Abstract
Objective:
To characterize cardiovascular (CV) risk factors and outcomes among incident cases of systemic sclerosis (SSc) in a population-based cohort.
Methods:
Medical records of patients diagnosed with SSc in Olmsted County, MN, between January 1, 1980 and December 31, 2016 were reviewed to identify 78 incident SSc cases. The comparators were 156 sex- and age-matched individuals from the same population. Data on SSc characteristics, traditional CV risk factors, and CV events were collected. Cumulative incidence was adjusted for the competing risk of death.
Results:
During a median follow up of 9.8 (SSc) and 9.2 years (non-SSc), 21 SSc and 17 non-SSc patients developed CV events, corresponding to 10-year cumulative incidence of 22.4% (SSc) and 12.1% (non-SSc). The risk of incident CV disease was increased by 3 fold (HR:2.97; 95% CI:1.56–5.66) in SSc patients vs. comparators, predominately due to coronary artery disease (HR:2.35; 95% CI:1.17–4.71). The mean body mass index and prevalence of diabetes mellitus were lower in SSc vs. non-SSc. There was no significant difference in smoking, hypertension or hyperlipidemia. Observed CV events were increased compared to CV events predicted by the Framingham Risk Score (FRS) and American College of Cardiology/American Heart Association (ACC/AHA) scores with standardized incident ratios of 4.16 (95% CI: 2.16–7.99) and 5.69 (95% CI:2.71–11.94), respectively.
Conclusion:
Patients with SSc are at a three-fold increased risk of experiencing a CV event compared to persons without SSc. FRS and ACC/AHA scores dramatically underestimate CV risk in SSc.
Keywords: systemic sclerosis, scleroderma, cardiovascular risk, cardiovascular events, cardiovascular risk scores, cardiovascular disease, heart disease, cardio-rheumatology
Introduction
Systemic Sclerosis (SSc) is a complex systemic chronic inflammatory autoimmune disease characterized by vasculopathy, widespread fibrosis of the skin and visceral organs, and evidence of immune system activation.[1, 2] Involvement of the microvasculature, clinically mirrored by Raynaud’s phenomenon is one of the earliest features of SSc. Pathological changes include endothelial disruption, mononuclear cell infiltration of the vessel wall, frank obliterative lesions, and progressive loss of capillaries. These microvascular abnormalities, along with chronic inflammation, circulating autoantibodies and pro-inflammatory cytokines also contribute to the pathogenesis of pulmonary arterial hypertension (PAH), scleroderma renal crisis, and digital ulceration in SSc.
The heart is one of the major organs affected in SSc,[3],[4] although its presence is probably underestimated due to the occult nature and variable reports of prevalence based on the definition employed. Clinically evident cardiac involvement is associated with a poor prognosis, with up to 70% mortality reported at 5 years.[3, 4] Approximately 25% of SSc-related fatalities are attributable to cardiac causes.[5],[6] Cardiac involvement typically manifests as myocardial fibrosis[7] and myocarditis.[8] These patients can often have concurrent pericarditis, significant valvular abnormalities and/or life-threatening arrhythmias. The time-course, dynamics and extent of each of these pathologies, however, are poorly understood. In the absence of screening or treatment guidelines, management of SSc–cardiomyopathy is varied and often in response to a clinical event when prognosis is particularly poor.
Cardiovascular disease (CVD), including coronary artery disease (CAD), cerebrovascular events (stroke and transient ischemic attack) and peripheral vascular disease (PVD), is a leading cause of morbidity and mortality worldwide and responsible for 17% of the US healthcare expenditure.[9] Traditional risk factors for CVD include age, sex, hypertension, diabetes mellitus, hyperlipidemia and smoking.[10] Chronic inflammation is also a risk factor for CVD. Indeed, patients with chronic inflammatory diseases, such as rheumatoid arthritis (RA),[11] systemic lupus erythematosus (SLE), ankylosing spondylitis,[12] sarcoidosis,[13] and idiopathic inflammatory myopathies (IIM), [14] experience a higher incidence of premature atherosclerosis.
Cardiovascular (CV) risk scores including the American College of Cardiology (ACC) / American Heart Association (AHA) and Framingham risk scores (FRS) often underestimate CV risk in patients with RA, SLE and sarcoidosis.[15–18] It remains unclear how these scores perform in estimation of CV risk in patients with SSc. The goal of our study was to establish the prevalence of traditional CV risk factors among a population-based cohort of patients with SSc, estimate their CV risk through traditional scoring systems and compare these to observed incidence of atherosclerotic CV events during follow up. We also compared the risk factors and CV events to comparators with similar age and sex from the same geographic area.
