Abstract
We have previously reported social isolation induces anxiety-like behavior, cognition decline, and reduction in brain ATP levels in mice. These changes were ameliorated by treatment with dihydromyricetin (DHM), a compound that positively modulates γ-aminobutyric A (GABAA) receptor. To gain further insight into the subcellular mechanisms underlying these changes, we utilized a social isolation-induced anxiety mouse model and investigated changes in mitochondrial oxidative capacity via the electron transport chain. We found that 4 weeks of social isolation decreased ATP levels by 43% and succinate dehydrogenase capacity by 52% of the control, while daily DHM (2 mg/kg oral) administration restored succinate dehydrogenase capacity. These results suggest that social isolation decreased mitochondrial capacity to generate ATP. DHM can be developed to be a therapeutic against anxiety and mitochondrial stress.
Keywords: Dihydromyricetin, Succinate Dehydrogenase, Complex II, Social Isolation, Anxiety, Stress
1. INTRODUCTION
Stress provokes numerous physiological changes and responses as the body attempts to mitigate the stressor and restore homeostasis. Psychological stressors like social isolation induce responses that are associated with psychiatric disorders such as anxiety1. Anxiety disorders are one of the leading causes of disability in the United States and its prevalence has been on a significant upsurge since the 2019 pandemic and social isolation mandates2–5. Current treatments that aim to alleviate anxious symptoms are ineffective, and this challenge is compounded by the lack of disease mechanism understanding, which hinders effective anxiolytic development3,6. Thus, to develop a safe and effective novel anxiolytic, it is critical to uncover the cellular changes associated with anxiety pathogenesis.
Previously, we demonstrated that 4 weeks of social isolation induced significant anxiety-like behaviors and mild cognitive impairment in wild-type C57BL/6 mice7,8. In the same mice, we found reduction of γ-aminobutyric acid A receptors (GABAAR) function, gephyrin expression, and ATP levels9. Gephyrin is a key ATP-dependent scaffolding protein that assists in proper GABAAR clustering and functioning10. Thus, our findings indicate that social isolation induces anxiety and cognitive impairment via disruption in gephyrin-GABAergic neurotransmission and synapse formation. Dihydromyricetin [(2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one (DHM)], a compound that we have identified as a positive allosteric modulator (PAM) of GABAARs11,12, was able to improve anxiety-like and cognition-related behaviors, GABAAR function, ATP levels, and gephyrin protein levels7–9.
In recent years, the mitochondrion has been gaining research interest as modulators of and therefore a therapeutic target of anxiety13. Stemming from our findings that anxiety caused by social isolation leads to changes in ATP levels9, we questioned the functional involvement of the mitochondria in this mechanism. In the current study, we utilized our social isolation-induced anxiety mouse model to investigate the mechanisms of subcellular (i.e. mitochondrial) responses to DHM and therapeutic effects to restore these changes. ATP is produced via a proton-driven pump, with the proton gradient mainly generated by the electron transport chain (ETC)14–16. Thus, we performed an electron flow assay to determine the ETC functionality in the mitochondria.
2. MATERIALS AND METHODS
2.1. Animals
All animal experiments and sacrifice were performed according to the protocols approved by the University of Southern California (USC) Institutional Animal Care and Use Committee (IACUC). All methods were carried out in compliance with relevant guidelines, regulations, and recommendations, including the ARRIVE guidelines. Six-week-old male C57BL/6 mice (Charles River Laboratories, Hollister, CA) were housed in the vivarium under a 12 h light/dark cycle with free access to food and water.
Social isolation-induced anxiety mouse models were established as described earlier9. In short, 6- to 8-week-old C57BL/6 mice (Charles River Laboratories, Hollister, CA) were housed in the vivarium under a 12 h light/dark cycle with free access to food and water. Two to three mice were housed in each cage for group housing. Social isolation mice were housed singly in opaque cages, except for the top and bottom, to minimize interaction with neighboring cages. We minimally handled the mice and provided no environmental stimuli, such as toys, to prevent confounding stress. The groups were randomly divided as follows for a total of four weeks prior to sacrifice.
