimal ampicillin concentrations that led to the development of aberrant RB followed by complete reversion after the removal of ampicillin were 0.2 and 10 μg/ml for C. trachomatis and C. psittaci, respectively.
Aberrant forms were also produced via Trp starvation. Trp-deficient MEM was produced with a Selectamine kit (Gibco) and supplemented with FBS. The appropriate concentration of FBS was determined by culturing C. psittaci-infected HeLa cells in Trp-deficient MEM containing a range of FBS concentrations for 48 h. Aberrancy was evaluated by fluorescence microscopy. Optimal aberrant growth occurred in Trp-deficient MEM containing 1% FBS (Trp− MEM-1). To confirm that these forms developed from the lack of Trp and not from inappropriately low FBS concentrations, Trp− MEM-1 was supplemented with Trp (10 μg/ml), resulting in typical RB development (data not shown). Cycloheximide was not used in the Trp starvation experiments. Infected cells were cultured in Trp− MEM-1 for 46 hpi prior to methanol fixation. Typical developmental forms were recovered from Trp-starved cells by removing the Trp− MEM-1 and incubating the cells in MEM-10.
Electrophoresis and immunoblotting.
C. trachomatis-infected HeLa cell lysates were solubilized in polyacrylamide gel electrophoresis sample buffer prior to electrophoresis through a 12% polyacrylamide gel (23). Proteins were transferred to nitrocellulose filters, and immunoblots were probed with guinea pig anti-SEP antibodies or the mouse anti-HSP60 hybridoma A57B9 (36). 35S-labeled staphylococcal protein A (124 nCi/ml; Amersham) or a chicken anti-mouse antibody–peroxidase conjugate (Pierce, Rockford, Ill.) was used as the secondary reagent for the guinea pig or mouse antibody, respectively. Signals were visualized by autoradiography or chemiluminescence.
Adsorption of antisera.
To determine if anti-SEP antibodies were directed at MCWS, an adsorption experiment was undertaken. Purified MCWS (a generous gift from Terry Ullrich of Ribi Immunochemical, Hamilton, Mont.) was suspended in 2% BSA–PBS to 5 mg/ml and incubated with a mixture of anti-HSP60 and anti-SEP antibodies for 1 h at RT. The anti-HSP60 was included as a control to eliminate the possibility that the very hydrophobic MCWS was nonspecifically binding antibodies in the adsorption. The concentration of anti-HSP60 used in this experiment was qualitatively determined by serially diluting the antibody until a reduction in