Methods
The resources of the Rochester Epidemiology Project (REP) were used to identify potential cases of SSc, using diagnosis codes related to SSc, allowing identification of essentially all clinically recognized cases of SSc in Olmsted County, Minnesota, USA from Jan 1, 1980 to Dec 31, 2016. The REP is well suited to investigate the epidemiology of CVD in SSc because comprehensive medical records for all residents in Olmsted County, MN, seeking medical care are available through a medical record linkage system.[19, 20] The study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center. Olmsted County residents with incident SSc in 1980–2010 were previously identified.[21] To extend this cohort, medical records of patients with a diagnosis or suspicion of SSc in 2011–2016 were reviewed to identify additional incident cases of physician diagnosed SSc. Fulfillment of the 1980 and 2013 American College of Rheumatology (ACR)/European League against Rheumatism (EULAR) Classification criteria for SSc was ascertained. [22]
The index date for cases was defined as the first date of physician diagnosis of SSc. Prevalent cases with SSc prior to residency in Olmsted County were not included in the incidence cohort. For each patient with SSc, two comparators without SSc of the same age (±1 year) and sex were randomly selected from all Olmsted County residents in the index year. Each non-SSc subject was assigned an index date corresponding to the SSc diagnosis date of the matched case. The medical records of cases and comparators were manually reviewed to abstract data on traditional CV risk factors collected at index date (closest to index date within 12 months prior to 3 months after index date): age (years), sex, systolic and diastolic blood pressure (mmHg), antihypertensive treatment, anti-lipemic treatment cigarette smoking status and diabetes mellitus. Body mass index (BMI) was calculated and obesity was defined as BMI ≥30 kg/m2. Laboratory test results, including SSc-specific antibodies, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides were collected. Family history of premature CV events was collected based on physician-documented or patient-provided data in the medical record. Physician diagnosed incident CV events (i.e., myocardial infarction (MI), angina, cardiovascular death, congestive heart failure (CHF), peripheral vascular disease (PVD) including peripheral arterial disease (PAD) and abdominal aortic aneurysm (AAA)) and cerebrovascular accidents (CVA) (including transient ischemic attack (TIA), ischemic and hemorrhagic stroke) before and after index date was abstracted.
The 10 year general FRS for cardiovascular disease was calculated and the office-based 10 year FRS, which does not include laboratory values, was used when lipid values were unavailable.[23]
The ACC/AHA atherosclerotic CVD (ASCVD) pooled risk score was also calculated using the ACC/AHA risk calculator.[24] Follow-up was continued until death, migration from Olmsted County or June 30, 2017.
Statistical analysis
Descriptive statistics (percentages, mean, etc.) were used to summarize patient characteristics for each cohort. Baseline comparisons between cohorts were performed using Chi-square and rank sum tests. The prevalence of prior CVD overall and by type was compared between cohorts using Fisher’s exact tests. Excluding subjects with prior CVD, the cumulative incidence adjusted for the competing risk of death was estimated for CVD overall and by type.[25] For overall CVD, the first date of occurrence of the any of the individual CVD events was used. Cause-specific Cox proportional hazards models were used to compare the development of CVD during follow-up between the SSc and the non-SSc. These were also used to evaluate the association of age and sex on the development of CVD events among patients with SSc.
The FRS and ACC/AHA risk scores were converted to the predicted number of CVD events and the predicted number of events was adjusted proportionately for patients with <10 years of follow-up to ensure comparability between the observed and expected events. In addition, the observed CVD events were defined in accordance with the methods used in the development of each risk score. The ratio of the observed to predicted number of CVD events is referred to as the standardized incidence ratio (SIR). For each SIR, 95% confidence intervals (CI) were calculated by assuming that the observed rates followed a Poisson distribution and the expected rates were fixed and. An SIR of >1 indicated the predicted risk underestimated the observed risk and an SIR of <1 indicated that the predicted risk overestimated the observed risk. Some of these methods have been described previously.[26] For all comparisons, a p-value of less than 0.05 was considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the 78 incident SSc cases and 156 age and sex-matched non-SSc comparators at SSc diagnosis/index date are displayed in table 1. The mean (± SD) age in both cohorts was 56 ± 16 years at diagnosis or index date, respectively. In both cohorts, 90% were women, 87% SSc and 93% non-SSc patients were Caucasian. Mean (± SD) BMI was significantly lower in SSc (26.5 ± 5.9 kg/m2) patients than non-SSc comparators (29.4 ± 7.6 kg/m2; P=.004). Diabetes mellitus was also less common in SSc than non-SSc (3% vs. 12%, P=.015). There were no differences between the groups in the presence of other CV risk factors such as smoking, hypertension or hyperlipidemia at index date.
Table 1.