Group-housed mice with sucrose agar treatment (G2+Veh2)
Group-housed mice with DHM 2 mg/kg treatment (G2+D2)
Isolated mice with sucrose agar treatment (SI2+Veh2)
Isolated mice with DHM 2 mg/kg treatment (SI2+D2)
In the first two weeks of isolation or group housing, all groups did not receive any oral treatment, and then added daily oral administration of sucrose agar (vehicle) or DHM 2 mg/kg for two weeks of isolation or group housing. Mice were humanely euthanized via carbon dioxide (CO2) exposure followed by rapid decapitation.
2.2. Drug preparation and administration
Sucrose agar and 2 mg/kg DHM (HPLC purified ≥98%, Master Herbs Inc., Pomona, CA) preparation as well as their administration were followed as described earlier7–9. In short, 3% agar was dissolved in ~90°C water, followed by DHM + 5% sucrose or 5% sucrose only until cooled and solidified. One small cube (0.5 cm3) per mouse was administered orally in a small weigh boat, once a day for the last two weeks. Treatments were administered during the dark period of the 12-hour light/dark cycle with minimal disturbance to the animal. Complete consumption of the respective treatment was observed each day.
2.3. Behavioral assays
2.3.1. Elevated Plus Maze (EPM)
The EPM apparatus consists of two open arms and two closed arms surrounded by high opaque walls. The arms intersect perpendicularly with an open central area. The maze is elevated around 20–30 cm and illuminated with red light. Testing started by placing the mouse in the center of the maze facing one of the open arms and behavior recorded for 5 mins. Each mouse was scored offline for time spent in the open arm and closed arm.
2.3.2. Open Field (OF) Test
The OF chamber measured 50 cm (length) × 50 cm (width) × 38 cm (height) and was made from white acrylic plastic. The floor was divided (drawn) into 10 × 10 cm squares for a total of 25 squares. Mouse activity was assessed in open field for 10 min. The following parameters were scored offline: pathlength (cm) traveled in the apparatus and freezing time.
All scoring was conducted manually in a double-blind manner, with each recording being observed three times to minimize error.
2.3. ATP bioluminescence assay
Mouse hippocampus was homogenized in ice-cold Tris-acetate EDTA buffer (0.1 M Tris-acetate + 2 mM EDTA, pH 7.8) using Branson Sonifier 150 (Emerson, St. Louis, MO). The homogenate was centrifuged at 10,000 × g in 4°C for 10 minutes. Supernatant was collected, ice-cold Tris-HCl EDTA buffer (pH 7.8) added, and pH readjusted to 7.8. ATP bioluminescence was measured using 100 μl of supernatant, standards, or water with 100 μl of ATP assay mix, following the ATP luciferin bioluminescence assay kit according to the manufacturer’s protocol (Thermo). Luciferase light production was measured using the Synergy H1 Hybrid Multi-Mode Reader (BioTek). Relative luminescence of three measurements from each group were normalized, percentage was calculated relative to the control, and mean and SEM were calculated.
2.4. Seahorse assay
Methods were adapted from recently published protocols for measuring mitochondrial respiration from previously frozen tissues17,18 as well as Seahorse assay guidelines by Agilent.
2.4.1. Preparation of solutions
Mitochondria isolation buffer (MSHE+BSA) was prepared with 210 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EGTA, and 0.5% (w/v) fatty acid-free BSA and pH was adjusted to 7.4. 1X mitochondria assay solution (MAS) was prepared with 200 mM mannitol, 70 mM sucrose, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA, and 0.2% w/v fatty acid-free BSA and pH was adjusted to 7.4. The buffer used in the 96-well assay plate was 1X MAS supplemented with 10 μg/ml cytochrome c and 10 μg/ml alamethicin (MASCA).
Stock injection solutions were prepared as follows: 10 mM NADH in 1X MAS, 0.5 M succinate in 1X MAS, 4 mM rotenone in DMSO, 2 mM antimycin A in DMSO, 10 mM ascorbate in 1X MAS, 5 mM TMPD in 10 mM ascorbate, and 0.5 M azide in 1X MAS. All stock injection solutions were stored in −20°C except for NADH, ascorbate, and TMPD, which were prepared fresh on the day of the assay. All chemicals used for the Seahorse assay were purchased from Millipore Sigma.