signal was observed by fluorescence microscopy. The lowest concentration of anti-HSP60 giving a fully positive fluorescent image was used for control adsorptions. Following incubation with antibodies, the MCWS suspension was removed from the mixture by centrifugation (10,145 × g for 5 min). The supernatant was removed and used in fluorescence microscopy of C. psittaci-infected HeLa cells that were methanol fixed 18 hpi.
RESULTS
Identification of SEP.
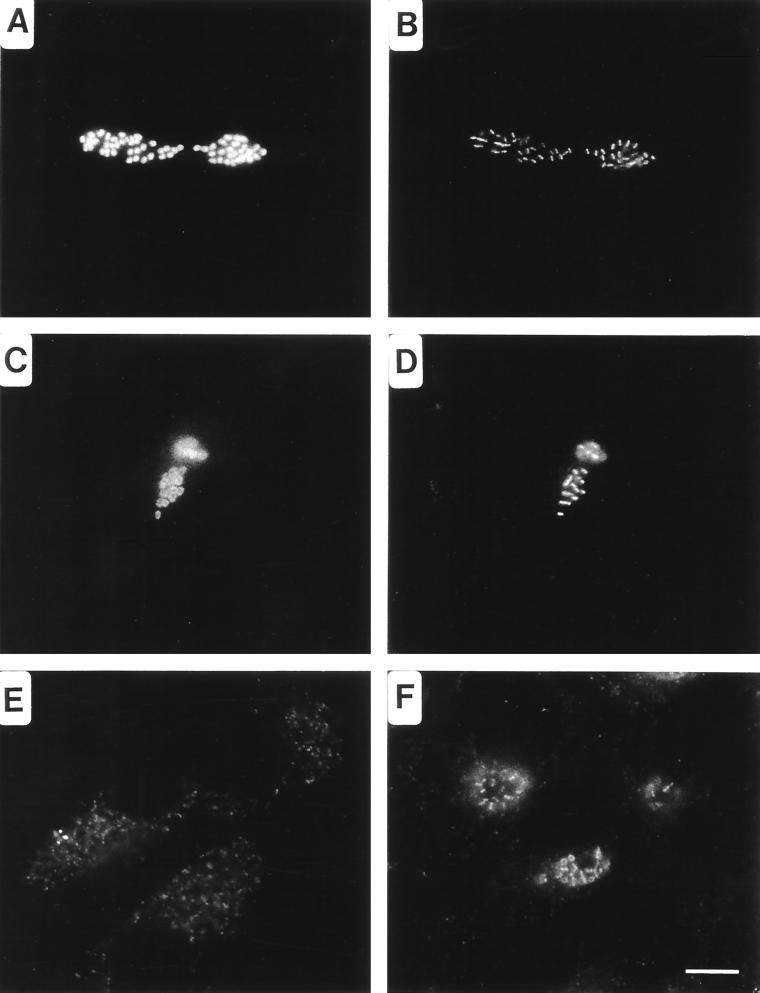
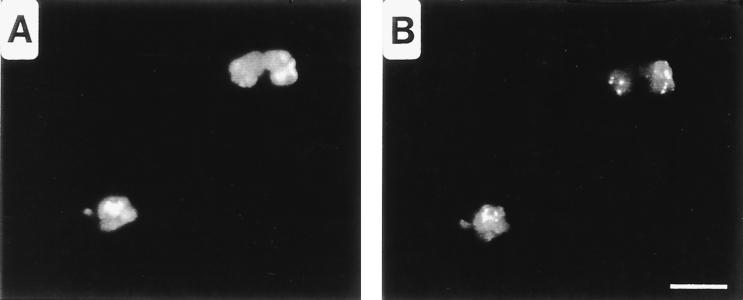
Antisera produced in guinea pigs after injection with Ribi trivalent adjuvant, along with monoclonal antibodies directed against chlamydial lipopolysaccharide (LPS) or heat shock protein (HSP60), were used as probes for fluorescent microscopic analysis of methanol-fixed, Chlamydia-infected HeLa cells. Antisera raised against the adjuvant alone reacted with antigens localizing to the apparent plane of bacterial division in C. psittaci (Fig. 1B and D), C. trachomatis (Fig. 1F), and Chlamydia pneumoniae (not shown) RB. The antigen was distributed as bars or ring structures within or between developing RB, reminiscent of FtsZ rings observed in other species of bacteria (5). This staining pattern is distinct from classical labeling of chlamydial antigens that localize to the cytoplasm, as seen with anti-HSP60 (Fig. 1A) or the chlamydial periphery, as seen with anti-LPS (Fig. 1C). In addition, the distribution patterns of SEP in RB and EB are distinct. As the transition from RB to EB occurs, SEP becomes diffuse and irregular (Fig. 1E). These findings suggest that SEP is a developmentally distinct structure that is distributed uniquely in the RB.
FIG. 1.
Distribution of SEP in C. psittaci- and C. trachomatis-infected HeLa cells visualized by fluorescence microscopy. C. psittaci-infected cells fixed with methanol 18 hpi were doubly labeled with anti-HSP60 (A) and anti-SEP (B) antibodies or with anti-LPS (C) and anti-SEP (D) antibodies. Notice that SEP localizes to the midpoint within dividing RB. SEP distribution is altered in chlamydiae found in late inclusions. (E) SEP distribution within C. psittaci-infected cells fixed 30 hpi. (F) SEP is a genus-common antigen, as is shown by labeling of C. trachomatis-infected cells fixed 18 hpi with anti-SEP antibodies. Note that in these cells the antigen is distributed in both bar and ring structures. Bar, 5 μm.
SEP redistribution in aberrant RB.