Clinical characteristics of 78 incident systemic sclerosis (SSc) cohort patients and 156 non-SSc comparators in Olmsted County (1980– 2016) at Baseline
| Characteristic | SSc (N=78) | Non-SSc (N=156) | p value |
|---|---|---|---|
| Age at diagnosis/index, years, mean (SD) | 56.1 (15.7) | 56.0 (15.6) | .97 |
| Female Sex | 71 (91%) | 142 (91%) | .99 |
| Caucasian | 68 (88%) | 147 (95%) | .24 |
| Length of follow-up, years, mean (SD) | 11.2 (8.7) | 11.4 (7.6) | -- |
| Body mass index, kg/m2, mean (SD) | 26.5 (5.9) | 29.4 (7.6) | .004 |
| Obesity (BMI≥30 kg/m2) | 15 (21%) | 61 (39%) | .008 |
| Smoking Status | .54 | ||
| Never smoked | 41 (53%) | 84 (55%) | |
| Former smoker | 24 (31%) | 38 (25%) | |
| Current smoker | 12 (16%) | 30 (20%) | |
| Prior diabetes mellitus | 2 (3%) | 19 (12%) | .015 |
| Prior hypertension | 30 (38%) | 57 (37%) | .77 |
| Anti-hypertensive use | 24 (31%) | 52 (33%) | .69 |
| Prior Dyslipidemia | 27 (35%) | 55 (35%) | .92 |
| Antilipemic use | 22 (28%) | 34 (22%) | .28 |
| Aspirin use | 13 (17%) | 38 (24%) | .18 |
| Fulfill 2013 ACR Criteria | 63 (81%) | -- | |
| Skin involvement | |||
| Limited cutaneous | 63 (81%) | -- | |
| Diffuse cutaneous | 11 (14%) | -- | |
| Sine scleroderma | 2 (3%) | -- | |
| Telangiectasias | 39 (50%) | -- | |
| Calcinosis | 18/75 (24%) | -- | |
| Interstitial Lung Disease | 7 (9%) | -- | |
| Pulmonary Arterial Hypertension | 8 (10%) | -- | |
| Inflammatory arthritis | 36/74 (49%) | -- | |
| Myositis | 11/75 (15%) | -- | |
| Scleroderma Renal Crisis | 6/73 (8%) | -- | |
| Gastrointestinal involvement | |||
| GERD | 58/76 (76%) | -- | |
| GI dysmotility | 38/75 (51%) | -- | |
| Chronic Intestinal Pseudo-obstruction | 6/75 (8%) | -- | |
| Gastric Antral Vascular Ectasia | 5/74 (7%) | -- | |
| Positive Anti-Nuclear Antibodies (ANA) | 70/75 (93%) | -- | |
| SSc specific Antibodies | 38/75 (51%) | -- | |
| Scl-70+ | 7/35 (20%) | -- | |
| Centromere + | 29/35 (83%) | -- | |
| RNA Pol III+ | 2/35 (6%) | -- |
Before SSc diagnosis or index date, there was no difference in the prevalence of any CV events (15 SSc vs. 22 non-SSc; P=.17; table 2). The cohorts were followed up for a median of 9.8 and 9.2 years in the SSc and non-SSc cohort, respectively, corresponding to 874 and 1,778 person-years of observation. During this time-period, 21 SSc and 17 non-SSc patients developed CV events, corresponding to 10-year cumulative incidence of 22.4% among SSc and 12.1% among non-SSc (figure 1). This represented a three-fold increased risk (hazard ratio [HR]: 2.97; 95% confidence interval [CI]: 1.56–5.66) in SSc vs non-SSc patients (table 3). This increased risk was predominately due to coronary artery disease (CAD) including MI and angina (HR: 2.35; 95% CI: 1.17–4.71). PVD risk, including PAD or AAA, also appeared to be increased in SSc compared to non-SSc (HR: 3.88; 95% CI: 0.91–16.55). Congestive heart failure (CHF) was diagnosed in 8 SSc-cases and in only 2 comparators prior to diagnosis/ index date (P=.003). After SSc diagnosis or index date heart failure was diagnosed in 10 SSc cases and in12 non-SSc comparators respectively. Leading to a cumulative incident after diagnosis of 12.8 events in SSc per 10 years and 7.2 in comparators respectively (HR: 2.10; 95% CI: 0.90–4.89). Only 5 SSc-cases and 3 comparators developed PVD during the follow-up period. The possible increased risk of cerebrovascular events also did not reach statistical significance due to the small number of events (HR: 1.66; 95% CI: 0.65–4.25).
Table 2.
Cardiovascular disease at baseline, preexistent to SSc diagnosis/index date
| SSc (n=78) | Non-SSc (n=156) | p-value | |
|---|---|---|---|
|
| |||
| Any CVD (CAD, CHF, CVE, PVD) | 15 (19%) | 23 (15%) | .45 |
|
| |||
| CAD | 11 (14%) | 17 (11%) | .52 |
| Myocardial Infarction | 2 (3%) | 3 (2%) | .99 |
| Angina | 3 (4%) | 11 (7%) | .40 |
|
| |||
| CHF | 8 (10%) | 2 (1%) | .003 |
|
| |||
| CVE (Stroke or TIA) | 1 (1%) | 6 (4%) | .43 |
|
| |||
| PVD (PAD or AAA) | 0 (0%) | 5 (3%) | .17 |
AAA: Abdominal aortic aneurysm; CAD: Coronary artery disease; CHF: Congestive heart failure; CI: Confidence Interval; CVD: Cardiovascular disease; CVE: Cerebrovascular event (Stroke or TIA); PAD: Peripheral artery disease; PVD: Peripheral vascular disease; SSc: Systemic Sclerosis; TIA: Transient ischemic attack.
Figure 1.