2.4.2. Seahorse XFe96 assay
Mice hippocampi were collected and immediately homogenized in ice-cold MSHE+BSA. The homogenates were centrifuged 800 × g in 4°C for 15 minutes, and supernatants were collected and centrifuged an additional 10,000 × g in 4°C for 15 minutes to isolate the mitochondria. Isolated mitochondria were resuspended in minimal amounts of MSHE+BSH, and protein concentration was determined by BCA Protein Assay kit (Thermo) according to the manufacturer’s protocol. The samples were stored in −80°C until further analysis.
Oxygen consumption rates (OCR) were measured using the Seahorse XFe96 Analyzer (Agilent Technologies, Santa Clara, CA). 10 μg of mitochondria (in 20 μL of MASCA) were seeded per well, except for the blanks in which only MASCA was added, and the microplate was centrifuged at 2,000 × g for 20 minutes. Additional 130 μl of pre-warmed (37°C) MASCA was added to each well. The loaded injection ports were A) 20 μl of 1 mM NADH for complex I or 5 mM succinate + 2 μM rotenone for complex II; B) 22 μl of 4μM antimycin A + 2 μM rotenone; C) 24 μl of 5 mM TMPD + 10 mM ascorbate; and D) 26 μl of 50 mM azide.
Following calibration and equilibration of the XFe96 instrument, customized mix/measure protocol was followed (Table 1). The protocol was designed on Agilent Wave Software.
Table 1.
Seahorse XFe96 protocol
| Action | Time (mins) |
|---|---|
|
| |
| Mix | 0.5 |
| Measure | 4 |
| INJECTION A | |
| Mix | 0.5 |
| Measure | 4 |
| Mix | 0.5 |
| Measure | 4 |
| INJECTION B | |
| Mix | 0.5 |
| Measure | 4 |
| INJECTION C | |
| Mix | 0.5 |
| Measure | 4 |
| Mix | 0.5 |
| Measure | 4 |
| INJECTION D | |
| Mix | 0.5 |
| Measure | 4 |
| Mix | 0.5 |
| Measure | 4 |
Measurements after antimycin A injection represent respiratory activity not produced by the non-mitochondrial respiration of the ETC complexes, while the measurements after azide injection represent any non-mitochondrial respiratory activity. These two measurements serve as correction values for NADH/succinate + rotenone and TMPD + ascorbate measurements, respectively17.
2.5. Western blot
2.5.1. SDS-PAGE gel
Mice hippocampus was homogenized in pre-cooled lysis buffer (RIPA + 1% protease inhibitor) using Branson Digital Sonifier 150. Homogenized samples were centrifuged, and supernatant was collected and quantified using the BCA Protein Assay kit (Thermo) according to the manufacturer’s guidance. 20 μg of hippocampal lysate was loaded into 10% precast polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA) and gel electrophoresis was performed in Mini-PROTEAN (Bio-Rad) under constant voltage.
2.5.2. Transfer and imaging
Proteins were transferred to 0.2 μm PVDF Trans-Blot Turbo membranes (Bio-Rad) using Trans-Blot Turbo (Bio-Rad). Membranes were blocked with 6% (w/v) skim milk (Bio-Rad) in PBST and incubated with primary antibody diluted 1:1000 in PBST + 3% (w/v) BSA overnight at 4°C. Membranes were probed with respective HRP-conjugated secondary antibodies (Bio-Rad 1706515 and 1706516) diluted 1:3000 in PBST + 6% (w/v) skim milk. Blots were detected using Pierce ECL Western Blotting substrate (Thermo) and iBright FL1000 Imager (Thermo). Band density was quantified using FIJI Gel Plugin (NIH). The following primary antibodies were used: rabbit anti-mouse TOM20 mAb (Cell Signaling 42406) and mouse anti-mouse β-actin mAb (Cell Signaling 4970).