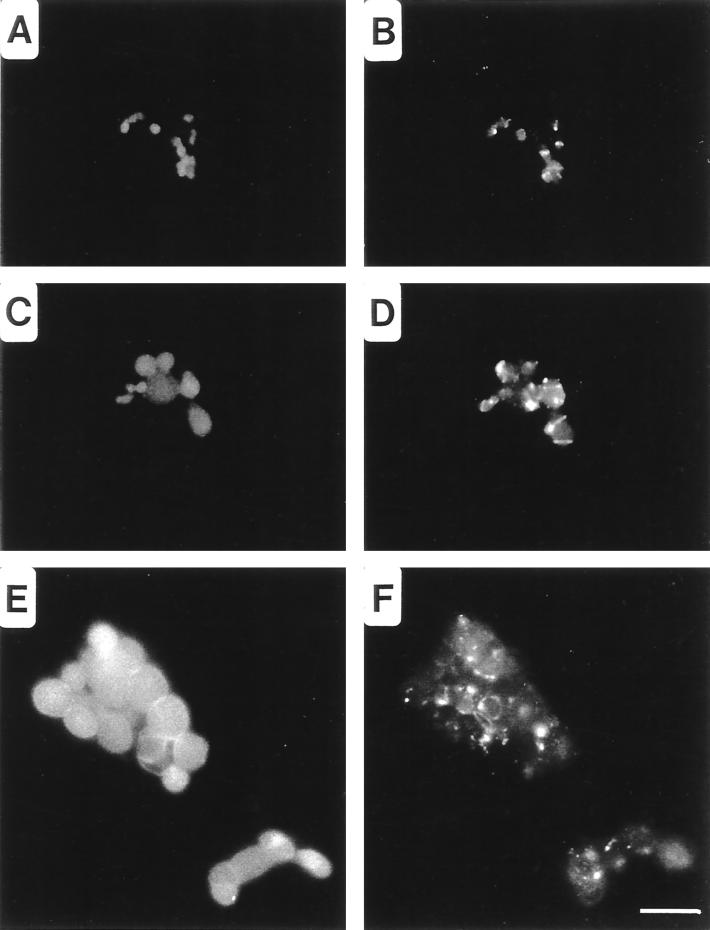
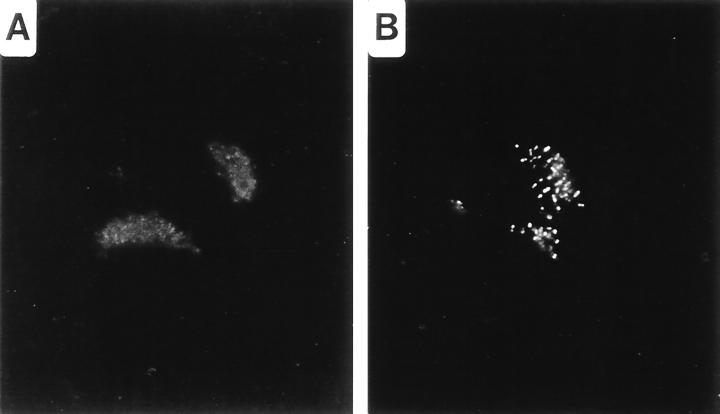
Treatment of Chlamydia-infected cells with β-lactam antibiotics inhibits chlamydial division, resulting in enlarged, or aberrant, RB (21, 28). To observe the distribution of SEP under conditions in which chlamydial division is inhibited, infected cells were cultured in MEM-10 containing 100 μg of ampicillin/ml. In these cells, SEP was distributed away from the apparent plane of division to distinct sites along the periphery of the RB. The progression of this shift can be observed in micrographs of C. psittaci-infected cells fixed at different times post-addition of ampicillin. Four hours after ampicillin addition, the chlamydiae began to enlarge but SEP remained closely associated with the central plane of the RB (Fig. 2B). At later times, SEP distribution was modified as the nondividing RB continued to enlarge (Fig. 2D). Labeling of SEP remained strong, but the antigen was no longer found at the center of the RB. Twenty-four hours after the addition of ampicillin, SEP appeared exclusively as distinct spots along the margins of aberrant RB (Fig. 2F). C. psittaci-infected HeLa cells treated with d-cycloserine, an inhibitor of the transglycosylation event of PG synthesis (25), produced a similar result (data not shown). A distinctly different distribution of SEP was observed following treatment of C. trachomatis-infected cells with ampicillin. SEP was similarly distributed at the peripheries of these aberrant forms, but the antigen accumulated to markedly higher concentrations (Fig. 3). This difference in SEP accumulation in ampicillin-treated C. trachomatis- and C. psittaci-infected cells was observed regardless of the ampicillin concentration used.
FIG. 2.