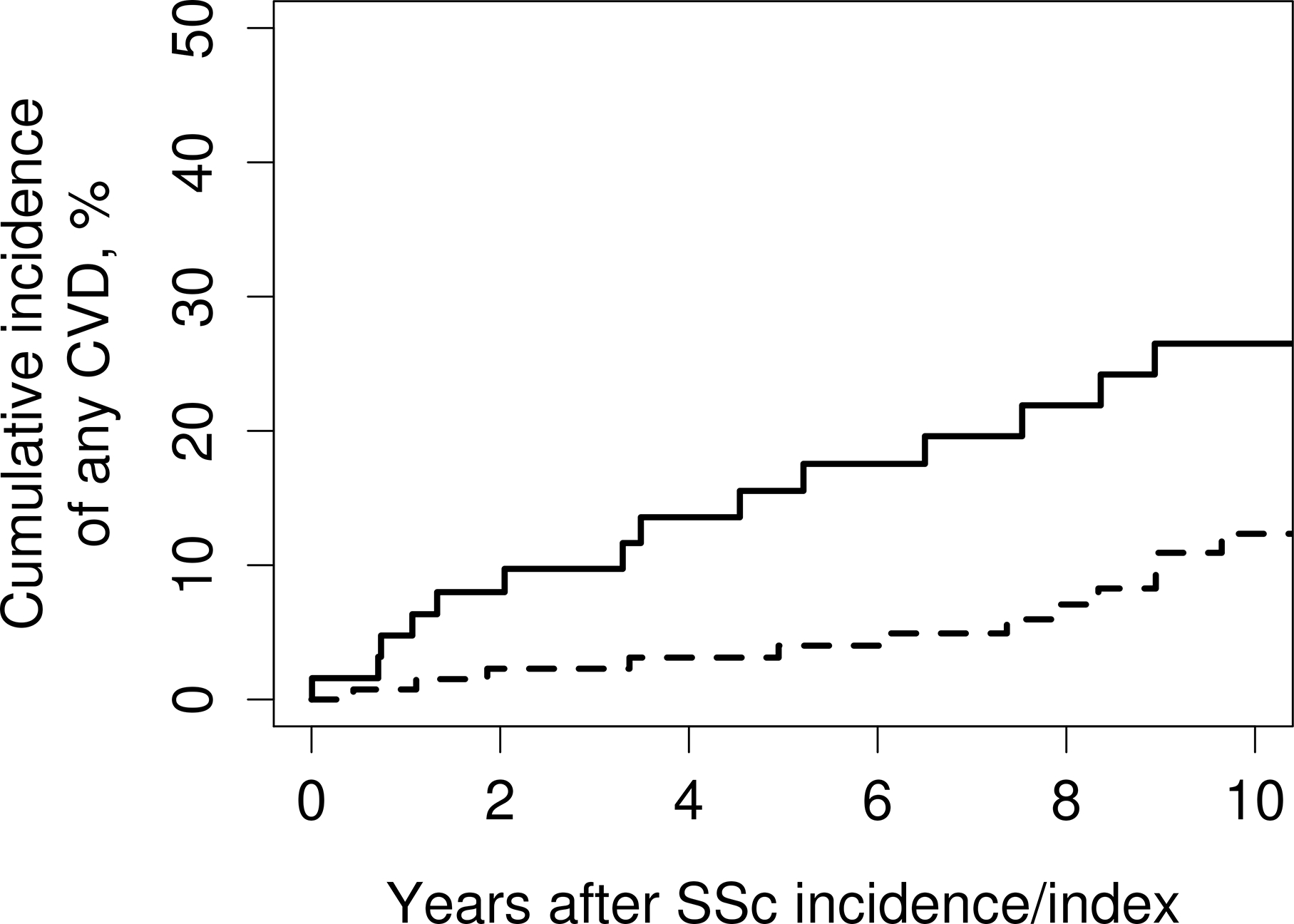
Cumulative incidence of cardiovascular disease after systemic sclerosis diagnosis/index date among patients with SSc (solid line) and non-SSc comparators (dashed line). CVD: Cardiovascular disease; SSc: Systemic sclerosis.
Table 3.
Cardiovascular events following SSc diagnosis:
| Number of events | Cumulative incidence at 10 years, % (95% CI)a | Hazard ratio (95% CI)b | Hazard ratio (95% CI)c | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Outcome | SSc | Non-SSc | SSc | Non-SSc | ||
|
| ||||||
| Any CVD | 21 | 20 | 24.4 (15.4– 38.7) | 15.2 (9.4– 24.6) | 2.38 (1.28– 4.43) | 2.66 (1.39– 5.11) |
|
| ||||||
| CAD | 18 | 15 | 19.6 (11.7– 32.6) | 9.2 (5.0– 17.0) | 2.35 (1.17– 4.71) | 2.60 (1.25– 5.41) |
| MI | 7 | 5 | 5.9 (2.2– 15.3) | 3.9 (1.6– 9.5) | 3.14 (0.97– 10.14) | 4.88 (1.21– 19.72) |
| Angina | 7 | 6 | 8.8 (4.1– 19.0) | 4.9 (2.0– 11.8) | 2.37 (0.79– 7.10) | 2.11 (0.68– 6.56) |
|
| ||||||
| CHF | 10 | 12 | 12.8 (6.7–24.6) | 7.2 (3.8– 13.6) | 2.10 (0.90– 4.89) | 3.60 (1.34– 9.71) |
|
| ||||||
| Cerebrovascular (Stroke or TIA) | 8 | 10 | 7.6 (3.3– 17.7) | 6.6 (3.3– 13.1) | 1.66 (0.65– 4.25) | 1.92 (0.69– 5.32) |
|
| ||||||
| PVD (PAD or AAA) | 5 | 3 | 5.7 (2.2– 15.0) | 1.9 (0.5– 7.7) | 3.88 (0.91– 16.55) | 3.72 (0.81– 17.11) |
Cumulative incidence is adjusted for the competing risk of death
adjusted for age, sex and calendar year of SSc/index date
adjusted for age, sex and calendar year of SSc/index date, smoking status, hypertension, diabetes mellitus and dyslipidemia
AAA: Abdominal aortic aneurysm; CAD: Coronary artery disease; CHF: Congestive heart failure; CI: Confidence Interval; CVD: Cardiovascular Disease; MI: Myocardial Infarction; PAD: Peripheral artery disease; PVD: Peripheral vascular disease; SSc: Systemic Sclerosis; TIA: Transient ischemic attack.