2.6. Statistical analysis
The following equations were used to analyze results from the Seahorse assay:
Maximal respiratory capacity (MRC) thru complex I = OCR NADH − OCR antimycin A
MRC through complex II = OCR succinate/rotenone − OCR antimycin A
MRC through complex IV = OCR TMPD/ascorbate − OCR azide
The ratio of MRC through complex I or II to MRC through complex IV was calculated for additional relative measure of respiratory capacity.
Bars in the graphs represent mean ± SEM. Significance for all data obtained was determined by two-way ANOVA with multiple comparisons, Holm-Sidak method. P ≤ 0.05 was considered as significant with the asterisks denoting statistical significance. Data were analyzed with Agilent Wave Software and SigmaPlot V14.5.
3. RESULTS
3.1. Social isolation induces anxiety-like behavior
Consistent with our previous findings, the animals in our social isolation anxiety model exhibited anxiety-like behavior, based on elevated plus maze (EPM) and open field (OF) tests (Fig. 1).
Fig. 1. Anxiety behavioral tests.
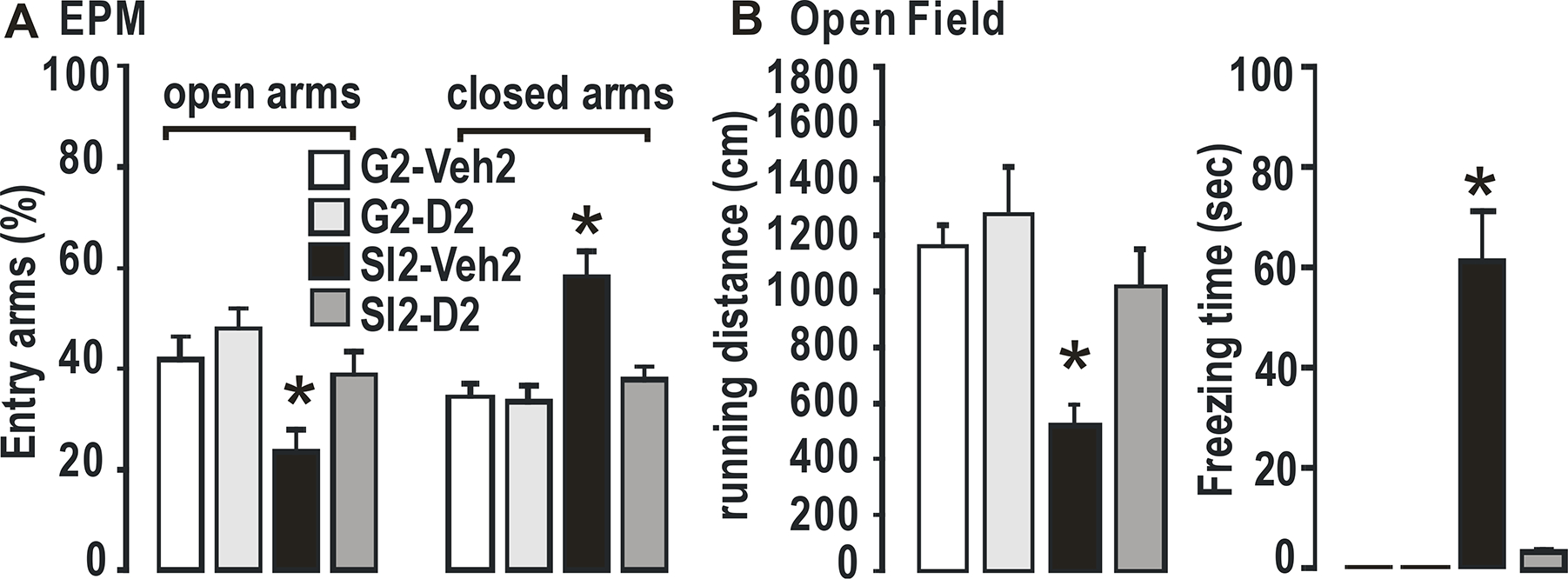
A. Elevated plus maze. B. Open field. N = 4 per group. Two-way ANOVA with multiple comparisons, Holm Sidak method, *, p ≤ 0.01 vs G2+Veh2.