Redistribution of SEP in aberrant C. psittaci RB produced following the addition of ampicillin to the culture medium. Fluorescence microscopy was performed by double-labeling cells with anti-HSP60 (A, C, and E) and anti-SEP (B, D, and F) antibodies. C. psittaci-infected HeLa cells were cultured in MEM-10 for 10 hpi and then cultured in MEM-10 containing 10 μg of ampicillin/ml for an additional 4 (A and B) or 16 (C and D) h prior to methanol fixation. In addition, C. psittaci-infected HeLa cells were treated with 10 μg of ampicillin/ml immediately postinfection for 24 h prior to methanol fixation (E and F). Bar, 5 μm.
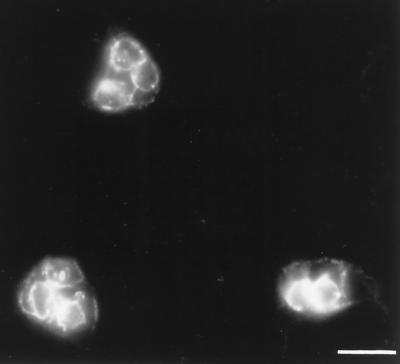
FIG. 3.
Redistribution of SEP in aberrant C. trachomatis RB produced following the addition of ampicillin to the culture medium. C. trachomatis-infected HeLa cells were treated with 0.2 μg of ampicillin/ml for 24 h prior to methanol fixation. Fluorescence microscopy was performed by labeling cells with anti-SEP antibodies. Note the greatly enlarged RB and the anti-SEP staining at the peripheries. Bar, 5 μm.
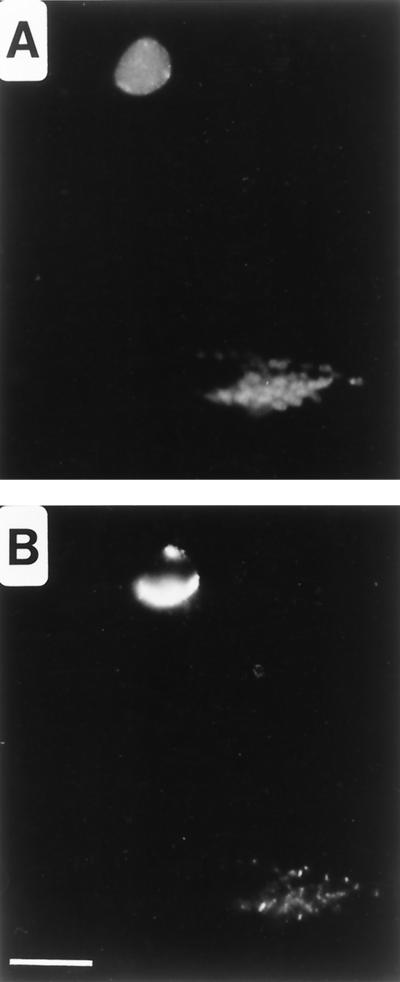
At low ampicillin concentrations, removal of the drug from infected cells restores chlamydial division, resulting in typical RB growth (21). C. psittaci-infected HeLa cells were cultured in the presence of 10 μg of ampicillin/ml for 24 h prior to culture in MEM-10 lacking antibiotics. Within 8 h after the removal of ampicillin, chlamydial division began to occur, although many aberrant forms remained in the culture. As RB reverted from aberrancy, SEP localized back to the apparent plane of RB division (Fig. 4B). These observations were consistent in cells infected with either C. psittaci (Fig. 4) or C. trachomatis (data not shown), although the ampicillin concentration required to facilitate reversion for C. trachomatis was lower (0.2 μg/ml). Collectively, these observations suggest that SEP distribution is associated with the process of chlamydial division.
FIG. 4.
Distribution of SEP after removal of MEM-10 containing ampicillin. C. psittaci-infected HeLa cells were cultured in MEM-10 containing 10 μg of ampicillin/ml for 24 h, after which the medium was removed and replaced with MEM-10 lacking ampicillin for 14 h prior to methanol fixation. Fluorescence microscopy was performed by double-labeling cells with anti-HSP60 (A) and anti-SEP (B) antibodies. Bar, 5 μm.
The distribution of SEP was also examined in both C. psittaci- and C. trachomatis-infected cells cultured in Trp-deficient medium. In C. psittaci, SEP redistributed to the peripheries of aberrant RB, as was seen in ampicillin-treated cells (Fig. 5B). In contrast, culture of C. trachomatis in HeLa cells starved for Trp resulted in a distinctly different pattern. SEP was virtually undetectable in C. trachomatis-infected cells cultured in Trp-deficient medium (data not shown).
