Abstract
Objective
This study aimed to evaluate the effectiveness and safety of transarterial chemoembolization (TACE) in combination with immune checkpoint inhibitors (ICIs) plus tyrosine kinase inhibitors (TKIs) (TACE+IT) versus ICIs plus TKIs (IT) for advanced hepatocellular carcinoma (HCC).
Materials and Methods
Data of consecutive advanced HCC patients receiving TACE+IT or IT between January 2019 and December 2021 were included and were retrospectively analyzed. Propensity score matching (PSM) was performed to reduce bias due to confounding variables. The primary outcome of the study was overall survival (OS). The secondary outcomes were progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and adverse events (AEs), respectively.
Results
Sixty-four patients were enrolled in the study, among which 24 and 40 received TACE+IT and IT, respectively. The PSM cohort included 24 patients receiving TACE+IT (TACE+IT group) and 24 patients receiving IT (IT group) alone. During a median follow-up of 23 months, patients in TACE+IT group had significantly longer OS (median, 17.3 vs 11.8 months, P = 0.023), better ORR (41.7% vs 12.5%, P = 0.023) and DCR (79.2% vs 50.0%, P = 0.035) than those in the IT group, whereas a non-significant trend in PFS (median, 7.4 vs 6.7 months, P = 0.23) was observed. According to multivariable cox regression analysis, it was found that treatment modality was the only independent risk factor for OS (HR = 0.404, 95% CI = 0.179–0.911, P < 0.05). There were no remarkable differences in AEs associated with ICIs and TKIs between the two groups, with the exception of gastrointestinal reaction.
Conclusion
TACE combined with ICIs plus TKIs significantly improved OS, ORR, and DCR and showed a relatively longer PFS trend over ICIs combined with TKIs for advanced HCC.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, immune checkpoint inhibitors, tyrosine kinase inhibitors
Introduction
Hepatocellular carcinoma (HCC), accounting for 75–85% of primary liver cancer, is the third leading cause of cancer-related mortality globally.1 Despite advances in early detection, most HCC patients are still found at advanced stage, which are unresponsive to curative therapies, and have unfavorable outcomes. Transarterial chemoembolization (TACE) is the recommended treatment for intermediate HCC in accordance with the Barcelona Clinic Liver Cancer (BCLC) staging system. In addition, it is also a frequently applied approach for advanced HCC in real-world setting.2–5
Recently, immune checkpoint inhibitors (ICIs), particularly programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors, were shown to be clinically beneficial for advanced HCC patients.6 ICIs combined with anti VEGF antibodies/tyrosine kinase inhibitors (TKIs) have revealed clinical benefit in advanced HCC. The IMbrave150 trial, the ORIENT-32 trial, and the Phase III trial of camrelizumab plus apatinib revealed that ICIs combined with anti VEGF antibodies/TKIs had remarkably improved overall survival (OS) as well as progression-free survival (PFS) than previous first-line therapy sorafenib in advanced HCC patients who had not undergone previous systemic therapy.7–9 Some of included patients in those trials had regional treatments history such as TACE. However, the COSMIC-312 trial10 and the LEAP-002 trial11 failed to reveal the benefits of ICIs combined with TKIs.
In addition to combinations of ICIs with TKIs, a mounting body of evidence suggests the effect of TACE in potentiating tumor immunity and enhancing the effect of ICIs.12–14 TACE can lead to local tumour cell necrosis after occlusion of tumor-feeding arteries and release tumoral neoantigens, facilitating recruitment and activation of dendritic cells into the microenvironment. As a result, an immunosuppressive microenvironment not conducive to ICIs can be transformed into an immunosupportive microenvironment, in which systemic therapies might be more effective.15 To date, several studies with relatively small sample size have identified treatment benefit of TACE combined with ICIs.16–19 In addition, some phase III trials testing the combination of TACE with TKIs/ICIs/anti VEGF are underway (LEAP-012, EMERALD-1, CheckMate 74W). Limited studies have demonstrated that TACE plus ICIs plus TKIs significantly improved OS over ICIs plus TKIs in patients with unresectable HCC.20 However, there has been no detailed investigation of TACE in combination with ICIs plus TKIs versus ICIs plus TKIs for HCC of BCLC stage C. Thus, we carried out this retrospective study to evaluate the effectiveness and safety of TACE in combination with ICIs plus TKIs against ICIs plus TKIs alone for advanced HCC.
Materials and Methods
Patient Information
The Institutional Ethics Review Board of the First Affiliated Hospital of Soochow University (Suzhou, Jiangsu Province, China) approved the study, which was carried out based on the Declaration of Helsinki. Requirement to obtain written informed consent was waived due to its retrospective nature and we stated that patient data was strictly confidential. Data of consecutive advanced HCC patients receiving TACE in combination with ICIs plus TKIs (TACE+IT) or ICIs plus TKIs (IT) at our hospital between January 2019 and December 2021 were included and were retrospectively analyzed. The inclusion criteria included the following: 1) age from 18 to 75 years old; 2) HCC diagnosed based on the current practice guidelines;2,4,21 3) BCLC stage C; 4) an identifiable lesion, including multinodular HCC; 5) after curative resection or ablation, recurrence of tumors was permitted; 6) Within one week of receiving treatment, the Eastern Cooperative Oncology Group Performance Status (ECOG PS) must be 0 or 1; 7) Child-Pugh class A/B; 8) ICIs and TKIs were started within one month before or after TACE in TACE+IT group and ICIs were started within one month before or after TKIs in the IT group; 9) At least one cycle of combination therapy in each group. The exclusion criteria were as follows: 1) diffuse HCC or approximately 70% of the liver is covered by tumors; 2) history of organ transplantation; 3) the presence of severe medical comorbidities such as cardiopulmonary, renal or coagulation disorders; and 4) incomplete data.