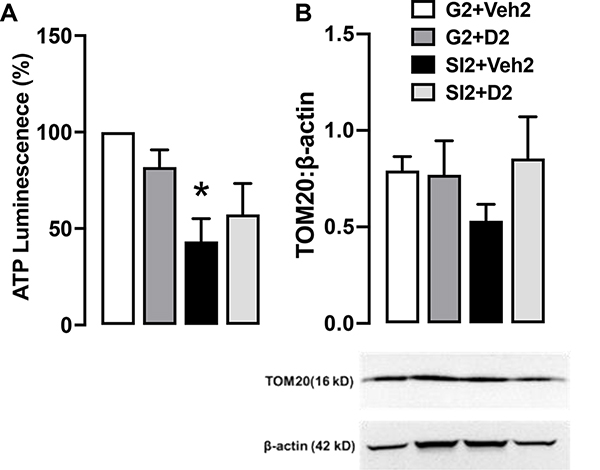
3.2. Social isolation reduces ATP levels
Previous work in our lab has shown a reduction in hippocampal ATP levels in socially-isolated mice and recovery with DHM treatment9. Mice in this present study also displayed lower ATP levels to 43.431% of control (G2+Veh2 group) (Fig. 2A), confirming the effect of social isolation-induced anxiety on ATP levels. We indirectly measured and compared the number of mitochondria across the groups by probing for translocase of the outer membrane (TOM20). Based on this protein, we found no differences in TOM20 expression and therefore no differences in mitochondrial density (Fig. 2B).
Fig. 2. ATP and TOM20.
A. Normalized hippocampal ATP bioluminescence shown as percentage in comparison to the control. N = 3 per group. B. TOM20 blotted on the same gel as β-actin. Blot is shown as different parts of the same gel. Bars show quantification of TOM20 with β-actin as the loading control. N = 4 per group. Two-way ANOVA with multiple comparisons, Holm Sidak method, *, p ≤ 0.01 vs G2+Veh2.
3.3. DHM restores the oxidative capacity of succinate dehydrogenase in socially isolated mice
Next, we utilized an electron flow assay to determine any dysfunctions among the complexes that may be leading to ATP reduction. The electron flow assay protocol was based on findings reported by Acin-Perez, R. (2020) and Osto, C. (2020). Seahorse XF instruments measure oxygen consumption and can therefore indirectly measure ETC activity from complex I (supplied by NADH) or complex II (supplied by succinate/rotenone) through complex IV. By sequentially supplying electrons to each complex of the ETC while inhibiting preceding ones, we can assess their oxidizing ability18.
Maximal respiratory capacity (MRC) through complex II was decreased in isolated mice (15.189 ± 12.806 pmol/min) compared to control (29.310 ± 9.115 pmol/min), and isolated DHM (30.135 ± 10.531) (Fig. 3A, right). These changes were somewhat reflected in the ratio with complex IV, where capacity was decreased in isolated mice (1.243 ± 1.273) compared to control (4.343 ± 4.027, P = 0.064) and isolated DHM (4.613 ± 1.268, P=0.036) (Fig. 3B, right). Based on this assay, there were no functional deficits in complex I (Fig. 3A, B, left).
Fig. 3.