FIG. 5.
Distribution of SEP in aberrant RB of C. psittaci produced by Trp starvation. C. psittaci-infected HeLa cells were cultured in Trp− MEM-1 for 46 hpi prior to methanol fixation. Fluorescence microscopy was performed by double-labeling cells with anti-HSP60 (A) and anti-SEP (B) antibodies. Bar, 5 μm.
Characterization of the antigen recognized by anti-SEP.
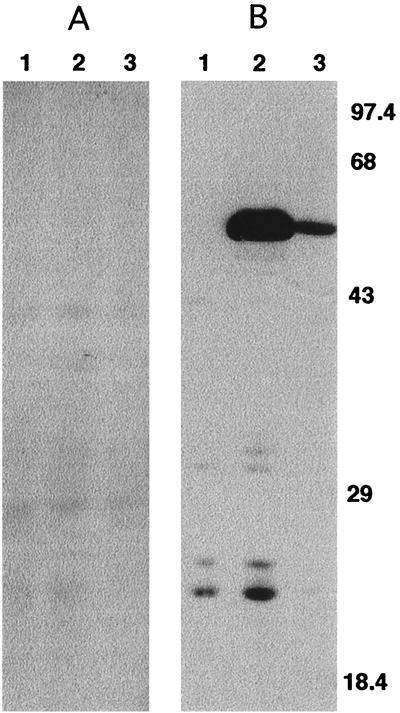
Immunoblotting with anti-SEP antisera was performed to potentially identify a protein that was a target of anti-SEP. Immunoblotting was performed on C. trachomatis-infected HeLa cell lysates collected during optimal SEP accumulation: at 18 hpi, when RB are actively dividing, and at 40 h post-ampicillin addition, when, as shown by immunofluorescence microscopy, SEP is very abundant. The chlamydial HSP60 protein was detected in these lysates by using monoclonal anti-HSP60 as a probe (Fig. 6B). In contrast, no proteins were identified in these lysates when parallel immunoblots were probed with anti-SEP (Fig. 6A). These results were consistent through different production lots of anti-SEP antisera and suggest that SEP may be nonproteinaceous.
FIG. 6.
Western blot analysis of SEP and HSP60 from C. trachomatis-infected HeLa cell lysates. Anti-SEP (A) and anti-HSP60 (B) antibodies were used to probe lysates collected from 18-h mock-infected HeLa cells cultured in MEM-10 (lanes 1), 40-h C. trachomatis-infected HeLa cells cultured in MEM-10 containing 0.2 μg of ampicillin/ml (lanes 2), or 18-h C. trachomatis-infected HeLa cells cultured in MEM-10 (lanes 3). Molecular mass standards (in kilodaltons) are shown on the right.
The Ribi adjuvant used to produce anti-SEP antisera is composed of three components: Salmonella enterica serovar Typhimurium MPL, synthetic Corynebacterium TDM, and MCWS. A commercial adjuvant that contains only MPL and TDM is also available. In preliminary experiments this adjuvant lacking MCWS was used to immunize guinea pigs, and the resulting antisera were used as probes of C. psittaci-infected HeLa cells. Sera from each of three different guinea pigs were negative for anti-SEP labeling (data not shown). Additionally, purified MCWS was used as an immunoadsorbant to remove anti-MCWS antibodies from preparations of anti-SEP. The resulting adsorbed antisera were used to probe C. psittaci-infected HeLa cells. Fluorescence microscopy showed a marked decrease in the intensity of SEP staining when antisera were absorbed with MCWS (Fig. 7A) compared to that with control antisera mock absorbed with BSA (Fig. 7B). To address the possibility that the MCWS was nonspecifically adsorbing the anti-SEP antibodies from the guinea pig antisera, mouse monoclonal anti-HSP60 was included with the anti-SEP in these adsorptions. There was no evidence that the MCWS absorbed any of the anti-HSP60 antibodies in these assays (data not shown). The affinity of anti-SEP antibodies for MCWS suggests that this antigen may be the immunogen stimulating the anti-SEP antibody response.
FIG. 7.
Immunoadsorption of MCWS with anti-SEP antibodies. Anti-SEP antibodies were absorbed with purified MCWS (A) or mock absorbed with BSA (B). Absorbed antisera were used to probe 18-h methanol-fixed C. psittaci-infected HeLa cells.