TACE Procedure
The TACE procedure was carried out according to standard procedures4,22 and details on it had been provided in our previous studies.23 A team of interventional radiologists with over 10 years of experience conducted TACE on patients. Repeat TACE was considered and provided according to the “on demand” model: contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) was performed one to three months after the prior procedure. In the absence of vital active tumor lesion(s) on follow-up CT/MRI, TACE was discontinued and the patient received subsequent contrast-enhanced CT/MRI along with alpha-fetoprotein follow-up. In cases where contrast-enhanced CT/MRI images revealed new lesions or vital active tumor lesion(s), the patients were assessed for repeated TACE.
ICIs and TKIs Administration
All patients received sorafenib plus camrelizumab or lenvatinib plus sintinimab as combination of ICIs and TKIs. Camrelizumab or sintilimab (200 mg) every 3 weeks intravenously was applied as ICIs therapy. Sorafenib (800 mg) or lenvatinib (8 mg for patients weighing less than 60kg or 12mg for those weighing more than 60kg) orally daily was applied as TKIs therapy. ICIs and TKIs could be given in different doses and intervals according to their toxicity and disease conditions. TACE was performed while systematic therapy was suspended and resumed 3–7 days afterwards. When disease progression occurred, switch to regorafenib or apatinib treatment depending on the patient’s disease condition and wishes.
Study Outcomes
Multiphase enhanced contrast-enhanced CT/MRI was carried out before treatment and every two to three months after treatment. The primary outcome is OS, determined as the time from the beginning of initial treatment to death or last contact. Secondary outcomes in this study included PFS, objective response rate (ORR), disease control rate (DCR) in addition to adverse events (AEs). PFS was determined as the period of time between the beginning of initial treatment and the first occurrence of progressive disease (PD), death, or last follow-up. Tumor response was evaluated by two interventional radiologists with no less than ten years’ experience using the modified Response Evaluation Criteria in Solid Tumors (mRECIST),24 consisting of complete response (CR), partial response (PR), stable disease (SD) in addition to PD. ORR consisted of CR as well as PR, while DCR was calculated as CR and PR as well as SD. Treatment-related AEs were documented and evaluated in accordance with the US National Cancer Institute Common Terminology Criteria for AEs (CTCAE) version 5.0. Following up was completed on June 30, 2022. AEs were recorded by fixed follow-up visits every 3 weeks and promptly if there were serious AEs.
Statistical Analyses
A logistic regression model was used to calculate propensity scores for all patients. The covariates in the analysis included Child-Pugh class, etiology, and main portal vein invasion. The optimal match in accordance with 1:1 ratio balanced the baseline characteristics of the patients. In the case of continuous variables, means ± standard deviations are presented, whereas in the case of categorical variables, frequencies (percentages) are expressed. To test continuous variables, Student’s t-tests or Mann–Whitney U-tests were applied, whereas to test categorical variables, Fisher’s exact tests or chi-square tests were applied. The significance level was defined as a double-tailed P-value ≤0.05. Kaplan–Meier analyses were used to calculate OS and PFS, and Log rank tests were used to compare differences in OS and PFS. The variables (P ≤ 0.1) in the univariate model and the variables that might affect the outcomes of patients were included in the multivariate Cox regression model. Statistical analyses were conducted with SPSS version 26.0 software for Mac (IBM, New York, USA).
Results
Patient Characteristics
Overall, 64 advanced HCC patients were included in this study. TACE combined with ICIs plus TKIs group included 24 patients, whereas the group receiving ICIs and TKIs included 40 patients (Figure 1). An overview of patient characteristics is provided in Table 1. After matching by the optimal match method (1:1), based on the number of 24 patients receiving TACE+IT (TACE+IT group), 24 patients receiving IT (IT group) were matched for the analyses. In the PSM cohort, the baseline characteristics were comparable among the two groups. Median follow-up period was 23 months. The median number of TACE sessions per patient was 2 (range: 1–8) in the TACE+IT group. The treatment duration in the TACE+IT group was 7.65 months (Range: 1.4–18.7). The treatment duration in the IT group was 7.0 months (range: 1.0–19.2 months).
Figure 1.
Study flow.
Abbreviations: HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; ICIs, immune checkpoint inhibitors; TKIs, tyrosine kinase inhibitors.
Table 1.