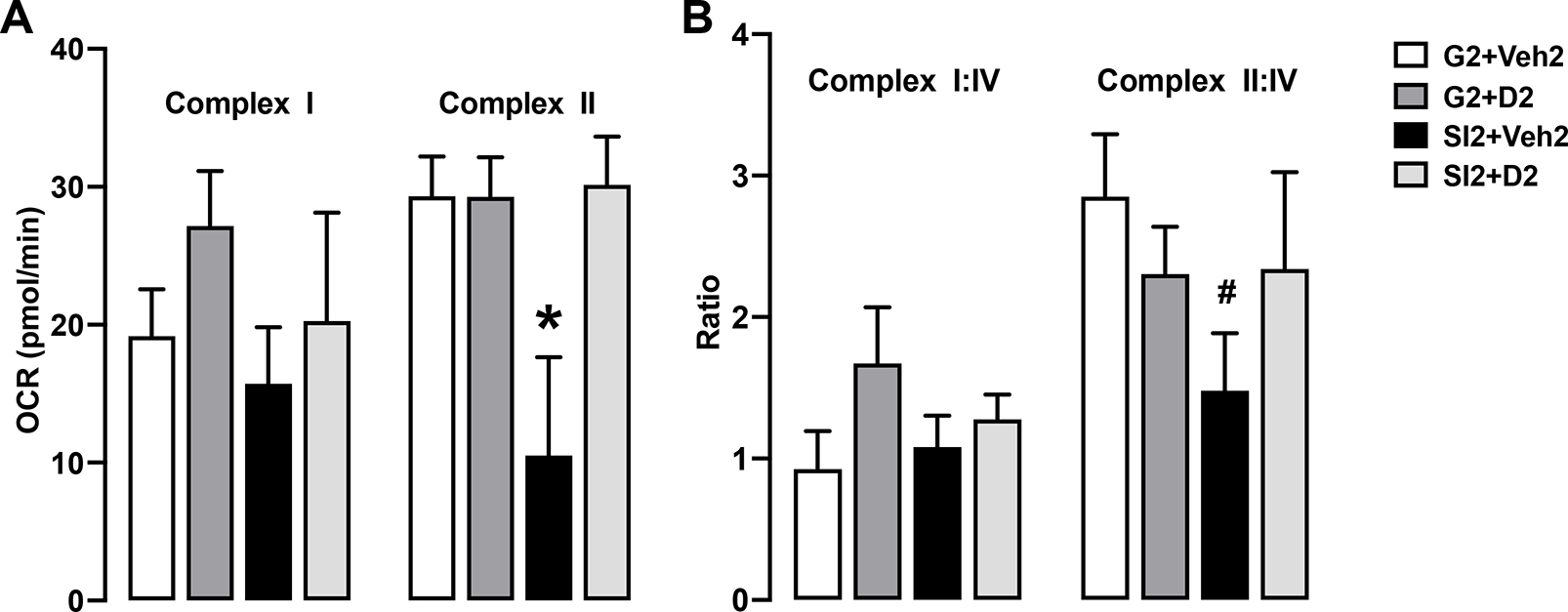
A, B. Maximal respiratory capacity (MRC) of isolated hippocampal mitochondria for complex I and complex II. N = 7 per group. C, D. MCR ratio to complex IV. N = 7 per group. Two-way ANOVA with multiple comparisons, Holm-Sidak method. *, p ≤ 0.01 vs. G2+Veh2; #, p ≤ 0.10 vs G2+Veh2.
4. DISCUSSION
In this study, even with just four weeks of social isolation, we could detect significant reductions in ATP levels and succinate dehydrogenase capacity. ATP bioluminescence assay confirmed that social isolation-induced anxiety mice exhibited reduced ATP (Fig. 2A). This reduction was not due to reduction in mitochondrial density (Fig. 2B), but likely a reduction in mitochondrial function. Relatedly, our present data demonstrate mitochondrial damage in socially isolated mice, with a normal oxidizing function in respiratory chain complex I, but an impairment in complex II (succinate dehydrogenase) (Fig. 3B, C).
Succinate dehydrogenase is a unique enzyme that participates in both the ETC and citric acid cycle16, and therefore plays an important role in ATP production and mitochondrial health19,20. This enzyme is the only membrane-bound component of the citric acid cycle, and is the smallest and only complex that is not involved in directly pumping protons21. In a similar study, high-anxious Wistar rats displayed lower mitochondrial complex I and II levels and respiratory capacity as well as lower ATP levels compared to low-anxious counterparts13. Another study reported the degeneration of succinate dehydrogenase following stress 22. Thus, reduced capability of succinate dehydrogenase is likely to contribute to the reduction in ATP.