A final experiment addressing the possible target of the anti-SEP antibodies involved fluorescent microscopy of methanol-fixed, E. coli-infected cells probed with the anti-SEP antisera. E. coli BM2711 containing pGB2inv encodes the Yersinia InvA protein, which facilitates its uptake into HeLa cells (12). The entire peripheries of these bacteria were labeled with the anti-SEP antibodies (data not shown), suggesting that an antigenic structure similar to SEP may be found within the cell walls of other bacterial species.
DISCUSSION
Many aspects of chlamydial division remain undiscovered. The sequenced genome reveals that chlamydiae lack many of the proteins required for septation in other bacterial species, suggesting a unique mechanism for cytokinesis. This work describes a unique chlamydial antigenic structure, termed SEP, which localizes to an apparent septum in dividing chlamydiae. SEP is developmental-stage dependent, localizing to a ring-like structure at the plane of RB division and becoming punctate and irregular after differentiation to EB. SEP is redistributed to distinct sites along the bacterial periphery following treatment of Chlamydia-infected cells with ampicillin or following incubation of infected cells in medium lacking Trp. Removal of these stressors leads to reversion of aberrant forms to typical RB and results in redistribution of SEP back to the apparent plane of bacterial division. This antigen is present in C. trachomatis, C. psittaci, and C. pneumoniae, suggesting that the structure is conserved within the genus. Collectively these results suggest a possible role for SEP in the chlamydial division process.
Recently much has been established surrounding the molecular biology of bacterial cell division. At least nine proteins which localize to the septal ring (a ring of proteins at the site of cytokinesis) are required for bacterial division in E. coli: FtsZ, ZipA, FtsW, FtsA, FtsL, FtsN, FtsQ, FtsK, and FtsI (PBP3) (6, 18, 24). Other Fts proteins, FtsH, FtsJ, FtsY, FtsX, and FtsE, may play an indirect role in septation. Essential to activating the septal protein assembly pathway is FtsZ ring formation at the division site. Paradoxically, chlamydiae lack an ftsZ homolog but do encode predicted proteins that are likely homologous to proteins involved in septation, including FtsK, FtsW, FtsY, FtsH, and FtsI (27). Of these proteins, FtsI, FtsW, and FtsK show immunofluorescence staining patterns in E. coli similar to that seen with SEP localization to the ring in chlamydiae (1, 19, 31, 33, 35). However, immunoblot analyses with anti-SEP antisera did not identify candidate proteins that might be the target antigen, suggesting that the SEP antigen may be nonproteinaceous.
Of the few fts genes present in the chlamydial genome, homologs to ftsI and possibly ftsW are involved in PG biosynthesis at the septal plane in E. coli (16, 26). These findings, along with the facts that the chlamydial genome contains all genes necessary for PG synthesis and that Chlamydia is highly sensitive to inhibitors of PG synthesis, are contradictory to other studies which conclude an absence of PG within the chlamydial cell.
Cell wall inhibitors block PG assembly through several mechanisms (25). In some cases this leads to accumulation of PG precursors within the bacterial cell. Treatment of E. coli with moenomycin, which inhibits the transglycosylation reaction, promotes the accumulation of several PG precursors prior to cell lysis. In contrast, treatment with penicillin G, which inhibits the transpeptidation reaction, results in unchanged or decreased concentrations of such precursors (17). In the present study, while treatment with ampicillin and culture in Trp-deficient medium both led to SEP redistribution, there were differences in the observed phenotype among the different chlamydial species. Within C. psittaci GPIC, SEP distribution patterns following ampicillin treatment and following Trp starvation were very similar. However, within C. trachomatis L2, SEP was very abundant following aberrancy produced by ampicillin treatment but virtually undetectable following Trp starvation. The observed differences in SEP accumulation between these two species are perplexing. Because ampicillin treatment has no effect on chlamydial protein synthesis, production and apparent accumulation of enzymes and possibly PG precursors may occur. This effect is markedly more evident in C. trachomatis than in C. psittaci. In contrast, depletion of available intracellular Trp affects the production of many proteins; 15 of the 18 PG biosynthesis proteins contain Trp. This may result in only small amounts of critical enzymes accumulating within treated chlamydiae. The difference in SEP accumulation observed between Trp-starved C. psittaci and Trp-starved C. trachomatis may reflect differences in their needs for Trp in the synthesis of various PG precursors.