After adjusting for traditional cv risk factors, such as smoking status, hypertension, diabetes mellitus and dyslipidemia, cardiovascular events following SSc diagnosis remained significant with a 3-fold increased risk (HR: 2.66; 95% CI: 1.39– 5.11). Risk for CAD, including MI and angina increased slightly after CV risk adjustment (HR: 2.60; 95% CI 1.25–5.41). Risk for CHF after adjusting for CV risk factors reached significance with an about 3 fold increased risk in SSc compared to Non-SSc (HR: 3.60; 95% CI; 1.34– 9.71). Other outcomes such as cerebrovascular events (Stroke and TIA) and PVD remained unchanged and did not reach statistical significance (HR: 1.92; 95% CI: 0.69– 5.32, and HR: 3.72; 95% CI 0.81– 17.11), respectively.
To apply the FRS in our cohort of 78 SSc patients, we excluded cases out of the FRS age limits (30–74 years) leaving us with 57 patients. Seven patients with prior CVD (MI, cardiovascular death, angina, stroke, intermittent claudication or heart failure) were excluded. Therefore, data was available in 44 of 50 cases. The mean FRS for SSc cases was 7.1% (SD: 5.2%). Among SSc cases 2.2 CV events were predicted and 9 CV events were observed, resulting in an SIR of 4.16 (95% CI: 2.16–7.99). The ACC/AHA risk score was designed for subjects age 40 to 79 years, so only subjects in this age range were included. Two patients with prior MI or stroke were excluded. Because of missing data on lipids, the ACC/AHA risk score was calculated in 22 out of the remaining 42 SSc cases. The mean ACC/AHA risk score was 8.9% (SD 7.8%). The predicted number of CV events was 1.2 in this SSc cohort; 7 CV events were actually observed, corresponding to an SIR of 5.69 (95% CI: 2.71–11.94; table 4).
Table 4.
Comparison of observed and predicted 10 year cardiovascular risk using ACC/AHA ASCVD pooled equation and Framingham Risk Score
| N | Observed CV events | Predicted CV events | Standardized incidence ratio | p-value | |
|---|---|---|---|---|---|
| AHA/ACC ASCVD Risk Score | 22 | 7 | 1.2 | 5.69 (2.71, 11.94) | <.001 |
| Framingham Risk Score | 44 | 9 | 2.2 | 4.16 (2.16, 7.99) | <.001 |
ACC: American College of Cardiology; AHA: American Heart Association; ASCVD: Atherosclerotic Cardiovascular Disease
In this study, 90% were women, similar to the general sex prevalence of SSc. Due to the small numbers of men; sex-specific analyses were not warranted.
Discussion
In the current population-based cohort study, incident CV events among SSc patients were three-fold higher compared to non-SSc comparators, with the risk of MI being greater than the risk for AAA or PVD. Despite adjustment for traditional risk factors, these associations persisted. Patients with SSc are expected to have an elevated atherosclerotic burden and potentially higher risk for associated cardiac events by virtue of the inflammatory nature of the disease, the associated vasculopathy and the significant endothelial and microvascular dysfunction that characterizes SSc. To date, only limited data are available to evaluate the atherosclerotic risk related to SSc.
While microcirculatory dysfunction is key in SSc, macrovascular pathology was not originally considered a feature of this disease. Several recent studies have revealed an increased prevalence of large-vessel disease of the extremities in patients with SSc.[27, 28] In a systematic review and meta-analysis of observational studies, Ungprasert et al reported a pooled risk ratio of 1.82 (95 % CI: 1.40–2.36) for CAD in patients with SSc compared to non-SSc comparators, although only 4 studies could be included for analysis.[29] Large population-based cohort studies of the incidence and prevalence of CV events in SSc relative to the general population are lacking. Our study findings are in general agreement with other population-based studies about CV risk in SSc. A population-based cohort study using a UK primary care database of 865 SSc individuals found a 2-fold increased CAD risk compared to non-SSc comparators.[30] A cross-sectional study from the Australian Scleroderma Cohort Study comparing 850 SSc patients to non-SSc comparators showed a three times higher prevalence of coronary heart disease,[31] although this study relied on self-reported CAD.