Baseline Characteristics of Patients Before and After Matching on the Propensity Score
| Characteristics | Before Matching | P value | After Matching | P value | ||
|---|---|---|---|---|---|---|
| TACE+IT n=24 | IT n=40 | TACE+IT n=24 | IT n=24 | |||
| Age [years] | 58.0±10.7 | 57.8±13.1 | 0.939 | 58.0±10.7 | 56.5±14.0 | 0.678 |
| Sex | 0.926 | 1.0 | ||||
| Male | 20 (83.3) | 35 (87.5) | 20 (83.3) | 21 (87.5) | ||
| Female | 4 (16.7) | 5 (12.5) | 4 (16.7) | 3 (12.5) | ||
| EGOG PS | 0.795 | 0.558 | ||||
| 0 | 11 (45.8) | 17 (42.5) | 11 (45.8) | 9 (37.5) | ||
| 1 | 13 (54.2) | 23 (57.5) | 13 (54.2) | 15 (62.5) | ||
| Child-Pugh class | 0.667 | 0.221 | ||||
| A | 18 (75.0) | 28 (70.0) | 18 (75.0) | 14 (58.3) | ||
| B | 6 (25.0) | 12 (30.0) | 6 (25.0) | 10 (41.7) | ||
| Etiology | 0.077 | 1.0 | ||||
| Hepatitis B | 20 (83.3) | 25 (62.5) | 20 (83.3) | 20 (83.3) | ||
| Non-B | 4 (16.7) | 15 (37.5) | 4 (16.7) | 4 (16.7) | ||
| Tumor distribution | 0.435 | 0.383 | ||||
| single | 12 (50.0) | 16 (40.0) | 12 (50.0) | 9 (37.5) | ||
| Multiple | 12 (50.0) | 24 (60.0) | 12 (50.0) | 15 (62.5) | ||
| Largest tumor size [cm] | 7.6±4.0 | 9.1±4.0 | 0.165 | 7.6±4.0 | 9.2±4.0 | 0.155 |
| Extrahepatic metastasis | 0.081 | 0.247 | ||||
| Yes | 9 (37.5) | 24 (60.0) | 9 (37.5) | 13 (54.2) | ||
| No | 15 (62.5) | 16 (40.0) | 15 (62.5) | 11 (45.8) | ||
| Main portal vein invasion | 0.221 | 1.0 | ||||
| Yes | 18 (75.0) | 24 (60.0) | 18 (75.0) | 18 (75.0) | ||
| No | 6 (25.0) | 16 (40.0) | 6 (25.0) | 6 (25.0) | ||
| Laboratory tests | ||||||
| AFP [ng/mL] | 0.846 | 0.383 | ||||
| >400 | 12 (50.0) | 21 (52.5) | 12 (50.0) | 15 (62.5) | ||
| ≤400 | 12 (50.0) | 19 (47.5) | 12 (50.0) | 9 (37.5) | ||
| TBIL [umol/L] | 19.5±9.7 | 30.2±34.6 | 0.142 | 19.5±9.7 | 27.2±19.4 | 0.088 |
| AST [U/L] | 59.5±27.1 | 76.4±76.8 | 0.211 | 59.5±27.1 | 75.3±52.3 | 0.195 |
| ALT [U/L] | 45.0±36.6 | 42.3±32.0 | 0.754 | 45.0±36.6 | 46.4±23.0 | 0.876 |
| ALB [g/L] | 35.6±4.9 | 36.0±5.4 | 0.731 | 35.6±4.9 | 35.5±5.4 | 0.982 |
| Hb [g/L] | 135.2±25.1 | 124.1±21.4 | 0.064 | 135.2±25.1 | 123.8±22.4 | 0.102 |
| WBC [109/L] | 5.5±2.0 | 5.7±3.2 | 0.782 | 5.5±2.0 | 5.8±3.8 | 0.764 |
| Platelet [109/L] | 144.9±58.0 | 154.4±87.5 | 0.638 | 144.9±58.0 | 165.0±101.7 | 0.406 |
| Neutrophils [109/L] | 3.6±1.7 | 4.1±2.9 | 0.406 | 3.6±1.7 | 4.2±3.5 | 0.478 |
| Lymphocyte [109/L] | 1.3±0.6 | 1.1±0.5 | 0.173 | 1.3±0.6 | 1.2±0.4 | 0.358 |
| NLR | 3.4±2.5 | 4.2±2.9 | 0.275 | 3.4±2.5 | 3.6±2.4 | 0.745 |
| Times of IT | 5.8±3.5 | 5.8±4.0 | 0.98 | 5.8±3.5 | 5.8±3.7 | 1.0 |
Notes: All results are reported in number of patients (%) or mean with standard deviation.
Abbreviations: TACE, transcatheter arterial chemoembolization; IT, immune checkpoint inhibitors and tyrosine kinase inhibitors; ECOG PS, Eastern Cooperative Oncology Group Performance Status; AFP, alpha-fetoprotein; TBIL, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; Hb, hemoglobin; WBC, white blood cell; NLR, neutrophils/lymphocyte.