In most walled bacteria PG serves two purposes. It forms a structural sacculus providing osmotic stability to the organism, and it forms a scaffold during initiation of the septation process (20, 24). There is considerable evidence that the chlamydiae probably do not require PG for structural stability within the cell envelope, as this function is provided by disulfide-linked outer membrane proteins. However, the second function remains a possible role for chlamydial PG. We hypothesize that small amounts of PG may function during septum formation within dividing RB. Our studies identify an antigen that either may be this PG or may colocalize with this theoretical structure during growth. The ability of MCWS to adsorb anti-SEP activity and the affinity of anti-SEP for the E. coli cell wall suggests that anti-SEP may be binding directly to an antigen in common between MCWS and the E. coli cell wall. Since E. coli is contained within a murein sacculus, one such candidate antigen is PG. Further experiments are in progress to more clearly identify the target of anti-SEP and to examine the function of SEP in chlamydial growth.
ACKNOWLEDGMENTS
We thank Terry Ullrich of Ribi Immunochemical for his generous gift of purified MCWS. We thank Ben Simon of Oregon State University for providing us with E. coli BM2711 pGB2inv.
This research is supported by grants from the Medical Research Foundation of Oregon (9823) and the National Institutes of Health (1R29AI42869-01).
Footnotes
Oregon Agricultural Experiment Station technical paper 11543.
REFERENCES
- 1.Addinall S G, Lutkenhaus J. FtsA is localized to the septum in an FtsZ-dependent manner. J Bacteriol. 1996;178:7167–7172. doi: 10.1128/jb.178.24.7167-7172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beall B, Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 1991;5:447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- 3.Beatty W L, Belanger T A, Desai A A, Morrison R P, Byrne G I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty W L, Byrne G I, Morrison R P. Morphologic and antigenic characterization of interferon-gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 6.Bi E, Lutkenhaus J. Genetics of bacterial cell division. In: Mohan S, Dow C, Cole J A, editors. Prokaryotic structure and function: a new perspective. Cambridge, United Kingdom: Cambridge University Press; 1991. pp. 123–152. [Google Scholar]
- 7.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chopra I, Storey C, Falla T J, Pearce J H. Antibiotics, peptidoglycan synthesis and genomics: the chlamydial anomaly revisited. Microbiology. 1998;144:2673–2678. doi: 10.1099/00221287-144-10-2673. [DOI] [PubMed] [Google Scholar]
- 9.Coles A M, Reynolds D J, Harper A, Devitt A, Pearce J H. Low-nutrient induction of abnormal chlamydial development: a novel component of chlamydial pathogenesis? FEMS Microbiol Lett. 1993;106:193–200. doi: 10.1111/j.1574-6968.1993.tb05958.x. [DOI] [PubMed] [Google Scholar]
- 10.Dai K, Lutkenhaus J. ftsZ is an essential cell division gene in Escherichia coli. J Bacteriol. 1991;173:3500–3506. doi: 10.1128/jb.173.11.3500-3506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox A, Rogers J C, Gilbart J, Morgan S, Davis C H, Knight S, Wyrick P B. Muramic acid is not detectable in Chlamydia psittaci or Chlamydia trachomatis by gas chromatography-mass spectrometry. Infect Immun. 1990;58:835–837. doi: 10.1128/iai.58.3.835-837.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grillot-Courvalin C, Goussard S, Huetz F, Ojcius D M, Courvalin P. Functional gene transfer from intracellular bacteria to mammalian cells. Nat Biotechnol. 1998;16:862–866. doi: 10.1038/nbt0998-862. [DOI] [PubMed] [Google Scholar]
- 13.Hatch T P. Disulfide cross-linked envelope proteins: the functional equivalent of peptidoglycan in chlamydiae. J Bacteriol. 1996;178:1–5. doi: 10.1128/jb.178.1.1-5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillis S D, Owens L M, Marchbanks P A, Amsterdam L F, MacKenzie W R. Recurrent chlamydial infections increase the risks of hospitalization for ectopic pregnancy and pelvic inflammatory disease. Am J Obstet Gynecol. 1997;176:103–107. doi: 10.1016/s0002-9378(97)80020-8. [DOI] [PubMed] [Google Scholar]
- 15.How S J, Hobson D, Hart C A. Studies in vitro of the nature and synthesis of the cell wall of Chlamydia trachomatis. Curr Microbiol. 1984;10:269–274. [Google Scholar]