In the past decades, mortality related to direct organ involvement in chronic autoimmune disease declined due to improvement in medical therapy. Therefore, patients with chronic inflammatory rheumatic diseases (CID) have longer survival but experience more CV related disease.[29] The burden of CVD in CID is well understood, but mostly studied in RA and SLE,[32, 33] where studies in RA and SLE report a 1.5 to 5-fold increased risk of incident CVD compared to a non-CID population.[14, 34]
Using a population-based cohort with expansive medical record review, our study shows an increased incidence of CVD occurrence among patients with SSc. CV involvement in SSc remains under explored, but can affect a wide variety of cardiac structures, such as coronary arteries, pericardium, myocardium, valves and conduction system. Chronic inflammation is known to contribute to accelerated atherogenesis by activating inflammatory cytokines, oxidative stress, and leucocytes, and causing vascular injury and endothelial dysfunction, as well as increase intravascular coagulation.[35, 36] Similar to other autoimmune diseases, like SLE and RA, chronic inflammation is probably a key driver for accelerated atherosclerotic cardiovascular disease (ASCVD) in SSc patients. Nevertheless, SSc is not a classic inflammatory condition and response to treatment is not tracked by inflammatory markers as in majority of the other inflammatory rheumatic conditions. Additionally, microvascular disease and endothelial dysfunction are central pathomechanisms in SSc, but up until now not definitively a proven cause or driver for ischemic heart disease. In our cohort, patients with incident SSc have a significantly lower BMI and prevalence of DM than non-SSc comparators. Differences in BMI might be driven by gastrointestinal (GI) involvement in SSc patients, as has been reported previously.[37, 38] Nevertheless, specific investigations regarding GI symptoms were not evaluated in this setting.
FRS and ACC/AHA risk scores have been developed to guide the clinician in appropriate risk factor modification to reduce an individual’s likelihood of an atherosclerotic event. The more current ASCVD risk algorithm is primarily utilized to determine the benefit of statin medications to reduce CVD risk in the general population. The present study is the first population-based study to assess the performance of the ACC/AHA and FRS risk scores in patients with SSc. Both risk scores underestimated the risk of CVD events by 4 to 5-fold. These findings suggest that the poor performance of these risk scores extends beyond RA and SLE to other chronic inflammatory disorders. Chronic inflammation, which is not accounted for by either risk score, could explain the underestimation of CVD risk in these patient groups.
While C-reactive protein (CRP) is considered a surrogate marker for inflammation, it is often normal in patients with SSc and hence not a reliable indicator of disease activity in this condition. Moreover, its addition to these risk scores has also not demonstrated any significant improvement in their performance for predicting CVD in the general population.[39] It thus seems doubtful that CRP would add any value to the risk calculators for CVD risk prediction in SSc in SSc.[40]
Immunosuppressive medications such as glucocorticoids that are often used in SSc patients and may also affect CVD risk. These medications are also not accounted for in the risk scores built for use in the general population.
In comparison to other CID, such as RA or SLE, our results show a high and comparable risk of incident CVD.
The major strengths of our study include the use of a population-based cohort, followed over decades, using a comprehensive record-linkage system that allows capturing every clinically relevant case of SSc in the community ranging from mild to severe, reflecting the real spectrum of the disease, unlike referral cohorts. This approach reduces referral bias and allows including a wide spectrum of the disease. By manually reviewing every medical record, we were able to confirm diagnosis and minimize the eventuality of disease misdiagnosis, which is a major concern in studies which are based on administrative claims data. The relatively long follow up in our cohort is important, as it may take several years to develop a CV event after diagnosis/index date.
The limitations of our study are its retrospective study design and limited numbers of participants. Clinical information was not prospectively collected. The analysis did not account for several potential CV confounders such as nonsteroidal anti-inflammatory drugs (NSAID), glucocorticoids, disease-modifying antirheumatic drugs (DMARDs), depression, and access to healthcare or socioeconomic status. Surveillance bias might play a major role in SSc, because these patients are usually closely monitored for pulmonary hypertension, and therefore undergo more frequent medical examinations and laboratory testing. This study was conducted in Olmsted County, Minnesota, USA where the population is primarily of Northern European heritage, with a high level of health-care employees and highly educated people, which can affect the use of health system and detection of SSc. The generalizability of this study to other ethnic cohorts might vary.[41] An additional factor which might hinder the correct CVD prediction in SSc is the high female predominance in CID in general. Sex differences in CV risk factors and CV outcome, independent of underlying CID, have been thoroughly studied in population-based cohorts.[42] [43] However, the impact of differences based on sex on traditional CV risk factors in SSc patients is unknown. The small sample size in our cohort limited statistical power for some comparisons, but the effect sizes were large enough to reach statistical significance for most of the results. Nevertheless, the major difference between predicted and observed CVD are highly important findings that need to be addressed with further studies in larger population based cohorts with longer follow up.
Conclusion
The increased rate of CV events in SSc patients despite the absence of traditional CV risk factors warrants a higher vigilance for CVD in patients with SSc. It may potentially be related to the increased risk of endothelial dysfunction, microvascular injury and chronic inflammation characteristic of the disease, but warrants further detailed study into the pathogenic mechanisms. SSc patients should be counseled and educated about this higher risk. We suggest close follow up for optimal control of disease activity, and early identification and treatment of modifiable risk factors. Screening for subclinical cardiac involvement may help provide an opportunity for early intervention, which is key for optimal outcomes. Further, prospective studies on larger SSc cohorts are needed to better understand CV risk and cardiovascular involvement in SSc patients better, although the best screening modality and frequency are however, yet unclear and also warrant further investigation.