Efficacy
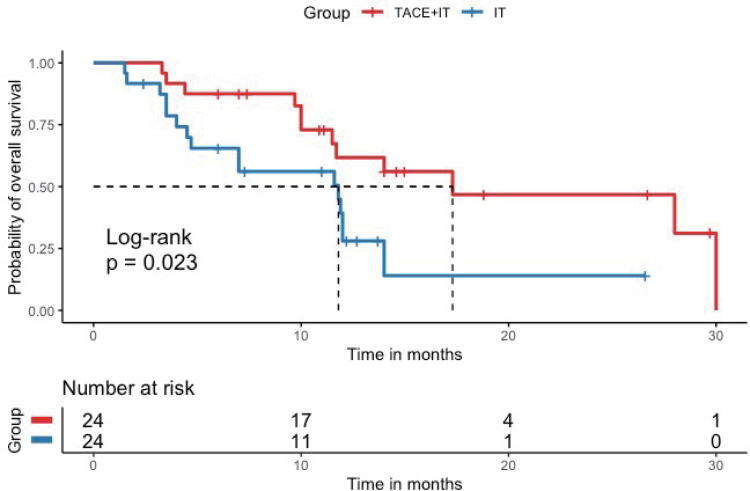
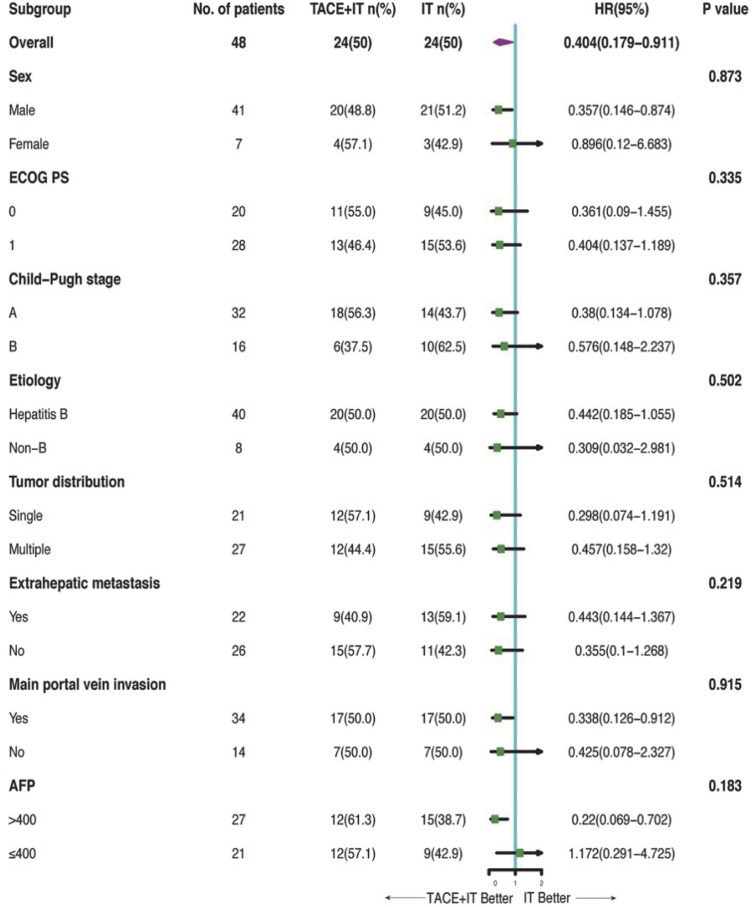
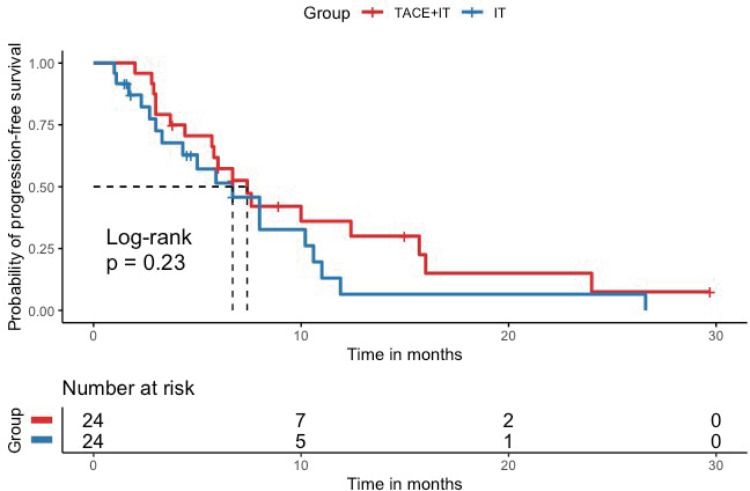
Twelve patients (50.0%) in the TACE+IT group died during the follow-up period, compared to 16 patients (66.7%) in the IT group. Median OS of the TACE+IT group was remarkably better compared to that of the IT group by the end of follow-up (17.3 vs 11.8 months; P = 0.023; Figure 2). Meanwhile, the only independent risk factor for OS was treatment modality, according to multivariable Cox regression (HR = 0.404, 95% CI = 0.179–0.911, P < 0.05) (Table 2). The median OS from last TACE was 9.4 months (range: 1.5–18.5). Figure 3 describes the subgroup analysis of OS. TACE+IT showed obvious survival benefits for OS in the following subgroups: male, main portal vein invasion, and AFP > 400 ng/mL. Nevertheless, TACE+IT did not significantly prolong median PFS compared with IT but a relatively non-significant PFS trend was observed (7.4 vs 6.7 months; P = 0.23; Figure 4).