- 16.Ikeda M, Sato T, Wachi M, Jung H K, Ishino F, Kobayashi Y, Matsuhashi M. Structural similarity among Escherichia coli FtsW and RodA proteins and Bacillus subtilis SpoVE protein, which function in cell division, cell elongation, and spore formation, respectively. J Bacteriol. 1989;171:6375–6378. doi: 10.1128/jb.171.11.6375-6378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohlrausch U, Holtje J. Analysis of murein and murein precursors during antibiotic-induced lysis of Escherichia coli. J Bacteriol. 1991;173:3425–3431. doi: 10.1128/jb.173.11.3425-3431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutkenhaus J, Addinall S G. Bacterial cell division and the Z ring. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 19.Ma X, Ehrhardt D W, Margolin W. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc Natl Acad Sci USA. 1996;93:12998–13003. doi: 10.1073/pnas.93.23.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacAlister T J, Cook W R, Weigand R, Rothfield L I. Membrane-murein attachment at the leading edge of the division septum: a second membrane-murein structure associated with morphogenesis of the gram-negative bacterial division septum. J Bacteriol. 1987;169:3945–3951. doi: 10.1128/jb.169.9.3945-3951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto A, Manire G P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970;101:278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patton D L, Rank R G. Animal models for the study of pelvic inflammatory disease. In: Gallin J I, Fauci A S, editors. Advances in host defense mechanisms. New York, N.Y: Raven Press, Ltd.; 1992. pp. 85–111. [Google Scholar]
- 23.Rockey D D, Rosquist J L. Protein antigens of Chlamydia psittaci present in infected cells but not detected in the infectious elementary body. Infect Immun. 1994;62:106–112. doi: 10.1128/iai.62.1.106-112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothfield L I, Justice S S. Bacterial cell division: the cycle of the ring. Cell. 1997;88:581–584. doi: 10.1016/s0092-8674(00)81899-1. [DOI] [PubMed] [Google Scholar]
- 25.Russell A D, Chopra I. Understanding antibacterial action and resistance. 2nd ed. London, United Kingdom: Ellis Horwood; 1996. [Google Scholar]
- 26.Spratt B G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977;131:293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens R S, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 28.Tamura A, Manire G P. Effect of penicillin on the multiplication of meningopneumonitis organisms (Chlamydia psittaci) J Bacteriol. 1968;96:875–880. doi: 10.1128/jb.96.4.875-880.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor M W, Feng G. Relationship between interferon-γ, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2521. [PubMed] [Google Scholar]
- 30.Thylefors B, Negral A D. Global data on blindness. Bull W H O. 1995;73:115–121. [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Khattar M K, Donachie W D, Lutkenhaus J. FtsI and FtsW are localized to the septum in Escherichia coli. J Bacteriol. 1998;180:2810–2816. doi: 10.1128/jb.180.11.2810-2816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Lutkenhaus J. Characterization of the ftsZ gene from Mycoplasma pulmonis, an organism lacking a cell wall. J Bacteriol. 1996;178:2314–2319. doi: 10.1128/jb.178.8.2314-2319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss D S, Pogliano K, Carson M, Guzman L, Fraipont C, Nguyen-Disteche M, Losick R, Beckwith J. Localization of the Escherichia coli cell division protein FtsI (PBP3) to the division site and cell pole. Mol Microbiol. 1997;25:671–681. doi: 10.1046/j.1365-2958.1997.5041869.x. [DOI] [PubMed] [Google Scholar]
- 34.Weiss E. The effect of antibiotics on agents of the psittacosis-lymphogranuloma group. I. The effect of penicillin. J Infect Dis. 1950;87:249–263. doi: 10.1093/infdis/87.3.249. [DOI] [PubMed] [Google Scholar]
- 35.Yu X C, Tran A H, Sun Q, Margolin W. Localization of cell division protein FtsK to the Escherichia coli septum and identification of a potential N-terminal targeting domain. J Bacteriol. 1998;180:1296–1304. doi: 10.1128/jb.180.5.1296-1304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Y, Lyng K, Zhang Y-X, Rockey D D, Morrison R P. Monoclonal antibodies define genus-specific, species-specific, and cross-reactive epitopes of the chlamydial 60-kilodalton heat shock protein (hsp60): specific immunodetection and purification of chlamydial hsp60. Infect Immun. 1992;60:2288–2296. doi: 10.1128/iai.60.6.2288-2296.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]