Acknowledgments
Funding Statement
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health. The study was also supported by the John M. Nasseff, Sr. Clinician Career Development Award in Rheumatology to Dr. Makol and Mayo Clinic CTSA through grant number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Kurmann received funding from Research Funds of the Department of Rheumatology and Clinical Immunology, University of Bern, Switzerland
Abbreviations
- AAA
Abdominal aortic aneurysm
- ACR
American College of Rheumatology
- BMI
Body mass index
- CAD
Coronary artery disease
- CHF
Congestive heart failure
- CI
Confidence Interval
- CID
Chronic inflammatory diseases
- CV
Cardiovascular
- CVD
Cardiovascular Disease
- DMARDs
Disease-modifying anti-rheumatic drugs
- EULAR
European League against Rheumatism
- GI
Gastrointestinal
- HDL
High-density lipoprotein
- HR
Hazard ratio
- LDL
Low-density lipoprotein
- MI
Myocardial Infarction
- NSAIDs
Non-steroidal anti-inflammatory drugs
- PAD
Peripheral artery disease
- PVD
Peripheral vascular disease
- SSc
Systemic Sclerosis
- TIA
Transient ischemic attack
Footnotes
The above study was presented at the American College of Rheumatology 2018 National Meeting in Chicago, IL (Sandhu AS, Kurmann R, Crowson CS, Mankad R, Matteson EL, Osborn T, Warrington KJ, Makol A. Cardiovascular (CV) Risk Factors and Atherosclerotic CV Events Among Incident Cases of Systemic Sclerosis: Results from a Population Based Cohort (1980–2016) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 10).)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Abraham DJ, Krieg T, Distler J, Distler O: Overview of pathogenesis of systemic sclerosis. Rheumatology (Oxford, England) 2009, 48 Suppl 3:iii3–7. [DOI] [PubMed] [Google Scholar]
- 2.Campbell PM, LeRoy EC: Pathogenesis of systemic sclerosis: a vascular hypothesis. Seminars in arthritis and rheumatism 1975, 4(4):351–368. [DOI] [PubMed] [Google Scholar]
- 3.Ashida R, Ihn H, Mimura Y, Jinnin M, Asano Y, Kubo M, Tamaki K: Clinical and laboratory features of Japanese patients with scleroderma and telangiectasia. Clinical and experimental dermatology 2009, 34(7):781–783. [DOI] [PubMed] [Google Scholar]
- 4.Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La Montagna G, Bullo A, Cazzato M, Tirri E, Storino F et al. : Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine 2002, 81(2):139–153. [DOI] [PubMed] [Google Scholar]
- 5.Hachulla E, Carpentier P, Gressin V, Diot E, Allanore Y, Sibilia J, Launay D, Mouthon L, Jego P, Cabane J et al. : Risk factors for death and the 3-year survival of patients with systemic sclerosis: the French ItinérAIR-Sclérodermie study. Rheumatology (Oxford, England) 2009, 48(3):304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, Bancel DF, Allanore Y, Muller-Ladner U, Distler O et al. : Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Annals of the rheumatic diseases 2010, 69(10):1809–1815. [DOI] [PubMed] [Google Scholar]
- 7.Bulkley BH, Ridolfi RL, Salyer WR, Hutchins GM: Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction. Circulation 1976, 53(3):483–490. [DOI] [PubMed] [Google Scholar]
- 8.Liangos O, Neure L, Kühl U, Pauschinger M, Sieper J, Distler A, Schwimmbeck PL, Braun J: The possible role of myocardial biopsy in systemic sclerosis. Rheumatology 2000, 39(6):674–679. [DOI] [PubMed] [Google Scholar]
- 9.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A et al. : Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011, 123(8):933–944. [DOI] [PubMed] [Google Scholar]
- 10.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM: Lifetime Risks of Cardiovascular Disease. New England Journal of Medicine 2012, 366(4):321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE: Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis & Rheumatism 2005, 52(3):722–732. [DOI] [PubMed] [Google Scholar]
- 12.Verma I, Krishan P, Syngle A: Predictors of Atherosclerosis in Ankylosing Spondylitis. Rheumatology and therapy 2015, 2(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ungprasert P, Matteson EL, Crowson CS: Increased Risk of Multimorbidity in Patients With Sarcoidosis: A Population-Based Cohort Study 1976 to 2013. Mayo Clinic proceedings 2017, 92(12):1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ungprasert P, Suksaranjit P, Spanuchart I, Leeaphorn N, Permpalung N: Risk of coronary artery disease in patients with idiopathic inflammatory myopathies: a systematic review and meta-analysis of observational studies. Seminars in arthritis and rheumatism 2014, 44(1):63–67. [DOI] [PubMed] [Google Scholar]
- 15.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE: Usefulness of Risk Scores to Estimate the Risk of Cardiovascular Disease in Patients With Rheumatoid Arthritis. The American Journal of Cardiology 2012, 110(3):420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg RJ, Urowitz MB, Ibanez D, Nikpour M, Gladman DD: Risk factors for development of coronary artery disease in women with systemic lupus erythematosus. The Journal of rheumatology 2009, 36(11):2454–2461. [DOI] [PubMed] [Google Scholar]
- 17.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Cote R, Grover SA, Fortin PR, Clarke AE et al. : Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis and rheumatism 2001, 44(10):2331–2337. [DOI] [PubMed] [Google Scholar]