Figure 2.
Kaplan–Meier curve for overall survival. Kaplan–Meier survival curve showing overall survival (OS) with combination of TACE and immune checkpoint inhibitors and tyrosine kinase inhibitors (TACE + IT group) compared to immune checkpoint inhibitors and tyrosine kinase inhibitors (IT group). Median OS was significantly improved in the TACE + IT group compared with the IT group.
Table 2.
Univariate and Multivariate Analysis of Risk Factors for Overall Survival
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Treatment modality (TACE+IT vs IT) | 0.404 | 0.179–0.911 | 0.029 | 0.404 | 0.179–0.911 | 0.029 |
| Age | 1.027 | 0.991–1.064 | 0.146 | |||
| Sex (female vs male) | 0.916 | 0.315–2.668 | 0.873 | |||
| ECOG PS (1 vs 0) | 1.490 | 0.662–3.354 | 0.335 | |||
| Child–Pugh class (B vs A) | 1.438 | 0.664–3.115 | 0.357 | – | ||
| Etiology (hepatitis B vs non-B) | 1.456 | 0.487–4.353 | 0.502 | – | ||
| Tumor distribution (multiple vs single) | 1.292 | 0.598–2.791 | 0.514 | |||
| Largest tumor size (per 1 point increase) | 1.049 | 0.952–1.156 | 0.334 | |||
| Extrahepatic metastasis (yes vs no) | 1.622 | 0.750–3.507 | 0.219 | – | ||
| Main portal vein invasion (yes vs no) | 0.956 | 0.418–2.188 | 0.915 | – | ||
| AFP (>400 vs ≤400) | 1.706 | 0.778–3.742 | 0.183 | |||
| TBIL | 1.011 | 0.987–1.035 | 0.378 | |||
| AST | 1.005 | 0.997–1.014 | 0.223 | |||
| ALT | 0.996 | 0.985–1.008 | 0.538 | |||
| ALB | 0.963 | 0.896–1.035 | 0.306 | |||
| Hb | 0.994 | 0.979–1.010 | 0.458 | |||
| WBC | 0.934 | 0.798–1.093 | 0.393 | |||
| Platelet | 1.001 | 0.996–1.005 | 0.770 | |||
| Neutrophils | 0.953 | 0.811–1.119 | 0.555 | |||
| Lymphocyte | 0.754 | 0.325–1.748 | 0.510 | |||
| NLR | 0.991 | 0.846–1.162 | 0.915 | |||
Abbreviations: TACE, transcatheter arterial chemoembolization; IT, immune checkpoint inhibitors and tyrosine kinase inhibitors; TACE+IT, TACE plus immune checkpoint inhibitors and tyrosine kinase inhibitors; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; AFP, alpha-fetoprotein; TBIL, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; Hb, hemoglobin; WBC, white blood cell; NLR, neutrophils/lymphocyte.
Figure 3.
Forest plot of overall survival in subgroups of patients treated with TACE plus immune checkpoint inhibitors plus tyrosine kinase inhibitors and immune checkpoint inhibitors plus tyrosine kinase inhibitors.
Abbreviations: TACE, transcatheter arterial chemoembolization; ECOG PS, Eastern Cooperative Oncology Group Performance Status; AFP, alpha-fetoprotein.
Figure 4.
Kaplan–Meier curve for progression-free survival. Kaplan–Meier survival curve showing progression-free survival (PFS) with combination of TACE and immune checkpoint inhibitors and tyrosine kinase inhibitors (TACE + IT group) compared to immune checkpoint inhibitors and tyrosine kinase inhibitors (IT group). Median PFS was significantly improved in the TACE + IT group compared with the IT group.
Table 3 shows the results of evaluating the best response in each group. One patient (4.2%) presented with CR, nine patients (37.5%) presented with PR, nine patients (37.5%) presented with SD, and five patients (20.8%) presented with PD in the TACE+IT group. Three patients (12.5%) presented with PR, 9 patients (37.5%) presented with SD, and 12 patients (50.0%) presented with PD in the IT group. Tumor response differed significantly between the two groups of patients according to mRECIST. In comparison to the IT group, the TACE+IT group had a better ORR [10 (41.7%) vs 3 (12.5%), P = 0.023]. Meanwhile, the TACE+IT group showed a better DCR [19 (79.2%) vs 12 (50.0%), P = 0.035] compared to the IT group.
Table 3.
Best Overall Response According to mRECIST
| TACE+IT Group (N=24) | IT Group (N=24) | P-value | |
|---|---|---|---|
| Best response | |||
| CR | 1 | 0 | |
| PR | 9 | 3 | |
| SD | 9 | 9 | |
| PD | 5 | 12 | |
| ORR | 10 (41.7%) | 3 (12.5%) | 0.023 |
| DCR | 19 (79.2%) | 12 (50%) | 0.035 |
Abbreviations: TACE, transcatheter arterial chemoembolization; IT, immune checkpoint inhibitors plus tyrosine kinase inhibitors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate, DCR, disease control rate; ORR=CR+PR; DCR=CR+PR+SD.