Since ATP is involved in many important functions in cells23, its reduction can cause damage at all levels, including proper neurotransmission23. From our previous studies utilizing the same anxiety mouse model, we found a reduction of GABAergic neurotransmission, gephyrin (a scaffolding protein that programs and supports GABAAR clustering) protein levels, and cognitive function7,8. Combined with the present findings, succinate dehydrogenase dysfunction could explain the ATP reduction, which leads to reduced ATP-dependent gephyrin function and therefore reduced GABAAR scaffolding and neurotransmission. Reduced GABAergic neurotransmission then leads to mild cognitive impairment as well as anxiety-like behaviors9.
This study may be subject to some limitations. Future studies will incorporate a larger sample size to increase the confidence of these findings. While DHM recovered ATP levels in our previous study9, the current findings did not show a significant recovery, but it could be seen that the tendency of recovery is consistent with previously discovery. This discrepancy is likely also related to the small sample size, and future studies will verify the effect of DHM on ATP levels.
Additionally, the findings in this study provide only a small snapshot of mitochondrial dysfunction in anxiety induced by social isolation. While we found complex II dysfunction via Seahorse assay, ongoing studies are incorporating other aspects of mitochondrial function and viability to provide a bigger picture of the role of this organelle in the mechanism of anxiety. However, our findings reinforce the notion that social isolation, even short-term, can induce changes in mitochondrial function and that this organelle is a critical player in proper neurotransmission.
5. CONCLUSION
In this report, we present important findings that social isolation induces substantial cellular changes, that cause damage to complex II of the electron transport chain. Decreased mitochondrial function could lead to the disruption of gephyrin-mediated GABAAR clustering, resulting in loss of neurotransmission and cognition decline. Our findings suggest that mitochondrial functions play a critical role in the development of anxiety.
ACKNOWLEDGEMENTS
This continuing research was supported by the National Institute of Health grants AA017991 (to J.L.), University of Southern California Good-Neighbor Foundation (to J.L.), and Army Health Professions Scholarship Program (HPSP) (to A.S.S.), Saudi Arabian Cultural Mission (SACM) (to A.J.A). We would like to thank Dr. Richard W. Olsen, Distinguished Professor at the David Geffen School of Medicine at UCLA, for his comments. We would like to thank Dr. Ronald Irwin at the Department of Pharmacology and Pharmaceutical Sciences at USC for his advice on isolated mitochondrial work. We would also like to thank Dr. Xuesi (Max) Shao at the Department of Neurobiology at UCLA for his comments.
Footnotes
COMPETING INTERESTS
The authors declare no financial interests or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mumtaz F, Khan MI, Zubair M, Dehpour AR. Neurobiology and consequences of social isolation stress in animal model—A comprehensive review. Biomedicine & Pharmacotherapy. 2018-09-01 2018;105:1205–1222. doi: 10.1016/j.biopha.2018.05.086 [DOI] [PubMed] [Google Scholar]
- 2.Kasper S, den Boer J, Ad Sitsen J. Handbook of Depression and Anxiety: A Biological, Approach Handbook of Depression and Anxiety: A Biological, Approach Marcel Dekker Inc., New York: 2003. [Google Scholar]
- 3.Craske MG, Stein MB, Eley TC, et al. Anxiety disorders. Nature Reviews Disease Primers. 2017-12-21 2017;3(1):17024. doi: 10.1038/nrdp.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossain MM, Rahman M, Trisha NF, et al. Prevalence of anxiety and depression in South Asia during COVID-19: A systematic review and meta-analysis. Heliyon. 2021-04-01 2021;7(4):e06677. doi: 10.1016/j.heliyon.2021.e06677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain, Behavior, and Immunity. 2020-08-01 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NIMH. Anxiety Disorders. NIMH; 2018. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml [Google Scholar]