- 18.Ungprasert P, Crowson CS, Matteson EL: Risk of cardiovascular disease among patients with sarcoidosis: a population-based retrospective cohort study, 1976–2013. The European respiratory journal 2017, 49(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA: Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. American journal of epidemiology 2011, 173(9):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd: History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings 2012, 87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer PR, Schiavo DN, Osborn TG, Levin DL, St Sauver J, Hanson AC, Schroeder DR, Ryu JH: Influence of interstitial lung disease on outcome in systemic sclerosis: a population-based historical cohort study. Chest 2013, 144(2):571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA Jr., Carreira PE et al. : 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Annals of the rheumatic diseases 2013, 72(11):1747–1755. [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB: General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008, 117(6):743–753. [DOI] [PubMed] [Google Scholar]
- 24.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ et al. : 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2014, 63(25 Part B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gooley TA, Leisenring W, Crowley J, Storer BE: Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine 1999, 18(6):695–706. [DOI] [PubMed] [Google Scholar]
- 26.Ungprasert P, Matteson EL, Crowson CS: Reliability of Cardiovascular Risk Calculators to Estimate Accurately the Risk of Cardiovascular Disease in Patients With Sarcoidosis. Am J Cardiol 2017, 120(5):868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veale DJ, Collidge TA, Belch JJ: Increased prevalence of symptomatic macrovascular disease in systemic sclerosis. Annals of the rheumatic diseases 1995, 54(10):853–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho M, Veale D, Eastmond C, Nuki G, Belch J: Macrovascular disease and systemic sclerosis. Annals of the rheumatic diseases 2000, 59(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ungprasert P, Charoenpong P, Ratanasrimetha P, Thongprayoon C, Cheungpasitporn W, Suksaranjit P: Risk of coronary artery disease in patients with systemic sclerosis: a systematic review and meta-analysis. Clinical rheumatology 2014, 33(8):1099–1104. [DOI] [PubMed] [Google Scholar]
- 30.Man A, Zhu Y, Zhang Y, Dubreuil M, Rho YH, Peloquin C, Simms RW, Choi HK: The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Annals of the rheumatic diseases 2013, 72(7):1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S: Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Annals of the rheumatic diseases 2012, 71(12):1980–1983. [DOI] [PubMed] [Google Scholar]
- 32.Crowson CS, Liao KP, Davis Iii JM, Solomon DH, Matteson EL, Knutson KL, Hlatky MA, Gabriel SE: Rheumatoid arthritis and cardiovascular disease. American Heart Journal 2013, 166(4):622–628.e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Tong Q, Guo L, Yu S, Li Y, Cao Q, Li J, Li F: Risk of Coronary Artery Disease in Patients With Systemic Lupus Erythematosus: A Systematic Review and Meta-analysis. The American journal of the medical sciences 2018, 356(5):451–463. [DOI] [PubMed] [Google Scholar]
- 34.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, Canning C, Schneeweiss S: Patterns of cardiovascular risk in rheumatoid arthritis. Annals of the rheumatic diseases 2006, 65(12):1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frostegard J: Atherosclerosis in patients with autoimmune disorders. Arteriosclerosis, thrombosis, and vascular biology 2005, 25(9):1776–1785. [DOI] [PubMed] [Google Scholar]
- 36.Libby P: Inflammation in atherosclerosis. Nature 2002, 420(6917):868–874. [DOI] [PubMed] [Google Scholar]
- 37.Harrison E, Herrick AL, McLaughlin JT, Lal S: Malnutrition in systemic sclerosis. Rheumatology (Oxford, England) 2012, 51(10):1747–1756. [DOI] [PubMed] [Google Scholar]
- 38.Skare T, Culpi M, Yokoo P, Dias M: Gastrointestinal symptoms in scleroderma patients and its influence in body mass index and quality of life. Acta reumatologica portuguesa 2014, 39(3):242–247. [PubMed] [Google Scholar]
- 39.Cooper JA, Sofat R, Humphries SE, Shah T, Hingorani AD, Casas JP, Smeeth L, McCormack V, Tzoulaki I, Deanfield JE et al. : Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. International Journal of Epidemiology 2008, 38(1):217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muangchan C, Harding S, Khimdas S, Bonner A, Group CSR, Baron M, Pope J: Association of C-reactive protein with high disease activity in systemic sclerosis: Results from the Canadian Scleroderma Research Group. Arthritis Care & Research 2012, 64(9):1405–1414. [DOI] [PubMed] [Google Scholar]
- 41.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, Rocca WA: Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012, 41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S: Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. European heart journal 2008, 29(7):932–940. [DOI] [PubMed] [Google Scholar]
- 43.Low TT, Chan SP, Wai SH, Ang Z, Kyu K, Lee KY, Ching A, Comer S, Tan NQP, Thong E et al. : The women’s heart health programme: a pilot trial of sex-specific cardiovascular management. BMC women’s health 2018, 18(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]