Safety
No therapy-related deaths and unexpected AEs were identified (Table 4). Common AEs related to TACE included fever (58.3%), pain (50.0%), liver dysfunction (50.0%), nausea and vomiting (20.8%), and gastrointestinal reaction (41.7%) in the TACE+IT group. Hand-foot skin reaction (41.7%) was the most prevalent AE related to ICIs plus TKIs found in the IT group, followed by hypertension (29.2%), proteinuria (25.0%) in addition to thyroid dysfunction [hypothyroidism (16.7%) and hyperthyroidism (16.7%)]. Since TACE treatment was only involved in the TACE+IT group, there was no remarkable difference in the occurrence of AEs associated with ICIs plus TKIs among the two groups, with the exception of gastrointestinal reaction (P < 0.05). The TACE+IT group included TACE treatment, so the occurrence of AEs associated with ICIs plus TKIs was not significantly different among the two group, with the exception of gastrointestinal reaction. The majority of AEs were grade 1/2 based on CTCAE 5.0, including 10 patient who were observed to experience fatigue [4 (16.7%) of IT group vs 6 (25.0%) of TACE+IT group, P > 0.05], 5 patients who developed anorexia [2 (8.3%) vs 3 (12.5%), P > 0.05], 3 patients who encountered skin rash [1 (4.2%) vs 2 (8.3%), P > 0.05], and 6 patients who occurred reactive cutaneous capillary endothelial proliferation (RCCEP) [3 (12.5%) vs 3 (12.5%), P > 0.05]. In addition, one patient of the TACE+IT group occurred hepatic abscess, which was successfully treated with a drainage tube and effective antimicrobial. One patient of the TACE+IT group encountered grade 3 immune-related hypophysitis that were responsible for discontinuation of ICIs follow-up therapy. Each patient was alleviated following conservative treatment. Each patient’s AEs alleviated after symptomatic treatment.
Table 4.
Outcomes and Adverse Events
| Adverse Events | TACE+IT Group (N=24) | IT Group (N=24) | ||
|---|---|---|---|---|
| Toxicity Grade | I/II | III/IV | I/II | III/IV |
| Liver dysfunction (transaminitis) | 11 (45.8) | 1 (4.2) | 2 (8.3) | 0 |
| Proteinuria | 6 (25.0) | 0 (0) | 6 (25.0) | 0 (0) |
| Fatigue | 6 (25.0) | 0 (0) | 4 (16.7) | 0 (0) |
| Gastrointestinal reaction | 9 (37.5) | 1 (4.2) | 2 (8.3) | 1 (4.2) |
| Anorexia | 3 (12.5) | 0 (0) | 2 (8.3) | 0 (0) |
| Hypertension | 6 (25.0) | 0 (0) | 7 (29.2) | 0 (0) |
| Hand-foot syndrome | 9 (37.5) | 1 (4.2) | 8 (33.3) | 2 (8.3) |
| Skin rash | 2 (8.3) | 0 (0) | 1 (4.2) | 0 (0) |
| Hyperthyroidism | 4 (16.7) | 0 (0) | 4 (16.7) | 0 (0) |
| Hypothyroidism | 5 (20.8) | 0 (0) | 4 (16.7) | 0 (0) |
| RCCEP | 3 (12.5) | 0 (0) | 3 (12.5) | 0 (0) |
| Liver abscess | 1 (4.2) | 0 (0) | 0 (0) | 0 (0) |
| Fever | 13 (54.2) | 1 (4.2) | 1 (2.5) | 0 (0) |
| Nausea and vomiting | 5 (20.8) | 0 (0) | 0 (0) | 0 (0) |
| Pain | 10 (41.7) | 2 (8.3) | 0 (0) | 0 (0) |
| Immune-related hypophysitis | 0 (0) | 1 (4.2) | 0 (0) | 0 (0) |
Abbreviations: TACE, transcatheter arterial chemoembolization; IT, immune checkpoint inhibitors plus tyrosine kinase inhibitors; RCCEP, reactive cutaneous capillary endothelial proliferation.
Discussion
In our study, patients in the TACE + IT group revealed remarkably prolonged OS (median, 17.3 vs 11.8 months, P = 0.023), higher ORR (41.7% vs 12.5%, P = 0.023) and DCR (79.2% vs 50.0%, P = 0.035) than the IT group, whereas a non-significant trend in PFS (median, 7.4 vs 6.7 months, P = 0.23) was observed. There were no remarkable differences in AEs associated with ICIs and TKIs between the two groups, with the exception of gastrointestinal reaction.