- 7.Watanabe S, Omran AA, Shao AS, et al. Dihydromyricetin improves social isolation-induced cognitive impairments and astrocytic changes in mice. Scientific Reports. 2022/04/07 2022;12(1):5899. doi: 10.1038/s41598-022-09814-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Omran AJ, Shao AS, Watanabe S, et al. Social isolation induces neuroinflammation and microglia overactivation, while dihydromyricetin prevents and improves them. Journal of Neuroinflammation. 2022/01/04 2022;19(1):2. doi: 10.1186/s12974-021-02368-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva J, Shao A, Shen Y, et al. Modulation of Hippocampal GABAergic Neurotransmission and Gephyrin Levels by Dihydromyricetin Improves Anxiety. Front Pharmacol, https://doiorg/103389/fphar202001008. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choii G, Ko J. Gephyrin: a central GABAergic synapse organizer. Experimental & Molecular Medicine. 2015/04/01 2015;47(4):e158–e158. doi: 10.1038/emm.2015.5 [DOI] [PubMed] [Google Scholar]
- 11.Shen Y, Lindemeyer AK, Gonzalez C, et al. Dihydromyricetin As a Novel Anti-Alcohol Intoxication Medication. Journal of Neuroscience. 2012-01-04 2012;32(1):390–401. doi: 10.1523/jneurosci.4639-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang J, Kerstin Lindemeyer A, Shen Y, et al. Dihydromyricetin Ameliorates Behavioral Deficits and Reverses Neuropathology of Transgenic Mouse Models of Alzheimer’s Disease. Neurochemical Research. 2014-06-01 2014;39(6):1171–1181. doi: 10.1007/s11064-014-1304-4 [DOI] [PubMed] [Google Scholar]
- 13.Filiou MD, Sandi C. Anxiety and Brain Mitochondria: A Bidirectional Crosstalk. Trends in Neurosciences. 2019;42(9):573–588. doi: 10.1016/j.tins.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 14.Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nature Cell Biology. 2018-07-01 2018;20(7):745–754. doi: 10.1038/s41556-018-0124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patergnani S, Suski JM, Agnoletto C, et al. Calcium signaling around Mitochondria Associated Membranes (MAMs). Cell Communication and Signaling. 2011-01-01 2011;9(1):19. doi: 10.1186/1478-811x-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutter J, Winge DR, Schiffman JD. Succinate dehydrogenase - Assembly, regulation and role in human disease. Mitochondrion. 2010;10(4):393–401. doi: 10.1016/j.mito.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osto C, Benador IY, Ngo J, et al. Measuring Mitochondrial Respiration in Previously Frozen Biological Samples. Current Protocols in Cell Biology. 2020-12-01 2020;89(1)doi: 10.1002/cpcb.116 [DOI] [PubMed] [Google Scholar]
- 18.Acin-Perez R, Benador IY, Petcherski A, et al. A novel approach to measure mitochondrial respiration in frozen biological samples. The EMBO Journal. 2020-07-01 2020;39(13)doi: 10.15252/embj.2019104073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rustin P, Munnich A, Rötig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. European Journal of Human Genetics. 2002/05/01 2002;10(5):289–291. doi: 10.1038/sj.ejhg.5200793 [DOI] [PubMed] [Google Scholar]
- 20.Jardim-Messeder D, Caverzan A, Rauber R, de Souza Ferreira E, Margis-Pinheiro M, Galina A. Succinate dehydrogenase (mitochondrial complex II) is a source of reactive oxygen species in plants and regulates development and stress responses. 10.1111/nph.13515. New Phytologist. 2015/11/01 2015;208(3):776–789. doi: 10.1111/nph.13515 [DOI] [PubMed] [Google Scholar]
- 21.Moosavi B, Berry EA, Zhu X-L, Yang W-C, Yang G-F. The assembly of succinate dehydrogenase: a key enzyme in bioenergetics. Cellular and Molecular Life Sciences. 2019/10/01 2019;76(20):4023–4042. doi: 10.1007/s00018-019-03200-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchi S, Patergnani S, Missiroli S, et al. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018-01-01 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 23.Patel A, Malinovska L, Saha S, et al. ATP as a biological hydrotrope. Science. 2017-05-19 2017;356(6339):753–756. doi: 10.1126/science.aaf6846 [DOI] [PubMed] [Google Scholar]