The phase III trials testing the combination of TACE plus systemic therapy in HCC patients of BCLC stage B are underway (LEAP-012, EMERALD-1, CheckMate 74W). TACE is not the standard of care in HCC patients of BCLC stage C according to international guidelines.2,3 However, according to the BRIDGE study, TACE is the most widely applied approach not only for intermediate but also for advanced HCC in real-world clinical practice.5 In addition, the LAUNCH trial showed that TACE combined with lenvatinib improved clinical outcomes and was a potential first-line therapy for advanced HCC.25 Few studies have compared the effectiveness and safety between TACE+IT treatment and IT treatment for advanced HCC. The study identified that TACE+IT offered a remarkable survival advantage compared to IT for advanced HCC patients. This result was linked to an improvement in median OS from 11.8 months to 17.3 months, which was probably due to the better ORR and DCR in addition to the relatively longer PFS trend in patients treated with TACE+IT but not IT. Based on the efficacy results, the triple combination of TACE+IT was potentially an effective management option for advanced HCC. It could be explained by the synergistic antitumor activity of TACE, ICIs, and TKIs. TACE results in extensive necrosis in the tumor, which may ultimately result in immunogenic cell death in HCC cells while synergizing with ICIs;26,27 The antiproliferative and antiangiogenic activities of TKIs may offset the hypoxia-induced angiogenesis following TACE, elicit antitumor immunity and boost ICIs immunity in HCC.15
Previous studies16–20,28 have evaluated the combination of TACE, ICIs, and TKIs for advanced HCC patients and revealed an OS of 23.3–24.8 months as well as a PFS of 8.5–16.3 months, which appeared to be longer compared to patients treated with TACE+IT in the present study. However, it should be noted that these studies included a large percentage (23.2–58.0%) of HCC patients with BCLC stage B, predicted to have more promising prognoses than the patients with BCLC stage C of our study. In addition, patients presenting with higher tumor burdens, including main portal vein invasion as well as extrahepatic metastasis, might also result in reduced survival benefits in our study. Based on the results of Cai et al, the combination treatment of TACE, ICIs, and TKIs provided an OS of 16.9 months as well as a PFS of 7.3 months in advanced HCC patients, which was comparable to the results of patients treated with TACE + IT in the study.29 The OS of patients in the IT group was lower than in the IMbrave150 trial7 (19.2 months), the COSMIC-312 trial10 (15.4 months), the LEAP-002 trial11 (21.2 months), and the phase III trial9 of camrelizumab plus apatinib (22.1 months), which might be explained by the fact that in our study only patients with BCLC stage C HCC were allowed to be enrolled.
In this study, the ORR of patients in the TACE+IT group was remarkably better in comparison to the PT group (41.7% vs 12.5%, P = 0.023) according to mRECIST 1.1. The ORR of patients in the TACE+IT group was outperformed or similar to the IMbrave150 trial (33.2%), the ORIENT-32 trial (24%), the phase III trial of camrelizumab plus apatinib (25.4%), and the LEAP-002 trial (26.1%), while the ORR of patients in the IT group was less than the outcomes of these trials.7–9,11 In previous studies, HCC from BCLC stages A and B in addition to stage C was included, which likely contributed to this result. However, in our study, only BCLC stage C HCC with a greater tumour burden (macrovascular invasion and/or extrahepatic metastasis) was eligible for enrolment, accompanied by poorer baseline characteristic. In addition, it might also be attributed to the different populations in those studies. Patients in the IMBrave150 trial (49%) as well as LEAP-002 trial (48.6%) had lower rates of hepatitis B than in the IT group of our study. A lower rate of patients had poor liver function in the IMbrave150 trial, the LEAP-002 trial, the III phase trial of camrelizumab plus apatinib (Child–Pugh B = 0%, 0.5%, and 0% respectively); A higher rate of participants (48%) involved in the IMbrave150 trial had undergone local treatment, 59.2% of participants involved in the III phase trial of camrelizumab plus apatinib in addition to 66% of participants involved in the ORIENT-32 trial had been treated with interventional treatment. Study revealed that poor hepatic function as well as hepatitis B-related HCC were associated with unfavorable outcomes following tumor treatment.8
In the subgroup analyses of men, main portal vein invasion, and AFP > 400 ng/mL, TACE+IT provided a higher OS whereas the other subgroups did not, which might be attributed to insufficient sample size. According to cox multivariate regression analysis, we discovered that treatment modality was the only independent risk factor that influenced patient outcome in the study. Therefore, we concluded that TACE might improve the clinically beneficial effects of these patients.
As we found in our study, the safety profile of TACE+IT and IT was manageable and tolerable. There was a greater incidence of postembolization syndrome (fever, nausea and vomiting in addition to pain), hepatic abscess, and liver dysfunction among the TACE+IT group compared to the IT group, as these AEs were probably associated with TACE. Hand-foot syndrome, hypertension, fatigue, proteinuria, and skin rash might be linked to TKIs, whereas the development of thyroid dysfunction, RCCEP in addition to immune-related hypophysitis might be linked to ICIs, and the occurrence of these AEs was largely comparable among both groups. The combination therapy might have been responsible for the gastrointestinal reaction, as there was a higher incidence among the group receiving TACE+IT. In the present study, combining PD-1 inhibitors and TKIs with/without TACE led to similar AEs as previously reported with two-drug combinations.7,8,30,31 There was no evidence of any new toxic effects or safety signals. According to these results, both TACE+IT and IT treatments were tolerable, and the combination of TACE and IT did not increase the risk of adverse events significantly over IT alone, which showed a safety profile of TACE+IT.
The main reason why camrelizumab and sintilimab were applied as PD-l inhibitors therapy in our study was that the two agents were covered by health insurance or complimentary drug policies during the time period of the patients included in this study. The financial burden on the patients was much less compared to atezolizumab in combination with bevacizumab. Furthermore, the effectiveness and safety of these two PD-1 inhibitors had been demonstrated in the first-line applications of randomized controlled trials in advanced HCC.8,30
There was a limitation to this study due to its small sample size and retrospective nature, which might reduce its statistical power. In addition, a selection bias inevitably resulted from the physician and patient determining the treatment option based on their preferences. Moreover, it was possible that our findings might be affected by the substantial heterogeneity of the patient population and treatment regimen used in this study.
In conclusion, the study identifies promising efficacy and safety of TACE+IT compared to those of IT in advanced HCC. Compared to IT, TACE+IT significantly improved OS and tumor response rate outcomes for advanced HCC. The results may have some significance for clinical practice. It is imperative that future prospective studies are conducted to determine whether such a combination therapy is efficacious and safe for advanced HCC.
Funding Statement
This study was funded by the Key R&D Program (Social Development) Project of Jiangsu Province (BE2021648).
Author Contributions
The manuscript has been read and approved by all the authors. The requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 3.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi: 10.1159/000509424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 7.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 8.Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2–3 study. Lancet Oncol. 2021;22(7):977–990. doi: 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 9.Qin S, Chan LS, Gu S, et al. LBA35-Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Annals Oncol. 2022;33(suppl_7):S808–S869. doi: 10.1016/annonc/annonc1089 [DOI] [Google Scholar]
- 10.Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, Phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6 [DOI] [PubMed] [Google Scholar]
- 11.Finn RS, Kudo M, Merle P, et al. LBA34 - Primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Annals Oncol. 2022;33(suppl_7):S808–S869. doi: 10.1016/annonc/annonc1089 [DOI] [Google Scholar]
- 12.Yue Y, Ren Z, Liu Y, Zhang Y. Changes in the frequency of myeloid-derived suppressor cells after transarterial chemoembolization with gelatin sponge microparticles for hepatocellular carcinoma. J Interv Med. 2019;2(1):21–26. doi: 10.1016/j.jimed.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JX, Chen P, Liu S, Zu QQ, Shi HB, Zhou CG. Safety and efficacy of transarterial chemoembolization and immune checkpoint inhibition with camrelizumab for treatment of unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:265–272. doi: 10.2147/JHC.S358658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiaochen M, Xiangyang S, Fubo X, et al. The influence of transarterial chemoembolization on serum levels of soluble programmed cell death ligand-1 in advanced hepatocellular carcinoma patients. Asia Pac J Clin Oncol. 2022;18(5). doi: 10.1111/ajco.13687 [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi: 10.1038/s41575-020-00395-0 [DOI] [PubMed] [Google Scholar]
- 16.Yang F, Yang J, Xiang W, et al. Safety and efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors and tyrosine kinase inhibitors for hepatocellular carcinoma. Front Oncol. 2021;11:657512. doi: 10.3389/fonc.2021.657512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao F, Yang Y, Si T, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2021;11:783480. doi: 10.3389/fonc.2021.783480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Li Z, Zhang W, et al. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol. 2021;12:709060. doi: 10.3389/fphar.2021.709060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng Y, Ding X, Li W, Sun W, Chen J. A retrospective study on therapeutic efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors plus lenvatinib in patients with unresectable hepatocellular carcinoma. Technol Cancer Res Treat. 2022;21:15330338221075174. doi: 10.1177/15330338221075174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju S, Zhou C, Yang C, et al. Apatinib plus camrelizumab with/without chemoembolization for hepatocellular carcinoma: a real-world experience of a single center. Front Oncol. 2021;11:835889. doi: 10.3389/fonc.2021.835889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Zhao M, Arai Y, et al. Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of International Society of Multidisciplinary Interventional Oncology (ISMIO). Hepatobiliary Surg Nutr. 2021;10(5):661–671. doi: 10.21037/hbsn-21-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F, Xu GL, Huang JT, et al. Transarterial chemoembolization combined with immune checkpoint inhibitors and tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: efficacy and systemic immune response. Front Immunol. 2022;13:847601. doi: 10.3389/fimmu.2022.847601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a Phase III, randomized clinical trial (LAUNCH). J Clin Oncol;2022. JCO2200392. doi: 10.1200/JCO.22.00392 [DOI] [PubMed] [Google Scholar]
- 26.Cheu JW, Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. 2021;74(4):2264–2276. doi: 10.1002/hep.31840 [DOI] [PubMed] [Google Scholar]
- 27.Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolization in hepatocellular carcinoma. Int J Mol Sci. 2020;21(21). doi: 10.3390/ijms21218165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng L, Fang S, Wu F, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced TACE-refractory hepatocellular carcinoma: a retrospective study. Front Mol Biosci. 2020;7:609322. doi: 10.3389/fmolb.2020.609322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 Inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi: 10.3389/fimmu.2022.848387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi: 10.1158/1078-0432.CCR-20-2571 [DOI] [PubMed] [Google Scholar]
- 31.Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi: 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]